Uneven Hydrophilic–Hydrophobic Nanoflowers Enhancing Solar Interface Evaporation: Se-Doped Carbon Loaded with Gradient Distribution of CoSe/Co
Abstract
1. Introduction
2. Materials and Methods
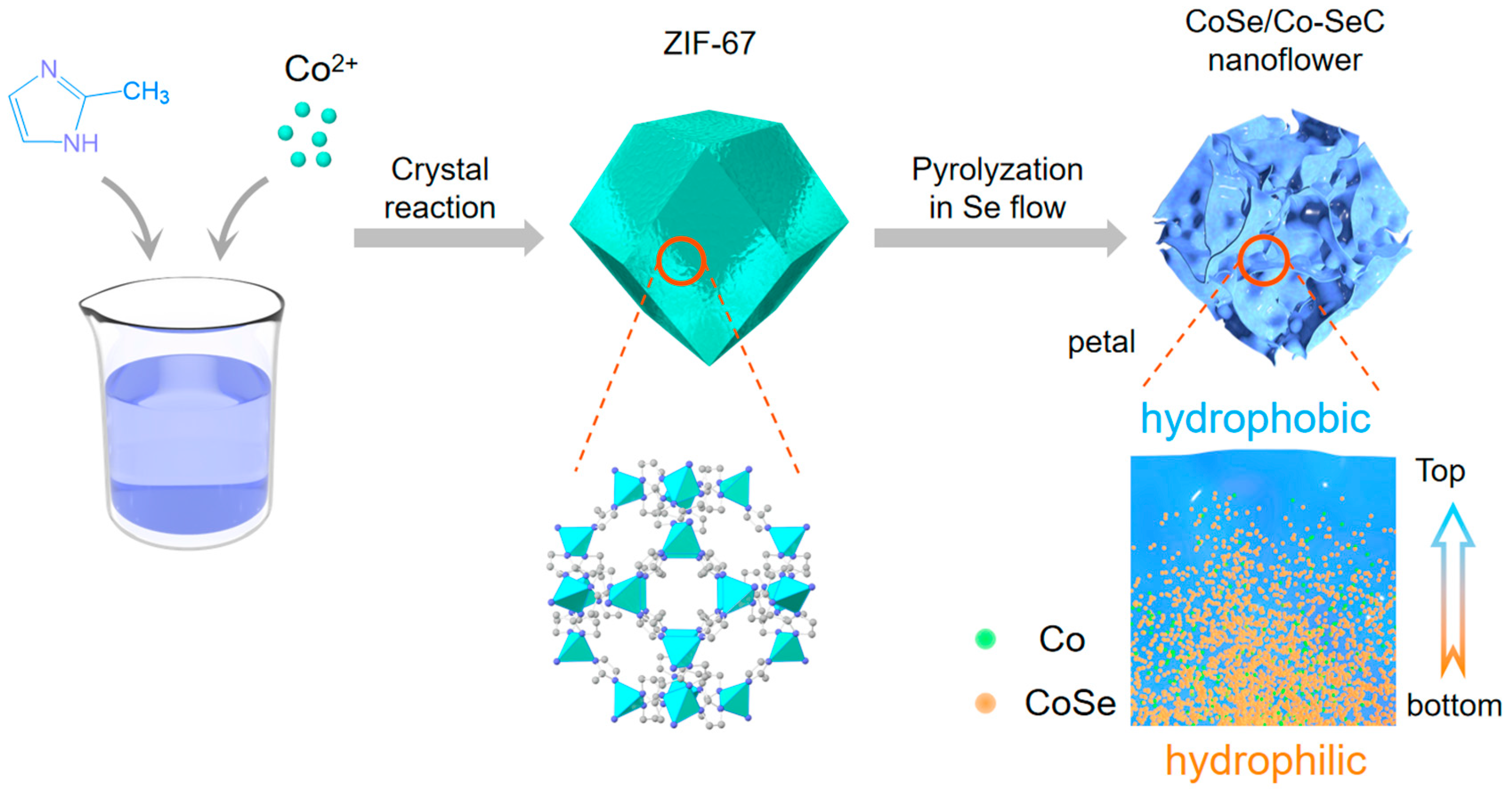
2.1. CoSe/Co-SeC Nanoflowers
2.2. Methods
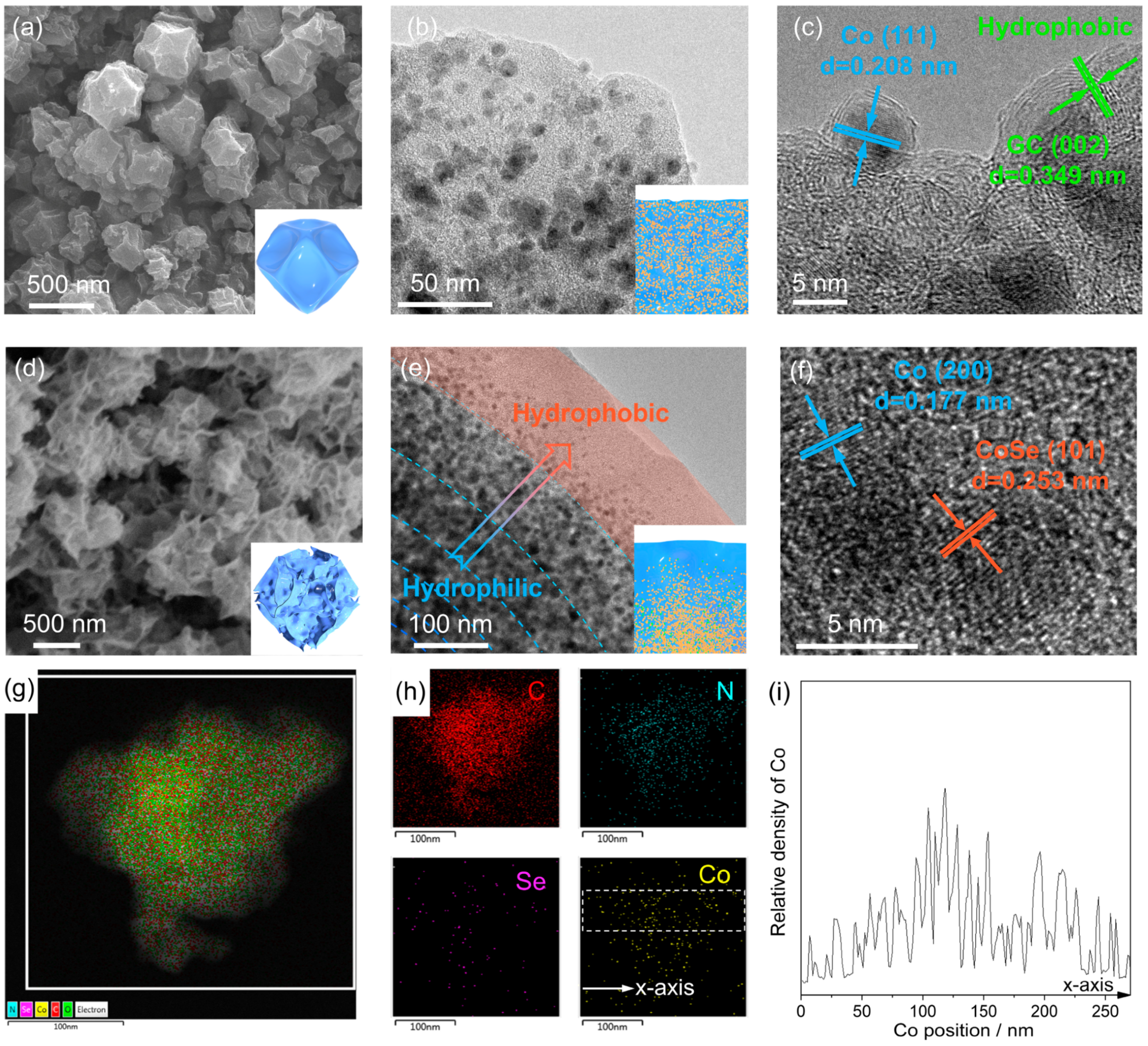
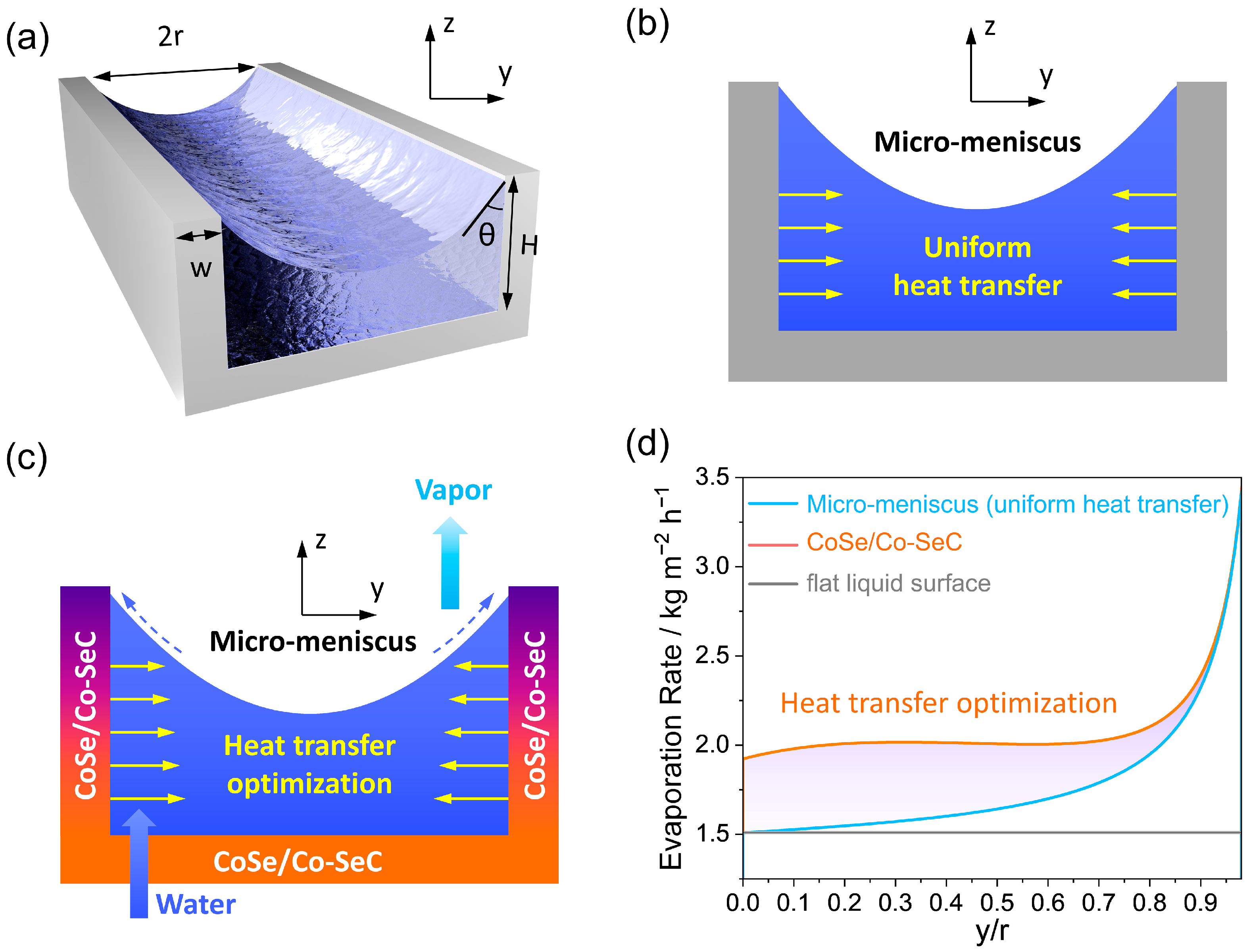
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, C.; Kuang, Y.; Hu, L. Challenges and opportunities for solar evaporation. Joule 2019, 3, 683–718. [Google Scholar] [CrossRef]
- Tao, P.; Ni, G.; Song, C.; Shang, W.; Wu, J.; Zhu, J.; Chen, G.; Deng, T. Solar-driven interfacial evaporation. Nat. Energy 2018, 3, 1031–1041. [Google Scholar] [CrossRef]
- Zhu, L.; Gao, M.; Peh, C.K.N.; Ho, G.W. Recent progress in solar-driven interfacial water evaporation: Advanced designs and applications. Nano Energy 2019, 57, 507–518. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Z.; Zhao, L.; Bhatia, B.; Zhong, Y.; Gong, S.; Wang, E.N. Passive, high-efficiency thermally-localized solar desalination. Energy Environ. Sci. 2021, 14, 1771–1793. [Google Scholar] [CrossRef]
- Zhang, H.; Ou, J.; Fang, X.; Lei, S.; Wang, F.; Li, C.; Li, W.; Hu, Y.; Amirfazli, A.; Wang, P. Robust superhydrophobic fabric via UV-accelerated atmospheric deposition of polydopamine and silver nanoparticles for solar evaporation and water/oil separation. Chem. Eng. J. 2022, 429, 132539. [Google Scholar] [CrossRef]
- Zhou, L.; Tan, Y.; Wang, J.; Xu, W.; Yuan, Y.; Cai, W.; Zhu, S.; Zhu, J. 3D self-assembly of aluminium nanoparticles for plasmon-enhanced solar desalination. Nat. Photonics 2016, 10, 393–398. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, H.; Zhang, X.; Jiang, H.; Geng, X.; Li, S.; Tu, H.; Cheng, X.; Yang, P.; Wan, Y. High-absorption solar steam device comprising Au@ Bi2MoO6-CDs: Extraordinary desalination and electricity generation. Nano Energy 2020, 68, 104298. [Google Scholar] [CrossRef]
- Sun, L.; Li, Z.; Su, R.; Wang, Y.; Li, Z.; Du, B.; Sun, Y.; Guan, P.; Besenbacher, F.; Yu, M. Phase-Transition Induced Conversion into a Photothermal Material: Quasi-Metallic WO2.9 Nanorods for Solar Water Evaporation and Anticancer Photothermal Therapy. Angew. Chem. Int. Ed. 2018, 57, 10666–10671. [Google Scholar] [CrossRef]
- Hu, T.; Chen, K.; Li, L.; Zhang, J. Carbon nanotubes@ silicone solar evaporators with controllable salt-tolerance for efficient water evaporation in a closed system. J. Mater. Chem. A 2021, 9, 17502–17511. [Google Scholar] [CrossRef]
- Dong, X.; Cao, L.; Si, Y.; Ding, B.; Deng, H. Cellular structured CNTs@ SiO2 nanofibrous aerogels with vertically aligned vessels for salt-resistant solar desalination. Adv. Mater. 2020, 32, 1908269. [Google Scholar] [CrossRef]
- Wu, Y.; Shen, L.; Zhang, C.; Gao, H.; Chen, J.; Jin, L.; Lin, P.; Zhang, H.; Xia, Y. Polyacid doping-enabled efficient solar evaporation of polypyrrole hydrogel. Desalination 2021, 505, 114766. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, J.; Han, Y.; Xu, H.; Wang, Y.; Qi, D.; Wang, W. A simple and universal strategy to deposit Ag/polypyrrole on various substrates for enhanced interfacial solar evaporation and antibacterial activity. Chem. Eng. J. 2020, 384, 123379. [Google Scholar] [CrossRef]
- Ibrahim, I.; Seo, D.H.; McDonagh, A.M.; Shon, H.K.; Tijing, L. Semiconductor photothermal materials enabling efficient solar steam generation toward desalination and wastewater treatment. Desalination 2021, 500, 114853. [Google Scholar] [CrossRef]
- Liu, P.; Li, X.Y.; Xu, L.; Chen, C.; Yuan, B.; Labiadh, L.; Hu, Y.B.; Fu, M.L. Recent progress in interfacial photo-vapor conversion technology using metal sulfide-based semiconductor materials. Desalination 2022, 527, 115532. [Google Scholar] [CrossRef]
- Chen, X.; Yang, N.; Wang, Y.; He, H.; Wang, J.; Wan, J.; Jiang, H.; Xu, B.; Wang, L.; Yu, R.; et al. Highly Efficient Photothermal Conversion and Water Transport during Solar Evaporation Enabled by Amorphous Hollow Multishelled Nanocomposites. Adv. Mater. 2022, 34, 2270052. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, Z.; Wu, N.; Zhang, R.; Zhu, B.; Jin, H.; Zhang, Y.; Zhu, M.; Chen, Z. Hierarchical Photothermal Fabrics with Low Evaporation Enthalpy as Heliotropic Evaporators for Efficient, Continuous, Salt-Free Desalination. ACS Nano 2021, 15, 13007–13018. [Google Scholar] [CrossRef]
- Ye, M.; Jia, J.; Wu, Z.; Qian, C.; Chen, R.; O’Brien, P.G.; Sun, W.; Dong, Y.; Ozin, G.A. Synthesis of Black TiOx Nanoparticles by Mg Reduction of TiO2 Nanocrystals and their Application for Solar Water Evaporation. Adv. Energy Mater. 2017, 7, 1601811. [Google Scholar] [CrossRef]
- Lu, Q.; Yang, Y.; Feng, J.; Wang, X. Oxygen-Defected Molybdenum Oxides Hierarchical Nanostructure Constructed by Atomic-Level Thickness Nanosheets as an Efficient Absorber for Solar Steam Generation. Solar RRL 2019, 3, 1800277. [Google Scholar] [CrossRef]
- Chen, R.; Wang, X.; Gan, Q.; Zhang, T.; Zhu, K.; Ye, M. A bifunctional MoS2-based solar evaporator for both efficient water evaporation and clean freshwater collection. J. Mater. Chem. A 2019, 7, 11177–11185. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, H.; Shan, X.; Di, Y.; Zhao, A.; Hu, Y.; Gan, Z. Solar steam generation based on the photothermal effect: From designs to applications, and beyond. J. Mater. Chem. A 2019, 7, 19203–19227. [Google Scholar] [CrossRef]
- Ren, P.; Yang, X. Bifunctional Cu2-xSe-decorated hierarchical TiO2 nanotube mesh with solar water evaporation and photodegradation effects for clean water generation. Water Supply 2019, 19, 2001–2008. [Google Scholar] [CrossRef]
- Shir, A.; Debasis, D.; Carlo, R.; Sergio, S.; Marco, A.; Anna, C.; Jessica, O.; Francesco, L.R.M.; Barun, G.; Daniel, F.; et al. NiSe and CoSe Topological Nodal-Line Semimetals: A Sustainable Platform for Efficient Thermoplasmonics and Solar-Driven Photothermal Membrane Distillation. Small 2022, 18, 2201473. [Google Scholar]
- Yao, J.; Zheng, Z.; Yang, G. Layered tin monoselenide as advanced photothermal conversion materials for efficient solar energy-driven water evaporation. Nanoscale 2018, 10, 2876–2886. [Google Scholar] [CrossRef]
- Jia, G.; Wang, P.; Zhang, Y.; Chang, K. Localized surface plasmon enhanced photothermal conversion in Bi2Se3 topological insulator nanoflowers. Sci. Rep. 2016, 6, 25884. [Google Scholar]
- Wang, K.; Li, Y.; Xie, L.H.; Li, X.; Li, J.R. Construction and application of base-stable MOFs: A critical review. Chem. Soc. Rev. 2022, 51, 6417–6441. [Google Scholar] [CrossRef]
- Kirchon, A.; Feng, L.; Drake, H.F.; Joseph, E.A.; Zhou, H.C. From fundamentals to applications: A toolbox for robust and multifunctional MOF materials. Chem. Soc. Rev. 2018, 47, 8611–8638. [Google Scholar] [CrossRef]
- Qin, J.; Wang, S.; Wang, X. Visible-light reduction CO2 with dodecahedral zeolitic imidazolate framework ZIF-67 as an efficient co-catalyst. Appl. Catal. B Environ. 2017, 209, 476–482. [Google Scholar] [CrossRef]
- Han, B.; Chakraborty, A. Advanced cooling heat pump and desalination employing functional UiO-66 (Zr) metal-organic frameworks. Energy Convers. Manag. 2020, 213, 112825. [Google Scholar] [CrossRef]
- Hall, J.N.; Bollini, P. Metal–Organic Framework MIL-100 Catalyzed Acetalization of Benzaldehyde with Methanol: Lewis or Brønsted Acid Catalysis? ACS Catal. 2020, 10, 3750–3763. [Google Scholar] [CrossRef]
- Lee, G.; Lee, M.; Jeong, Y.; Jang, E.; Baik, H.; Jung, J.C.; Choi, J. Synthetic Origin-Dependent catalytic activity of Metal-Organic Frameworks: Unprecedented demonstration with ZIF-8 s on CO2 cycloaddition reaction. Chem. Eng. J. 2022, 435, 134964. [Google Scholar] [CrossRef]
- Scheurle, P.I.; Mähringer, A.; Biewald, A.; Hartschuh, A.; Bein, T.; Medina, D.D. MOF-74 (M) Films Obtained through Vapor-Assisted Conversion—Impact on Crystal Orientation and Optical Properties. Chem. Mater. 2021, 33, 5896–5904. [Google Scholar] [CrossRef]
- Hou, S.; Zhang, A.; Zhou, Q.; Wen, Y.; Zhang, S.; Su, L.; Huang, X.; Wang, T.; Rui, K.; Wang, C.; et al. Designing heterostructured FeP—CoP for oxygen evolution reaction: Interface engineering to enhance electrocatalytic performance. Nano Res. 2023, 16, 6601–6607. [Google Scholar] [CrossRef]
- Xin, X.; Song, Y.; Guo, S.; Zhang, Y.; Wang, B.; Yu, J.; Li, X. In-situ growth of high-content 1T phase MoS2 confined in the CuS nanoframe for efficient photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2020, 269, 118773. [Google Scholar] [CrossRef]
- Wang, B.; Sun, D.; Ren, Y.; Zhou, X.; Ma, Y.; Tang, S.; Meng, X. MOFs derived ZnSe/N-doped carbon nanosheets as multifunctional interlayers for ultralong-Life lithium-sulfur batteries. J. Mater. Sci. Technol. 2022, 125, 97–104. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Wu, Y.; Guan, C.; Cheetham, A.K.; Wang, J. MOF-derived nanohybrids for electrocatalysis and energy storage: Current status and perspectives. Chem. Commun. 2018, 54, 5268–5288. [Google Scholar] [CrossRef]
- Li, J.; Li, W.; Fang, H.; Zhang, J. Dynamics of evaporation from confined water in an SWCNT in the presence of an external field. J. Phys. Chem. C 2016, 120, 6493–6501. [Google Scholar] [CrossRef]
- Duffey, K.C.; Shih, O.; Wong, N.L.; Drisdell, W.S.; Saykally, R.J.; Cohen, R.C. Evaporation kinetics of aqueous acetic acid droplets: Effects of soluble organic aerosol components on the mechanism of water evaporation. Phys. Chem. Chem. Phys. 2013, 15, 11634–11639. [Google Scholar] [CrossRef]
- Brovchenko, I.; Oleinikova, A. Effect of pore size on the condensation/evaporation transition of confined water in equilibrium with saturated bulk water. J. Phys. Chem. B 2011, 115, 9990–10000. [Google Scholar] [CrossRef]
- Zhao, F.; Guo, Y.; Zhou, X.; Shi, W.; Yu, G. Materials for solar-powered water evaporation. Nat. Rev. Mater. 2020, 5, 388–401. [Google Scholar] [CrossRef]
- Zou, M.; Zhang, Y.; Cai, Z.; Li, C.; Sun, Z.; Yu, C.; Dong, Z.; Wu, L.; Song, Y. 3D printing a biomimetic bridge-arch solar evaporator for eliminating salt accumulation with desalination and agricultural applications. Adv. Mater. 2021, 33, 2102443. [Google Scholar] [CrossRef]
- Morciano, M.; Fasano, M.; Boriskina, S.V.; Chiavazzo, E.; Asinari, P. Solar passive distiller with high productivity and Marangoni effect-driven salt rejection. Energy Environ. Sci. 2020, 13, 3646–3655. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, X.; Zhao, F.; Jiao, Z.; Zhou, X.; Yu, G. Tailoring surface wetting states for ultrafast solar-driven water evaporation. Energy Environ. Sci. 2020, 13, 2087–2095. [Google Scholar] [CrossRef]
- Yu, Z.; Gu, R.; Tian, Y.; Xie, P.; Jin, B.; Cheng, S. Enhanced Interfacial Solar Evaporation through Formation of Micro-Meniscuses and Microdroplets to Reduce Evaporation Enthalpy. Adv. Funct. Mater. 2022, 32, 2108586. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, A.; Ding, L.; Zhou, Z.; Wang, Y.; Niu, S.; Liang, S.; Cao, G. Nitrogen-Doped Yolk-Shell-Structured CoSe/C Dodecahedra for High-Performance Sodium Ion Batteries. Acs Appl. Mater. Interfaces 2017, 9, 3624–3633. [Google Scholar] [CrossRef]
- Murugadoss, V.; Wang, N.; Tadakamalla, S.; Wang, B.; Guo, Z.; Angaiah, S. In situ grown cobalt selenide/graphene nanocomposite counter electrodes for enhanced dye-sensitized solar cell performance. J. Mater. Chem. A 2017, 5, 14583–14594. [Google Scholar] [CrossRef]
- Yang, H.; Gao, S.; Rao, D.; Yan, X. Designing superior bifunctional electrocatalyst with high-purity pyrrole-type CoN4 and adjacent metallic cobalt sites for rechargeable Zn-air batteries. Energy Storage Mater. 2022, 46, 553–562. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Y.; Wang, Z.; Bai, Y.; Zhang, K.; Dong, R.; Gao, Y.; Ni, Q.; Wu, F.; Liu, Y.; et al. Stable Carbon–Selenium Bonds for Enhanced Performance in Tremella-Like 2D Chalcogenide Battery Anode. Adv. Energy Mater. 2018, 8, 1800927. [Google Scholar] [CrossRef]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Schuepfer, D.B.; Badaczewski, F.; Guerra-Castro, J.M.; Hofmann, D.M.; Heiliger, C.; Smarsly, B.; Klar, P.J. Assessing the structural properties of graphitic and non-graphitic carbons by Raman spectroscopy. Carbon 2020, 161, 359–372. [Google Scholar] [CrossRef]
- Gao, L.; Ma, X.; Cheng, J.; Liu, Y. The effect of preparation process on combustibility of Robinia pseudoacacia charcoal and its structure-performance relationship. Sci. Rep. 2025, 15, 14563. [Google Scholar] [CrossRef]
- Moseenkov, S.I.; Kuznetsov, V.L.; Zolotarev, N.A.; Kolesov, B.A.; Prosvirin, I.P.; Ishchenko, A.V.; Zavorin, A.V. Investigation of Amorphous Carbon in Nanostructured Carbon Materials (A Comparative Study by TEM, XPS, Raman Spectroscopy and XRD). Materials 2023, 16, 1112. [Google Scholar] [CrossRef] [PubMed]
- Perazzolo, V.; Durante, C.; Pilot, R.; Paduano, A.; Zheng, J.; Rizzi, G.A.; Martucci, A.; Granozzi, G.; Gennaro, A. Nitrogen and sulfur doped mesoporous carbon as metal-free electrocatalysts for the in situ production of hydrogen peroxide. Carbon 2015, 95, 949–963. [Google Scholar] [CrossRef]
- Jing, S.; Zhang, Y.; Chen, F.; Liang, H.; Yin, S.; Tsiakaras, P. Novel and highly efficient cathodes for Li-O2 batteries: 3D self-standing NiFe@NC-functionalized N-doped carbon nanonet derived from Prussian blue analogues/biomass composites. Appl. Catal. B 2019, 245, 721–732. [Google Scholar] [CrossRef]
- Vélez-Cordero, J.R.; Hernandez-Cordero, J. Heat generation and conduction in PDMS-carbon nanoparticle membranes irradiated with optical fibers. Int. J. Therm. Sci. 2015, 96, 12–22. [Google Scholar] [CrossRef]
- Li, Y.; Feng, Y.; Qin, M.; Chen, K.; An, Y.; Liu, P.; Jiang, Y.; Shen, Z.; Chen, X. Co-anchored Hollow Carbonized Kapok Fiber Encapsulated Phase Change Materials for Upgrading Photothermal Utilization. Small 2025, 2500479. [Google Scholar] [CrossRef]
- Chen, X.; Wang, L.; Gao, Y.; Li, Y.; Zhang, X.; Jiang, Y.; Wang, G. Co/N co-doped flower-like carbon-based phase change materials toward solar energy harvesting. Aggregate 2024, 5, e413. [Google Scholar] [CrossRef]
- Weng, Y.; Guan, S.; Wang, L.; Lu, H.; Meng, X.; Waterhouse, G.I.; Zhou, S. Defective Porous Carbon Polyhedra Decorated with Copper Nanoparticles for Enhanced NIR-Driven Photothermal Cancer Therapy. Small 2020, 16, 1905184. [Google Scholar] [CrossRef]
- Jena, K.C.; Hore, D.K. Water structure at solid surfaces and its implications for biomolecule adsorption. Phys. Chem. Chem. Phys. 2010, 12, 14383–14404. [Google Scholar] [CrossRef]
- Zhao, F.; Zhou, X.; Shi, Y.; Qian, X.; Alexander, M.; Zhao, X.; Mendez, S.; Yang, R.; Qu, L.; Yu, G. Highly efficient solar vapour generation via hierarchically nanostructured gels. Nat. Nanotechnol. 2018, 13, 489–495. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, F.; Guo, Y.; Rosenberger, B.; Yu, G. Architecting highly hydratable polymer networks to tune the water state for solar water purification. Sci. Adv. 2019, 5, eaaw5484. [Google Scholar] [CrossRef]
- Yang, J.; Pang, Y.; Huang, W.; Shaw, S.K.; Schiffbauer, J.; Pillers, M.A.; Mu, X.; Luo, S.; Zhang, T.; Huang, Y.; et al. Functionalized graphene enables highly efficient solar thermal steam generation. ACS Nano 2017, 11, 5510–5518. [Google Scholar] [CrossRef]
- Farokhnia, N.; Irajizad, P.; Sajadi, S.M.; Ghasemi, H. Rational Micro-Nano Structuring for Thin Film Evaporation. J. Phys. Chem. C 2016, 120, 8742–8750. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, L.; Liu, Z.; Hao, H.; Liu, Z. Uneven Hydrophilic–Hydrophobic Nanoflowers Enhancing Solar Interface Evaporation: Se-Doped Carbon Loaded with Gradient Distribution of CoSe/Co. Materials 2025, 18, 2409. https://doi.org/10.3390/ma18102409
Jia L, Liu Z, Hao H, Liu Z. Uneven Hydrophilic–Hydrophobic Nanoflowers Enhancing Solar Interface Evaporation: Se-Doped Carbon Loaded with Gradient Distribution of CoSe/Co. Materials. 2025; 18(10):2409. https://doi.org/10.3390/ma18102409
Chicago/Turabian StyleJia, Linhui, Zhenhao Liu, Hongxun Hao, and Zhongxin Liu. 2025. "Uneven Hydrophilic–Hydrophobic Nanoflowers Enhancing Solar Interface Evaporation: Se-Doped Carbon Loaded with Gradient Distribution of CoSe/Co" Materials 18, no. 10: 2409. https://doi.org/10.3390/ma18102409
APA StyleJia, L., Liu, Z., Hao, H., & Liu, Z. (2025). Uneven Hydrophilic–Hydrophobic Nanoflowers Enhancing Solar Interface Evaporation: Se-Doped Carbon Loaded with Gradient Distribution of CoSe/Co. Materials, 18(10), 2409. https://doi.org/10.3390/ma18102409








