Unveiling the Nitrogen-Doping Mechanism in Carbon Catalysts for Oxidative Dehydrogenation of Ethanol to Acetaldehyde
Abstract
1. Introduction
2. Experiment Section
3. Results and Discussion
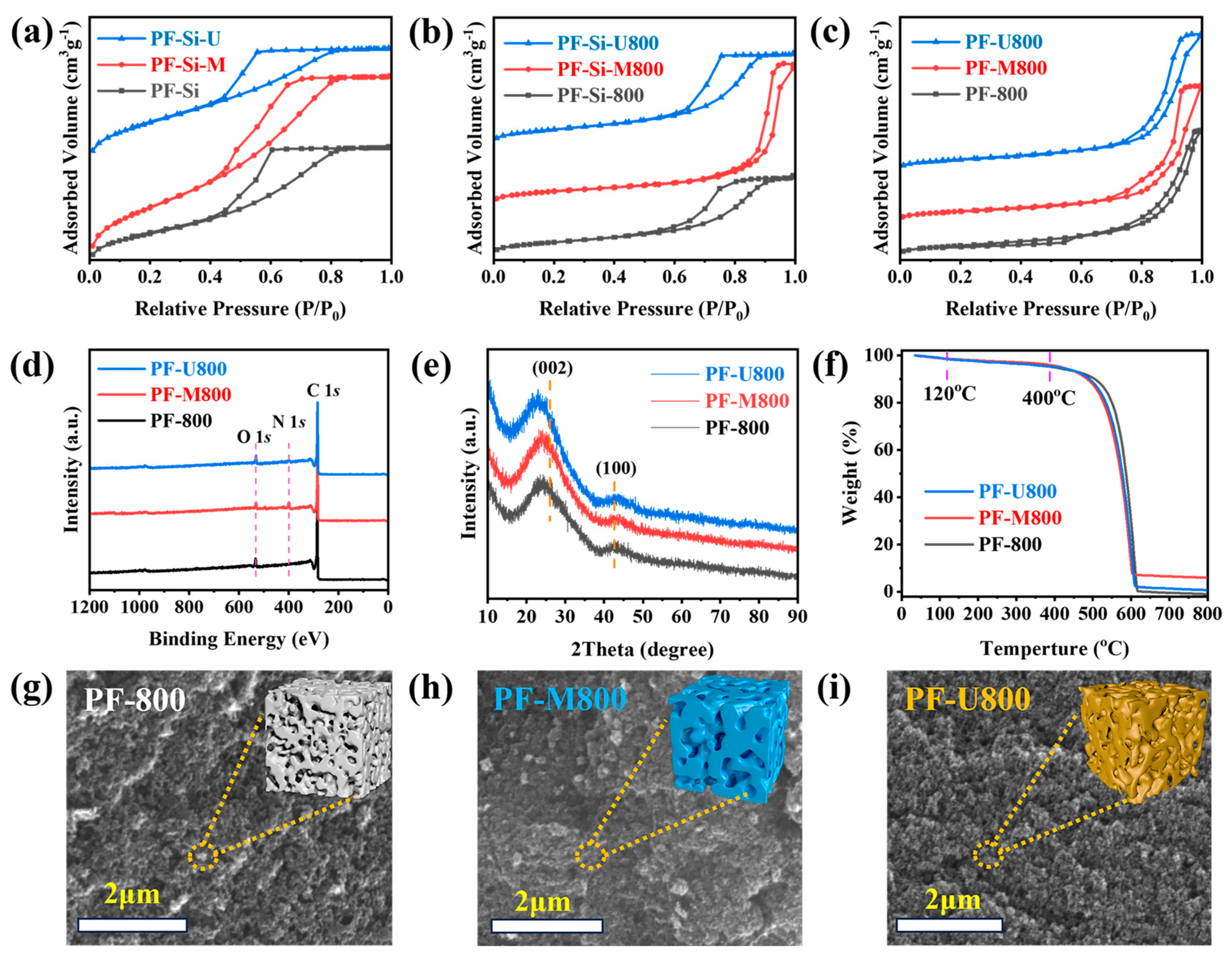
3.1. Synthesis and Characterization of N-Doped Carbon Catalysts
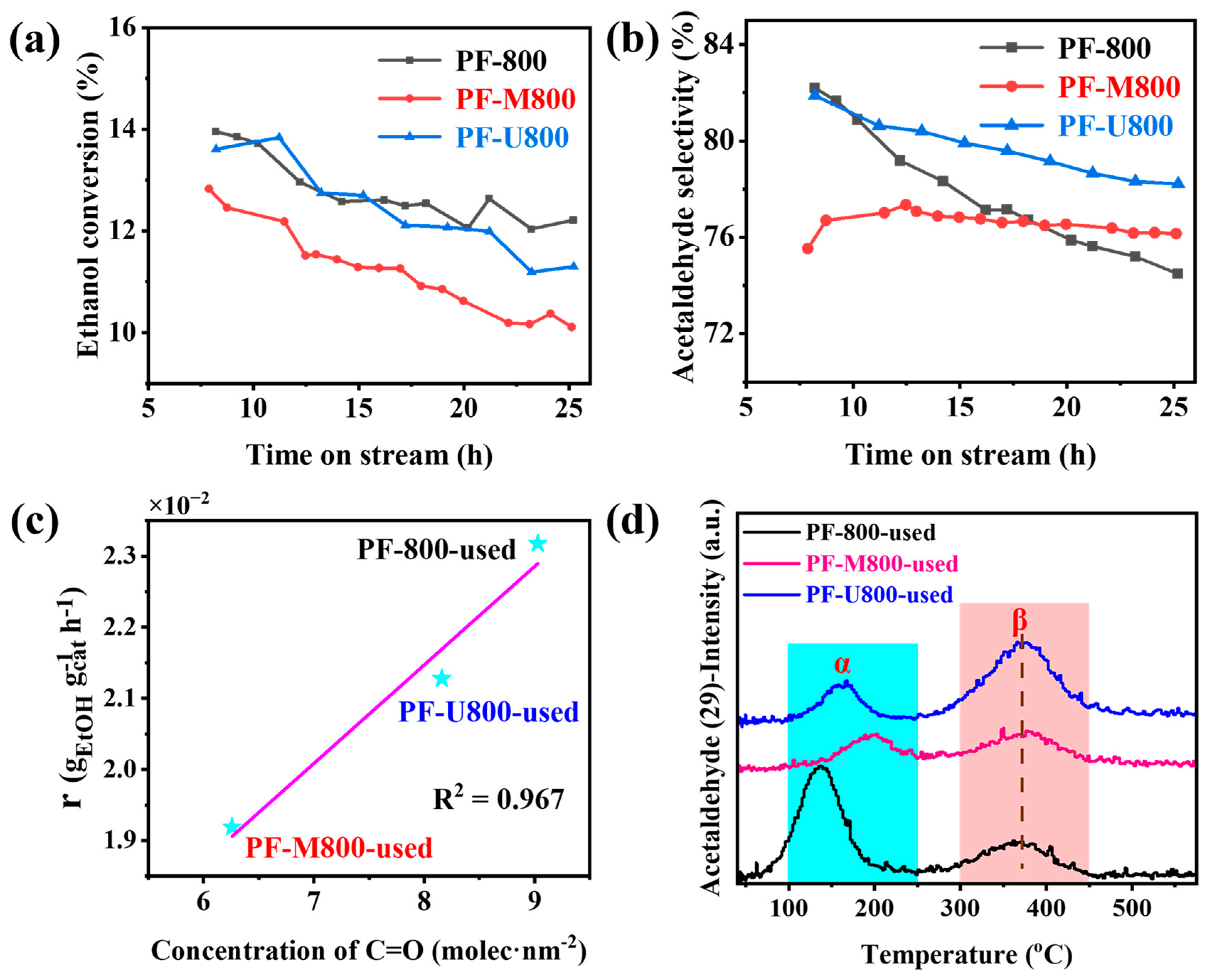
3.2. Structure–Activity Relationship
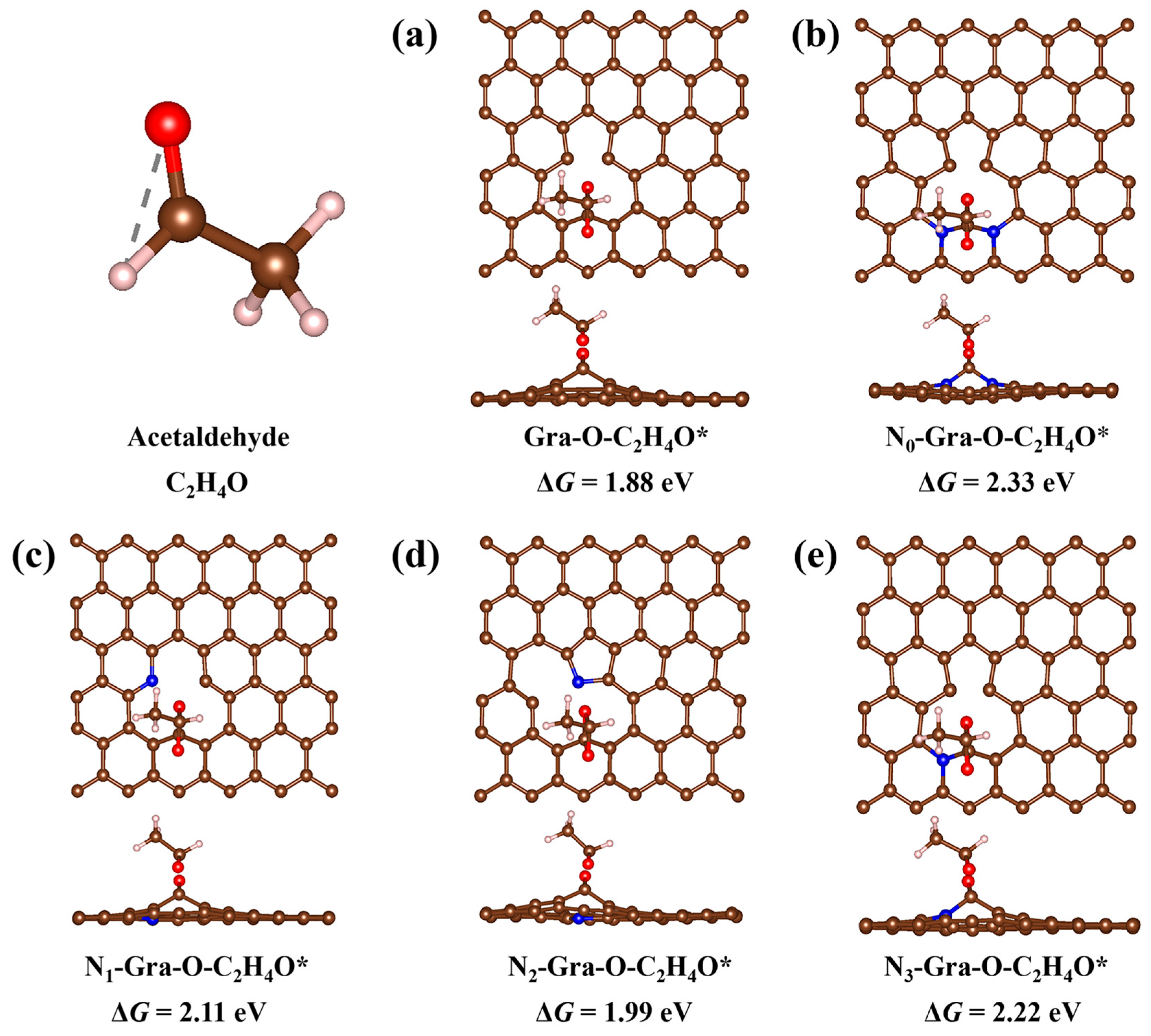
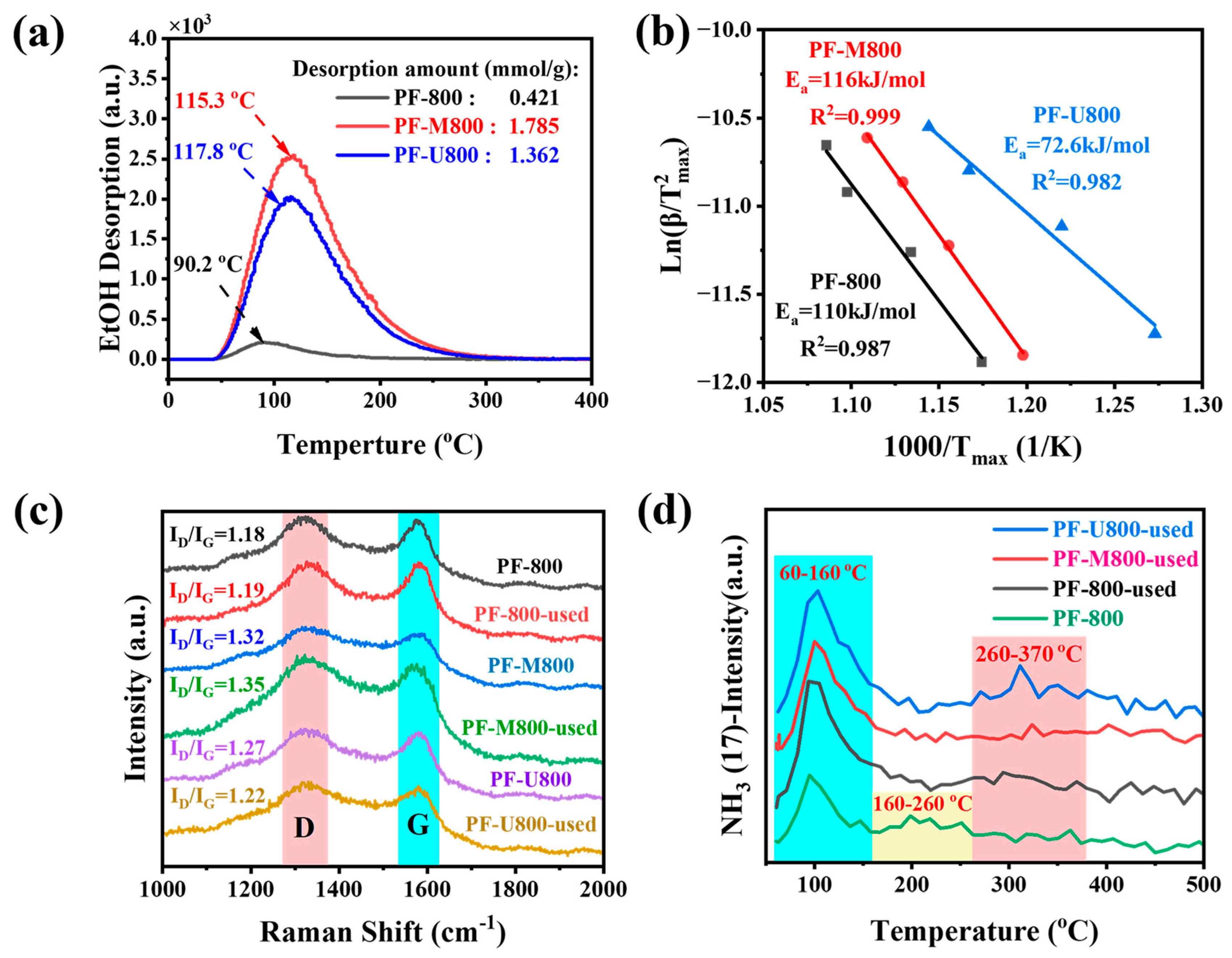
3.3. Chemical Properties of Nitrogen Doping Catalyst
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pang, J.; Zheng, M.; He, L.; Li, L.; Pan, X.; Wang, A.; Wang, X.; Zhang, T. Upgrading Ethanol to N-butanol over Highly Dispersed Ni–MgAlO Catalysts. J. Catal. 2016, 344, 184. [Google Scholar] [CrossRef]
- Pang, J.; Yin, M.; Wu, P.; Li, X.; Li, H.; Zheng, M.; Zhang, T. Advances in Catalytic Dehydrogenation of Ethanol to Acetaldehyde. Green Chem. 2021, 23, 7902. [Google Scholar] [CrossRef]
- He, L.; Zhou, B.C.; Sun, D.H.; Li, W.C.; Lv, W.L.; Wang, J.; Liang, Y.Q.; Lu, A.H. Catalytic Conversion of Ethanol to Oxygen-Containing Value-Added Chemicals. ACS Catal. 2023, 13, 11291. [Google Scholar] [CrossRef]
- Keith, J.A.; Henry, P.M. The Mechanism of the Wacker Reaction: A Tale of Two Hydroxypalladations. Angew. Chem. Int. Ed. 2009, 48, 9038. [Google Scholar] [CrossRef]
- Wang, J.; Huang, R.; Zhang, Y.; Diao, J.; Zhang, J.; Liu, H.; Su, D. Nitrogen-Doped Carbon Nanotubes as Bifunctional Catalysts with Enhanced Catalytic Performance for Selective Oxidation of Ethanol. Carbon 2017, 111, 519. [Google Scholar] [CrossRef]
- Jun, Z.T.; Zhen, S.W.; Yi, G.X.; Magnus, R.; De, C.; Chun, D.Y.; Kang, Y.W.; Anders, H. Rational Design of the Carbon Nanofiber Catalysts for Oxidative Dehydrogenation of Ethylbenzene. Appl. Catal. A Gen. 2007, 323, 135. [Google Scholar] [CrossRef]
- Weinstein, R.D.; Ferens, A.R.; Orange, R.J.; Lemaire, P. Oxidative Dehydrogenation of Ethanol to Acetaldehyde and Ethyl Acetate by Graphite Nanofibers. Carbon 2011, 49, 701. [Google Scholar] [CrossRef]
- Schwartz, V.; Fu, W.; Tsai, Y.T.; Meyer, H.M.; Rondinone, A.J.; Chen, J.; Wu, Z.; Overbury, S.H.; Liang, C. Oxygen-Functionalized Few-Layer Graphene Sheets as Active Catalysts for Oxidative Dehydrogenation Reactions. ChemSusChem 2013, 6, 840. [Google Scholar] [CrossRef]
- Liu, W.; Wang, C.; Herold, F.; Etzold, B.J.M.; Su, D.; Qi, W. Oxidative Dehydrogenation on Nanocarbon: Effect of Heteroatom Doping. Appl. Catal. B Environ. 2019, 258, 117982. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, P.; Xu, J.; Li, F.; Herold, F.; Etzold, B.J.M.; Wang, P.; Su, D.S.; Lin, S.; Qi, W.; et al. Methanol Conversion on Borocarbonitride Catalysts: Identification and Quantification of Active Sites. Sci. Adv. 2020, 6, eaba5778. Available online: https://www.science.org/doi/10.1126/sciadv.aba5778 (accessed on 15 May 2025). [CrossRef] [PubMed]
- Qi, W.; Su, D. Metal-Free Carbon Catalysts for Oxidative Dehydrogenation Reactions. ACS Catal. 2014, 4, 3212. [Google Scholar] [CrossRef]
- Cao, Y.; Yu, H.; Tan, J.; Peng, F.; Wang, H.; Li, J.; Zheng, W.; Wong, N.B. Nitrogen-, Phosphorous- and Boron-Doped Carbon Nanotubes as Catalysts for the Aerobic Oxidation of Cyclohexane. Carbon 2013, 57, 433. [Google Scholar] [CrossRef]
- Wang, J.; Huang, R.; Feng, Z.; Liu, H.Y.; Su, D. Multi-Walled Carbon Nanotubes as a Catalyst for Gas-Phase Oxidation of Ethanol to Acetaldehyde. ChemSusChem 2016, 9, 1820. [Google Scholar] [CrossRef]
- Huang, X.; Wu, S.; Xiao, Z.; Kong, D.; Liang, T.; Li, X.; Luo, B.; Wang, B.; Zhi, L. Predicting the Optimal Chemical Composition of Functionalized Carbon Catalysts Towards Oxidative Dehydrogenation of Ethanol to Acetaldehyde. Nano Today 2022, 44, 101508. [Google Scholar] [CrossRef]
- Yan, P.; Zhang, X.; Herold, F.; Li, F.; Dai, X.; Cao, T.; Etzoldc, B.J.M.; Qi, W. Methanol Oxidative Dehydrogenation and Dehydration on Carbon Nanotubes: Active Sites and Basic Reaction Kinetics. Catal. Sci. Technol. 2020, 10, 4952. [Google Scholar] [CrossRef]
- Gläsel, J.; Diao, J.; Feng, Z.; Hilgart, M.; Wolker, T.; Su, D.S.; Etzold, B.J.M. Mesoporous and Graphitic Carbide-Derived Carbons as Selective and Stable Catalysts for the Dehydrogenation Reaction. Chem. Mater. 2015, 27, 5719. [Google Scholar] [CrossRef]
- Xiao, N.; Zhou, Y.; Ling, Z.; Zhao, Z.; Qiu, J. Carbon Foams Made of in Situ Produced Carbon Nanocapsules and the Use as a Catalyst for Oxidative Dehydrogenation of Ethylbenzene. Carbon 2013, 60, 514. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.; Yang, L.; Xu, T.; Wu, H.; Zhang, J.; He, L. Effect of Pre-heat Treatment and Carbonization Temperature on Comprehensive Properties of Melamine-Derived Carbon Foam. Ceram. Int. 2024, 50, 31548. [Google Scholar] [CrossRef]
- Xiao, Q.; Wen, J.; Guo, Y.; Hu, J.; Wang, J.; Zhang, F.; Tu, G.; Zhong, Y.; Zhu, W. Synthesis, Carbonization, and CO2 Adsorption Properties of Phloroglucinol–Melamine–Formaldehyde Polymeric Nanofibers. Ind. Eng. Chem. Res. 2016, 55, 12667. [Google Scholar] [CrossRef]
- Peng, J.; Chen, X.; Zhang, J.; Essawy, H.; Du, G.; Zhou, X. Characterization on the Copolymerization Resin between Bayberry (Myrica rubra) Tannin and Pre-Polymers of Conventional Urea–Formaldehyde Resin. Forests 2022, 13, 624. [Google Scholar] [CrossRef]
- Bacigalupe, A.; Molinari, F.; Eisenberg, P.; Escobar, M.M. Adhesive Properties of Urea-Formaldehyde Resins Blended with Soy Protein Concentrate. Adv. Compos. Hybrid Mater. 2020, 3, 213. [Google Scholar] [CrossRef]
- Zhao, Y.; Kong, L.; Xu, Y.; Huang, H.; Yao, Y.; Zhang, J.; Wang, S.; Ma, X. Deactivation Mechanism of Cu/SiO2 Catalysts in the Synthesis of Ethylene Glycol via Methyl Glycolate Hydrogenation. Ind. Eng. Chem. Res. 2020, 59, 12381. [Google Scholar] [CrossRef]
- Cao, T.; Dai, X.; Liu, W.; Fu, Y.; Qi, W. Carbon Nanotubes Modified by Multi-Heteroatoms Polymer for Oxidative Dehydrogenation of Propane: Improvement of Propene Selectivity and Oxidation Resistance. Carbon 2022, 189, 199. [Google Scholar] [CrossRef]
- Shi, L.A.; Ge, J.; Hu, B.C.; Ma, T.; Zhao, H.; Song, Y.H.; Li, C.; Yu, S.H. Joule-Heated Carbonized Melamine Sponge for High-Speed Absorption of Viscous Oil Spills. Nano Res. 2021, 14, 2697. [Google Scholar] [CrossRef]
- Yang, Q.; Huang, X.; Zhang, J.; Xiao, Z.; Duan, X.; Zhou, S.; Niu, Y.; Sun, H.; Zhi, L.; Wang, S. Unzipping MWCNTs for Controlled Edge- and Heteroatom-Defects in Revealing Their Roles in Gas-Phase Oxidative Dehydrogenation of Ethanol to Acetaldehyde. Chem. Eng. J. 2022, 446, 137150. [Google Scholar] [CrossRef]
- Bai, Y.L.; Dai, X.; Cao, T.L.; Qi, W. N,O-Codoped Onion-like Carbon Catalyzed Selective Oxidation of Methanol to Dimethoxymethane: Structure-Activity Relationship. ChemCatChem 2023, 15, e202300813. [Google Scholar] [CrossRef]
- Nieto, E.A.; Cadenas, M.P.; Morales, M.V.; Baeza, B.B.; Suarez, E.G.; Ramos, I.R.; Ruiz, A.G. High Nitrogen Doped Graphenes and Their Applicability as Basic Catalysts. Diamond Relat. Mater. 2014, 44, 26. [Google Scholar] [CrossRef]
- Gao, Y.; Hu, G.; Zhong, J.; Shi, Z.; Zhu, Y.; Su, D.S.; Wang, J.; Bao, X.; Ma, D. Nitrogen-Doped sp2-Hybridized Carbon as a Superior Catalyst for Selective Oxidation. Angew. Chem. Int. Ed. 2013, 52, 2109. [Google Scholar] [CrossRef]
- Hu, X.; Wu, Y.; Li, H.; Zhang, Z. Adsorption and Activation of O2 on Nitrogen-Doped Carbon Nanotubes. J. Phys. Chem. C 2010, 114, 9603. [Google Scholar] [CrossRef]
- Eckmann, A.; Felten, A.; Mishchenko, A.; Britnell, L.; Krupke, R.; Novoselov, K.S.; Casiraghi, C. Probing the Nature of Defects in Graphene by Raman Spectroscopy. Nano Lett. 2012, 12, 3925. [Google Scholar] [CrossRef]
- Phung, T.K. Copper-Based Catalysts for Ethanol Dehydrogenation and Dehydrogenative Coupling into Hydrogen, Acetaldehyde and Ethyl acetate. Int. J. Hydrogen Energy 2022, 47, 42234. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmiiller, J. Efficiency of Ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set. Comput. Mater. Sci. 1996, 6, 15. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmiiller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, L.; Guo, C.; Song, W.; Liu, Y.; Luo, G.; Xu, Y.; Zhao, Y.; Jin, P. Unveiling the Nitrogen-Doping Mechanism in Carbon Catalysts for Oxidative Dehydrogenation of Ethanol to Acetaldehyde. Materials 2025, 18, 2345. https://doi.org/10.3390/ma18102345
Kong L, Guo C, Song W, Liu Y, Luo G, Xu Y, Zhao Y, Jin P. Unveiling the Nitrogen-Doping Mechanism in Carbon Catalysts for Oxidative Dehydrogenation of Ethanol to Acetaldehyde. Materials. 2025; 18(10):2345. https://doi.org/10.3390/ma18102345
Chicago/Turabian StyleKong, Lingxin, Chenxi Guo, Wenkai Song, Yujie Liu, Guiyao Luo, Yan Xu, Yujun Zhao, and Peng Jin. 2025. "Unveiling the Nitrogen-Doping Mechanism in Carbon Catalysts for Oxidative Dehydrogenation of Ethanol to Acetaldehyde" Materials 18, no. 10: 2345. https://doi.org/10.3390/ma18102345
APA StyleKong, L., Guo, C., Song, W., Liu, Y., Luo, G., Xu, Y., Zhao, Y., & Jin, P. (2025). Unveiling the Nitrogen-Doping Mechanism in Carbon Catalysts for Oxidative Dehydrogenation of Ethanol to Acetaldehyde. Materials, 18(10), 2345. https://doi.org/10.3390/ma18102345





