Effect of Brazing Temperature and Holding Time on the Interfacial Microstructure and Properties of TC4-Brazed Joints with Ti-Zr-Cu-Ni Amorphous Filler
Abstract
1. Introduction
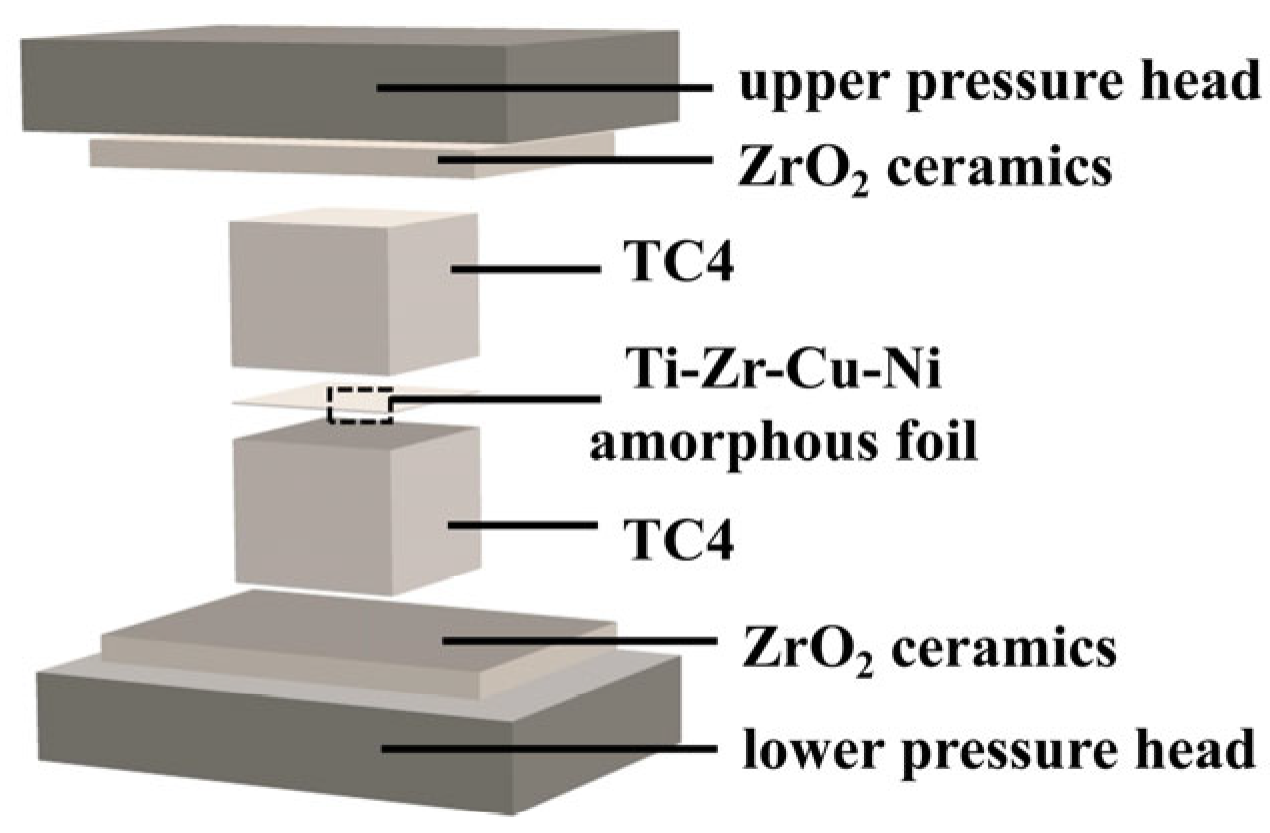
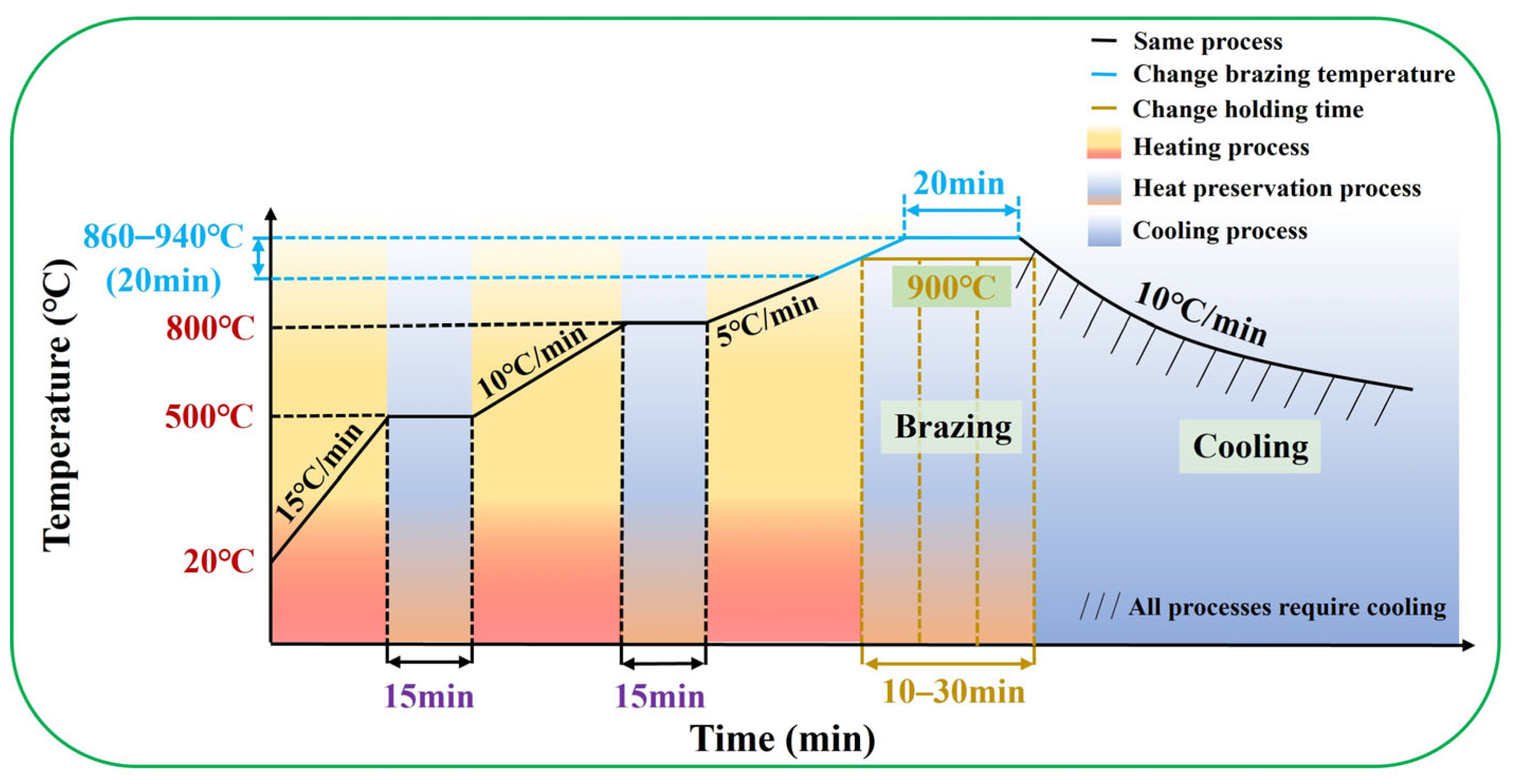
2. Materials and Methods
3. Results and Discussion
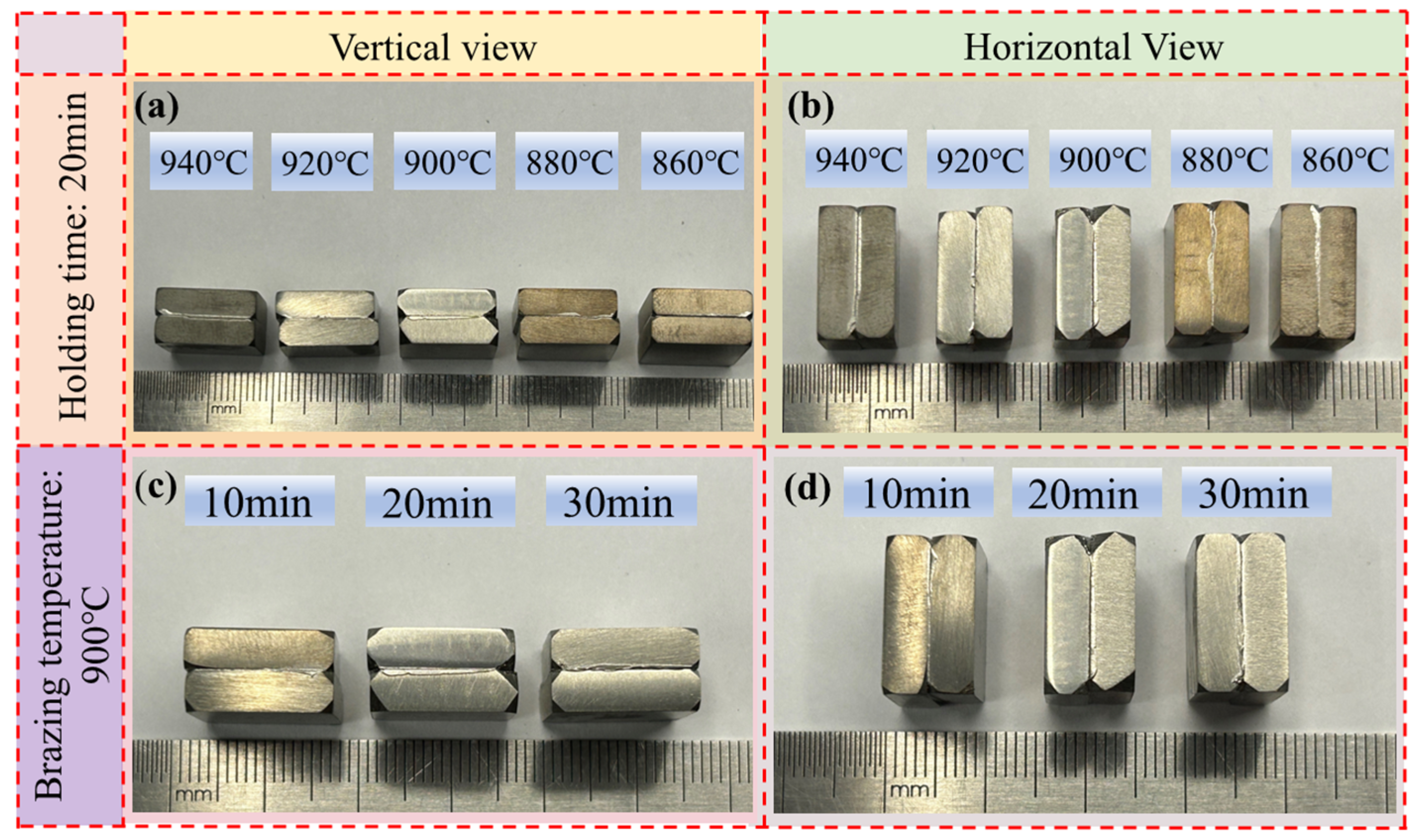
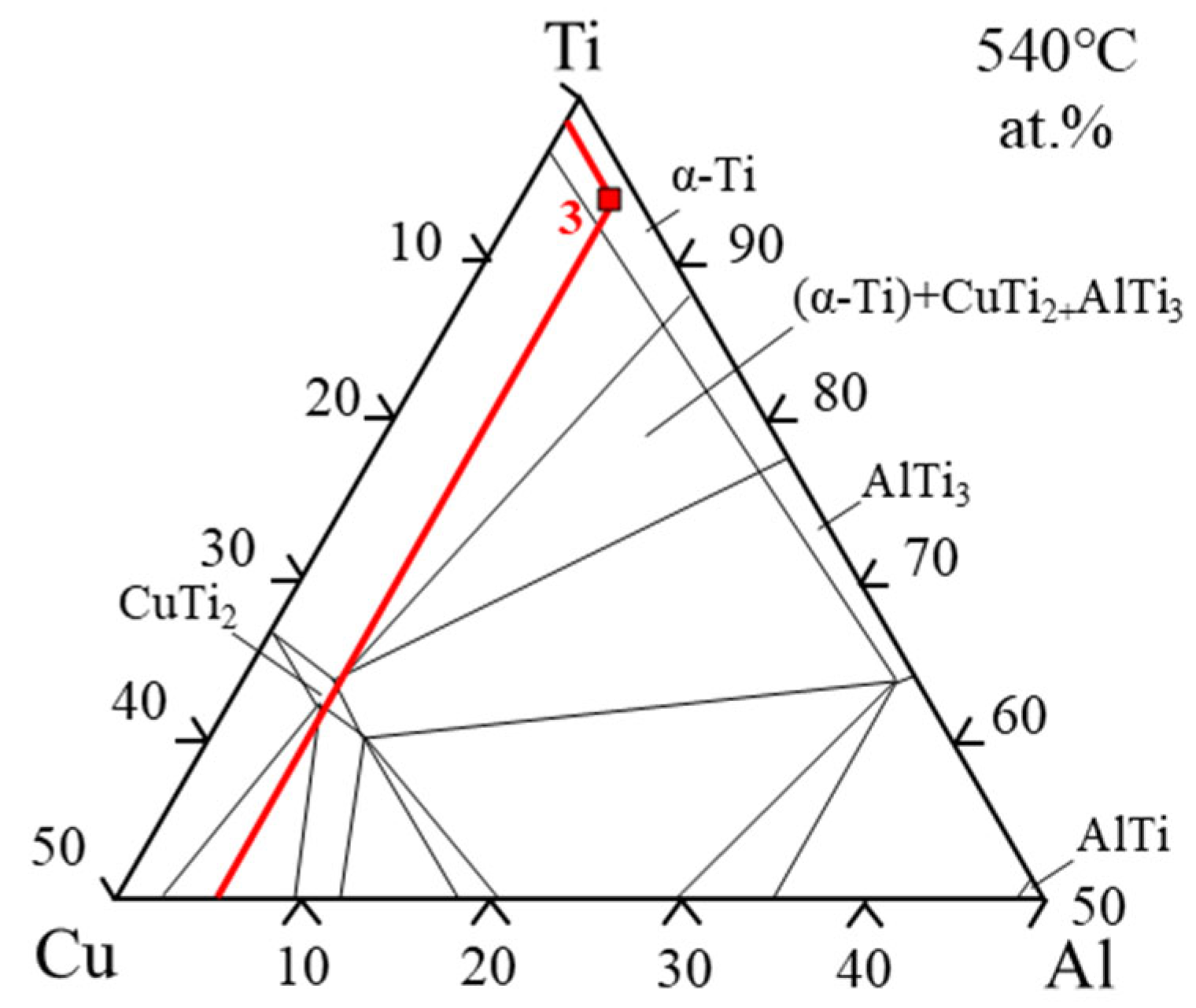
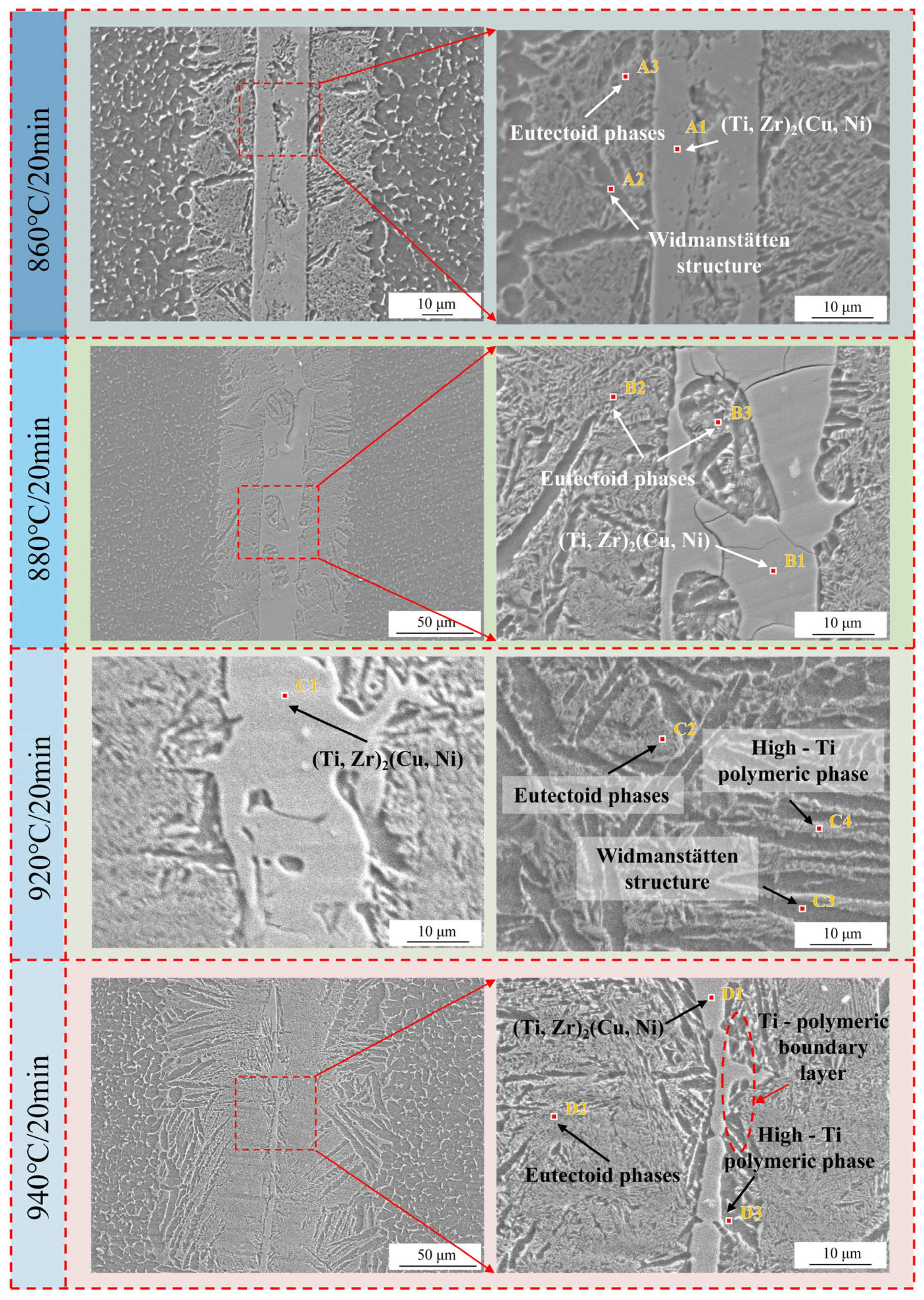
3.1. The Effect of Different Brazing Temperatures on Brazed Joint
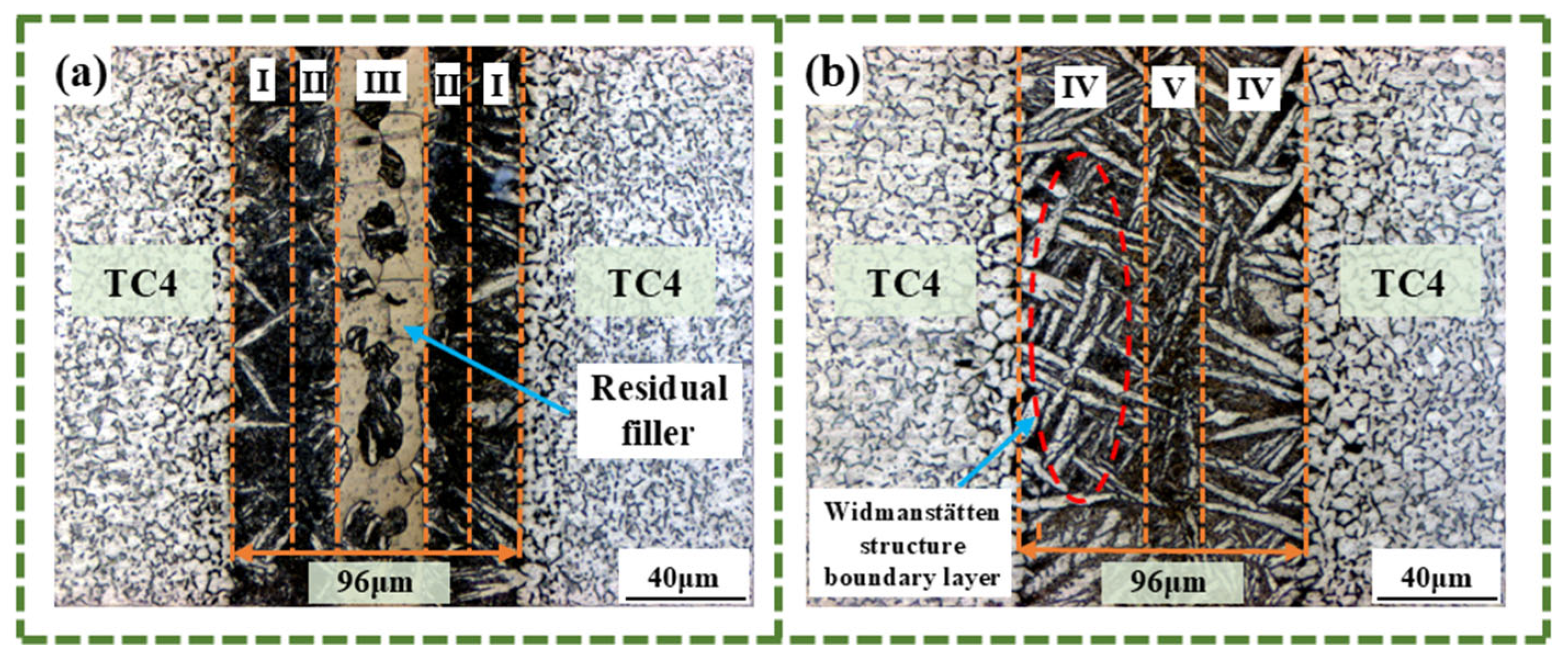
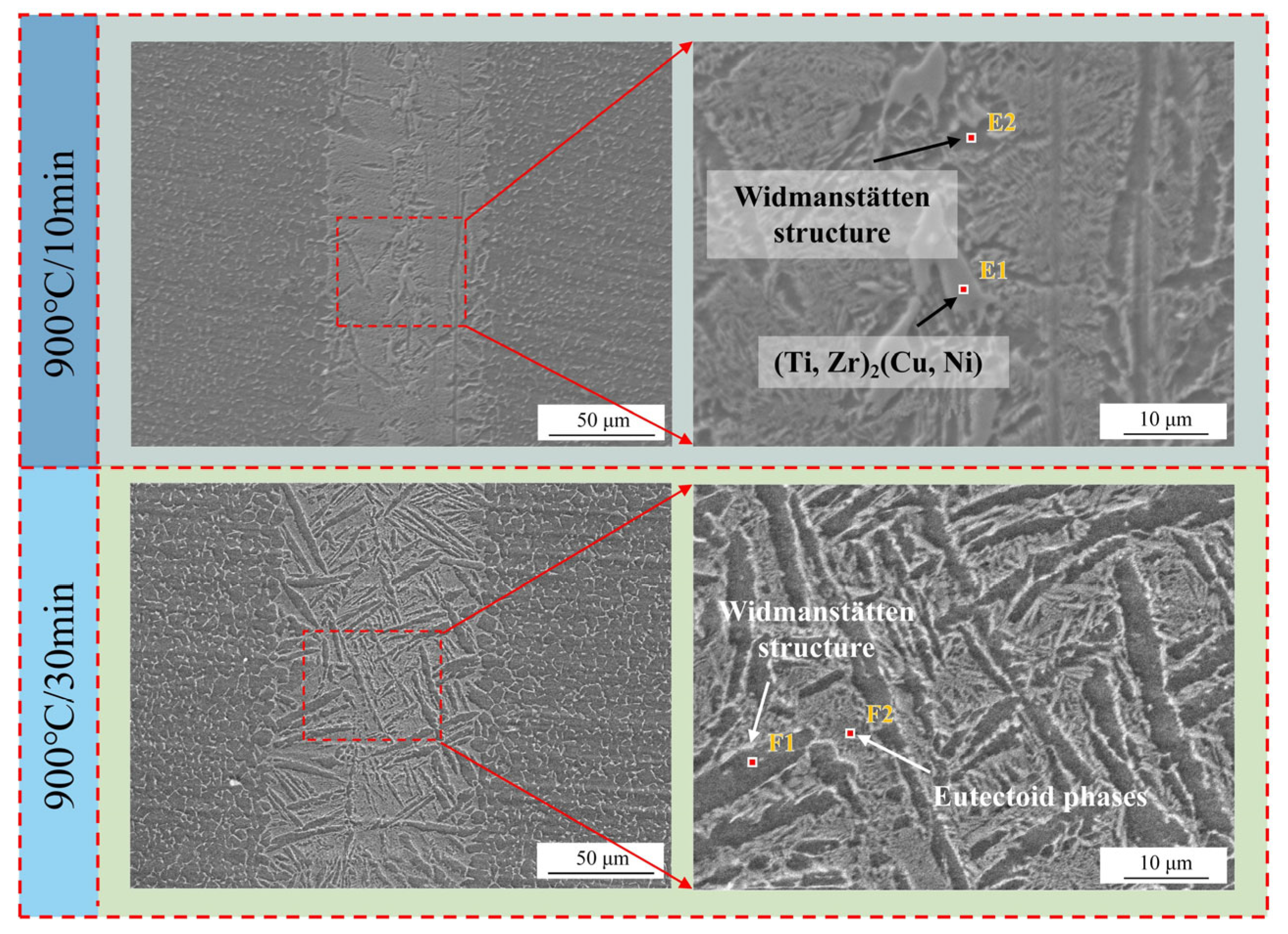
3.2. The Effect of Different Holding Times on Brazed Joints
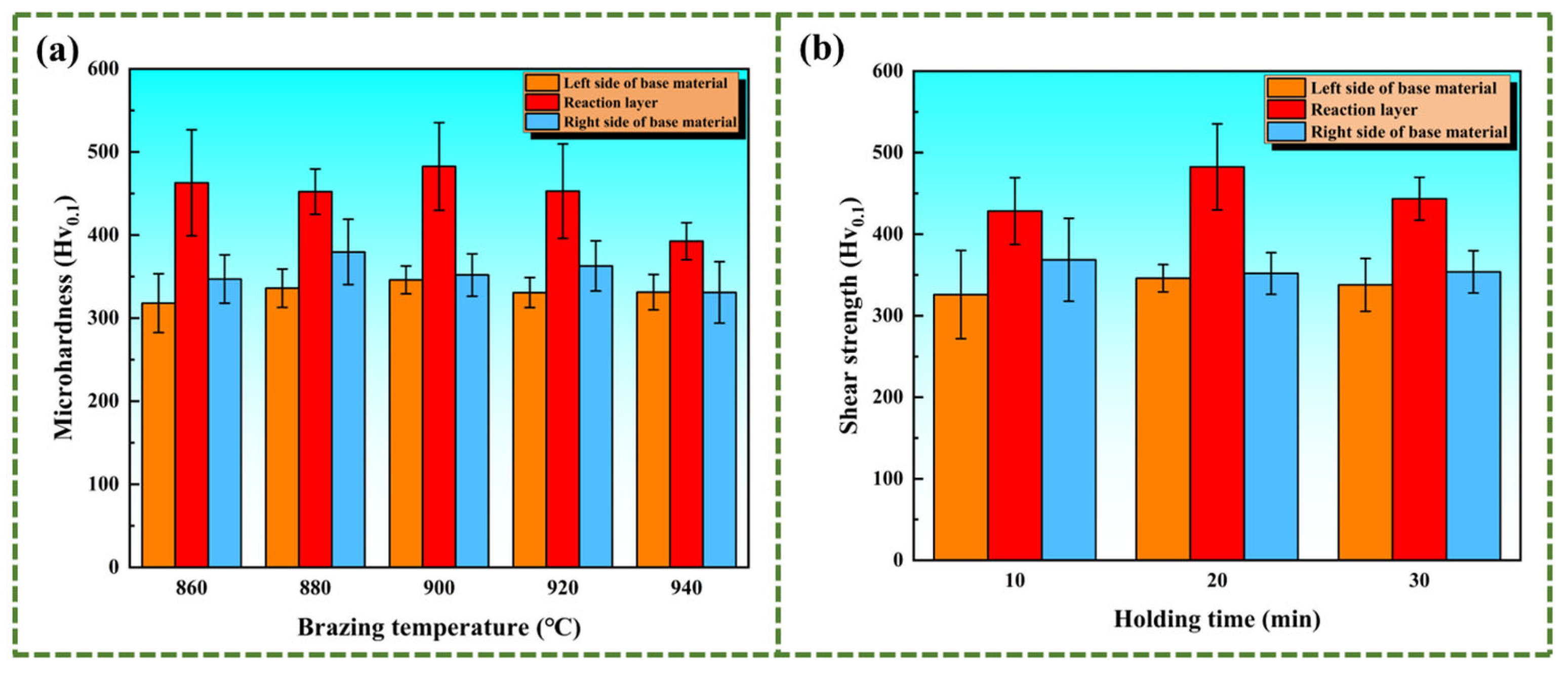
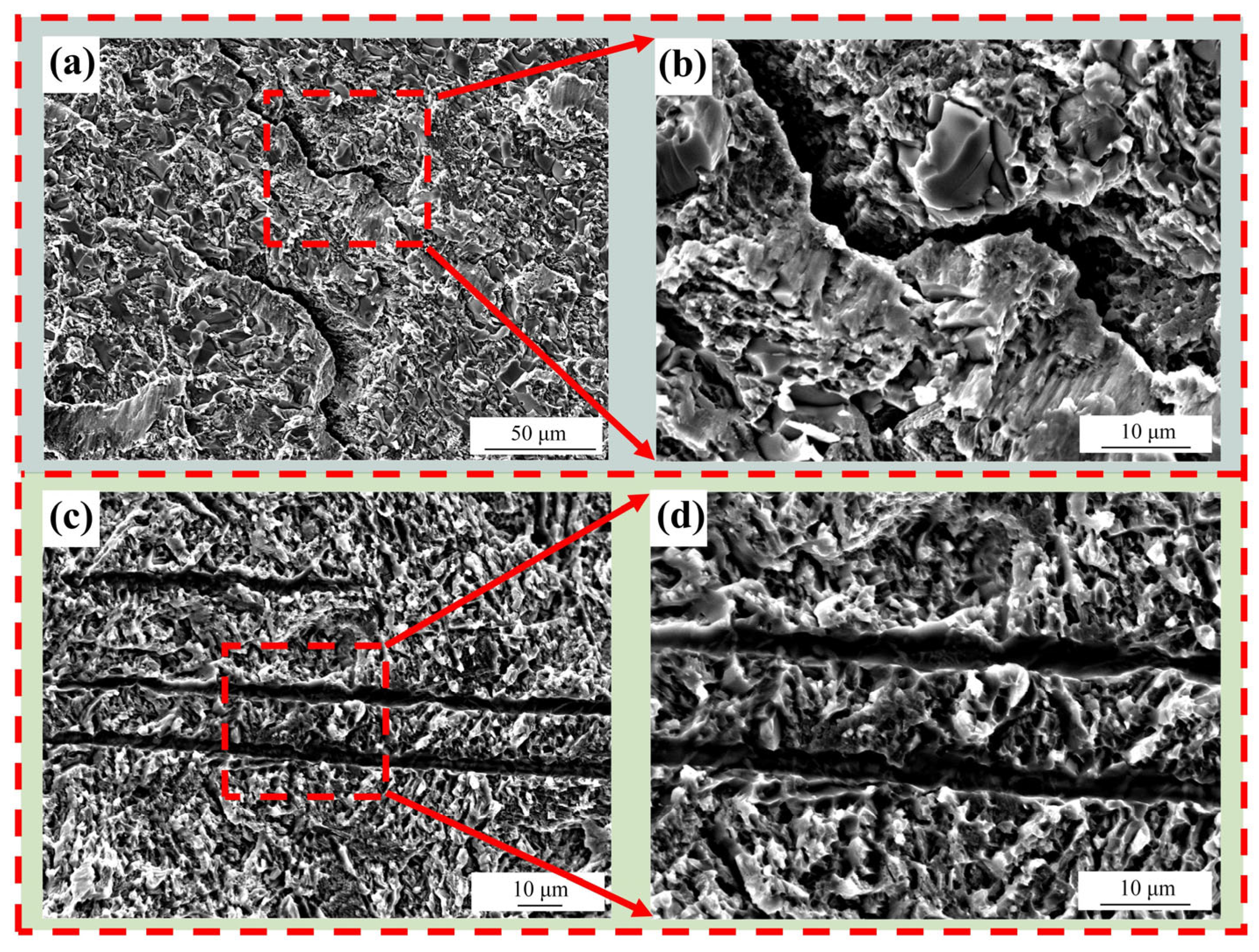
3.3. Microhardness and Shear Strength of the Joint
4. Conclusions
- (1)
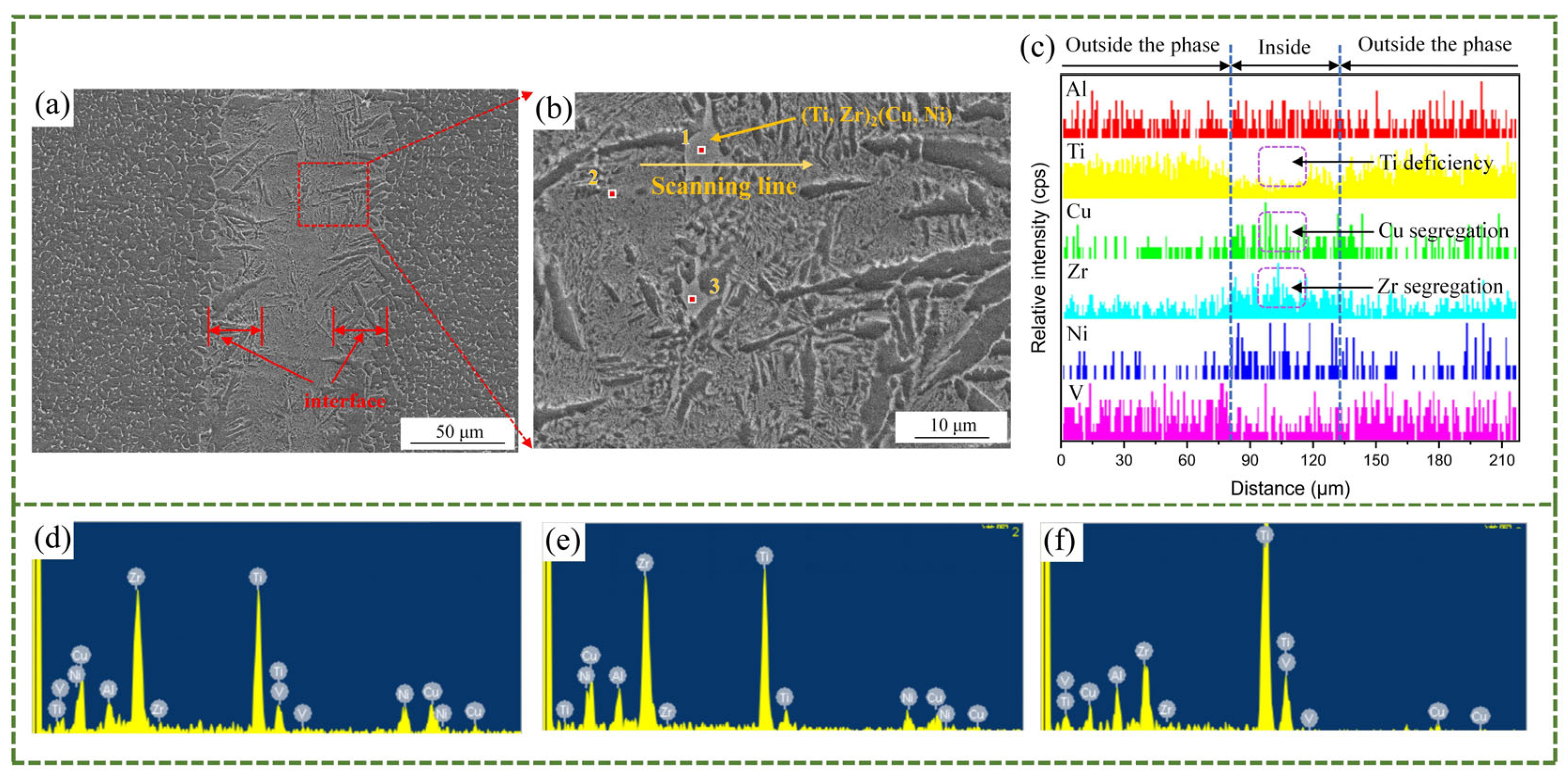
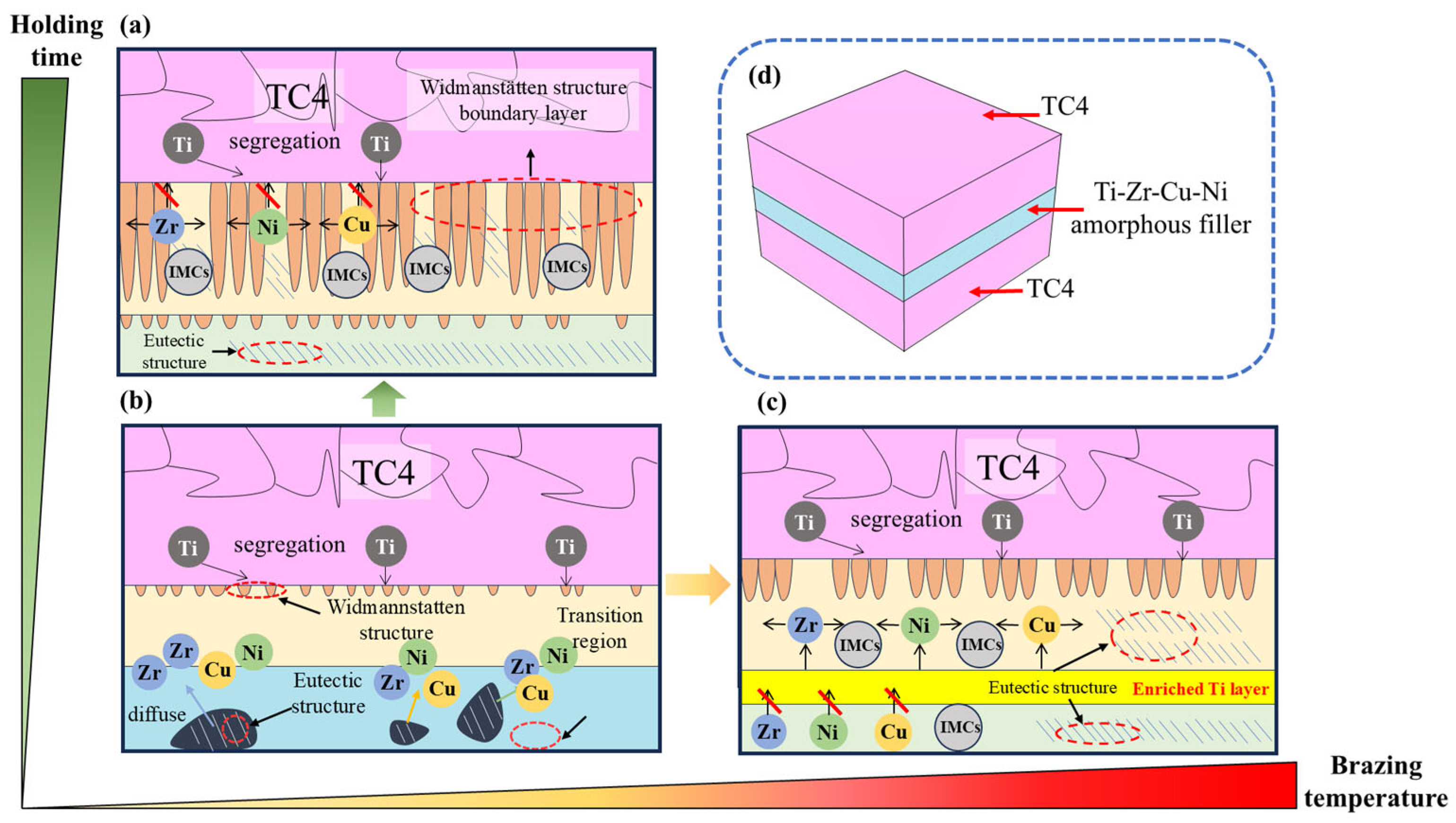
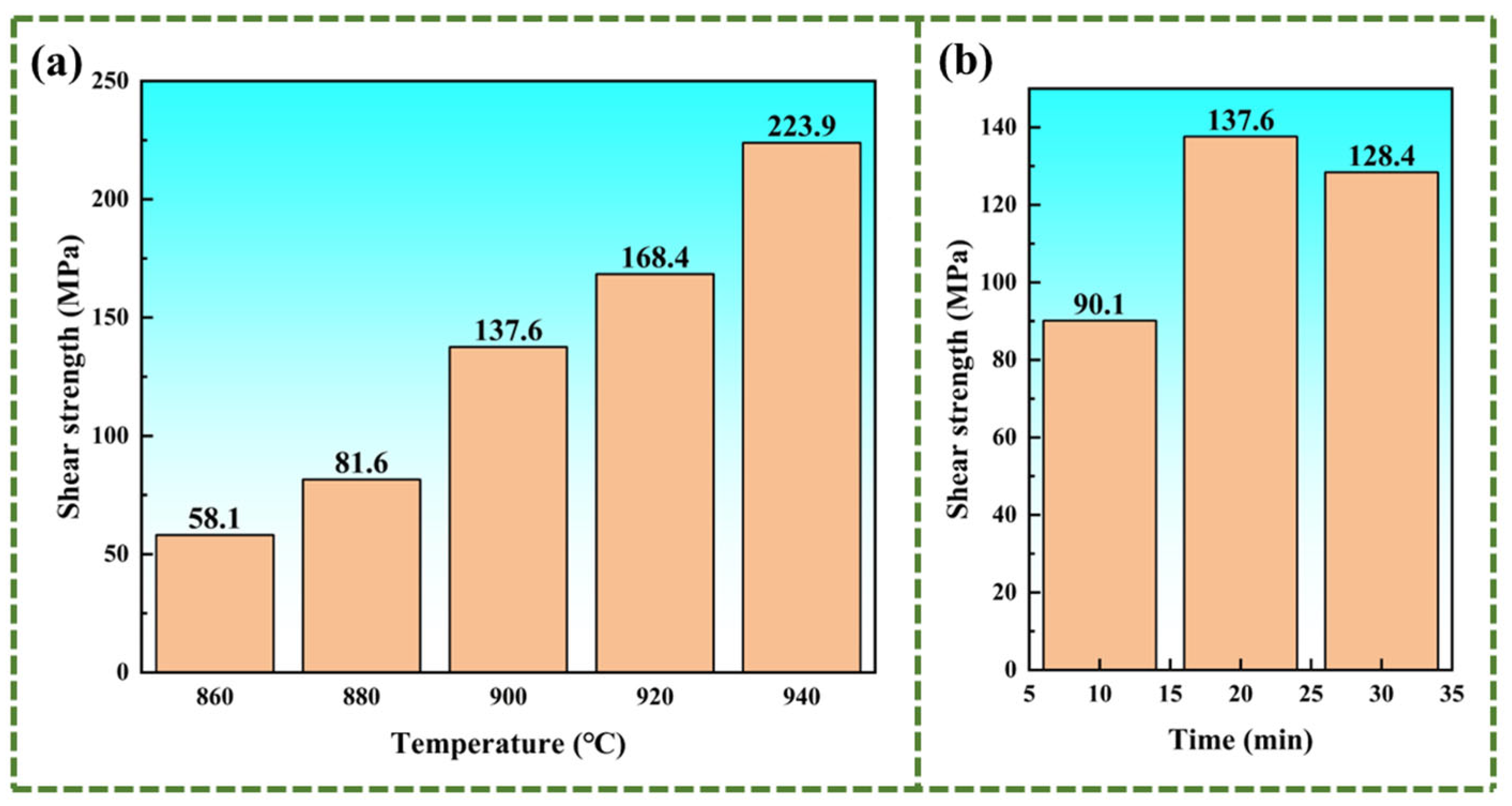
- The increase in the brazing temperature leads to an intensification of the atomic thermal motion and a decrease in the diffusion activation energy, accelerating the diffusion rate of elements, thus widening the width of the joint interface. Additionally, with the increase in the brazing temperature, the Widmanstätten structure, influenced by the diffusion of titanium out of the base metal, continuously grows towards the center of the joint. When elements such as Zr, Cu, and Ni diffuse towards the base metal, (Ti, Zr)2(Cu, Ni) will be formed in the diffusion transition zone, making the joint more brittle and harder. Moreover, affected by the loss of Zr, Cu, and Ni elements and the relatively low diffusion rate of Ti elements, a Ti-rich zone is formed, which hinders the continuous diffusion of elements. At a brazing temperature of 900 °C, the brazing filler metal exhibits good diffusion, and the Widmanstätten structure does not yet become coarse, resulting in relatively good performance.
- (2)
- Prolonging the holding time does not increase the width of the joint interface. Since extending the holding time does not accelerate the diffusion rate of Zr, Ni, and Cu elements, when the diffusion in the joint reaches a certain level, the diffusion of Ti elements towards the joint center will form a boundary layer of the Widmanstätten structure on the side of the base metal. In turn, the continuous diffusion of Zr, Ni, and Cu elements encounters more resistance. At a holding time of 30 min, the Widmanstätten structure continuously grows from the side of the base metal and extends to the central area of the joint, and the (Ti, Zr)2(Cu, Ni) keep growing, which is detrimental to the performance of the joint. Therefore, when the holding time is chosen to be 20 min, the comprehensive performance of the joint is the best.
- (3)
- At a brazing temperature of 900 °C with a holding time extended to 20 min, the elements diffuse sufficiently, and (Ti, Zr)2(Cu, Ni) is evenly distributed in the transition zone. In addition, the Widmanstätten structure has not experienced coarsening, which makes the microhardness of the central area of the brazed joint reach the highest value, hitting 482.6 MPa.
- (4)
- The shear strength is mainly affected by the width of the joint interface. The brazing temperature causes an increase in the width of the joint interface, thereby resulting in a higher shear strength. Increasing the brazing temperature to 940 °C raises the shear strength to 224 MPa. When the brazing temperature is held constant, the joint interface width remains unchanged, while prolonging the holding time promotes the development of the Widmanstätten structure. At a holding time of 20 min, elements diffuse sufficiently, leading to the highest shear strength of 137.6 MPa.
- (5)
- This work investigated the effects of brazing temperature and holding time on the microstructure and properties of brazed joints, and guidance was provided for practical industrial applications. Since the size of sheets in this work is small, the holding time did not need to be kept too long. However, for large-scale parts in industrial practice, higher brazing temperatures or longer holding times are required compared to the small-sized samples in this work to ensure complete heat conduction to the joint center and full diffusion of atoms. Therefore, this study establishes the minimum thresholds for brazing temperature and holding time for industrial production.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shao, L.; Xie, G.; Liu, X.; Wu, Y.; Yu, J.; Hao, Z.; Lu, W.; Liu, X. Combustion behaviour and mechanism of TC4 and TC11 alloys. Corros. Sci. 2020, 168, 108564. [Google Scholar] [CrossRef]
- Liu, Y.; Qu, N.; Li, H.; Zhang, Z. Boundary fluid constraints during electrochemical jet machining of large size emerging titanium alloy aerospace parts in gas–liquid flows: Experimental and numerical simulation. Chin. J. Aeronaut. 2025, 38, 103024. [Google Scholar] [CrossRef]
- Qu, Z.; Zhang, Z.; Liu, R.; Xu, L.; Zhang, Y.; Li, X.; Zhao, Z.; Duan, Q.; Wang, S.; Li, S.; et al. High fatigue resistance in a titanium alloy via near-void-free 3D printing. Nature 2024, 626, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Kluy, L.; Groche, P.; Klinge, L.; Siemers, C.; Spiegel, C. Nanostructuring of the titanium alloy Ti-13Nb-13Zr (NanoTNZ) for osteosynthesis implants by continuous multidirectional swaging. CIRP J. Manuf. Sci. Technol. 2025, 58, 47–61. [Google Scholar] [CrossRef]
- Jian, S.; Liu, K.; Li, J.; Wang, H.; Chen, Z.; Bokuchava, G. Effect of interlayer on interfacial microstructure and properties of Ni80Cr20/TC4 vacuum diffusion bonded joint. Vacuum 2023, 208, 111738. [Google Scholar] [CrossRef]
- Song, T.; Chen, Z.; Cui, X.; Lu, S.; Chen, H.; Wang, H.; Dong, T.; Qin, B.; Chan, K.C.; Brandt, M.; et al. Strong and ductile titanium–oxygen–iron alloys by additive manufacturing. Nature 2023, 618, 63–68. [Google Scholar] [CrossRef]
- Wang, L.; Liu, K.; Li, J.; Chen, Z.; Wang, J.; Okulov, A. Interfacial microstructure and mechanical properties of diffusion bonded joints of additive manufactured 17-4 PH stainless steel and TC4 titanium alloy. Vacuum 2024, 219, 112709. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, J.; Li, J.; Wang, F.; Ding, Y.; Xiong, J.; Guo, W. Microstructure evolution and mechanical properties of Ti2AlNb/TC17 joints brazed with Ti–Zr–Cu–Ni filler metal. Vacuum 2023, 215, 112365. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Xu, Q.; Liu, K.; Qin, G. Microstructure evolution and shear strength in CoCrFeNi/AISI 304 joint by vacuum brazing with BNi-2 filler metal. J. Mater. Res. Technol. 2024, 33, 8425–8434. [Google Scholar] [CrossRef]
- Li, H.; Zhuo, L.; Guo, W.; Pang, S.; Bai, X.; Xiong, H.; Zhang, T. Effect of Nb addition in novel amorphous/nanocrystalline Ti-Zr-Cu-Ni-Nb brazing fillers on improving microstructure and mechanical properties of TiAl alloy joints. Intermetallics 2025, 179, 108673. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, X.; Shi, J.; Pan, K.; Zhang, L.; Peng, Y.; Chen, X.; Zhang, Y. Multiscale consideration of mechanism revealing in diamond brazing with a novel NiTiZrCrVAl amorphous filler alloy: First-principles and experimental perspective. J. Mater. Res. Technol. 2025, 35, 3811–3824. [Google Scholar] [CrossRef]
- Ling, L.; Teng, J.; Chen, M. Microstructure evolution, diffusion behavior and fatigue properties of TC4 titanium alloy joints brazed with Ti–Zr-based filler. Weld. World 2022, 66, 2625–2638. [Google Scholar] [CrossRef]
- Yang, B.; Li, C.; Si, X.; Yuan, H.; Li, M.; Gao, J.; Qi, J.; Cao, J. Brazing Zircaloy-4 to Ti3AlC2 using Ti–Zr–Cu–Ni amorphous alloys: Microstructural evolution and mechanical properties. Mat. Sci. Eng. A Struct. 2024, 913, 147058. [Google Scholar] [CrossRef]
- Liu, S.; Miao, J.; Zhang, W.; Wei, R.; Chen, C.; Wang, T.; Zhao, W.; Jiang, Z.; Li, F. Interfacial microstructure and shear strength of TC4 alloy joints vacuum brazed with Ti–Zr–Ni–Cu filler metal. Mat. Sci. Eng. A Struct. 2020, 775, 138990. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Liu, K.; Lu, S.; Wang, Z.; Tang, J.; Wang, J.; Zhu, H. Study on microstructure and mechanical properties of vacuum brazing TC4 titanium alloy with Ti-37.5Zr-15Cu-10Ni amorphous filler metal. Mater. Today Commun. 2024, 41, 110552. [Google Scholar] [CrossRef]
- Yuan, L.; Xiong, J.T.; Du, Y.J.; Wang, Y.; Shi, J.M.; Li, J.L. Effects of pure Ti or Zr powder on microstructure and mechanical properties of Ti6Al4V and Ti2AlNb joints brazed with TiZrCuNi. Mat. Sci. Eng. A Struct. 2020, 788, 139602. [Google Scholar] [CrossRef]
- Song, J.; Guo, W.; Xu, S.; Hao, D.; Du, Y.; Xiong, J.; Li, J. Interfacial Microstructure Evolution and Mechanical Properties of TC4/MgAl2O4 Joints Brazed with Ti–Zr–Cu–Ni Filler Metal. Acta Metall. Sin. 2024, 37, 2057–2067. [Google Scholar] [CrossRef]
- Xia, Y.; Dong, H.; Hao, X.; Li, P.; Li, S. Vacuum brazing of Ti6Al4V alloy to 316L stainless steel using a Ti-Cu-based amorphous filler metal. J. Mater. Process. Technol. 2019, 269, 35–44. [Google Scholar] [CrossRef]
- Cao, X.; Dong, K.; Zhu, R.; Wang, Q.; Kong, J. A high-strength vacuum brazed TC4/316L joint with a Ti–Zr-based amorphous ribbon as the filler metal. Vacuum 2021, 187, 110070. [Google Scholar] [CrossRef]
- Ganjeh, E.; Sarkhosh, H.; Bajgholi, M.E.; Khorsand, H.; Ghaffari, M. Increasing Ti–6Al–4V brazed joint strength equal to the base metal by Ti and Zr amorphous filler alloys. Mater. Charact. 2012, 71, 31–40. [Google Scholar] [CrossRef]
- Cai, Y.S.; Liu, R.C.; Zhu, Z.W.; Cui, Y.Y.; Yang, R. Effect of brazing temperature and brazing time on the microstructure and tensile strength of TiAl-based alloy joints with Ti-Zr-Cu-Ni amorphous alloy as filler metal. Intermetallics 2017, 91, 35–44. [Google Scholar] [CrossRef]
- Jing, Y.; Gao, X.; Su, D.; Zhao, C.; Jiang, J. The effects of Zr level in Ti-Zr-Cu-Ni brazing fillers for brazing Ti-6Al-4V. J. Manuf. Process. 2018, 31, 124–130. [Google Scholar] [CrossRef]
- Ganjeh, E.; Sarkhosh, H. Microstructural, mechanical and fractographical study of titanium-CP and Ti–6Al–4V similar brazing with Ti-based filler. Mat. Sci. Eng. A Struct. 2013, 559, 119–129. [Google Scholar] [CrossRef]
- Liu, K.; Li, Y.J.; Xia, C.Z.; Wang, J. Effect of bonding time on interfacial microstructure and shear strength of vacuum diffusion bonding super-Ni/NiCr laminated composite to Ti-6Al-4V joint without interlayer. Vacuum 2017, 143, 195–198. [Google Scholar] [CrossRef]
- Liang, M.; Qin, Y.; Zhang, D.; Zhao, F. Effect of Brazing Clearance on the Microstructure and Mechanical Properties of TC4/TC4 Joints Brazed in Vacuum with Ti–Zr–Ni Filler Metal. J. Mater. Eng. Perform. 2023, 32, 2973–2982. [Google Scholar] [CrossRef]
- Xia, Y.; Li, P.; Hao, X.; Dong, H. Interfacial microstructure and mechanical property of TC4 titanium alloy/316L stainless steel joint brazed with Ti-Zr-Cu-Ni-V amorphous filler metal. J. Manuf. Process. 2018, 35, 382–395. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, B.; Li, D.; Zhu, X.; Chang, Q.; Zhang, B.; Zhang, L.; Long, W.; Zhong, S. Brazing of TC4 Alloy Using Ti-Zr-Ni-Cu-Sn Amorphous Braze Fillers. Materials 2024, 17, 3745. [Google Scholar] [CrossRef]
- Liang, M.; Qin, Y.; Zhang, D.; Zhao, F. Microstructural Evolution and Mechanical Properties of Vacuum Brazed TC4 Titanium Alloy Joints with Ti-Zr-Ni Filler Metal. J. Mater. Eng. Perform. 2022, 31, 9340–9348. [Google Scholar] [CrossRef]
- Wang, B.; Shi, H.; Yu, Z.; Zhang, P.; Cheng, Q.; Song, N.; Yan, H. Study on the microstructure and mechanical properties of Ti-Zr-Cu-Ni amorphous filler brazed Inconel 718 and 3D printed TC4 alloy. Weld. World 2023, 67, 455–471. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, Y.; Niu, S.; Wang, Y.; Han, Y.; Cai, X.; Wang, D. Phase transition, microstructural evolution and mechanical properties of Ti–6Al–4V and Ti–6.5Al–3.5Mo–1.5Zr–0.3Si joints brazed with Ti–Zr–Ni–Cu filler metal. Arch. Civ. Mech. Eng. 2020, 20, 88. [Google Scholar] [CrossRef]



| Materials | Ti | Al | V | Zr | Cu | Ni |
|---|---|---|---|---|---|---|
| TC4 | Bal. | 5.5~6.75 | 3.5 | |||
| Ti-Zr-Cu-Ni | Bal. | 37.5 | 15 | 10 |
| Brazing Parameters for TC4 (Sample#) | Brazing Temperature (°C) | Holding Time (min) |
|---|---|---|
| 1# | 860 | 20 |
| 2# | 880 | 20 |
| 3# | 900 | 20 |
| 4# | 920 | 20 |
| 5# | 940 | 20 |
| 6# | 900 | 10 |
| 7# | 900 | 30 |
| Points | Elements (at%) | Possible Phase | |||||
|---|---|---|---|---|---|---|---|
| Al | Ti | Cu | Zr | Ni | V | ||
| 1 | 7.0 | 35.5 | 16.8 | 24.3 | 14.7 | 1.7 | (Ti, Zr)2(Cu, Ni) |
| 2 | 10.3 | 42.6 | 9.4 | 28.1 | 9.7 | eutectic phases | |
| 3 | 8.4 | 74.4 | 3.6 | 11.7 | 1.9 | α-Ti (high Ti concentration) | |
| Points | Elements (at%) | Possible Phase | |||||
|---|---|---|---|---|---|---|---|
| Al | Ti | Cu | Zr | Ni | V | ||
| A1 | 2.0 | 38.6 | 14.7 | 30.5 | 14.3 | (Ti, Zr)2(Cu, Ni) | |
| A2 | 6.2 | 81.8 | 2.4 | 5.1 | 1.8 | 2.7 | α-Ti (Widmanstätten structure) |
| A3 | 6.5 | 76.0 | 4.1 | 9.5 | 3.4 | 0.6 | eutectic phases |
| B1 | 5.0 | 37.8 | 16.2 | 28.9 | 12.1 | (Ti, Zr)2(Cu, Ni) | |
| B2 | 6.2 | 73.0 | 4.7 | 11.1 | 3.1 | 2.0 | eutectic phases |
| B3 | 3.3 | 76.6 | 3.7 | 12.2 | 1.3 | 3.0 | eutectic phases |
| C1 | 37.5 | 11.6 | 35.1 | 15.8 | (Ti, Zr)2(Cu, Ni) | ||
| C2 | 6.2 | 74.2 | 2.9 | 10.8 | 2.0 | 4.0 | eutectic phases |
| C3 | 11.5 | 80.6 | 1.0 | 6.0 | 0.9 | α-Ti (Widmanstätten structure) | |
| C4 | 85.6 | 2.0 | 7.8 | 4.5 | α-Ti | ||
| D1 | 13.1 | 36 | 11.6 | 27.5 | 10.0 | 1.9 | (Ti, Zr)2(Cu, Ni) |
| D2 | 7.0 | 71.4 | 6.5 | 10.4 | 4.1 | eutectic phases | |
| D3 | 6.6 | 84.7 | 4.6 | 1.0 | 2.8 | α-Ti | |
| Points | Elements (at%) | Possible Phase | |||||
|---|---|---|---|---|---|---|---|
| Al | Ti | Cu | Zr | Ni | V | ||
| E1 | 9.0 | 41.3 | 11.7 | 26.5 | 11.4 | (Ti, Zr)2(Cu, Ni) | |
| E2 | 6.6 | 79.5 | 3.2 | 9.0 | 1.7 | α-Ti (Widmanstätten structure) | |
| F1 | 10.7 | 78.1 | 2.5 | 6.1 | 1.0 | 1.5 | α-Ti (Widmanstätten structure) |
| F2 | 1.6 | 78.9 | 3.4 | 6.3 | 2.7 | 7.0 | eutectic phases |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Li, J.; Wang, Z.; Lu, S.; Liu, K. Effect of Brazing Temperature and Holding Time on the Interfacial Microstructure and Properties of TC4-Brazed Joints with Ti-Zr-Cu-Ni Amorphous Filler. Materials 2025, 18, 2471. https://doi.org/10.3390/ma18112471
Wu Y, Li J, Wang Z, Lu S, Liu K. Effect of Brazing Temperature and Holding Time on the Interfacial Microstructure and Properties of TC4-Brazed Joints with Ti-Zr-Cu-Ni Amorphous Filler. Materials. 2025; 18(11):2471. https://doi.org/10.3390/ma18112471
Chicago/Turabian StyleWu, Yibin, Jie Li, Zexin Wang, Sheng Lu, and Kun Liu. 2025. "Effect of Brazing Temperature and Holding Time on the Interfacial Microstructure and Properties of TC4-Brazed Joints with Ti-Zr-Cu-Ni Amorphous Filler" Materials 18, no. 11: 2471. https://doi.org/10.3390/ma18112471
APA StyleWu, Y., Li, J., Wang, Z., Lu, S., & Liu, K. (2025). Effect of Brazing Temperature and Holding Time on the Interfacial Microstructure and Properties of TC4-Brazed Joints with Ti-Zr-Cu-Ni Amorphous Filler. Materials, 18(11), 2471. https://doi.org/10.3390/ma18112471






