Stability Analysis of Polymer Flooding-Produced Liquid in Oilfields Based on Molecular Dynamics Simulation
Abstract
1. Introduction
2. Molecular Model
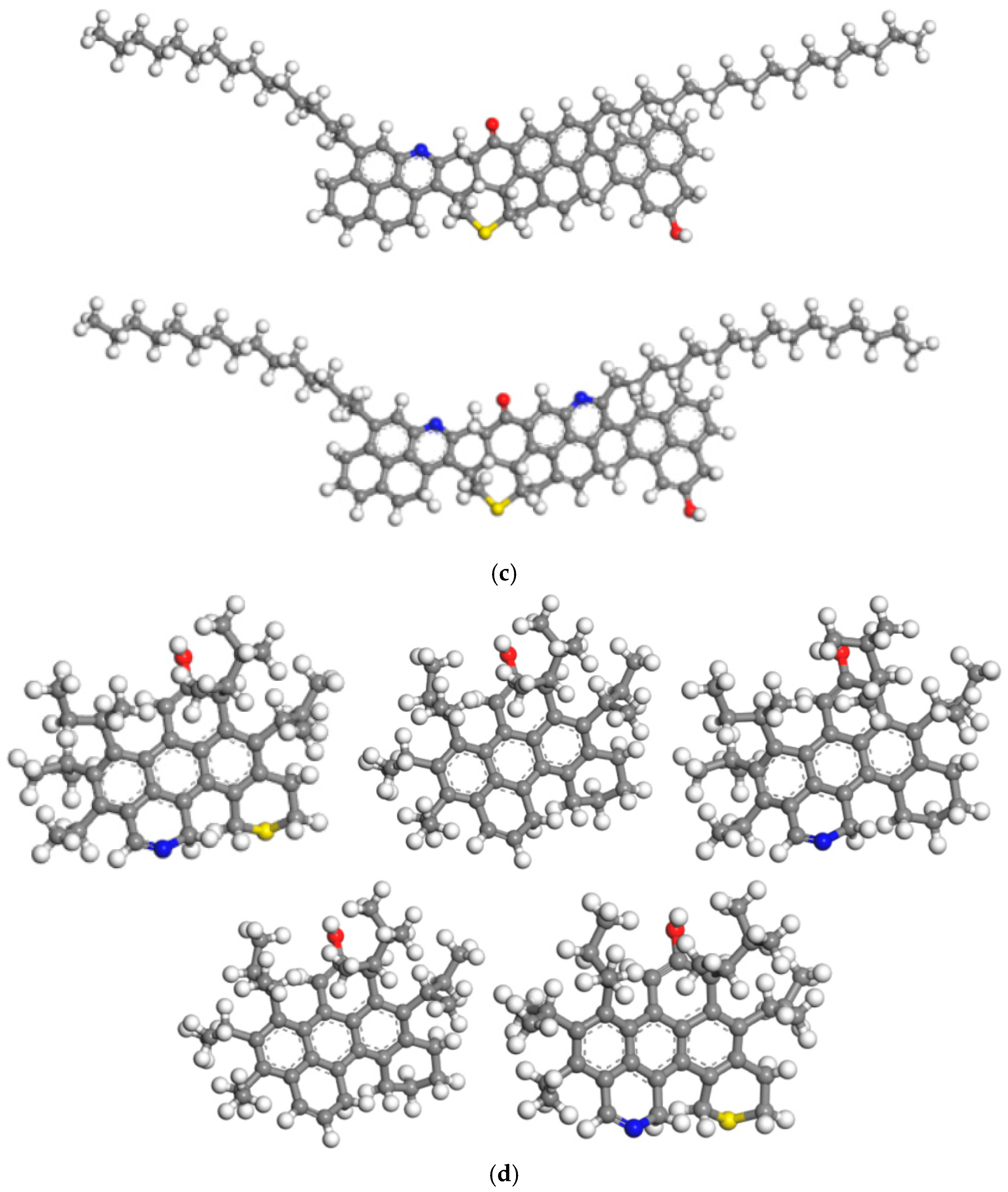
2.1. Molecular Model of Heavy Oil
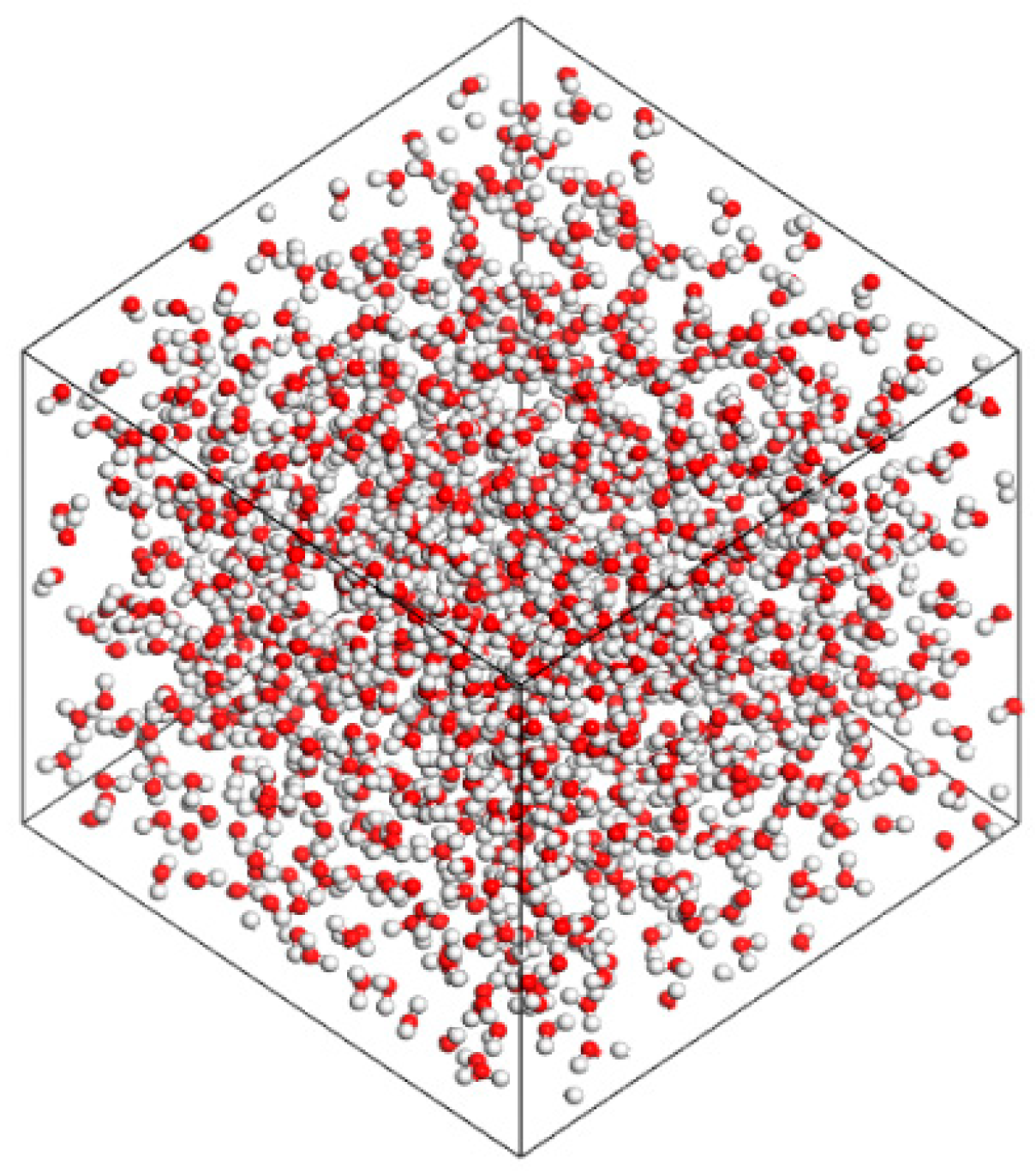
2.2. Establishment of the Mineralized Water Molecular Model
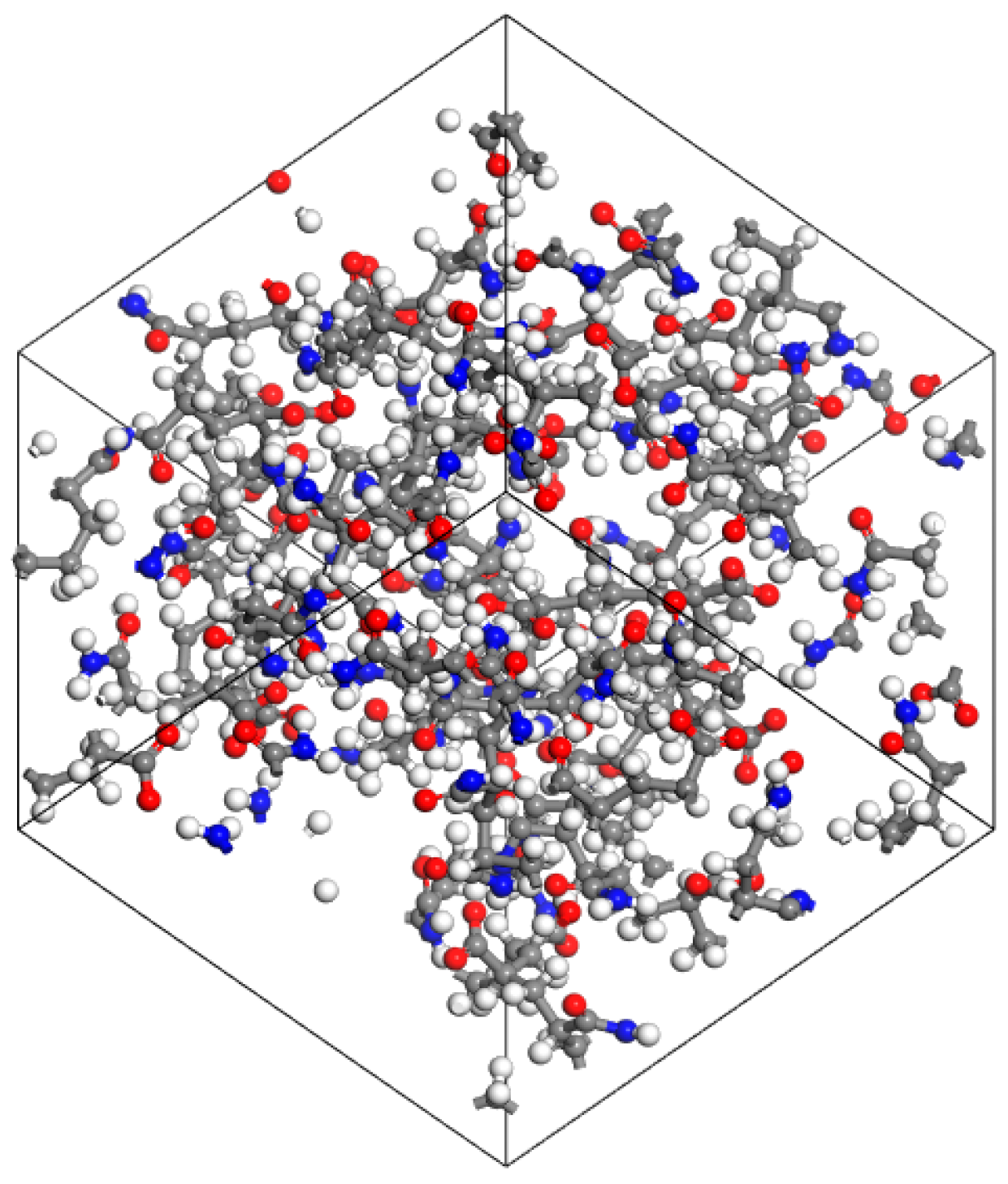
2.3. Polymer Molecular Model
3. Molecular Dynamics Simulation for Stability Analysis of Produced Liquids
3.1. Molecular Dynamics Simulation of Oil–Pure Water System
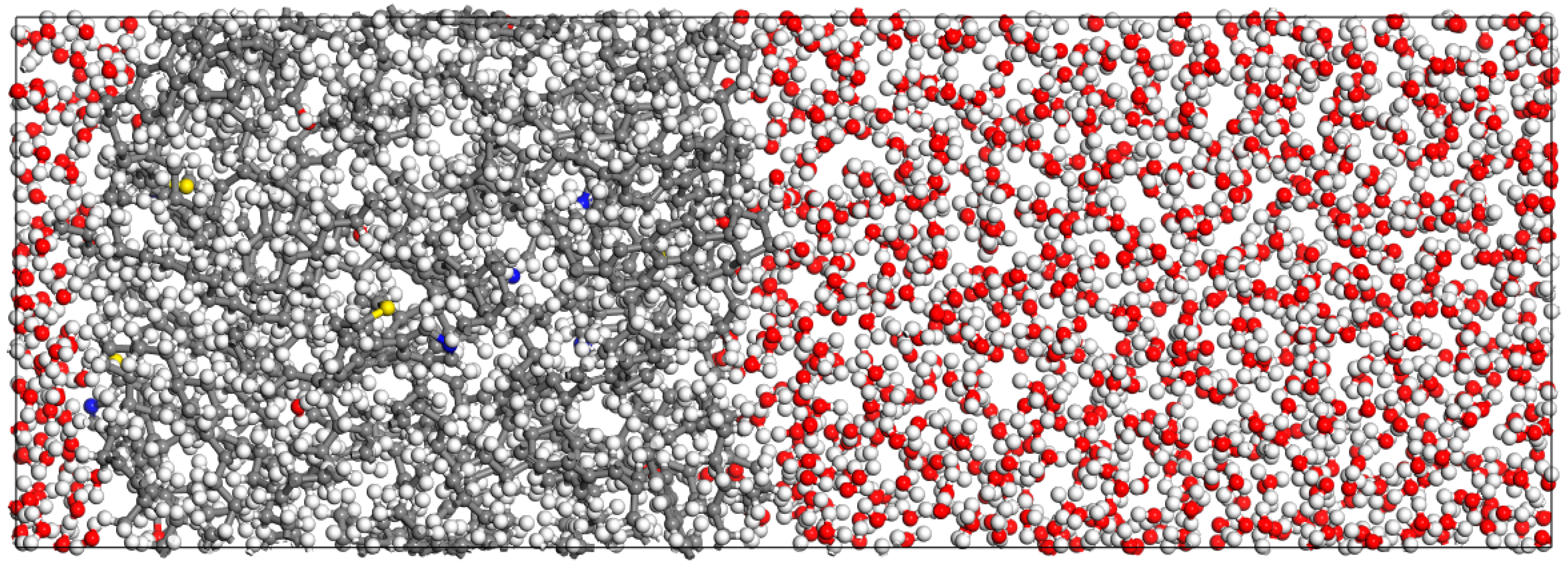
3.1.1. Oil–Pure Water Model Construction
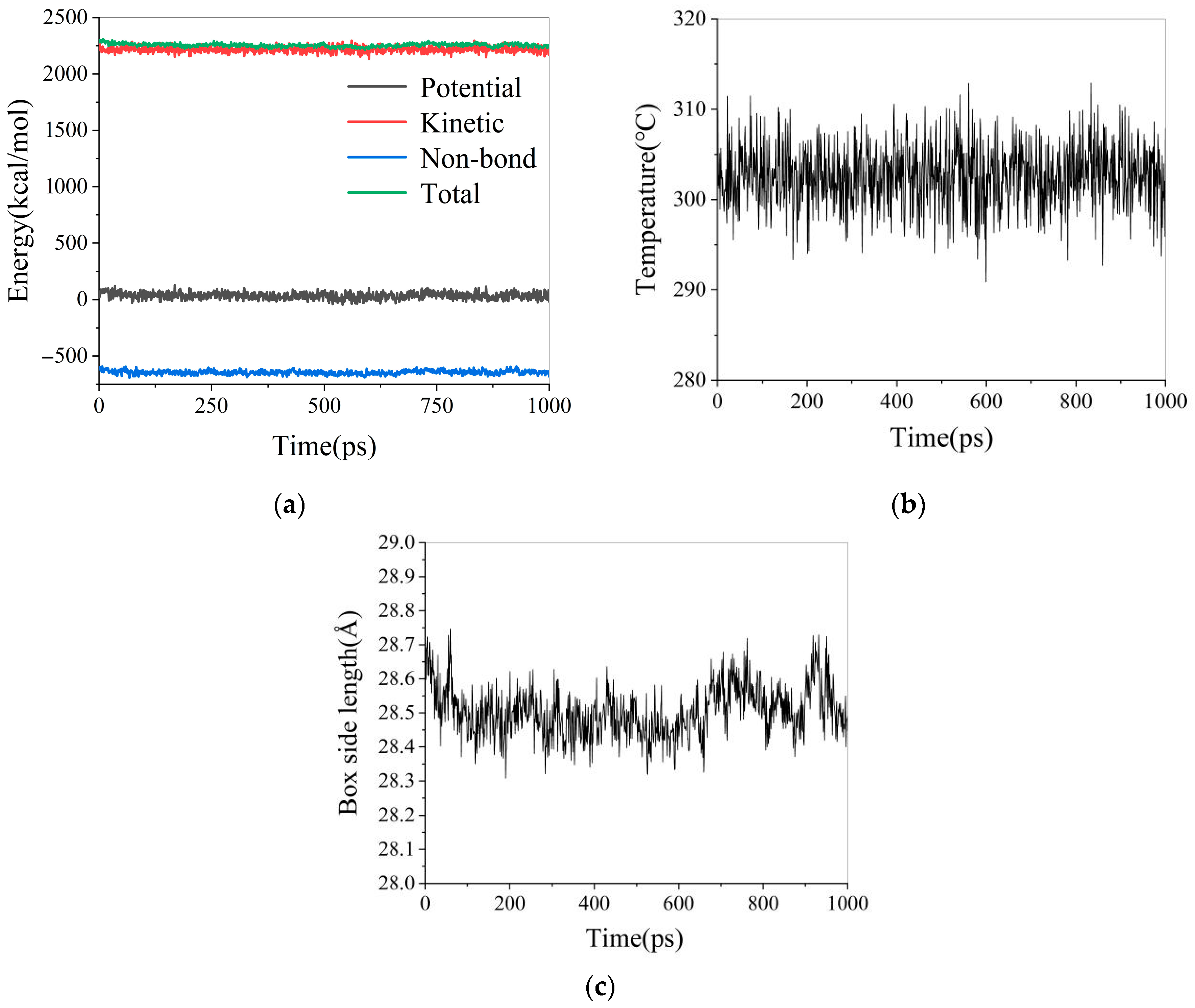
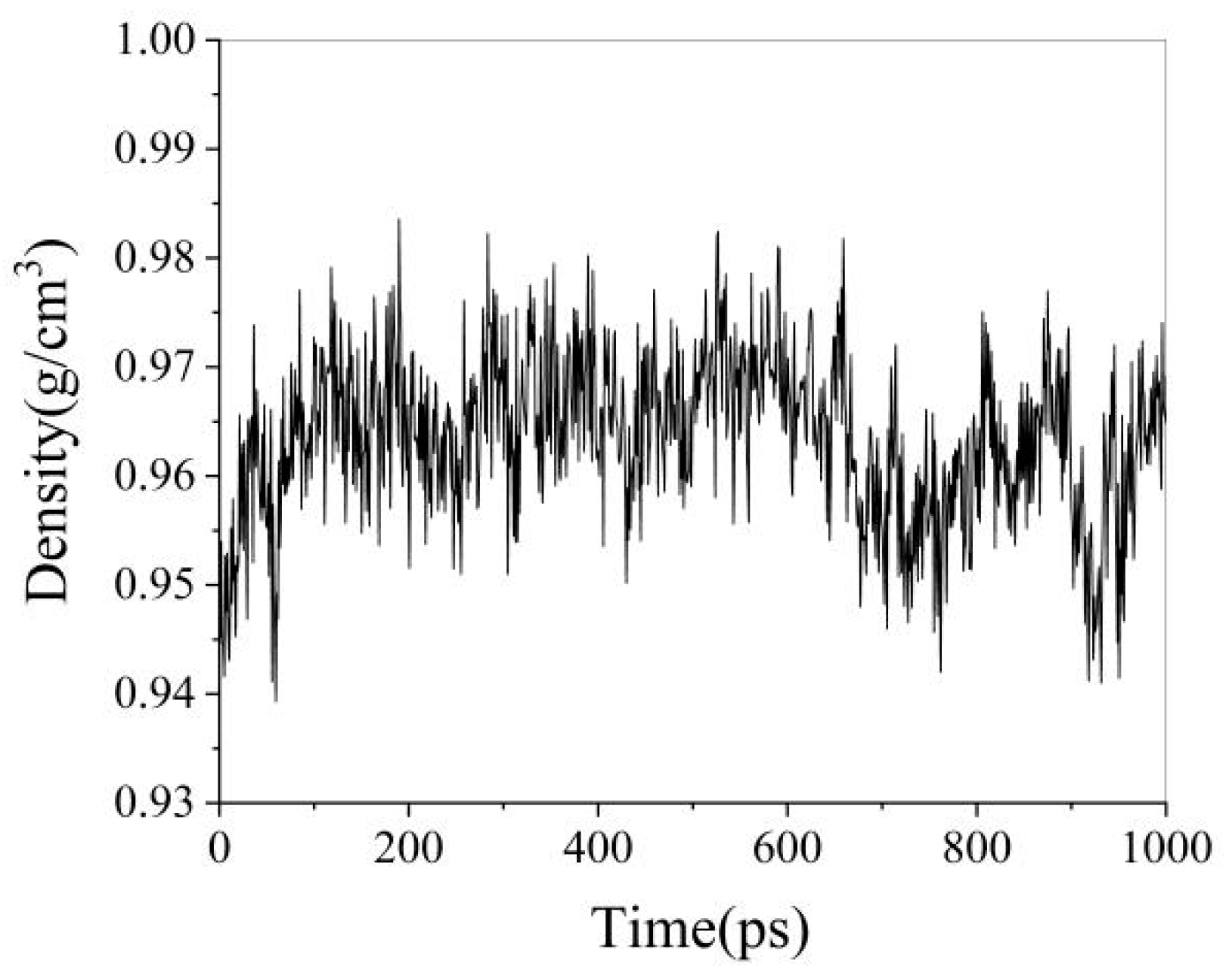
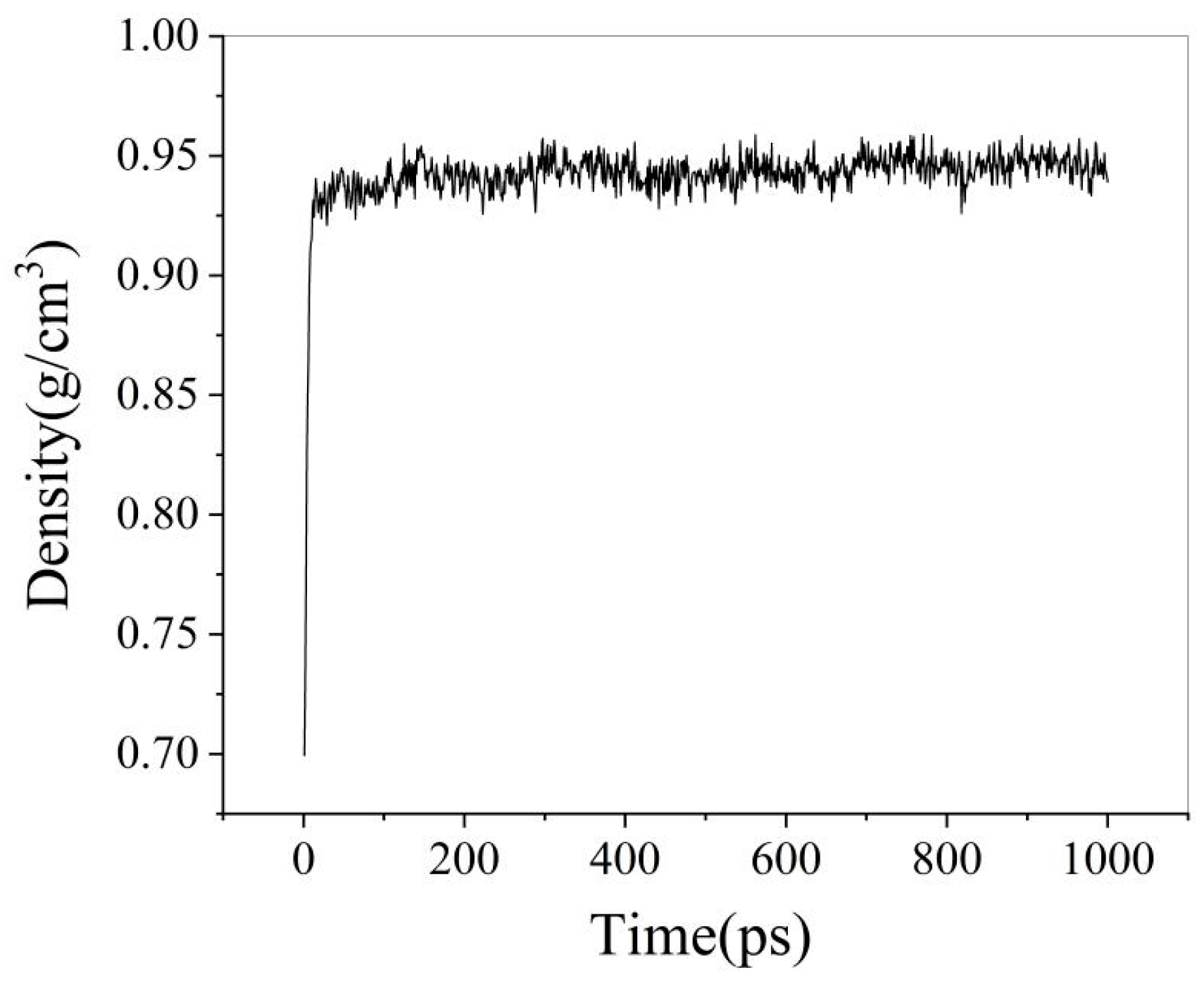
3.1.2. Molecular Dynamics Calculation Process
3.1.3. Analysis of Simulation Results
- Mean square displacement (MSD) and diffusion coefficient of water molecules
- 2.
- Interfacial tension
- 3.
- Interface interaction energy
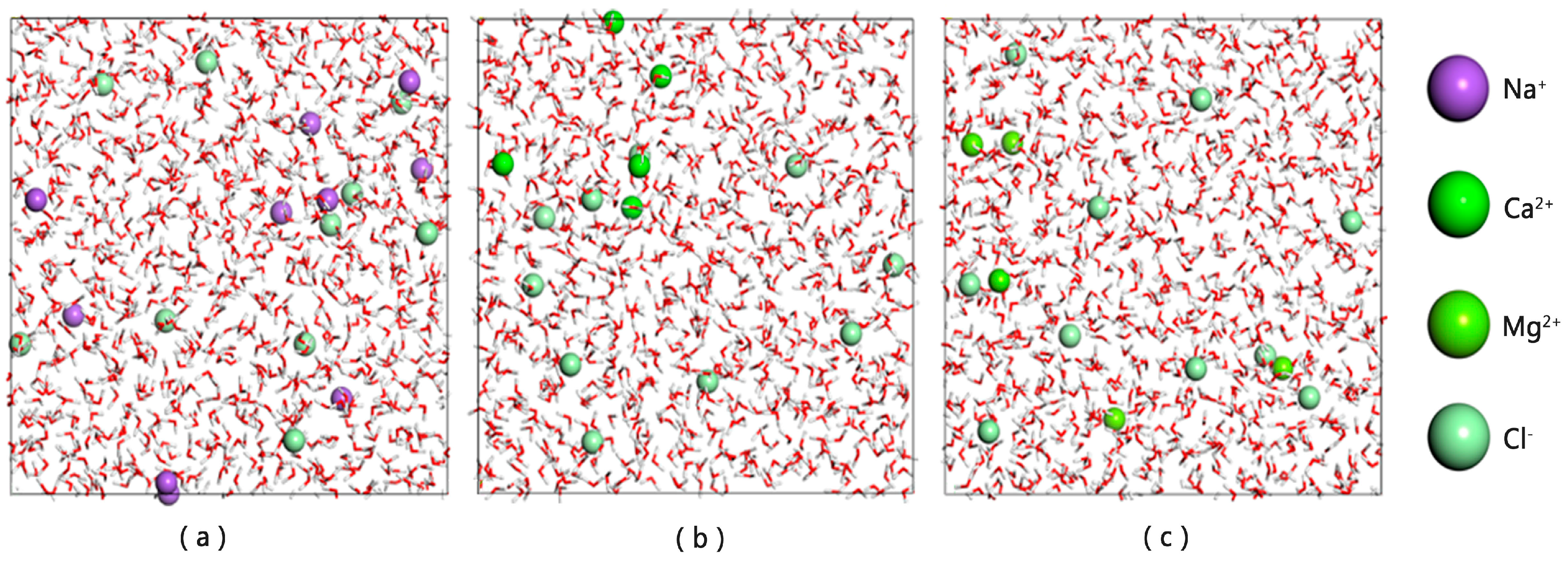
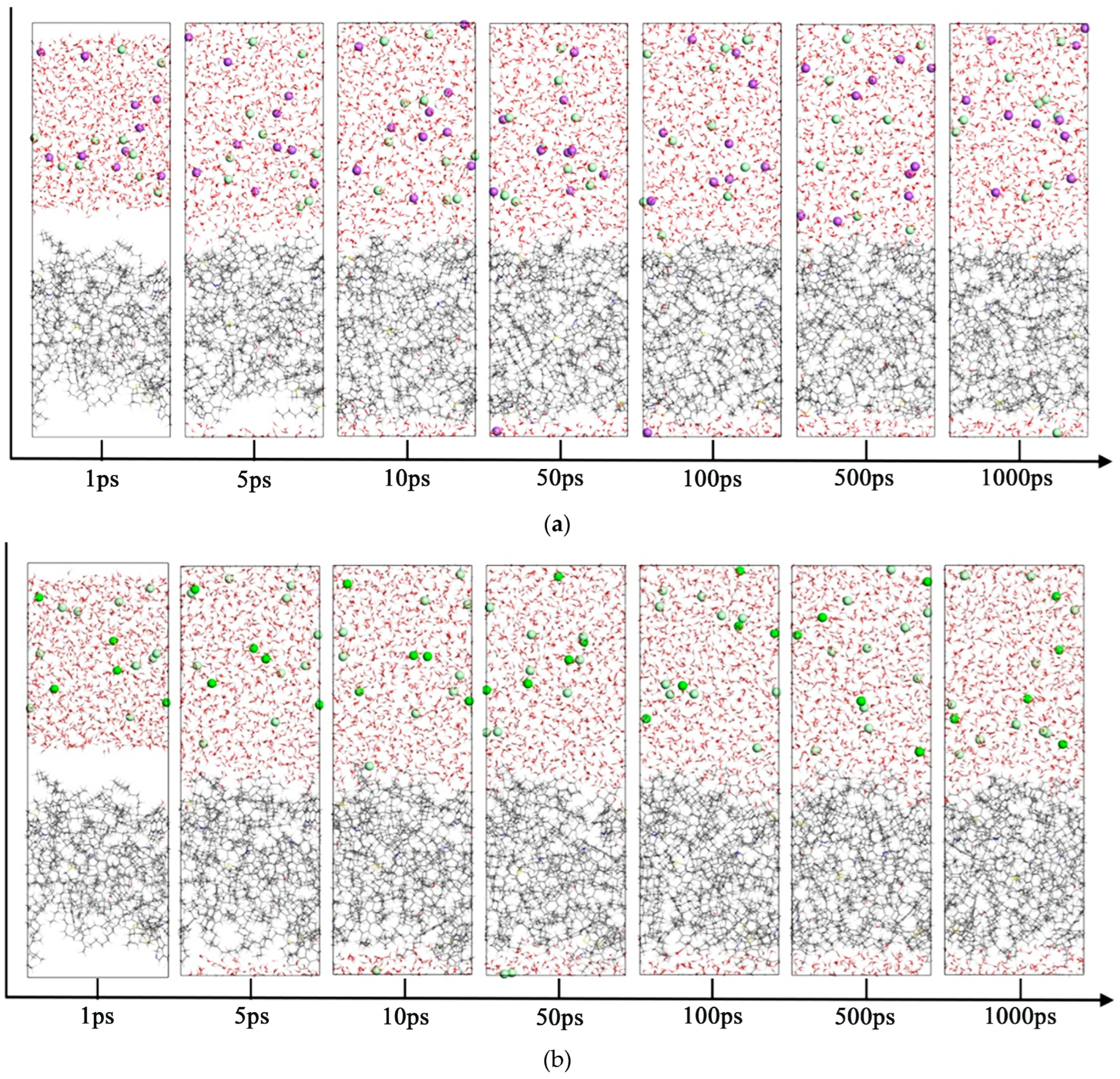
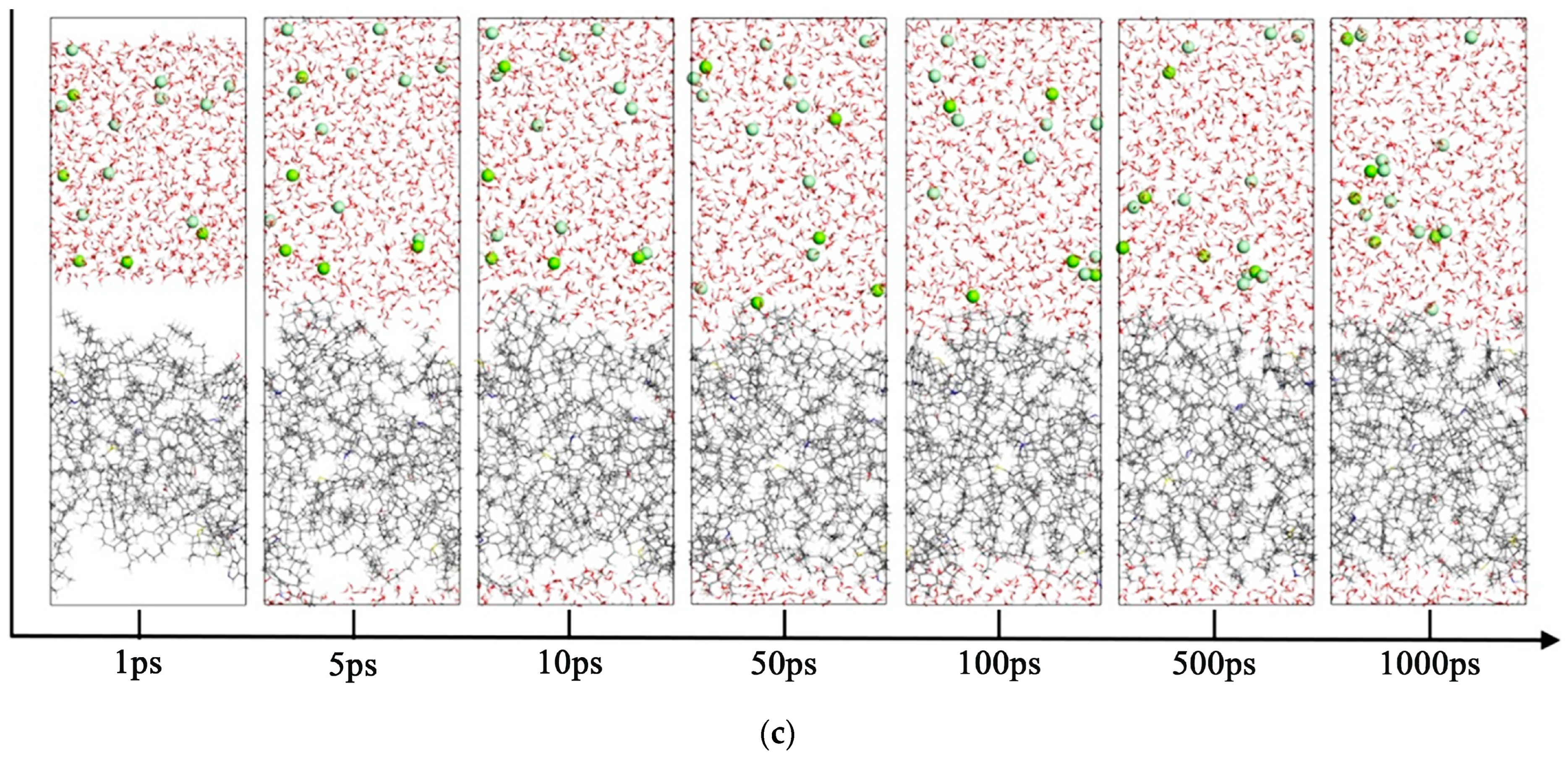
3.2. Molecular Dynamics Simulation of the Oil–Mineralized Water System
- 4.
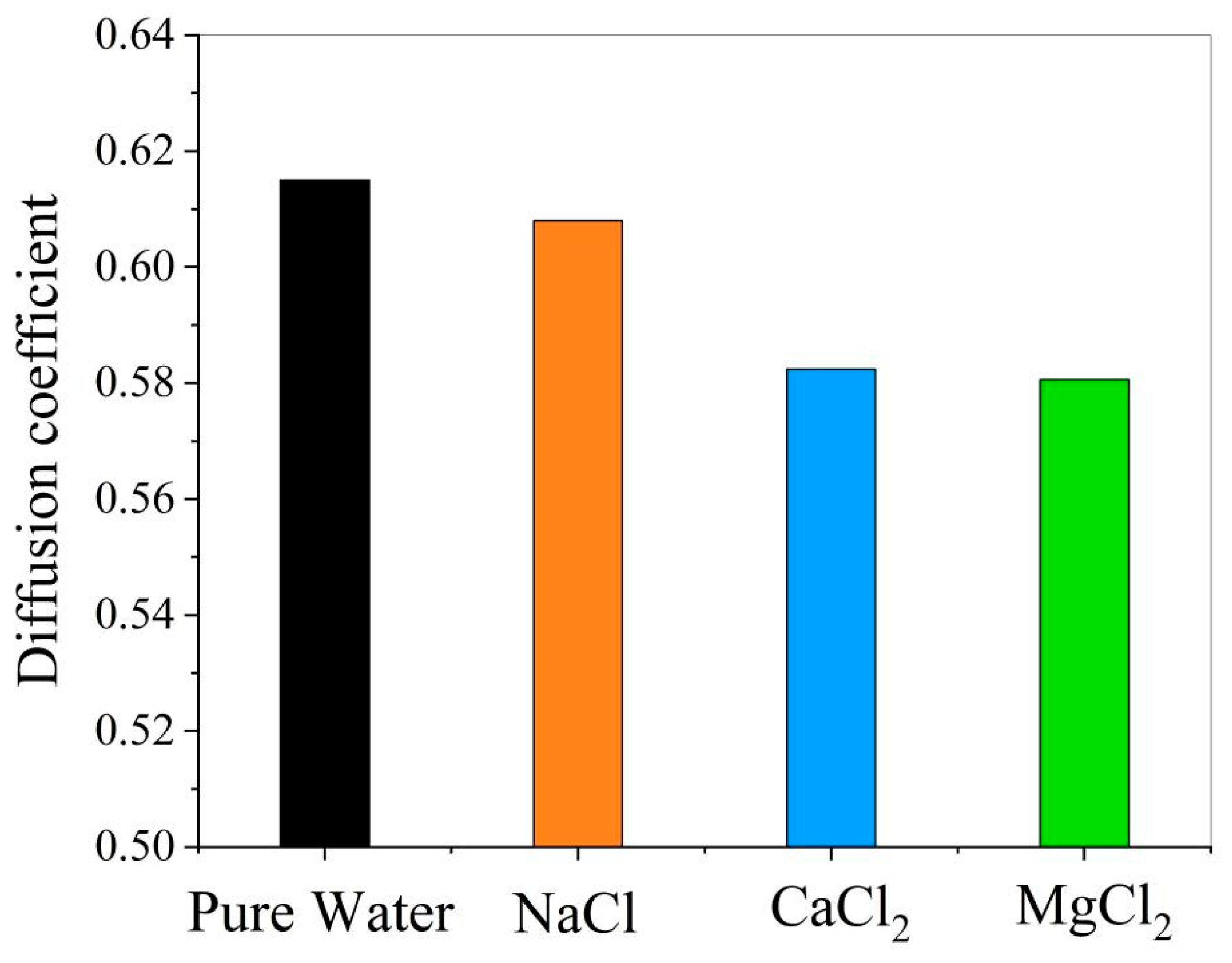
- Effect of ions on the diffusion coefficient of water molecules
- 5.
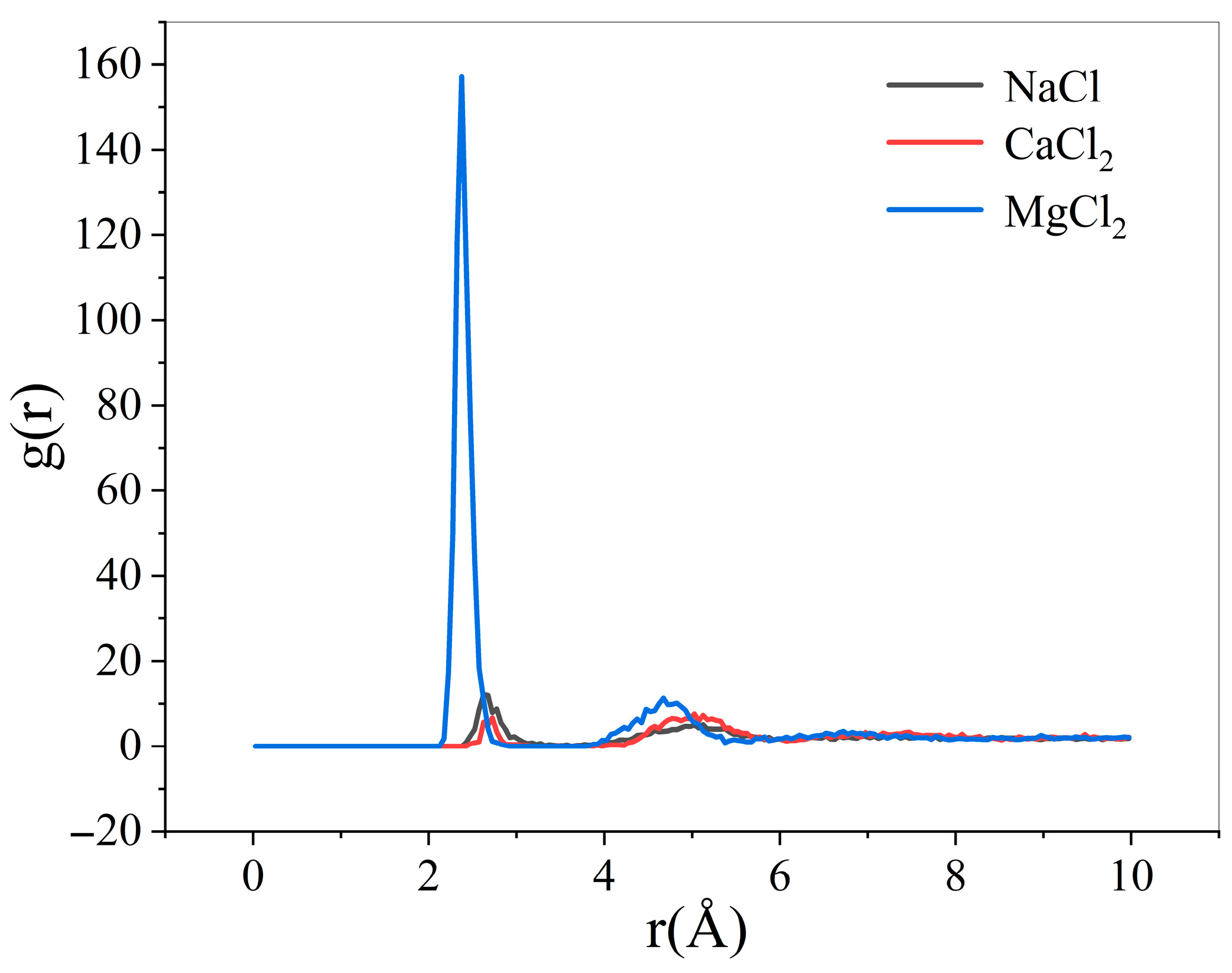
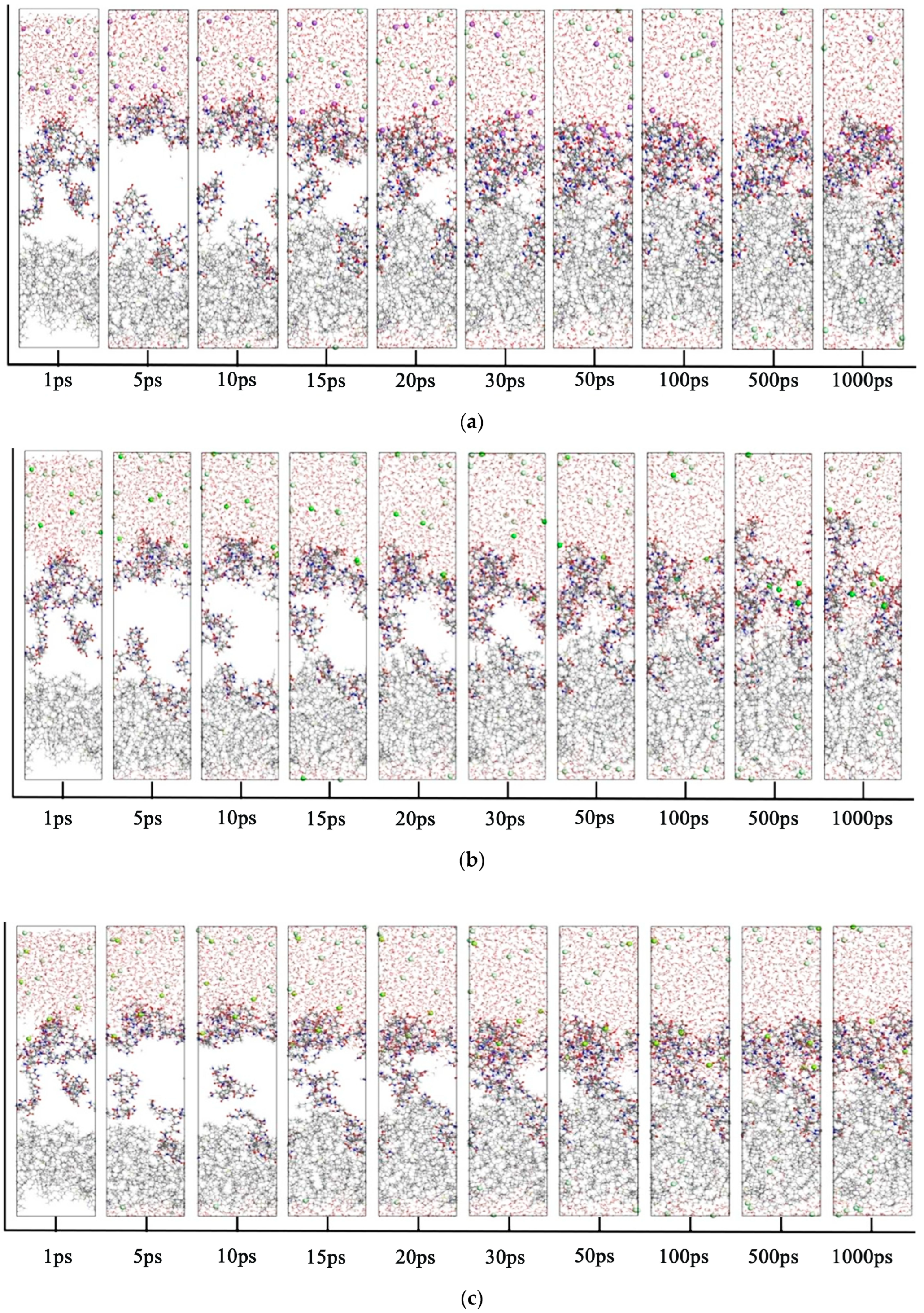
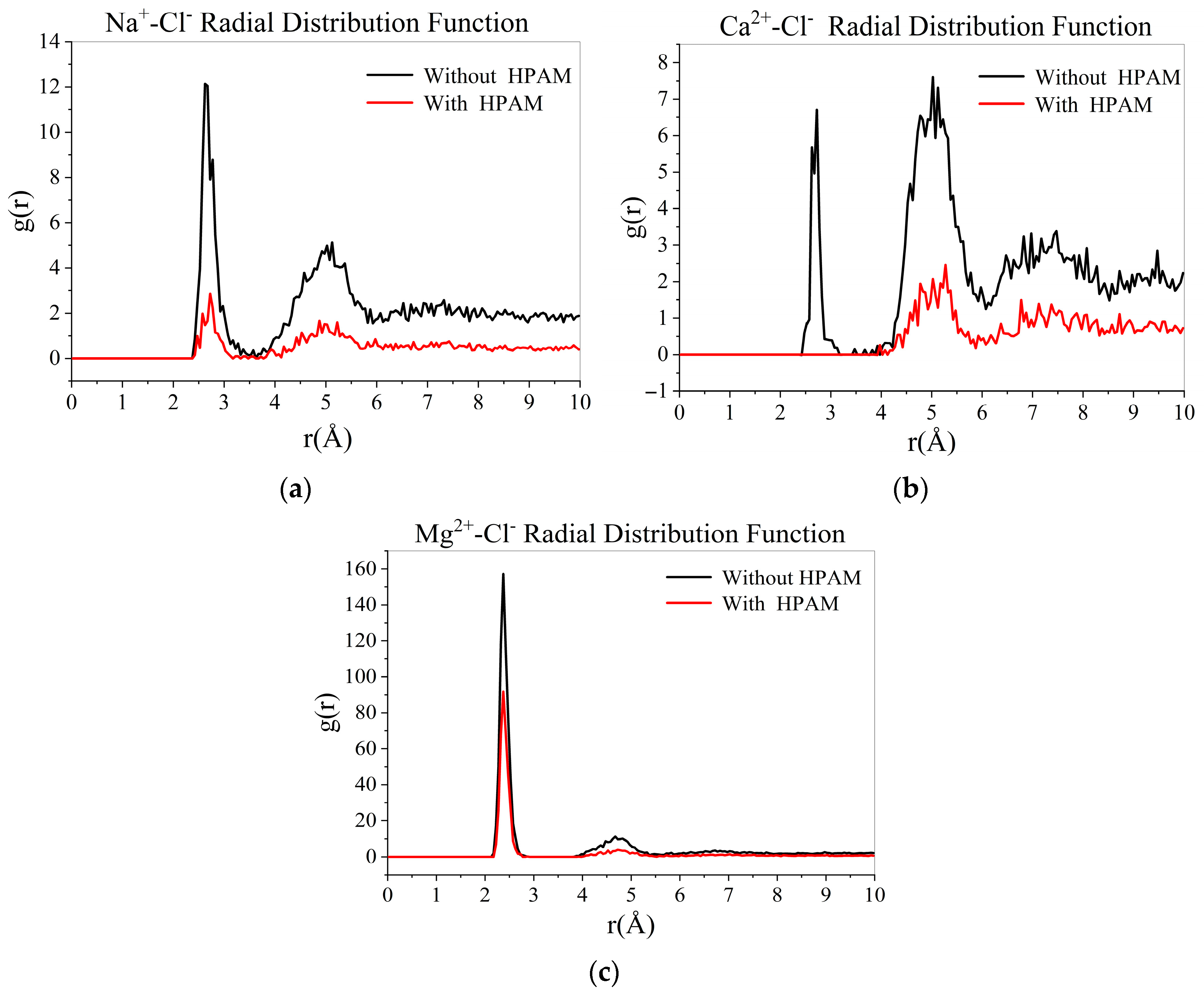
- Comparison of radial distribution of ions in different mineralized water systems
- 6.
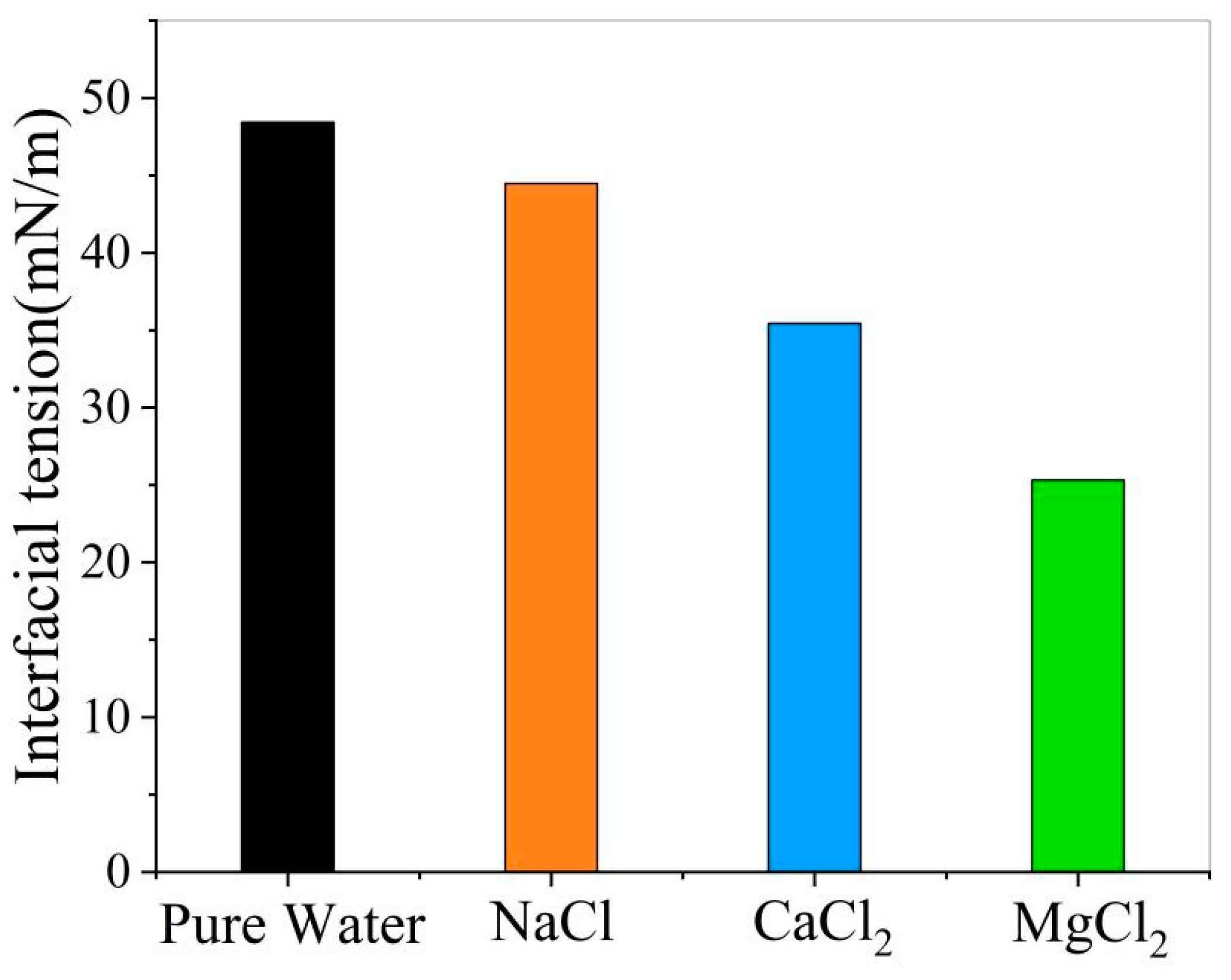
- Effect of ions on the interfacial tension of oil and water
- 7.
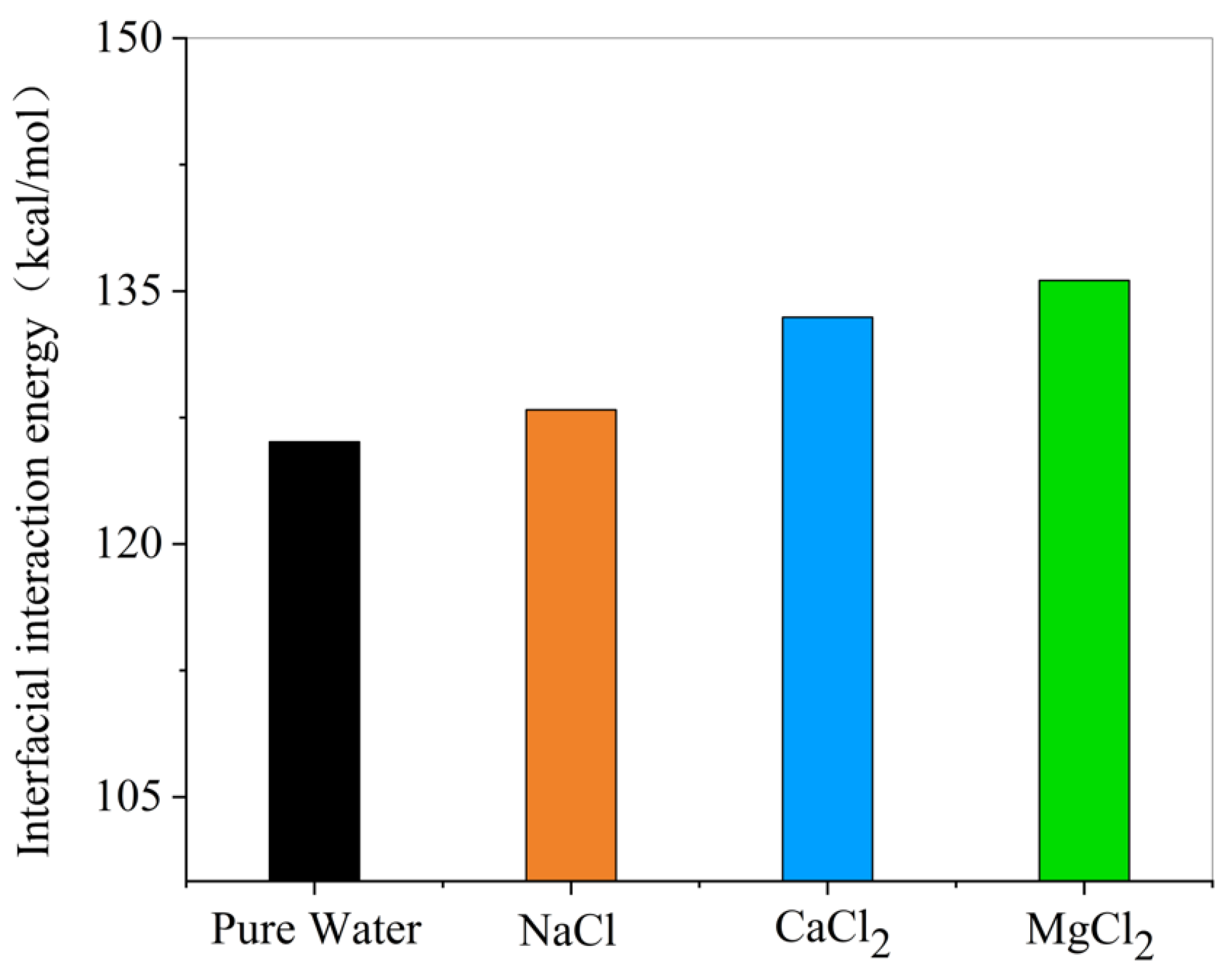
- Effect of ions on interfacial interaction energy
3.3. Molecular Dynamics Simulation of Oil–Polymer–Mineralized Water System
- 8.
- Effect of polymers on the radial distribution of cations in water
- 9.
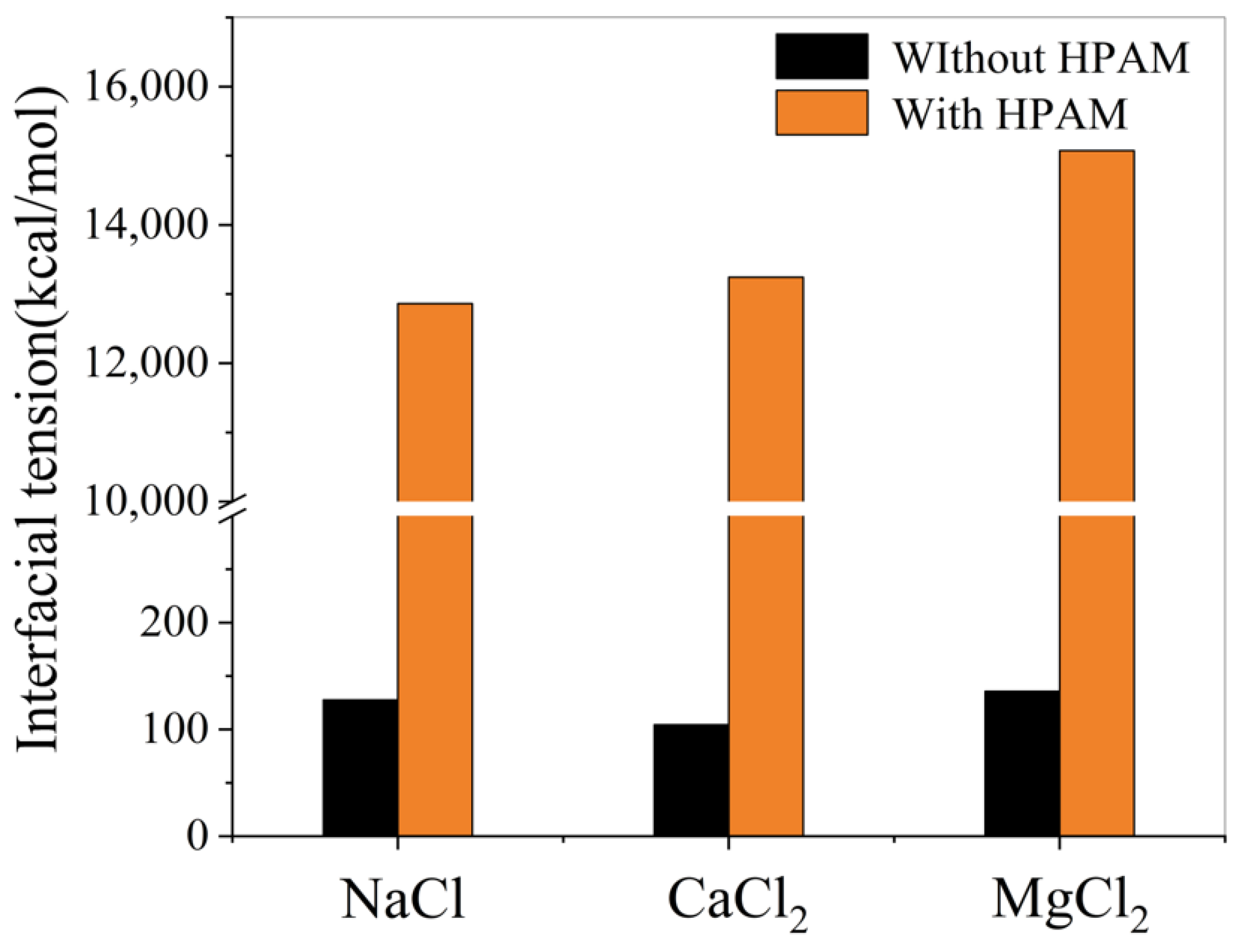
- Effect of polymers on oil–water interfacial tension
- 10.
- Effect of polymers on interfacial interaction energy
4. Conclusions
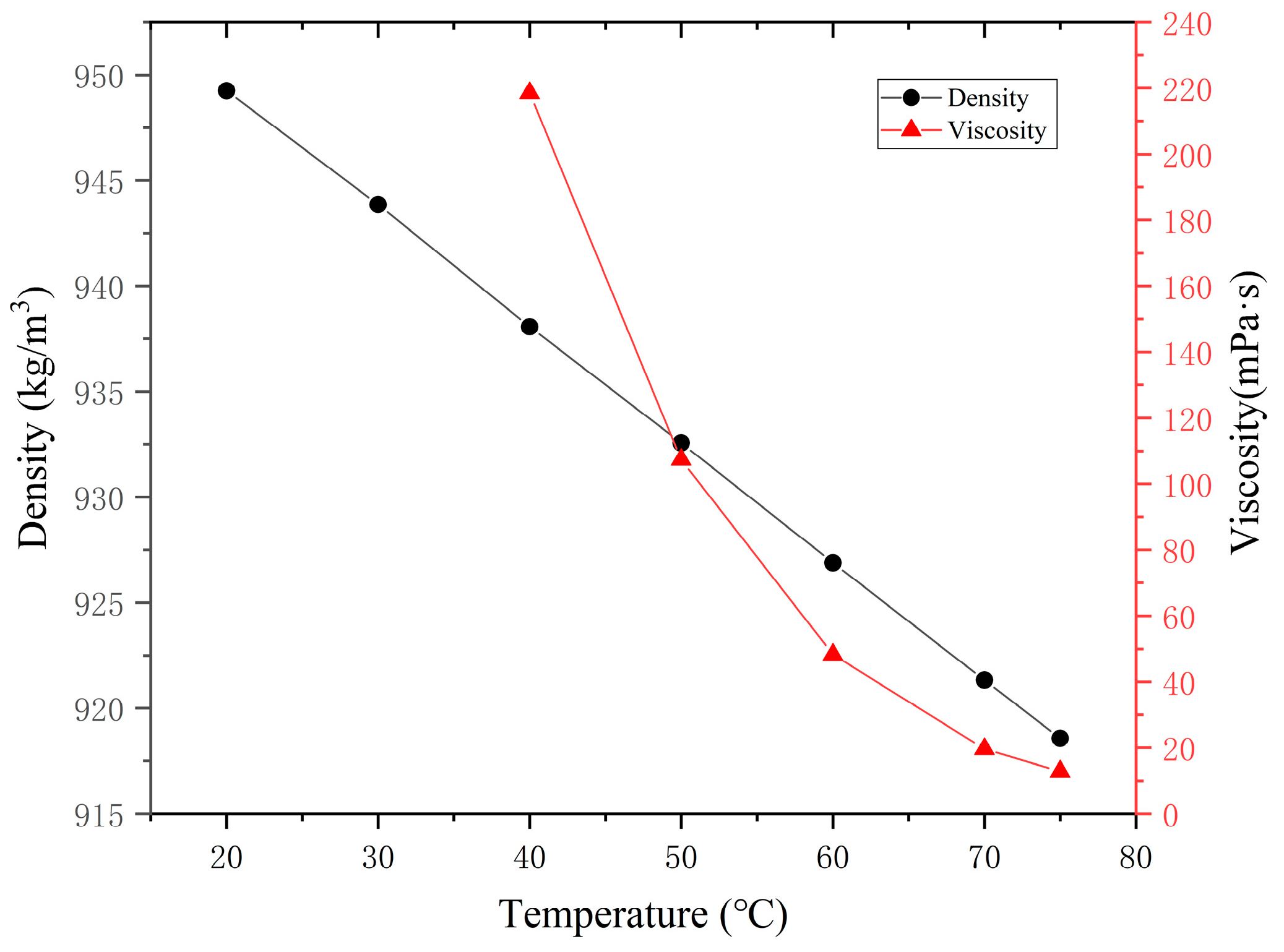
- In molecular modeling, fundamental models of heavy oil, water and polymers were successfully established. The accuracy of these molecular models was verified through comparative analysis between experimental measurements and simulation results. Specifically, the experimental density of heavy oil was determined to be 0.944 g/cm3, while the simulated value yielded 0.958 g/cm3, showing a relative error of only 1.46%. For the water box model, the actual ionic composition of field water samples was incorporated, with particular emphasis on investigating the interfacial effects of cations such as Na+, Ca2+, and Mg2+ at oil–water interfaces.
- The presence of ions (NaCl, CaCl2, MgCl2) reduces oil–water interfacial tension and enhances interfacial interactions, thereby improving emulsion stability. The degree to which cations reduce the interfacial tension between oil and water is Mg2+ > Ca2+ > Na+.
- In the simulation of the oil/polymer/water system, it was observed that the cations in the water attract the anionic groups hydrolyzed from the HPAM polymer chains, thereby enhancing the hydrophilicity of the HPAM polymer. Meanwhile, the lipophilic segments of the polymer dissolve into the heavy oil, forming an interfacial film at the oil–water interface. This film reduces the interfacial tension of the emulsion and improves its stability.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Guo, J.; Li, C.; Xiong, R.; Chen, X.; Zhang, X. Advancements and future prospects in in-situ catalytic technology for heavy oil reservoirs in China: A review. Fuel 2024, 374, 132376. [Google Scholar] [CrossRef]
- Fakher, S.; Ahdaya, M.; Elturki, M.; Imqam, A. Critical review of asphaltene properties and factors impacting its stability in crude oil. J. Pet. Explor. Prod. Technol. 2020, 10, 1183–1200. [Google Scholar] [CrossRef]
- Jia, C. Development challenges and future scientific and technological researches in China’s petroleum industry upstream. Acta Pet. Sin. 2020, 41, 1445. [Google Scholar]
- Liu, X.; Wu, T.; Ge, T.; Du, C.; Wang, D.; Gen, Z. Analysis on in-situ enhanced oil recovery technology for heavy oil and extra-heavy oil. Contemp. Chem. Ind. 2023, 52, 1224–1230+1235. [Google Scholar]
- Cao, X.; Ji, Y.; Zhu, Y.; Zhao, F. Research advance and technology outlook of polymer flooding. Pet. Reserv. Eval. Dev. 2020, 10, 8–16. [Google Scholar]
- Faisal, W.; Almomani, F. A critical review of the development and demulsification processes applied for oil recovery from oil in water emulsions. Chemosphere 2022, 291, 133099. [Google Scholar] [CrossRef]
- Yang, B. Research Progress of Polymer Flooding and Mobility Regulation Mechanism in Heavy Oil Reservoirs. Shandong Chem. Ind. 2023, 52, 87–89. [Google Scholar]
- Liu, B.; Chen, B.; Ling, J.; Matchinski, E.J.; Dong, G.; Ye, X.; Wu, F.; Shen, W.; Liu, L.; Lee, K.; et al. Development of advanced oil/water separation technologies to enhance the effectiveness of mechanical oil recovery operations at sea: Potential and challenges. J. Hazard. Mater. 2022, 437, 129340. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, Z. Biomimetic materials in oil/water separation: Focusing on switchable wettabilities and applications. Adv. Colloid Interface Sci. 2023, 320, 103003. [Google Scholar] [CrossRef]
- Fan, L.; Liu, Y.; Huang, S.; Li, J. Effects of proteins on emulsion stability: The role of proteins at the oil-water interface. Food Chem. 2022, 397, 133726. [Google Scholar]
- Ahmadi, M.; Aliabadian, E.; Liu, B.; Lei, X.; Khalilpoorkordi, P.; Hou, Q.; Wang, Y.; Chen, Z. Comprehensive review of the interfacial behavior of water/oil/surfactant systems using dissipative particle dynamics simulation. Adv. Colloid Interface Sci. 2022, 309, 102774. [Google Scholar] [CrossRef] [PubMed]
- Goual, L.; Zhang, B.; Rahham, Y. Nanoscale characterization of thin films at oil/water interfaces and implications to emulsion stability. Energy Fuels 2020, 35, 444–455. [Google Scholar] [CrossRef]
- Liu, D.; Li, C.; Yang, F.; Sun, G.; You, J.; Cui, K. Synergetic effect of resins and asphaltenes on water/oil interfacial properties and emulsion stability. Fuel 2019, 252, 581–588. [Google Scholar] [CrossRef]
- Ese, M.H.; Kilpatrick, P.K. Stabilization of water-in-oil emulsions by naphthenic acids and their salts: Model compounds, role of pH, and soap: Acid ratio. J. Dispers. Sci. Technol. 2004, 25, 253–261. [Google Scholar] [CrossRef]
- Mohamed, A.I.A.; Sultan, A.S.; Hussein, I.A.; Al-Muntasheri, G.A. Influence of surfactant structure on the stability of water-in-oil emulsions under high-temperature high-salinity conditions. J. Chem. 2017, 2017, 5471376. [Google Scholar] [CrossRef]
- Prašnikar, E.; Ljubič, M.; Perdih, A.; Borišek, J. Machine learning heralding a new development phase in molecular dynamics simulations. Artif. Intell. Rev. 2024, 57, 102. [Google Scholar] [CrossRef]
- Rogel, E. Studies on asphaltene aggregation via computational chemistry. Colloids Surf. A Physicochem. Eng. Asp. 1995, 104, 85–93. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.; Ren, S. Molecular dynamics simulation of self-aggregation of asphaltenes at an oil/water interface: Formation and destruction of the asphaltene protective film. Energy Fuels 2015, 29, 1233–1242. [Google Scholar] [CrossRef]
- Lan, T.; Zeng, H.; Tang, T. Molecular dynamics study on the mechanism of graphene oxide to destabilize oil/water emulsion. J. Phys. Chem. C 2019, 123, 22989–22999. [Google Scholar] [CrossRef]
- Sun, H.; Li, X.; Liu, D.; Li, X. Synergetic adsorption of asphaltenes and oil displacement surfactants on the oil-water interface: Insights on stabilization mechanism of the interfacial film. Chem. Eng. Sci. 2021, 245, 116850. [Google Scholar] [CrossRef]
- Salem, K.G.; Salem, A.M.; Tantawy, M.A.; Gawish, A.A.; Gomaa, S.; El-hoshoudy, A.E. A comprehensive investigation of nanocomposite polymer flooding at reservoir conditions: New insights into enhanced oil recovery. J. Polym. Environ. 2024, 32, 5915–5935. [Google Scholar] [CrossRef]
- SH/T 0509-2010; Test Method for Separation of Asphalt into Four Fractions. National Energy Administration: Beijing, China, 2010.
- Cao, Y. Study on Molecular Structure Characteristics of Fractions and Catalytic Aquathermolysis of Shengli Heavy Oil. Ph.D. Thesis, China University of Petroleum (East China), Qingdao, China, 2017. [Google Scholar]
- Kunieda, M.; Nakaoka, K.; Liang, Y.; Miranda, C.R.; Ueda, A.; Takahashi, S.; Okabe, H.; Matsuoka, T. Self-accumulation of aromatics at the oil-water interface through weak hydrogen bonding. Am. Chem. Soc. 2010, 132, 18281–18286. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.D.; Chen, J.C.; Feng, H.; Yang, Y.C. Molecular dynamics simulation for microstructure of cold-drawn Cu-Sn alloy conductor. Mater. Mech. Eng. 2012, 36, 88–91+101. [Google Scholar] [CrossRef]








| Composition | Mass Fraction (%) |
|---|---|
| Saturated fraction | 26.6 |
| Aromatic hydrocarbons | 35.2 |
| Resins | 18.1 |
| Asphaltenes | 20.1 |
| Composition | Quantity | Mass Fraction (%) |
|---|---|---|
| Saturates | 6 | 26.22 |
| Aromatics | 5 | 34.74 |
| Asphaltenes1 | 1 | 4.44 |
| Asphaltenes2 | 1 | 4.29 |
| Asphaltenes3 | 1 | 4.30 |
| Asphaltenes4 | 1 | 4.29 |
| Asphaltenes5 | 1 | 4.44 |
| Resins1 | 1 | 8.64 |
| Resins2 | 1 | 8.65 |
| Pxx (GPa) | Pyy (GPa) | Pzz (GPa) | Lz (Å) | Interfacial Tension (mN/m) |
|---|---|---|---|---|
| −0.036 | −0.037 | −0.031 | 84.721 | 48.439 |
| EA−B (kcal/mol) | EA (kcal/mol) | EB (kcal/mol) | Eint (kcal/mol) |
|---|---|---|---|
| −7689.527 | 206.521 | −7769.993 | −126.055 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Q.; Shen, M.; Mu, L.; Tian, Y.; Huang, H.; Long, X. Stability Analysis of Polymer Flooding-Produced Liquid in Oilfields Based on Molecular Dynamics Simulation. Materials 2025, 18, 2349. https://doi.org/10.3390/ma18102349
Huang Q, Shen M, Mu L, Tian Y, Huang H, Long X. Stability Analysis of Polymer Flooding-Produced Liquid in Oilfields Based on Molecular Dynamics Simulation. Materials. 2025; 18(10):2349. https://doi.org/10.3390/ma18102349
Chicago/Turabian StyleHuang, Qian, Mingming Shen, Lingyan Mu, Yuan Tian, Huirong Huang, and Xueyuan Long. 2025. "Stability Analysis of Polymer Flooding-Produced Liquid in Oilfields Based on Molecular Dynamics Simulation" Materials 18, no. 10: 2349. https://doi.org/10.3390/ma18102349
APA StyleHuang, Q., Shen, M., Mu, L., Tian, Y., Huang, H., & Long, X. (2025). Stability Analysis of Polymer Flooding-Produced Liquid in Oilfields Based on Molecular Dynamics Simulation. Materials, 18(10), 2349. https://doi.org/10.3390/ma18102349






