Bioactive Glass and Melittin Thin Films Deposited by MAPLE for Titanium Implant Functionalization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Thin Films Characterization Methods
2.3.1. Scanning Electron Microscopy and Elemental Analysis
2.3.2. Atomic Force Microscopy
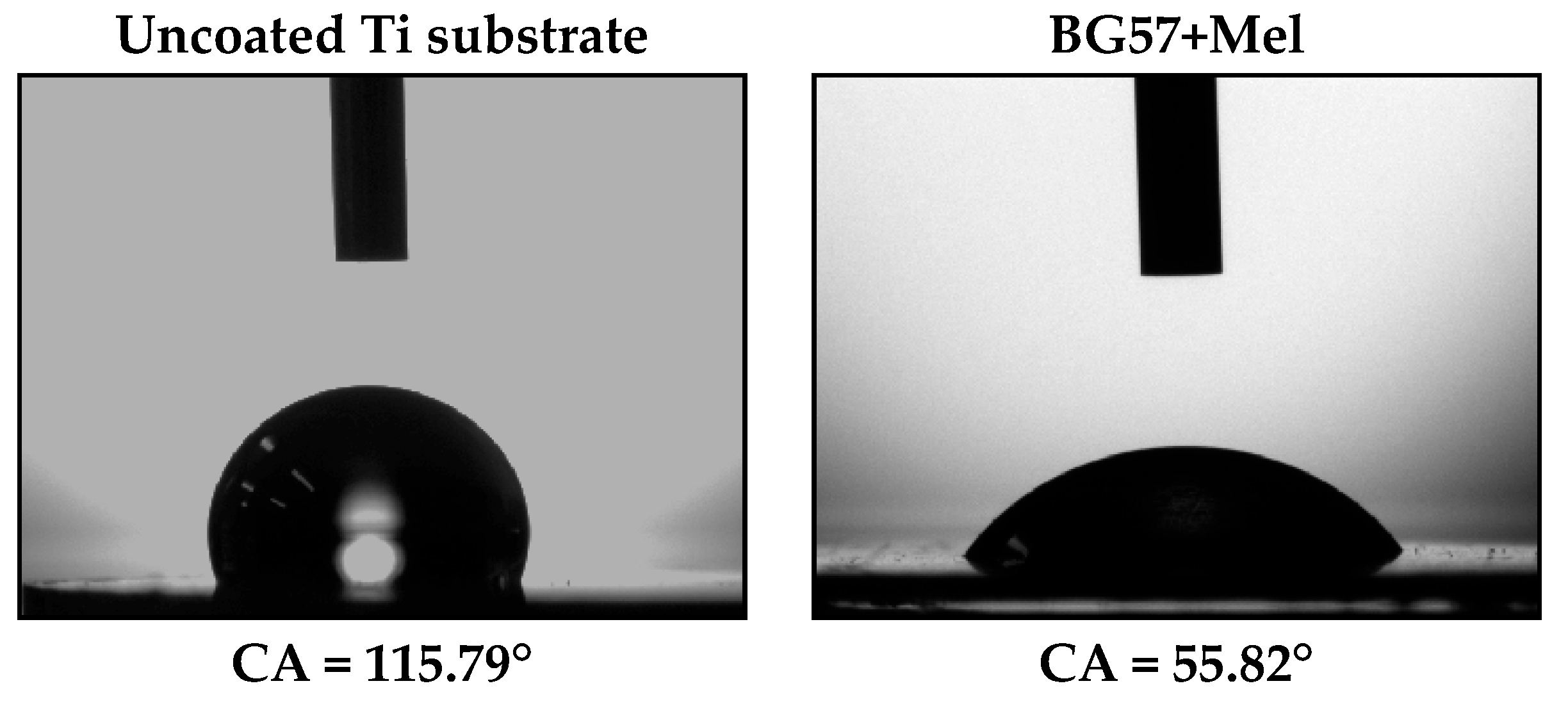
2.3.3. Contact Angle Measurements and Surface Wettability
2.3.4. Electrochemical Behavior of the Samples
2.3.5. Fourier Transform Infrared Spectroscopy
3. Results
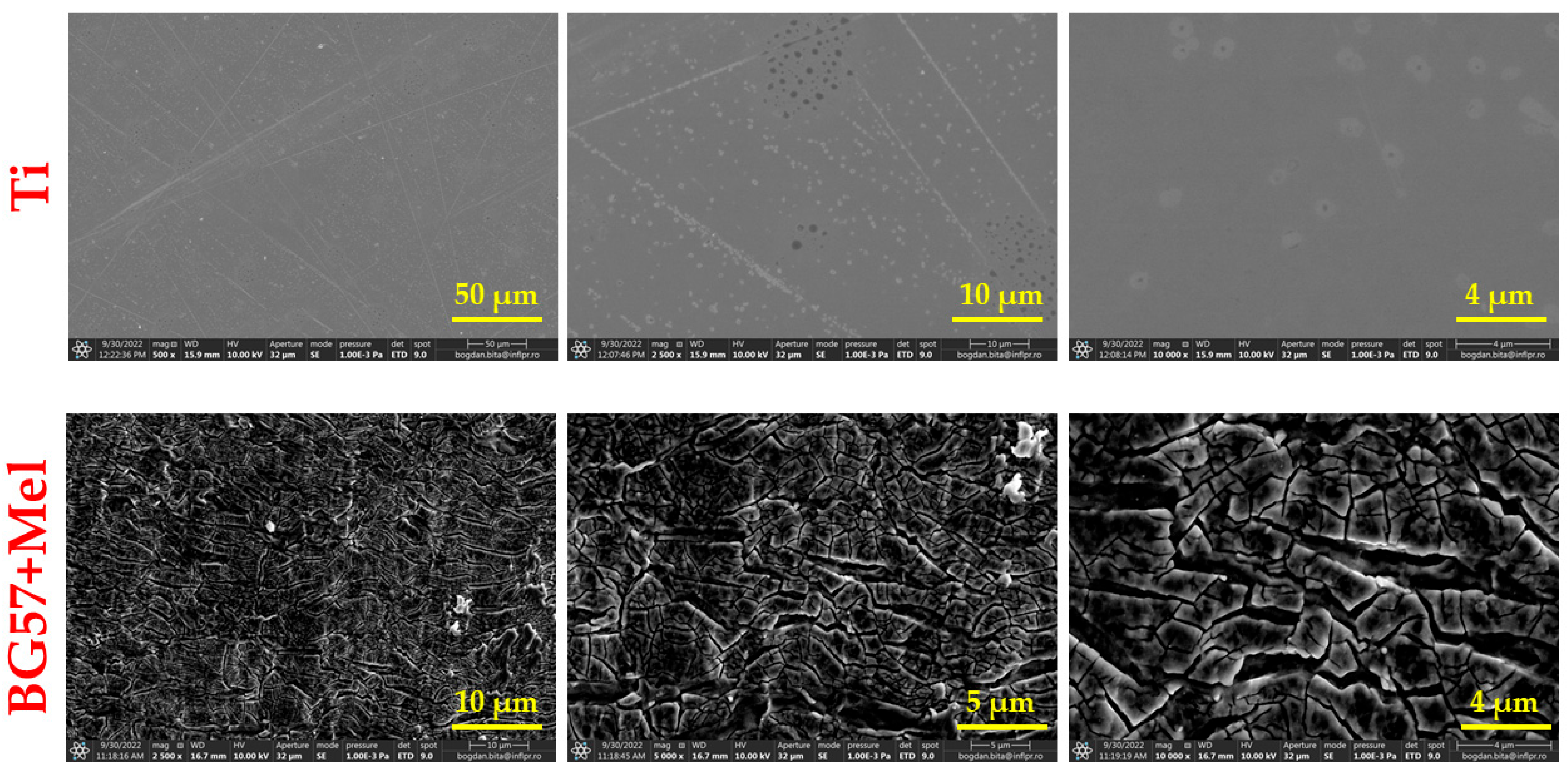
3.1. Surface Investigation of As-Deposited Thin Films
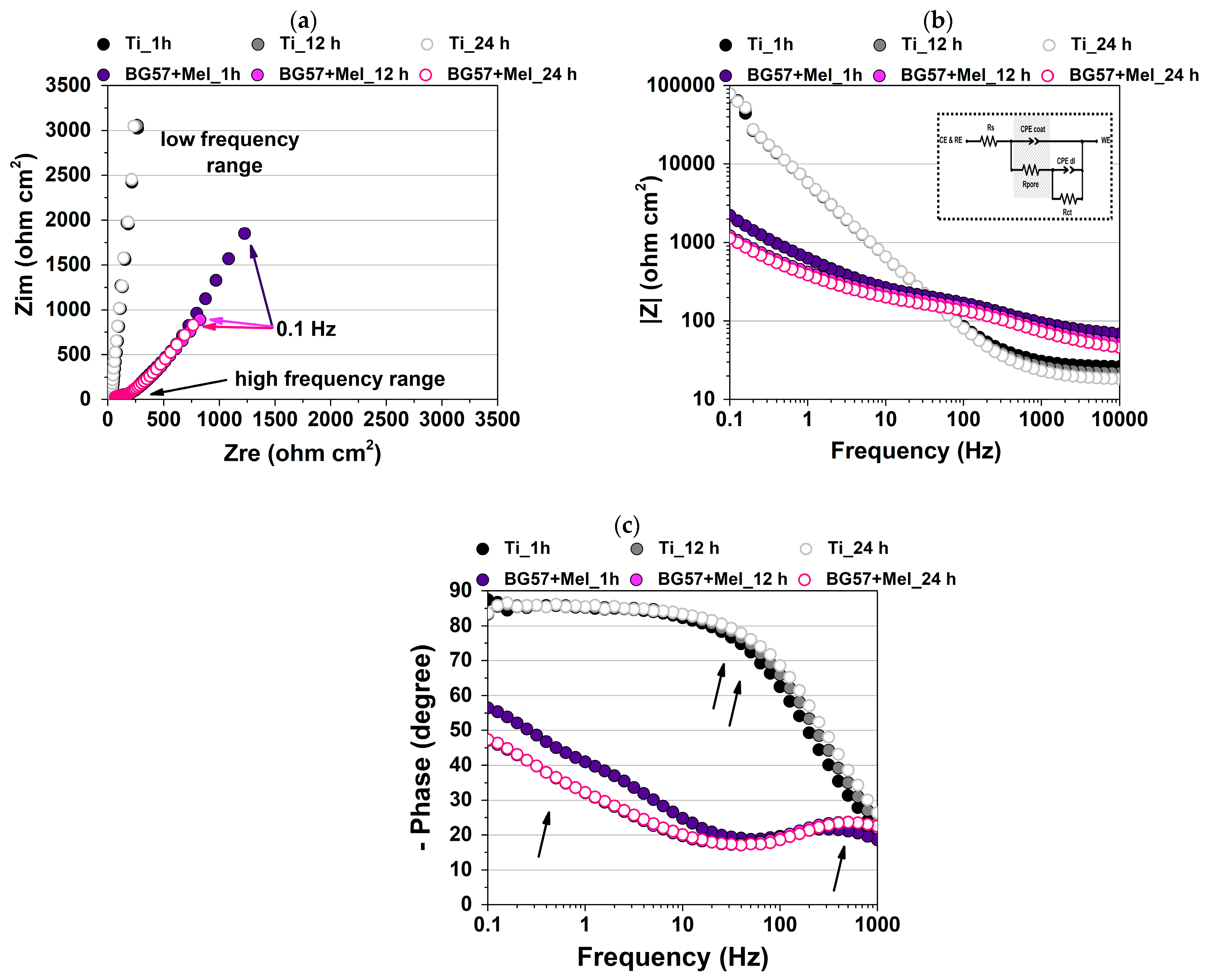
3.2. Electrochemical Performance of the Tested Samples
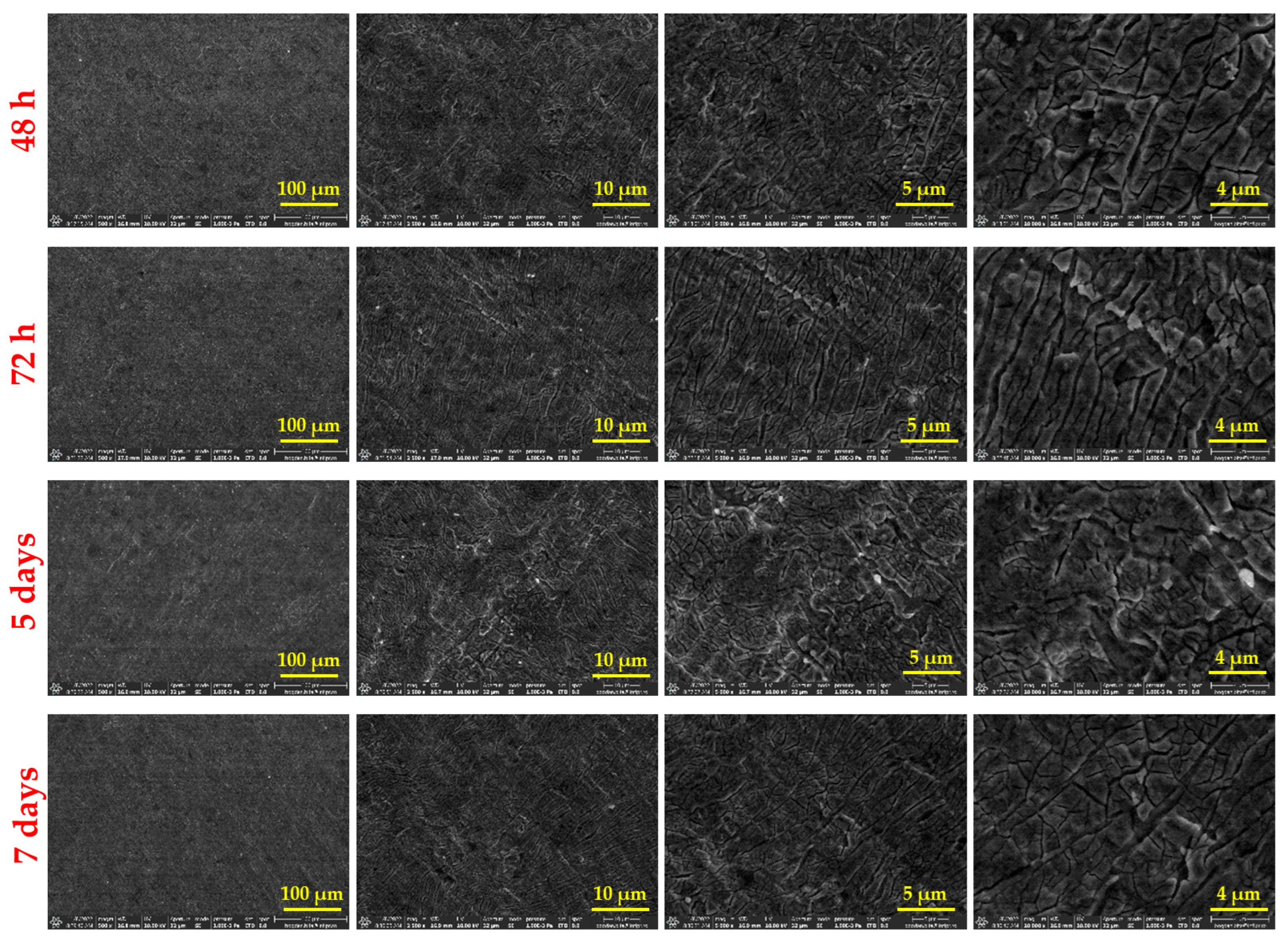
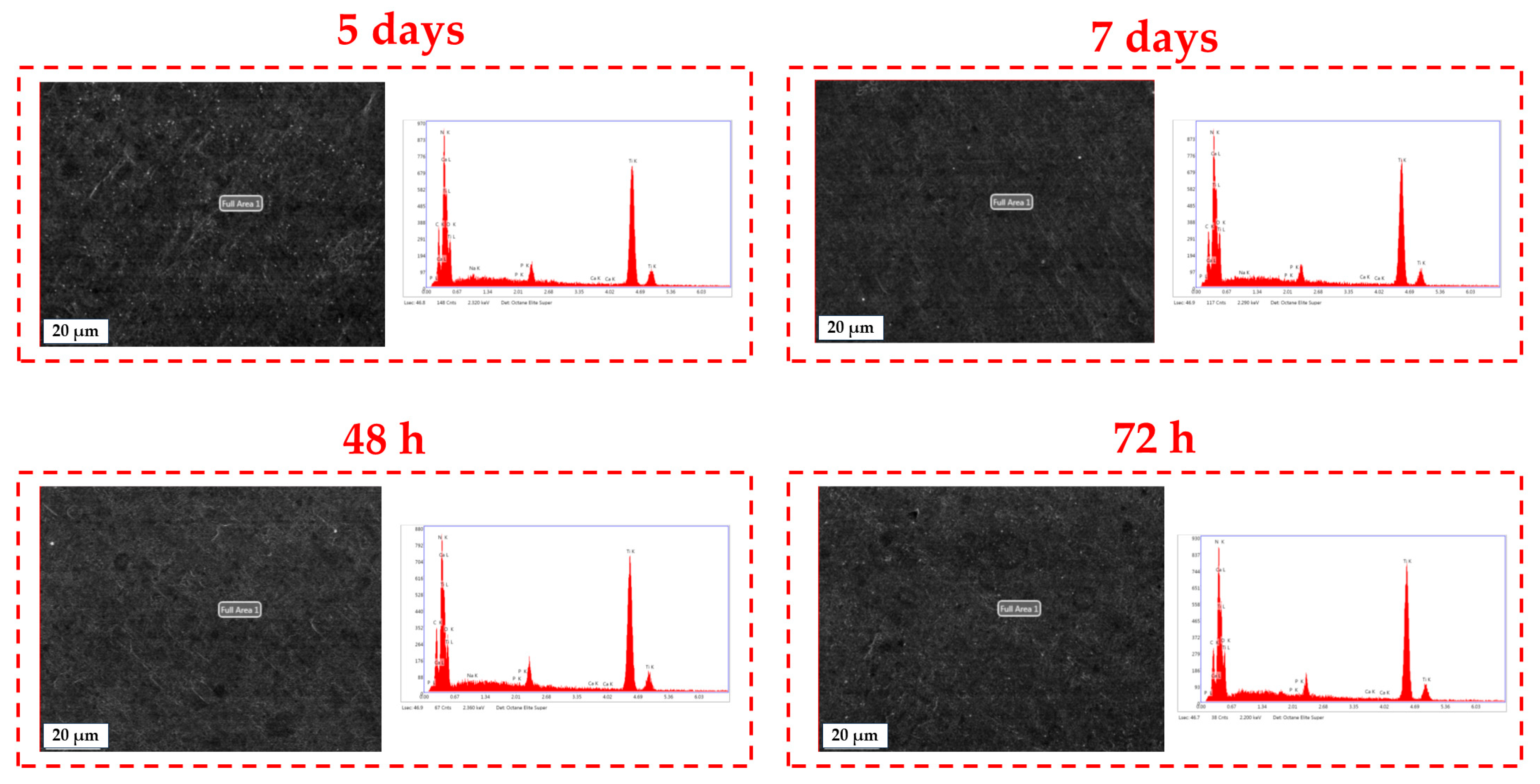
3.3. Surface Investigation of Thin Films After SBF Immersion
4. Discussion
4.1. Surface Investigation of As-Deposited Thin Films
4.2. Electrochemical Performance of the Tested Samples
4.3. Surface Investigation of Thin Films After SBF Immersion
5. Conclusions
- MAPLE-deposited composite coatings composed of BG57 and Mel significantly enhance the surface properties and bioactivity of Ti substrates, making them promising candidates for biomedical implant applications.
- The resulting thin films exhibited a rough, nanoscale-textured morphology, which is favorable for supporting cell adhesion and tissue integration.
- Contact angle measurements confirmed a marked improvement in surface wettability, with the coatings transitioning from hydrophobic to moderately hydrophilic behavior.
- EIS demonstrated enhanced corrosion resistance in SBF, indicating the protective effect of the BG57+Mel coating.
- FTIR and EDS analyses confirmed the gradual formation of a carbonated apatite layer over time, supporting the material’s osteoconductive potential.
- The integration of Mel successfully functionalized the surface without compromising the structural integrity of the films, highlighting the benefit of MAPLE for depositing thermally sensitive organic–inorganic composites.
- The study’s limitations include the absence of direct antimicrobial and cytocompatibility testing, the exclusive use of SBF immersion to assess bioactivity, and testing under static in vitro conditions only. These limitations emphasize the need for future research, which should focus on cytocompatibility and cell viability assays, following established methodologies as outlined in systematic reviews such as Valenti et al., to fully validate the coatings’ biocompatibility [89].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chakraborty, A.; Bodhak, S.; Tah, I.; Kant, S.; Saha, D.; Dey, K.K.; Gupta, N.; Ghosh, M.; Tripathy, S.; Allu, A.R.; et al. Tailored Bioactive Glass Coating: Navigating Devitrification Toward a Superior Implant Performance. ACS Biomater. Sci. Eng. 2024, 10, 5300–5312. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Miao, L.; Meng, X.; Sui, J.; Chen, M.; Zheng, Z.; Huo, S.; Liu, S.; Zhang, H. Strontium-Doped Bioactive Glass-Functionalized Polyetheretherketone Enhances Osseointegration by Facilitating Cell Adhesion. Colloids Surf. B Biointerfaces 2024, 241, 114042. [Google Scholar] [CrossRef] [PubMed]
- Avelino, S.d.O.M.; Alvares Sobral-Silva, L.; Thim, G.P.; de Almeida-Silva, L.A.; dos Santos Lupp, J.; Campos, T.M.B.; de Vasconcellos, L.M.R. Development, Characterization, and Biological Study of Bioglass Coatings 45S5 and BioK on Zirconia Implant Surfaces. J. Biomed. Mater. Res. B Appl. Biomater. 2024, 112, e35380. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Lai, J.-Y. Synthesis, Bioactive Properties, and Biomedical Applications of Intrinsically Therapeutic Nanoparticles for Disease Treatment. Chem. Eng. J. 2022, 435, 134970. [Google Scholar] [CrossRef]
- Manivasagam, V.K.; Popat, K.C. Hydrothermally Treated Titanium Surfaces for Enhanced Osteogenic Differentiation of Adipose Derived Stem Cells. Mater. Sci. Eng. C 2021, 128, 112315. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, X.; Hsu, Y.; He, Y.; Wang, F.; Yang, F.; Yan, F.; Xia, D.; Liu, Y. Surface Modification of Titanium Implants with Mg-Containing Coatings to Promote Osseointegration. Acta Biomater. 2023, 169, 19–44. [Google Scholar] [CrossRef]
- Homa, K.; Zakrzewski, W.; Dobrzyński, W.; Piszko, P.J.; Piszko, A.; Matys, J.; Wiglusz, R.J.; Dobrzyński, M. Surface Functionalization of Titanium-Based Implants with a Nanohydroxyapatite Layer and Its Impact on Osteoblasts: A Systematic Review. J. Funct. Biomater. 2024, 15, 45. [Google Scholar] [CrossRef]
- Tuikampee, S.; Chaijareenont, P.; Rungsiyakull, P.; Yavirach, A. Titanium Surface Modification Techniques to Enhance Osteoblasts and Bone Formation for Dental Implants: A Narrative Review on Current Advances. Metals 2024, 14, 515. [Google Scholar] [CrossRef]
- Deng, J.; Van Duyn, C.; Cohen, D.J.; Schwartz, Z.; Boyan, B.D. Strategies for Improving Impaired Osseointegration in Compromised Animal Models. J. Dent. Res. 2024, 103, 467–476. [Google Scholar] [CrossRef]
- Liang, J.; Lu, X.; Zheng, X.; Li, Y.R.; Geng, X.; Sun, K.; Cai, H.; Jia, Q.; Jiang, H.B.; Liu, K. Modification of Titanium Orthopedic Implants with Bioactive Glass: A Systematic Review of in Vivo and in Vitro Studies. Front. Bioeng. Biotechnol. 2023, 11, 1269223. [Google Scholar] [CrossRef]
- Costa, R.C.; Souza, J.G.S.; Cordeiro, J.M.; Bertolini, M.; de Avila, E.D.; Landers, R.; Rangel, E.C.; Fortulan, C.A.; Retamal-Valdes, B.; da Cruz, N.C.; et al. Synthesis of Bioactive Glass-Based Coating by Plasma Electrolytic Oxidation: Untangling a New Deposition Pathway toward Titanium Implant Surfaces. J. Colloid Interface Sci. 2020, 579, 680–698. [Google Scholar] [CrossRef] [PubMed]
- AlMaimouni, Y.K.; Benrashed, M.A.; Alyousef, N.I.; Shah, A.T.; Khan, A.S. 6—Bioactive Glass Coated Dental Implants. In Dental Implants; Zafar, M.S., Khurshid, Z., Khan, A.S., Najeeb, S., Sefat, F., Eds.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2020; pp. 93–115. ISBN 978-0-12-819586-4. [Google Scholar]
- Beltrán, A.M.; Begines, B.; Alcudia, A.; Rodríguez-Ortiz, J.A.; Torres, Y. Biofunctional and Tribomechanical Behavior of Porous Titanium Substrates Coated with a Bioactive Glass Bilayer (45S5–1393). ACS Appl. Mater. Interfaces 2020, 12, 30170–30180. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Q.; He, Y.; Geng, X.; Wang, C.; Li, Y.; Li, Z.; Wang, C.; Qiu, D.; Tian, H. A pH-Neutral Bioactive Glass Coated 3D-Printed Porous Ti6Al4V Scaffold with Enhanced Osseointegration. J. Mater. Chem. B 2023, 11, 1203–1212. [Google Scholar] [CrossRef]
- Zarghami, V.; Ghorbani, M.; Pooshang Bagheri, K.; Shokrgozar, M.A. Melittin Antimicrobial Peptide Thin Layer on Bone Implant Chitosan-Antibiotic Coatings and Their Bactericidal Properties. Mater. Chem. Phys. 2021, 263, 124432. [Google Scholar] [CrossRef]
- Zarghami, V.; Ghorbani, M.; Bagheri, K.P.; Shokrgozar, M.A. Improving Bactericidal Performance of Implant Composite Coatings by Synergism between Melittin and Tetracycline. J. Mater. Sci. Mater. Med. 2022, 33, 46. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.A. Enhance the Biological Properties of Commercial Pure Titanium with Bioactive Glass Coating by Pulsed Laser Deposition. J. Biomim. Biomater. Biomed. Eng. 2021, 51, 29–37. [Google Scholar] [CrossRef]
- Negut, I.; Gradisteanu-Pircalabioru, G.; Dinu, M.; Bita, B.; Parau, A.C.; Grumezescu, V.; Ristoscu, C.; Chifiriuc, M.C. Bioglass and Vitamin D3 Coatings for Titanium Implants: Osseointegration and Corrosion Protection. Biomedicines 2023, 11, 2772. [Google Scholar] [CrossRef] [PubMed]
- Askari, P.; Namaei, M.H.; Ghazvini, K.; Hosseini, M. In Vitro and in Vivo Toxicity and Antibacterial Efficacy of Melittin against Clinical Extensively Drug-Resistant Bacteria. BMC Pharmacol. Toxicol. 2021, 22, 42. [Google Scholar] [CrossRef]
- Norisada, K.; Javkhlantugs, N.; Mishima, D.; Kawamura, I.; Saitô, H.; Ueda, K.; Naito, A. Dynamic Structure and Orientation of Melittin Bound to Acidic Lipid Bilayers, As Revealed by Solid-State NMR and Molecular Dynamics Simulation. J. Phys. Chem. B 2017, 121, 1802–1811. [Google Scholar] [CrossRef]
- Kargozar, S.; Baino, F.; Hamzehlou, S.; Hill, R.G.; Mozafari, M. Bioactive Glasses: Sprouting Angiogenesis in Tissue Engineering. Trends Biotechnol. 2018, 36, 430–444. [Google Scholar] [CrossRef]
- Kowalski, J.; Rylska, D.; Januszewicz, B.; Konieczny, B.; Cichomski, M.; Matinlinna, J.P.; Radwanski, M.; Sokolowski, J.; Lukomska-Szymanska, M. Corrosion Resistance of Titanium Dental Implant Abutments: Comparative Analysis and Surface Characterization. Materials 2023, 16, 6624. [Google Scholar] [CrossRef] [PubMed]
- Robles, D.; Brizuela, A.; Fernández-Domínguez, M.; Gil, J. Corrosion Resistance and Titanium Ion Release of Hybrid Dental Implants. Materials 2023, 16, 3650. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions Able to Reproduce in Vivo Surface-Structure Changes in Bioactive Glass-Ceramic A-W3. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef]
- Gyorgy, E.; Grigorescu, S.; Socol, G.; Mihailescu, I.N.; Janackovic, D.; Dindune, A.; Kanepe, Z.; Palcevskis, E.; Zdrentu, E.L.; Petrescu, S.M. Bioactive Glass and Hydroxyapatite Thin Films Obtained by Pulsed Laser Deposition. Appl. Surf. Sci. 2007, 253, 7981–7986. [Google Scholar] [CrossRef]
- Tanaskovic, D.; Jokic, B.; Socol, G.; Popescu, A.; Mihailescu, I.N.; Petrovic, R.; Janackovic, D. Synthesis of Functionally Graded Bioactive Glass-Apatite Multistructures on Ti Substrates by Pulsed Laser Deposition. Appl. Surf. Sci. 2007, 254, 1279–1282. [Google Scholar] [CrossRef]
- Jacobs, Z.; Schipani, R.; Pastrama, M.; Ahmadi, S.M.; Sajadi, B. Evaluation of Biocompatibility and Osseointegration of Multi-Component TiAl6V4 Titanium Alloy Implants. J. Orthop. Res. 2025, 43, 139–152. [Google Scholar] [CrossRef]
- Wu, H.; Chen, X.; Kong, L.; Liu, P. Mechanical and Biological Properties of Titanium and Its Alloys for Oral Implant with Preparation Techniques: A Review. Materials 2023, 16, 6860. [Google Scholar] [CrossRef] [PubMed]
- Rohith, A.S.; Gupta, P.; Fernandes, A.; Khan, F.A.; Ashok, A.; Vijaya, S. The Titanium Triumph: Exploring the Transformative World of Dental Implants. IP Ann. Prosthodont. Restor. Dent. 2024, 10, 124–128. [Google Scholar] [CrossRef]
- Rai, A.; Pinto, S.; Evangelista, M.B.; Gil, H.; Kallip, S.; Ferreira, M.G.S.; Ferreira, L. High-Density Antimicrobial Peptide Coating with Broad Activity and Low Cytotoxicity against Human Cells. Acta Biomater. 2016, 33, 64–77. [Google Scholar] [CrossRef]
- Maher, S.; McClean, S. Melittin Exhibits Necrotic Cytotoxicity in Gastrointestinal Cells Which Is Attenuated by Cholesterol. Biochem. Pharmacol. 2008, 75, 1104–1114. [Google Scholar] [CrossRef]
- Wu, X.; Singh, A.K.; Wu, X.; Lyu, Y.; Bhunia, A.K.; Narsimhan, G. Characterization of Antimicrobial Activity against Listeria and Cytotoxicity of Native Melittin and Its Mutant Variants. Colloids Surf. B Biointerfaces 2016, 143, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.-T.; Cheng, H.-H.; Huang, C.-J.; Chang, H.-C.; Chi, C.-C.; Su, H.-H.; Hsu, S.-S.; Wang, J.-L.; Chen, I.-S.; Liu, S.-I.; et al. Phospholipase A2-Independent Ca2+ Entry and Subsequent Apoptosis Induced by Melittin in Human MG63 Osteosarcoma Cells. Life Sci. 2007, 80, 364–369. [Google Scholar] [CrossRef]
- Ye, R.; Zheng, Y.; Chen, Y.; Wei, X.; Shi, S.; Chen, Y.; Zhu, W.; Wang, A.; Yang, L.; Xu, Y.; et al. Stable Loading and Delivery of Melittin with Lipid-Coated Polymeric Nanoparticles for Effective Tumor Therapy with Negligible Systemic Toxicity. ACS Appl. Mater. Interfaces 2021, 13, 55902–55912. [Google Scholar] [CrossRef]
- Üretmen, A.; Demir, C.; Odabaşıoğlu, V.; Kılıç, A. Imprinting Bone Surface Topography on Cast Metal. Bull. Mater. Sci. Metall. 2024, 1, 16–22. [Google Scholar] [CrossRef]
- Feng, J.; Liu, J.; Wang, Y.; Diao, J.; Kuang, Y.; Zhao, N. Beta-TCP Scaffolds with Rationally Designed Macro-Micro Hierarchical Structure Improved Angio/Osteo-Genesis Capability for Bone Regeneration. J. Mater. Sci. Mater. Med. 2023, 34, 36. [Google Scholar] [CrossRef]
- Özcolak, B.; Erenay, B.; Odabaş, S.; Jandt, K.D.; Garipcan, B. Effects of Bone Surface Topography and Chemistry on Macrophage Polarization. Sci. Rep. 2024, 14, 12721. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.; Spiro, R.C.; Jain, H.; Falk, M.M. Effects of Titanium Implant Surface Topology on Bone Cell Attachment and Proliferation in Vitro. Med. Devices Evid. Res. 2022, 15, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.H.; Wang, S.; Moawad, H.M.; Falk, M.M.; Jain, H. Glass Bone Implants: The Effect of Surface Topology on Attachment and Proliferation of Osteoblast Cells on 45S Bioactive Glass. MRS Online Proc. Libr. OPL 2009, 1235, 1235. [Google Scholar] [CrossRef]
- Dai, W.; Li, S.; Jia, H.; Zhao, X.; Liu, C.; Zhou, C.; Xiao, Y.; Guo, L.; Fan, Y.; Zhang, X. Indirect 3D Printing CDHA Scaffolds with Hierarchical Porous Structure to Promote Osteoinductivity and Bone Regeneration. J. Mater. Sci. Technol. 2025, 207, 295–307. [Google Scholar] [CrossRef]
- Liu, C.; Xue, C.; Zhang, B. Cell Migration Behavior Regulation by Nanopillars and Oxide Coating. AIP Adv. 2024, 14, 115215. [Google Scholar] [CrossRef]
- Qi, X.; Liu, Y.; Yin, X.; Zhao, R.; Zhang, W.; Cao, J.; Wang, W.; Jia, W. Surface-Based Modified 3D-Printed BG/GO Scaffolds Promote Bone Defect Repair through Bone Immunomodulation. Compos. Part B Eng. 2023, 257, 110673. [Google Scholar] [CrossRef]
- Hariharan, A.; Goldberg, P.; Schell, F.; Hempel, U.; Striggow, F.; Hantusch, M.; Medina-Sánchez, M.; Lasagni, A.F.; Gebert, A. Single- and Multiscale Laser Patterning of 3D Printed Biomedical Titanium Alloy: Toward an Enhanced Adhesion and Early Differentiation of Human Bone Marrow Stromal Cells. Adv. Funct. Mater. 2024, 34, 2310607. [Google Scholar] [CrossRef]
- Hadady, H.; Alam, A.; Khurana, I.; Mutreja, I.; Kumar, D.; Shankar, M.R.; Dua, R. Optimizing Alkaline Hydrothermal Treatment for Biomimetic Smart Metallic Orthopedic and Dental Implants. J. Mater. Sci. Mater. Med. 2024, 35, 31. [Google Scholar] [CrossRef]
- Xiang, Y.; Lin, D.; Zhou, Q.; Luo, H.; Zhou, Z.; Wu, S.; Xu, K.; Tang, X.; Ma, P.; Cai, C.; et al. Elucidating the Mechanism of Large-Diameter Titanium Dioxide Nanotubes in Protecting Osteoblasts Under Oxidative Stress Environment: The Role of Fibronectin and Albumin Adsorption. Int. J. Nanomed. 2024, 19, 10639–10659. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhao, Y.; Wang, M.; Liu, C.; Luo, J. Theoretical Modeling Approach for Adsorption of Fibronectin on the Nanotopographical Implants. Proc. Inst. Mech. Eng. Part H 2023, 237, 1102–1115. [Google Scholar] [CrossRef]
- Wu, H.; Ueno, T.; Nozaki, K.; Xu, H.; Nakano, Y.; Chen, P.; Wakabayashi, N. Lithium-Modified TiO2 Surface by Anodization for Enhanced Protein Adsorption and Cell Adhesion. ACS Appl. Mater. Interfaces 2023, 15, 55232–55243. [Google Scholar] [CrossRef]
- Li, K.; Liu, S.; Li, J.; Yi, D.; Shao, D.; Hu, T.; Zheng, X. Manganese Supplementation of Orthopedic Implants: A New Strategy for Enhancing Integrin-Mediated Cellular Responses. Biomater. Sci. 2023, 11, 3893–3905. [Google Scholar] [CrossRef]
- Gough, J.E.; Notingher, I.; Hench, L.L. Osteoblast Attachment and Mineralized Nodule Formation on Rough and Smooth 45S5 Bioactive Glass Monoliths. J. Biomed. Mater. Res. A 2004, 68A, 640–650. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, C.; Fu, S.; Wang, S.; Ma, S.; Du, J.; Li, W.; Zhang, H. In Situ Assembly of Melittin-PHA Microspheres for Enhancing Therapeutic Efficacy in Cancer Treatment. Int. J. Pept. Res. Ther. 2024, 30, 30. [Google Scholar] [CrossRef]
- Jafari, Z.; Sadeghi, S.; Dehaghi, M.M.; Bigham, A.; Honarmand, S.; Tavasoli, A.; Hoseini, M.H.M.; Varma, R.S. Immunomodulatory Activities and Biomedical Applications of Melittin and Its Recent Advances. Arch. Pharm. 2024, 357, 2300569. [Google Scholar] [CrossRef]
- Bolaño Alvarez, A.; Pino, M.; Petersen, S.B.; Rodríguez, P.E.A.; Fidelio, G.D. Structural and Conductivity Properties of Lipid-Coated Melittin Peptide Nanowires Molded at Air-Water Interface. J. Mol. Liq. 2024, 397, 124129. [Google Scholar] [CrossRef]
- Latifi, S.M.; Shankar, R.; Donahue, H.J. Polydopamine Coating on Titanium Affects Osteoblastic Differentiation to a Greater Degree than Does Surface Roughness. Adv. Mater. Phys. Chem. 2020, 10, 339–349. [Google Scholar] [CrossRef]
- Xiang, Y.; Fulmek, P.; Sauer, M.; Foelske, A.; Schmid, U. Characterization of Surface Modifications in Oxygen Plasma-Treated Teflon AF1600. Langmuir 2024, 40, 4779–4788. [Google Scholar] [CrossRef]
- Dwivedi, S.; Dixit, A.R.; Das, A.K.; Srivastava, A.K. Time-Dependent Wetting Behavior of the Micro-Textured Stainless Steel 316L Using the Mechanical Indentation Method. Proc. Inst. Mech. Eng. Part E 2023. [Google Scholar] [CrossRef]
- Gao, P.; MacKay, I.; Gruber, A.; Krantz, J.; Piccolo, L.; Lucchetta, G.; Pelaccia, R.; Orazi, L.; Masato, D. Wetting Characteristics of Laser-Ablated Hierarchical Textures Replicated by Micro Injection Molding. Micromachines 2023, 14, 863. [Google Scholar] [CrossRef]
- Lazauskas, A.; Andrulevičius, M.; Abakevičienė, B.; Jucius, D.; Grigaliūnas, V.; Guobienė, A.; Meškinis, Š. Hydrophilic Surface Modification of Amorphous Hydrogenated Carbon Nanocomposite Films via Atmospheric Oxygen Plasma Treatment. Nanomaterials 2023, 13, 1108. [Google Scholar] [CrossRef]
- Surmeneva, M.; Nikityuk, P.; Hans, M.; Surmenev, R. Deposition of Ultrathin Nano-Hydroxyapatite Films on Laser Micro-Textured Titanium Surfaces to Prepare a Multiscale Surface Topography for Improved Surface Wettability/Energy. Materials 2016, 9, 862. [Google Scholar] [CrossRef]
- Allain, J.P.; Echeverry-Rendón, M.; Pavón, J.J.; Arias, S.L. Nanostructured Biointerfaces. In Nanopatterning and Nanoscale Devices for Biological Applications; CRC Press: Boca Raton, FL, USA, 2015; ISBN 978-1-315-21580-8. [Google Scholar]
- Lu, N.; Wu, H.-M.; Wang, J.-L.; Jiang, W.-Z.; Deng, Z.-P.; Deng, J.-J. A Hydrophilic Nanocomposite Coating with Enhanced Durability and Antifouling Properties. J. Sol-Gel Sci. Technol. 2024, 110, 267–279. [Google Scholar] [CrossRef]
- de Oliveira, D.P.; Micocci, K.C.; de Almeida, G.F.B.; Otuka, A.J.G.; Mendonça, C.R.; Selistre-de-Araujo, H.S.; Bolfarini, C. Comparison of Hydrophilic and Hydrophobic Nano Topographic Surfaces of Titanium Alloys on Pre-Osteoblastic Cell Interaction. Biomed. Phys. Eng. Express 2023, 9, 045020. [Google Scholar] [CrossRef]
- Al-Noaman, A.; Rawlinson, S.C.F. A Novel Bioactive Glass/Graphene Oxide Composite Coating for a Polyether Ether Ketone-Based Dental Implant. Eur. J. Oral Sci. 2023, 131, e12915. [Google Scholar] [CrossRef]
- Maciąg, F.; Moskalewicz, T.; Cholewa-Kowalska, K.; Hadzhieva, Z.; Dziadek, M.; Dubiel, B.; Łukaszczyk, A.; Boccaccini, A.R. Influence of Mesoporous Bioactive Glass Particles Doped with Cu and Mg on the Microstructure and Properties of Zein-Based Coatings Obtained by Electrophoretic Deposition. J. Electrochem. Soc. 2023, 170, 082501. [Google Scholar] [CrossRef]
- Spriano, S.; Riccucci, G.; Örlygsson, G.; Ng, C.H.; Vernè, E.; Sehn, F.P.; de Oliveira, P.T.; Ferraris, S. Coating of Bioactive Glasses with Chitosan: The Effects of the Glass Composition and Coating Method on the Surface Properties, Including Preliminary in Vitro Results. Surf. Coat. Technol. 2023, 470, 129824. [Google Scholar] [CrossRef]
- Milošev, I.; Metikoš-Huković, M.; Strehblow, H.-H. Passive Film on Orthopaedic TiAlV Alloy Formed in Physiological Solution Investigated by X-Ray Photoelectron Spectroscopy. Biomaterials 2000, 21, 2103–2113. [Google Scholar] [CrossRef]
- Turdean-Ionescu, C.; Stevensson, B.; Izquierdo-Barba, I.; García, A.; Arcos, D.; Vallet-Regí, M.; Edén, M. Surface Reactions of Mesoporous Bioactive Glasses Monitored by Solid-State NMR: Concentration Effects in Simulated Body Fluid. J. Phys. Chem. C 2016, 120, 4961–4974. [Google Scholar] [CrossRef]
- Zurita-Méndez, N.N.; Carbajal-De la Torre, G.; Flores-Merino, M.V.; Espinosa-Medina, M.A. Development of Bioactive Glass-Collagen-Hyaluronic Acid-Polycaprolactone Scaffolds for Tissue Engineering Applications. Front. Bioeng. Biotechnol. 2022, 10, 825903. [Google Scholar] [CrossRef] [PubMed]
- Edathazhe, A.B.; Shashikala, H.D. Corrosion Resistance and In-Vitro Bioactivity of BaO Containing Na2O-CaO-P2O5 Phosphate Glass-Ceramic Coating Prepared on 316 L, Duplex Stainless Steel 2205 and Ti6Al4V. Mater. Res. Express 2018, 5, 035404. [Google Scholar] [CrossRef]
- Chen, H.; Shi, Q.; Zheng, K. Mathematical Modeling of Bioactive Glass Degradation. J. Non-Cryst. Solids 2024, 646, 123265. [Google Scholar] [CrossRef]
- Córdoba-Torres, P. Relationship between Constant-Phase Element (CPE) Parameters and Physical Properties of Films with a Distributed Resistivity. Electrochim. Acta 2017, 225, 592–604. [Google Scholar] [CrossRef]
- Meng, Y.; Yang, Y.; Zhang, C.; Cui, X.; Liu, E.; Jin, G.; Kang, J.; She, P. Influence of Bioactive Glass Addition on TC4 Laser Cladding Coatings: Microstructure and Electrochemical Properties. Coatings 2023, 13, 1621. [Google Scholar] [CrossRef]
- Randviir, E.P.; Banks, C.E. Electrochemical Impedance Spectroscopy: An Overview of Bioanalytical Applications. Anal. Methods 2013, 5, 1098–1115. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: From Concept to Clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Civan, L.; Nurbas, M. Characterization of Sodium Alginate Containing Bioactive Glass Coatings Prepared by Sol–Gel Processing. Polym. Bull. 2021, 78, 6473–6491. [Google Scholar] [CrossRef]
- Mattos, A.S.; da Costa, R.B.; Inocente, J.M.; Pereira, F.R.; Arcaro, S.; Montedo, O.R.K. Mesoporous Bioactive Scaffolds Based on the 14.6Li2O⋅8.6ZrO2⋅67.3SiO2⋅9.5Al2O3 Glass-Ceramic as Drug Delivery for Bone Regeneration. Ceram. Int. 2024, 50, 19084–19094. [Google Scholar] [CrossRef]
- Andersson, Ö.H.; Vähätalo, K.; Yli-Urpo, A.; Happonen, R.-P.; Karlsson, K.H. Short-Term Reaction Kinetics of Bioactive Glass in Simulated Body Fluid and in Subcutaneous Tissue. In Bioceramics; Andersson, Ö.H., Happonen, R.-P., Yli-Urpo, A., Eds.; Pergamon: Oxford, UK, 1994; pp. 67–72. ISBN 978-0-08-042144-5. [Google Scholar]
- Kawai, T. The Bioactivity Evaluation of Bioacitive Glass Coated Titanium Surface with Simulated Body Fluid. J. Adv. Sci. 2006, 18, 43–45. [Google Scholar] [CrossRef]
- Hamagami, J.-I.; Yamaguchi, G.; Kanamura, K.; Umegaki, T. Direct Observation of Apatite Formation on Bioglass in Simulated Body Fluid by Atomic Force Microscopy. Phosphorus Sulfur Silicon Relat. Elem. 2002, 177, 1921–1922. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Romero, A.M.; Ragel, C.V.; LeGeros, R.Z. XRD, SEM-EDS, and FTIR Studies of in Vitro Growth of an Apatite-like Layer on Sol-Gel Glasses. J. Biomed. Mater. Res. 1999, 44, 416–421. [Google Scholar] [CrossRef]
- Tirri, T.; Rich, J.; Wolke, J.; Seppälä, J.; Yli-Urpo, A.; Närhi, T.O. Bioactive Glass Induced in Vitro Apatite Formation on Composite GBR Membranes. J. Mater. Sci. Mater. Med. 2008, 19, 2919–2923. [Google Scholar] [CrossRef]
- Thian, E.S.; Huang, J.; Best, S.; Barber, Z.H.; Bonfield, W. Nanostructured Apatite Coatings for Rapid Bone Repair. Key Eng. Mater. 2006, 309–311, 519–522. [Google Scholar] [CrossRef]
- Costantino, L.; Gandolfi, F.; Bossynobs, L.; Tosi, G.; Gurny, R.; Rivasi, F.; Angelavandelli, M.; Forni, F. Nanoparticulate Drug Carriers Based on Hybrid Poly(d,l-Lactide-Co-Glycolide)-Dendron Structures. Biomaterials 2006, 27, 4635–4645. [Google Scholar] [CrossRef]
- Chang, C.C.; Boland, E.D.; Williams, S.K.; Hoying, J.B. Direct-Write Bioprinting Three-Dimensional Biohybrid Systems for Future Regenerative Therapies. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 98B, 160–170. [Google Scholar] [CrossRef]
- Floroian, L.; Florescu, M.; Munteanu, D.; Badea, M.; Ristoscu, C.; Sima, F.; Chifiriuc, M.C. A New Concept of Stainless Steel Medical Implant Based upon Composite Nanostructures Coating. Dig. J. Nanomater. Biostruct 2014, 9, 1555–1568. [Google Scholar]
- Floroian, L.; Sima, F.; Florescu, M.; Badea, M.; Popescu, A.C.; Serban, N.; Mihailescu, I.N. Double Layered Nanostructured Composite Coatings with Bioactive Silicate Glass and Polymethylmetacrylate for Biomimetic Implant Applications. J. Electroanal. Chem. 2010, 648, 111–118. [Google Scholar] [CrossRef]
- Park, C.; Lee, D.G. Melittin Induces Apoptotic Features in Candida albicans. Biochem. Biophys. Res. Commun. 2010, 394, 170–172. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Narsimhan, G.; Wu, X.; Du, F. Pore Formation in 1,2-Dimyristoyl-Sn-Glycero-3-Phosphocholine/Cholesterol Mixed Bilayers by Low Concentrations of Antimicrobial Peptide Melittin. Colloids Surf. B Biointerfaces 2014, 123, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Mamontov, E.; Anunciado, D.B.; O’Neill, H.; Urban, V.S. Effect of Antimicrobial Peptide on the Dynamics of Phosphocholine Membrane: Role of Cholesterol and Physical State of Bilayer. Soft Matter 2015, 11, 6755–6767. [Google Scholar] [CrossRef]
- Valenti, C.; Billi, M.; Pancrazi, G.L.; Calabria, E.; Armogida, N.G.; Tortora, G.; Pagano, S.; Barnaba, P.; Marinucci, L. Biological Effects of Cannabidiol on Human Cancer Cells: Systematic Review of the Literature. Pharmacol. Res. 2022, 181, 106267. [Google Scholar] [CrossRef]

| Sample | 2D Image | 3D Image | Roughness Parameters (nm) | |
|---|---|---|---|---|
| Ra | Rrms | |||
| BG57+Mel | 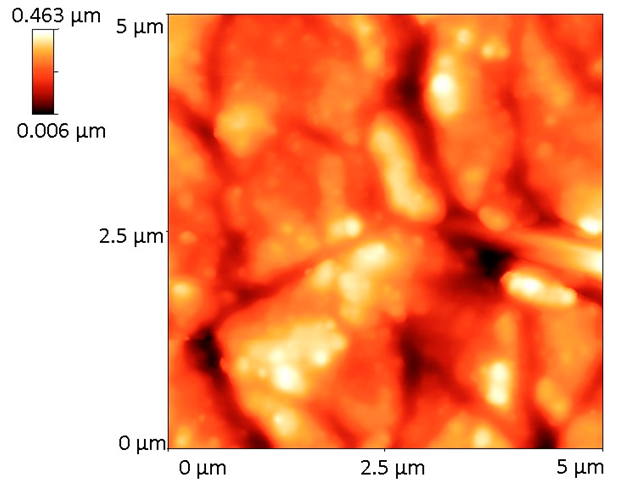 |  | 62.0 | 78.4 |
| Samples | lZl (Ω) @0.1 Hz | Stability in Time (% Decrease @0.1 Hz) |
|---|---|---|
| Ti_1h | 75,163 | - |
| Ti_12h | 72,218 | 4 |
| Ti_24h | 78,868 | -5 |
| BG57+Mel_1h | 2221 | - |
| BG57+Mel_12h | 1213 | 45 |
| BG57+Mel_24h | 1127 | 49 |
| Samples | Time | Ti | BG57+Mel |
|---|---|---|---|
| Rs (Ω cm2) | 1 h | 27 | 69 |
| 12 h | 23 | 47 | |
| 24 h | 19 | 41 | |
| Qcoat (μF s(α−1) cm−2) | 1 h | 19.89 | 28.636 |
| 12 h | 19.95 | 47.089 | |
| 24 h | 20.25 | 49.223 | |
| αcoat | 1 h | 0.96 | 0.77 |
| 12 h | 0.96 | 0.70 | |
| 24 h | 0.96 | 0.69 | |
| Rpore (Ω cm2) | 1 h | 87 | 132 |
| 12 h | 75 | 139 | |
| 24 h | 70 | 122 | |
| Qdl (μF s(α−1) cm−2) | 1 h | 9.90 | 623.16 |
| 12 h | 9.68 | 1199.70 | |
| 24 h | 9.13 | 1300.70 | |
| αdl | 1 h | 0.93 | 0.60 |
| 12 h | 0.93 | 0.56 | |
| 24 h | 0.93 | 0.56 | |
| Rct (Ω cm2) | 1 h | - | - |
| 12 h | - | - | |
| 24 h | - | - | |
| χ2 | 1 h | 2 × 10−4 | 3 × 10−4 |
| 12 h | 2 × 10−4 | 1 × 10−4 | |
| 24 h | 2 × 10−4 | 1 × 10−4 |
| Immersion Time | Key Elements in BG57+Mel Films | |||||
|---|---|---|---|---|---|---|
| %C | %N | %O | %Na | %P | %Ca | |
| 24 h | 9.37 | 13.16 | 8.85 | 0.02 | 0.27 | 0.22 |
| 48 h | 10.27 | 12.88 | 7.79 | 0.02 | 0.33 | 0.43 |
| 5 days | 9.71 | 14.46 | 7.6 | 0.15 | 0.13 | 0.25 |
| 7 days | 9.92 | 13.92 | 9.77 | 0.11 | 0.26 | 0.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinu, M.; Bita, B.; Parau, A.C.; Ristoscu, C.; Negut, I. Bioactive Glass and Melittin Thin Films Deposited by MAPLE for Titanium Implant Functionalization. Materials 2025, 18, 2410. https://doi.org/10.3390/ma18102410
Dinu M, Bita B, Parau AC, Ristoscu C, Negut I. Bioactive Glass and Melittin Thin Films Deposited by MAPLE for Titanium Implant Functionalization. Materials. 2025; 18(10):2410. https://doi.org/10.3390/ma18102410
Chicago/Turabian StyleDinu, Mihaela, Bogdan Bita, Anca Constantina Parau, Carmen Ristoscu, and Irina Negut. 2025. "Bioactive Glass and Melittin Thin Films Deposited by MAPLE for Titanium Implant Functionalization" Materials 18, no. 10: 2410. https://doi.org/10.3390/ma18102410
APA StyleDinu, M., Bita, B., Parau, A. C., Ristoscu, C., & Negut, I. (2025). Bioactive Glass and Melittin Thin Films Deposited by MAPLE for Titanium Implant Functionalization. Materials, 18(10), 2410. https://doi.org/10.3390/ma18102410








