Abstract
The development of highly effective natural-based adsorbents to face the increasing rates of CO2 production and their delivery to the atmosphere are a big concern nowadays. For such purposes, synthetic and natural zeolites were modified via an ion exchange procedure to enhance the CO2 uptake. Samples were characterized by SEM, EDS, TGA and nitrogen adsorption at 77 K, showing the correct incorporation of the new metals; in addition, the CO2 adsorption isotherms were determined using a gas analyser. During the first stage, the role of the compensation cations for CO2 adsorption was assessed by modifying a pure ZSM-5 synthetic zeolite with different metal precursors present in salt solutions via an ion exchange procedure. Then, five samples were studied; the samples modified with bivalent cation precursors (Zn2+ and Cu2+) presented a higher adsorption uptake than those modified with a monovalent cation (Na+ and K+). Specifically, the substitution of the compensation cations for Cu2+ increased the CO2 capture uptake without affecting the surface properties of the zeolite. The results depict the prevalence of π-cation interactions enhanced by the field gradient induced by divalent cations and their lower ionic radii, if compared to monovalent ones. Subsequently, a natural zeolite was modified considering the best results of the previous phase. This Surface Response Methodology was implemented considering 11 samples by varying the concentration of the copper precursor and the time of the ion exchange procedure. A quantitative quadratic model to predict the adsorption uptake with an R2 of 0.92 was obtained. The results depicted the optimal conditions to modify the used natural zeolite for CO2 capture. The modification procedure implemented increased the CO2 adsorption capacity of the natural zeolite more than 20%, reaching an adsorption capacity of 75.8 mg CO2/g zeolite.
1. Introduction
The increasing demands of human activities and industrial development necessitate intensive energy consumption, primarily through the combustion of fossil fuels. However, these processes lead to significant environmental challenges, particularly due to the high production rates of carbon dioxide (CO2) and other greenhouse gases. CO2, which accounts for approximately 65% of the global radiative forcing caused by long-lived greenhouse gases, acts as a significant absorber and emitter of infrared radiation []. Over the past 50 years, atmospheric CO2 concentrations have risen by more than 100 ppm, contributing to global warming and ocean acidification []. CO2 alone accounts for approximately 74% of global anthropogenic greenhouse gas (GHG) emissions and is the major contributor to climate-related impacts []. According to the Intergovernmental Panel on Climate Change (IPCC) [] and recent economic analyses [], the global economic losses resulting from climate change, predominantly driven by GHGs such as CO2, could reach 11–14% of GDP under a scenario of 2 °C warming by 2050, meaning potential losses higher than USD 11 trillion.
Carbon capture and storage (CCS) technologies have emerged as effective methods for mitigating CO2 emissions. Projections indicate that CCS technologies could sequester up to 240 billion tonnes of CO2 by 2050 []. Among the existing CCS, the traditional approaches, such as amine-based absorption, could achieve over 90% efficiency [,]. Nevertheless, operational challenges, including high energy consumption, solvent degradation, and equipment corrosion, underscore the necessity for improved technologies []. Alternative technologies, particularly solid adsorbents, are emerging as promising solutions. These systems offer lower energy consumption, reduced corrosion risks, and adsorption efficiencies of up to 95% [,]. Notably, replacing amine-based absorption systems with solid adsorbents can reduce the energy required for solvent regeneration by approximately 40% [].
Among the available solid adsorbents, zeolites and activated carbons stand out due to their exceptional properties []. Specifically, zeolites are crystalline aluminosilicates with a highly ordered pore structure, ranging from 0.3 to 1 nm in diameter, that allow for selective adsorption and permeation based on molecular size and polarity []. Their thermal stability, resistance to acids and bases, and modifiable surface properties make them versatile for several applications, such as gas stream purification and natural gas upgrading []. Additionally, adsorbents based on zeolites can be reactivated by chemical and thermal processes, allowing them to be used several times []. Moreover, the high mechanical stability of zeolites, attributed to their advanced nanomechanical properties, makes these materials suitable for large-scale processes. In this sense, zeolite can be used as a component for concrete for building, using functional materials for industrial applications []. For practical, large-scale application, the production costs should allow the adsorption to be competitive with other CO2 separation processes.
The unique structure of zeolites comprises a three-dimensional tetrahedral framework of SiO4 and AlO4 units. The substitution of Si with Al induces a negative charge, which is balanced by exchangeable cations, often alkali cations, within the porous network. These compensating cations maintain the structure’s electro-neutrality and confer specific sorption characteristics, including ion exchange and selective adsorption [,]. The functionalization of zeolite surfaces enables the incorporation of new chemical and physical properties into these materials. In particular, supporting transition and noble metals on zeolites is a widely employed functionalization strategy for adsorbent and catalyst synthesis, as it yields materials with high surface areas and enhanced chemical activity [].
Carbon dioxide adsorption in zeolites is predominantly influenced by the interaction between CO2 molecules and the electric field generated by compensating cations []. In the last few years, several studies concerning CO2 remotion have been conducted using zeolites, both synthetic zeolites, such as Y [,], 13X [,], LTA [], SSZ13 [], and natural zeolites such as clinoptilolite [,,,] and mordenite [,,]. Different experiments have been performed to determine the main structural and experimental factors influencing the carbon dioxide adsorption onto zeolites. In this sense, the influence of parameters, such as the temperature [,,], pressure [,,], Si/Al ratio [,,], compensation cation [,,], metal loading [,,], among other properties, has been assessed. Insights drawn from research reviews [,] depict that CO2 adsorption is principally governed by the inclusion of exchangeable cations within the cavities of zeolites, which induce the electric field interacting with the adsorbate molecule. Research also suggests that basic compensating cations enhance the electrostatic field on the zeolite surface, improving CO2 adsorption []. Interestingly, acidic zeolites have also demonstrated promising results, underscoring the need for further investigation into the role of cations in enhancing the adsorption uptake.
Zeolites offer a promising pathway toward sustainable industrial practices. Their availability, particularly of natural zeolites, reduces production costs, enhancing their applicability []. Even when previous studies have reported insights into the role of the compensation cations of zeolites and other parameters in CO2 adsorption, few reports have checked the optimal conditions to modify natural zeolites to obtain a cost-effective CO2 adsorbent. This work investigates this potential through a two-stage approach. Initially, the effect of modifying synthetic zeolites by ion exchange with various compensating cations on surface physicochemical properties and CO2 adsorption capacity was evaluated. Building on these findings, the second stage focused on modifying natural Chilean zeolites with salts of specific compensating cations to improve CO2 adsorption, aiming to develop efficient and cost-effective adsorbent materials. This research contributes to advancing carbon capture technologies and promoting green industrial processes as the CO2 trapped in these materials can be further valorised to interesting energetic resources, such as Methanol and Dimethyl ether, via a catalytic process. Thus, this work aligns with Sustainable Development Goal (SDG) 7 “affordable and clean energy” and Goal 13 “climate action”, specifically by integrating climate change-related measures by diminishing the GHGs emitted by the industrial process and the conventional energy production systems, among others [].
2. Materials and Methods
2.1. Materials and Reagents
Synthetic ZSM-5 zeolite provided by TOSOH Corporation (Grove City, OH, USA) was employed. Specifically, the 840NHA variant containing NH4⁺ ions as compensating cations was utilised. In addition, the natural zeolite was sourced from a natural extraction site in Maule, located in Chile’s VII Region, and provided by the company Zeolita del Maule (Maule, Chile). According to the technical datasheet, this zeolite consists of a mixture of Clinoptilolite and Mordenite.
For the chemical modification of the zeolite samples, the following salts were used as precursors: copper (II) nitrate trihydrate [Cu(NO3)2·3H2O], zinc nitrate hexahydrate [Zn(NO3)2·6H2O], sodium nitrate [NaNO3], and potassium nitrate [KNO3]. These salts were supplied, respectively, by Merck S.A. (Darmstadt, Germany), Sigma Aldrich (Burlington, MA, USA), Winkler (Santiago, Chile), and Merck S.A. (Darmstadt, Germany), all with analytical-grade purity.
The use of a synthetic and mono-cationic zeolite of known composition was aimed at investigating the role of the compensation cations and their interaction with the CO2 molecule. This effect is particularly difficult to investigate in poly-cationic zeolites, such as natural zeolites, where the ion exchange equilibrium is more complex as well as the final composition after modification []. Therefore, once the cations that enhanced CO2 capture were identified, the incorporation of this specific cation into the synthetic zeolite was studied to optimize the surface.
2.2. Physicochemical Modification of Zeolites
The parent zeolites were modified by chemical and thermal processes, considering an ion exchange procedure and a thermal outgassing in an inert atmosphere. Before the modifications, the zeolites were washed several times with deionized water and dried in an oven (WGLL-BE, FAITHFUL, Cangzhou, China) at 363 K for 24 h.
2.2.1. Chemical Modifications by Ion Exchange Process
The precursor solutions used for modifying the parent zeolites were prepared using a nominal mass percentage, calculated as grams of the target cation () per gram of the final modified zeolite ( + ). In this sense, the weight of the metal cation within the precursor salt molecule was determined considering the ratio of their molar mass. Consequently, the theoretical percentage of the metal cation within the modified zeolite was calculated using Equation (1).
In this work, Na⁺, K⁺, Cu2⁺, and Zn2⁺ were selected as compensating cations based on their specific physicochemical characteristics, such as ionic radius, charge density, and their ability to promote interactions with CO2 molecules [,,]. In addition, the selection considered cost-effective salt precursors among the possible cations. For the initial phase of this study, a nominal percentage of 4% was selected.
Afterward, the zeolite was added to the precursor solutions at a ratio of 10:1 (cm3 of solution per gram of modified zeolite), and the samples were placed in a thermostatically controlled water bath at 363 K using an EV400H rotary evaporator (Labtex, Jinan, China). The ion exchange process was conducted under constant stirring at 120 rpm for 4 h. Subsequently, the samples were washed in deionized water and oven dried (Labtex, China) at 363 K for 24 h and stored in a vacuum desiccator for future use. Values of metal loading, exchange temperature and the procedure time were determined based on prior investigations involving natural and ZSM-5 zeolites modified with copper cations [,]. The modification parameter selection also considered information from other researchers [,,].
For a better understanding, samples are labelled as indicated in Table 1.

Table 1.
Synthetic zeolites modified by ion exchange procedure.
It is noteworthy that, during the second phase of this study, the precursor solutions were prepared at varying concentrations, and the ion exchange process was conducted for different durations.
2.2.2. Thermal Outgassing of Chemically Modified Zeolites
To remove residues from the ion exchange process and other volatile impurities from the zeolite surface, the pre-modified samples were placed in a tubular reactor supported by a quartz frit, under a continuous argon flow of 300 cm3/min. The setup was subjected to a gradual heating process in an SZGL-1200C furnace (SIOMM, Shanghai, China) at a constant temperature increase rate of 5 K per minute, reaching 623 K, where it was held for one hour. The cooling phase was subsequently performed while maintaining the argon flow.
2.3. Characterization of the Adsorbent Materials
2.3.1. Porosimetry Measurements
The textural characteristics of the parent zeolites and the prepared adsorbents were evaluated through nitrogen adsorption–desorption isotherms at 77 K using a Nova 800 porosimeter (Anton Paar, Graz, Austria). The surface area was calculated using the Brunauer–Emmett–Teller (BET) method, while the BJH (Barrett–Joyner–Halenda) method for pore size distribution, H-K (Horvath–Kawazoe) method for micropore volume and t-plot method for micropore surface area estimation were applied. Prior to analysis, the samples were degassed at 623 K for 2 h to eliminate potential interference with pore accessibility.
2.3.2. Scanning Electron Microscopy
The morphology of the samples was examined through scanning electron microscopy (SEM) with an SU-3500 microscope (Hitachi, Tokyo, Japan), operated under high vacuum (30 Pa) at an accelerating voltage of 10.0 kV. Images were captured at magnifications ranging from 5 μm to 100 μm. Energy-dispersive spectroscopy (EDS) was employed alongside SEM to confirm the incorporation of copper nanoparticles within the zeolite framework.
2.3.3. Thermogravimetric Measurements
Thermogravimetric Analysis (TGA) was conducted using an STA 6000 analyser (PerkinElmer, Shelton, CT, USA) to evaluate the thermal stability of the samples and quantify the mass loss associated with the degassing process. During the assay, the samples were heated from room temperature to 873 K at a constant heating rate of 15 K per minute. Furthermore, mass variations were recorded in parallel with heat flow measurements using a Differential Scanning Calorimeter (DSC) coupled to the STA 6000 analyser.
2.4. Determination of the Maximum CO2 Adsorption Capacity
2.4.1. Experimental CO2 Isotherms
The CO2 adsorption isotherms were obtained using a NOVA 800 gas analyser (Anton Paar, Graz, Austria). For this analysis, 0.06 g of zeolite were pre-activated through an outgassing procedure under vacuum. The temperature was increased at a rate of 10 K/min from room temperature to 573 K, held for 3 h, and then cooled to 298 K. Subsequently, a flow of pure CO2 was applied through the cell containing the samples at relative pressures (p/p0) ranging from 0.001 to 1. The temperature remained constant at 298 K throughout the experiment. The precision for gas adsorption measurements is given as ±1% of reading and reproducibility of 2% as reported by the manufacturer.
2.4.2. Isotherm Modelling
The experimental data of CO2 adsorption isotherms were analysed using the linearised Langmuir and Freundlich models to determine the adsorption capacity and the affinity of CO2 for the original and modified zeolites. The Langmuir isotherm was considered appropriate due to the presumed monolayer adsorption and surface homogeneity of the synthetic zeolite, while the Freundlich model accounted for the potential heterogeneity and multilayer adsorption effects, particularly at higher concentrations, mainly because the study involved the use of natural zeolites []. Table 2 displays the isotherm models used.

Table 2.
General and linearized equations of theoretical adsorption models.
2.5. Determination of the Optimised Modification Conditions for Natural Zeolites
Response Surface Methodology (RSM) was applied employing a Central Composite Design (CCD) to optimise the conditions that enhance the adsorption capacity of the natural zeolite (NZ_0). This experimental design incorporated a factorial approach with two levels, three central points and four-star points, yielding a total of 11 samples. The analysis was conducted using MODDE PRO-13 software. The software randomized the order of the experiments to reduce bias in the execution of the measurements []. The statistical analysis will be performed considering the correlation coefficient (R2) and cross validation correlation coefficient (Q2) referring the adjustment of the experimental data to those predicted by the model.
The independent variables considered were the theoretical copper concentration to be incorporated onto the zeolite surface (2% to 8%), calculated using Equation (1), and the duration of the ion exchange process (4 to 12 h). Table 3 presents the experimental design, which comprised eleven samples with theoretical copper concentrations ranging from 0.34% to 11% and ion exchange durations from 2.34 to 13.7 h. It is worth noting that, to generate the response surface, the CCD included modification conditions that extended beyond the initially defined variable limits.

Table 3.
Experimental design for natural zeolite modification.
3. Results and Discussion
3.1. On the Influence of the Compensating Cation on the CO2 Adsorption in Synthetic Zeolite
In the first experimental stage, a pure synthetic zeolite was used to assess how the incorporation of new compensation cations affects the CO2 adsorption behaviour. In this sense a commercial ZSM-5 zeolite synthetized with NH4+ as compensating cation was used. Then, ion exchange procedures in identical conditions but with different metal precursors were performed.
3.1.1. Physical, Chemical and Surface Characterization of Raw and Modified Synthetic Zeolites
Table 4 displays information about the physical-chemical composition and surface properties of the synthetic zeolite and the samples generated by modifying their compensation cations. As expected, the parent zeolite (SZ) presents only Si, Al and O (Nitrogen and Hydrogen cannot be detected by the EDS apparatus) whose, purity allowed to verify the incorporation of the desired metallic cations via ion exchange and their influence in the zeolite properties. In addition, the incorporation of the new compensation cations was verified in all the samples at concentrations ranging from 1.17% (SZ_Na) to 2.03% (SZ_K) without affecting the Si/Al ratio.

Table 4.
Physical-chemical and surface properties of parent and modified synthetic zeolites.
The nitrogen adsorption assays yielded interesting results. A slight decrease in the surface area was observed across samples exchanged with divalent cation precursors, while, for the sample prepared using KNO3 and NaNO3 as the precursor, the decrease in the total surface was slightly higher. Moreover, in the cases using Cu(NO3)2 and Zn(NO3)2 as cation precursors, the microporous surface remained similar and a slight decrease in the mesoporous area occurred in both samples. The samples modified with monovalent cations presented decreases in both the microporous and mesoporous surfaces. However, based on the lower decrease of the microporous and mesoporous surfaces for all the samples, it can be inferred that the modification procedure was successfully implemented without affecting the main structure of the zeolite. Regarding the pore volume, the sample presented similar results with a lower effect on the samples modified with Cu2+ and Zn2+ precursors. Finally, it is noting worth that the average pore size is similar for all the studied samples.
SEM images and the Elemental Mapping performed using EDS (Figure 1) confirmed the successful incorporation of the metals in the zeolite. At first inspection, SEM image of SZ (Figure 1A) looks similar to the modified samples (Figure 1C,E,G,I) suggesting that the external surface was not affected. EDX spectra is displayed in the Supplementary Materials (Figure S1). The Peaks referring to carbon element correspond to the material used to deposit the samples during the sample preparation for imaging.
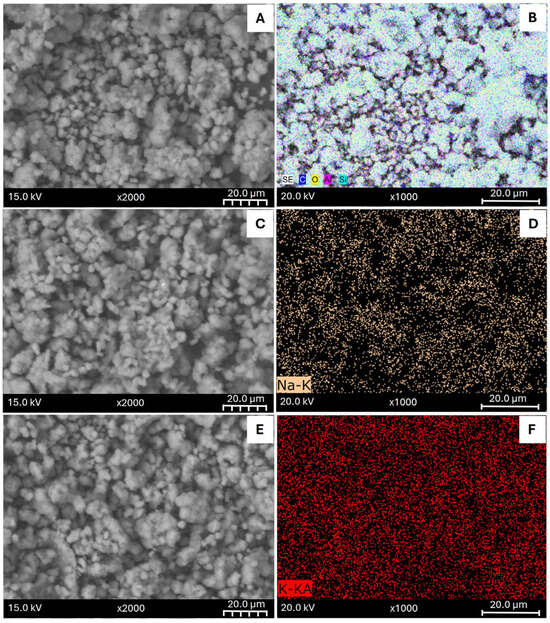
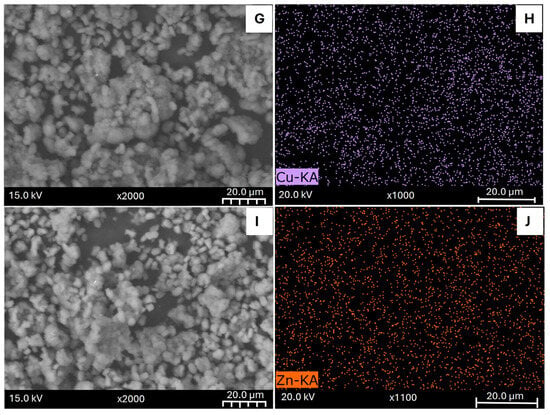
Figure 1.
SEM Images (left) and Elemental Mapping (right) of parent synthetic zeolite (A,B) and modified samples: SZ_Na (C,D), SZ_K (E,F), SZ_Cu (G,H) and SZ_Zn (I,J).
Besides, Figure 1D,F,H,J displays the metal dispersion of the metal elements in the modified samples. As it can be observed, all the samples present a uniform distribution of the metals added by ion exchange, specifically, the formation of metal clusters or zones without the used cation was not observed. It is interesting to note that, in the sample modified with KNO3 (Figure 1F), it can be appreciated a higher particle density consistent with the higher concentration of K element obtained by EDS analysis.
3.1.2. On the Determination of the Temperature for the Outgassing Procedure Using Thermogravimetric Information
The results of the Thermogravimetric Analysis are displayed in Figure 2. For all samples, the first peaks of weight loss occur around 363 K. This result can be attributed to the desorption of water from the zeolite surface []. Consequently, aligned with the observations of Sun et al. [], water seems to be mostly desorbed during the initial degassing stage, in a temperature range from 300 K to 523 K. Moreover, further changes that occurred at temperatures higher than 523 K can be attributed to the desorption of volatile compounds such as the compound formed by the anion that accompanies the metallic precursor deposited as a compensation cation on the zeolite surface. The desorption at these higher temperatures could also be attributed to more tightly bound water molecules located in the micropores as well as the dihydroxylation by the remotion of some OH groups from the zeolite framework [].
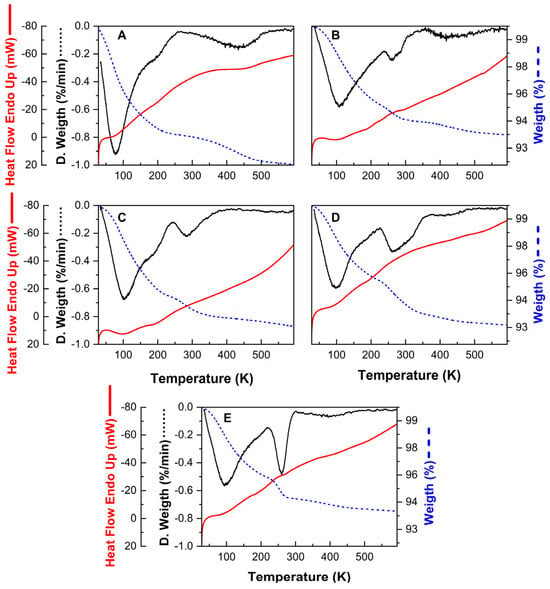
Figure 2.
Thermogravimetric Analysis of the parent synthetic zeolite (A) and modified samples: SZ_Na (B), SZ_K (C), SZ_Cu (D) and SZ_Zn (E).
The parent zeolite (Figure 2A) exhibits a second desorption peak around 573 K to 773 K, characteristic of the NH3 desorption from strongly acidic sites [], allowing for the zeolite provided of Brönsted acidic sites by creating new Si-O-H terminals []. On the other hand, the second desorption peak occurred at lower temperatures on the modified samples (Figure 2B–D), with almost negligible weight loss after 623 K, suggesting that NH4+ cations were correctly replaced during the ion exchange process. Thus, the released substances are likely the nitrate used during the modification process. In this sense, it was checked that the minimum temperature required to activate the modified zeolites was approximately 623 K, since around 80% of the species were desorbed from the surface at this temperature.
Likewise, the heat flow graphs confirm that the mass loss processes are of an exothermic nature [,,], in agreement with those discussed above. Specifically, an endothermic transition is observed at lower temperatures, like the weight loss attributed to water desorption. In addition, at higher temperatures, broader peaks can be observed, also suggesting endothermic transitions, centred at temperatures similar to those at which the mass loss peaks were reported. Finally, glass transition signals were not detected, either in the pristine or modified samples, which is also a typical behaviour of highly crystalline zeolites such as the ZSM-5 zeolite used. It is worth noting that the heat flow graphs are reported with an inverted axis (endothermic up) for a better visualization.
3.1.3. Assessing the Role of the Zeolite Compensation Cations for CO2 Adsorption
As expected, the CO2 adsorption isotherms at 298 K suggest that the cation exchange procedure affects the interaction between the target molecule and the adsorbent. Figure 3 indicates that modifying synthetic zeolite with divalent cations, within the studied concentration range, could enhance the interaction between the CO2 molecule and the zeolite surface. According to the shape of the adsorption curves, a typical type 1 isotherm is formed, suggesting the formation of an adsorbate monolayer. In the low-concentration range, where pressures are below 10 kPa, the adsorption capacity increases almost linearly with the CO2 concentration. As the CO2 concentration rises further, the surface appears to reach saturation, resulting in a minimal increase in absorbed volume, which ultimately levels off. This behaviour is characteristic of the adsorption process that primarily occurs through chemical interactions in materials with a regular porous structure, such as the synthetic zeolites utilized in this study [].
Figure 3.
Adsorption isotherms (dots) and Langmuir model representation (lines) of the parent synthetic zeolite (•) and modified samples: SZ_Na (•), SZ_K (•), SZ_Cu (•) and SZ_Zn (•).
As suggested by the adsorption curve shape, the experimental data closely align with the Langmuir model for the raw ZSM-5 zeolite and the modified samples, demonstrated by a coefficient of determination (R2) greater than 0.99. It can, therefore, be stated that localized chemical adsorption mainly takes place, meaning that the CO2 molecules are retained as a monolayer on the zeolite surface []. In contrast, the limited alignment of the data with the Freundlich model, with R2 values ranging from 0.93 to 0.977 in the most accurately fitting datasets, suggests that the formation of multiple adsorbate layers on the zeolite surface could be discarded [].
Interesting results were obtained from the Langmuir model. Although the CO2 uptake by zeolites modified with monovalent and divalent cations presents clear differences at the experimental concentration range, the maximum CO2 uptake () predicted by the Langmuir (Table 5) model remains similar for all samples (105.4–105.9 mg/g). However, the value obtained is calculated at the curve asymptote, which may not necessarily correspond to the adsorbate concentration range relevant in applying the adsorbent materials.

Table 5.
Models for CO2 adsorption isotherms at 293K on ZSM-5 zeolite, raw and modified.
The fact that approximately 70% of CO2 emissions originate from anthropogenic sources represents a global issue. Kumar and Nagendra et al. (2015) investigated CO2 emissions in commercial, industrial, and urban sectors of Chennai, India, reporting values of 467 ± 33.45 ppm, 464 ± 31.68 ppm, and 448 ± 33.45 ppm, respectively []. A similar case has been documented in the Hsinchu Industrial Park, Taiwan, where a daily average of nearly 600 ppm was recorded []. Another significant source of CO2 emissions is vehicular transport, with an annual average concentration of 533 ± 105 ppm in Delhi, India, highlighting the urgent need for cost-effective CO2 capture solutions []. In this concentration range, the zeolite samples modified with copper and zinc precursors present higher adsorption uptakes than the other samples applicable to the CO2 uptake from contaminant sources.
The results obtained are concordant with previous works that reported high adsorption capacities for molecules with a high quadrupolar moment when using zeolites modified with divalent cations, as opposed to those modified with monovalent cations, particularly in the case of copper-modified zeolites [,].
According to this, the behaviour of the adsorption can be explained by two main aspects. On the one hand, the field gradient within the zeolite and quadrupole interaction seems to dominate the adsorption of CO2, as the adsorbate possesses a strong quadrupole moment but no dipole []. In this sense, Cu2+ and Zn2+ as divalent cations have twice the amount of charge than Na+ and K+ with monovalent cations. On the other hand, previous studies have reported that the π-cation interactions present an inverse proportionality with the ionic radii [,]. In this sense, higher adsorption was obtained using the Cu2+- and Zn2+-modified samples, with ionic radii of 73 and 74 pm, respectively, lower than Na+ and K+ with ionic radii of 102 pm and 138 pm, respectively. Accordingly, molecular dynamics simulations have also suggested that the interactions among the CO2 (or other quadrupolar molecules) and zeolite can be improved by exchanging zeolites with cations with lower ionic radii [,,]. This phenomenon can be explained by a shorter distance between the centre of mass of the CO2 molecule and the cation, increasing the Van der Waals interactions [,].
3.2. On the Development of a CO2 Adsorbent Material Based on Natural Zeolite
Considering the results obtained in the previous sections, the lower price of natural zeolites compared to synthetics and their availability in Chile, in the second phase of this study, natural Chilean zeolites were modified with copper cation precursors.
3.2.1. Physicochemical and Surface Characterization of Raw and Modified Natural Zeolite Samples
Five representative samples of natural zeolites were selected from the RSM experimental design (Table 3) to study the influence of chemical modification on the physical, chemical, and surface properties. For a better understanding, samples were ordered considering the amount of copper salts used in the precursor solutions. In this sense, the selected samples were NZ (0%), NZ_5 (0.34%), NZ_3 (2%), NZ_8 (6%), NZ_4 (10%).
Table 6 displays information about the physicochemical composition and surface properties of the natural zeolite and the samples generated. As has been reported in previous investigations using natural Chilean zeolite, its chemical composition differs from the synthetic zeolite with several compensation cations, such as Na, Mg, K, Ca and Fe. It is important to note that in all the modified samples, copper was detected; however, the mass percentage of this metal was lower than the calculated concentration. Nevertheless, this is normal behaviour for ion exchange procedures because the zeolite has a limited quantity of compensating cations and also because some of the compensating cations preset in the natural zeolite possess a high affinity to the surface, becoming difficult to remove []. In this sense, in all the modified samples, the mass percentage of the initial cations diminished, while the copper concentration reached 2.76% in the sample with higher copper loading.

Table 6.
Physical-chemical and surface properties of parent and modified natural zeolites.
Regarding the surface properties, all the modified natural zeolites presented higher surface areas and pore volumes than the raw natural zeolite. This result was also expected because the chemical and physical modification can clean the zeolite surface from impurities and previously adsorbed compounds in their natural reservoirs. Similar to what occurred with synthetic zeolites, the variation in the surface areas was low, and there were no evident variations in the external surface, as can be seen in Figure 4, suggesting that the incorporation of the metal within the zeolite does not block the pore network.
Figure 4.
SEM images of parent natural zeolite (A) and modified samples: NZ_5 (B), NZ_3 (C), NZ_8 (D) and NZ_4 (E).
3.2.2. CO2 Adsorption Isotherms onto Raw and Modified Natural Zeolites
Table 7 presents a summary of the obtained parameters for Langmuir and Freundlich mathematical models applied to the CO2 adsorption onto the natural zeolite (Z0) and selected samples, of natural zeolites modified with copper (Z5, Z3, Z8 and Z4). The experimental data adjusted to the Langmuir model for the selected samples are shown in Figure 5. Here, it can be observed that the parameter of the Langmuir model, which indicates the maximum adsorption capacity of CO2 in the zeolites, increases in most of the modified samples compared to the unmodified sample (Z0). The Langmuir model parameters for all the samples are presented in Supplementary Information Table S1.

Table 7.
Models for CO2 adsorption isotherms at 293 K on natural zeolite, raw and modified.

Figure 5.
Adsorption isotherms (dots) and Langmuir model representation (lines) of the parent natural zeolite NZ (•) and modified samples: NZ_5 (•), NZ_3 (•), NZ8 (•) and NZ_4 (•).
Specifically, the values for samples Z3, Z4 and Z8 are higher than those of Z0, indicating an improvement in the adsorption capacity due to the modification of the zeolites. However, sample Z5 presented a slightly lower adsorption capacity than Z0, which could be due to the low concentration of copper added, which might not be sufficient to improve this capacity significantly.
As expected, the experimental data fit the Langmuir model well. Similar to what occurred with the synthetic zeolites, in the explored concentrations, the CO2 uptake and the maximum adsorption capacity increase, indicated by the Langmuir model, which increases from 61.72 mg/g in the natural unmodified zeolite (NZ) to 73.5 mg/g in the sample NZ_4. This result can be attributed to the higher amounts of the copper precursor, if compared to those used to modify synthetic zeolite. Additionally, contrary to the results obtained using synthetic zeolites, the CO2 adsorption isotherms using the raw and modified natural zeolite samples also fit the Freundlich model. In this sense, the Freundlich model parameter Kf, which is directly related to the adsorption capacity in heterogeneous systems, presented results reaffirming that copper modifications in natural zeolites increase their CO2 adsorption capacity. Furthermore, the values of the parameter n in all samples suggest the occurrence of physisorption processes [], in which the active sites of natural zeolites (raw and modified) may have the capacity to adsorb one or more CO2 molecules, with a non-parallel orientation, thus indicating a multi-molecular adsorption process for each sample [].
3.2.3. Finding the Optimized Conditions to Modify Natural Zeolites for CO2 Capture: SRM Results
Table 8 presents the results of the maximum CO2 adsorption capacity for each sample, obtained directly from the analysis of experimental isotherms according to the Langmuir model as well as the model parameters.

Table 8.
Results of the experimental design for natural zeolite modification.
Figure 6A shows the Surface Response as a bidimensional representation of the polynomial response when analysing how ion exchange time and the amount of copper in the precursor solution affect the CO2 adsorption capacity of natural zeolite. The interaction between a high ion exchange time and an increase in copper concentration is indicated in the reddish area of the graph as the optimal conditions with the highest CO2 capture efficiency. In addition, the model parameters presented in Figure 6B allow for exploring the relationships between the copper concentration in the precursor solution (Cu) and the ion exchange time (t) with the CO2 adsorption capacity (Qe).
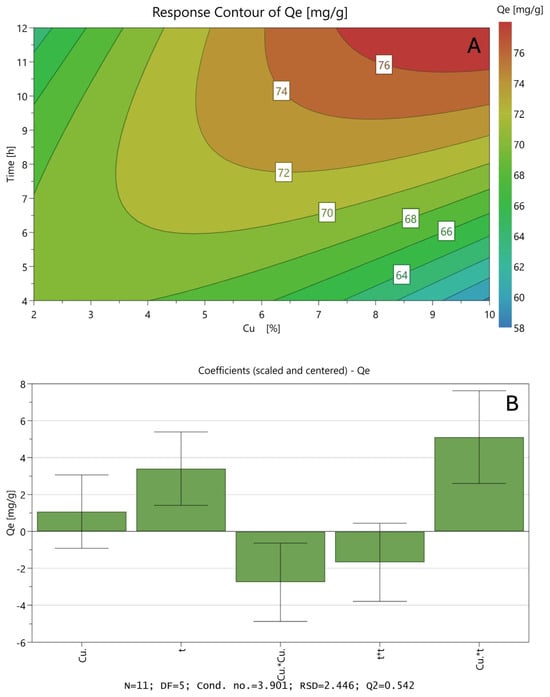
Figure 6.
Results of the SRM using a CCD experimental design for CO2 adsorption onto natural modified zeolites: Surface Response for the adsorption uptake (A) and model coefficient values (B).
Similar to the preliminary analysis, the square parameter that indicates the interactions between both variables presents a positive value, so it can be assumed that the best modification conditions are obtained by combining high values in both variables, at least in the experimental range. It is well known that, when carrying out ion exchange processes with very saturated precursor solutions, it can lead to the precipitation of these metals as oxides on the surface of the support, blocking the pores, decreasing the surface area and finally affecting the removal capacity.
It is worth noting that the statistical parameters related to the obtained model presented show a high level of fit, considering that it is a quadratic model (R2 = 0.92). This can be corroborated in Figure S1 (see Supplementary Information), where it can be observed that the values obtained by the model are similar to the values obtained experimentally.
According to the data obtained, the optimized conditions to modify the natural zeolite for the remotion of CO2 are those considering a precursor solution with a theoretical copper concentration of 9.06% (wCu/wZeolite+Cu) and performing the modification process by ion exchange by 10.9 h, predicting a maximum adsorption uptake of 76.3 mg/g, increasing the adsorption uptake of the natural zeolite at 23.6%. The optimized sample was also developed in this investigation. The maximum adsorption uptake of CO2 obtained by fitting the CO2 adsorption data to the Langmuir model was 75.8 mg/g, which closely aligns with the predicted value, supporting the validity of the model within the studied range. To facilitate a better understanding of the model, a three-dimensional Response Surface Plot is included in the Supplementary Information (Figure S3).
Finally, a comparison between the removal capacity of the natural zeolites and the synthetic zeolite studied is necessary. In this sense, although the removal capacity of the synthetic zeolite is indeed more significant than the CO2 adsorption capacity of the natural zeolite, the surface area of the synthetic zeolite almost triplicates the values of the natural one. Table 9 shows a weighted comparison for the surface area.

Table 9.
CO2 maximum adsorption uptakes per exposed surface area.
It is well known that several aspects different from the Surface Area could affect the adsorption uptake, such as the pore size, pore volume, the polarity and the nature and distribution of the active sites [], among another parameters. In this case, it is evident that the amount of CO2 adsorbed per m2 of surface is more significant in the case of natural zeolites, and such results can be attributed to the presence of diverse monovalent and divalent compensation cations present in the natural zeolite and the possibility of multilayer adsorption, as suggested by the good fit to the Freundlich model. Furthermore, the existence of bigger zeolite channels in the natural zeolite, specifically the site pocket of mordenite, could alter cation localization. It has been suggested that the allocation of cations in the existing larger pores leaves channels of zeolites free for absorbate transport, which is beneficial for adsorption [].
A possible modification strategy could involve physicochemical treatments to the natural zeolite to increase its surface area before the ion exchange procedure. The zeolites developed using this methodology could also be investigated for the remotion of other hazardous compounds from gaseous streams and/or their valorization to valuable chemical products.
4. Conclusions
Ion exchange methodology has proven to be an efficient technique to modify the compensating cations of synthetic and natural zeolites, without affecting their structure or morphological characteristics. This methodology promotes a new charge balance in the original zeolite structure, allowing for the incorporation of more adequate cations for specific processes such as CO2 adsorption. Using precursor solutions with salts of divalent cations such as Cu(NO3)2 and Zn(NO3)2 improves the CO2 adsorption capacity of zeolites by increasing the field gradient on their surface. In this way, cations that present more significant interaction with the pi bond electrons in the CO2 molecule would be incorporated. The adsorption uptake was also favoured by the lower ionic radius in the case of Cu2+ and Zn2+ compared to monovalent cations such as Na+, K+ and NH4+. At high CO2 concentrations, the adsorption uptake of all the samples reaches 105–106 mg of CO2 per zeolite gram.
The Response Surface Methodology allowed us to determine the influence of the concentration of the copper precursor solutions and the modification times on the CO2 adsorption capacity of natural zeolites, showing that, in the range of variables studied, the increase in copper contraction in the precursor solution and the ion exchange times favour the process; however, it is observed that, above certain values in both variables, the performance of the material is affected. Finally, the optimization of these parameters allowed for obtaining a catalyst with a removal capacity of 75 mgCO2/gads, which increased the maximum adsorption capacity of the natural zeolite by 23.6%. The modified zeolites developed in this investigation are promising and cost-effective adsorbent materials suitable for use in filters for CO2 capture. Furthermore, future studies could explore their application in the removal of other hazardous compounds from gaseous streams and/or the valorization of these compounds into valuable chemical products.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ma18102403/s1, Table S1: Langmuir isotherm parameters for raw and modified natural zeolites; Figure S1: EDX Spectra of parent synthetic zeolite (A) and modified samples: SZ_Na (B), SZ_K (C), SZ_Cu (D) and SZ_Zn (E). Figure S2: Values predicted from the quadratic model versus observed data; Figure S3: Response Surface Plot for CO2 adsorption uptake onto modified natural zeolites.
Author Contributions
N.J.A., A.F.J., D.F.A.B.-G. and M.M.P. Developed the investigation, experimentation, and wrote the manuscript draft. N.J.A. and A.F.J.: funding acquisition. N.J.A., A.F.J., J.Ñ. and D.F.A.B.-G.: carried out the adsorption tests and their analysis. M.C., A.F.J. and N.J.A. performed the characterization of the structural, physicochemical, and morphological properties. D.F.A.B.-G., J.A.M.-R., J.Ñ. and R.M.-R.: carried out data curation, graphs design and mathematical modelling. A.F.J., D.F.A.B.-G., M.C., R.M.-R., C.A. and J.A.M.-R. reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FONDECYT POSTDOCTORADO project 3210158 from the National Agency for Research and Development (ANID), under the Ministry of Science, Government of Chile.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
N.J.A. extends thanks to ANID for the support received through the FONDECYT POSTDOCTORADO project (grant 3210158) and FONDECYT INICIACION project (grant 11250677). A.F.J. acknowledges ANID for their support through FONDECYT REGULAR project (grant 1231376). M.C. acknowledges ANID for their support through FONDECYT REGULAR project (grant 1231242). Special thanks to Eng. Claudia Campos for her valuable collaboration.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BET | Brunauer–Emmett–Teller |
| CCD | Central Composite Design |
| CCS | Carbon Capture and Storage |
| DSC | Differential Scanning Calorimeter |
| NZ | Natural Zeolite |
| SEM | Scanning Electron Microscopy |
| SRM | Surface Response Methodology |
| SZ | Synthetic Zeolite |
| TGA | Thermogravimetric Analysis |
References
- Wei, C.; Wang, M.; Fu, Q.; Dai, C.; Huang, R.; Bao, Q. Temporal Characteristics of Greenhouse Gases (CO2 and CH4) in the Megacity Shanghai, China: Association with Air Pollutants and Meteorological Conditions. Atmos. Res. 2020, 235, 104759. [Google Scholar] [CrossRef]
- Dinda, S.; Murge, P.; Chakravarthy Paruchuri, B. A Study on Zeolite-Based Adsorbents for CO2 Capture. Bull. Mater. Sci. 2019, 42, 240. [Google Scholar] [CrossRef]
- Olivier, J.G.J.; Peters, J.A.H.W. Trends in global CO2 and total greenhouse gas emissions. In 2020 Report Trends in Global CO2 and Total Greenhouse Gas Emissions: 2020 Report; PBL Netherlands Environmental Assessment Agency: Den Haag, The Netherlands, 2020. [Google Scholar]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2022—Impacts, Adaptation and Vulnerability; Cambridge University Press: Singapore, 2023. [Google Scholar]
- Swiss Re Partnering for Progress Financial Highlights. Available online: https://www.swissre.com/dam/jcr:59cd3668-b9ef-4746-b9d3-132139c461a6/ar-2021-shareholders-letter-doc-en.pdf (accessed on 19 April 2025).
- Murge, P.; Dinda, S.; Roy, S. Zeolite-Based Sorbent for CO2 Capture: Preparation and Performance Evaluation. Langmuir 2019, 35, 14751–14760. [Google Scholar] [CrossRef] [PubMed]
- Gunawardene, O.H.P.; Gunathilake, C.A.; Vikrant, K.; Amaraweera, S.M. Carbon Dioxide Capture through Physical and Chemical Adsorption Using Porous Carbon Materials: A Review. Atmosphere 2022, 13, 397. [Google Scholar] [CrossRef]
- Loachamin, D.; Casierra, J.; Calva, V.; Palma-Cando, A.; Ávila, E.E.; Ricaurte, M. Amine-Based Solvents and Additives to Improve the CO2 Capture Processes: A Review. ChemEngineering 2024, 8, 129. [Google Scholar] [CrossRef]
- Allangawi, A.; Alzaimoor, E.F.H.; Shanaah, H.H.; Mohammed, H.A.; Saqer, H.; El-Fattah, A.A.; Kamel, A.H. Carbon Capture Materials in Post-Combustion: Adsorption and Absorption-Based Processes. C 2023, 9, 17. [Google Scholar] [CrossRef]
- Davarpanah, E.; Armandi, M.; Hernández, S.; Fino, D.; Arletti, R.; Bensaid, S.; Piumetti, M. CO2 Capture on Natural Zeolite Clinoptilolite: Effect of Temperature and Role of the Adsorption Sites. J. Environ. Manag. 2020, 275, 111229. [Google Scholar] [CrossRef]
- Kennedy, D.A.; Mujčin, M.; Abou-Zeid, C.; Tezel, F.H. Cation Exchange Modification of Clinoptilolite –Thermodynamic Effects on Adsorption Separations of Carbon Dioxide, Methane, and Nitrogen. Microporous Mesoporous Mater. 2019, 274, 327–341. [Google Scholar] [CrossRef]
- Espinal, L.; Poster, D.L.; Wong-Ng, W.; Allen, A.J.; Green, M.L. Measurement, Standards, and Data Needs for CO2 Capture Materials: A Critical Review. Environ. Sci. Technol. 2013, 47, 11960–11975. [Google Scholar] [CrossRef]
- Petrovic, B.; Gorbounov, M.; Masoudi Soltani, S. Influence of Surface Modification on Selective CO2 Adsorption: A Technical Review on Mechanisms and Methods. Microporous Mesoporous Mater. 2021, 312, 110751. [Google Scholar] [CrossRef]
- Bayrakdar Ates, E. Exploring the Impact of NaOH Pre-Treatment for H2 and CO2 Adsorption on Clinoptilolite. Int. J. Hydrogen Energy 2024, 50, 990–1003. [Google Scholar] [CrossRef]
- Yu, T.; Zhang, Y.; Cao, L.; Cao, P.; Zhou, C.; Gu, S. Freeze–Thaw Cycle Durability and Mechanism Analysis of Zeolite Powder-Modified Recycled Concrete. Materials 2024, 17, 2671. [Google Scholar] [CrossRef]
- Solińska, A.; Bajda, T. Modified Zeolite as a Sorbent for Removal of Contaminants from Wet Flue Gas Desulphurization Wastewater. Chemosphere 2022, 286, 131772. [Google Scholar] [CrossRef]
- Pérez-Botella, E.; Valencia, S.; Rey, F. Zeolites in Adsorption Processes: State of the Art and Future Prospects. Chem. Rev. 2022, 122, 17647–17695. [Google Scholar] [CrossRef]
- Walton, K.S.; Abney, M.B.; LeVan, M.D. CO2 Adsorption in y and X Zeolites Modified by Alkali Metal Cation Exchange. Microporous Mesoporous Mater. 2006, 91, 78–84. [Google Scholar] [CrossRef]
- Rao, F.; Liu, M.; Liu, C.; Deng, W.; Huang, R.; Liao, C.; Qi, T.; Hu, G. Synthesis of Binder-Free Pelletized Y Zeolite for CO2 Capture. Carbon Capture Sci. Technol. 2024, 10, 100166. [Google Scholar] [CrossRef]
- Drenchev, N.L.; Ivanova, E.Z.; Mihaylov, M.Y.; Aleksandrov, H.A.; Vayssilov, G.N.; Hadjiivanov, K.I. One Ca2+ Site in CaNaY Zeolite Can Attach Three CO2 Molecules. J. Phys. Chem. Lett. 2023, 14, 1564–1569. [Google Scholar] [CrossRef]
- Henrotin, A.; Heymans, N.; Duprez, M.E.; Mouchaham, G.; Serre, C.; Wong, D.; Robinson, R.; Mulrooney, D.; Casaban, J.; De Weireld, G. Lab-Scale Pilot for CO2 Capture Vacuum Pressure Swing Adsorption: MIL-160(Al) vs Zeolite 13X. Carbon Capture Sci. Technol. 2024, 12, 100224. [Google Scholar] [CrossRef]
- Erguvan, M.; Amini, S. Parametric Investigation of CO2 Desorption of Zeolite 13X Under Microwave Condition. Carbon Capture Sci. Technol. 2024, 11, 100189. [Google Scholar] [CrossRef]
- Tao, Z.; Tian, Y.; Hanif, A.; Chan, V.; Gu, Q.; Shang, J. Metal Cation-Exchanged LTA Zeolites for CO2/N2 and CO2/CH4 Separation: The Roles of Gas-Framework and Gas-Cation Interactions. Carbon Capture Sci. Technol. 2023, 8, 100126. [Google Scholar] [CrossRef]
- Athayde, D.D.; dos Santos, G.M.; de Faria, A.C.B.; de Lima Ribeiro, C.; Aiube, C.M.; Ladislau, D.A.A.; Paulo da Silva, E.; de Sousa Lima, L.F.; de Souza, R.N.; Pereira da Silva, S.L.; et al. Investigation of the Reaction Time and Hydrothermal Synthesis Route on the SSZ-13 Zeolite Particle Crystallization and CO2 Adsorption. Microporous Mesoporous Mater. 2025, 384, 113428. [Google Scholar] [CrossRef]
- Kencana, K.S.; Hong, S.B. Nanocrystalline Sodium Mordenite as an Efficient Low-Concentration CO2 Adsorbent. Sep. Purif. Technol. 2024, 350, 128018. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, J.; Wei, J.; Cui, X.; Wan, C.; Liu, M.; Bai, S.; Sun, J.; Wang, J. Performance Evaluation of Bimetallic Ion Exchanged Clinoptilolite for Potential Equilibrium and Kinetic Adsorption Separations of CO2 from CO2/CH4 Mixture. Sep. Purif. Technol. 2024, 331, 125563. [Google Scholar] [CrossRef]
- Wahono, S.K.; Stalin, J.; Addai-Mensah, J.; Skinner, W.; Vinu, A.; Vasilev, K. Physico-Chemical Modification of Natural Mordenite-Clinoptilolite Zeolites and Their Enhanced CO2 Adsorption Capacity. Microporous Mesoporous Mater. 2020, 294, 109871. [Google Scholar] [CrossRef]
- Cabana, N.C.; Serra, R.M.; Boix, A.V.; Bolcatto, P.G. SLAFES XXIII CO2 Adsorption on Cs-and Na-Doped Mordenites. Mater. Today Proc. 2019, 14, 185–188. [Google Scholar] [CrossRef]
- Calleja, G.; Pau, J.; Calles, J.A. Pure and Multicomponent Adsorption Equilibrium of Carbon Dioxide, Ethylene, and Propane on ZSM-5 Zeolites with Different Si/Al Ratios. J. Chem. Eng. Data 1998, 43, 994–1003. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, J.H.; Kim, J.T.; Suh, J.K.; Lee, J.M.; Lee, C.H. Adsorption Equilibria of CO2 on Zeolite 13X and Zeolite X/Activated Carbon Composite. J. Chem. Eng. Data 2002, 47, 1237–1242. [Google Scholar] [CrossRef]
- Boscherini, M.; Miccio, F.; Papa, E.; Medri, V.; Landi, E.; Doghieri, F.; Minelli, M. The Relevance of Thermal Effects during CO2 Adsorption and Regeneration in a Geopolymer-Zeolite Composite: Experimental and Modelling Insights. Chem. Eng. J. 2021, 408, 127315. [Google Scholar] [CrossRef]
- Hadi, E.; ba Karim Ghani, W.; Azlina, W. High-Pressure CO2 Adsorption onto NaX Zeolite: Effect of Li+, K+, Mg2+, and Zn2+ and Equilibrium Isotherms Study. Iran. J. Chem. Chem. Eng. 2021, 40, 1195–1215. [Google Scholar]
- Hauchhum, L.; Mahanta, P. Carbon Dioxide Adsorption on Zeolites and Activated Carbon by Pressure Swing Adsorption in a Fixed Bed. Int. J. Energy Environ. Eng. 2014, 5, 349–356. [Google Scholar] [CrossRef]
- Hernández-Huesca, R.; Díaz, L.; Aguilar-Armenta, G. Adsorption Equilibria and Kinetics of CO2, CH4 and N2 in Natural Zeolites. Sep. Purif. Technol. 1999, 15, 163–173. [Google Scholar] [CrossRef]
- Bonenfant, D.; Kharoune, M.; Niquette, P.; Mimeault, M.; Hausler, R. Advances in Principal Factors Influencing Carbon Dioxide Adsorption on Zeolites. Sci. Technol. Adv. Mater. 2008, 9, 13007. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, S.J. A Review on Solid Adsorbents for Carbon Dioxide Capture. J. Ind. Eng. Chem. 2015, 23, 1–11. [Google Scholar] [CrossRef]
- Bahmanzadegan, F.; Ghaemi, A. Modification and Functionalization of Zeolites to Improve the Efficiency of CO2 Adsorption: A Review. Case Stud. Chem. Environ. Eng. 2024, 9, 100564. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, B.; Xu, L.; Chen, M.; Wu, C.-E.; Qiu, J.; Cheng, G.; Wang, N.; Xu, J.; Hu, X. CO2 Methanation over the Ni-Based Catalysts Supported on the Hollow ZSM-5 Zeolites: Effects of the Hollow Structure and Alkaline Treatment. Fuel 2023, 334, 126783. [Google Scholar] [CrossRef]
- Spataru, D.; Canastreiro, D.; Świrk Da Costa, K.; Quindimil, A.; Lopes, J.M.; Da Costa, P.; Henriques, C.; Bacariza, C. Doping Ni/USY Zeolite Catalysts with Transition Metals for CO2 Methanation. Int. J. Hydrogen Energy 2024, 53, 468–481. [Google Scholar] [CrossRef]
- Gao, X.; Wang, Z.; Chen, T.; Hu, L.; Yang, S.; Kawi, S. State-of-Art Designs and Synthesis of Zeolite Membranes for CO2 Capture. Carbon Capture Sci. Technol. 2022, 5, 100073. [Google Scholar] [CrossRef]
- United Nation. Edición Especial 2023 Informe de Los Objetivos de Desarrollo Sostenible; United Nation: Manhattan, NY, USA, 2023. [Google Scholar]
- Król, M. Natural vs. Synthetic Zeolites. Crystals 2020, 10, 622. [Google Scholar] [CrossRef]
- Gęsikiewicz-Puchalska, A.; Zgrzebnicki, M.; Michalkiewicz, B.; Kałamaga, A.; Narkiewicz, U.; Morawski, A.W.; Wrobel, R. Changes in Porous Parameters of the Ion Exchanged x Zeolite and Their Effect on CO2 Adsorption. Molecules 2021, 26, 7520. [Google Scholar] [CrossRef]
- Huenuvil-Pacheco, I.; Jaramillo, A.F.; Abreu, N.J.; Garrido-Miranda, K.; Sánchez-Sanhueza, G.; González-Rocha, G.; Medina, C.; Montoya, L.F.; Sanhueza, J.P.; Melendrez, M.F. Biocidal Effects of Organometallic Materials Supported on ZSM-5 Zeolite: Influence of the Physicochemical and Surface Properties. Heliyon 2024, 10, e27182. [Google Scholar] [CrossRef]
- Abreu, N.J.; Valdés, H.; Zaror, C.A.; Azzolina-Jury, F.; Meléndrez, M.F. Ethylene Adsorption onto Natural and Transition Metal Modified Chilean Zeolite: An Operando DRIFTS Approach. Microporous Mesoporous Mater. 2019, 274, 138–148. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Song, H.; Zhang, L.; Wu, Y.; He, Y.; Ma, L.; Hong, J.; Tayal, A.; Marinkovic, N.; et al. Tuning Metal-Support Interactions in Nickel-Zeolite Catalysts Leads to Enhanced Stability during Dry Reforming of Methane. Nat. Commun. 2024, 15, 8566. [Google Scholar] [CrossRef] [PubMed]
- Isa, M.A.; Halim, M.H. Cation-Exchanged NaY Zeolite: Effect of Temperature and Ion Concentration to Membrane Performance. J. Phys. Conf. Ser. 2019, 1349, 012072. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Mohamed, A.S. Adsorption Removal of Safranin Dye Contaminants from Water Using Various Types of Natural Zeolite. Silicon 2019, 11, 1635–1647. [Google Scholar] [CrossRef]
- Mera, A.C.; Contreras, D.; Escalona, N.; Mansilla, H.D. BiOI Microspheres for Photocatalytic Degradation of Gallic Acid. J. Photochem. Photobiol. A Chem. 2016, 318, 71–76. [Google Scholar] [CrossRef]
- Xu, D.; Feng, J.; Che, S. An Insight into the Role of the Surfactant CTAB in the Formation of Microporous Molecular Sieves. Dalton Trans. 2014, 43, 3612–3617. [Google Scholar] [CrossRef]
- Sun, Z.; Xu, B.; Rony, A.H.; Toan, S.; Chen, S.; Gasem, K.A.M.; Adidharma, H.; Fan, M.; Xiang, W. Thermogravimetric and Kinetics Investigation of Pine Wood Pyrolysis Catalyzed with Alkali-Treated CaO/ZSM-5. Energy Convers. Manag. 2017, 146, 182–194. [Google Scholar] [CrossRef]
- Król, M.; Dechnik, J.; Szymczak, P.; Handke, B.; Szumera, M.; Stoch, P. Thermal Behavior of Clinoptilolite. Crystals 2024, 14, 646. [Google Scholar] [CrossRef]
- Jia, Y.; Shi, Q.; Wang, J.; Ding, C.; Zhang, K. Synthesis, Characterization, and Catalytic Application of Hierarchical Nano-ZSM-5 Zeolite. RSC Adv. 2020, 10, 29618–29626. [Google Scholar] [CrossRef]
- Król, M.K.; Jeleń, P. The Effect of Heat Treatment on the Structure of Zeolite a. Materials 2021, 14, 4642. [Google Scholar] [CrossRef]
- Pires, J. Simultaneous Thermogravimetry-Differential Scanning Calorimetry (TG-DSC) in Nanoporous Materials: Examples of Data for Zeolites, Metal–Organic Frameworks (MOFs), Clay Based and Mesostructured Solids. J. Inorg. Organomet. Polym. Mater. 2024, 34, 3346–3359. [Google Scholar] [CrossRef]
- Babatunde, K.A.; Negash, B.M.; Jufar, S.R.; Ahmed, T.Y.; Mojid, M.R. Adsorption of Gases on Heterogeneous Shale Surfaces: A Review. J. Pet. Sci. Eng. 2022, 208, 109466. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Sanzana, S.; Abreu, N.J.; Levío-Raimán, M.; Proal-Nájera, J.; Osorio, A.; Maza, S.; Daniele, L.; Castro-Rojas, J.; Soto, V.; González, C.; et al. Enhancing Manganese Sorption: Batch and Fixed-Bed Column Studies on Activated Zeolite. Environ. Technol. Innov. 2024, 33, 103495. [Google Scholar] [CrossRef]
- Kishore Kumar, M.; Shiva Nagendra, S.M. Characteristics of Ground Level CO2 Concentrations over Contrasting Land Uses in a Tropical Urban Environment. Atmos. Environ. 2015, 115, 286–294. [Google Scholar] [CrossRef]
- Wang, K.Y.; Wang, J.L.; Liu, W.T. Ambient Carbon Dioxide Concentrations in Industrial Park Areas: A Monitoring and Modeling Study. Atmos. Pollut. Res. 2014, 5, 179–188. [Google Scholar] [CrossRef]
- Komal; Soni, D.; Singh, K.; Aggarwal, S.G. Comparative Measurement of CO2, CH4 and CO at Two Traffic Interjunctions Having Inflated Vehicular Flow in Delhi. J. Environ. Sci. 2024, 141, 314–329. [Google Scholar] [CrossRef]
- Abreu, N.J.; Valdés, H.; Zaror, C.A.; de Oliveira, T.F.; Azzolina-Jury, F.; Thibault-Starzyk, F. Evidence of Synergy Effects between Zinc and Copper Oxides with Acidic Sites on Natural Zeolite during Photocatalytic Oxidation of Ethylene Using Operando DRIFTS Studies. Catalysts 2023, 13, 610. [Google Scholar] [CrossRef]
- Yang, R.T. Zeolites and Molecular Sieves. In Adsorbents: Fundamentals and Applications; Yang, R., Ed.; Wiley-Interscience: Hoboken, NJ, USA, 2003; p. 410. ISBN 0471297410. [Google Scholar]
- Chen, S.; Zhu, M.; Tang, Y.; Fu, Y.; Li, W.; Xiao, B. Molecular Simulation and Experimental Investigation of CO2 Capture in a Polymetallic Cation-Exchanged 13X Zeolite. J. Mater. Chem. A 2018, 6, 19570–19583. [Google Scholar] [CrossRef]
- Maurin, G.; Bell, R.; Kuchta, B.; Poyet, T.; Llewellyn, P. Adsorption of Non Polar and Quadrupolar Gases in Siliceous Faujasite: Molecular Simulations and Experiments. Adsorption 2005, 11, 331–336. [Google Scholar] [CrossRef]
- Smykowski, D.; Szyja, B.; Szczygieł, J. DFT Modeling of CO2 Adsorption on Cu, Zn, Ni, Pd/DOH Zeolite. J. Mol. Graph. Model. 2013, 41, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Townsend, R.P.; Coker, E.N. Ion Exchange in Zeolites. In Studies in Surface Science and Catalysis; van Bekkum, H., Flanigen, E.M., Jacobs, P.A., Jansen, J.C., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2001; Volume 7. [Google Scholar]
- Tsiotsias, A.I.; Georgiadis, A.G.; Charisiou, N.D.; Goula, M.A. CO2 Physisorption over an Industrial Molecular Sieve Zeolite: An Experimental and Theoretical Approach. Materials 2023, 16, 6656. [Google Scholar] [CrossRef]
- Feng, L.; Shen, Y.; Wu, T.; Liu, B.; Zhang, D.; Tang, Z. Adsorption Equilibrium Isotherms and Thermodynamic Analysis of CH4, CO2, CO, N2 and H2 on NaY Zeolite. Adsorption 2020, 26, 1101–1111. [Google Scholar] [CrossRef]
- Bahmanzadegan, F.; Ghaemi, A. Exploring the Effect of Zeolite’s Structural Parameters on the CO2 Capture Efficiency Using RSM and ANN Methodologies. Case Stud. Chem. Environ. Eng. 2024, 9, 100595. [Google Scholar] [CrossRef]
- Sánchez-López, P.; Kotolevich, Y.; Antúnez-García, J.; Chávez-Rivas, F.; Khramov, E.; Berlier, G.; Moreno-Ruiz, L.; Zubavichus, Y.; Petranovskii, V.; Fuentes-Moyado, S.; et al. Influence of Components Deposition Order on Silver Species Formation in Bimetallic Ag-Fe System Supported on Mordenite. Catalysts 2022, 12, 1453. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).