Biodegradability of Bioplastics in Managed and Unmanaged Environments: A Comprehensive Review
Abstract
1. Introduction
2. Biodegradation Mechanism
- Microorganism interaction with the polymer surface: The first phase involves the attachment of microorganisms to the polymer surface, resulting in the formation of a biofilm. When microorganisms infiltrate the amorphous region of the polymer, the primary polymer chain is broken down into low molecular weight oligomers, dimers, and monomers through the action of the extracellular enzymes released by microorganisms (lipase, proteinase K, andhydrogenase, etc.). The polymer porosity is a crucial factor for the duration of this stage.
- Microorganisms’ growth: After adhering to the polymer, the microbes multiply by breaking down the polymers and lowering their molecular weight. Another name for this procedure is “biofragmentation”.
- Ultimate mineralization of the polymer: Finally, if the plastic’s molecular weight has been sufficiently lowered to produce water-soluble intermediates, these products can be introduced into the microorganisms and find a conduit into the relevant metabolic pathways (bio-assimilation). Only when these low molecular weight polymers are subsequently utilized by microorganisms as carbon sources does the polymer ultimately degrade. The production of metabolites, such as CO2, H2O, or CH4, is referred to as mineralization. After that, these products are used as energy and carbon sources. When either the oligomers or monomers are no longer present, it is considered that the biodegradation process has finished.
3. Biodegradability Standards
| Standard | Environment | Volume of the Reactor | Temperature | Duration | Sample/Media | Validity Criteria |
|---|---|---|---|---|---|---|
| ASTM D5338 [43] | Compost | 2–5 L | 58 °C | ≥45 d | 1/6 | >70% biodegradation of the reference material after 45 days <20% deviation of the percentage of biodegradation within the reference replicates at the end of the test |
| ISO 14855-1 [41] | Compost | >2 L | 58 °C | ≤6 m | 1/6 | >70% biodegradation of the reference material after 45 days <20% deviation of the percentage of biodegradation within the reference replicates at the end of the test 50 < mg CO2/g VS < 150 after 10 days for the blank |
| ISO 14855-2 [42] | Compost | >500 mL | 58 °C | ≤6 m | 1/10 | >70% biodegradation of the reference material after 45 days <20% deviation of the percentage of biodegradation within the reference replicates at the end of the test 50 < mg CO2/g VS < 150 after 10 days for the blank |
| ISO 17556 [44] | Soil | 20–28 °C | ≤24 m | 100–300 mg/100–330 g | >60% biodegradation of the reference material at the end of the test <20% deviation of the percentage of biodegradation within the blank replicates at the end of the test | |
| ASTM D5988 [58] | Soil | 2–4 L | 20–28 °C | 200–1000 mg/500 g * | >70% biodegradation of the reference material after 6 months <20% deviation of the amount of CO2 (or BOD) within the blanks at the end of the test | |
| ISO 14851 [46] | Water | 20–25 °C | ≤6 m | 100–2000 mg/L * | >60% biodegradation of the reference material at the end of the test | |
| ISO 14852 [47] | Water | 20–25 °C | ≤6 m | 100–2000 mg/L * | >60% biodegradation of the reference material at the end of the test <20% deviation of the amount of CO2 within the blank and test replicates at the end of the test | |
| ASTM D6691 [48] | Marine | 125 mL | 30 °C | ≤90 d | 20 mg | >70% biodegradation of the reference material at the end of the test |
| ASTM D5511 [51] | Landfill and anaerobic digestion | 52 °C | ≥15 d | 15–100 g/1000 g ** | >70% biodegradation of the reference material after 15 days <20% deviation of the percentage of biodegradation within the reference replicates | |
| ISO 15985 [52] | Landfill and anaerobic digestion | >750 mL | 52 °C | >20 g | >70% biodegradation of the reference material after 15 days <20% deviation of the percentage of biodegradation within the reference replicates | |
| ISO 14853 [50] | Anaerobic digestion | 0.1–1 L | 35 °C | ≤90 d | 20–200 mg/L * | > 70% biodegradation of the reference material after 60 days 6 < pH < 8 <20% deviation of the percentage of biodegradation within the reference replicates |
| ISO 13975 [59] | Anaerobic digestion | >1.5 L | 55–35 °C | ≤90 d | 7–10 g/L ** | >70% biodegradation reference after 15 days <20% deviation of the percentage of biodegradation within the reference replicates at the end of the test |
- Chemical test: identification of all constituents, organic matter content expressed by volatile solids (minimum 50%), and threshold values for heavy metals (tabulated values);
- Biodegradability: at least 90% of the organic material is converted into CO2 within 6 months;
- Disintegration: after 3 months of composting and subsequent sifting through a 2 mm sieve, no more than 10% residue may remain compared to the original mass.
- Compost quality: final compost quality should not be negatively influenced by the addition of a biodegradable plastic into the original substrate that is to be composted.
4. Biodegradability in Managed Environments
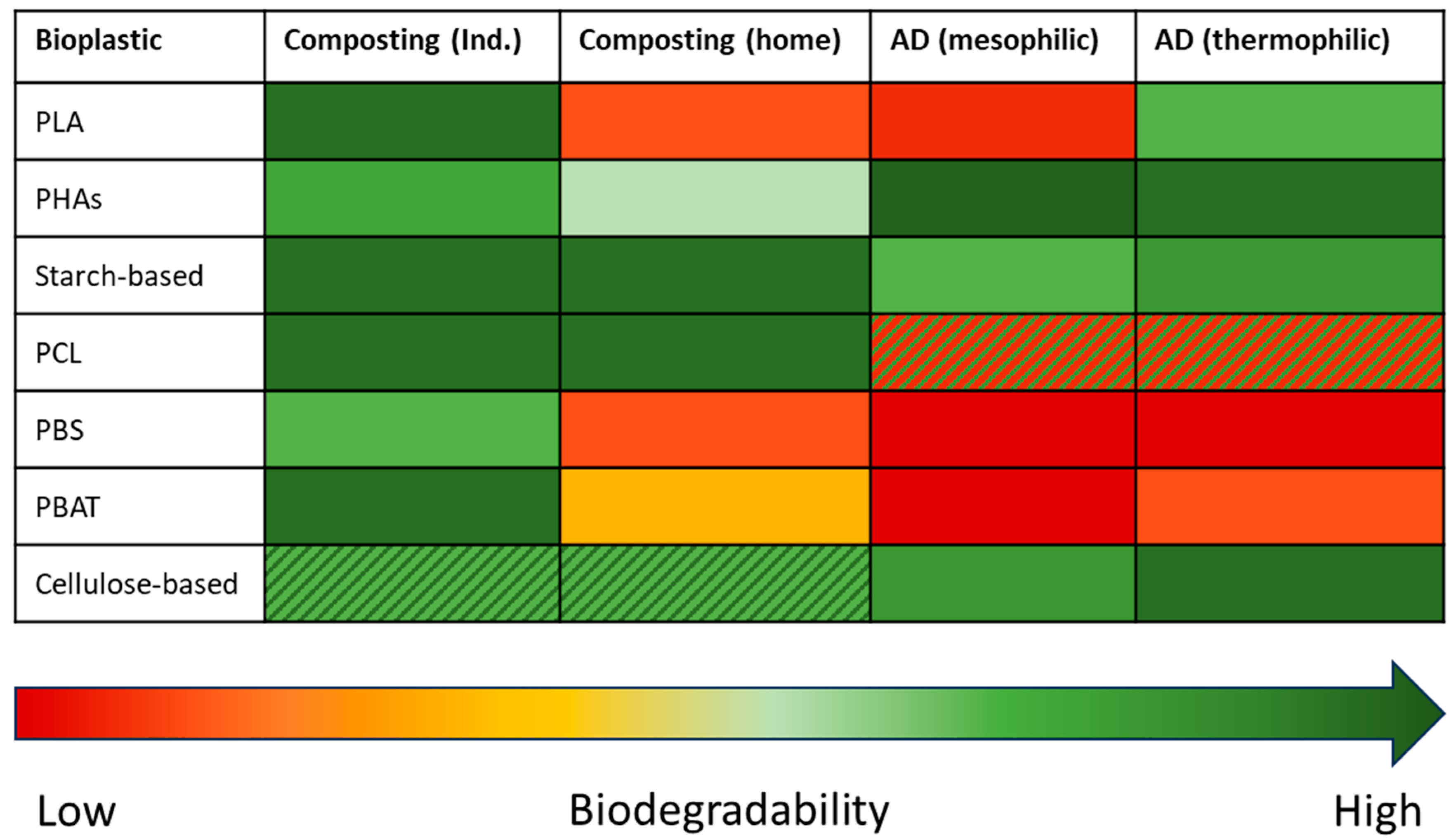
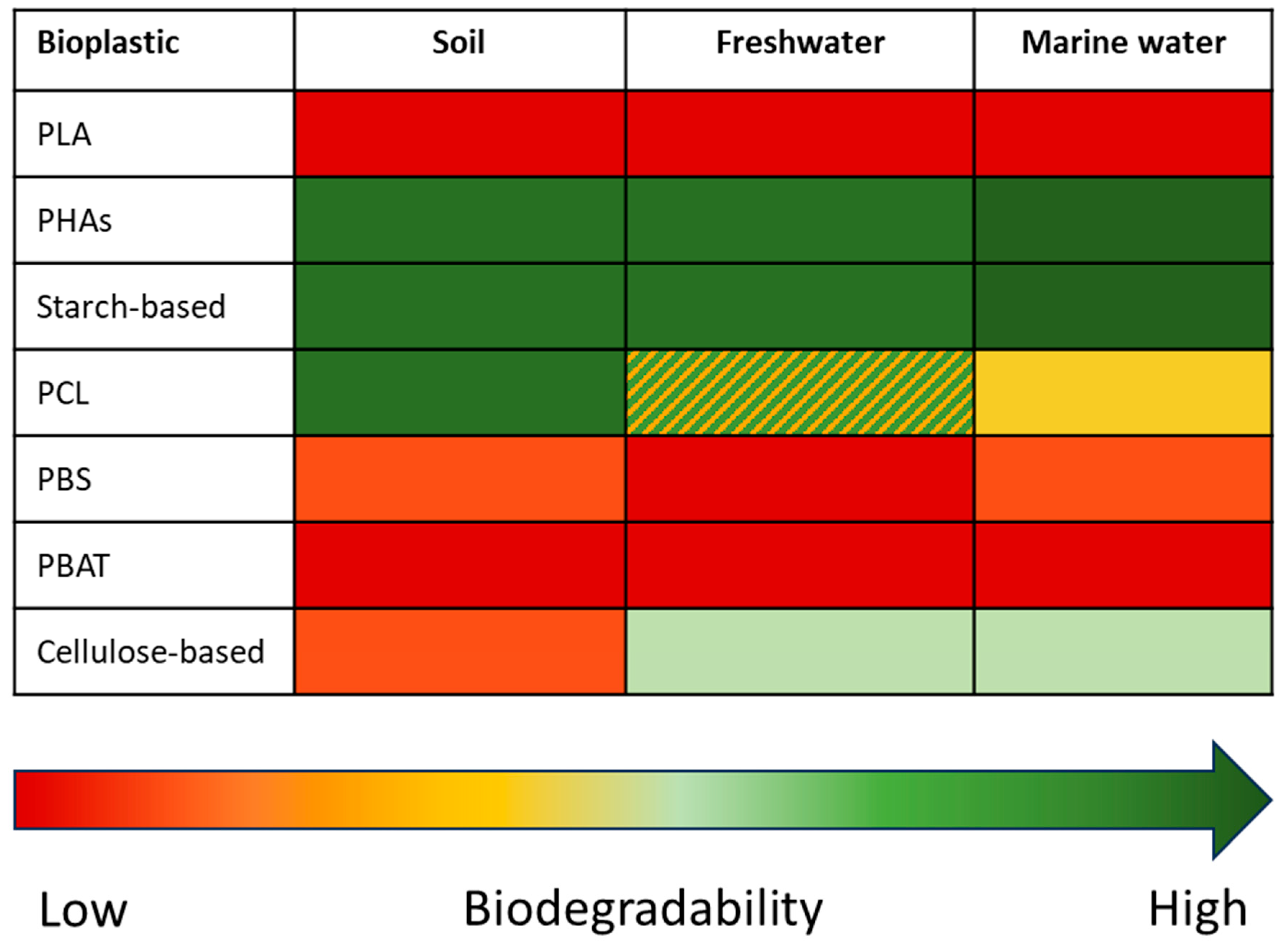
5. Biodegradability in Unmanaged Environments
6. Challenges and Future Perspectives
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Borrelle, S.B.; Ringma, J.; Law, K.L.; Monnahan, C.C.; Lebreton, L.; McGivern, A.; Murphy, E.; Jambeck, J.; Leonard, G.H.; Hilleary, M.A.; et al. Predicted Growth in Plastic Waste Exceeds Efforts to Mitigate Plastic Pollution. Science 2020, 369, 1515–1518. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- European Bioplastics Renewable Feedstock. Available online: https://www.european-bioplastics.org/bioplastics/Feedstock/ (accessed on 24 April 2025).
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Jacquot, M.; Desobry, S. Poly-Lactic Acid: Production, Applications, Nanocomposites, and Release Studies. Compr. Rev. Food Sci. Food Saf. 2010, 9, 552–571. [Google Scholar] [CrossRef]
- Steinbüchel, A.; Valentin, H.E. Diversity of Bacterial Polyhydroxyalkanoic Acids. FEMS Microbiol. Lett. 1995, 128, 219–228. [Google Scholar] [CrossRef]
- Elmowafy, E.; Abdal-Hay, A.; Skouras, A.; Tiboni, M.; Casettari, L.; Guarino, V. Polyhydroxyalkanoate (PHA): Applications in Drug Delivery and Tissue Engineering. Expert Rev. Med. Devices 2019, 16, 467–482. [Google Scholar] [CrossRef]
- Barletta, M.; Aversa, C.; Ayyoob, M.; Gisario, A.; Hamad, K.; Mehrpouya, M.; Vahabi, H. Poly(Butylene Succinate) (PBS): Materials, Processing, and Industrial Applications. Prog. Polym. Sci. 2022, 132, 101579. [Google Scholar] [CrossRef]
- Siddiqui, N.; Asawa, S.; Birru, B.; Baadhe, R.; Rao, S. PCL-Based Composite Scaffold Matrices for Tissue Engineering Applications. Mol. Biotechnol. 2018, 60, 506–532. [Google Scholar] [CrossRef]
- Yu, Y.; Flury, M. Unlocking the Potentials of Biodegradable Plastics with Proper Management and Evaluation at Environmentally Relevant Concentrations. NPJ Mater. Sustain. 2024, 2, 9. [Google Scholar] [CrossRef]
- Cheng, S.; Jiang, Y.; Yin, J.; Zhang, L.; Han, L.; Zhu, G.; Zhang, Y. PBSeT/Lignin: A Complete Bio-Based Biodegradable Plastic with Excellent Mechanical and Anti-UV Properties. Eur. Polym. J. 2024, 203, 112638. [Google Scholar] [CrossRef]
- Kumar, V.; Sehgal, R.; Gupta, R. Blends and Composites of Polyhydroxyalkanoates (PHAs) and Their Applications. Eur. Polym. J. 2021, 161, 110824. [Google Scholar] [CrossRef]
- Jian, J.; Zeng, X.; Huang, X. An Overview on Synthesis, Properties and Applications of Poly(Butylene-Adipate-Co-Terephthalate)–PBAT. Adv. Ind. Eng. Polym. Res. 2020, 3, 19–26. [Google Scholar] [CrossRef]
- Paola Bracciale, M.; De Gioannis, G.; Falzarano, M.; Muntoni, A.; Polettini, A.; Pomi, R.; Rossi, A.; Sarasini, F.; Tirillò, J.; Zonfa, T. Disposable Mater-Bi® Bioplastic Tableware: Characterization and Assessment of Anaerobic Biodegradability. Fuel 2024, 355, 129361. [Google Scholar] [CrossRef]
- Vatanpour, V.; Pasaoglu, M.E.; Barzegar, H.; Teber, O.O.; Kaya, R.; Bastug, M.; Khataee, A.; Koyuncu, I. Cellulose Acetate in Fabrication of Polymeric Membranes: A Review. Chemosphere 2022, 295, 133914. [Google Scholar] [CrossRef]
- Fischer, S.; Thümmler, K.; Volkert, B.; Hettrich, K.; Schmidt, I.; Fischer, K. Properties and Applications of Cellulose Acetate. Macromol. Symp. 2008, 262, 89–96. [Google Scholar] [CrossRef]
- Bilo, F.; Pandini, S.; Sartore, L.; Depero, L.E.; Gargiulo, G.; Bonassi, A.; Federici, S.; Bontempi, E. A Sustainable Bioplastic Obtained from Rice Straw. J. Clean. Prod. 2018, 200, 357–368. [Google Scholar] [CrossRef]
- Polman, E.M.N.; Gruter, G.-J.M.; Parsons, J.R.; Tietema, A. Comparison of the Aerobic Biodegradation of Biopolymers and the Corresponding Bioplastics: A Review. Sci. Total Environ. 2021, 753, 141953. [Google Scholar] [CrossRef]
- Tang, X.; Alavi, S. Recent Advances in Starch, Polyvinyl Alcohol Based Polymer Blends, Nanocomposites and Their Biodegradability. Carbohydr. Polym. 2011, 85, 7–16. [Google Scholar] [CrossRef]
- Mohammadi Nafchi, A.; Moradpour, M.; Saeidi, M.; Alias, A.K. Thermoplastic Starches: Properties, Challenges, and Prospects. Starch-Stärke 2013, 65, 61–72. [Google Scholar] [CrossRef]
- García-Depraect, O.; Bordel, S.; Lebrero, R.; Santos-Beneit, F.; Börner, R.A.; Börner, T.; Muñoz, R. Inspired by Nature: Microbial Production, Degradation and Valorization of Biodegradable Bioplastics for Life-Cycle-Engineered Products. Biotechnol. Adv. 2021, 53, 107772. [Google Scholar] [CrossRef]
- Ruggero, F.; Gori, R.; Lubello, C. Methodologies to Assess Biodegradation of Bioplastics during Aerobic Composting and Anaerobic Digestion: A Review. Waste Manag. Res. 2019, 37, 959–975. [Google Scholar] [CrossRef]
- Folino, A.; Karageorgiou, A.; Calabrò, P.S.; Komilis, D. Biodegradation of Wasted Bioplastics in Natural and Industrial Environments: A Review. Sustainability 2020, 12, 6030. [Google Scholar] [CrossRef]
- Vardar, S.; Demirel, B.; Onay, T.T. Degradability of Bioplastics in Anaerobic Digestion Systems and Their Effects on Biogas Production: A Review. Rev. Environ. Sci. Biotechnol. 2022, 21, 205–223. [Google Scholar] [CrossRef]
- Bher, A.; Mayekar, P.C.; Auras, R.A.; Schvezov, C.E. Biodegradation of Biodegradable Polymers in Mesophilic Aerobic Environments. Int. J. Mol. Sci. 2022, 23, 12165. [Google Scholar] [CrossRef]
- Ahsan, W.A.; Hussain, A.; Lin, C.; Nguyen, M.K. Biodegradation of Different Types of Bioplastics through Composting—A Recent Trend in Green Recycling. Catalysts 2023, 13, 294. [Google Scholar] [CrossRef]
- Gioia, C.; Giacobazzi, G.; Vannini, M.; Totaro, G.; Sisti, L.; Colonna, M.; Marchese, P.; Celli, A. End of Life of Biodegradable Plastics: Composting versus Re/Upcycling. ChemSusChem 2021, 14, 4167–4175. [Google Scholar] [CrossRef]
- Awasthi, S.K.; Kumar, M.; Kumar, V.; Sarsaiya, S.; Anerao, P.; Ghosh, P.; Singh, L.; Liu, H.; Zhang, Z.; Awasthi, M.K. A Comprehensive Review on Recent Advancements in Biodegradation and Sustainable Management of Biopolymers. Environ. Pollut. 2022, 307, 119600. [Google Scholar] [CrossRef]
- Ojeda, T.F.M.; Dalmolin, E.; Forte, M.M.C.; Jacques, R.J.S.; Bento, F.M.; Camargo, F.A.O. Abiotic and Biotic Degradation of Oxo-Biodegradable Polyethylenes. Polym. Degrad. Stab. 2009, 94, 965–970. [Google Scholar] [CrossRef]
- Kyrikou, I.; Briassoulis, D. Biodegradation of Agricultural Plastic Films: A Critical Review. J. Polym. Environ. 2007, 15, 125–150. [Google Scholar] [CrossRef]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological Degradation of Plastics: A Comprehensive Review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef]
- Bátori, V.; Åkesson, D.; Zamani, A.; Taherzadeh, M.J.; Sárvári Horváth, I. Anaerobic Degradation of Bioplastics: A Review. Waste Manag. 2018, 80, 406–413. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, W.; Xing, R.; Xie, S.; Yang, X.; Cui, P.; Lü, J.; Liao, H.; Yu, Z.; Wang, S.; et al. Enhanced in Situ Biodegradation of Microplastics in Sewage Sludge Using Hyperthermophilic Composting Technology. J. Hazard. Mater. 2020, 384, 121271. [Google Scholar] [CrossRef] [PubMed]
- Larrañaga, A.; Lizundia, E. A Review on the Thermomechanical Properties and Biodegradation Behaviour of Polyesters. Eur. Polym. J. 2019, 121, 109296. [Google Scholar] [CrossRef]
- Goel, V.; Luthra, P.; Kapur, G.S.; Ramakumar, S.S.V. Biodegradable/Bio-Plastics: Myths and Realities. J. Polym. Environ. 2021, 29, 3079–3104. [Google Scholar] [CrossRef]
- Gorrasi, G.; Sorrentino, A.; Pantani, R. Modulation of Biodegradation Rate of Poly(Lactic Acid) by Silver Nanoparticles. J. Polym. Environ. 2015, 23, 316–320. [Google Scholar] [CrossRef]
- Emadian, S.M.; Onay, T.T.; Demirel, B. Biodegradation of Bioplastics in Natural Environments. Waste Manag. 2017, 59, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Díaz, A.; Franco, L.; Estrany, F.; del Valle, L.J.; Puiggalí, J. Poly(Butylene Azelate-Co-Butylene Succinate) Copolymers: Crystalline Morphologies and Degradation. Polym. Degrad. Stab. 2014, 99, 80–91. [Google Scholar] [CrossRef]
- Kumar, S.; Maiti, P. Understanding the Controlled Biodegradation of Polymers Using Nanoclays. Polymer 2015, 76, 25–33. [Google Scholar] [CrossRef]
- Ruggero, F.; Onderwater, R.C.A.; Carretti, E.; Roosa, S.; Benali, S.; Raquez, J.-M.; Gori, R.; Lubello, C.; Wattiez, R. Degradation of Film and Rigid Bioplastics During the Thermophilic Phase and the Maturation Phase of Simulated Composting. J. Polym. Environ. 2021, 29, 3015–3028. [Google Scholar] [CrossRef]
- Funabashi, M.; Ninomiya, F.; Kunioka, M. Method of Producing Biodegradable Reference Material and Its Biodegradability Based on International Standard Evaluation Method (ISO 14855-2). J. Polym. Environ. 2007, 15, 245–250. [Google Scholar] [CrossRef]
- ISO 14855-1:2012; Determination of the Ultimate Aerobic Biodegradability of Plastic Materials Under Controlled Composting Conditions Method by Analysis of Evolved Carbon Dioxide Part 1: General Method. ISO: Geneva, Switzerland, 2012.
- ISO 14855-2:2018; Determination of the Ultimate Aerobic Biodegradability of Plastic Materials Under Controlled Composting Conditions Method by Analysis of Evolved Carbon Dioxide Part 2: Gravimetric Measurement of Carbon Dioxide Evolved in a Laboratory-Scale Test. ISO: Geneva, Switzerland, 2018; pp. 1–16.
- ASTM D5338; Standard Test Method for Determining Aerobic Biodegradation of Plastic Materials Under Controlled Composting Conditions. ASTM: West Conshohocken, PA, USA, 2021; pp. 1–6.
- ISO 17556:2019; Plastics—Determination of the Ultimate Aerobic Biodegradability of Plastic Materials in Soil by Measuring the Oxygen Demand in a Respirometer or the Amount of Carbon Dioxide Evolved. ISO: Geneva, Switzerland, 2019.
- EN 17033; Plastics—Biodegradable Mulch Films for Use in Agriculture and Horticulture—Requirements and Test Methods. EN: Brussels, Belgium, 2018.
- ISO 14851:2019; Determination of the Ultimate Aerobic Biodegradability of Plastic Materials in an Aqueous Medium—Method by Measuring the Oxygen Demand in a Closed Respirometer. ISO: Geneva, Switzerland, 2019.
- ISO 14852:2021; Determination of the Ultimate Aerobic Biodegradability of Plastic Materials in an Aqueous Medium—Method by Analysis of Evolved Carbon Dioxide. ISO: Geneva, Switzerland, 2021.
- ASTM D6691-17; Standard Test Method for Determining Aerobic Biodegradation of Plastic Materials in the Marine Environment by a Defined Microbial Consortium or Natural Sea Water Inoculum. ASTM: West Conshohocken, PA, USA, 2018.
- ASTM D7473/D7473M-21; Standard Test Method for Weight Attrition of Non-Floating Plastic Materials by Open System Aquarium Incubations. ASTM: West Conshohocken, PA, USA, 2021.
- ISO 14853:2016; Plastics—Determination of the Ultimate Anaerobic Biodegradation of Plastic Materials in an Aqueous System Method by Measurement of Biogas Production. ISO: Geneva, Switzerland, 2021.
- ASTM D5511-18; Standard Test Method for Determining Anaerobic Biodegradation of Plastic Materials Under High-Solids Anaerobic-Digestion Conditions. ASTM: West Conshohocken, PA, USA, 2018.
- ISO 15985:2014; Plastics—Determination of the Ultimate Anaerobic Biodegradation Under High-Solids Anaerobic-Digestion Conditions—Method by Analysis of Released Biogas. ISO: Geneva, Switzerland, 2019.
- ASTM D7475-20; Standard Test Method for Determining the Aerobic Degradation and Anaerobic Biodegradation of Plastic Materials Under Accelerated Bioreactor Landfill Conditions. ASTM: West Conshohocken, PA, USA, 2020.
- ISO 20200:2023; Plastics—Determination of the Degree of Disintegration of Plastic Materials Under Composting Conditions in a Laboratory-Scale Test. ISO: Geneva, Switzerland, 2023.
- EN 14806:2006; Packaging—Preliminary Evaluation of the Disintegration of Packaging Materials Under Simulated Composting Conditions in a Laboratory Scale Test. EN: Brussels, Belgium, 2006.
- ISO 16929:2021; Plastics—Determination of the Degree of Disintegration of Plastic Materials Under Defined Composting Conditions in a Pilot-Scale Test. ISO: Geneva, Switzerland, 2021.
- EN 14045:2003; Packaging—Evaluation of the Disintegration of Packaging Materials in Practical Oriented Tests Under Defined Composting Conditions. EN: Brussels, Belgium, 2003.
- ASTM D5988-18; Standard Test Method for Determining Aerobic Biodegradation of Plastic Materials in Soil. ASTM: West Conshohocken, PA, USA, 2018; pp. 1–15.
- ISO 13975:2219; Plastics—Determination of the Ultimate Anaerobic Biodegradation of Plastic Materials in Controlled Slurry Digestion Systems—Method by Measurement of Biogas Production. ISO: Geneva, Switzerland, 2019.
- Arikan, E.B.; Ozsoy, H.D. A Review: Investigation of Bioplastics. J. Civ. Eng. Archit. 2015, 9, 188–192. [Google Scholar] [CrossRef]
- EN 14995:2006; Plastics. Evaluation of Compostability. Test Scheme and Specifications. EN: Brussels, Belgium, 2006.
- EN 13432:2001; Requirements for Packaging Recoverable Through Composting and Biodegradation. Test Scheme and Evaluation Criteria for the Final Acceptance of Packaging. EN: Brussels, Belgium, 2001.
- ASTM D6400-21; Standard Specification for Labeling of Plastics Designed to Be Aerobically Composted in Municipal or Industrial Facilities. ASTM: West Conshohocken, PA, USA, 2022; pp. 1–21.
- OECD Guidelines for the Testing of Chemicals. Test No. 208—Terrestrial Plant Test: Seedling Emergence and Seedling Growth Test; OECD: Paris, France, 2010; ISBN 9789264090910. [Google Scholar]
- Zumstein, M.T.; Narayan, R.; Kohler, H.-P.E.; McNeill, K.; Sander, M. Dos and Do Nots When Assessing the Biodegradation of Plastics. Environ. Sci. Technol. 2019, 53, 9967–9969. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, G.; Gray, K.; Wilson, S.P.; Muniyasamy, S.; Vincent, S.G.T.; Bush, R.; Palanisami, T. Benchmarking Bioplastics: A Natural Step Towards a Sustainable Future. J. Polym. Environ. 2020, 28, 3055–3075. [Google Scholar] [CrossRef]
- ISO 17088:2021; Plastics—Organic Recycling—Specifications for Compostable Plastics. ISO: Geneva, Switzerland, 2021.
- EN 14987:2006; Plastics—Evaluation of Disposability in Waste Water Treatment Plants—Test Scheme for Final Acceptance and Specifications. CEN: Brussels, Belgium, 2006.
- BNQ 9011-911/2007; Compostable Plastics – Certification Program. Bureau de normalisation du Québec (BNQ): Québec, Canada, 2007.
- Stloukal, P.; Pekařová, S.; Kalendova, A.; Mattausch, H.; Laske, S.; Holzer, C.; Chitu, L.; Bodner, S.; Maier, G.; Slouf, M.; et al. Kinetics and Mechanism of the Biodegradation of PLA/Clay Nanocomposites during Thermophilic Phase of Composting Process. Waste Manag. 2015, 42, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Satti, S.M.; Shah, A.A.; Marsh, T.L.; Auras, R. Biodegradation of Poly(Lactic Acid) in Soil Microcosms at Ambient Temperature: Evaluation of Natural Attenuation, Bio-Augmentation and Bio-Stimulation. J. Polym. Environ. 2018, 26, 3848–3857. [Google Scholar] [CrossRef]
- Yagi, H.; Ninomiya, F.; Funabashi, M.; Kunioka, M. Mesophilic Anaerobic Biodegradation Test and Analysis of Eubacteria and Archaea Involved in Anaerobic Biodegradation of Four Specified Biodegradable Polyesters. Polym. Degrad. Stab. 2014, 110, 278–283. [Google Scholar] [CrossRef]
- Zhang, W.; Heaven, S.; Banks, C.J. Degradation of Some EN13432 Compliant Plastics in Simulated Mesophilic Anaerobic Digestion of Food Waste. Polym. Degrad. Stab. 2018, 147, 76–88. [Google Scholar] [CrossRef]
- Yagi, H.; Ninomiya, F.; Funabashi, M.; Kunioka, M. Thermophilic Anaerobic Biodegradation Test and Analysis of Eubacteria Involved in Anaerobic Biodegradation of Four Specified Biodegradable Polyesters. Polym. Degrad. Stab. 2013, 98, 1182–1187. [Google Scholar] [CrossRef]
- Wang, F.; Hidaka, T.; Tsuno, H.; Tsubota, J. Co-Digestion of Polylactide and Kitchen Garbage in Hyperthermophilic and Thermophilic Continuous Anaerobic Process. Bioresour. Technol. 2012, 112, 67–74. [Google Scholar] [CrossRef]
- Rosa, D.S.; Filho, R.P.; Chui, Q.S.H.; Calil, M.R.; Guedes, C.G.F. The Biodegradation of Poly-β-(Hydroxybutyrate), Poly-β-(Hydroxybutyrate-Co-β-Valerate) and Poly(ε-Caprolactone) in Compost Derived from Municipal Solid Waste. Eur. Polym. J. 2003, 39, 233–237. [Google Scholar] [CrossRef]
- Meereboer, K.W.; Misra, M.; Mohanty, A.K. Review of Recent Advances in the Biodegradability of Polyhydroxyalkanoate (PHA) Bioplastics and Their Composites. Green Chem. 2020, 22, 5519–5558. [Google Scholar] [CrossRef]
- Cafiero, L.M.; Canditelli, M.; Musmeci, F.; Sagnotti, G.; Tuffi, R. Assessment of Disintegration of Compostable Bioplastic Bags by Management of Electromechanical and Static Home Composters. Sustainability 2020, 13, 263. [Google Scholar] [CrossRef]
- Calabro, P.S.; Folino, A.; Fazzino, F.; Komilis, D. Preliminary Evaluation of the Anaerobic Biodegradability of Three Biobased Materials Used for the Production of Disposable Plastics. J. Hazard. Mater. 2020, 390, 121653. [Google Scholar] [CrossRef]
- Bandini, F.; Frache, A.; Ferrarini, A.; Taskin, E.; Cocconcelli, P.S.; Puglisi, E. Fate of Biodegradable Polymers Under Industrial Conditions for Anaerobic Digestion and Aerobic Composting of Food Waste. J. Polym. Environ. 2020, 28, 2539–2550. [Google Scholar] [CrossRef]
- Camacho-Muñoz, R.; Villada-Castillo, H.S.; Solanilla-Duque, J.F. Anaerobic Biodegradation under Slurry Thermophilic Conditions of Poly(Lactic Acid)/Starch Blend Compatibilized by Maleic Anhydride. Int. J. Biol. Macromol. 2020, 163, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Anstey, A.; Muniyasamy, S.; Reddy, M.M.; Misra, M.; Mohanty, A. Processability and Biodegradability Evaluation of Composites from Poly(Butylene Succinate) (PBS) Bioplastic and Biofuel Co-Products from Ontario. J. Polym. Environ. 2014, 22, 209–218. [Google Scholar] [CrossRef]
- Altieri, R.; Seggiani, M.; Esposito, A.; Cinelli, P.; Stanzione, V. Thermoplastic Blends Based on Poly(Butylene Succinate-Co-Adipate) and Different Collagen Hydrolysates from Tanning Industry—II: Aerobic Biodegradation in Composting Medium. J. Polym. Environ. 2021, 29, 3375–3388. [Google Scholar] [CrossRef]
- Cho, H.S.; Moon, H.S.; Kim, M.; Nam, K.; Kim, J.Y. Biodegradability and Biodegradation Rate of Poly(Caprolactone)-Starch Blend and Poly(Butylene Succinate) Biodegradable Polymer under Aerobic and Anaerobic Environment. Waste Manag. 2011, 31, 475–480. [Google Scholar] [CrossRef]
- Gadaleta, G.; De Gisi, S.; Picuno, C.; Heerenklage, J.; Kuchta, K.; Sorrentino, A.; Notarnicola, M.; Oliviero, M. Assessment of Methane Production, Disintegration, and Biodegradation Potential of Bioplastic Waste in Anaerobic Digestion Systems. J. Environ. Chem. Eng. 2024, 12, 111658. [Google Scholar] [CrossRef]
- Nakasaki, K.; Matsuura, H.; Tanaka, H.; Sakai, T. Synergy of Two Thermophiles Enables Decomposition of Poly-ɛ-Caprolactone under Composting Conditions. FEMS Microbiol. Ecol. 2006, 58, 373–383. [Google Scholar] [CrossRef]
- Kale, G.; Kijchavengkul, T.; Auras, R.; Rubino, M.; Selke, S.E.; Singh, S.P. Compostability of Bioplastic Packaging Materials: An Overview. Macromol. Biosci. 2007, 7, 255–277. [Google Scholar] [CrossRef]
- García-Depraect, O.; Lebrero, R.; Rodriguez-Vega, S.; Bordel, S.; Santos-Beneit, F.; Martínez-Mendoza, L.J.; Aragão Börner, R.; Börner, T.; Muñoz, R. Biodegradation of Bioplastics under Aerobic and Anaerobic Aqueous Conditions: Kinetics, Carbon Fate and Particle Size Effect. Bioresour. Technol. 2022, 344, 126265. [Google Scholar] [CrossRef] [PubMed]
- Abou-Zeid, D.-M.; Müller, R.-J.; Deckwer, W.-D. Degradation of Natural and Synthetic Polyesters under Anaerobic Conditions. J. Biotechnol. 2001, 86, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Yagi, H.; Ninomiya, F.; Funabashi, M.; Kunioka, M. Anaerobic Biodegradation Tests of Poly(Lactic Acid) and Polycaprolactone Using New Evaluation System for Methane Fermentation in Anaerobic Sludge. Polym. Degrad. Stab. 2009, 94, 1397–1404. [Google Scholar] [CrossRef]
- Kijchavengkul, T.; Auras, R.; Rubino, M.; Selke, S.; Ngouajio, M.; Fernandez, R.T. Biodegradation and Hydrolysis Rate of Aliphatic Aromatic Polyester. Polym. Degrad. Stab. 2010, 95, 2641–2647. [Google Scholar] [CrossRef]
- Cazaudehore, G.; Monlau, F.; Gassie, C.; Lallement, A.; Guyoneaud, R. Active Microbial Communities during Biodegradation of Biodegradable Plastics by Mesophilic and Thermophilic Anaerobic Digestion. J. Hazard. Mater. 2023, 443, 130208. [Google Scholar] [CrossRef]
- Svoboda, P.; Dvorackova, M.; Svobodova, D. Influence of Biodegradation on Crystallization of Poly (Butylene Adipate-co-terephthalate). Polym. Adv. Technol. 2019, 30, 552–562. [Google Scholar] [CrossRef]
- Phuong, V.T.; Verstiche, S.; Cinelli, P.; Anguillesi, I.; Coltelli, M.-B.; Lazzeri, A. Cellulose Acetate Blends—Effect of Plasticizers on Properties and Biodegradability. J. Renew. Mater. 2014, 2, 35–41. [Google Scholar] [CrossRef]
- Gadaleta, G.; De Gisi, S.; Chong, Z.K.; Heerenklage, J.; Notarnicola, M.; Kuchta, K.; Cafiero, L.; Oliviero, M.; Sorrentino, A.; Picuno, C. Degradation of Thermoplastic Cellulose Acetate-Based Bioplastics by Full-Scale Experimentation of Industrial Anaerobic Digestion and Composting. Chem. Eng. J. 2023, 462, 142301. [Google Scholar] [CrossRef]
- Dolci, G.; Venturelli, V.; Catenacci, A.; Ciapponi, R.; Malpei, F.; Romano Turri, S.E.; Grosso, M. Evaluation of the Anaerobic Degradation of Food Waste Collection Bags Made of Paper or Bioplastic. J. Environ. Manag. 2022, 305, 114331. [Google Scholar] [CrossRef]
- Narancic, T.; Verstichel, S.; Reddy Chaganti, S.; Morales-Gamez, L.; Kenny, S.T.; De Wilde, B.; Babu Padamati, R.; O’Connor, K.E. Biodegradable Plastic Blends Create New Possibilities for End-of-Life Management of Plastics but They Are Not a Panacea for Plastic Pollution. Environ. Sci. Technol. 2018, 52, 10441–10452. [Google Scholar] [CrossRef]
- Muniyasamy, S.; Ofosu, O.; John, M.J.; Anandjiwala, R.D. Mineralization of Poly(Lactic Acid) (PLA), Poly(3-Hydroxybutyrate-Co-Valerate) (PHBV) and PLA/PHBV Blend in Compost and Soil Environments. J. Renew. Mater. 2016, 4, 133–145. [Google Scholar] [CrossRef]
- Jeszeová, L.; Puškárová, A.; Bučková, M.; Kraková, L.; Grivalský, T.; Danko, M.; Mosnáčková, K.; Chmela, Š.; Pangallo, D. Microbial Communities Responsible for the Degradation of Poly(Lactic Acid)/Poly(3-Hydroxybutyrate) Blend Mulches in Soil Burial Respirometric Tests. World J. Microbiol. Biotechnol. 2018, 34, 101. [Google Scholar] [CrossRef]
- Touchaleaume, F.; Martin-Closas, L.; Angellier-Coussy, H.; Chevillard, A.; Cesar, G.; Gontard, N.; Gastaldi, E. Performance and Environmental Impact of Biodegradable Polymers as Agricultural Mulching Films. Chemosphere 2016, 144, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Lunt, J. Large-Scale Production, Properties and Commercial Applications of Polylactic Acid Polymers. Polym. Degrad. Stab. 1998, 59, 145–152. [Google Scholar] [CrossRef]
- Šerá, J.; Serbruyns, L.; De Wilde, B.; Koutný, M. Accelerated Biodegradation Testing of Slowly Degradable Polyesters in Soil. Polym. Degrad. Stab. 2020, 171, 109031. [Google Scholar] [CrossRef]
- Briassoulis, D.; Mistriotis, A.; Mortier, N.; Tosin, M. A Horizontal Test Method for Biodegradation in Soil of Bio-Based and Conventional Plastics and Lubricants. J. Clean. Prod. 2020, 242, 118392. [Google Scholar] [CrossRef]
- Thellen, C.; Cheney, S.; Ratto, J.A. Melt Processing and Characterization of Polyvinyl Alcohol and Polyhydroxyalkanoate Multilayer Films. J. Appl. Polym. Sci. 2013, 127, 2314–2324. [Google Scholar] [CrossRef]
- Thellen, C.; Coyne, M.; Froio, D.; Auerbach, M.; Wirsen, C.; Ratto, J.A. A Processing, Characterization and Marine Biodegradation Study of Melt-Extruded Polyhydroxyalkanoate (PHA) Films. J. Polym. Environ. 2008, 16, 1–11. [Google Scholar] [CrossRef]
- Deroiné, M.; César, G.; Le Duigou, A.; Davies, P.; Bruzaud, S. Natural Degradation and Biodegradation of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) in Liquid and Solid Marine Environments. J. Polym. Environ. 2015, 23, 493–505. [Google Scholar] [CrossRef]
- Guo, W.; Tao, J.; Yang, C.; Song, C.; Geng, W.; Li, Q.; Wang, Y.; Kong, M.; Wang, S. Introduction of Environmentally Degradable Parameters to Evaluate the Biodegradability of Biodegradable Polymers. PLoS ONE 2012, 7, e38341. [Google Scholar] [CrossRef]
- Zee, M.; Stoutjesdijk, J.H.; Heijden, P.A.A.W.; Wit, D. Structure-Biodegradation Relationships of Polymeric Materials. 1. Effect of Degree of Oxidation on Biodegradability of Carbohydrate Polymers. J. Environ. Polym. Degrad. 1995, 3, 235–242. [Google Scholar] [CrossRef]
- Nevoralová, M.; Koutný, M.; Ujčić, A.; Starý, Z.; Šerá, J.; Vlková, H.; Šlouf, M.; Fortelný, I.; Kruliš, Z. Structure Characterization and Biodegradation Rate of Poly(ε-Caprolactone)/Starch Blends. Front. Mater. 2020, 7. [Google Scholar] [CrossRef]
- Bettas Ardisson, G.; Tosin, M.; Barbale, M.; Degli-Innocenti, F. Biodegradation of Plastics in Soil and Effects on Nitrification Activity. A Laboratory Approach. Front. Microbiol. 2014, 5, 710. [Google Scholar] [CrossRef]
- van der Zee, M. Biodegradability of Biodegradable Mulch Film: A Review of the Scientific Literature on the Biodegradability of Materials Used for Biodegradable Mulch Film; Wageningen Food & Biobased Research: Wageningen, The Netherlands, 2021. [Google Scholar]
- van der Zee, M.; Sijtsma, L.; Tan, G.B.; Tournois, H.; de Wit, D. Assessment of Biodegradation of Water Insoluble Polymeric Materials in Aerobic and Anaerobic Aquatic Environments. Chemosphere 1994, 28, 1757–1771. [Google Scholar] [CrossRef]
- Julinová, M.; Vaňharová, L.; Jurča, M.; Minařík, A.; Duchek, P.; Kavečková, J.; Rouchalová, D.; Skácelík, P. Effect of Different Fillers on the Biodegradation Rate of Thermoplastic Starch in Water and Soil Environments. J. Polym. Environ. 2020, 28, 566–583. [Google Scholar] [CrossRef]
- Modelli, A.; Calcagno, B.; Scandola, M. Kinetics of Aerobic Polymer Degradation in Soil by Means of the ASTM D 5988-96 Standard Method. J. Polym. Environ. 1999, 7, 109–116. [Google Scholar] [CrossRef]
- Nakayama, A.; Yamano, N.; Kawasaki, N. Biodegradation in Seawater of Aliphatic Polyesters. Polym. Degrad. Stab. 2019, 166, 290–299. [Google Scholar] [CrossRef]
- Funabashi, M.; Ninomiya, F.; Kunioka, M. Biodegradation of Polycaprolactone Powders Proposed as Reference Test Materials for International Standard of Biodegradation Evaluation Method. J. Polym. Environ. 2007, 15, 7–17. [Google Scholar] [CrossRef]
- Moura, I.; Machado, A.V.; Duarte, F.M.; Nogueira, R. Biodegradability Assessment of Aliphatic Polyesters-Based Blends Using Standard Methods. J. Appl. Polym. Sci. 2011, 119, 3338–3346. [Google Scholar] [CrossRef]
- Nabeoka, R.; Suzuki, H.; Akasaka, Y.; Ando, N.; Yoshida, T. Evaluating the Ready Biodegradability of Biodegradable Plastics. Environ. Toxicol. Chem. 2021, 40, 2443–2449. [Google Scholar] [CrossRef]
- Kim, Y.; Choe, S.; Cho, Y.; Moon, H.; Shin, H.; Seo, J.; Myung, J. Biodegradation of Poly(Butylene Adipate Terephthalate) and Poly(Vinyl Alcohol) within Aquatic Pathway. Sci. Total Environ. 2024, 953, 176129. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.-X.; Jin, Y.-J.; Meng, Q.-Y.; Wang, L.; Zhang, M.; Wang, Y.-Z. Biodegradation Behavior of Poly(Butylene Adipate-Co-Terephthalate) (PBAT), Poly(Lactic Acid) (PLA), and Their Blend under Soil Conditions. Polym. Test. 2013, 32, 918–926. [Google Scholar] [CrossRef]
- Dissanayake, P.D.; Withana, P.A.; Sang, M.K.; Cho, Y.; Park, J.; Oh, D.X.; Chang, S.X.; Lin, C.S.K.; Bank, M.S.; Hwang, S.Y.; et al. Effects of Biodegradable Poly(Butylene Adipate-co-terephthalate) and Poly(Lactic Acid) Plastic Degradation on Soil Ecosystems. Soil Use Manag. 2024, 40, e13055. [Google Scholar] [CrossRef]
- Francioni, M.; Kishimoto-Mo, A.W.; Tsuboi, S.; Takada Hoshino, Y. Evaluation of the Mulch Films Biodegradation in Soil: A Methodological Review. Ital. J. Agron. 2021, 17, 1936. [Google Scholar] [CrossRef]
- Guo, W.; Tao, J.; Yang, C.; Zhao, Q.; Song, C.; Wang, S. The Rapid Evaluation of Material Biodegradability Using an Improved ISO 14852 Method with a Microbial Community. Polym. Test. 2010, 29, 832–839. [Google Scholar] [CrossRef]
- Kasuya, K.; Takagi, K.; Ishiwatari, S.; Yoshida, Y.; Doi, Y. Biodegradabilities of Various Aliphatic Polyesters in Natural Waters. Polym. Degrad. Stab. 1998, 59, 327–332. [Google Scholar] [CrossRef]
- Doi, Y.; Kasuya, K.; Abe, H.; Koyama, N.; Shin-ichi, I.; Koichi, T.; Yoshida, Y. Evaluation of Biodegradabilities of Biosynthetic and Chemosynthetic Polyesters in River Water. Polym. Degrad. Stab. 1996, 51, 281–286. [Google Scholar] [CrossRef]
- Ahn, B.D.; Kim, S.H.; Kim, Y.H.; Yang, J.S. Synthesis and Characterization of the Biodegradable Copolymers from Succinic Acid and Adipic Acid with 1,4-butanediol. J. Appl. Polym. Sci. 2001, 82, 2808–2826. [Google Scholar] [CrossRef]
- Hayase, N.; Yano, H.; Kudoh, E.; Tsutsumi, C.; Ushio, K.; Miyahara, Y.; Tanaka, S.; Nakagawa, K. Isolation and Characterization of Poly(Butylene Succinate-Co-Butylene Adipate)-Degrading Microorganism. J. Biosci. Bioeng. 2004, 97, 131–133. [Google Scholar] [CrossRef]
- Afshar, S.V.; Boldrin, A.; Astrup, T.F.; Daugaard, A.E.; Hartmann, N.B. Degradation of Biodegradable Plastics in Waste Management Systems and the Open Environment: A Critical Review. J. Clean. Prod. 2024, 434, 140000. [Google Scholar] [CrossRef]
- Yadav, N.; Hakkarainen, M. Degradable or Not? Cellulose Acetate as a Model for Complicated Interplay between Structure, Environment and Degradation. Chemosphere 2021, 265, 128731. [Google Scholar] [CrossRef]
- Ach, A. Biodegradable Plastics Based on Cellulose Acetate. J. Macromol. Sci. Part A 1993, 30, 733–740. [Google Scholar] [CrossRef]
- Joly, F.-X.; Coulis, M. Comparison of Cellulose vs. Plastic Cigarette Filter Decomposition under Distinct Disposal Environments. Waste Manag. 2018, 72, 349–353. [Google Scholar] [CrossRef]
- Gómez, E.F.; Michel, F.C. Biodegradability of Conventional and Bio-Based Plastics and Natural Fiber Composites during Composting, Anaerobic Digestion and Long-Term Soil Incubation. Polym. Degrad. Stab. 2013, 98, 2583–2591. [Google Scholar] [CrossRef]
- Fei, Z.; Huang, S.; Yin, J.; Xu, F.; Zhang, Y. Preparation and Characterization of Bio-Based Degradable Plastic Films Composed of Cellulose Acetate and Starch Acetate. J. Polym. Environ. 2015, 23, 383–391. [Google Scholar] [CrossRef]
- Serbruyns, L.; Van de Perre, D.; Hölter, D. Biodegradability of Cellulose Diacetate in Aqueous Environments. J. Polym. Environ. 2023, 32, 1326–1341. [Google Scholar] [CrossRef]
- Buchanan, C.M.; Gardner, R.M.; Komarek, R.J. Aerobic Biodegradation of Cellulose Acetate. J. Appl. Polym. Sci. 1993, 47, 1709–1719. [Google Scholar] [CrossRef]
- Allen, A.L.; Mayer, J.; Stote, R.; Kaplan, D.L. Simulated Marine Respirometry of Biodegradable Polymers. J. Environ. Polym. Degrad. 1994, 2, 237–244. [Google Scholar] [CrossRef]
- Folino, A.; Pangallo, D.; Calabrò, P.S. Assessing Bioplastics Biodegradability by Standard and Research Methods: Current Trends and Open Issues. J. Environ. Chem. Eng. 2023, 11, 109424. [Google Scholar] [CrossRef]
- Kosheleva, A.; Gadaleta, G.; De Gisi, S.; Heerenklage, J.; Picuno, C.; Notarnicola, M.; Kuchta, K.; Sorrentino, A. Co-Digestion of Food Waste and Cellulose-Based Bioplastic: From Batch to Semi-Continuous Scale Investigation. Waste Manag. 2023, 156, 272–281. [Google Scholar] [CrossRef]
- De Gisi, S.; Gadaleta, G.; Gorrasi, G.; La Mantia, F.P.; Notarnicola, M.; Sorrentino, A. The Role of (Bio)Degradability on the Management of Petrochemical and Bio-Based Plastic Waste. J. Environ. Manag. 2022, 310, 114769. [Google Scholar] [CrossRef]
- Cucina, M.; de Nisi, P.; Tambone, F.; Adani, F. The Role of Waste Management in Reducing Bioplastics’ Leakage into the Environment: A Review. Bioresour. Technol. 2021, 337, 125459. [Google Scholar] [CrossRef] [PubMed]
- Gadaleta, G.; Ferrara, C.; De Gisi, S.; Notarnicola, M.; De Feo, G. Life Cycle Assessment of End-of-Life Options for Cellulose-Based Bioplastics When Introduced into a Municipal Solid Waste Management System. Sci. Total Environ. 2023, 871, 161958. [Google Scholar] [CrossRef] [PubMed]
- Urbanek, A.K.; Rymowicz, W.; Strzelecki, M.C.; Kociuba, W.; Franczak, Ł.; Mirończuk, A.M. Isolation and Characterization of Arctic Microorganisms Decomposing Bioplastics. AMB Express 2017, 7, 148. [Google Scholar] [CrossRef] [PubMed]
- Mihai, M.; Legros, N.; Alemdar, A. Formulation-Properties Versatility of Wood Fiber Biocomposites Based on Polylactide and Polylactide/Thermoplastic Starch Blends. Polym. Eng. Sci. 2014, 54, 1325–1340. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Yunus, W.M.Z.W.; Othman, M.; Abdan, K.; Hadithon, K.A. Poly(Lactic Acid) (PLA)-Reinforced Kenaf Bast Fiber Composites: The Effect of Triacetin. J. Reinf. Plast. Compos. 2010, 29, 1099–1111. [Google Scholar] [CrossRef]
- Wu, C. Preparation, Characterization, and Biodegradability of Renewable Resource-based Composites from Recycled Polylactide Bioplastic and Sisal Fibers. J. Appl. Polym. Sci. 2012, 123, 347–355. [Google Scholar] [CrossRef]
- Wu, C.-S. Preparation and Characterization of Polyhydroxyalkanoate Bioplastic-Based Green Renewable Composites from Rice Husk. J. Polym. Environ. 2014, 22, 384–392. [Google Scholar] [CrossRef]
- Iwańczuk, A.; Kozłowski, M.; Łukaszewicz, M.; Jabłoński, S. Anaerobic Biodegradation of Polymer Composites Filled with Natural Fibers. J. Polym. Environ. 2015, 23, 277–282. [Google Scholar] [CrossRef]
- Orhan, Y.; Hrenovic, J.; Büyükgüngor, H. Biodegradation of Plastic Compost Bags under Controlled Soil Conditions. Acta Chim. Slov. 2004, 51, 579–588. [Google Scholar]
- Harmaen, A.S.; Khalina, A.; Azowa, I.; Hassan, M.A.; Tarmian, A.; Jawaid, M. Thermal and Biodegradation Properties of Poly(Lactic Acid)/Fertilizer/Oil Palm Fibers Blends Biocomposites. Polym. Compos. 2015, 36, 576–583. [Google Scholar] [CrossRef]
- Jain, R.; Tiwari, A. Biosynthesis of Planet Friendly Bioplastics Using Renewable Carbon Source. J. Environ. Health Sci. Eng. 2015, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Uribe-Echeverría, T.; Beiras, R. Acute Toxicity of Bioplastic Leachates to Paracentrotus Lividus Sea Urchin Larvae. Mar. Environ. Res. 2022, 176, 105605. [Google Scholar] [CrossRef]
- Savva, K.; Borrell, X.; Moreno, T.; Pérez-Pomeda, I.; Barata, C.; Llorca, M.; Farré, M. Cytotoxicity Assessment and Suspected Screening of PLASTIC ADDITIVES in Bioplastics of Single-Use Household Items. Chemosphere 2023, 313, 137494. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, L.; Dombrowski, A.; Völker, C.; Wagner, M. Are Bioplastics and Plant-Based Materials Safer than Conventional Plastics? In Vitro Toxicity and Chemical Composition. Environ. Int. 2020, 145, 106066. [Google Scholar] [CrossRef]
- Rosenboom, J.G.; Langer, R.; Traverso, G. Bioplastics for a Circular Economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef]
- Findrik, E.; Meixner, O. Drivers and Barriers for Consumers Purchasing Bioplastics—A Systematic Literature Review. J. Clean. Prod. 2023, 410, 137311. [Google Scholar] [CrossRef]
- Notaro, S.; Lovera, E.; Paletto, A. Consumers’ Preferences for Bioplastic Products: A Discrete Choice Experiment with a Focus on Purchase Drivers. J. Clean. Prod. 2022, 330, 129870. [Google Scholar] [CrossRef]
- Filho, W.L.; Barbir, J.; Abubakar, I.R.; Paço, A.; Stasiskiene, Z.; Hornbogen, M.; Fendt, M.T.C.; Voronova, V.; Klõga, M. Consumer Attitudes and Concerns with Bioplastics Use: An International Study. PLoS ONE 2022, 17, e0266918. [Google Scholar] [CrossRef]
- Dilkes-Hoffman, L.; Ashworth, P.; Laycock, B.; Pratt, S.; Lant, P. Public Attitudes towards Bioplastics—Knowledge, Perception and End-of-Life Management. Resour. Conserv. Recycl. 2019, 151, 104479. [Google Scholar] [CrossRef]
| Certifying Body | Country | Label | Standard | Acceptance |
|---|---|---|---|---|
| DIN Certco | DE |  | EN 13432 [62] ASTM D6400 [63] EN 14995 [61] ISO 17088 [67] | Europe |
 | ISO 17556 [44] | |||
| ORG | UK |  | EN 13432 | Europe |
| KEURMERK INSTITUTE | NL |  | EN 13432 | Europe |
| COBRO | PL |  | EN 13432 | Europe |
| ABA | AU |  | EN 13432 | Europe/Australia |
| TÜV Austria | BE |  | EN 13432 | Europe |
 | ISO 17556 | |||
 | ASTM D6691 [48] | |||
 | EN 14987 [68] | |||
| Finnish Solid Waste Association (FSWA) | FI |  | EN 13432 | Finland |
| CIC | IT |  | EN 13432 | Italy |
| Avfall Norge | NO |  | EN 13432 | Norway |
| Generalitat Catalunya | SP |  | EN 13432 | Spain |
| Biodegradable Products Institute (BPI)/US Composting Council (USCC) | US |  | ASTM D6400 | USA |
| BNQ | CA |  | BNQ 9011-911/2007 [69] | Canada |
| BPS | JP |  | Green Scheme Pla | Japan |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gadaleta, G.; Andrade-Chapal, J.C.; López-Ibáñez, S.; Mozo-Toledo, M.; Navarro-Calderón, Á. Biodegradability of Bioplastics in Managed and Unmanaged Environments: A Comprehensive Review. Materials 2025, 18, 2382. https://doi.org/10.3390/ma18102382
Gadaleta G, Andrade-Chapal JC, López-Ibáñez S, Mozo-Toledo M, Navarro-Calderón Á. Biodegradability of Bioplastics in Managed and Unmanaged Environments: A Comprehensive Review. Materials. 2025; 18(10):2382. https://doi.org/10.3390/ma18102382
Chicago/Turabian StyleGadaleta, Giovanni, Johana Carolina Andrade-Chapal, Sara López-Ibáñez, María Mozo-Toledo, and Ángela Navarro-Calderón. 2025. "Biodegradability of Bioplastics in Managed and Unmanaged Environments: A Comprehensive Review" Materials 18, no. 10: 2382. https://doi.org/10.3390/ma18102382
APA StyleGadaleta, G., Andrade-Chapal, J. C., López-Ibáñez, S., Mozo-Toledo, M., & Navarro-Calderón, Á. (2025). Biodegradability of Bioplastics in Managed and Unmanaged Environments: A Comprehensive Review. Materials, 18(10), 2382. https://doi.org/10.3390/ma18102382








