New Biodegradable Carboxymethyl Cellulose-Based Films with Liquid Products of Wood Pine Pyrolysis with Antibacterial and Antioxidant Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Products of Pyrolysis
2.2. Film Preparation
2.3. SEM Analysis
2.4. Uniaxial Tensile Tests
2.5. Oxygen Permeability
2.6. Evaluation of the Antioxidant Properties of Films
2.7. Assessment of the Antibacterial Properties of the Film
2.8. Cytotoxicity Analysis of Film
2.9. Biodegradation of CMC and CMC-LPP Films
2.10. Statistical Analysis
3. Results and Discussion
3.1. Obtained Films
3.2. Antioxidant Properties of Films
3.3. Antibacterial Properties
3.4. Cytotoxicity Analysis of Films
3.5. Biodegradability of Obtained Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Kock, L.; Sadan, Z.; Arp, R.; Upadhyaya, P. A circular economy response to plastic pollution: Current policy landscape and consumer perception. S. Afr. J. Sci. 2020, 116. [Google Scholar] [CrossRef] [PubMed]
- Janczak, K.; Hrynkiewicz, K.; Znajewska, Z.; Dąbrowska, G. Use of rhizosphere microorganisms in the biodegradation of PLA and PET polymers in compost soil. Int. Biodeterior. Biodegrad. 2018, 130, 65–75. [Google Scholar] [CrossRef]
- Janczak, K.; Dąbrowska, G.B.; Raszkowska-Kaczor, A.; Kaczor, D.; Hrynkiewicz, K.; Richert, A. Biodegradation of the plastics PLA and PET in cultivated soil with the participation of microorganisms and plants. Int. Biodeterior. Biodegrad. 2020, 155, 105087. [Google Scholar] [CrossRef]
- Sozer, N.; Kokini, J.L. Nanotechnology and its applications in the food sector. Trends Biotechnol. 2009, 27, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, A.; Gorrasi, G.; Vittoria, V. Potential perspectives of bio-nanocomposites for food packaging applications. Trends Food Sci. Technol. 2007, 18, 84–95. [Google Scholar] [CrossRef]
- Heinze, T.; El Seoud, O.A.; Koschella, A. Production and Characteristics of Cellulose from Different Sources. In Cellulose Derivatives; Springer: Cham, Switzerland, 2018; pp. 1–38. [Google Scholar]
- Turhan, K.N.; Şahbaz, F. Water vapor permeability, tensile properties and solubility of methylcellulose-based edible films. J. Food Eng. 2004, 61, 459–466. [Google Scholar] [CrossRef]
- Westlake, J.R.; Tran, M.W.; Jiang, Y.; Zhang, X.; Burrows, A.D.; Xie, M. Biodegradable biopolymers for active packaging: Demand, development and directions. Sustain. Food Technol. 2023, 1, 50–72. [Google Scholar] [CrossRef]
- Verma, S.K.; Prasad, A.; Sonika; Katiyar, V. State of art review on sustainable biodegradable polymers with a market overview for sustainability packaging. Mater. Today Sustain. 2024, 26, 100776. [Google Scholar] [CrossRef]
- Šešlija, S.; Nešić, A.; Škorić, M.L.; Krušić, M.K.; Santagata, G.; Malinconico, M. Pectin/carboxymethylcellulose films as a potential food packaging material. Macromol. Symp. 2018, 378, 1600163. [Google Scholar] [CrossRef]
- Yao, Q.-B.; Huang, F.; Lu, Y.-H.; Huang, J.-M.; Ali, M.; Jia, X.Z.; Zeng, X.A.; Huang, Y.-Y. Polysaccharide-based food packaging and intelligent packaging applications: A comprehensive review. Trends Food Sci. Technol. 2024, 147, 104390. [Google Scholar] [CrossRef]
- Rahman, M.S.; Hasan, M.S.; Nitai, A.S.; Nam, S.; Karmakar, A.K.; Ahsan, M.S.; Shiddiky, M.J.A.; Ahmed, M.B. Recent developments of carboxymethyl cellulose. Polymers 2021, 13, 1345. [Google Scholar] [CrossRef]
- Mondal, M.I.H.; Yeasmin, M.S.; Rahman, M.S. Preparation of food grade carboxymethyl cellulose from corn husk agrowaste. Int. J. Biol. Macromol. 2015, 79, 144–150. [Google Scholar] [CrossRef]
- Akhtar, H.M.S.; Ahmed, S.; Olewnik-Kruszkowska, E.; Gierszewska, M.; Brzezinska, M.S.; Dembińska, K.; Kalwasińska, A. Carboxymethyl cellulose based films enriched with polysaccharides from mulberry leaves (Morus alba L.) as new biodegradable packaging material. Int. J. Biol. Macromol. 2023, 253, 127633. [Google Scholar] [CrossRef] [PubMed]
- Ahlfeld, T.; Guduric, V.; Duin, S.; Akkineni, A.R.; Schütz, K.; Kilian, D.; Emmermacher, J.; Cubo-Mateo, N.; Dani, S.; Witzleben, M.V.; et al. Methylcellulose—A versatile printing material that enables biofabrication of tissue equivalents with high shape fidelity. Biomater. Sci. 2020, 8, 2102–2110. [Google Scholar] [CrossRef] [PubMed]
- El Bouchtaoui, F.Z.; Ablouh, E.H.; Mhada, M.; Kassem, I.; Salim, M.H.; Mouhib, S.; Kassab, Z.; Sehaqui, H.; El Achaby, M. Methylcellulose/lignin biocomposite as an eco-friendly and multifunctional coating material for slow-release fertilizers: Effect on nutrients management and wheat growth. Int. J. Biol. Macromol. 2022, 221, 398–415. [Google Scholar] [CrossRef]
- Khater, E.S.; Bahnasawy, A.; Gabal, B.A.; Abbas, W.; Morsy, O. Effect of adding nano-materials on the properties of hydroxypropyl methylcellulose (HPMC) edible films. Sci. Rep. 2023, 13, 5063. [Google Scholar] [CrossRef] [PubMed]
- Jacob, C.; Melotto, M. Human pathogen colonization of lettuce dependent upon plant genotype and defense response activation. Front. Plant Sci. 2020, 10, 491866. [Google Scholar] [CrossRef]
- Luna-Guevara, J.J.; Arenas-Hernandez, M.M.P.; Martínez De La Peña, C.; Silva, J.L.; Luna-Guevara, M.L. The role of pathogenic E. coli in fresh vegetables: Behavior, contamination factors, and preventive measures. Int. J. Microbiol. 2019, 2019, 2894328. [Google Scholar] [CrossRef]
- Mogren, L.; Windstam, S.; Boqvist, S.; Vågsholm, I.; Söderqvist, K.; Rosberg, A.K.; Lindén, J.; Mulaosmanovic, E.; Karlsson, M.; Uhlig, E.; et al. The hurdle approach-A holistic concept for controlling food safety risks associated with pathogenic bacterial contamination of leafy green vegetables. A review. Front. Microbiol. 2018, 9, 391226. [Google Scholar] [CrossRef]
- Richert, A.; Olewnik-Kruszkowska, E.; Dąbrowska, G.B.; Dąbrowski, H.P. The role of birch tar in changing the physicochemical and biocidal properties of polylactide-based films. Int. J. Mol. Sci. 2021, 23, 268. [Google Scholar] [CrossRef]
- Hoffmann, S.; Devleesschauwer, B.; Aspinall, W.; Cooke, R.; Corrigan, T.; Havelaar, A.; Angulo, F.; Gibb, H.; Kirk, M.; Lake, R.; et al. Attribution of global foodborne disease to specific foods: Findings from a World Health Organization structured expert elicitation. PLoS ONE 2017, 12, e0183641. [Google Scholar] [CrossRef] [PubMed]
- Kwieciński, J.; Eick, S.; Wójcik, K. Effects of tea tree (Melaleuca alternifolia) oil on Staphylococcus aureus in biofilms and stationary growth phase. Int. J. Antimicrob. Agents 2009, 33, 343–347. [Google Scholar] [CrossRef]
- Monegro, A.F.; Muppidi, V.; Regunath, H. Hospital-acquired infections. In Patient Safety: A Case-Based Innovative Playbook for Safer Care; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 183–198. [Google Scholar]
- Grasselli, G.; Scaravilli, V.; Mangioni, D.; Scudeller, L.; Alagna, L.; Bartoletti, M.; Bellani, G.; Biagioni, E.; Bonfanti, P.; Bottino, N.; et al. Hospital-acquired infections in critically ill patients with COVID-19. Chest 2021, 160, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Raoofi, S.; Kan, F.P.; Rafiei, S.; Hosseinipalangi, Z.; Mejareh, Z.N.; Khani, S.; Abdollahi, B.; Talab, F.S.; Sanaei, M.; Zarabi, F.; et al. Global prevalence of nosocomial infection: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0274248. [Google Scholar] [CrossRef]
- Ahmed, J.; Hiremath, N.; Jacob, H. Antimicrobial, rheological, and thermal properties of plasticized polylactide films incorporated with essential oils to inhibit Staphylococcus aureus and Campylobacter jejuni. J. Food Sci. 2016, 81, E419–E429. [Google Scholar] [CrossRef]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 1999, 86, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Li, W.; Liu, D.; Yuan, M.; Li, L. Development of active packaging film made from poly (lactic acid) incorporated essential oil. Prog. Org. Coat. 2017, 103, 76–82. [Google Scholar] [CrossRef]
- Tarach, I.; Olewnik-Kruszkowska, E.; Richert, A.; Gierszewska, M.; Rudawska, A. Influence of tea tree essential oil and poly(ethylene glycol) on antibacterial and physicochemical properties of polylactide-based films. Materials 2020, 13, 4953. [Google Scholar] [CrossRef]
- Swiontek Brzezinska, M.; Kaczmarek-Szczepańska, B.; Dąbrowska, G.B.; Michalska-Sionkowska, M.; Dembińska, K.; Richert, A.; Pejchalová, M.; Kumar, S.B.; Kalwasińska, A. Application potential of Trichoderma in the degradation of phenolic acid-modified chitosan. Foods 2023, 12, 3669. [Google Scholar] [CrossRef]
- Basta, A.H.; El-Saied, H.; El-Deftar, M.M.; El-Henawy, A.A.; El-Sheikh, H.H.; Abdel-Shakour, E.H.; Hasanin, M.S. Properties of modified carboxymethyl cellulose and its use as bioactive compound. Carbohydr. Polymers 2016, 153, 641–651. [Google Scholar] [CrossRef]
- Turkan, S.; Mierek-Adamska, A.; Kulasek, M.; Konieczna, W.B.; Dabrowska, G.B. New seed coating containing Trichoderma viride with anti-pathogenic properties. PeerJ 2023, 11, e15392. [Google Scholar] [CrossRef] [PubMed]
- Bartkowiak, M.; Rosołowski, S.; Brózdowski, J.; Cofta, G.; Dąbrowska, G.; Zborowska, M. Comparison of the properties of birch bark tar obtained by the double-clay pot method and the laboratory method. Eur. J. Wood Prod. 2025, 102, 83. [Google Scholar]
- Hagner, M.; Penttinen, O.-P.; Pasanen, T.; Tiilikkala, K.; Setälä, H. Acute toxicity of birch tar oil on aquatic organisms. Agric. Food Sci. 2010, 19, 24–33. [Google Scholar] [CrossRef]
- Hagner, M.; Pasanen, T.; Lindqvist, B.; Lindqvist, I.; Tiilikkala, K.; Penttinen, O.P.; Setälä, H. Effects of birch tar oils on soil organisms and plants. Agric. Food Sci. 2010, 19, 13–23. [Google Scholar] [CrossRef]
- Dąbrowska, G.B.; Garstecka, Z.; Narbutt, O.; Dąbrowski, H.P.; Pyrkosz, W. Szczep Grzyba Trichoderma harzianum ZggD-19, Sposób Biostymulacji Wzrostu Rzepaku i Roślin Uprawnych z Rodziny Brassicaceae, Sposób Ochrony Rzepaku i Roślin Uprawnych z Rodziny Brassicaceae Oraz Roztwór Do Ochrony Rzepaku i Roślin Uprawnych z Rodziny Brassicaceae. 2021. Available online: https://omega.umk.pl/info/patent/UMK697fcbfdfc084b9ea2cebcdc991b971b/ (accessed on 8 May 2025).
- Richert, A.; Malinowski, R.; Ringwelska, M.; Dąbrowska, G.B. Birch tar introduced into polylactide and its influence on the barrier, thermal, functional and biological properties of the film obtained by industrial extrusion. Materials 2022, 15, 7382. [Google Scholar] [CrossRef] [PubMed]
- Richert, A.; Kalwasińska, A.; Brzezinska, M.S.; Dąbrowska, G.B. Biodegradability of novel polylactide and polycaprolactone materials with bacteriostatic properties due to embedded birch tar in different environments. Int. J. Mol. Sci. 2021, 22, 10228. [Google Scholar] [CrossRef]
- Mora, M.; Fàbregas, E.; Céspedes, F.; Rovira, P.; Puy, N. Dialysis and column chromatography for biomass pyrolysis liquids separation. Waste Manag. 2023, 168, 311–320. [Google Scholar] [CrossRef]
- Celeiro, M.; Lamas, J.P.; Arcas, R.; Lores, M. Wood processing industry by-products as a source of natural bioactive compounds. Energy Environ. 2021, 32, 981–1001. [Google Scholar] [CrossRef]
- Wei, Q.; Ma, X.; Zhao, Z.; Zhang, S.; Liu, S. Antioxidant activities and chemical profiles of pyroligneous acids from walnut shell. J. Anal. Appl. Pyrolysis 2010, 88, 149–154. [Google Scholar] [CrossRef]
- Hassan, E.B.; El-Giar, E.M.; Steele, P. Evaluation of the antioxidant activities of different bio-oils and their phenolic distilled fractions for wood preservation. Int. Biodeterior. Biodegrad. 2016, 110, 121–128. [Google Scholar] [CrossRef]
- Mathew, S.; Zakaria, Z.A. Pyroligneous acid-the smoky acidic liquid from plant biomass. Appl. Microbiol. Biotechnol. 2015, 99, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Brózdowski, J.; Witczak, M.; Sikorska, K.; Ratajczak, I.; Woźniak, M.; Bartkowiak, M.; Cofta, G.; Dąbrowska, G.B.; Zborowska, M. Valorization of forest biomass through pyrolysis: A study on the energy potential of wood tars. Energies 2025, 18, 1113. [Google Scholar] [CrossRef]
- ASTMF 1927; Standard Test Method for Determination of Oxygen Gas Transmission Rate, Permeability and Permeance at Controlled Relative Humidity Through Barrier Materials Using a Coulometric Detector. ASTM: West Conshohocken, PA, USA, 2020.
- Tymczewska, A.; Szydłowska-Czerniak, A.; Nowaczyk, J. Bioactive packaging based on gelatin incorporated with rapeseed meal for prolonging shelf life of rapeseed. Food Packag. Shelf Life 2021, 29, 100728. [Google Scholar] [CrossRef]
- ISO 10993-12; Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials. ISO: Geneva, Switzerland, 2021.
- PN-ISO 10390:1997; Soil Quality—Determination of pH. Polish Committee for Standardization: Warszawa, Poland, 1997.
- PN-R-04022:1996; Chemical and Agricultural Analysis—Determination of the Content Available Potasium in Mineral Soils. Polish Committee for Standardization: Warszawa, Poland, 1996.
- PN-R-04022:1996/Az1:2002; Chemical-Agricultural Analysis of Soil—Determination of Available Potassium in Mineral Soils. Polish Committee for Standardization: Warszawa, Poland, 2002.
- PN-R-04020:1994/Az1:2004; Chemical and Agricultural Analysis of Soil. Determining the Content of Available Magnesium. Polish Committee for Standardization: Warszawa, Poland, 2004.
- PN-R-04028:1997; Soil Chemical and Agricultural Analysis—Method of Sampling and Determination of Nitrate and Ammonium Ions in Mineral Soils. Polish Committee for Standardization: Warszawa, Poland, 1997.
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Yang, J.; Li, Y.; Liu, B.; Wang, K.; Li, H.; Peng, L. Carboxymethyl cellulose-based multifunctional film integrated with polyphenol-rich extract and carbon dots from coffee husk waste for active food packaging applications. Food Chem. 2024, 448, 139143. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, H.; Zhao, Q.S.; Zhao, B. Effect of DES lignin incorporation on physicochemical, antioxidant and antimicrobial properties of carboxymethyl cellulose-based films. Int. J. Biol. Macromol. 2024, 263, 130294. [Google Scholar] [CrossRef]
- Choi, I.; Chang, Y.; Shin, S.H.; Joo, E.; Song, H.J.; Eom, H.; Han, J. Development of biopolymer composite films using a microfluidization technique for carboxymethylcellulose and apple skin particles. Int. J. Mol. Sci. 2017, 18, 1278. [Google Scholar] [CrossRef]
- Bourbon, A.I.; Costa, M.J.; Maciel, L.C.; Pastrana, L.; Vicente, A.A.; Cerqueira, M.A. Active carboxymethylcellulose-based edible films: Influence of free and encapsulated curcumin on films’ properties. Foods 2021, 10, 1512. [Google Scholar] [CrossRef]
- Pirsa, S.; Bener, M.; Şen, F.B. Biodegradable film of carboxymethyl cellulose modified with red onion peel powder waste and boron nitride nanoparticles: Investigation of physicochemical properties and release of active substances. Food Chem. 2024, 445, 138721. [Google Scholar] [CrossRef]
- Pan, J.; Li, C.; Liu, J.; Jiao, Z.; Zhang, Q.; Lv, Z.; Yang, W.; Chen, D.; Liu, H. Polysaccharide-based packaging coatings and films with phenolic compounds in preservation of fruits and vegetables—A review. Foods 2024, 13, 3896. [Google Scholar] [CrossRef]
- Vidal, O.L.; Barros Santos, M.C.; Batista, A.P.; Andrigo, F.F.; Baréa, B.; Lecomte, J.; Figueroa-Espinoza, M.C.; Gontard, N.; Villeneuve, P.; Guillard, V.; et al. Active packaging films containing antioxidant extracts from green coffee oil by-products to prevent lipid oxidation. J. Food Eng. 2022, 312, 110744. [Google Scholar] [CrossRef]
- Regert, M.; Alexandre, V.; Thomas, N.; Lattuati-Derieux, A. Molecular characterisation of birch bark tar by headspace solid-phase microextraction gas chromatography–mass spectrometry: A new way for identifying archaeological glues. J. Chromatogr. A 2006, 1101, 245–253. [Google Scholar] [CrossRef]
- Takci, M.; Ucan Turkmen, F.; Sari, M. Effect of cedar (Cedrus libani A. Rich) tar on bacterial growth. J. Microbiol. Biotechnol. Food Sci. 2020, 9, 805–808. [Google Scholar] [CrossRef]
- Kizil, M.; Kizil, G.; Yavuz, M.; Aytekin, Ç. Antimicrobial activity of the tar obtained from the roots and stems of Pinus brutia. Pharm. Biol. 2002, 40, 135–138. [Google Scholar] [CrossRef]
- ISO 10993-5; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- Costa, E.M.; Silva, S.; Pereira, C.F.; Ribeiro, A.B.; Casanova, F.; Freixo, R.; Pintado, M.; Ramos, Ó.L. Carboxymethyl cellulose as a food emulsifier: Are its days numbered? Polymers 2023, 15, 2408. [Google Scholar] [CrossRef]
- Lee, L.S.; Lee, S.U.; Che, C.Y.; Lee, J.E. Comparison of cytotoxicity and wound healing effect of carboxymethylcellulose and hyaluronic acid on human corneal epithelial cells. Int. J. Ophthalmol. 2015, 8, 215–221. [Google Scholar]
- Hamzah, M.A.A.M.; Hasham, R.; Malek, N.A.N.N.; Hashim, Z.; Yahayu, M.; Razak, F.I.A.; Zakaria, Z.A. Beyond conventional biomass valorisation: Pyrolysis-derived products for biomedical applications. Biofuel Res. J. 2022, 9, 1648–1658. [Google Scholar] [CrossRef]
- de Souza, J.L.S.; Alves, T.; Camerini, L.; Nedel, F.; Campos, A.D.; Lund, R.G. Antimicrobial and cytotoxic capacity of pyroligneous extracts films of Eucalyptus grandis and chitosan for oral applications. Sci. Rep. 2021, 11, 21531. [Google Scholar] [CrossRef]
- Chatterjee, N.; Eom, H.J.; Jung, S.H.; Kim, J.S.; Choi, J. Toxic potentiality of bio-oils, from biomass pyrolysis, in cultured cells and Caenorhabditis elegans. Environ. Toxicol. 2014, 29, 1409–1419. [Google Scholar] [CrossRef]
- Putnam, K.P.; Bombick, D.W.; Avalos, J.T.; Doolittle, D.J. Comparison of the cytotoxic and mutagenic potential of liquid smoke food flavourings, cigarette smoke condensate and wood smoke condensate. Food Chem. Toxicol. 1999, 37, 1113–1118. [Google Scholar] [CrossRef]
- Cordella, M.; Torri, C.; Adamiano, A.; Fabbri, D.; Barontini, F.; Cozzani, V. Bio-oils from biomass slow pyrolysis: A chemical and toxicological screening. J. Hazard. Mater. 2012, 231, 26–35. [Google Scholar] [CrossRef]
- Garstecka, Z.; Antoszewski, M.; Mierek-Adamska, A.; Krauklis, D.; Niedojadło, K.; Kaliska, B.; Hrynkiewicz, K.; Dąbrowska, G.B. Trichoderma viride colonizes the roots of Brassica napus L., alters the expression of stress-responsive genes, and increases the yield of canola under field conditions during drought. Int. J. Mol. Sci. 2023, 24, 15349. [Google Scholar] [CrossRef] [PubMed]
- Antoszewski, M.; Mierek-Adamska, A.; Dąbrowska, G.B. The importance of microorganisms for sustainable agriculture—A review. Metabolites 2022, 12, 1100. [Google Scholar] [CrossRef]
- Lima, P.C.; Karimian, P.; Johnston, E.; Hartley, C.J.; Lima, P.C.; Karimian, P.; Johnston, E.; Hartley, C.J. The use of Trichoderma spp. for the bioconversion of agro-industrial waste biomass via fermentation: A review. Fermentation 2024, 10, 442. [Google Scholar] [CrossRef]
- Znajewska, Z.; Dąbrowska, G.B.; Hrynkiewicz, K.; Janczak, K. Biodegradation of polycaprolactone by Trichoderma viride fungi. Przemysł Chem. 2018, 97, 1676–1679. [Google Scholar]
- Dabrowska, G.B.; Garstecka, Z.; Olewnik-Kruszkowska, E.; Szczepańska, G.; Ostrowski, M.; Mierek-Adamska, A. Comparative study of structural changes of polylactide and poly(ethylene terephthalate) in the presence of Trichoderma viride. Int. J. Mol. Sci. 2021, 22, 3491. [Google Scholar] [CrossRef]
- Richert, A.; Kalwasińska, A.; Jankiewicz, U.; Brzezinska, M.S. Effect of birch tar embedded in polylactide on its biodegradation. Int. J. Biol. Macromol. 2023, 239, 124226. [Google Scholar] [CrossRef]


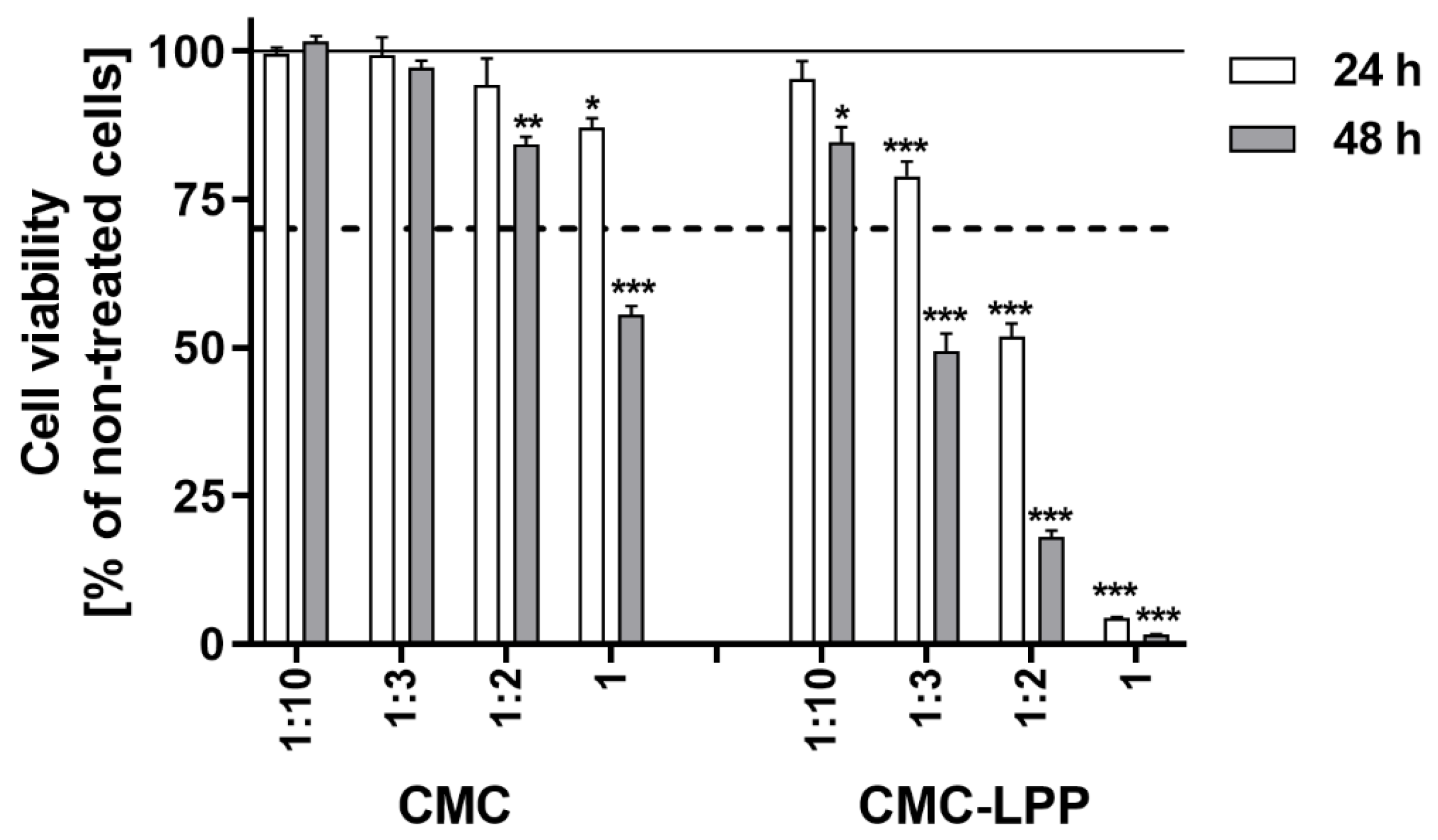
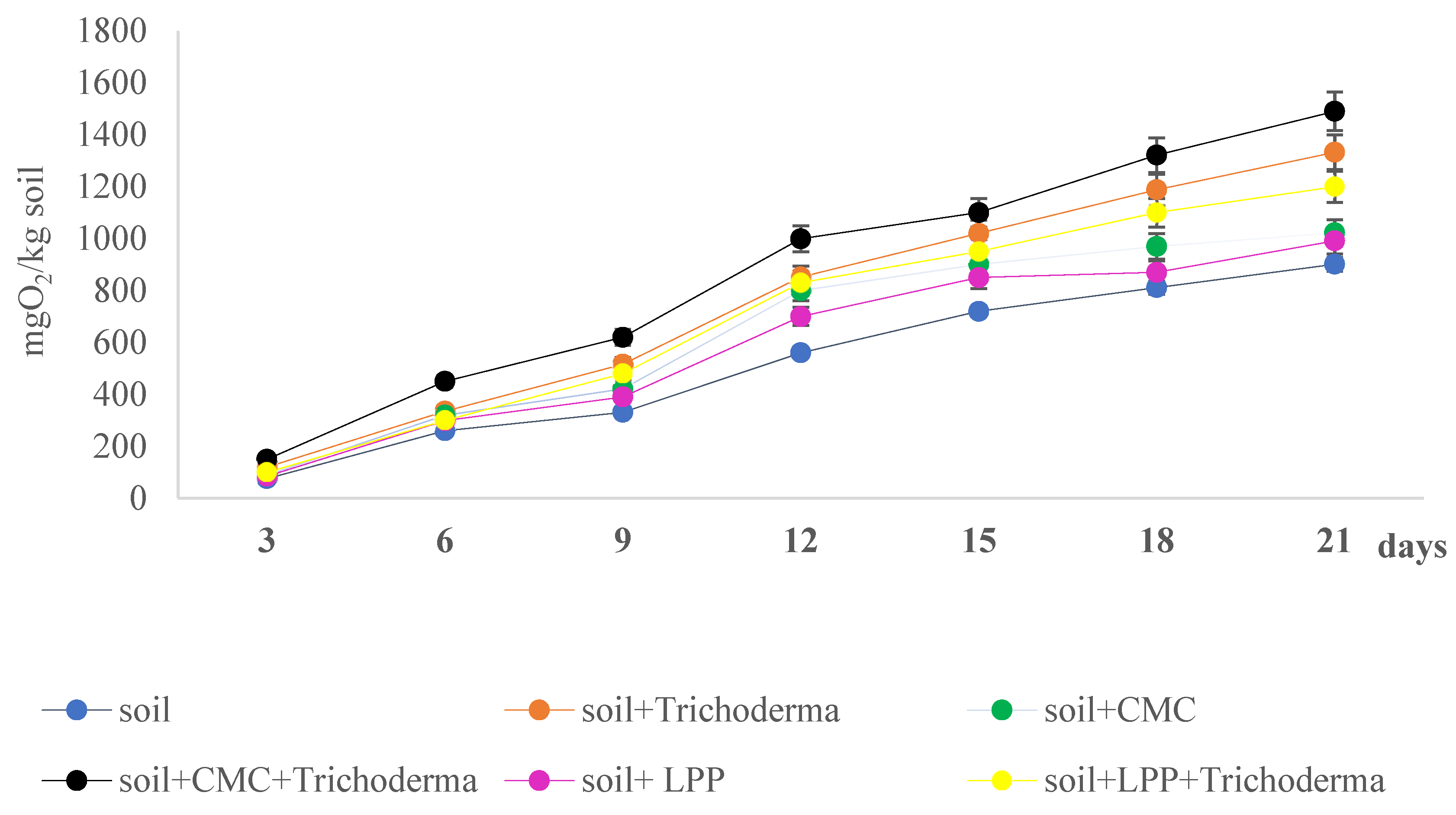
| Parameter | Abundance [CFU/g] |
|---|---|
| Heterotrophic bacteria | 55 × 105 |
| Actinomycetes | 42 × 104 |
| Fungi | 33 × 103 |
| Film Sample | E ± SD * (MPa) | σmax ± SD * (MPa) | ε ± SD * (%) |
|---|---|---|---|
| CMC | 0.053 ± 0.017 b | 0.136 ± 0.016 b | 621.38 ± 58.28 b |
| CMC-LPP | 0.0078 ± 0.0016 a | 0.026 ± 0.004 a | 372.78 ± 41.85 a |
| Film Sample | QUENCHERDPPH ± SD * [μmol TE/g] | QUENCHERCUPRAC ± SD * [μmol TE/g] |
|---|---|---|
| CMC | 2.26 ± 0.19 a | 0.92 ± 0.04 a |
| CMC + LPP | 10.48 ± 0.39 b | 16.82 ± 0.73 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dąbrowska, G.B.; Antoszewski, M.; Szydłowska-Czerniak, A.; Raszkowska-Kaczor, A.; Jędrzejewski, T.; Wrotek, S.; Bartkowiak, M.; Swiontek Brzezinska, M.; Zborowska, M. New Biodegradable Carboxymethyl Cellulose-Based Films with Liquid Products of Wood Pine Pyrolysis with Antibacterial and Antioxidant Properties. Materials 2025, 18, 2228. https://doi.org/10.3390/ma18102228
Dąbrowska GB, Antoszewski M, Szydłowska-Czerniak A, Raszkowska-Kaczor A, Jędrzejewski T, Wrotek S, Bartkowiak M, Swiontek Brzezinska M, Zborowska M. New Biodegradable Carboxymethyl Cellulose-Based Films with Liquid Products of Wood Pine Pyrolysis with Antibacterial and Antioxidant Properties. Materials. 2025; 18(10):2228. https://doi.org/10.3390/ma18102228
Chicago/Turabian StyleDąbrowska, Grażyna B., Marcel Antoszewski, Aleksandra Szydłowska-Czerniak, Aneta Raszkowska-Kaczor, Tomasz Jędrzejewski, Sylwia Wrotek, Monika Bartkowiak, Maria Swiontek Brzezinska, and Magdalena Zborowska. 2025. "New Biodegradable Carboxymethyl Cellulose-Based Films with Liquid Products of Wood Pine Pyrolysis with Antibacterial and Antioxidant Properties" Materials 18, no. 10: 2228. https://doi.org/10.3390/ma18102228
APA StyleDąbrowska, G. B., Antoszewski, M., Szydłowska-Czerniak, A., Raszkowska-Kaczor, A., Jędrzejewski, T., Wrotek, S., Bartkowiak, M., Swiontek Brzezinska, M., & Zborowska, M. (2025). New Biodegradable Carboxymethyl Cellulose-Based Films with Liquid Products of Wood Pine Pyrolysis with Antibacterial and Antioxidant Properties. Materials, 18(10), 2228. https://doi.org/10.3390/ma18102228









