Potential Function-Based Molecular Dynamics Simulation of Al-Cu-Li Alloys and Comparison with Experiments
Abstract
1. Introduction
2. Simulation and Experimental Method
2.1. MD Simulation Statement
2.2. Materials
2.3. Creep-Aging Tests and Mechanical Properties Tests
2.4. Microstructure Characterization
3. Results and Discussion
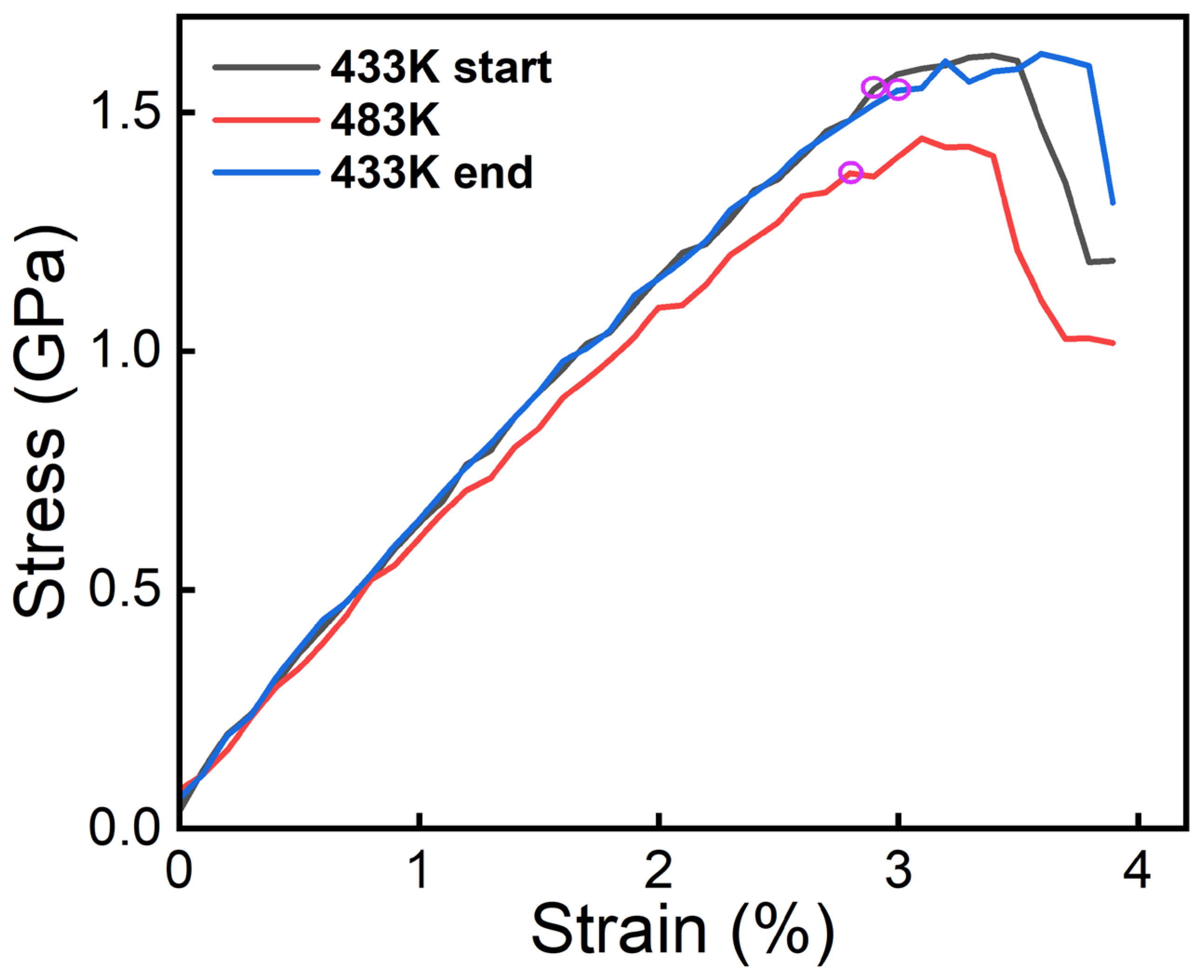
3.1. MD Simulation Results of Dislocation–Precipitate Interaction
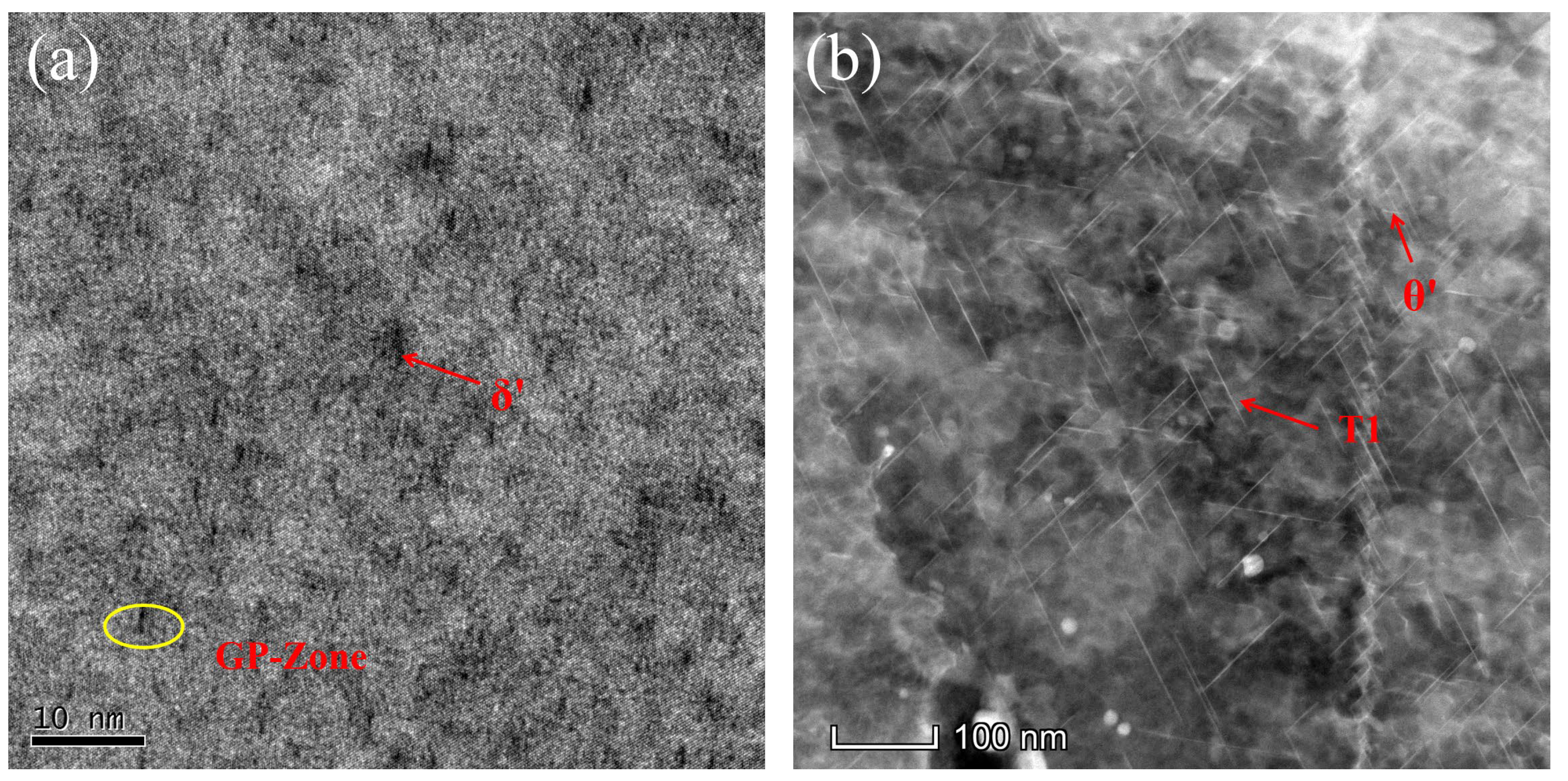
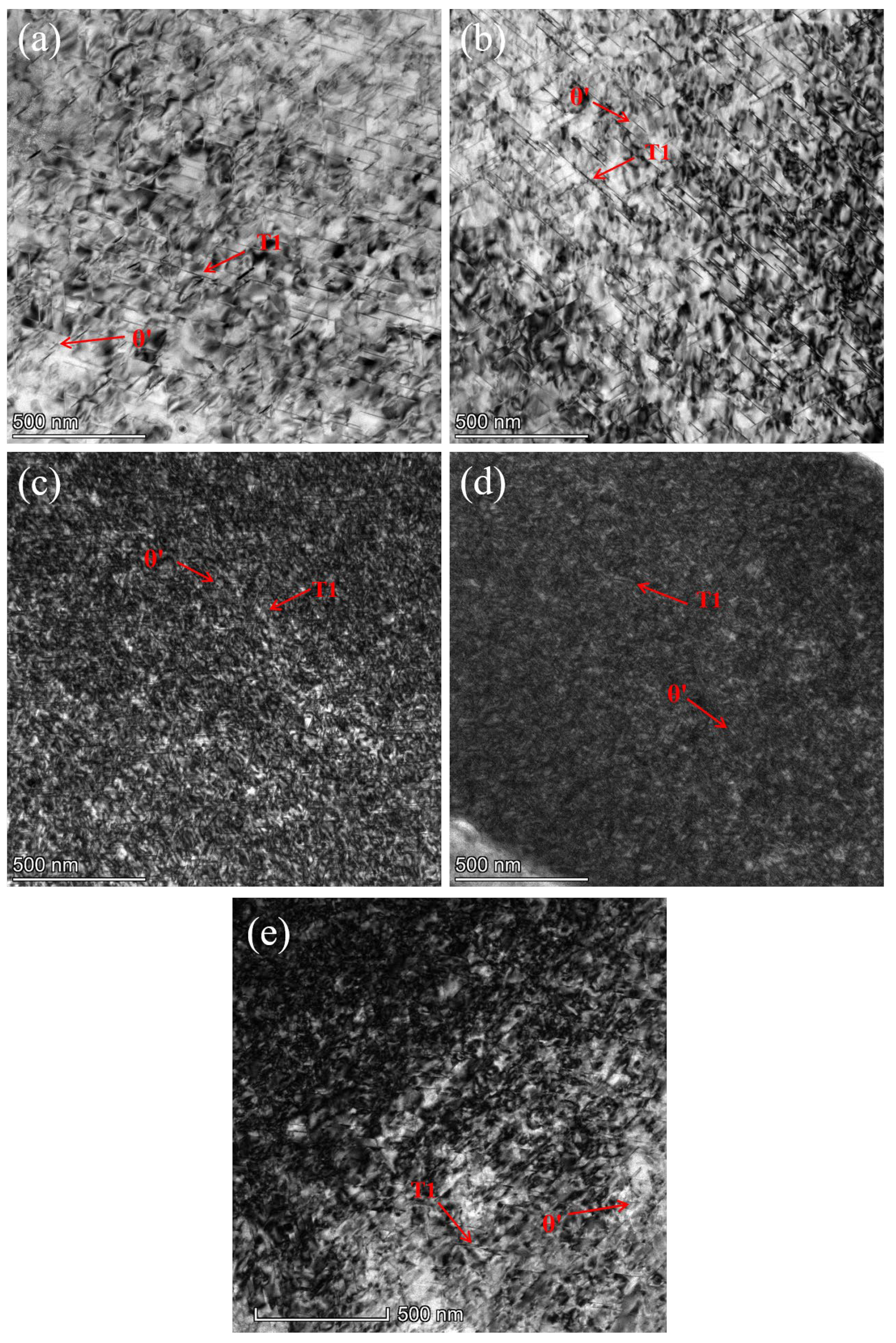
3.2. Mechanical Properties and Microstructure After Multi-Stage CA
4. Conclusions
- (1)
- After undergoing the low-temperature-high-temperature-low-temperature three-stage CA process, the specimens achieved a tensile strength of 598 MPa and a yield strength of 559 MPa, which are comparable to the peak performance of the traditional single-stage CA specimens. This result demonstrates that the multi-stage CA process not only ensures a high degree of creep deformation but also maintains excellent mechanical properties, achieving a synergistic optimization of both high plasticity and high strength.
- (2)
- When the temperature of the first-stage low-temperature CA is too low, it leads to the re-dissolution of precipitates during the high-temperature stage. Similarly, if the temperature of the second-stage high-temperature aging is too high, it will also cause the dissolution of a large amount of precipitates. Both scenarios result in a reduction in nucleation sites, thereby affecting the material’s performance.
- (3)
- The segregation behavior of Li atoms promotes the precipitation of the T1 strengthening phase in Al-Cu-Li alloys. However, as the temperature increases, the segregation of Li atoms gradually diminishes, leading to the re-dissolution of the precipitate phase and consequently causing a deterioration in the material’s mechanical properties. The excellent agreement between the MD simulation results based on the NEP function and the experimental results further validates the reliability of the NEP function in simulating complex alloy systems.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dursun, T.; Soutis, C. Recent developments in advanced aircraft aluminium alloys. Mater. Des. (1980–2015) 2014, 56, 862–871. [Google Scholar] [CrossRef]
- Honma, T.; Yanagita, S.; Hono, K.; Nagai, Y.; Hasegawa, M. Coincidence Doppler broadening and 3DAP study of the pre-precipitation stage of an Al–Li–Cu–Mg–Ag alloy. Acta Mater. 2004, 52, 1997–2003. [Google Scholar] [CrossRef]
- Zhang, L.; Heng, L.I.; Tianjun, B.I.A.N.; Changhui, W.U.; Yanfeng, Y.A.N.G. Significant reduction of anisotropy in stress relaxation aging and mechanical properties improvement for 2195 Al-Cu-Li alloy subjected to plastic loading. Chin. J. Aeronaut. 2025, 38, 103165. [Google Scholar] [CrossRef]
- Long, S.; Jiang, Y.P.; Xia, R.Z.; Peng, P.; Zhang, C.; Li, S.S.; Dai, Q.W.; Zhou, J. Recrystallization behavior and kinetic analysis of an Al-Cu-Li alloy during hot deformation and subsequent heat treatment. Mater. Charact. 2024, 208, 113640. [Google Scholar] [CrossRef]
- Song, L.; Li, H.; Zhao, G.; Bao, X.; Zheng, Z. Quantification of precipitate evolution in cast Al–Li alloys containing Cu and Mg additions using small-angle X-ray scattering. J. Mater. Res. Technol. 2024, 33, 7362–7370. [Google Scholar] [CrossRef]
- Gao, Z.; Chen, J.H.; Duan, S.Y.; Yang, X.B.; Wu, C.L. Complex precipitation sequences of Al-Cu-Li-(Mg) alloys characterized in relation to thermal ageing processes. Acta Metall. Sin. Engl. Lett. 2016, 29, 94–103. [Google Scholar] [CrossRef]
- Duan, S.; Liu, Z.; Guo, F.; Pan, Y.; Matsuda, K.; Zou, Y. Precipitates evolution during artificial aging and their influence on mechanical properties of a cast Al–Cu–Li alloy. J. Mater. Res. Technol. 2023, 22, 2502–2517. [Google Scholar] [CrossRef]
- Deschamps, A.; Decreus, B.; De Geuser, F.; Dorin, T.; Weyland, M. The influence of precipitation on plastic deformation of Al–Cu–Li alloys. Acta Mater. 2013, 61, 4010–4021. [Google Scholar] [CrossRef]
- Dorin, T.; Deschamps, A.; De Geuser, F.; Sigli, C. Quantification and modelling of the microstructure/strength relationship by tailoring the morphological parameters of the T1 phase in an Al–Cu–Li alloy. Acta Mater. 2014, 75, 134–146. [Google Scholar] [CrossRef]
- Xie, B.; Huang, L.; Xu, J.; Su, H.; Zhang, H.; Xu, Y.; Li, J.; Wang, Y. Effect of the aging process and pre-deformation on the precipitated phase and mechanical properties of 2195 Al–Li alloy. Mater. Sci. Eng. A 2022, 832, 142394. [Google Scholar] [CrossRef]
- Hu, W.; Chen, J.; Xu, J.; Ren, J.; Miao, J.; Xing, T.; Guan, R.; Ojo, O.A. Relationship between precipitation behavior and loading orientations of the creep-aged Al–Cu–Li single crystal. J. Mater. Res. Technol. 2023, 24, 689–702. [Google Scholar] [CrossRef]
- Wu, C.H.; Li, H.; Lei, C.; Zhang, D.; Bian, T.J.; Zhang, L.W. Origin and effect of anisotropy in creep aging behavior of Al–Cu–Li alloy. J. Mater. Res. Technol. 2023, 26, 3368–3382. [Google Scholar] [CrossRef]
- Wang, D.; Zhan, L.; Liu, C.; Zeng, Q.; Xu, Y.; Ma, B.; Gan, K.; Lai, R.; Li, Y.; Liu, C. Study on creep aging behavior of the friction stir welded Al-Li alloy joints under different stresses. Mater. Charact. 2024, 210, 113763. [Google Scholar] [CrossRef]
- Al-Motasem, A.T.; Posselt, M.; Bergner, F.; Birkenheuer, U. Structure, energetics and thermodynamics of copper–vacancy clusters in bcc-Fe: An atomistic study. J. Nucl. Mater. 2011, 414, 161–168. [Google Scholar] [CrossRef]
- Molnar, D.; Mukherjee, R.; Choudhury, A.; Mora, A.; Binkele, P.; Selzer, M.; Nestler, B.; Schmauder, S. Multiscale simulations on the coarsening of Cu-rich precipitates in α-Fe using kinetic Monte Carlo, molecular dynamics and phase-field simulations. Acta Mater. 2012, 60, 6961–6971. [Google Scholar] [CrossRef]
- Clouet, E.; Barbu, A.; Laé, L.; Martin, G. Precipitation kinetics of Al3Zr and Al3Sc in aluminum alloys modeled with cluster dynamics. Acta Mater. 2005, 53, 2313–2325. [Google Scholar] [CrossRef]
- Yi, D.; Wei, G. MD Simulation of Diffusion Behaviors in Collision Welding Processes of Al-Cu, Al-Al, Cu-Cu. Comput. Mater. Contin. 2024, 79, 3455. [Google Scholar]
- Ouyang, D.; Mao, R.; Zhang, L.; Liang, S.; Song, J. Study on the tensile properties of Al-Zn-Mg alloy based on molecular dynamics. Eng. Fail. Anal. 2024, 155, 107752. [Google Scholar] [CrossRef]
- Li, F.; Dong, T.; Zhang, C.; Chen, G.; Chen, S.; Chen, K.; Zhu, C. Controllable precipitation behavior near grain boundaries enabled by artificial strain concentration in age-hardening aluminum alloys. Mater. Sci. Eng. A 2024, 892, 146026. [Google Scholar] [CrossRef]
- Tang, L.; Guo, A.; Farid, W.; Wang, Z.; Kong, C.; Zhang, L.; Yu, H. Enhanced mechanical and corrosion properties of 2195 Al-Li alloy via cryogenic pre-rolling and aging. J. Alloys Compd. 2025, 1013, 178664. [Google Scholar] [CrossRef]
- Shuai, X.; Hong, M.A.O.; Sai, T.A.N.G.; Yi, K.O.N.G.; Yong, D.U. Atomic-scale insights into microscopic mechanisms of grain boundary segregation in Al−Cu alloys. Trans. Nonferrous Met. Soc. China 2025, 35, 1–12. [Google Scholar] [CrossRef]
- Liao, H.; Du, J.P.; Kimizuka, H.; Ogata, S. Atomistic simulation of Guinier–Preston zone nucleation kinetics in Al–Cu alloys: A neural network-driven kinetic Monte Carlo approach. Comput. Mater. Sci. 2025, 251, 113771. [Google Scholar] [CrossRef]
- Xu, L.; Tong, C.; Zhan, L.; Xu, Y.; Liu, C.; Huang, M.; Yang, Y.; Ma, B.; Wang, Y. Improved creep forming efficiency and retained performance via a novel two-stage creep aging process of Al–Zn–Mg–Cu alloys. Mater. Sci. Eng. A 2022, 851, 143581. [Google Scholar] [CrossRef]
- Chen, F.; Yu, Y.; Jiang, Y.; Zeng, Q.; Zhan, L. A new multi-stage creep aging process for improving creep age formability and maintaining performance of Al-Li alloy. Prog. Nat. Sci. Mater. Int. 2025; in press. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, Y.; Ying, P.; Song, K.; Wang, J.; Wang, Y.; Zeng, Z.; Xu, K.; Lindgren, E.; Rahm, J.M.; et al. GPUMD: A package for constructing accurate machine-learned potentials and performing highly efficient atomistic simulations. J. Chem. Phys. 2022, 157, 114801. [Google Scholar] [CrossRef]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO–the Open Visualization Tool. Model. Simul. Mater. Sci. Eng. 2009, 18, 015012. [Google Scholar] [CrossRef]
- Chen, F.; Wang, H.; Jiang, Y.; Zhan, L.; Yang, Y. Development of a Neuroevolution Machine Learning Potential of Al-Cu-Li Alloys. Metals 2025, 15, 48. [Google Scholar] [CrossRef]
- Fan, Z.; Zeng, Z.; Zhang, C.; Wang, Y.; Song, K.; Dong, H.; Chen, Y.; Ala-Nissila, T. Neuroevolution machine learning potentials: Combining high accuracy and low cost in atomistic simulations and application to heat transport. Phys. Rev. B 2021, 104, 104309. [Google Scholar] [CrossRef]
- Chen, F.; Wang, P.; Zhan, L.; Ruan, X.; Wu, H.; Xu, Y.; Ma, B.; Liu, C.; Zeng, Q.; Hu, Z.; et al. Creep behavior and microstructure evolution during two-stage creep aging of a 2195 Al–Li alloy. Mater. Sci. Eng. A 2022, 850, 143555. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, C.; Cheng, Z.; Zhao, H.; Meng, Z.; Chen, L.; Zhao, G. Dynamic evolution of the T1 phase and its effect on continuous dynamic recrystallization in Al–Cu–Li alloys. Int. J. Plast. 2024, 175, 103948. [Google Scholar] [CrossRef]
- Wu, M.; Yao, T.; Xiao, D.; Yuan, S.; Li, Z.; Wang, J.; Huang, L.; Liu, W. Precipitation behavior and properties of an Al-8.26 Zn-1.95 Mg-1.89 Cu-0.08 Sc-0.17 Zr alloy with different dislocation morphologies via pre-treatment. J. Mater. Res. Technol. 2024, 29, 4714–4727. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, S.; Guo, X.; He, X.; Liang, C.; Deng, Y. Modulation of precipitation behavior by dislocations and alloying for superior strength-ductility balance in Al-Cu-Li alloys. J. Alloys Compd. 2025, 1010, 177334. [Google Scholar] [CrossRef]
- Wang, X.M.; Li, G.A.; Jiang, J.T.; Shao, W.Z.; Zhen, L. Influence of Mg content on ageing precipitation behavior of Al-Cu-Li-x alloys. Mater. Sci. Eng. A 2019, 742, 138–149. [Google Scholar] [CrossRef]
- Wang, J.; Yang, K.; Lu, Y.; Cheng, X.; You, D.; Zhou, D.; Wang, M.; Zhu, B.; Li, J. Investigation on the microstructural evolution and corrosion behaviors of Zn-microalloyed Al-Cu-Li alloys. Mater. Today Commun. 2024, 41, 110464. [Google Scholar] [CrossRef]
- Deng, Y.; Bai, J.; Wu, X.; Huang, G.; Cao, L.; Huang, L. Investigation on formation mechanism of T1 precipitate in an Al-Cu-Li alloy. J. Alloys Compd. 2017, 723, 661–666. [Google Scholar] [CrossRef]
- Li, J.F.; Huang, J.L.; Liu, D.Y.; Chen, Y.L.; Zhang, X.H. Distribution and evolution of aging precipitates in Al-Cu-Li alloy with high Li concentration. Trans. Nonferrous Met. Soc. China 2019, 29, 15–24. [Google Scholar] [CrossRef]
- Firrao, D.; Scavino, G.; Doglione, R. Precipitation phenomena during an Al-Li-Cu alloy ageing. In Proceedings of the 5th International Conference on Aluminum-Lithium Alloys, Williamsburg, VA, USA, 27–31 March 1989; MCPE Ltd.: Birmingham, UK, 1989; Volume 2, pp. 671–680. [Google Scholar]
- Engler, O.; Marioara, C.D.; Aruga, Y.; Kozuka, M.; Myhr, O.R. Effect of natural ageing or pre-ageing on the evolution of precipitate structure and strength during age hardening of Al–Mg–Si alloy AA 6016. Mater. Sci. Eng. A 2019, 759, 520–529. [Google Scholar] [CrossRef]
- Xue, L.W.; Jia, H.L.; Wang, J.K.; Zha, M.; Jin, S.B.; Wang, H.Y. Superior strength-ductility synergy of Al-Si-Cu-Mg alloys achieved by regulating solute clusters and precipitates: Experimental validation and numerical simulation. Int. J. Plast. 2025, 188, 104320. [Google Scholar] [CrossRef]
- Yu, Z.; Li, H.; Cai, P.; Fu, X.; Feng, Z.; Zhang, L.; Wang, J.; Xiao, N. Effect of aging route on the precipitation behavior and thermal stability of Al–Cu–Mg–Ag alloy. J. Mater. Res. Technol. 2023, 23, 2010–2019. [Google Scholar] [CrossRef]
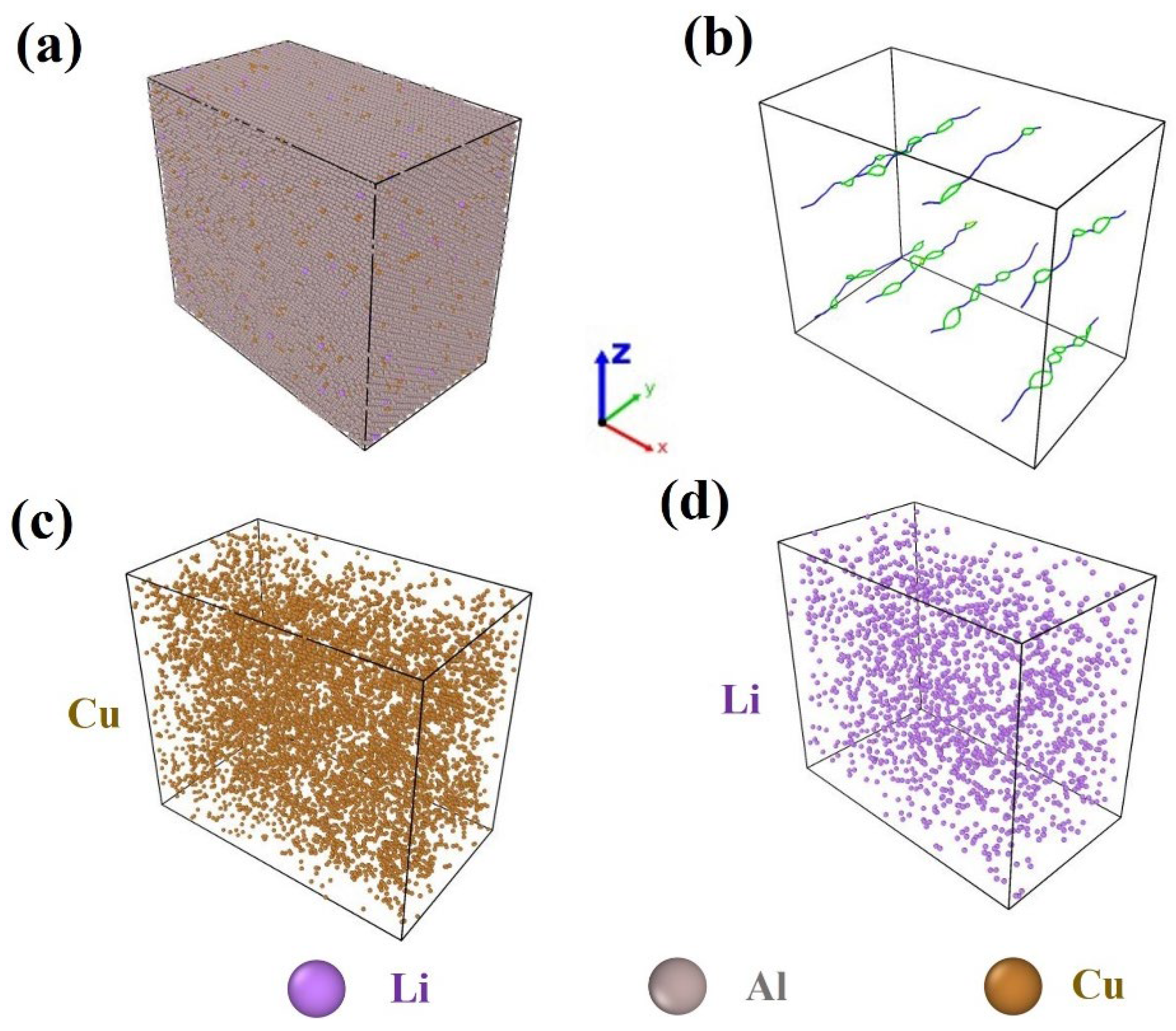


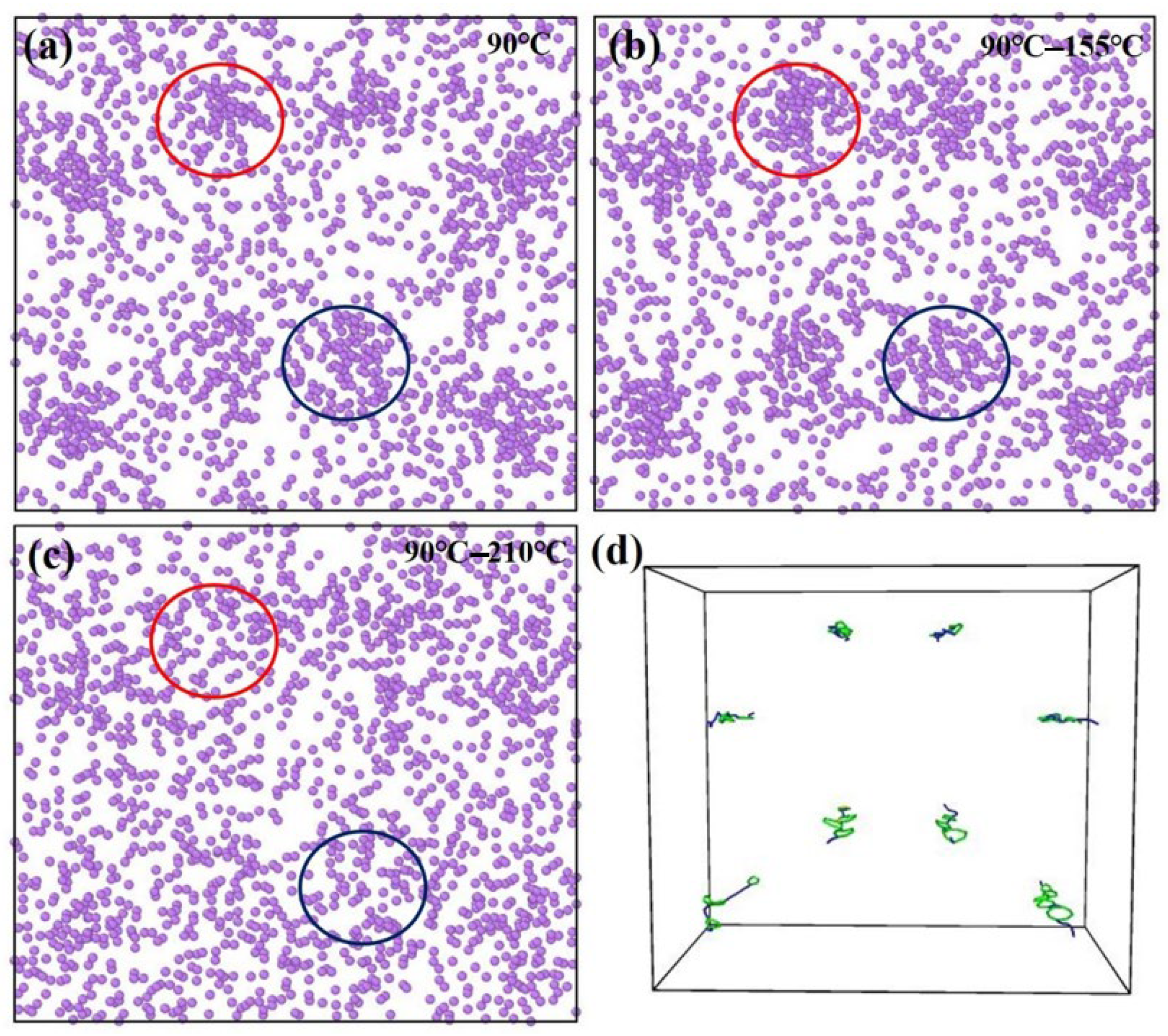


| Boundary Condition | Parameters |
|---|---|
| Model size | 181.61 Å × 105.92 Å × 159.78 Å |
| Boundary conditions (X, Y, Z) | P, P, P (P: Periodic boundary conditions) |
| Simulation pressure | 220 MPa |
| Simulation temperature | 90, 155, 210, 260 °C |
| Time step | 1 fs |
| Simulation steps | 2,000,000 |
| Cu | Li | Mg | Ag | Fe | Zr | Mn | Si | Ti | Al |
|---|---|---|---|---|---|---|---|---|---|
| 4.18 | 1.19 | 0.40 | 0.38 | 0.052 | 0.094 | 0.0047 | 0.014 | 0.018 | Bal. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Wang, H.; Liu, Y.; Qi, L.; Zeng, Q. Potential Function-Based Molecular Dynamics Simulation of Al-Cu-Li Alloys and Comparison with Experiments. Materials 2025, 18, 2420. https://doi.org/10.3390/ma18112420
Chen F, Wang H, Liu Y, Qi L, Zeng Q. Potential Function-Based Molecular Dynamics Simulation of Al-Cu-Li Alloys and Comparison with Experiments. Materials. 2025; 18(11):2420. https://doi.org/10.3390/ma18112420
Chicago/Turabian StyleChen, Fei, Han Wang, Yu Liu, Liangtao Qi, and Quanqing Zeng. 2025. "Potential Function-Based Molecular Dynamics Simulation of Al-Cu-Li Alloys and Comparison with Experiments" Materials 18, no. 11: 2420. https://doi.org/10.3390/ma18112420
APA StyleChen, F., Wang, H., Liu, Y., Qi, L., & Zeng, Q. (2025). Potential Function-Based Molecular Dynamics Simulation of Al-Cu-Li Alloys and Comparison with Experiments. Materials, 18(11), 2420. https://doi.org/10.3390/ma18112420




