Influence of Different Precursors on Properties and Photocatalytic Activity of g-C3N4 Synthesized via Thermal Polymerization
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of g-C3N4 Samples
2.2. Characterization of Prepared g-C3N4 Samples
2.3. Adsorption, Photolytic, and Photocatalytic Testing
2.4. Scavenger Test
3. Results and Discussion
3.1. Results of Characterization of Prepared g-C3N4 Samples
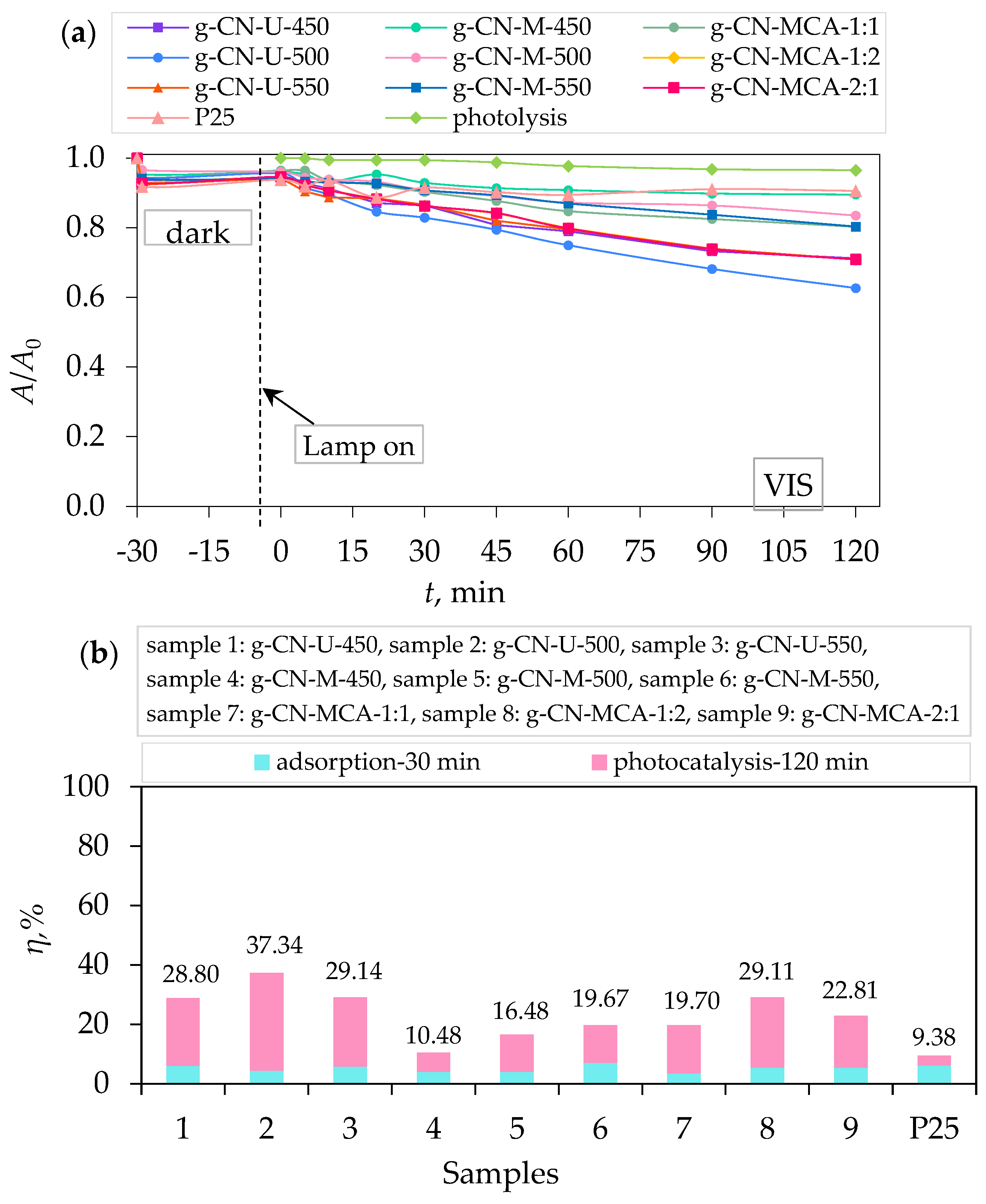
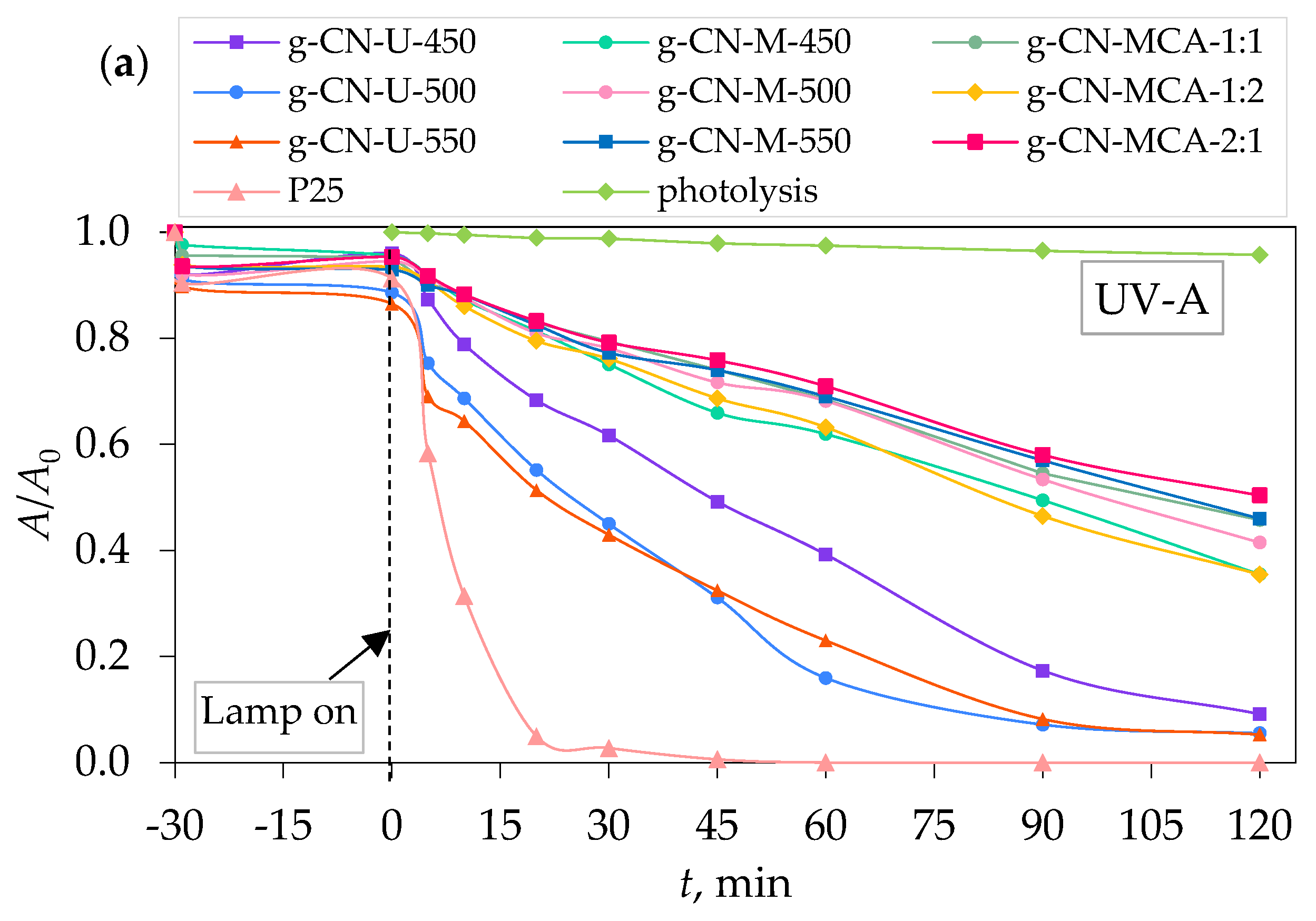
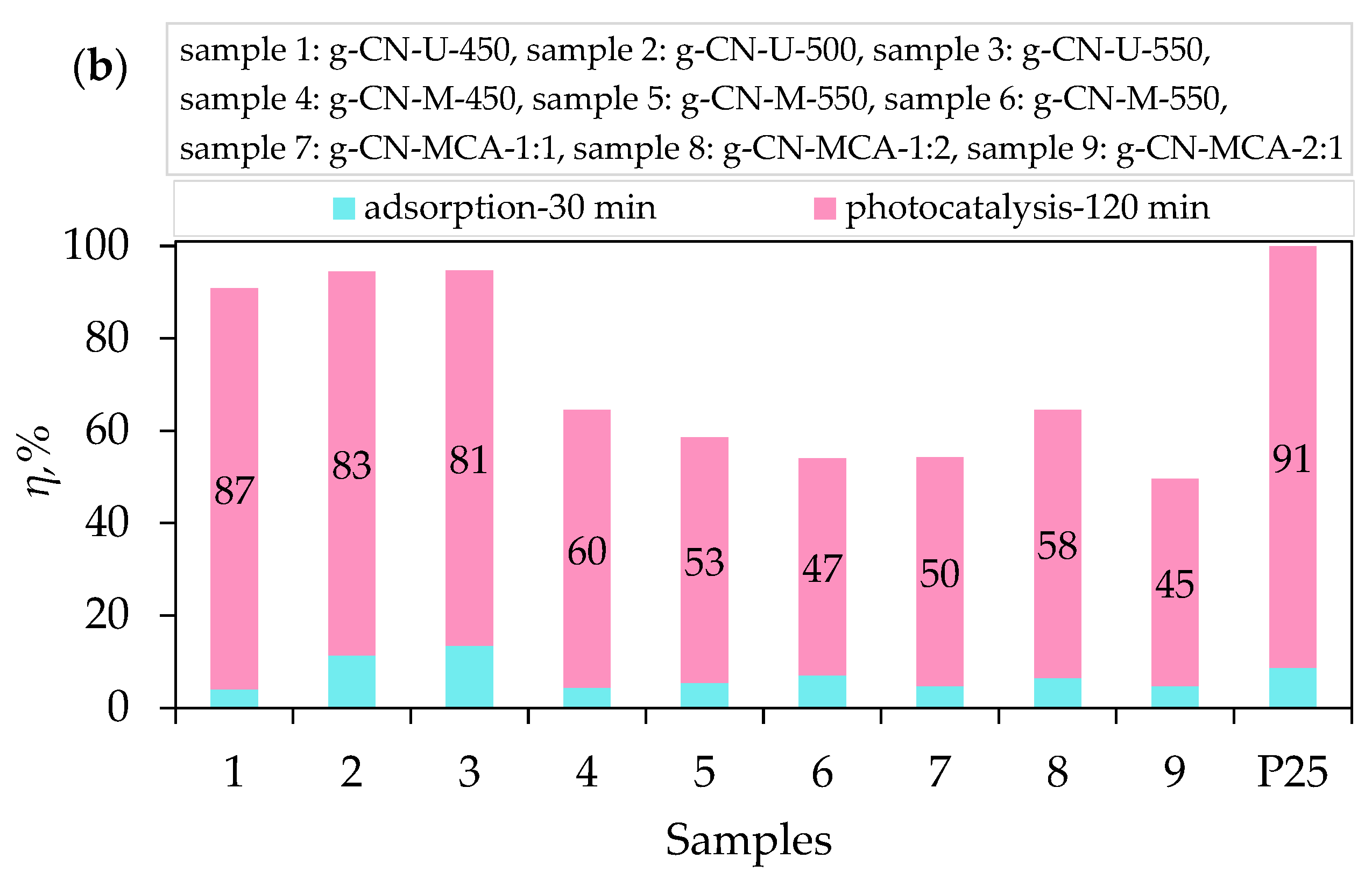
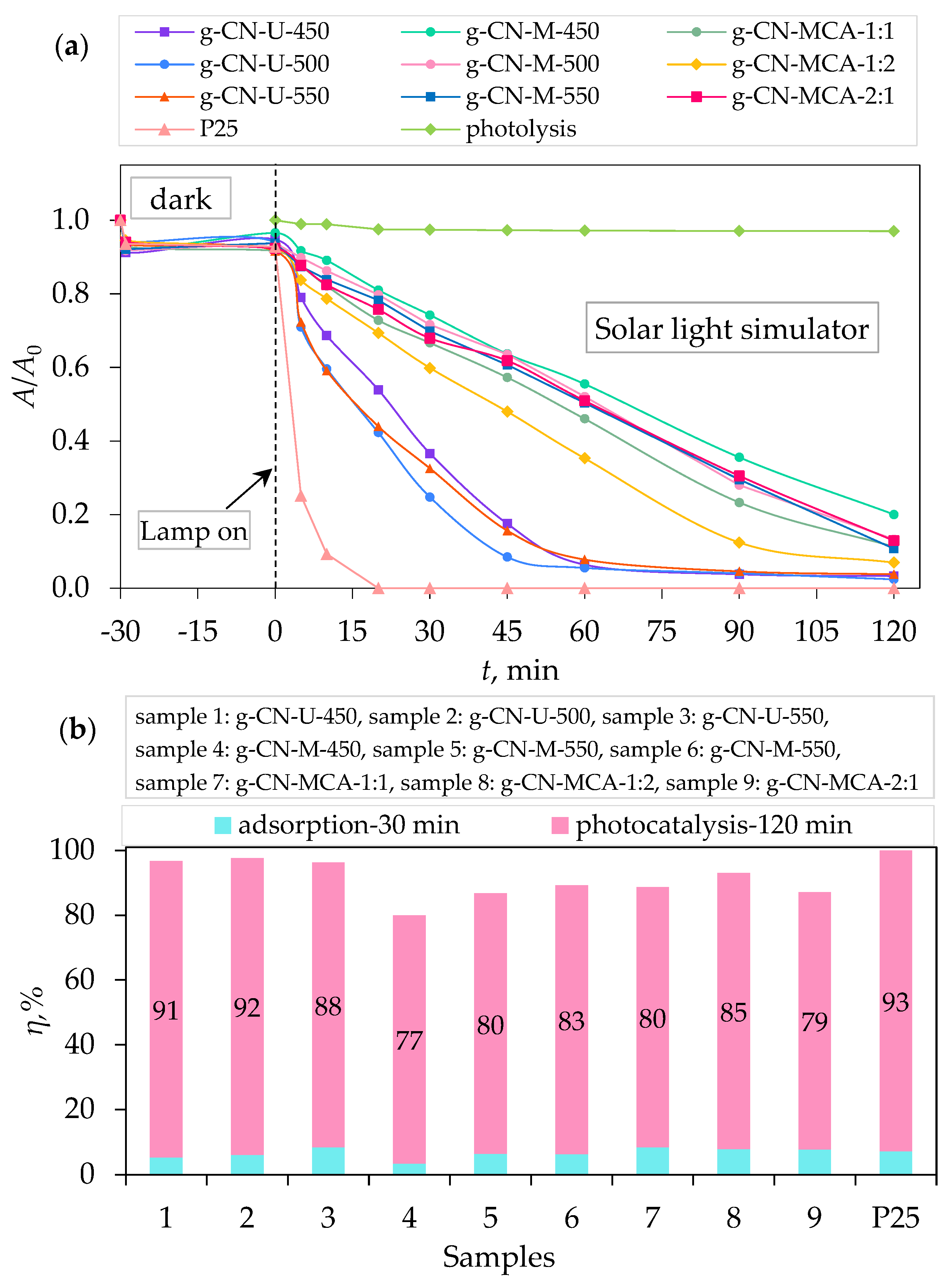
3.2. Adsorption, Photolysis, and Photocatalytic Degradation of Procaine
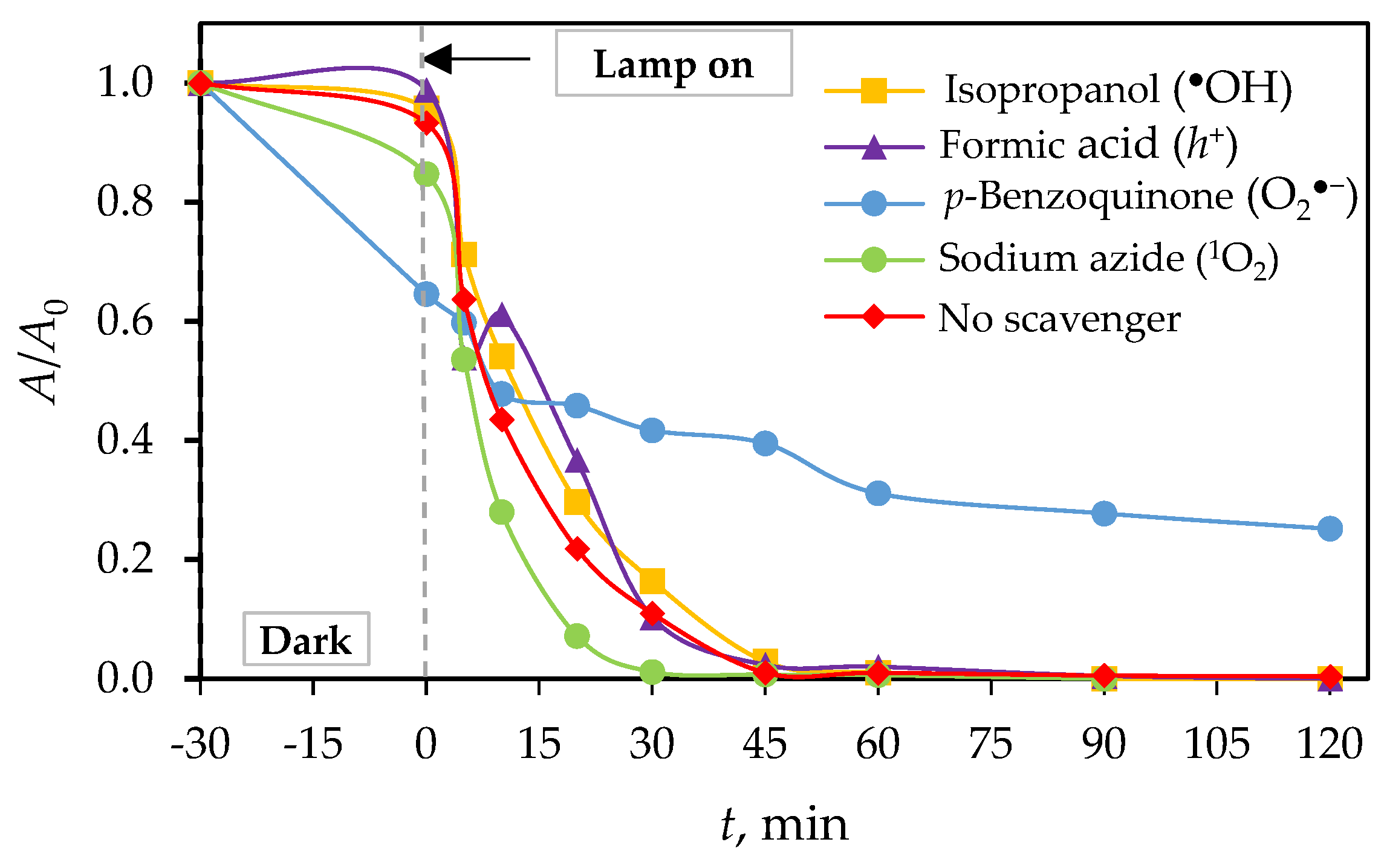
3.3. Photocatalytic Mechanisms of g-C3N4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ecke, F.; Golovko, O.; Hörnfeldt, B.; Ahrens, L. Trophic fate and biomagnification of organic micropollutants from staple food to a specialized predator. Environ. Res. 2024, 261, 119686. [Google Scholar] [CrossRef]
- El Hammoudani, Y.; Haboubi, K.; Bourjila, A.; Achoukhi, I.; Benaissa, C.; Faiz, H.; Touzani, A.; Moudou, M.; Esskifati, M.; El Boudammoussi, M.; et al. Assessing the impact of organic and inorganic micropollutants released from a wastewater treatment plant on humans and aquatic environment, Al-Hoceima city, Morocco. Toxicol. Rep. 2024, 13, 101699. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, N.M.P.; Sirés, I.; Zanta, C.L.P.S.; Tonholo, J.; Brillas, E. Removal of the drug procaine from acidic aqueous solutions using a flow reactor with a boron-doped diamond anode. Sep. Purif. Technol. 2019, 216, 65–73. [Google Scholar] [CrossRef]
- Zrnčić, M.; Ljubas, D.; Rozman, M.; Asperger, D.; Ćurković, L.; Babic, S. Photocatalytic Degradation of Azithromycin by Nanostructured TiO2 Film: Kinetics, Degradation Products, and Toxicity. Materials 2019, 12, 873. [Google Scholar] [CrossRef]
- Wudil, Y.S.; Ahmad, U.F.; Gondal, M.A.; Al-Osta, M.A.; Almohammedi, A.; Sa’id, R.S.; Hrahsheh, F.; Haruna, K.; Mohamed, M.J.S. Tuning of graphitic carbon nitride (g-C3N4) for photocatalysis: A critical review. Arab. J. Chem. 2023, 16, 104542. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Joseph, M.; Paulson, F.; Nasrin, C.; Aparna, S.; Remello, S.N.; Haridas, S.; Aravind, U.K. Layer-by-layer assembled graphitic carbon nitride membranes for water treatment. Chemosphere 2024, 353, 141544. [Google Scholar] [CrossRef]
- Singh, R.; Chauhan, M.; Garg, P.; Sharma, B.; Attri, P.; Sharma, R.K.; Sharma, D.; Chaudhary, G.R. A critical review on visible light active graphitic carbon nitride (g-CN) based photocatalyst for environment remediation application: A sustainable approach. J. Clean. Prod. 2023, 427, 138855. [Google Scholar] [CrossRef]
- Sathishkumar, K.; Li, Y.; Alsalhi, M.S.; Muthukumar, B.; Gaurav, G.K.; Devanesan, S.; Rajasekar, A.; Manikandan, R. Enhanced biological nitrate removal by gC3N4/TiO2 composite and role of extracellular polymeric substances. Environ. Res. 2022, 207, 112158. [Google Scholar] [CrossRef]
- Gogoi, D.; Shah, A.K.; Qureshi, M.; Golder, A.K.; Peela, N.R. Silver grafted graphitic-carbon nitride ternary hetero-junction Ag/gC3N4(Urea)-gC3N4(Thiourea) with efficient charge transfer for enhanced visible-light photocatalytic green H2 production. Appl. Surf. Sci. 2021, 558, 149900. [Google Scholar] [CrossRef]
- Iqbal, W.; Dong, C.; Xing, M.; Tan, X.; Zhang, J. Eco-friendly one-pot synthesis of well-adorned mesoporous g-C3N4 with efficiently enhanced visible light photocatalytic activity. Catal. Sci. Technol. 2017, 7, 1726–1734. [Google Scholar] [CrossRef]
- Hwang, S.; Lee, S.; Yu, J.S. Template-directed synthesis of highly ordered nanoporous graphitic carbon nitride through polymerization of cyanamide. Appl. Surf. Sci. 2007, 253, 5656–5659. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, M.; Ye, X.; Qiu, X.; Lin, S.; Wang, X. Iodine Modified Carbon Nitride Semiconductors as Visible Light Photocatalysts for Hydrogen Evolution. Adv. Mater. 2014, 26, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Li, J.; Qiu, X.; Wang, K.; Guo, L. One-step thermal polymerization synthesis of P and K co-doped two-dimensional porous g-C3N4 photocatalyst with enhanced visible light photocatalytic activity for RhB. Mater. Lett. 2024, 370, 136812. [Google Scholar] [CrossRef]
- Li, C.; Cao, C.B.; Zhu, H.S. Graphitic carbon nitride thin films deposited by electrodeposition. Mater. Lett. 2004, 58, 1903–1906. [Google Scholar] [CrossRef]
- Rubesh, A.K.S.; Vasvini, M.D.; Suganya, J.G.A.; Sivasamy, A. Hydrothermally synthesized WO3:CeO2 supported gC3N4 nanolayers for rapid photocatalytic degradation of azo dye under natural sunlight. Inorg. Chem. Commun. 2024, 164, 112366. [Google Scholar] [CrossRef]
- Villabona-Leal, E.G.; Flores-Zuñiga, G.; Pérez-Valverde, M.I.; Negrete-Durán, S.E.; Ojeda-Galván, H.J.; Alanis, J.; Velázquez-Galván, Y.G.; Ocampo-Pérez, R.; Ovando-Medina, V.M.; Navarro-Contreras, H.R.; et al. Facile solvothermal synthesis of Exfoliated-Corrugated g-C3N4@BiOBr heterojunction for fast visible light Photocatalyst: A structural and optical study. Appl. Surf. Sci. 2024, 642, 158506. [Google Scholar] [CrossRef]
- Torres-Pinto, A.; Silva, C.G.; Faria, J.L.; Silva, A.M.T. The effect of precursor selection on the microwave-assisted synthesis of graphitic carbon nitride. Catal. Today 2023, 424, 113868. [Google Scholar] [CrossRef]
- Pattanayak, D.S.; Surana, M.; Kumar, A.; Singh, D.; Pal, D. Graphitic carbon nitride(g-C3N4)-based photocatalysts for dye removal: Current status. Sustain. Chem. Environ. 2024, 7, 100141. [Google Scholar] [CrossRef]
- Raizada, P.; Sudhaik, A.; Singh, P.; Hosseini-Bandegharaei, A.; Gupta, V.K.; Agarwal, S. Silver-mediated Bi2O3 and graphitic carbon nitride nanocomposite as all solid state Z scheme photocatalyst for imidacloprid pesticide abatement from water. Desalin. Water Treat. 2019, 171, 344–355. [Google Scholar] [CrossRef]
- Zandipak, R.; Bahramifar, N.; Torabi, M.; Calero, M.; Muñoz-Batista, M.J.; Solís, R.R. Synergistic effect of graphitic-like carbon nitride and sulfur-based thiazole-linked organic polymer heterostructures for boosting the photocatalytic degradation of pharmaceuticals in water. Chem. Eng. J. 2024, 494, 152843. [Google Scholar] [CrossRef]
- Miao, Z.; Wu, G.; Wang, Q.; Yang, J.; Wang, Z.; Yan, P.; Sun, P.; Lei, Y.; Mo, Z.; Xu, H. Recent advances in graphitic carbon nitride-based photocatalysts for solar-driven hydrogen production. Mater. Rep. Energy 2023, 3, 100235. [Google Scholar] [CrossRef]
- do Carmo Batista, W.V.F.; Coelho, J.F.; de Oliveira, W.L.; Pereira Filho, N.G.; de Oliveira, E.F.; da Cruz, T.D.S.; da Silva, H.S.T.; Marques, G.N.; de Mesquita, J.P.; de Souza, R.F.; et al. Nickel supported on polymeric graphitic carbon nitride for electrocatalytic in reduction of carbon dioxide. J. CO2 Util. 2023, 77, 102614. [Google Scholar] [CrossRef]
- Ding, R.; Cao, S.; Chen, H.; Jiang, F.; Wang, X. Preparation of tellurium doped graphitic carbon nitride and its visible-light photocatalytic performance on nitrogen fixation. Colloids Surf. A Physicochem. Eng. Asp. 2019, 563, 263–270. [Google Scholar] [CrossRef]
- Vasu, D.; Meenakshi, G.A.; Akila, B.; You, Y.F.; Pichumani, M.; Chiu, T.W. Heterogeneous single-atom doped 2D-layered graphitic carbon nitride electrocatalyst for oxygen evolution reaction and removal of toxic heavy metal ion Cr from Wastewater. Mater. Res. Bull. 2024, 170, 112597. [Google Scholar] [CrossRef]
- Wang, F.; Li, W.; Zhang, W.; Ye, R.; Tan, X. Facile fabrication of the Ag nanoparticles decorated graphitic carbon nitride photocatalyst film for indoor air purification under visible light. Build. Environ. 2022, 222, 109402. [Google Scholar] [CrossRef]
- Wei, F.; Liu, Y.; Zhao, F.; Ren, X.; Liu, J.; Hasan, T.; Chen, L.; Li, Y.; Su, B.-L. Oxygen self-doped g-C3N4 with tunable electronic band structure for unprecedentedly enhanced photocatalytic performance. Nanoscale 2018, 10, 4515–4522. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, C.; Bandaru, S.; Pei, L.; Zheng, R.; Hau Ng, Y.; Arenas Esteban, D.; Bals, S.; Zhong, J.; Hofkens, J.; et al. Cs3Bi2Br9 nanoparticles decorated C3N4 nanotubes composite photocatalyst for highly selective oxidation of benzylic alcohol. J. Colloid Interface Sci. 2024, 672, 600. [Google Scholar] [CrossRef]
- Jun, Y.; Lee, E.Z.; Wang, X.; Won, H.H.; Stucky, G.; Arne, T. From Melamine-Cyanuric Acid Supramolecular Aggregates to Carbon Nitride Hollow Spheres. Advanced Functional Materials. Adv. Funct. Mater. 2013, 23, 3661–3667. [Google Scholar] [CrossRef]
- Niu, H.; Zhao, W.; Lv, H.; Yang, Y.; Cai, Y. Accurate design of hollow/tubular porous g-C3N4 from melamine-cyanuric acid supramolecular prepared with mechanochemical method. J. Chem. Eng. 2021, 411, 128400. [Google Scholar] [CrossRef]
- Paul, D.R.; Sharma, R.; Nehra, S.P.; Sharma, A. Effect of calcination temperature, pH and catalyst loading on photodegradation efficiency of urea derived graphitic carbon nitride towards methylene blue dye solution. RSC Adv. 2019, 9, 15381–15391. [Google Scholar] [CrossRef] [PubMed]
- Narkbuakaew, T.; Sujaridworakun, P. Synthesis of Tri-S-Triazine Based g-C3N4 Photocatalyst for Cationic Rhodamine B Degradation under Visible Light. Top. Catal. 2020, 63, 1086–1096. [Google Scholar] [CrossRef]
- Alaghmandfard, A.; Ghandi, K. A Comprehensive Review of Graphitic Carbon Nitride (g-C3N4)–Metal Oxide-Based Nanocomposites: Potential for Photocatalysis and Sensing. Nanomaterials 2022, 12, 294. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.; She, X.; Li, Y.; Liu, L.; Huang, L.; Chen, Z.; Zhang, Q.; Xu, H.; Li, H. Synthesis of g-C3N4 at different temperatures for superior visible/UV photocatalytic performance and photoelectrochemical sensing of MB solution. RSC Adv. 2015, 5, 101552–101562. [Google Scholar] [CrossRef]
- Vu, N.N.; Nguyen, C.; Kaliaguine, S. Synthesis of g-C3N4 Nanosheets by Using a Highly Condensed Lamellar Crystalline Melamine–Cyanuric Acid Supramolecular Complex for Enhanced Solar Hydrogen Generation. Chem. Sus. Chem. 2018, 12, 291–302. [Google Scholar] [CrossRef]
- Xu, H.-Y.; Wu, L.-C.; Zhao, H.; Jin, L.-G.; Qi, S.-Y. Synergic Effect between Adsorption and Photocatalysis of Metal-Free g-C3N4 Derived from Different Precursors. PLoS ONE 2015, 10, e0142616. [Google Scholar] [CrossRef]
- Praus, P.; Smýkalová, A.; Foniok, K. Comparison of Graphitic Carbon Nitrides Synthetized from Melamine and Melamine-Cyanurate Complex: Characterization and Photocatalytic Decomposition of Ofloxacin and Ampicillin. Materials 2021, 14, 1967. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.; Olivier, J.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Thommes, M. Physical Adsorption Characterization of Nanoporous Materials. Chem. Ing. Technik. 2010, 82, 1059–1073. [Google Scholar] [CrossRef]
- Landers, J.; Gor, G.Y.; Neimark, A.V. Density functional theory methods for characterization of porous materials. Colloids Surf. A Physicochem. Eng. Asp. 2013, 437, 3–32. [Google Scholar] [CrossRef]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density, 1st ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; Volume 16. [Google Scholar]
- Ćurković, L.; Ljubas, D.; Juretić, H. Photocatalytic decolorization kinetics of diazo dye Congo Red aqueous solution by UV/TiO2 nanoparticles. React. Kinet. Mech. Catal. 2010, 99, 201–208. [Google Scholar] [CrossRef]
- Praus, P.; Svoboda, L.; Ritz, M.; Troppová, I.; Šihor, M.; Kočí, K. Graphitic carbon nitride: Synthesis, characterization and photocatalytic decomposition of nitrous oxide. Mater. Chem. Phys. 2017, 193, 438–446. [Google Scholar] [CrossRef]
- He, Y.; Sutton, N.B.; Rijnaarts, H.H.H.; Langenhoff, A.A.M. Degradation of pharmaceuticals in wastewater using immobilized TiO2 photocatalysis under simulated solar irradiation. Appl. Catal. B. 2016, 182, 132–141. [Google Scholar] [CrossRef]
- Trawiński, J.; Skibiński, R. Rapid degradation of clozapine by heterogeneous photocatalysis. Comparison with direct photolysis, kinetics, identification of transformation products and scavenger study. Sci. Total Environ. 2019, 665, 557–567. [Google Scholar] [CrossRef]
- Evgenidou, E.; Chatzisalata, Z.; Tsevis, A.; Bourikas, K.; Torounidou, P.; Sergelidis, D.; Koltsakidou, A.; Lambropoulou, D.A. Photocatalytic degradation of a mixture of eight antibiotics using Cu-modified TiO2 photocatalysts: Kinetics, mineralization, antimicrobial activity elimination and disinfection. J. Environ. Chem. Eng. 2021, 9, 105295. [Google Scholar] [CrossRef]
- Rodríguez, E.M.; Márquez, G.; Tena, M.; Álvarez, P.M.; Beltrán, F.J. Determination of main species involved in the first steps of TiO2 photocatalytic degradation of organics with the use of scavengers: The case of ofloxacin. Appl. Catal. B 2015, 178, 44–53. [Google Scholar] [CrossRef]
- Nair, N.; Vimal, G.; Shukla, A.; Ghotekar, S.; Nguyen, V.H.; Varma, K. Mechanisms in the photocatalytic breakdown of persistent pharmaceutical and pesticide molecules over TiO2-based photocatalysts: A review. J. Phys. Condens. Matter. 2024, 36, 413003. [Google Scholar] [CrossRef]
- Ye, Y.; Feng, Y.; Bruning, H.; Yntema, D.; Rijnaarts, H.H.M. Photocatalytic degradation of metoprolol by TiO2 nanotube arrays and UV-LED: Effects of catalyst properties, operational parameters, commonly present water constituents, and photo-induced reactive species. Appl. Catal. B 2018, 220, 171–181. [Google Scholar] [CrossRef]
- Bairamis, F.; Konstantinou, I.; Petrakis, D.; Vaimakis, T. Enhanced Performance of Electrospun Nanofibrous TiO2/g-C3N4 Photocatalyst in Photocatalytic Degradation of Methylene Blue. Catalysts 2019, 9, 880. [Google Scholar] [CrossRef]
- Antonopoulou, M.; Papadaki, M.; Rapti, I.; Konstantinou, I. Photocatalytic Degradation of Pharmaceutical Amisulpride Using g-C3N4 Catalyst and UV-A Irradiation. Catalysts 2023, 13, 226. [Google Scholar] [CrossRef]
- Fónagy, O.; Szabó-Bárdos, E.; Horváth, O. 1,4-Benzoquinone and 1,4-hydroquinone based determination of electron and superoxide radical formed in heterogeneous photocatalytic systems. J. Photochem. Photobiol. A Chem. 2021, 407, 113057. [Google Scholar] [CrossRef]
- Martín-Gómez, J.; Pérez-Losada, M.; López-Tenllado, F.J.; Hidalgo-Carrillo, J.; Herrera-Beurnio, M.C.; Estévez, R.; Marinas, A.; Urbano, F.J. Insight into the reaction mechanism of photocatalytic production of solketal. Catal. Today 2024, 429, 114506. [Google Scholar] [CrossRef]
- Jiménez-Salcedo, M.; Monge, M.; Tena, M.T. The photocatalytic degradation of sodium diclofenac in different water matrices using g-C3N4 nanosheets: A study of the intermediate by-products and mechanism. J. Environ. Chem. Eng. 2021, 9, 105827. [Google Scholar] [CrossRef]
- Zhao, Y.; Shi, H.; Yang, D.; Fan, J.; Hu, X.; Liu, E. Fabrication of a Sb2MoO6/g-C3N4 Photocatalyst for Enhanced RhB Degradation and H2 Generation. J. Phys. Chem. C 2020, 124, 13771–13778. [Google Scholar] [CrossRef]
- Van, K.N.; Huu, H.T.; Thi, V.N.N.; Thi, T.L.L.; Truong, D.H.; Truong, T.T.; Dao, N.N.; Vo, V.; Tran, D.L.; Vasseghian, Y. Facile construction of S-scheme SnO2/g-C3N4 photocatalyst for improved photoactivity. Chemosphere 2022, 289, 133120. [Google Scholar] [CrossRef]
- Ge, L. Synthesis and photocatalytic performance of novel metal-free g-C3N4 photocatalysts. Mater. Lett. 2011, 65, 2652–2654. [Google Scholar] [CrossRef]
- Chidhambaram, N.; Ravichandran, K. Single step transformation of urea into metal-free g-C3N4 nanoflakes for visible light photocatalytic applications. Mater. Lett. 2017, 207, 44–48. [Google Scholar] [CrossRef]
- Yang, Y.; Geng, L.; Guo, Y.; Meng, J.; Guo, Y. Easy dispersion and excellent visible-light photocatalytic activity of the ultrathin urea-derived g-C3N4 nanosheets. Appl. Surf. Sci. 2017, 425, 535–546. [Google Scholar] [CrossRef]
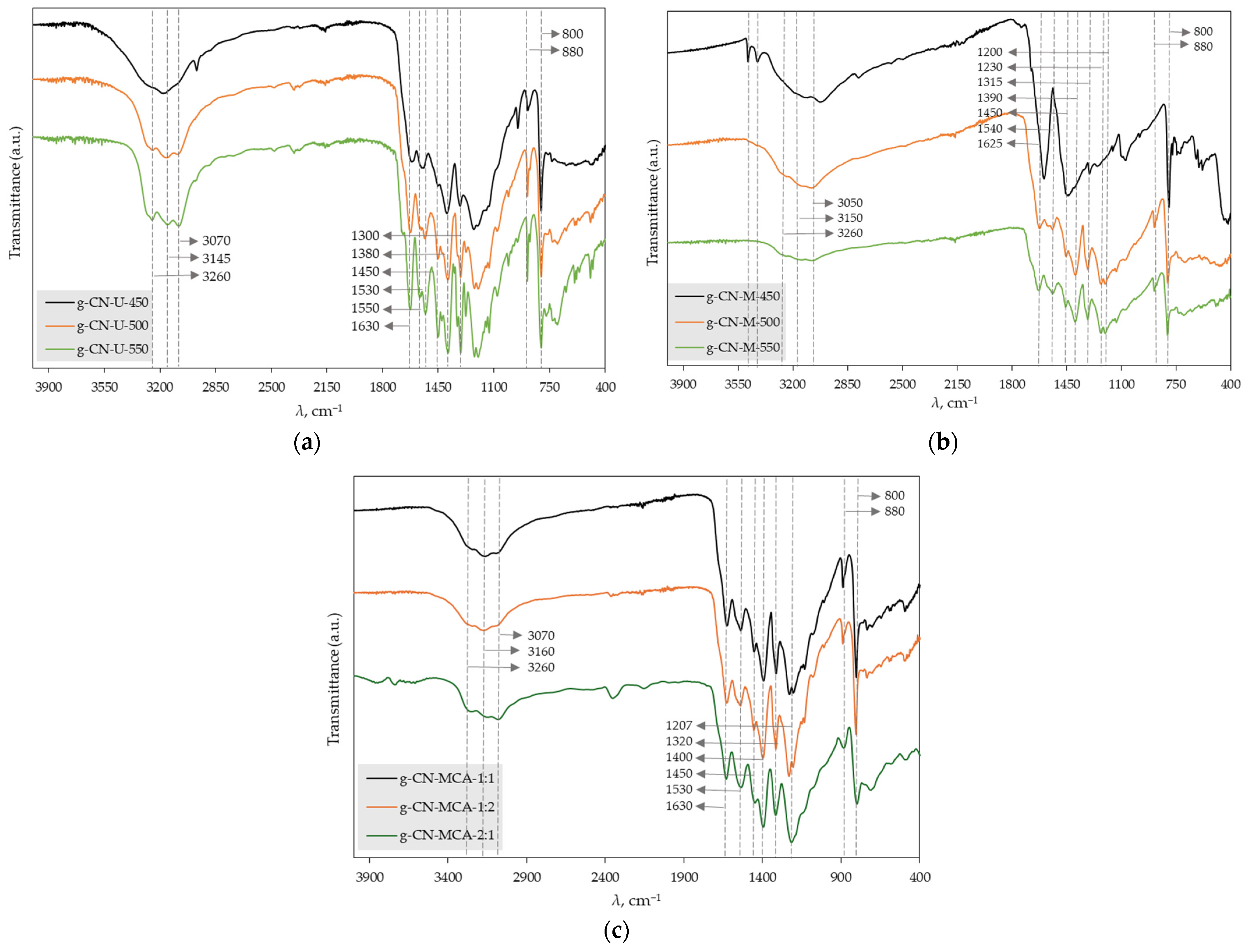
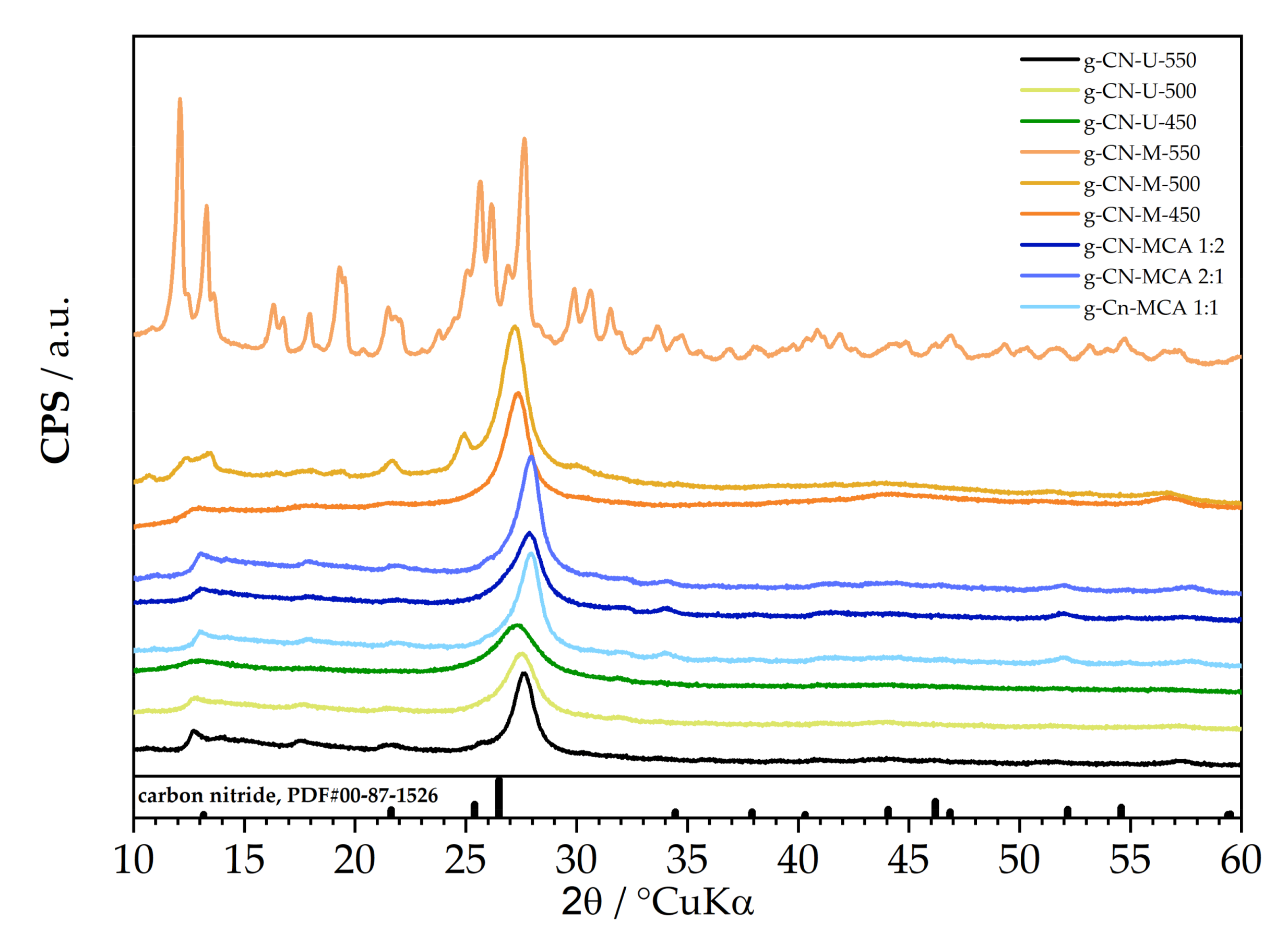


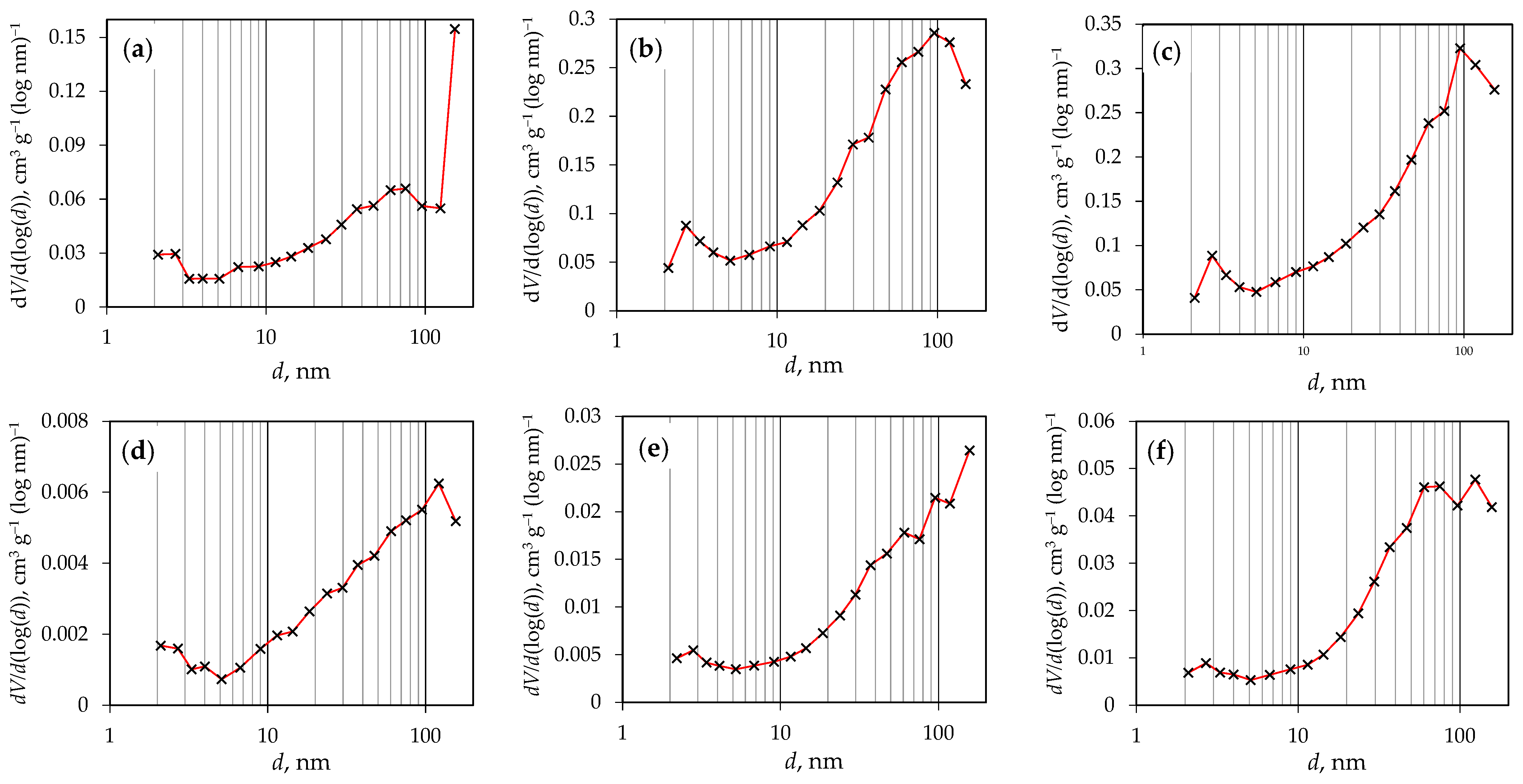
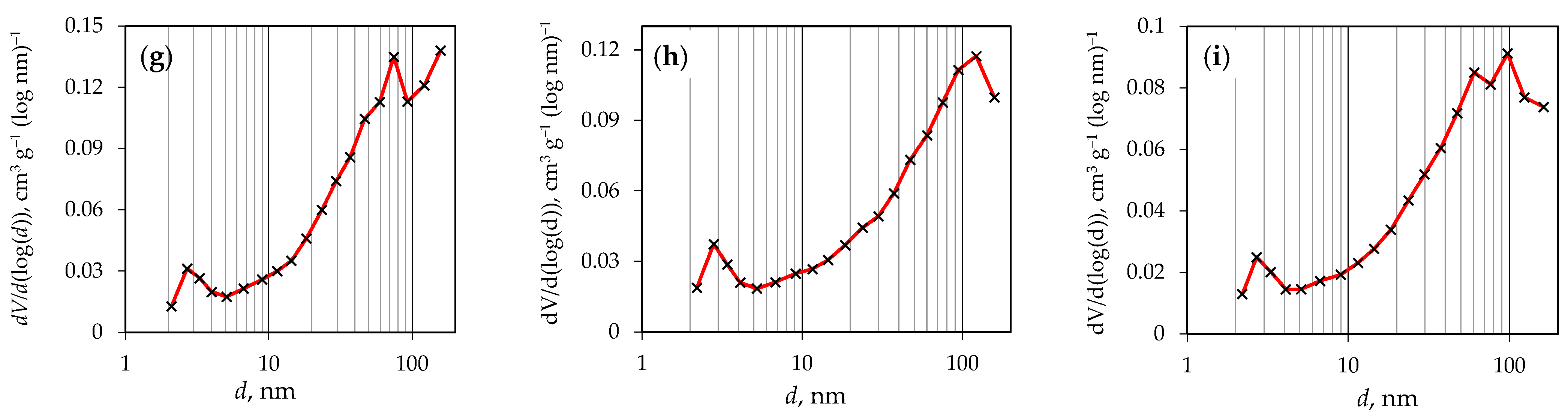
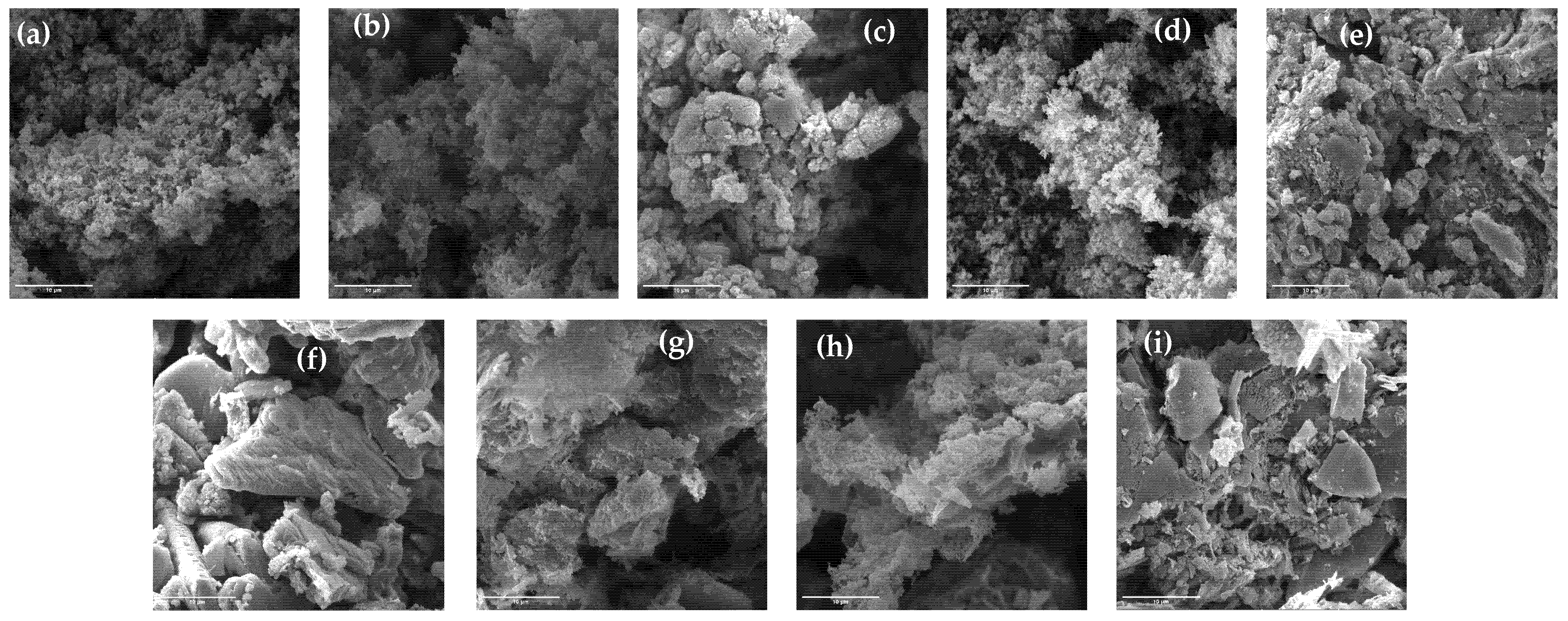
| Precursor | Sample Name | T, °C | t, min |
|---|---|---|---|
| Urea | g-CN-U-450 (1) | 450 | 120 min, 3 °C min−1 |
| g-CN-U-500 (2) | 500 | ||
| g-CN-U-550 (3) | 550 | ||
| Melamine | g-CN-M-450 (4) | 450 | |
| g-CN-M-500 (5) | 500 | ||
| g-CN-M-550 (6) | 550 | ||
| Melamine + cyanuric acid 1:1 | g-CN-MCA-1:1 (7) | 550 | |
| Melamine + cyanuric acid 1:2 | g-CN-MCA-1:2 (8) | 550 | |
| Melamine + cyanuric acid 2:1 | g-CN-MCA-2:1 (9) | 550 |
| g-C3N4 Sample | Indirect Band Gap (Eg, eV) |
|---|---|
| g-CN-U-450 | 2.81 |
| g-CN-U-500 | 2.81 |
| g-CN-U-550 | 2.88 |
| g-CN-M-450 | 2.82 |
| g-CN-M-500 | 2.72 |
| g-CN-M-550 | 2.66 |
| g-CN-MCA-1:1 | 2.69 |
| g-CN-MCA-1:2 | 2.72 |
| g-CN-MCA-2:1 | 2.65 |
| g-C3N4 Sample | Specific Surface Area | Average Pore Diameter |
|---|---|---|
| g-CN-U-450 | 36.0 | 8.0 |
| g-CN-U-500 | 73.7 | 13.3 |
| g-CN-U-550 | 74.2 | 13.0 |
| g-CN-M-450 | 2.1 | 10.1 |
| g-CN-M-500 | 4.7 | 14.4 |
| g-CN-M-550 | 8.3 | 17.5 |
| g-CN-MCA-1:1 | 24.8 | 16.4 |
| g-CN-MCA-1:2 | 25.1 | 13.7 |
| g-CN-MCA-2:1 | 19.1 | 16.2 |
| P25 1 | 48.1 [42] | 13.7 [42] |
| g-C3N4 Sample | k1, min−1 | t1/2, min | R2 |
|---|---|---|---|
| g-CN-U-450 | 0.0027 | 256.72 | 0.9797 |
| g-CN-U-500 | 0.0330 | 21.00 | 0.9930 |
| g-CN-U-550 | 0.0024 | 288.81 | 0.9836 |
| g-CN-M-450 | 0.0006 | 1155.25 | 0.7594 |
| g-CN-M-500 | 0.0017 | 407.73 | 0.9735 |
| g-CN-M-550 | 0.0011 | 630.13 | 0.9149 |
| g-CN-MCA-1:1 | 0.0014 | 495.11 | 0.9908 |
| g-CN-MCA-1:2 | 0.0026 | 266.60 | 0.9928 |
| g-CN-MCA-2:1 | 0.0017 | 407.73 | 0.9289 |
| P25 | 0.0002 | 3465.74 | 0.0631 |
| g-C3N4 Sample | k1, min−1 | t1/2, min | R2 |
|---|---|---|---|
| g-CN-U-450 | 0.0185 | 37.47 | 0.9645 |
| g-CN-U-500 | 0.0291 | 23.82 | 0.9886 |
| g-CN-U-550 | 0.0251 | 27.62 | 0.9774 |
| g-CN-M-450 | 0.0071 | 97.63 | 0.9949 |
| g-CN-M-500 | 0.0059 | 117.48 | 0.9807 |
| g-CN-M-550 | 0.0052 | 133.30 | 0.9878 |
| g-CN-MCA-1:1 | 0.0058 | 119.51 | 0.9862 |
| g-CN-MCA-1:2 | 0.0074 | 93.67 | 0.9803 |
| g-CN-MCA-2:1 | 0.0050 | 138.63 | 0.9813 |
| P25 | 0.0002 | 5.48 | 0.9698 |
| g-C3N4 Sample | k1, min−1 | t1/2, min | R2 |
|---|---|---|---|
| g-CN-U-450 | 0.0396 | 17.50 | 0.9611 |
| g-CN-U-500 | 0.0363 | 19.09 | 0.9006 |
| g-CN-U-550 | 0.0341 | 20.33 | 0.9698 |
| g-CN-M-450 | 0.0113 | 61.34 | 0.9858 |
| g-CN-M-500 | 0.0137 | 50.59 | 0.9557 |
| g-CN-M-550 | 0.0129 | 53.73 | 0.9741 |
| g-CN-MCA-1:1 | 0.0153 | 45.30 | 0.9591 |
| g-CN-MCA-1:2 | 0.0226 | 30.67 | 0.9498 |
| g-CN-MCA-2:1 | 0.0121 | 57.28 | 0.9676 |
| P25 | 0.2306 | 3.01 | 0.9942 |
| Scavenger | k, min−1 | t1/2, min | R2 |
|---|---|---|---|
| Isopropanol | 0.0772 | 8.98 | 0.9850 |
| Formic acid | 0.0708 | 9.79 | 0.9506 |
| p-benzoquinone | 0.0076 | 91.20 | 0.9121 |
| Sodium azide | 0.0908 | 7.63 | 0.8986 |
| No scavenger | 0.0836 | 8.29 | 0.9558 |
| Photocatalyst | Pollutant | Pollutant Concentration, mg L−1 | Light Source | Degradation Efficiency, % | Reference |
|---|---|---|---|---|---|
| g-C3N4 (melamine) | diclofenac | 3 | Visible light | 80 | [54] |
| g-C3N4 (melamine) | RhB | 10 | Visible light | 35 | [55] |
| g-C3N4 (melamine) | RhB | - | Visible light | 39.86 | [56] |
| g-C3N4 (melamine) | phenol | 10 | Visible light | 92.5 | [57] |
| g-C3N4 (urea) | MB | 3 | Visible light | 44 | [58] |
| g-C3N4 (urea) | p-nitrophenol | 10 | Visible light | 41 | [59] |
| g-C3N4 (melamine- cyanuric acid 1:1) | sulfamethazine | 10 | Visible light | 14 | [30] |
| g-CN-U-450 | PRO | 10 | Visible light | 28.8 | This work |
| g-CN-U-500 | 37.3 | ||||
| g-CN-U-550 | 29.1 | ||||
| g-CN-M-450 | 10.5 | ||||
| g-CN-M-500 | 16.5 | ||||
| g-CN-M-550 | 19.7 | ||||
| g-CN-MCA-1:1 | 19.7 | ||||
| g-CN-MCA-1:2 | 29.1 | ||||
| g-CN-MCA-2:1 | 22.8 | ||||
| P25 | 9.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Briševac, D.; Gabelica, I.; Radovanović-Perić, F.; Tolić Čop, K.; Matijašić, G.; Ljubas, D.; Ćurković, L. Influence of Different Precursors on Properties and Photocatalytic Activity of g-C3N4 Synthesized via Thermal Polymerization. Materials 2025, 18, 2522. https://doi.org/10.3390/ma18112522
Briševac D, Gabelica I, Radovanović-Perić F, Tolić Čop K, Matijašić G, Ljubas D, Ćurković L. Influence of Different Precursors on Properties and Photocatalytic Activity of g-C3N4 Synthesized via Thermal Polymerization. Materials. 2025; 18(11):2522. https://doi.org/10.3390/ma18112522
Chicago/Turabian StyleBriševac, Debora, Ivana Gabelica, Floren Radovanović-Perić, Kristina Tolić Čop, Gordana Matijašić, Davor Ljubas, and Lidija Ćurković. 2025. "Influence of Different Precursors on Properties and Photocatalytic Activity of g-C3N4 Synthesized via Thermal Polymerization" Materials 18, no. 11: 2522. https://doi.org/10.3390/ma18112522
APA StyleBriševac, D., Gabelica, I., Radovanović-Perić, F., Tolić Čop, K., Matijašić, G., Ljubas, D., & Ćurković, L. (2025). Influence of Different Precursors on Properties and Photocatalytic Activity of g-C3N4 Synthesized via Thermal Polymerization. Materials, 18(11), 2522. https://doi.org/10.3390/ma18112522







