Mixing Approaches in Enhancing the Capacitive Performance of rGO-Based Hybrid Electrodes
Abstract
1. Introduction
2. Materials and Methods
2.1. Composites Preparation
2.2. Structural and Morphological Characterization of Composites
2.3. Electrochemical Characterization of Composites
3. Results and Discussion
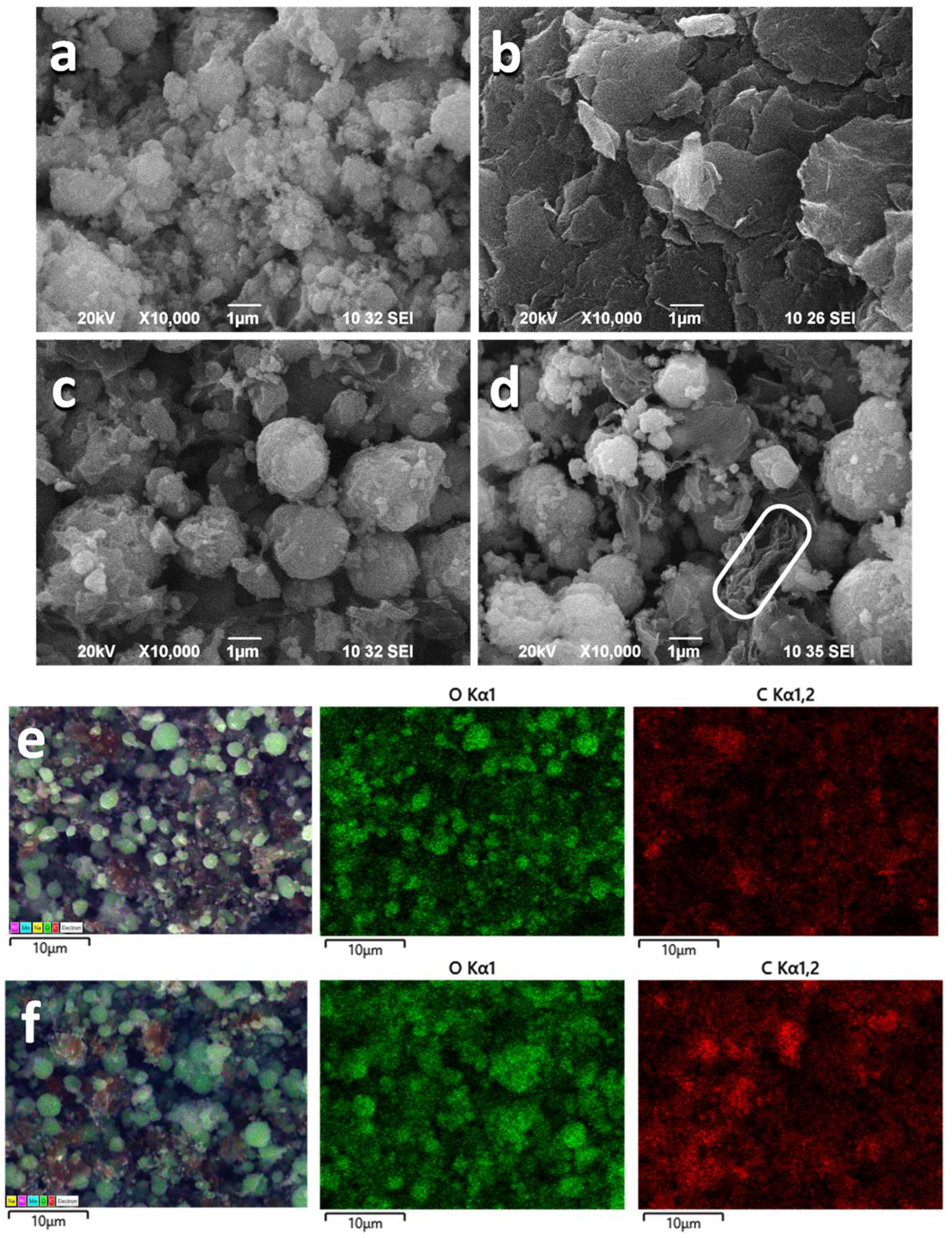
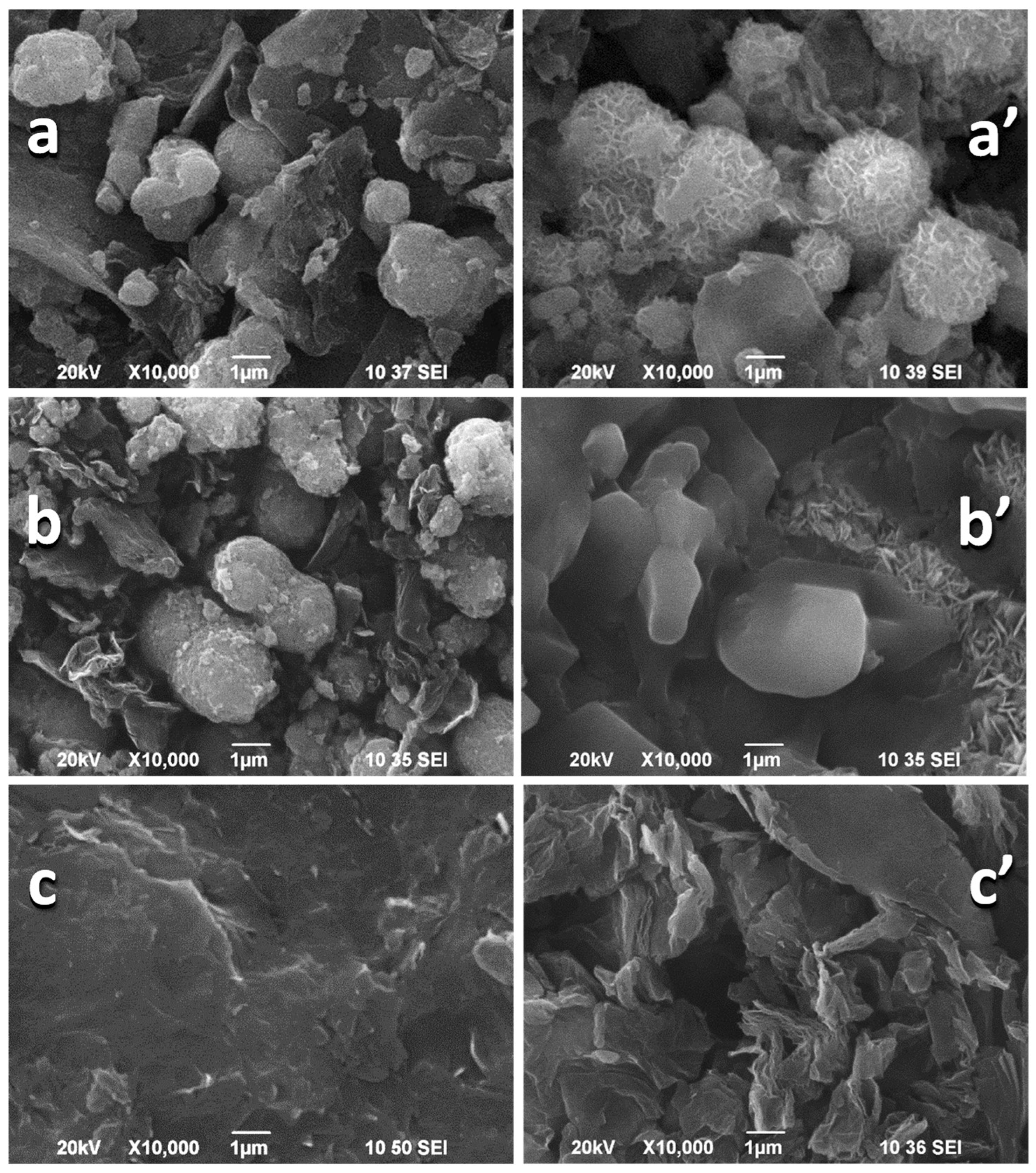
3.1. Structure and Morphology of NMO/rGO Composites
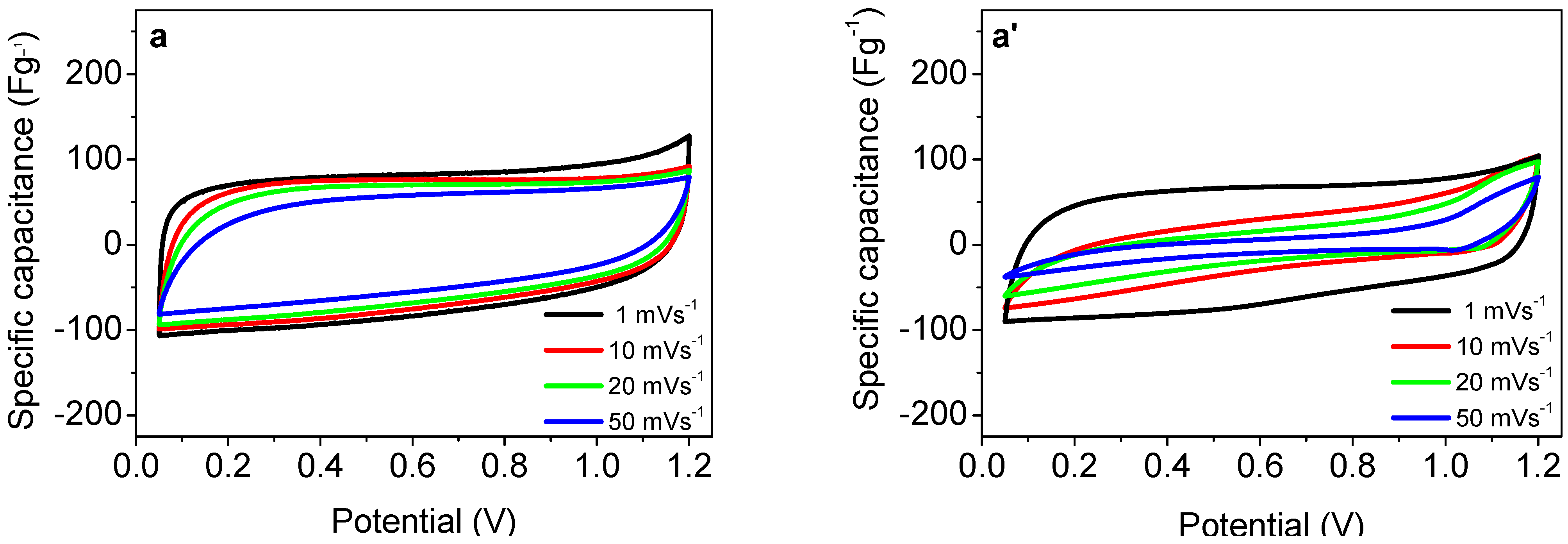
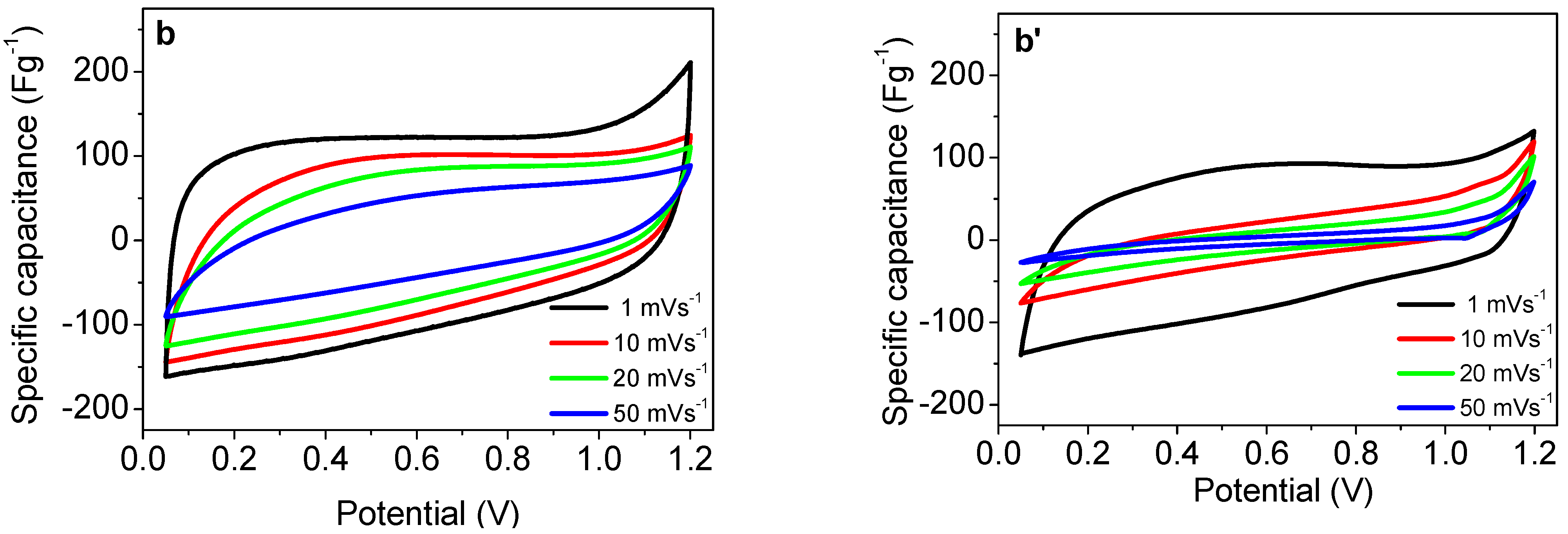
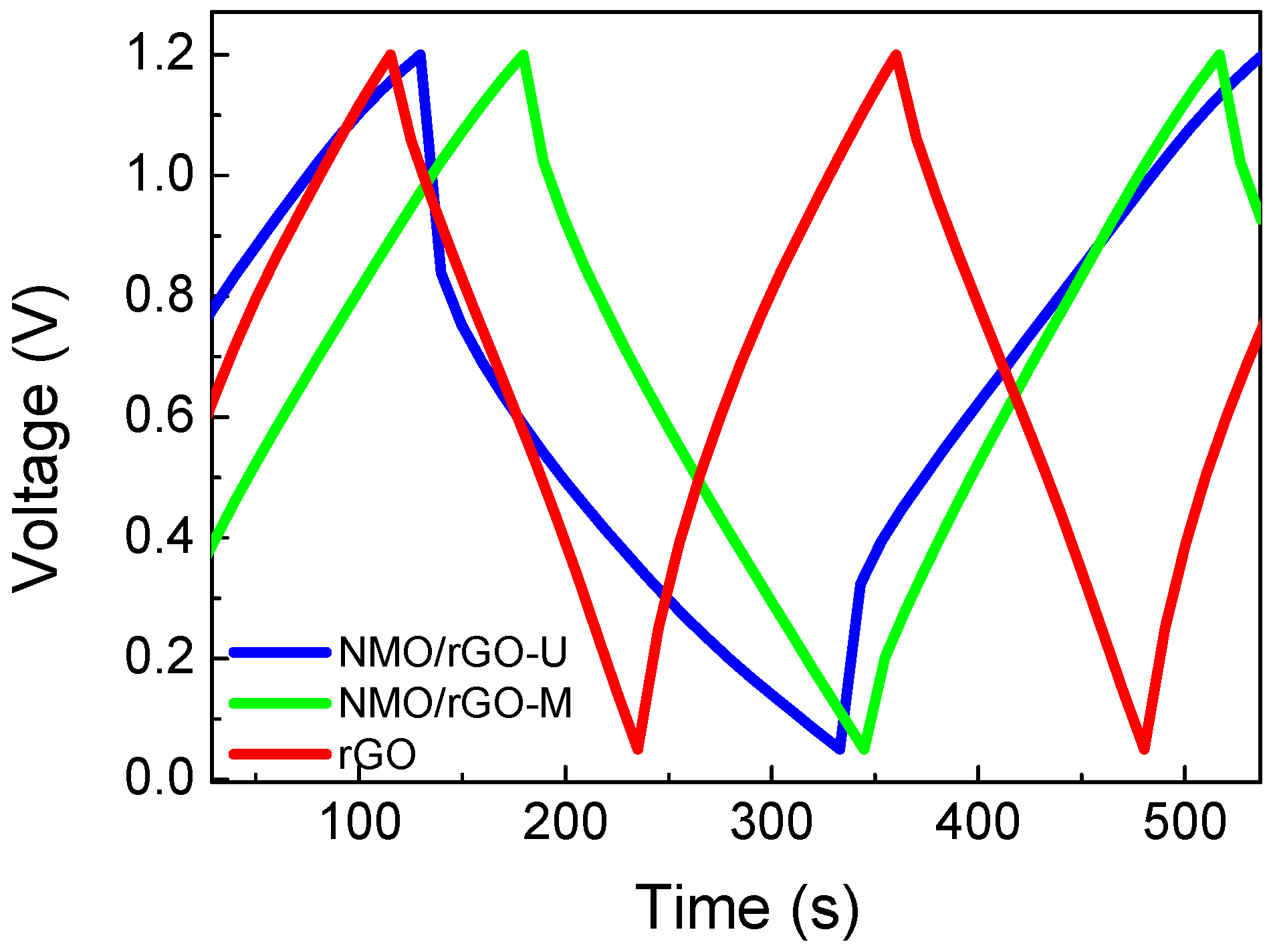
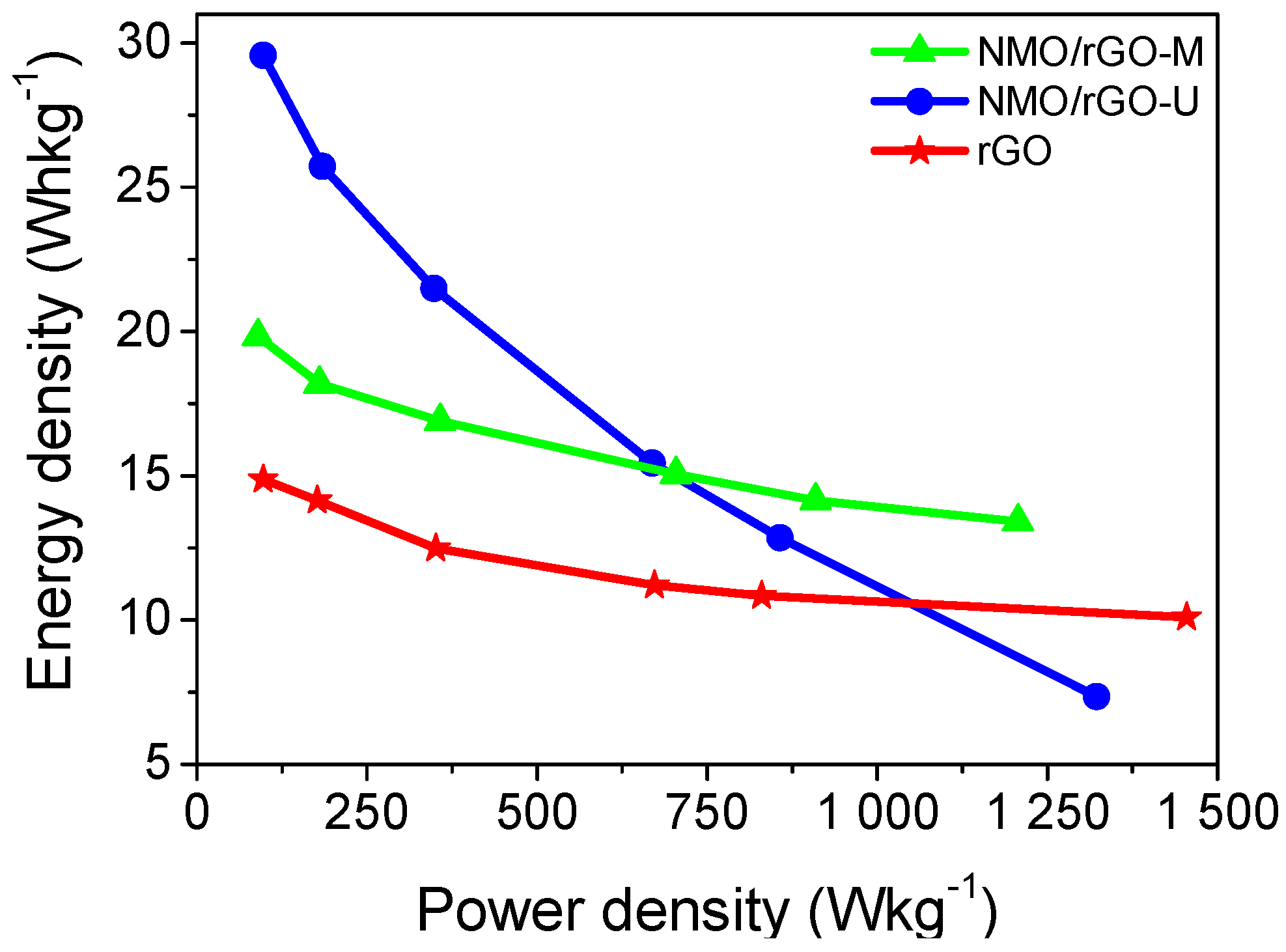
3.2. Supercapacitor Performance
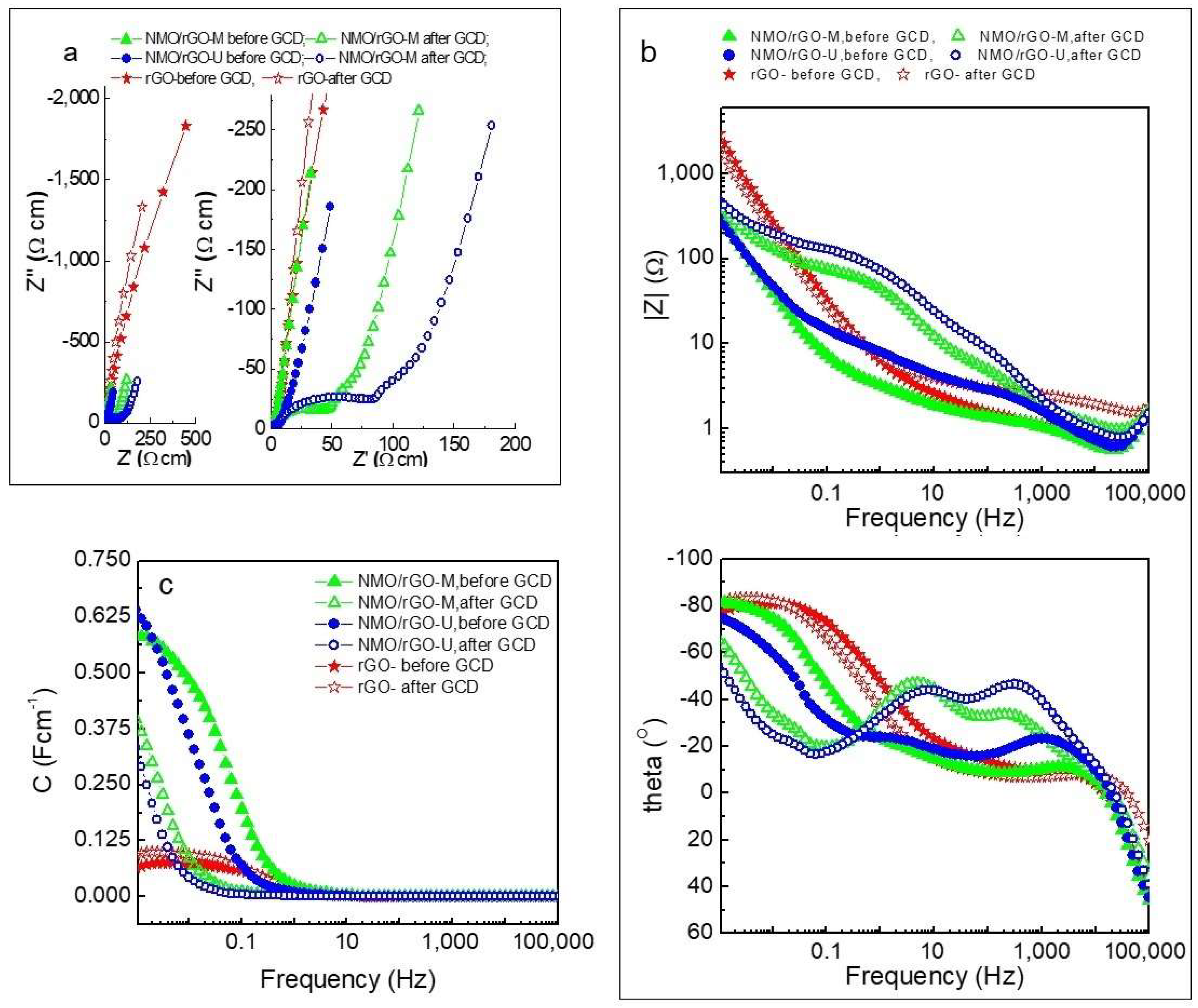
3.3. Ex Situ Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mathis, T.S.; Kurra, N.; Wang, X.; Pinto, D.; Simon, P.; Gogotsi, Y. Energy storage data reporting in perspective-guidelines for interpreting the performance of electrochemical energy storage systems. Adv. Energy Mater. 2019, 9, 1902007. [Google Scholar] [CrossRef]
- Gadipelli, S.; Guo, J.; Li, Z.; Howard, C.A.; Liang, Y.; Zhang, H.; Shearing, P.R.; Brett, D.J.L. Understanding and optimizing capacitance performance in reduced graphene-oxide based supercapacitors. Small 2023, 20, 2201557. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, K.; Kularatna-Abeywardana, D. A review of supercapacitors: Materials, technology, challenges, and renewable energy applications. J. Energy Storage 2024, 96, 112563. [Google Scholar] [CrossRef]
- He, X.; Zhang, X. A comprehensive review of supercapacitors: Properties, electrodes, electrolytes and thermal management systems based on phase change materials. J. Energy Storage 2022, 56 Pt C, 106023. [Google Scholar] [CrossRef]
- Gao, D.; Luo, Z.; Liu, C.; Fan, S. A survey of hybrid energy devices based on supercapacitors. Green Energy Environ. 2023, 8, 972–988. [Google Scholar] [CrossRef]
- Chatterjee, D.P.; Nandi, A.K. A review on the recent advances in hybrid supercapacitors. J. Mater. Chem. A 2021, 9, 15880–15918. [Google Scholar]
- El-Kady, M.F.; Shao, Y.; Kaner, R.B. Graphene for batteries, supercapacitors and beyond. Nat. Rev. Mater. 2016, 1, 16033. [Google Scholar] [CrossRef]
- Bokhari, S.W.; Siddique, A.H.; Sherrell, P.C.; Yue, X.; Karumbaiah, K.M.; Wei, S.; Ellis, A.V.; Gao, W. Advances in graphene-based supercapacitor electrodes. Energy Rep. 2020, 6, 2768–2784. [Google Scholar] [CrossRef]
- Malik, M.T.U.; Sarker, A.; Rahat, S.M.; Shuchi, S.B. Performance enhancement of graphene/GO/rGO based supercapacitors: A comparative review. Materials Today Commun. 2021, 28, 102685. [Google Scholar] [CrossRef]
- Soserov, L.; Marinova, D.; Koleva, V.; Stoyanova, A.; Stoyanova, R. Comparison of the properties of Ni–Mn hydroxides/oxides with Ni–Mn phosphates for the purpose of hybrid supercapacitors. Batteries 2022, 8, 51. [Google Scholar] [CrossRef]
- Ahuja, P.; Ujjain, S.K.; Sharma, S.R.; Singh, G. Enhanced supercapacitor performance by incorporating nickel in manganese oxide. RSC Adv. 2014, 4, 57192–57199. [Google Scholar] [CrossRef]
- Giri, S.; Ghosh, D.; Das, C.K. One pot synthesis of ilmenite-type NiMnO3–“nitrogen-doped” graphene nanocomposite as next generation supercapacitors. Dalton Trans. 2013, 42, 14361–14364. [Google Scholar] [CrossRef]
- Sanchez, J.S.; Pendashteh, A.; Palma, J.; Anderson, M.; Marcilla, R. Synthesis and application of NiMnO3-rGO nanocomposites as electrode materials for hybrid energy storage devices. Appl. Surf. Sci. 2018, 460, 74–83. [Google Scholar] [CrossRef]
- Al-khaykanee, A.H.; Ghorbani, S.R.; Arabi, H.; Ghanbari, R. NiMnO3-rGO nanocomposites in a cotton-based flexible yarn supercapacitor. J. Mater Sci. Mater. Electron. 2024, 35, 141. [Google Scholar] [CrossRef]
- Dhas, S.D.; Kim, G.; Thonge, P.N.; Patil, A.M.; Mendhe, A.C.; Batoola, R.; Kim, D. Enhanced electrochemical performance of CTAB-assisted Zn-doped perovskite NiMnO3 nanocrystals on reduced graphene oxide for supercapacitors and oxygen evolution reactions. J. Mater. Chem. A 2025, 13, 3735–3748. [Google Scholar] [CrossRef]
- Hsieh, H.L.; Wang, Y.H.; Tsai, C.Y.; Yang, C.H.; Lin, E.J.; Chen, C.C. Tailoring the morphology of Cu2O microcrystals in Cu2O/rGO composites for achieving enhanced supercapacitor performance. ACS Appl. Energy Mater. 2024, 7, 8869–8881. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, B.S. Balancing the anode and cathode using a reduced graphene binder for boosting both energy and power density of hybrid supercapacitors. Sustain. Energy Fuels 2021, 5, 1988–1994. [Google Scholar] [CrossRef]
- Kakvand, P.; Rahmanifar, M.S.; El-Kady, M.F.; Pendashteh, A.; Ali Kiani, M.; Hashami, M.; Najafi, M.; Abbasi, A.; Mousavi, M.F.; Kaner, R.B. Synthesis of NiMnO3/C nano-composite electrode materials for electrochemical capacitors. Nanotechnology 2016, 27, 315401. [Google Scholar] [CrossRef]
- Li, Y.; Kamdem, P.; Cheng, C.; Jin, X.-J.; Liu, X. Hydrophilic “bridge” H–C3N4 stabilizing CuO onto graphenes with enhanced energy density for asymmetric supercapacitors. Sustain. Energy Fuels 2020, 4, 4196–4206. [Google Scholar] [CrossRef]
- Pal, B.; Yang, S.; Ramesh, S.; Thangadurai, V.; Jose, R. Electrolyte selection for supercapacitive devices: A critical review. Nanoscale Adv. 2019, 1, 3807–3835. [Google Scholar] [CrossRef]
- Meena, J.; Sivasubramaniam, S.; David, E.; Santhakumar, K. Green supercapacitors: Review and perspectives on sustainable template-free synthesis of metal and metal oxide nanoparticles. RSC Sustain. 2024, 2, 1224–1245. [Google Scholar] [CrossRef]
- Dhas, S.D.; Thonge, P.N.; Mendhe, A.C.; Yun, J.; Kim, D. Nickel manganite-based materials for electrochemical supercapacitors: An overview. Renew. Sustain. Energy Rev. 2024, 204, 114819. [Google Scholar] [CrossRef]
- Soserov, L.; Stoyanova, A.; Boyadzhieva, T.; Koleva, V.; Kalapsazova, M.; Stoyanova, R. Nickel-manganese structured and multiphase composites as electrodes for hybrid supercapacitors. Electrochim. Acta 2018, 283, 1063–1071. [Google Scholar] [CrossRef]
- Ash, B.; Nalajala, V.S.; Popuri, A.K.; Subbaiah, T.; Minakshi, M. Perspectives on nickel hydroxide electrodes suitable for rechargeable batteries: Electrolytic vs. Chemical Synthesis Routes. Nanomater 2020, 10, 1878. [Google Scholar] [CrossRef]
- Fic, K.; Lota, G.; Meller, M.; Frackowiak, E. Novel insight into neutral medium as electrolyte for high-voltage supercapacitors. Energy Environ. Sci. 2012, 5, 5842–5850. [Google Scholar] [CrossRef]
- Rundla, A.; Priyanka Kumar, B.; Tripathi, P.; Kumar, P.; Singh, S. Ti3C2Tx MXene@rGO composite electrodes for high-performance supercapacitor applications. J. Power Sources 2025, 632, 236408. [Google Scholar] [CrossRef]
- Thomas, M.; Veleva, S.; Karamanova, B.; Brigandì, A.; Rey-Raap, N.; Arenillas, A.; Stoyanova, A.; Lufrano, F. Highly stable and reliable asymmetric solid-state supercapacitors with low self-discharge rates. Sustain. Mater. Technol. 2023, 38, e00770. [Google Scholar] [CrossRef]
- Harizanova, S.; Tushev, T.; Koleva, V.; Stoyanova, R. Carbon-based composites with mixed phosphate-pyrophosphates with improved electrochemical performance at elevated temperature. Materials 2023, 16, 6546. [Google Scholar] [CrossRef]
- Tamang, S.; Rai, S.; Bhujel, R.; Bhattacharyya, N.K.; Swain, B.P.; Biswas, J. A concise review on GO, rGO and metal oxide/rGO composites: Fabrication and their supercapacitor and catalytic applications. J. Alloys Compd. 2023, 947, 169588. [Google Scholar] [CrossRef]
- Ahmad, F.; Ahmed Khan, M.; Waqas, U.; Ramay, S.M.; Atiq, M. Elucidating an efficient super-capacitive response of a Sr2Ni2O5/rGO composite as an electrode material in supercapacitors. RSC Adv. 2023, 13, 25316. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Y.; Xia, Y. Electrochemical capacitors: Mechanism, materials, systems, characterization and applications. Chem. Soc. Rev. 2016, 45, 5925–5950. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Afkhami, A.; Madrakian, T.; Moazami, R.H. Hydrothermal synthesis of nanocages of Mn-Co Prussian blue analogue and charge storage investigation of the derived Mn-Co oxide@/rGO composites. FlatChem 2022, 32, 100350. [Google Scholar] [CrossRef]
- Rosaiah, P.; Maaouni, N.; Goddati, M.; Lee, J.; Sambasivam, S.; Rezaul Karim, M.; Alnaser, I.A.; Reddy, V.; Reddy, M.; Kim, K.K. Surface design engineering of ZnMn2O4/RGOcomposites for highly stable supercapacitor devices. J. Energy Storage 2024, 76, 109636. [Google Scholar] [CrossRef]
- Sankar, K.V.; Selvan, R.K. Improved electrochemical performances of reduced graphene oxide based supercapacitor using redox additive electrolyte. Carbon 2015, 90, 260–273. [Google Scholar] [CrossRef]
- Reiche, S.; Blume, R.; Zhao, X.C.; Su, D.; Kunkes, E.; Behrens, M.; Schlögl, R. Reactivity of mesoporous carbon against water—An in-situ XPS study. Carbon 2014, 77, 175–183. [Google Scholar] [CrossRef]
- Ganguly, A.; Sharma, S.; Papakonstantinou, P.; Hamilton, J. Probing the thermal deoxygenation of graphene oxide using high-resolution in-situ X-ray-based spectroscopies. J. Phys. Chem. C 2011, 115, 17009–17019. [Google Scholar] [CrossRef]
- Kundu, S.; Wang, Y.; Xia, W.; Muhler, M. Thermal stability and reducibility of oxygen-containing functional groups on multiwalled carbon nanotube surfaces: A quantitative high-resolution XPS and TPD/TPR study. J. Phys. Chem. C 2008, 112, 16869–16878. [Google Scholar] [CrossRef]
- Clark, D.T.; Dilks, A. ESCA applied to polymers XXIII. RF glow discharge modification of polymers in pure oxygen and helium oxygen mixtures. J. Polym. Sci. Pol. Chem. Ed. 1979, 17, 957–976. [Google Scholar]
- YFang, B.; Luo, Y.; Jia, X.; Li, B.; Wang, Q.; Song, F.; Kang, L.Z. Renewing functionalized graphene as electrodes for high-performance supercapacitors. Adv. Mater. 2012, 24, 6348–6355. [Google Scholar]
- Karamanova, B.; Stoyanova, A.; Shipochka, M.; Veleva, S.; Stoyanova, R. Effect of alkaline-basic electrolytes on the capacitance performance of biomass-derived carbonaceous materials. Materials 2020, 13, 2941. [Google Scholar] [CrossRef]
- Karamanova, B.; Stoyanova, A.; Shipochka, M.; Girginov, C.S.; Stoyanova, R. On the cycling stability of biomass-derived carbons as electrodes in supercapacitors. J. Alloys Compd. 2019, 803, 882–890. [Google Scholar] [CrossRef]
- Chen, C.M.; Zhang, Q.; Yang, M.C.; Huang, C.H.; Yang, Y.G.; Wang, M.Z. Structural evolution during annealing of thermally reduced graphene nanosheets for application in supercapacitors. Carbon 2012, 50, 3572–3584. [Google Scholar] [CrossRef]
- Frackowiak, E.; Beguin, F. Carbon materials for the electrochemical storage of energy in capacitors. Carbon 2009, 39, 937–950. [Google Scholar] [CrossRef]
- Bakandritsos, A.; Jakubec, P.; Pykal, M.; Otyepka, M. Covalently functionalized graphene as a supercapacitor electrode material. FlatChem 2019, 13, 25–33. [Google Scholar] [CrossRef]


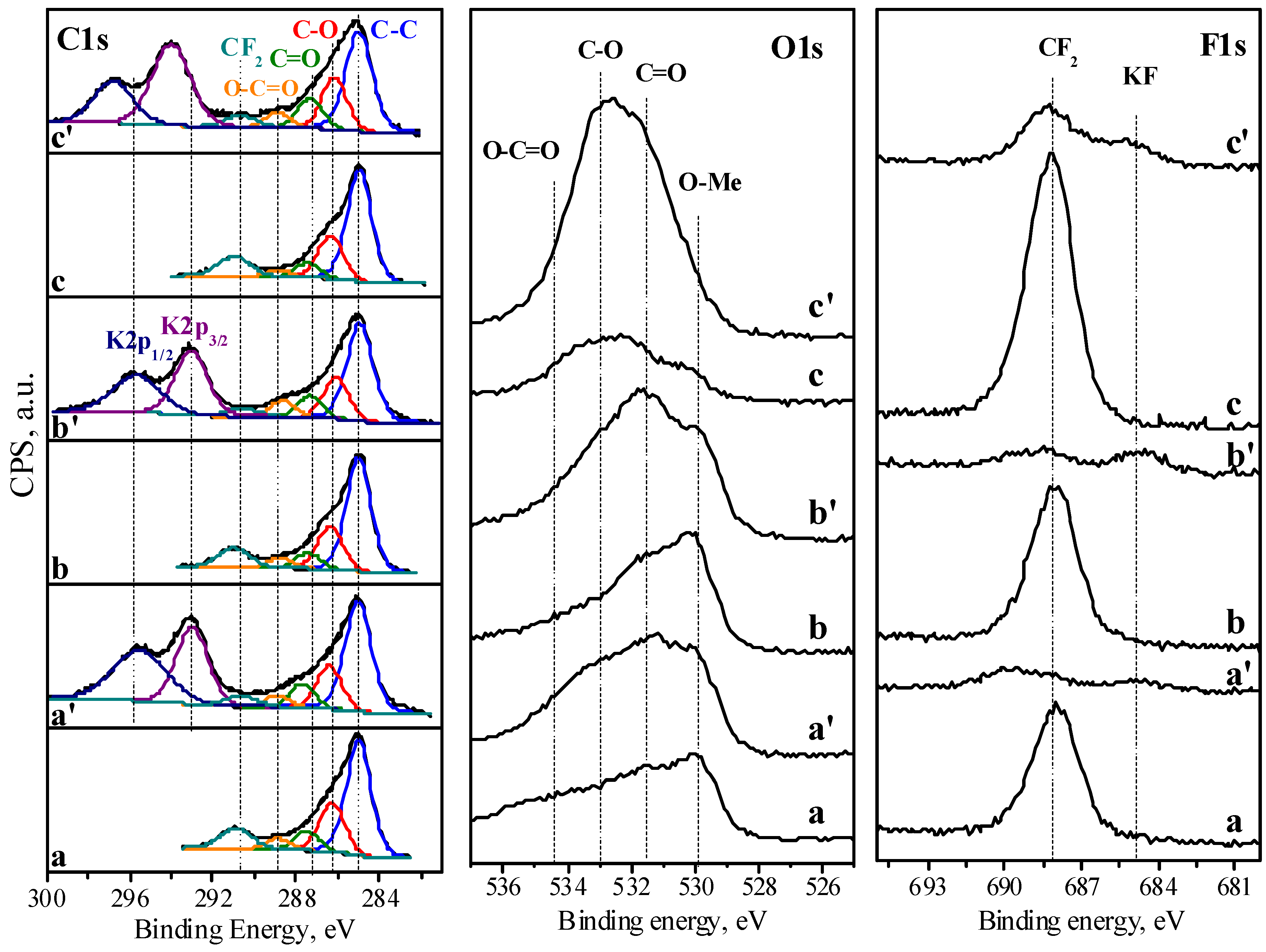
| Samples | C at.% | O at.% | K at.% | F at.% | C-O C-C | C=O C-C | O-C=O C-C |
|---|---|---|---|---|---|---|---|
| NMO/rGO-M | 63.6 | 23.9 | - | 12.5 | 0.42 | 0.17 | 0.09 |
| NMO/rGO-M (10,000 cycles) | 55.9 | 31.7 | 8.7 | 3.7 | 0.35 | 0.20 | 0.10 |
| NMO/rGO-U | 64.9 | 22.3 | - | 12.8 | 0.38 | 0.14 | 0.09 |
| NMO/rGO-U (10,000 cycles) | 56.1 | 33.2 | 7.2 | 3.5 | 0.39 | 0.20 | 0.15 |
| rGO | 74.3 | 9.9 | - | 15.8 | 0.38 | 0.13 | 0.06 |
| rGO (10,000 cycles) | 53.1 | 34.8 | 6.7 | 5.4 | 0.46 | 0.25 | 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veleva, S.; Marinova, D.; Harizanova, S.; Koleva, V.; Lefterova, E.; Shipochka, M.; Dimitrov, O.; Stoyanova, A.; Stoyanova, R. Mixing Approaches in Enhancing the Capacitive Performance of rGO-Based Hybrid Electrodes. Materials 2025, 18, 2460. https://doi.org/10.3390/ma18112460
Veleva S, Marinova D, Harizanova S, Koleva V, Lefterova E, Shipochka M, Dimitrov O, Stoyanova A, Stoyanova R. Mixing Approaches in Enhancing the Capacitive Performance of rGO-Based Hybrid Electrodes. Materials. 2025; 18(11):2460. https://doi.org/10.3390/ma18112460
Chicago/Turabian StyleVeleva, Svetlana, Delyana Marinova, Sonya Harizanova, Violeta Koleva, Elefteria Lefterova, Maria Shipochka, Ognian Dimitrov, Antonia Stoyanova, and Radostina Stoyanova. 2025. "Mixing Approaches in Enhancing the Capacitive Performance of rGO-Based Hybrid Electrodes" Materials 18, no. 11: 2460. https://doi.org/10.3390/ma18112460
APA StyleVeleva, S., Marinova, D., Harizanova, S., Koleva, V., Lefterova, E., Shipochka, M., Dimitrov, O., Stoyanova, A., & Stoyanova, R. (2025). Mixing Approaches in Enhancing the Capacitive Performance of rGO-Based Hybrid Electrodes. Materials, 18(11), 2460. https://doi.org/10.3390/ma18112460








