Zn Complex with Homovanillic Acid: Theoretical (B3LYP/6-311++G(d,p)), Structural (FT-IR, NMR), Thermal (TG, DTG, and DSC) and Biological (Antioxidant and Antimicrobial) Characteristics
Abstract
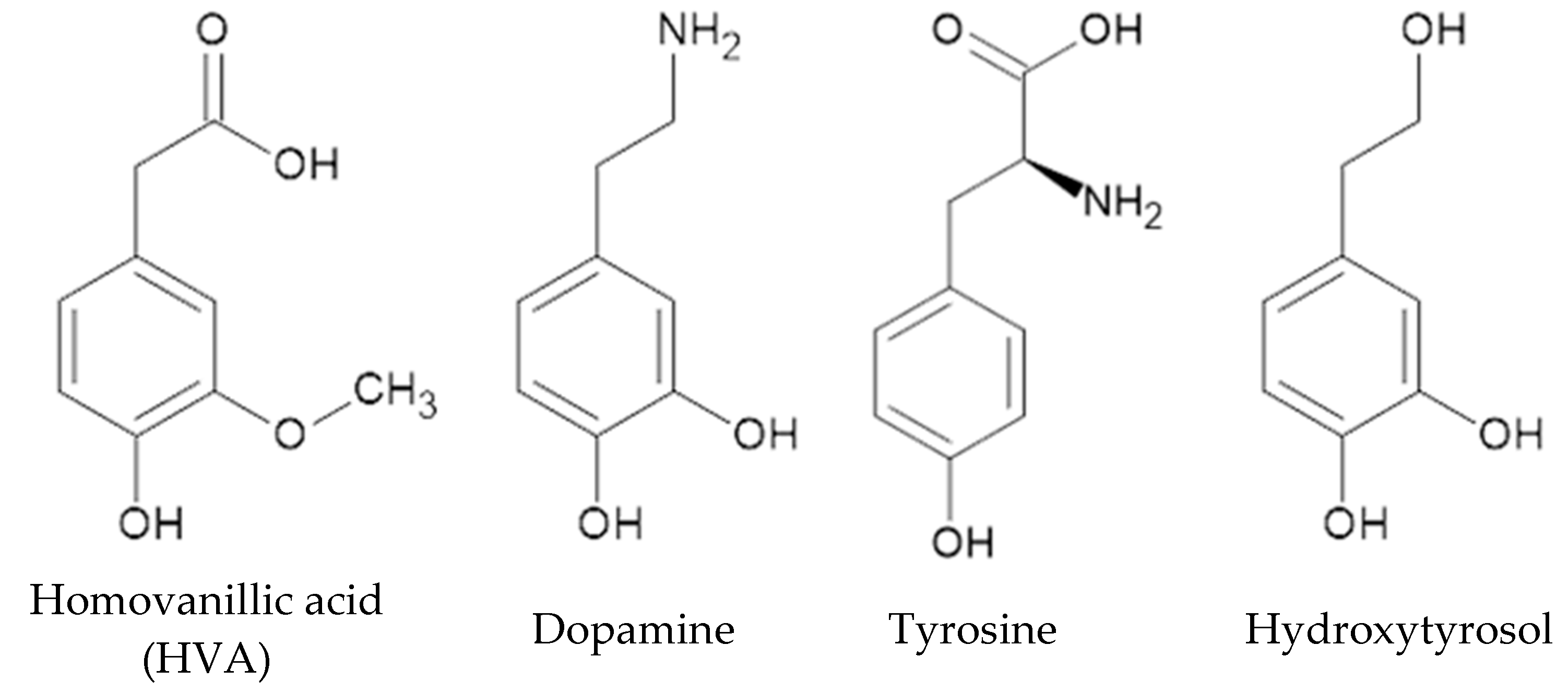
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.3. NMR Spectra
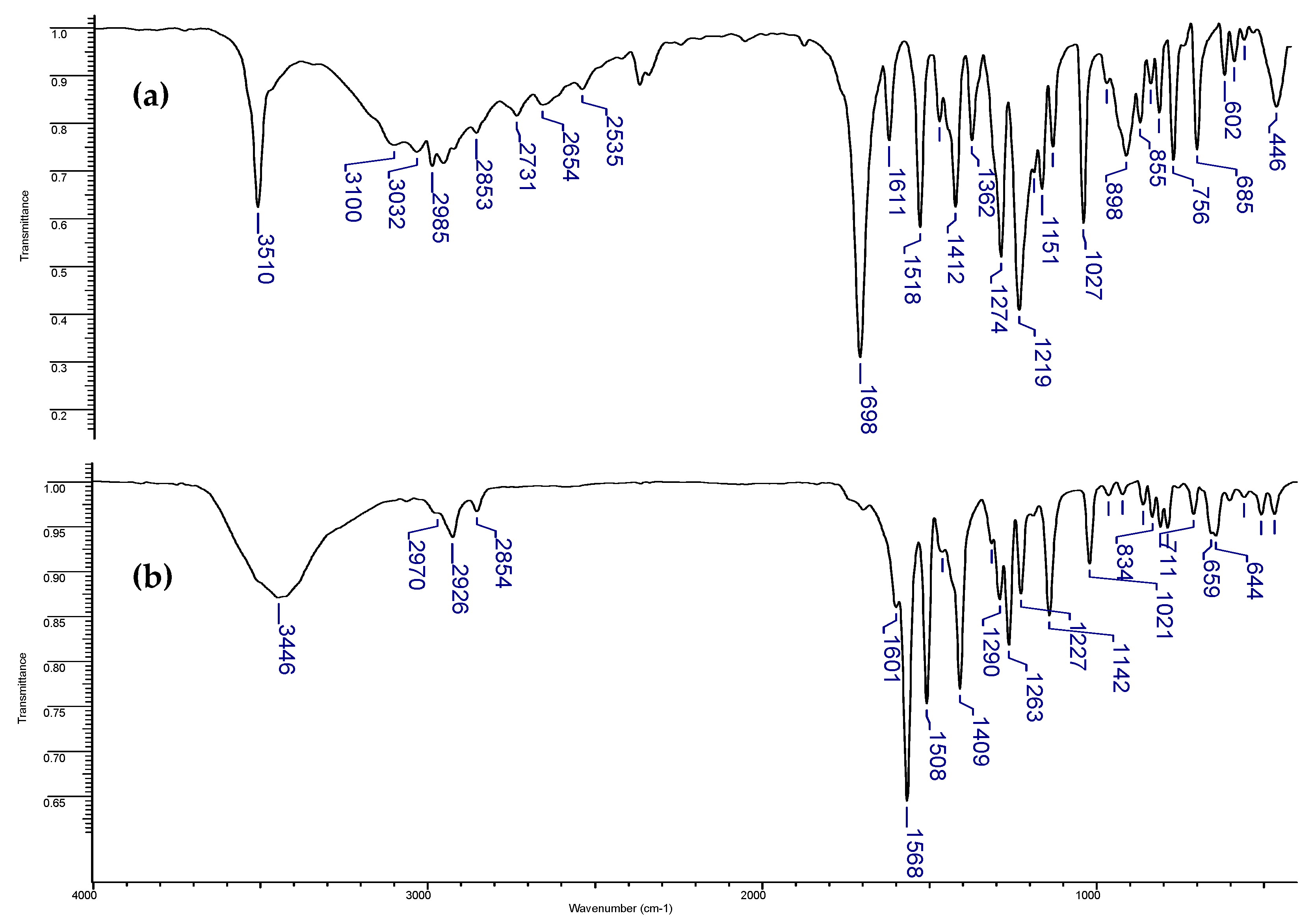
2.4. FT-IR Spectra
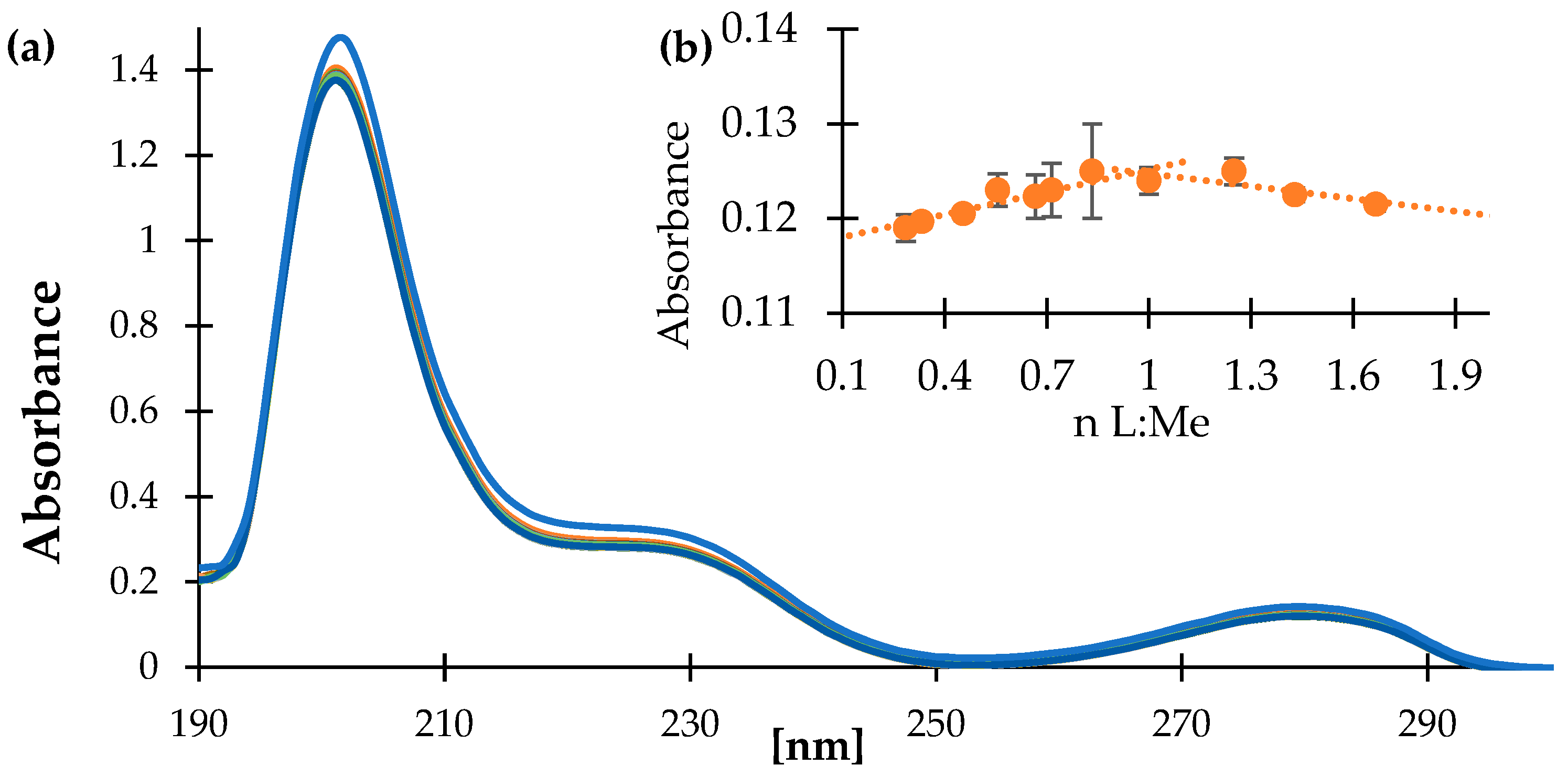
2.5. UV-Vis Study
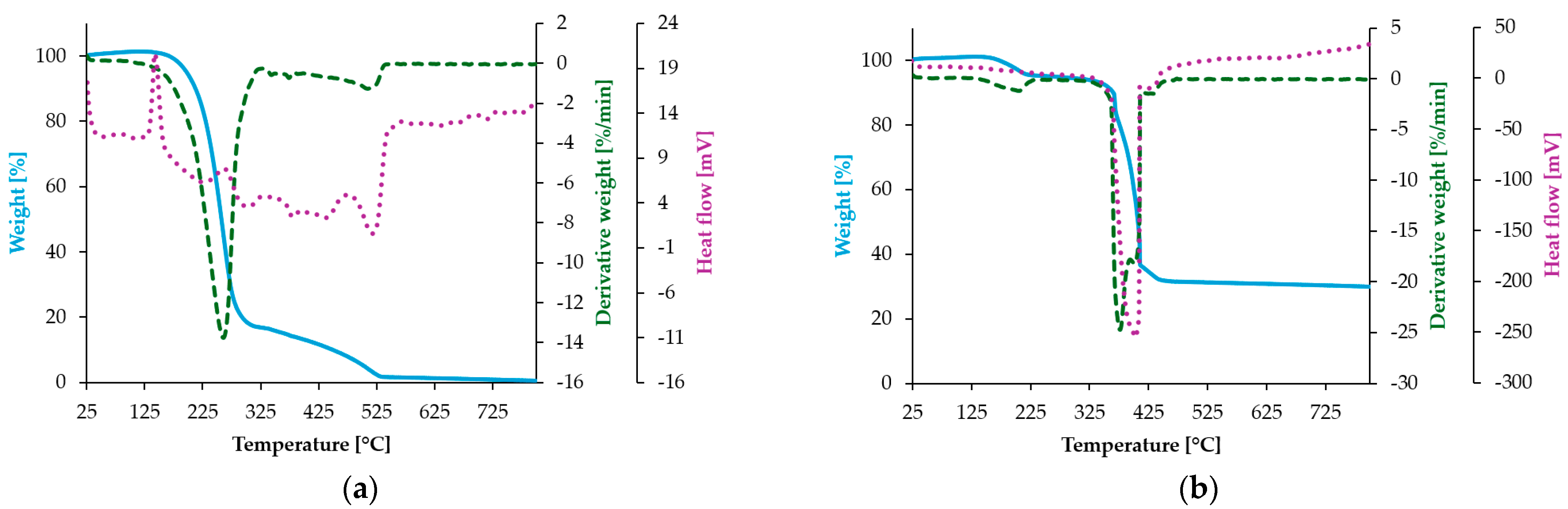
2.6. Elemental and Thermogravimetric Analyses
2.7. Antioxidant Activity
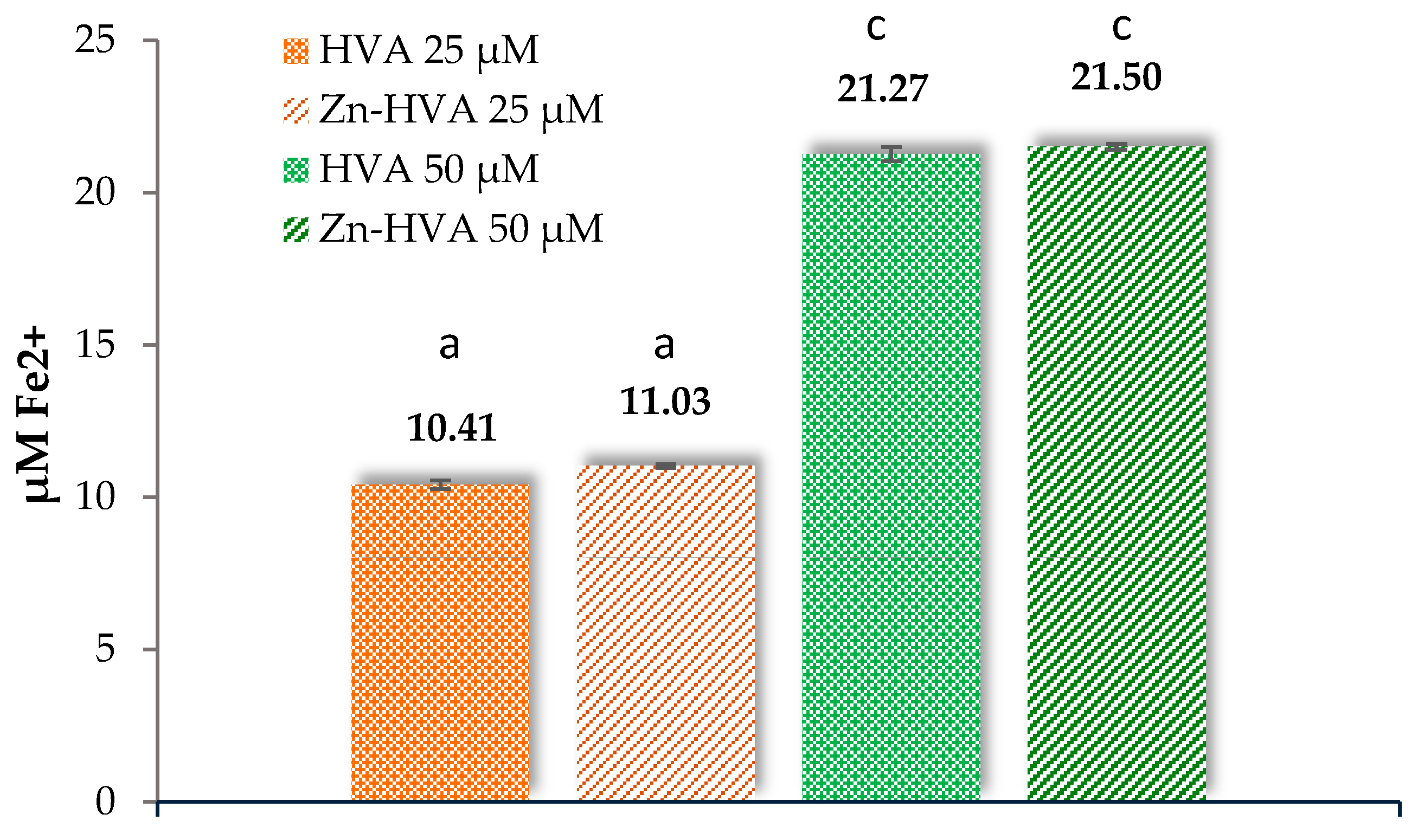
2.7.1. Ferric Reducing Antioxidant Power Assay (FRAP)
2.7.2. DPPH• Antiradical Activity Assay
2.7.3. ABTS•+ Antiradical Activity Assay
2.8. Antimicrobial Activity
2.9. Computational Details
3. Results
3.1. Quantum Chemical Calculations
3.1.1. Changes in Bond Lengths and Angles
3.1.2. Natural Bond Orbital (NBO)
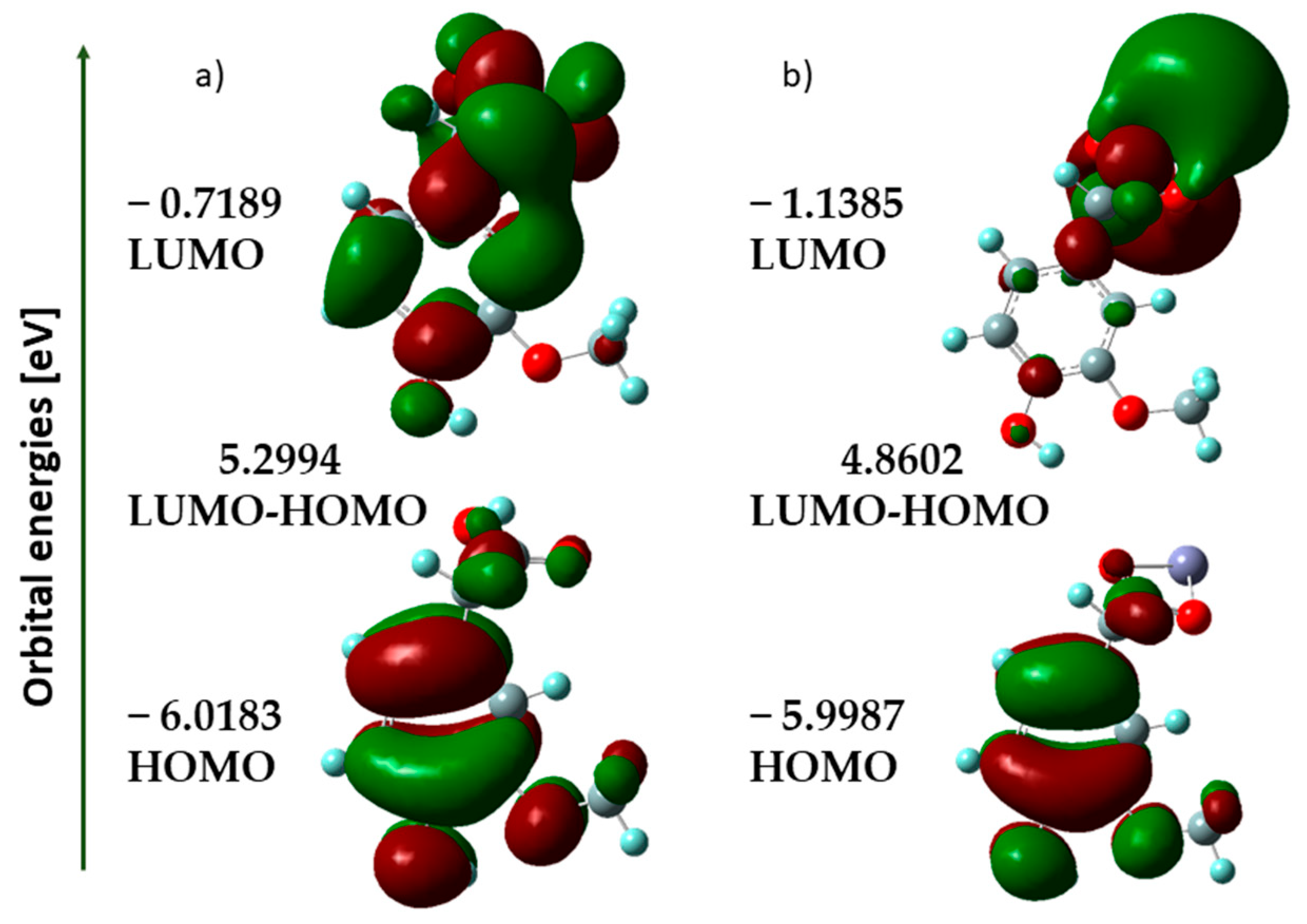
3.1.3. HOMO-LUMO Analysis
3.1.4. Thermodynamical Parameters
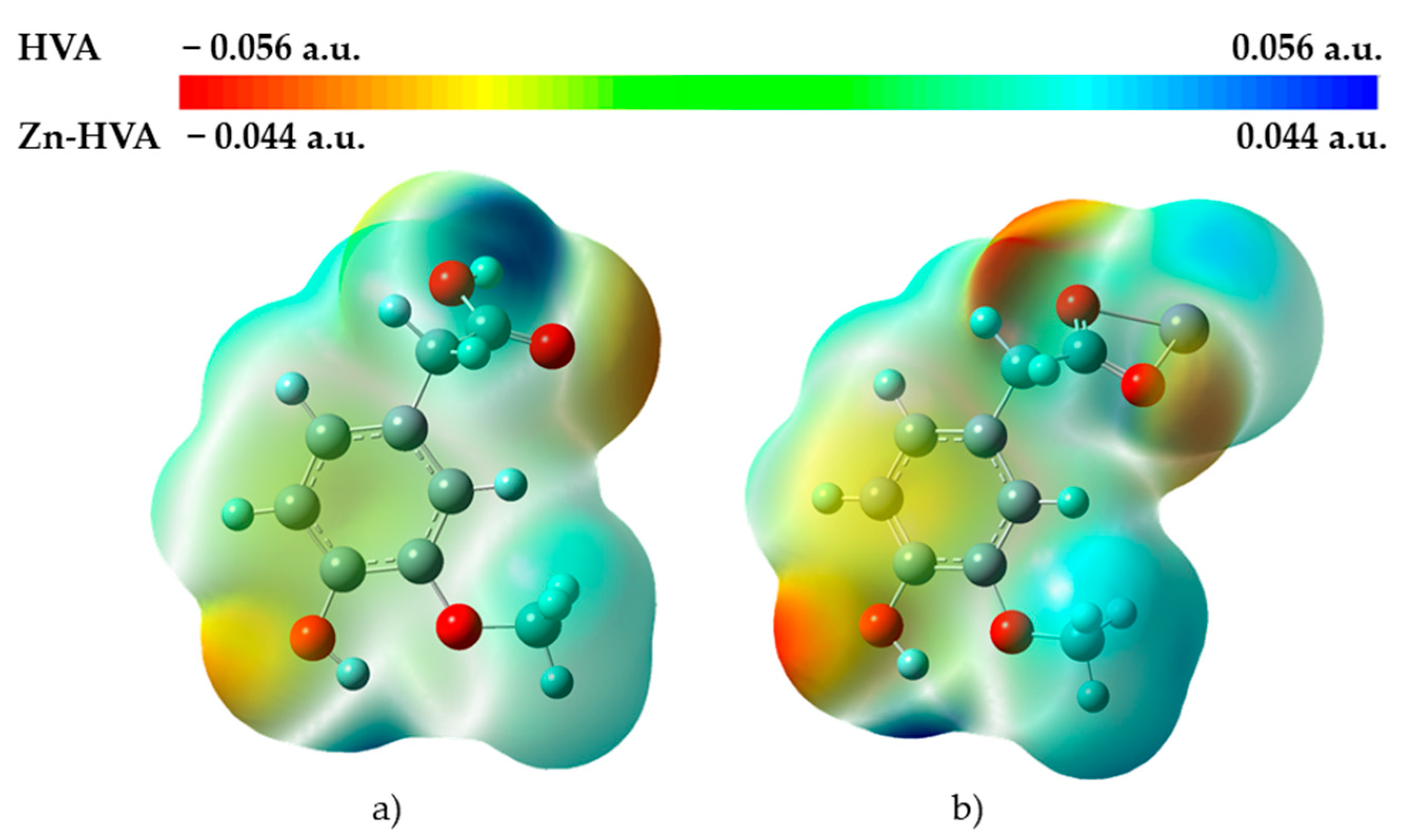
3.1.5. Molecular Electrostatic Potential
3.1.6. Fukui Function (FF) Analysis
3.2. UV-Vis Spectroscopy
3.3. FT-IR Spectroscopy
3.4. NMR
3.5. Elemental and Thermogravimetric Analysis
3.5.1. Elemental Analysis
3.5.2. Thermogravimetric Analysis
3.6. Antioxidant Assays
3.6.1. Reducing Activity Assays
3.6.2. DPPH• Antiradical Activity Assay
3.6.3. ABTS
3.7. Microbiological Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Serreli, G.; Deiana, M. Biological Relevance of Extra Virgin Olive Oil Polyphenols Metabolites. Antioxidants 2018, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- Dimić, D.; Milenković, D.; Dimitrić Marković, J.; Marković, Z. Antiradical Activity of Catecholamines and Metabolites of Dopamine: Theoretical and Experimental Study. Phys. Chem. Chem. Phys. 2017, 19, 12970–12980. [Google Scholar] [CrossRef]
- Mihai, R.A.; Espinoza Caiza, I.A.; Melo Heras, E.J.; Florescu, L.I.; Catana, R.D. Comparative Assessment of Antioxidant Activity and Functional Components of Chionanthus Virginicus and Chionanthus Pubescens from the Andean Region of Ecuador. Pharmaceutics 2023, 15, 1676. [Google Scholar] [CrossRef]
- Tuck, K.L.; Hayball, P.J. Major Phenolic Compounds in Olive Oil: Metabolism and Health Effects. J. Nutr. Biochem. 2002, 13, 636–644. [Google Scholar] [CrossRef]
- Samsonowicz, M.; Kowczyk-Sadowy, M.; Regulska, E.; Lewandowski, W. Molecular Structure and Spectroscopic Analysis of Homovanillic Acid and Its Sodium Salt-NMR, FT-IR and DFT Studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 118, 1068–1074. [Google Scholar] [CrossRef]
- Rodríguez-Morató, J.; Boronat, A.; Kotronoulas, A.; Pujadas, M.; Pastor, A.; Olesti, E.; Pérez-Mañá, C.; Khymenets, O.; Fitó, M.; Farré, M.; et al. Metabolic Disposition and Biological Significance of Simple Phenols of Dietary Origin: Hydroxytyrosol and Tyrosol. Drug Metab. Rev. 2016, 48, 218–236. [Google Scholar] [CrossRef] [PubMed]
- Szekely, G.; Didaskalou, C. 7-Biomimics of Metalloenzymes via Imprinting. In Molecularly Imprinted Catalysts; Li, S., Cao, S., Piletsky, S.A., Turner, A.P.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 121–158. ISBN 978-0-12-801301-4. [Google Scholar]
- Bianco, A.; Uccella, N. Biophenolic Components of Olives. Food Res. Int. 2000, 33, 475–485. [Google Scholar] [CrossRef]
- Floridi, S.; Montanari, L.; Marconi, O.; Fantozzi, P. Determination of Free Phenolic Acids in Wort and Beer by Coulometric Array Detection. J. Agric. Food Chem. 2003, 51, 1548–1554. [Google Scholar] [CrossRef]
- Shan, L.; Tyagi, A.; Ham, H.-J.; Oh, D.H. Uncovering the Antiinflammatory Potential of Lactiplantibacillus Plantarum Fermented Cannabis Sativa L Seeds. NPJ Sci. Food 2024, 8, 42. [Google Scholar] [CrossRef]
- Zhao, M.; Ren, Z.; Zhao, A.; Tang, Y.; Kuang, J.; Li, M.; Chen, T.; Wang, S.; Wang, J.; Zhang, H.; et al. Gut Bacteria-Driven Homovanillic Acid Alleviates Depression by Modulating Synaptic Integrity. Cell Metab. 2024, 36, 1000–1012.e6. [Google Scholar] [CrossRef]
- Ibero-Baraibar, I.; Perez-Cornago, A.; Ramirez, M.J.; Martínez, J.A.; Zulet, M.A. An Increase in Plasma Homovanillic Acid with Cocoa Extract Consumption Is Associated with the Alleviation of Depressive Symptoms in Overweight or Obese Adults on an Energy Restricted Diet in a Randomized Controlled Trial. J. Nutr. 2015, 146, 897S–904S. [Google Scholar] [CrossRef] [PubMed]
- Mihai, R.A.; Cubi-Insuaste, N.S.; Catana, R.D. Biological Activity and Phenolic Content of Kombucha Beverages Under the Influence of Different Tea Extract Substrates. Fermentation 2024, 10, 338. [Google Scholar] [CrossRef]
- Tuck, K.L.; Hayball, P.J.; Stupans, I. Structural Characterization of the Metabolites of Hydroxytyrosol, the Principal Phenolic Component in Olive Oil, in Rats. J. Agric. Food Chem. 2002, 50, 2404–2409. [Google Scholar] [CrossRef] [PubMed]
- Prashanth, M.; Revanasiddappa, H.; Rai, K.; Raveesha, K.; Jayalakshmi, B. Antioxidant and Antibacterial Activity of Ajwain Seed Extract Against Antibiotic Resistant Bacteria and Activity Enhancement by the Addition of Metal Salts. J. Pharm. Res. 2012, 5, 1952–1956. [Google Scholar]
- Samsonowicz, M.; Regulska, E.; Kalinowska, M. Hydroxyflavone Metal Complexes—Molecular Structure, Antioxidant Activity and Biological Effects. Chem.-Biol. Interact. 2017, 273, 245–256. [Google Scholar] [CrossRef]
- Samsonowicz, M.; Regulska, E.; Świsłocka, R.; Butarewicz, A. Molecular Structure and Microbiological Activity of Alkali Metal 3,4-Dihydroxyphenylacetates. J. Saudi Chem. Soc. 2018, 22, 896–907. [Google Scholar] [CrossRef]
- Samsonowicz, M.; Kalinowska, M.; Gryko, K. Enhanced Antioxidant Activity of Ursolic Acid by Complexation with Copper (II): Experimental and Theoretical Study. Materials 2021, 14, 264. [Google Scholar] [CrossRef]
- Świderski, G.; Gołębiewska, E.; Kowalczyk, N.; Kalinowska, M.; Świsłocka, R.; Wołejko, E.; Wydro, U.; Malinowski, P.; Pietryczuk, A.; Cudowski, A.; et al. Structure, Antioxidant Activity and Antimicrobial Study of Light Lanthanide Complexes with p-Coumaric Acid. Materials 2024, 17, 1324. [Google Scholar] [CrossRef]
- Kalinowska, M.; Sienkiewicz-Gromiuk, J.; Świderski, G.; Pietryczuk, A.; Cudowski, A.; Lewandowski, W. Zn(II) Complex of Plant Phenolic Chlorogenic Acid: Antioxidant, Antimicrobial and Structural Studies. Materials 2020, 13, 3745. [Google Scholar] [CrossRef]
- Samsonowicz, M.; Regulska, E.; Kowczyk-Sadowy, M.; Butarewicz, A.; Lewandowski, W. The Study on molecular Structure and Microbiological Activity of Alkali Metal 3-Hydroxyphenylycetates. J. Mol. Struct. 2017, 1146, 755–765. [Google Scholar] [CrossRef]
- Samsonowicz, M.; Gołębiewska, E.; Wołejko, E.; Wydro, U.; Świderski, G.; Zwolińska, J.; Kalinowska, M.; Lewandowski, W. Spectroscopic, Thermal, Microbiological, and Antioxidant Study of Alkali Metal 2-Hydroxyphenylacetates. Materials 2021, 14, 7824. [Google Scholar] [CrossRef] [PubMed]
- Kedare, S.B.; Singh, R.P. Genesis and Development of DPPH Method of Antioxidant Assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian, Inc.: Wallingford, UK, 2016. [Google Scholar]
- Gryko, K.; Kalinowska, M.; Ofman, P.; Choińska, R.; Świderski, G.; Świsłocka, R.; Lewandowski, W. Natural Cinnamic Acid Derivatives: A Comprehensive Study on Structural, Anti/Pro-Oxidant, and Environmental Impacts. Materials 2021, 14, 6098. [Google Scholar] [CrossRef]
- Weinhold, F.; Landis, C.R. Natural Bond Orbitals And Extensions of Localized Bonding Concepts. Chem. Educ. Res. Pract. 2001, 2, 91–104. [Google Scholar] [CrossRef]
- Tang, X.; Wang, L.; Zhang, Y.; Sun, C.; Huang, Z. Enhancing the Antioxidant Potential of ESIPT-Based Naringenin Flavonoids Based on Excited State Hydrogen Bond Dynamics: A Theoretical Study. J. Photochem. Photobiol. B Biol. 2024, 258, 112996. [Google Scholar] [CrossRef]
- Morell, C.; Grand, A.; Toro-Labbé, A. New Dual Descriptor for Chemical Reactivity. J. Phys. Chem. A 2005, 109, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Marković, Z.; Tošović, J.; Milenković, D.; Marković, S. Revisiting the Solvation Enthalpies and Free Energies of the Proton and Electron in Various Solvents. Comput. Theor. Chem. 2016, 1077, 11–17. [Google Scholar] [CrossRef]
- Szeląg, M.; Urbaniak, A.; Bluyssen, H.A.R. A Theoretical Antioxidant Pharmacophore for Natural Hydroxycinnamic Acids. Open Chem. 2015, 13, 000010151520150001. [Google Scholar] [CrossRef]
- Rahman, A. Frontiers in Clinical Drug Research—Hematology; Atta-Ur-Rahman, Ed.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2016; Volume 2. [Google Scholar]
- Amić, A.; Marković, Z.; Dimitrić Marković, J.M.; Milenković, D.; Stepanić, V. Antioxidative Potential of Ferulic Acid Phenoxyl Radical. Phytochemistry 2020, 170, 112218. [Google Scholar] [CrossRef]
- Wałejko, P.; Dobrzycki, Ł.; Ratkiewicz, A.; Socha, P.; Witkowski, S.; Cyrański, M.K. An X-Ray and Natural Bond Orbital (NBO) Structural Study of α-Tocopheryl and 2,2,5,7,8-Pentamethylchroman-6-Yl Succinates. J. Saudi Chem. Soc. 2019, 23, 365–377. [Google Scholar] [CrossRef]
- Förner, W.; Badawi, H.M. Analysis of the vibrational spectra of 3,4-dihydroxy- and 4-hydroxy-3-methoxyphenylacetic acids and the ring breathing mode in their raman spectra. J. Theor. Comput. Chem. 2011, 10, 711–730. [Google Scholar] [CrossRef]
- Costa-de-Oliveira, S.; Rodrigues, A.G. Candida Albicans Antifungal Resistance and Tolerance in Bloodstream Infections: The Triad Yeast-Host-Antifungal. Microorganisms 2020, 8, 154. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Gallardo, M.; Eckhard, U.; Delgado, L.M.; de Roo Puente, Y.J.D.; Hoyos-Nogués, M.; Gil, F.J.; Perez, R.A. Antibacterial Approaches in Tissue Engineering Using Metal Ions and Nanoparticles: From Mechanisms to Applications. Bioact. Mater. 2021, 6, 4470–4490. [Google Scholar] [CrossRef]
- Jafarirad, S.; Mehrabi, M.; Divband, B.; Kosari-Nasab, M. Biofabrication of Zinc Oxide Nanoparticles Using Fruit Extract of Rosa Canina and Their Toxic Potential Against Bacteria: A Mechanistic Approach. Mater. Sci. Eng. C 2016, 59, 296–302. [Google Scholar] [CrossRef]
- Nairn, B.L.; Lonergan, Z.R.; Wang, J.; Braymer, J.J.; Zhang, Y.; Calcutt, M.W.; Lisher, J.P.; Gilston, B.A.; Chazin, W.J.; de Crécy-Lagard, V.; et al. The Response of Acinetobacter Baumannii to Zinc Starvation. Cell Host Microbe 2016, 19, 826–836. [Google Scholar] [CrossRef]
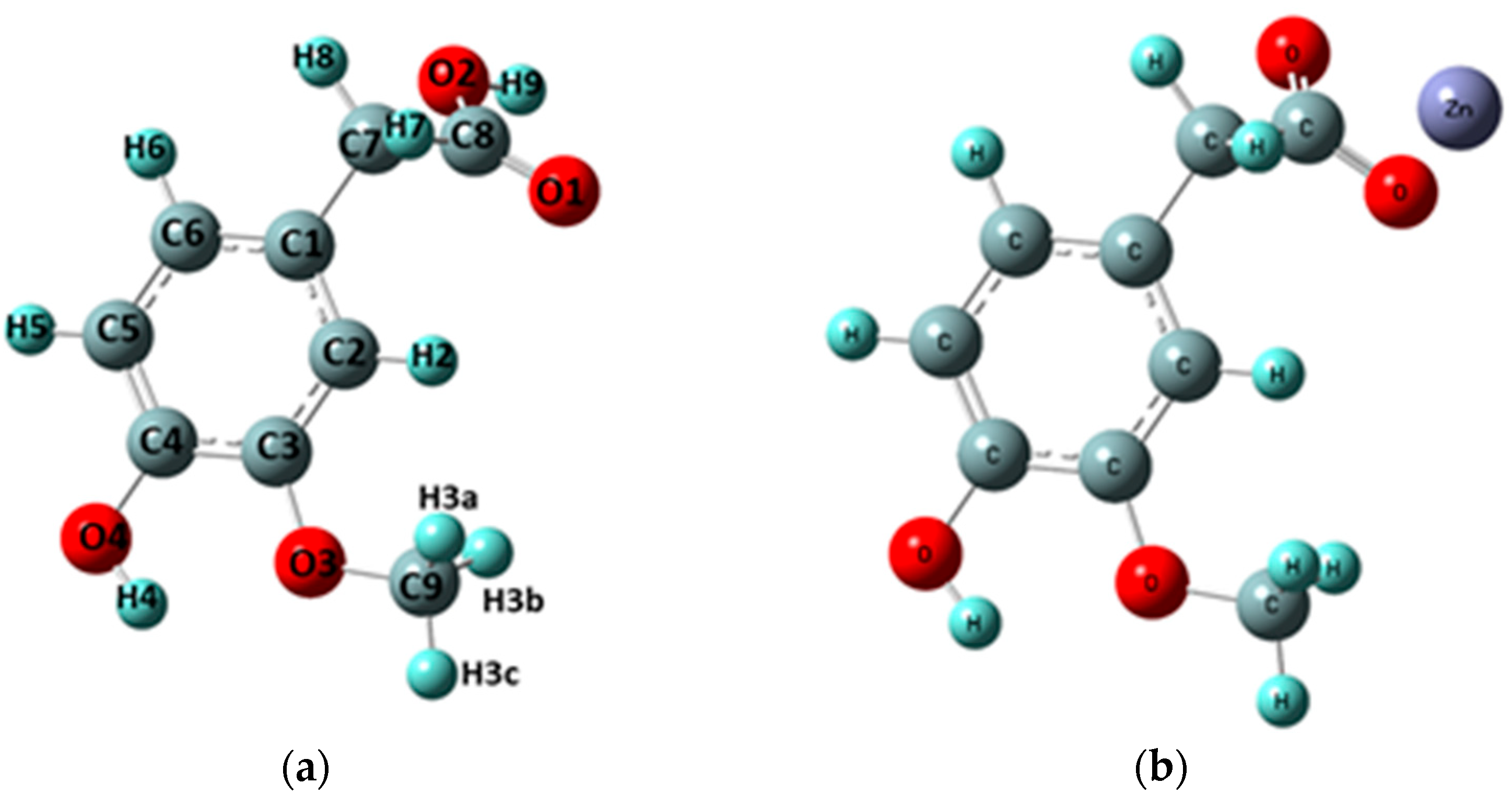



| Atom Numbers 1 | Distance Between Atoms [Å] | |
|---|---|---|
| HVA [5] | Zn-HVA | |
| C1-C7 | 1.521 | 1.519 |
| C7-C8 | 1.515 | 1.520 |
| C8-O1 | 1.207 | 1.272 |
| C8-O2 | 1.354 | 1.269 |
| O1-H9/Zn | 2.291 | 2.128 |
| O2-H9/Zn | 0.970 | 2.12 |
| Bond Angles (°) | ||
| HVA [5] | Zn-HVA | |
| C6-C1-C7 | 120.92 | 120.74 |
| C7-C1-C2 | 119.91 | 120.18 |
| C1-C7-C8 | 111.28 | 111.92 |
| C1-C7-H8 | 109.87 | 110.07 |
| C1-C7-H7 | 110.42 | 110.25 |
| H8-C7-C8 | 109.53 | 108.31 |
| H7-C7-C8 | 106.95 | 107.41 |
| C7-C8-O1 | 125.26 | 119.54 |
| C7-C8-O2 | 112.25 | 120.03 |
| C8-O1-H9/Zn | 55.22 | 88.32 |
| C8-O2-H9/Zn | 107.20 | 88.73 |
| C1-C2-2H | 119.30 | 110.17 |
| Atoms 1 | Charge [e] | |
|---|---|---|
| HVA | Zn-HVA | |
| O1 | −0.608 | −0.767 |
| O2 | −0.685 | −0.750 |
| C2 | −0.272 | −0.273 |
| C3 | −0.270 | −0.268 |
| C7 | −0.483 | −0.468 |
| C8 | 0.815 | 0.804 |
| H3a | 0.177 | 0.173 |
| H3b | 0.173 | 0.175 |
| H7 | 0.231 | 0.228 |
| H9/Zn | 0.482 | 1.333 |
| Parameter | HVA | Zn-HVA |
|---|---|---|
| Chemical reactivity parameters | ||
| Energy (Hartree *) | −650.08 | −2428.81 |
| Dipole moment (Debye) | 1.31 | 2.84 |
| Ionization potential, I = −EHOMO (eV) | 6.018 | 5.999 |
| Electron affinity, A = −A = −ELUMO (eV) | 0.719 | 1.139 |
| Electronegativity, (eV) | 3.369 | 3.569 |
| Electronic chemical potential, (eV) | −3.369 | −3.569 |
| Chemical hardness, (eV) | 2.650 | 2.430 |
| Chemical softness, S (eV) | 0.189 | 0.206 |
| Electrophilicity index, (eV) | 2.141 | 2.620 |
| Thermodynamical parameters (kcal/mol) | ||
| Bond dissociation enthalpy, BDE | 95.16 | 104.31 |
| Ionization potential, IP | 185.46 | 171.54 |
| Proton dissociation enthalpy, PDE | 223.80 | 246.88 |
| Proton affinity, PA | 359.67 | 344.75 |
| Electron transfer enthalpy, ETE | 49.59 | 73.67 |
| Compound | C [wt. %] | H [wt. %] | ||
|---|---|---|---|---|
| Calc | Exp | Calc | Exp | |
| Zn2(C9H8O4)3·2H2O | 45.85 | 45.77 ± 2.01 | 3.99 | 3.57 ± 0.19 |
| Compound | Stage | TG | DTG (DSC) | Process Nature | Mass Loss [wt. %] | Residue | |
|---|---|---|---|---|---|---|---|
| Trange [°C] | Tmax (Peaks) [°C] | Calc | Exp | ||||
| HVA | Decomposition | 165–535 | 261 (265) | Egzo | 100 | 99.5 | - |
| Zn-HVA | Dehydration | 150–232 | 206 | Endo | 5.09 | 4.88 | Zn2C27H24O12 |
| Decomposition | 340–460 | 376 (389) 402 (403) 434 (434) | egzo egzo egzo | 71.89 | 61.72 | ZnO; inorganic residue | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samsonowicz, M.; Kalinowska, M.; Dowbysz, A.; Koronkiewicz, K.; Kukfisz, B.; Pietryczuk, A. Zn Complex with Homovanillic Acid: Theoretical (B3LYP/6-311++G(d,p)), Structural (FT-IR, NMR), Thermal (TG, DTG, and DSC) and Biological (Antioxidant and Antimicrobial) Characteristics. Materials 2025, 18, 2374. https://doi.org/10.3390/ma18102374
Samsonowicz M, Kalinowska M, Dowbysz A, Koronkiewicz K, Kukfisz B, Pietryczuk A. Zn Complex with Homovanillic Acid: Theoretical (B3LYP/6-311++G(d,p)), Structural (FT-IR, NMR), Thermal (TG, DTG, and DSC) and Biological (Antioxidant and Antimicrobial) Characteristics. Materials. 2025; 18(10):2374. https://doi.org/10.3390/ma18102374
Chicago/Turabian StyleSamsonowicz, Mariola, Monika Kalinowska, Adriana Dowbysz, Kamila Koronkiewicz, Bożena Kukfisz, and Anna Pietryczuk. 2025. "Zn Complex with Homovanillic Acid: Theoretical (B3LYP/6-311++G(d,p)), Structural (FT-IR, NMR), Thermal (TG, DTG, and DSC) and Biological (Antioxidant and Antimicrobial) Characteristics" Materials 18, no. 10: 2374. https://doi.org/10.3390/ma18102374
APA StyleSamsonowicz, M., Kalinowska, M., Dowbysz, A., Koronkiewicz, K., Kukfisz, B., & Pietryczuk, A. (2025). Zn Complex with Homovanillic Acid: Theoretical (B3LYP/6-311++G(d,p)), Structural (FT-IR, NMR), Thermal (TG, DTG, and DSC) and Biological (Antioxidant and Antimicrobial) Characteristics. Materials, 18(10), 2374. https://doi.org/10.3390/ma18102374








