Abstract
Ammonia (NH3) is a crucial feedstock in chemical manufacturing. The electrocatalytic NO reduction reaction (eNORR) to NH3 represents a promising alternative method for the green production of NH3 and for environmental management. This study presents a comprehensive investigation of eNORR properties of single transition metal atoms deposited on WS2 nanosheets (TM@WS2). Our results indicate that 19 single TM atoms exhibit strong thermal stability. Among these, five specific TM@WS2 catalysts—Ti, Mn, Co, Zr, and Hf—demonstrate remarkable eNORR activity, with limiting potentials of 0, −0.19, −0.26, 0, and −0.15 V, respectively. These catalysts effectively suppress the formation of byproducts (N2O/N2) and the hydrogen evolution reaction (HER), thereby ensuring high NH3 selectivity. Our theoretical study confirms that TM@WS2 catalysts are highly promising for achieving high activity, selectivity, and stability in eNORR, providing valuable insights for future experimental investigations into efficient NH3 production.
1. Introduction
Ammonia (NH3) serves as a crucial chemical compound not only for the production of fertilizers, pharmaceuticals, and dyes, but also as a significant carrier of carbon-free energy [1,2,3]. Moreover, given its high hydrogen content (17.6 wt%) and its ability to easily condense into a liquid state, NH3 is considered an ideal medium for hydrogen energy storage [1,4]. As a result, the synthesis of NH3 is of great importance and has garnered widespread attention. Traditionally, the large-scale production of ammonia relies on the Haber–Bosch process, which necessitates extremely high temperatures (400–500 °C) and significant pressures (200–250 bar). However, this widely used process has significant drawbacks, as it depends on fossil fuel combustion and results in the emission of the greenhouse gas CO2 (accounting for approximately 3% of global CO2 emissions annually) [2,5]. The method of electrocatalytic ammonia synthesis has garnered widespread attention and thorough research as a viable alternative [2,6]. This method operates at room temperature and can utilize electric energy derived from solar or wind power [6]. Therefore, electrocatalytic NH3 synthesis is viewed as an environmentally friendly and flexible technique, with nitrogen (N2) or nitrogen oxides, such as nitrate (NO3−) and nitric oxide (NO) serving as its feedstocks [6,7,8,9,10,11,12,13,14,15,16,17].
Despite over a decade of research, the electrocatalytic nitrogen reduction reaction (eNRR) has not yet reached practical implementation, mainly attributed to its insufficient NH3 yield and poor Faradaic efficiency. The challenges arise from the robust nature of the N≡N triple bond, which has a bond energy of 914 kJ/mol and makes nitrogen molecules highly unreactive. Additionally, the eNRR faces issues with poor selectivity, as it often competes unfavorably with the hydrogen evolution reaction (HER) [12]. Consequently, there remains an urgent need and significant opportunity to develop exceptionally efficient and specific electrocatalysts to facilitate efficient NH3 synthesis.
In addition, flue gases from thermal power plants and industrial boilers typically contain high levels of NOx, with NO accounting for approximately 95% of these emissions. The significant presence of NO in flue gases poses a serious threat to both the environment and human health [18,19]. Therefore, electrocatalytic NO reduction (eNORR) offers a dual benefit by not only producing valuable NH3 but also effectively cleaning the flue gases.
To date, numerous types of catalysts have been investigated for the eNORR [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34]. Noble metal catalysts, in particular, have garnered significant interest due to their intrinsic properties. However, their application is often limited by insufficient activity or prohibitive cost [21,26,27]. In addition to noble metals, transition metal-based composites have also been investigated as potential catalysts for eNORR [20,23,24,25]. Despite these efforts, the durability and selectivity of these catalysts have not yet reached the desired standards. At present, the primary obstacle in the realm of eNORR is to develop catalysts that simultaneously exhibit high activity, excellent durability, and cost-effectiveness.
Single-atom catalysts (SACs) have emerged as promising candidates for the eNORR due to their exceptional atom utilization efficiency and unique geometric and electronic properties [32,33,34]. To date, many substrates are used as single-atom catalysts in eNORR, such as graphene [28,29,30,31,33], transition metal dichalgonides (TMDs) [32,35,36,37], metal mxides [38], carbides (including metal and non-metal carbides) [39,40,41], nitrides (including metal and non-metal nitrides) [42,43,44,45,46], and others [47,48,49,50,51]. However, the inherently high surface energy of single atoms on these substrates tends to drive metal atoms to aggregate into clusters, which, in turn, impedes the formation of highly dispersed single atoms [36,37,38]. To overcome this challenge, it is crucial to pinpoint the optimal substrates that can firmly immobilize and stabilize the single atoms. Recent research has demonstrated that vacancies in support materials can act as efficient binding sites for individual metal atoms, effectively inhibiting their nucleation and aggregation [22,38,39,40].
WS2 is an ideal substrate, owing to it is particularly prone to the formation of sulfur vacancies, which provide effective sites for the deposition of single metal atoms [52,53,54]. Experimental studies have shown that loading Fe, Co, Ni, and Cu atoms on WS2 at concentrations up to 10% does not induce nucleation [53]. Inspired by these findings, we have designed a WS2 nanosheet with a single sulfur vacancy modified by transition metal (TM) atoms, which can serve as highly efficient single-atom catalysts (SACs) for the eNORR.
In this study, we employed DFT calculations to evaluate the electrocatalytic nitric oxide reduction reaction (eNORR) performance of 27 TM single-atom catalysts deposited on WS2 (denoted as TM@WS2, where TM includes atoms from the 3 d to 5 d periods). First, we assessed the thermal stability of these catalysts by calculating their binding energies. Subsequently, we evaluated their practical viability by comparing the binding energies of synthesized TM@WS2 catalysts. After identifying the stable and viable candidates, we examined their ability to adsorb and activate NO. We further explored the eNORR mechanisms leading to NH3 production and evaluated their catalytic performance in competing reactions, such as the formation of N2O or N2 and the hydrogen evolution reaction (HER). Finally, we elucidated the origin of activity for the promising eNORR candidates through detailed electronic structure analysis.
2. Materials and Methods
We conducted spin-polarized periodic density functional theory (DFT) calculations using the Vienna ab initio simulation package (VASP version 5.4.4) [55]. The electron exchange and correlation effects were described using the generalized gradient approximation (GGA) with the Perdew–Burke–Ernzerhof (PBE) functional [56]. The projector augmented wave (PAW) method was applied, and a plane-wave cutoff energy of 450 eV was set [57]. To incorporate van der Waals interactions between adsorbates and substrates, the DFT-D3 correction was employed [58]. A 4 × 4 × 1 supercell was created, and a 15 Å vacuum layer was introduced to reduce interactions between WS2 nanosheets and their periodic images. The k-point grid of 4 × 4 × 1 was used for Brillouin zone sampling [58]. All atoms were allowed to relax freely without any constraints, and the calculations were converged to an energy tolerance of 10−4 eV and a residual force tolerance of 0.02 eV Å−1. The binding energies (Eb) of TM on WS2 were calculated using the following formula [59,60]:
Here, ETM@WS2 signifies the total energy of the transition metal (TM) atoms deposed on WS2, while Edefective-WS2 refers to the total energy of WS2 with a single sulfur vacancy. μTM denotes the chemical potential of the TM atoms. The Gibbs free energy (ΔG) for each step of eNORR was determined using the following equation [33,34]:
ΔG = ΔE + ΔEZPE − TΔS + ΔGpH
In this equation, ΔE denotes the energy difference between the two intermediates involved in each elementary reaction step. This value can be directly obtained from the DFT results. ΔEZPE and ΔS represent the changes in zero-point energy and entropy, respectively, at room temperature (T = 298.15 K). These values were calculated using vibrational frequencies through the VASPKIT program [61]. The ΔG correction for pH (ΔGpH) was determined by:
ΔGpH = kBT × pH × ln10
In this case, the pH was fixed at 0. The influence of the solvent (water) on the ΔG was assessed via implicit solvent model, which is integrated within the VASP computational framework [62].
The limiting potential (UL) acts as an activity indicator, representing the minimum electrical potential required to make the ΔG of all elementary steps in the reaction exothermic (i.e., to ensure that ΔG decreases for each step). It is calculated using the following equation [33,34]:
UL = −max (∆G1, ∆G2, ∆G3, ∆G4…, ∆Gi)/e
In this equation, ΔGi is the free energy variation corresponding to each specific intermediate step within the overall eNORR process.
3. Results
3.1. Stability of TM@WS2
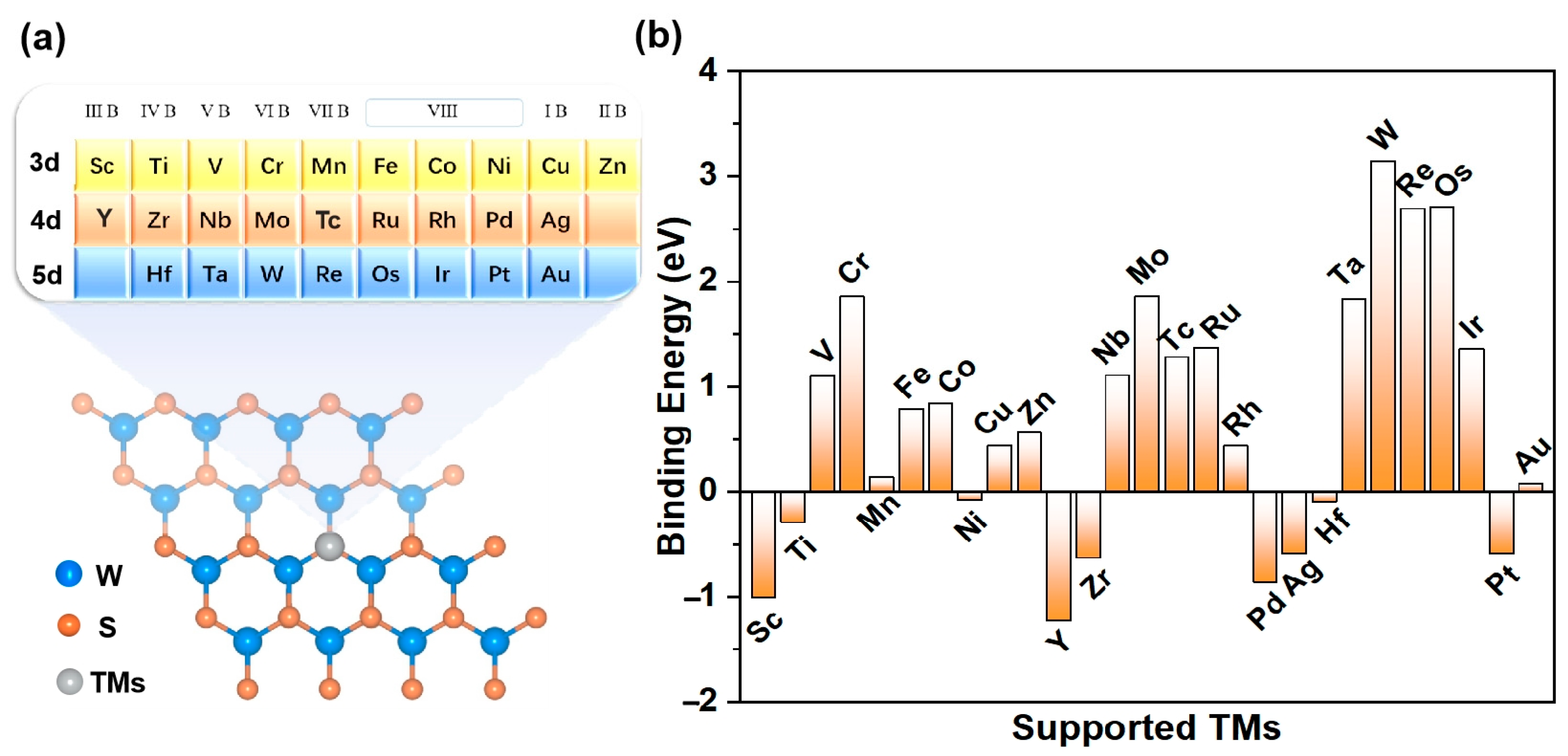
The specific TM@WS2 model under investigation is depicted in Figure 1a. In order to assess the stability and experimental viability of the TM@WS2 catalysts, we first calculated their binding energies (Eb). The Eb values of several synthesized TM@WS2 catalysts (where TM represents Fe, Co, and Cu) were used as reference values to estimate the stability of other catalysts [53]. Given these values, an Eb of 0.84 eV can be used as a preliminary benchmark to evaluate the stability of other unsynthesized TM@WS2 catalysts. Figure 1 also shows that, among the 27 TM@WS2 catalysts examined, 11 metal atoms —specifically, V, Cr, Nb, Mo, Tc, Ru, Ta, W, Re, Os, and Ir—exhibit Eb values greater than 0.84 eV, suggesting that they are considered unstable; these have been excluded from further analysis. Conversely, the remaining 16 TM atoms, which possess Eb values below 0.84 eV, are deemed more probable candidates for stable TM@WS2 catalysts.
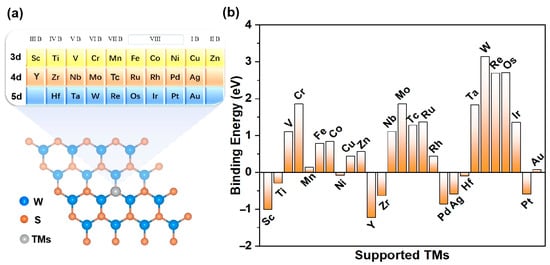
Figure 1.
(a) The modeled structure of TM@WS2 catalysts; (b) Eb values of TM@WS2 catalysts.
In order to conduct a more comprehensive evaluation of the thermodynamic stability of the 16 transition metal (TM) atoms on the WS2 surface, we chose the Zn@WS2 catalyst as a representative case for performing ab initio molecular dynamics (AIMD) simulations. This selection was based on the fact that the binding energy (Eb) of Zn@WS2 is 0.56 eV, which is above 0 eV. The AIMD results are presented in Figure S1. Upon reaching a stable state at 500 K, the Zn@WS2 catalyst exhibited no substantial structural distortions or displacements of the Zn atom, thereby confirming its robust thermodynamic stability.
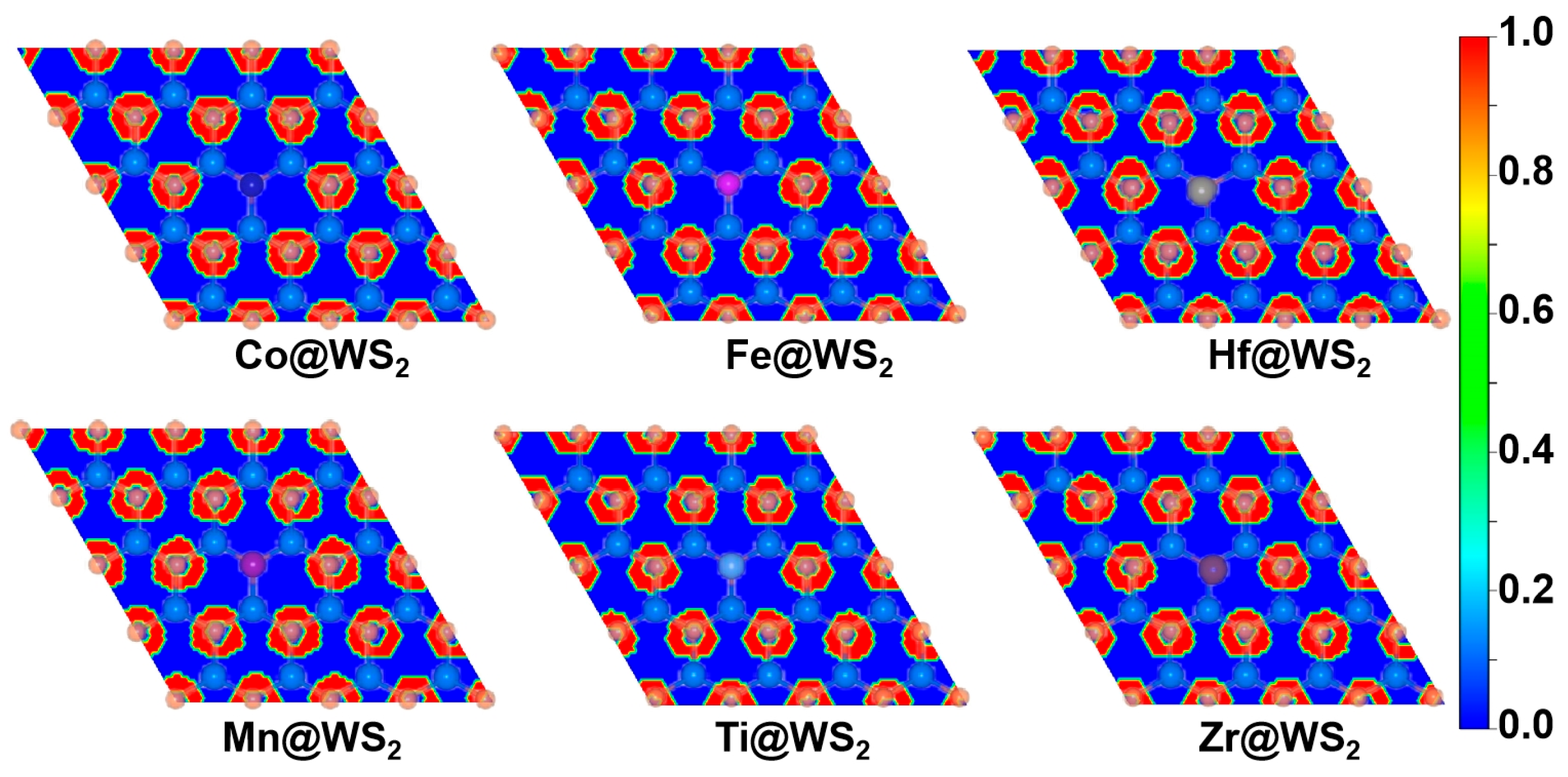
By calculating the electron localization function (ELF), we further reveal the bonding characteristics and stability. The closer the ELF value is to 1, the higher the density of covalent bonds and lone pairs of electrons. As shown in Figure 2, there is a high electron distribution between the metal atoms and sulfur atoms (S), indicating that the TM-S bond is a stable covalent bond. Therefore, the TM@WS2 catalysts possess good thermodynamic stability.
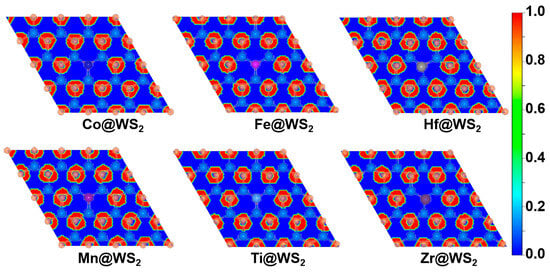
Figure 2.
Electron localization function (ELF) plots of TM@WS2 catalysts.
3.2. NO Adsorption
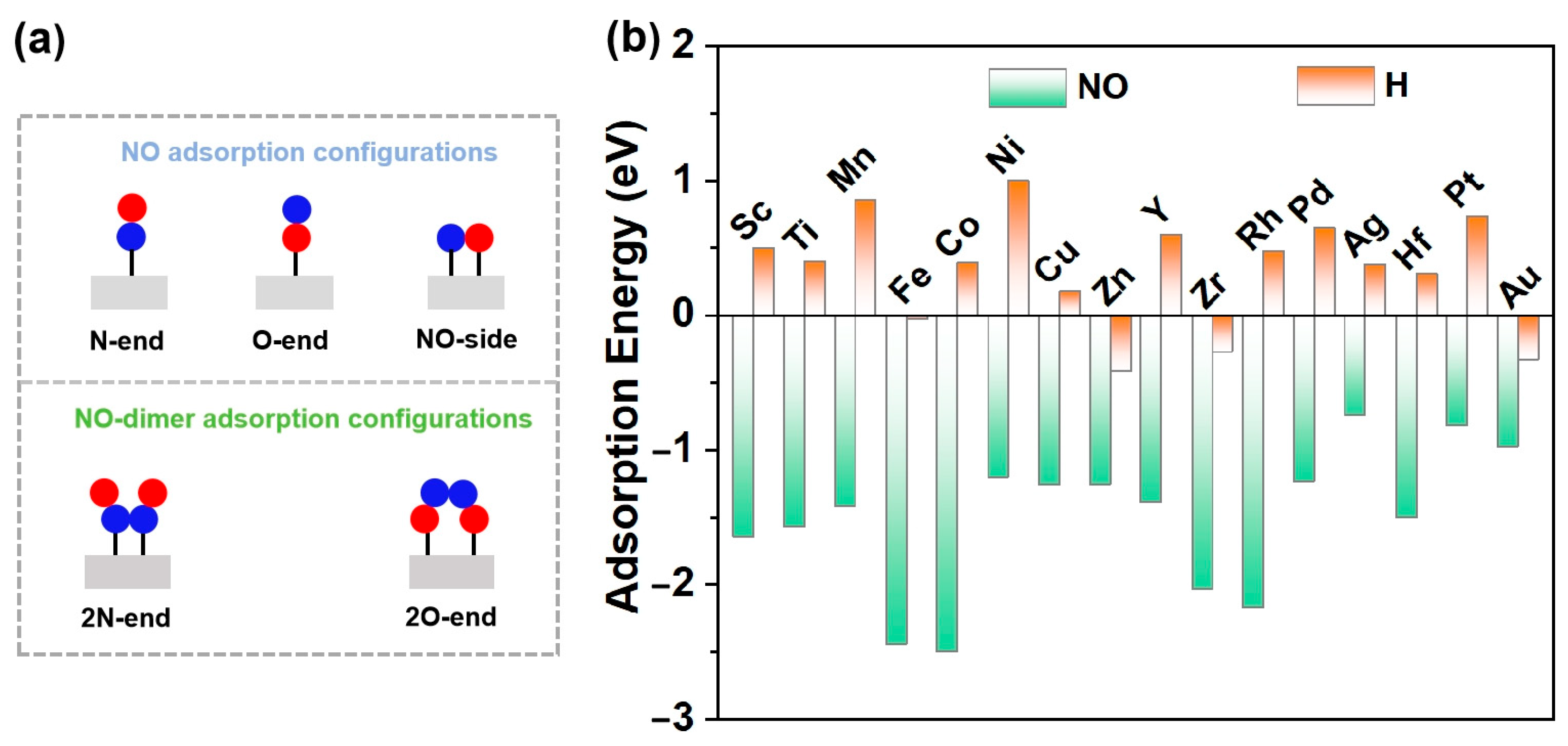
NO adsorption and activation are the first steps in eNORR and play a crucial role in the subsequent reaction [32]. There are three different adsorption configurations of the NO molecule: N-end, O-end, and NO-side (as shown in Figure 3). Among the 16 TM@WS2 catalysts screened, the N-end adsorption configuration exhibited the most negative adsorption energy compared to the other configurations (Table S1), indicating that it is the most stable configuration. Notably, the NO-side configuration was unstable and spontaneously converted to the N-end configuration on several TM@WS2 catalysts, including Co@WS2, Ni@WS2, Cu@WS2, Zn@WS2, Rh@WS2, Pd@WS2, Ag@WS2, Pt@WS2, and Au@WS2. Therefore, our focus is primarily on the N-end configuration.
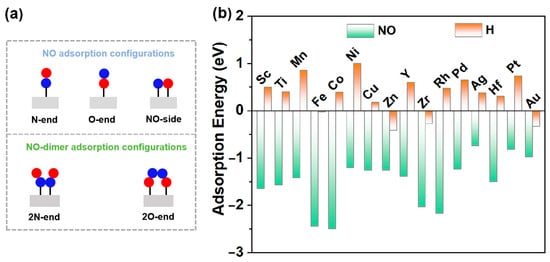
Figure 3.
(a) Possible NO adsorption configurations both high and low NO concentration; (b) NO and H adsorption over 19 TM@WS2 catalysts.
In an aqueous environment, the hydrogen evolution reaction (HER) emerges as a predominant competing process. In the first step, the reactants (here NO and H) are adsorbed on to the catalysts to initiate the reaction. This competition determines whether eNORR or HER is prioritized. Therefore, following a similar approach, we undertook a comparative analysis of the adsorption energies of of H and NO on 16 catalysts. As shown in Figure 3b, NO adsorption is significantly stronger than H adsorption across all 16 catalysts. This indicates that H adsorption is hindered, thereby favoring eNORR over HER.
3.3. eNORR Mechanism and Evaluation of Activity
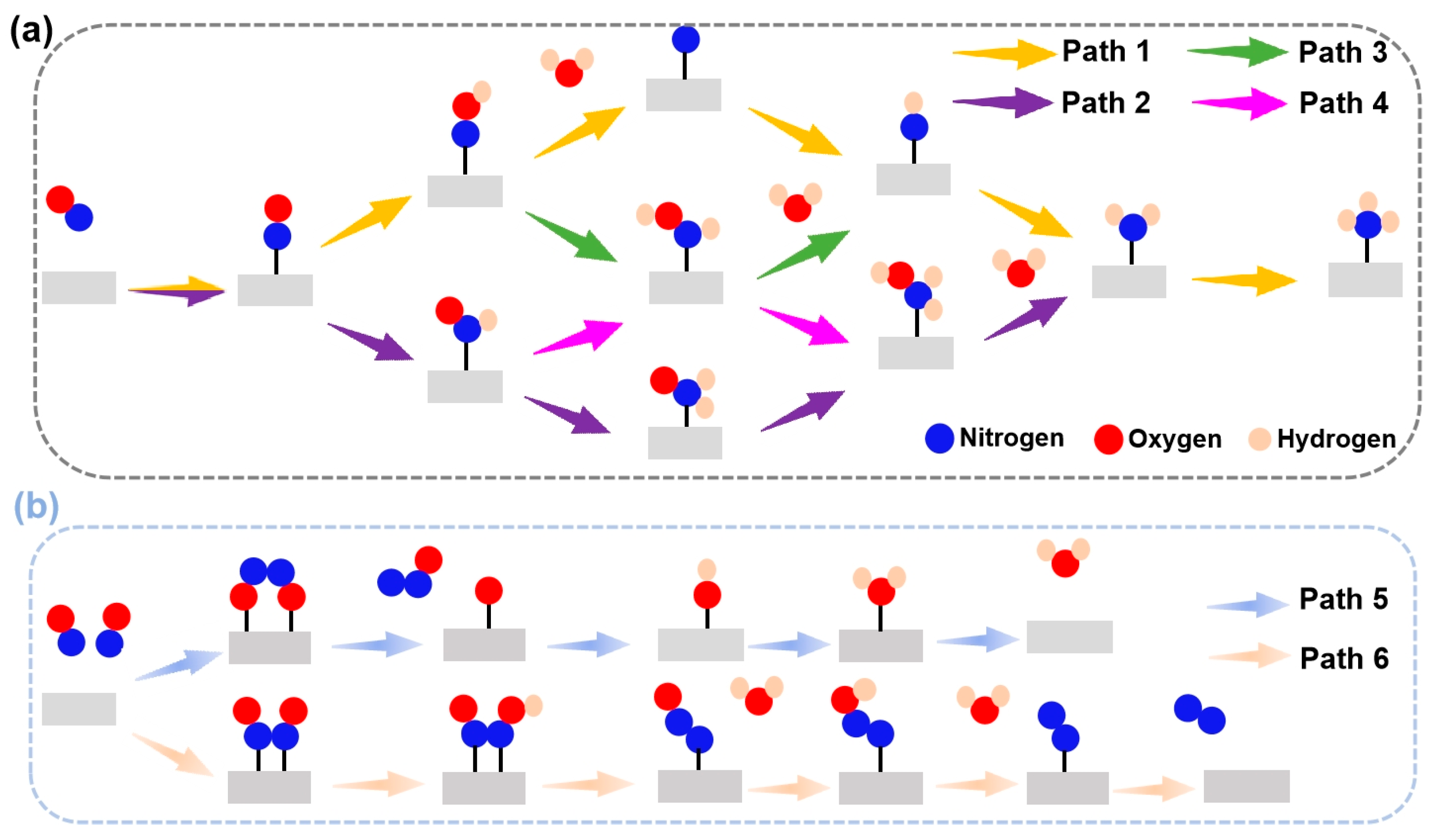
The eNORR reaction involves multiple reaction pathways (see Figure 4). The selectivity of the eNORR process is significantly influenced by NO concentration. At low NO concentrations, NH3 serves as the final product (as shown in Figure 4a), while at high NO concentrations, N2 and N2O are formed due to dimerization of NO (N2O2). In this scenario, since NH3 is the target product, a series of distinct pathways are followed and each pathway involves five protonation steps to yield NH3. At high NO concentrations, multiple two-pathway branches emerge, with N2 and N2O being formed through dimerization [26].
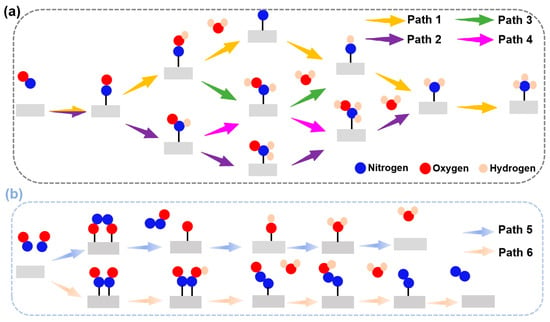
Figure 4.
Schematic depiction of the probable reaction pathways for the eNORR. (a) Pathways of NO reduction to NH3; (b) Pathways of NO reduction to N2/N2O.
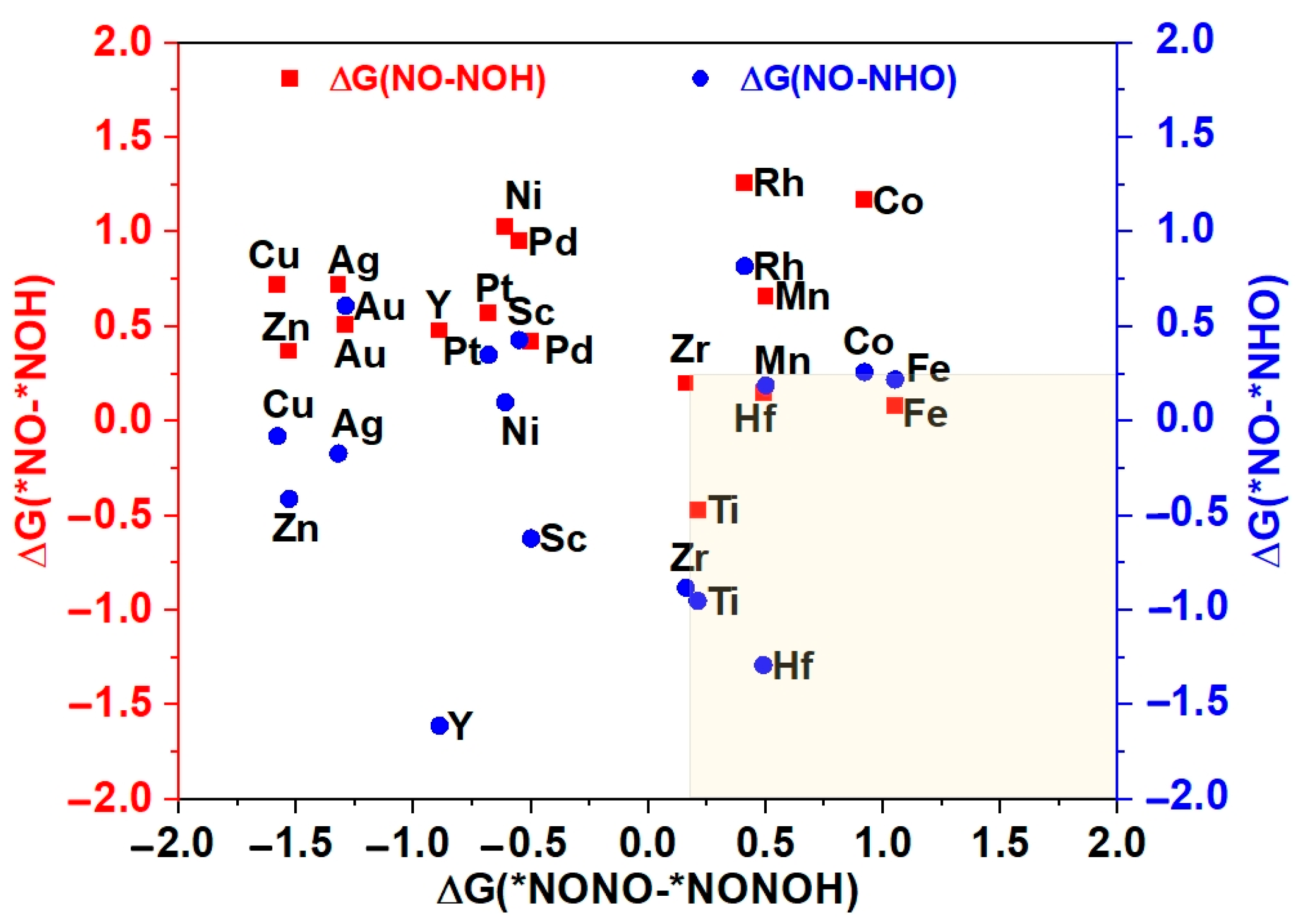
The potential and efficiency of the eNORR reaction is typically evaluated based on its potential determination step (PDS), which is marked by the most substantial increase in Gibbs free energy change (∆G) along the reaction pathway. In this context, processes such as the initial protonation of *NO to form *NOH or *NHO, and the protonation of *NONO to yield *NONOH, are commonly considered as the PDS [63]. To further evaluate the catalytic activity and product selectivity of the 16 catalysts, the ∆G values for the initial hydrogenation steps were calculated, specifically for the pathways from *NO→*NOH (*NO→*NHO) to NH3 and from *NONO→*NONOH to N2. As illustrated in Figure 5, for the Ti@WS2, Mn@WS2, Fe@WS2, Co@WS2, Zr@WS2, and Hf@WS2 catalysts, the ∆G values of PDS for the *NO→*NOH or *NO→*NHO steps are lower than those for the *NONO→*NONOH step, indicating that these six catalysts preferentially form NH3, making them potential candidate catalysts.
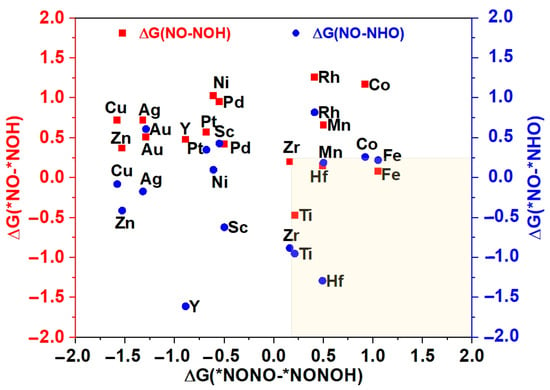
Figure 5.
The Gibbs free energy differences of first protonation steps for NH3 vs. N2. * represents the adsorption states of reaction species.
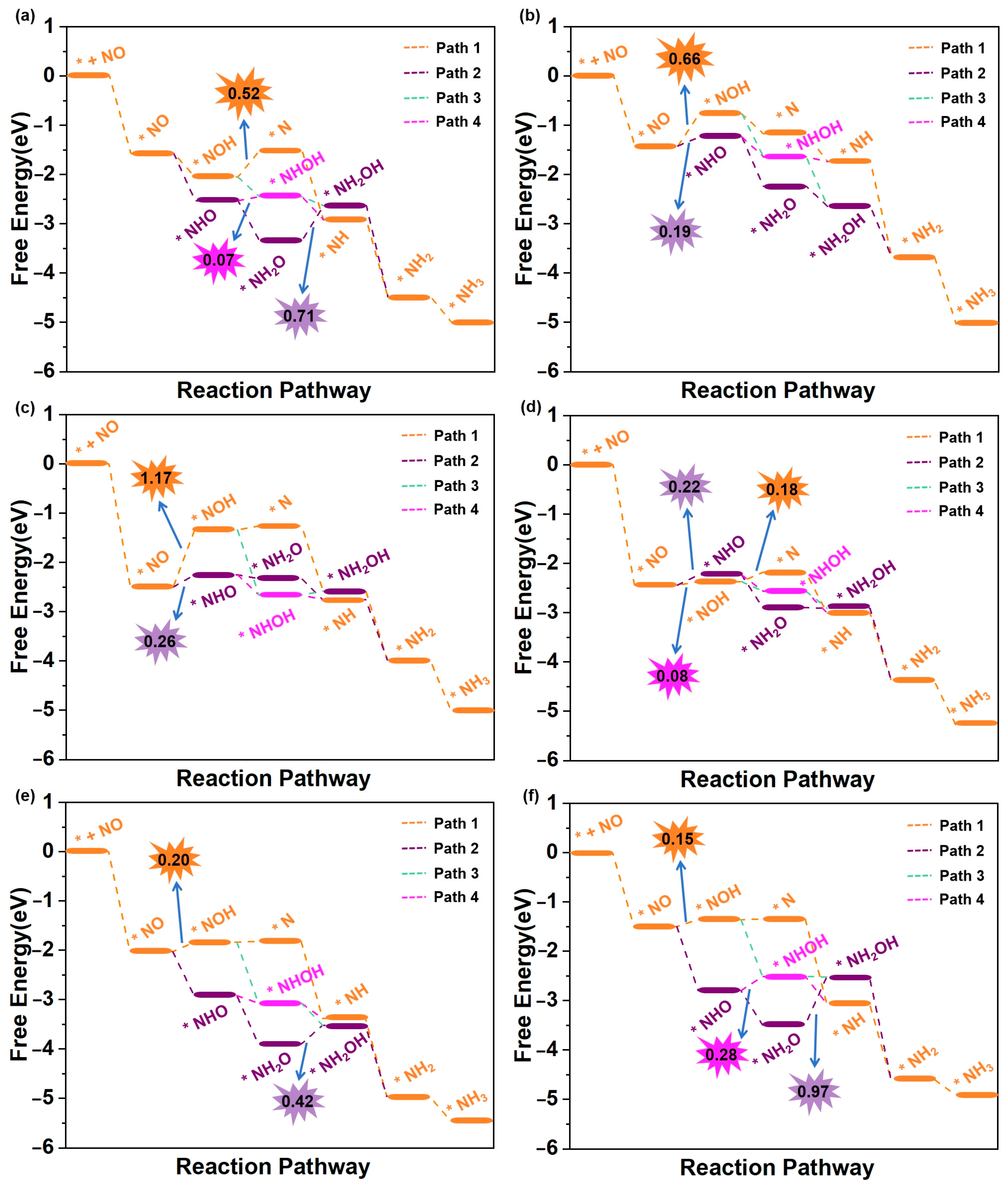
We then subsequently evaluated the eNORR activity of Ti@WS2, Mn@WS2, Fe@WS2, Co@WS2, Zr@WS2, and Hf@WS2 catalysts for NH3 synthesis. The eNORR free energy profiles for each reaction pathway of these six catalysts were constructed and are shown in Figure 6a–f. The potential determining step (PDS) for each pathway was identified and labelled, providing a clear indication of the most favorable reaction pathway and associated potential barriers.
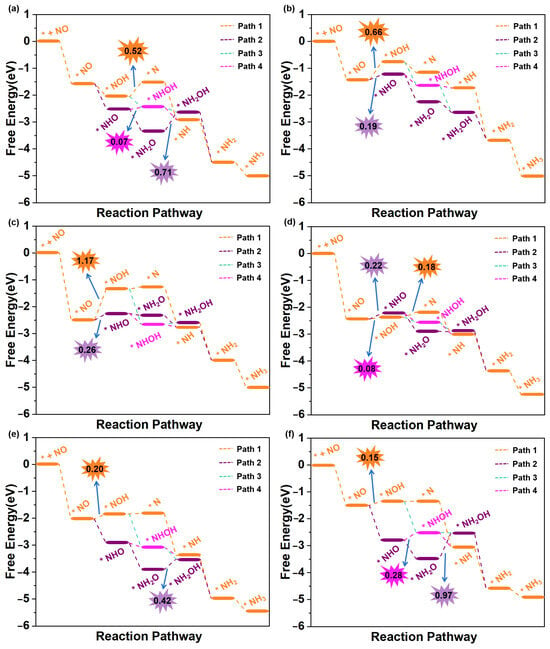
Figure 6.
Free energy diagrams of eNORR to NH3 over six TM@WS2 catalysts. (a) Ti@WS2; (b) Mn@WS2; (c) Co@WS2; (d) Fe@WS2; (e) Zr@WS2; (f) Hf@WS2.
The eNORR process is initiated by the adsorption of NO on the catalyst surface, resulting in the formation of the *NO species. Following this, protons from the electrolyte approach and interact with the *NO species, driving the formation of *HNO or *NOH. Subsequently, a series of hydrogenation steps occur, ultimately resulting in the formation of NH3. As shown in Figure 6a, the most favorable reaction pathway for the Ti@WS2 catalyst is Path3. Along this pathway, all elementary steps are exergonic (∆G < 0 eV), indicating that the eNORR process occurs spontaneously. Consequently, the UL of the Ti@WS2 catalyst is 0 V. As shown in Figure 6b,c, for both the Mn@WS2 and Co@WS2 catalysts, Path2 is selected as the most favorable reaction pathway. In this pathway, the eNORR process can occur after overcoming a potential barrier (corresponding to ∆G of *NO→*NHO) of 0.19 eV for Mn@WS2 and 0.26 eV for Co@WS2. For Fe@WS2 (Figure 6d), Path3 emerges as the most favorable pathway, featuring a minimal energy barrier of 0.08 eV during the transition from *NO to *NHO. For the Zr@WS2 catalyst (Figure 6e), the most favorable reaction pathway is Path4, along which all elementary steps are exergonic, suggesting that the eNORR proceeds spontaneously at 0 V electrolyte potential. For the Hf@WS2 catalyst, as shown in Figure 6f, the most favorable pathway is identified as Path4, with a barrier height of 0.15 eV. In particular, in an acidic environment, the transformation of NH3 into NH4+ proceeds rapidly, primarily because this process releases a significant amount of energy [22,26,35]. As a result, the desorption of NH3 is not taken into account when evaluating the performance of all six catalysts.
In summary, the limiting potentials (UL) of the Ti@WS2, Mn@WS2, Fe@WS2, Co@WS2, Zr@WS2, and Hf@WS2 catalysts are 0, −0.19, −0.26, −0.08, 0, and −0.15 V, respectively. These values are either equivalent to or significantly lower than those of reported for other single-atom catalysts. For ease of comparison, we have compiled the limiting potentials from our study and those reported in the literature in Table S2.
In order to further elucidate the effects of TM atoms modification, the eNORR activity of WS2 with single sulfur vacancies was investigated. As demonstrated in Figure S2, the most favorable reaction pathway is Path4, which involves a relatively low potential barrier of 0.96 eV for the *NHO→*NHOH step, corresponding to UL of −0.96 V. In comparison, TM depositing on sulfur vacancy site significantly enhances the catalytic activity of WS2. Specifically, the UL values for the TM@WS2 catalysts exhibit a notable increase from −0.96 V for WS2 to 0 V for Ti@WS2, −0.19 V for Mn@WS2, −0.26 V for Fe@WS2, −0.08 V for Co@WS2, 0 V for Zr@WS2, and −0.15 V for Hf@WS2. These observations demonstrate that TM atoms modification substantially improves the eNORR activity of WS2.
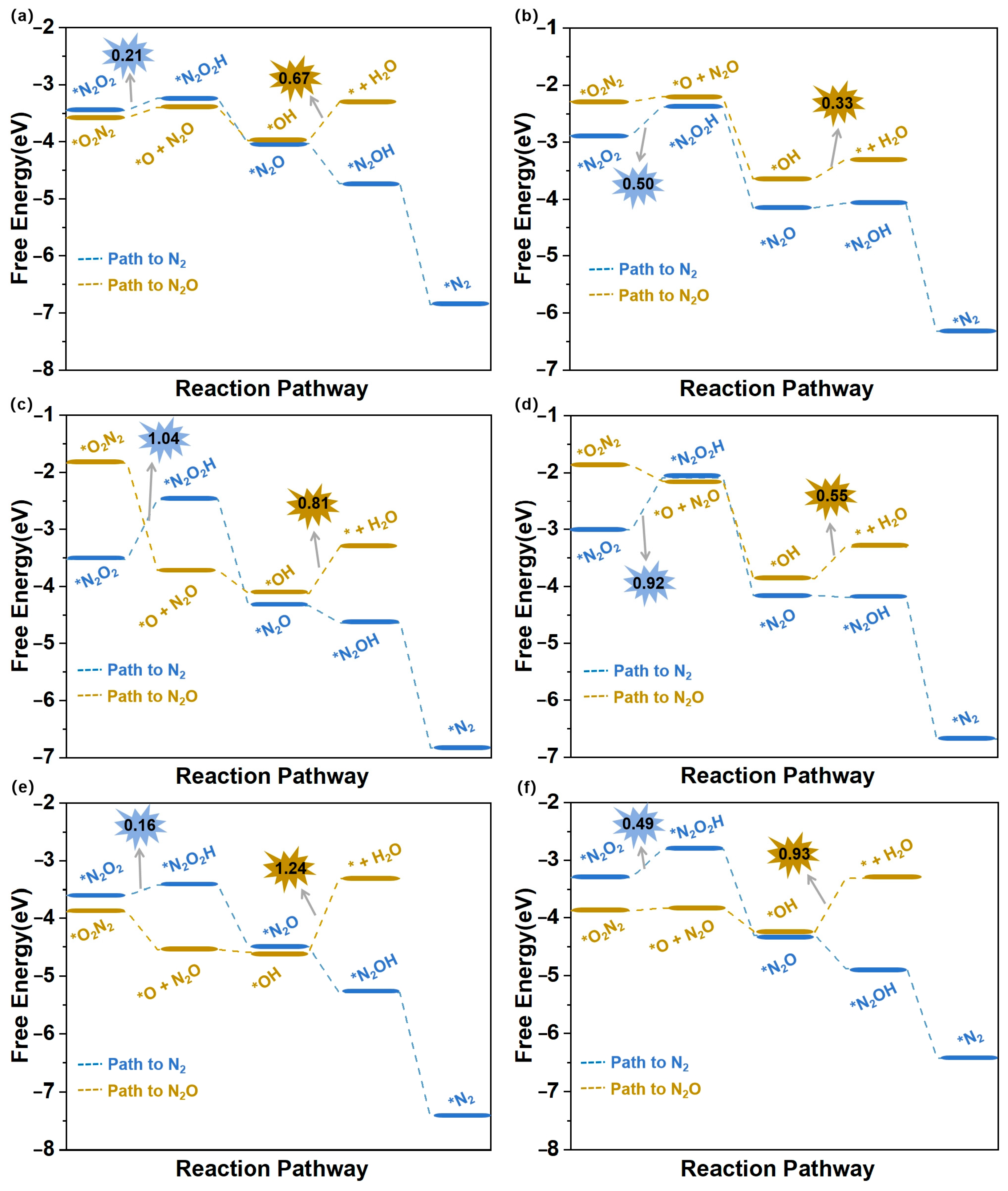
During the eNORR process, N2O and N2 can be produced as by-products due to the formation of the NO dimer (N2O2), as shown in Figure 3a. Therefore, we investigated the NO-to-N2O and NO-to-N2 pathways (Path5 and Path6 in Figure 4b) on the above six catalysts from the 2N-end (*N2O2) and 2O-end (*O2N2) configurations of N2O2, respectively. Figure 7a–f illustrate the energy diagrams for the formation of N2O and N2 on Ti@WS2, Mn@WS2, Fe@WS2, Co@WS2, Zr@WS2, and Hf@WS2 catalysts; during the NO-to-N2O process, starting from*O2N2 configurations, it is evident that all of these six catalysts face potential barriers (in the process of *OH→* + H2O step) of 0.67, 0.33, 0.81, 0.55, 1.24, and 0.93 eV, respectively. This indicates that the conversion of *OH to *+H2O is a rather challenging step for these catalysts. For the N2 formation process, over Ti@WS2, Mn@WS2, Fe@WS2, Co@WS2, Zr@WS2, and Hf@WS2 catalysts, the first hydrogenation of first step, namely the *N2O2-*N2O2H step, is the PDS step, and corresponding potential barriers are 0.21, 0.50, 1.04, 0.92, 0.16, and 0.49 eV, respectively. Based on the above results, the energy barriers of N2O and N2 formation are higher than those of NH3 formation. Consequently, although under conditions of high NO concentration, the formation of N2O and N2 can be effectively suppressed on these six catalysts. Hence, these catalysts demonstrate high selectivity for NH3 production.
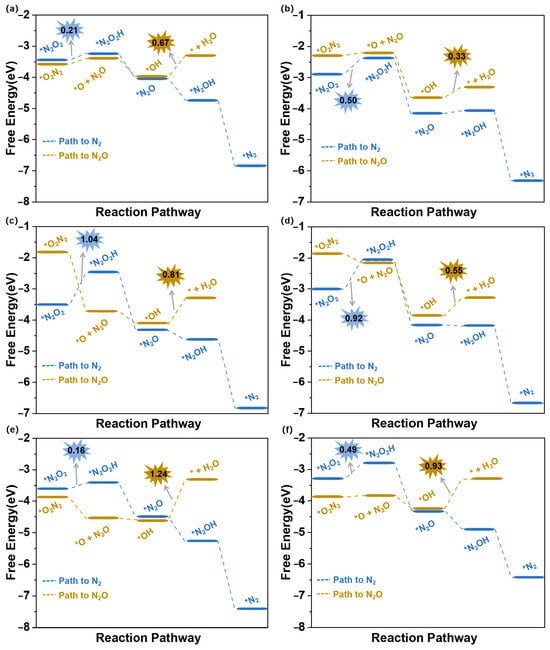
Figure 7.
Free energy diagrams of eNORR to N2O/N2 over six TM@WS2 catalysts. (a) Ti@WS2; (b) Mn@WS2; (c) Fe@WS2; (d) Co@WS2; (e) Zr@WS2; (f) Hf@WS2.
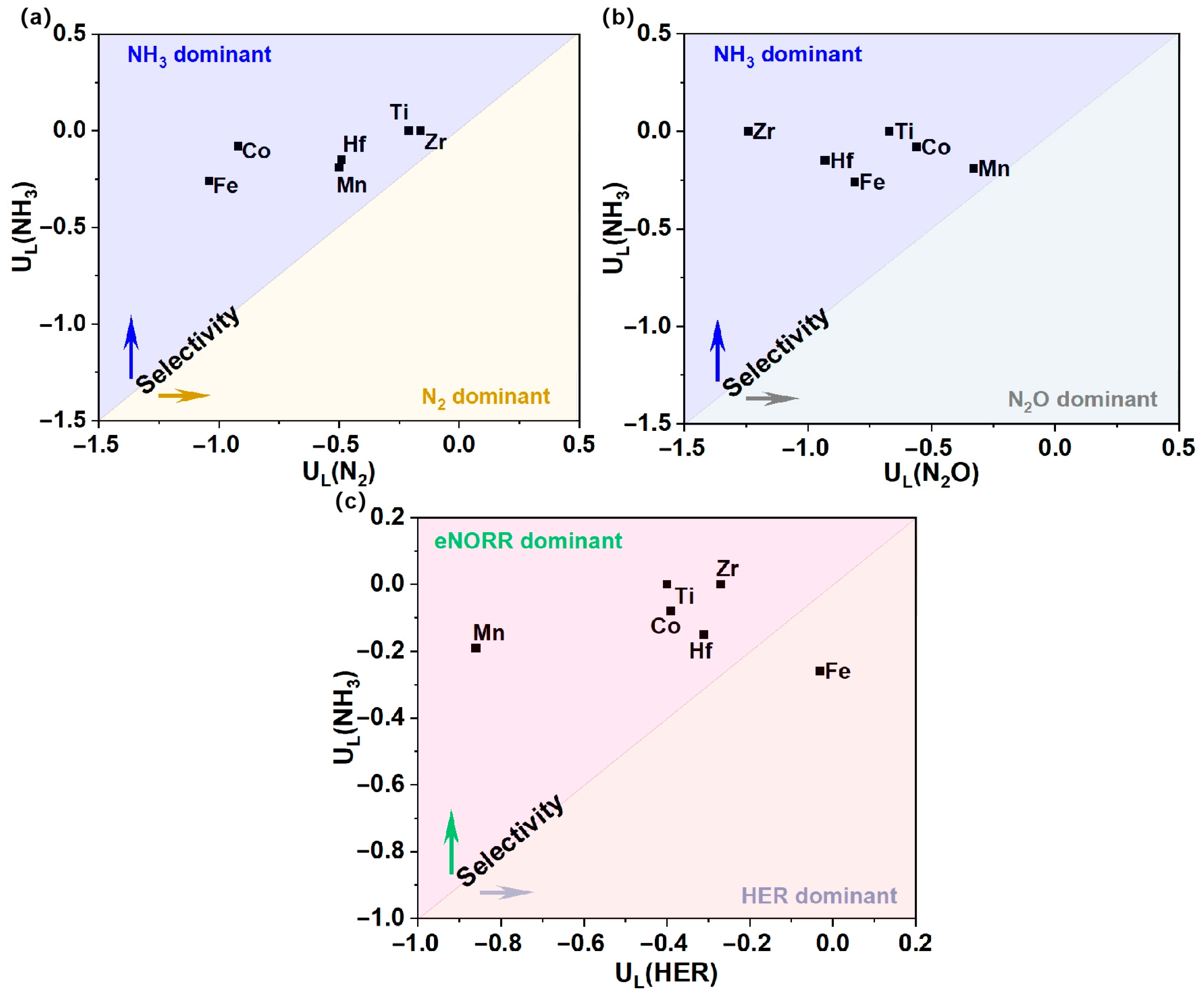
3.4. Selectivity Analysis
To more effectively elucidate the product selectivity of NH3 and N2O/N2, as well as the HER, for the Ti, Mn, Fe, Co, Zr, and Hf@WS2 catalysts, we have constructed a graph depicting the differences in the limiting potentials between NO reduction to NH3 and N2O/N2, as well as the HER. As shown in Figure 8a,b, the Ti, Mn, Fe, Co, Zr, and Hf@WS2 catalysts exhibit a pronounced preference for NH3 production over N2O and N2. Meanwhile, as depicted in Figure 8c, with the exception of the Fe@WS2 catalyst, the remaining five catalysts—Ti, Mn, Co, Zr, and Hf@WS2—maintain a pronounced selectivity for the eNORR over the HER, thereby highlighting their exceptional selectivity for NH3 production.
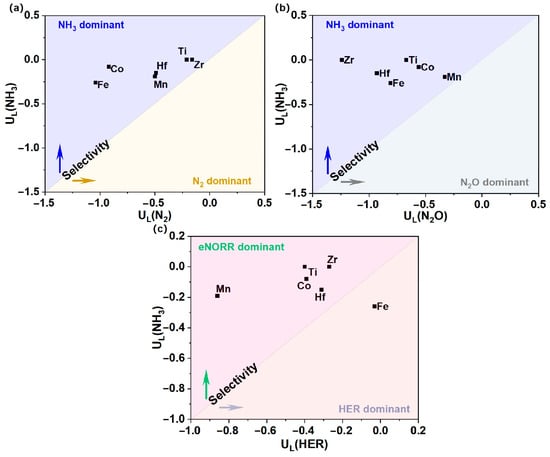
Figure 8.
Selectivity for NH3 production vs. N2O/N2 and HER on TM@WS2 catalysts. (a) UL(NH3) vs. UL(N2); (b) UL(NH3) vs. UL(N2O); (c) UL(NH3) vs. UL(HER).
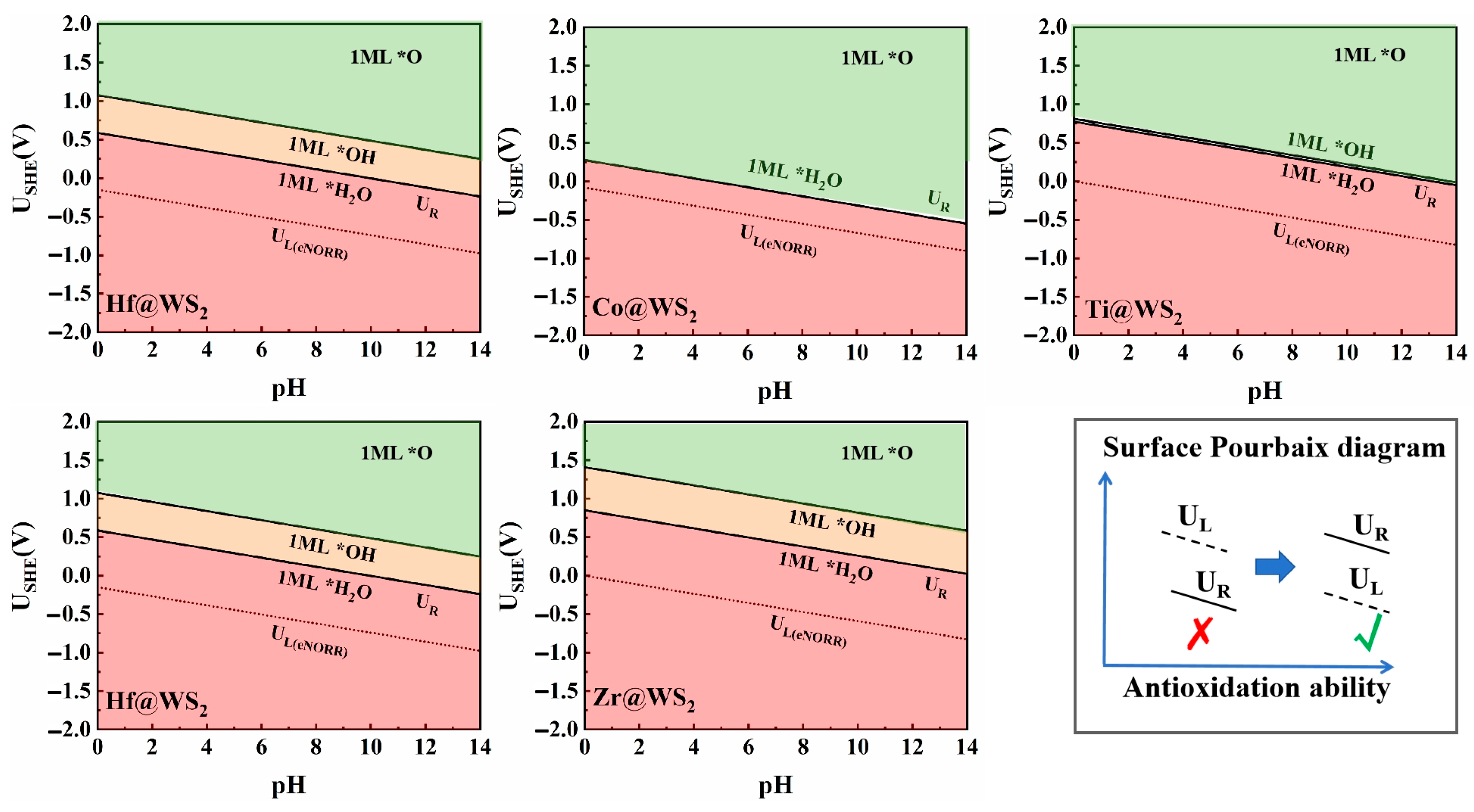
3.5. Pourbaix Diagram Analysis
Since eNORR typically occurs under solution conditions, hydroxyl groups (*OH), oxygen groups (*O), or water molecules (*H2O) present in the solution may adsorb onto the active sites, thereby reducing the activity of catalysts. The Pourbaix diagram, which provides detailed information about the catalyst surface under different pH values and applied voltages [64,65], is a valuable tool for evaluating the electrochemical stability of catalysts. Thus, we employed the Pourbaix diagram to evaluate the electrochemical stability of the TM@WS2 catalysts. As shown in the Figure 9, the minimum redox potentials (UR) required to eliminate *OH/*O on the surfaces of the Mn@WS2, Hf@WS2, Zr@WS2, Co@WS2, and Ti@WS2 catalysts are 0.36 V, 0.59 V, 0.85 V, 0.28 V, and 0.77 V, respectively. When the value of UR is greater than UL, the electrocatalysts exhibit significant oxidation resistance, preventing the adsorption of *OH/*O on the catalyst surface. This, in turn, facilitates the adsorption of NO molecules on the active sites, thereby promoting the subsequent eNORR process. Notably, for the Mn@WS2, Hf@WS2, Zr@WS2, Co@WS2, and Ti@WS2 catalysts, the value of UR is greater than the corresponding UL. This indicates that these catalysts possess both stability and high selectivity for eNORR. Therefore, they can serve as stable and promising candidates for eNORR applications.
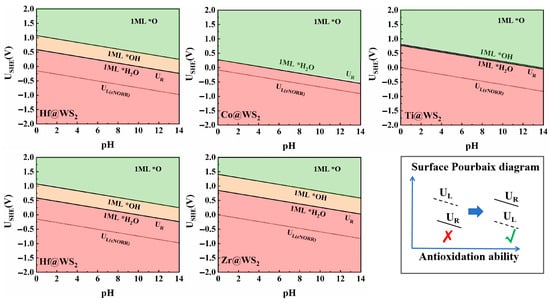
Figure 9.
Surface Pourbaix diagrams of TM@WS2 catalysts.
3.6. Revealing the Origin of Activity
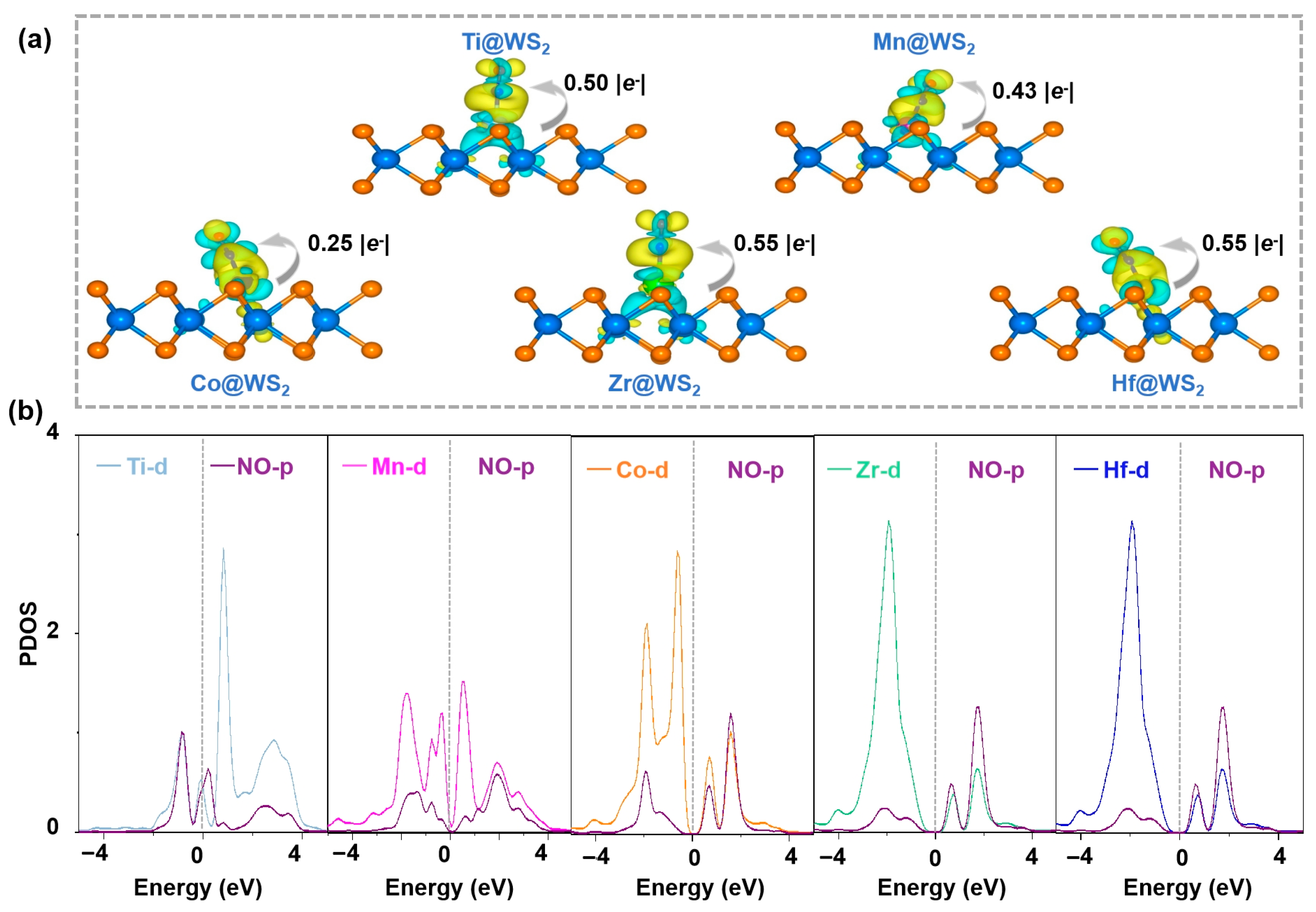
The initial stages of the eNORR process are highly contingent upon the adsorption and activation of NO, as these steps serve as the cornerstone for the entire reaction mechanism. To elucidate the origin of eNORR activity, we performed detailed electronic structure analyses to illustrate the activation of *NO. As depicted in Figure 10a, the analysis of electron density difference maps for five TM@WS2 catalysts reveals substantial charge redistribution occurring between the adsorbed *NO species and the catalyst substrates, primarily characterized by the accumulation of electrons on the *NO. The Bader charge analysis provides additional validation for this observation, demonstrating that the quantity of electrons transferred from the TM@WS2 catalysts to the *NO species varies among different transition metals: 0.50 for Ti, 0.43 for Mn, 0.25 for Co, 0.55 for Zr, and 0.55 for Hf.
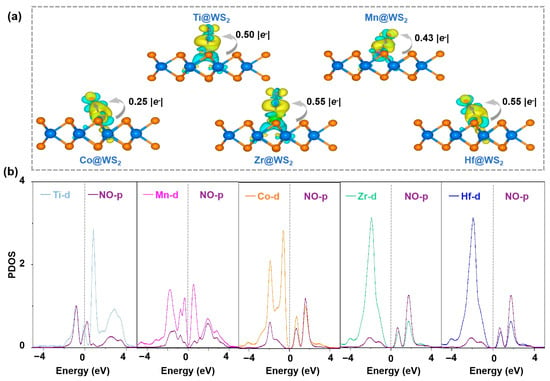
Figure 10.
(a) The charge density difference maps of five TM@WS2 catalysts. In these visualizations, the isosurface threshold is 0.003 e/Å3, yellow regions signify areas of charge accumulation, while cyan regions indicate charge depletion; (b) partial density of states (PDOS) of NO adsorbed on five TM@WS2 catalysts.
A detailed analysis was conducted on the partial density of states (PDOS) for the five catalyst candidates. As shown in Figure 10b, there is a clear overlap between the p orbital of the *NO species and the d orbitals of the Ti, Mn, Co, Zr, and Hf atoms. This overlap indicates a strong interaction between the d orbitals of these metal atoms and the p orbital of *NO, which ultimately promotes the activation (weakening) of the N-O bond in the NO molecule.
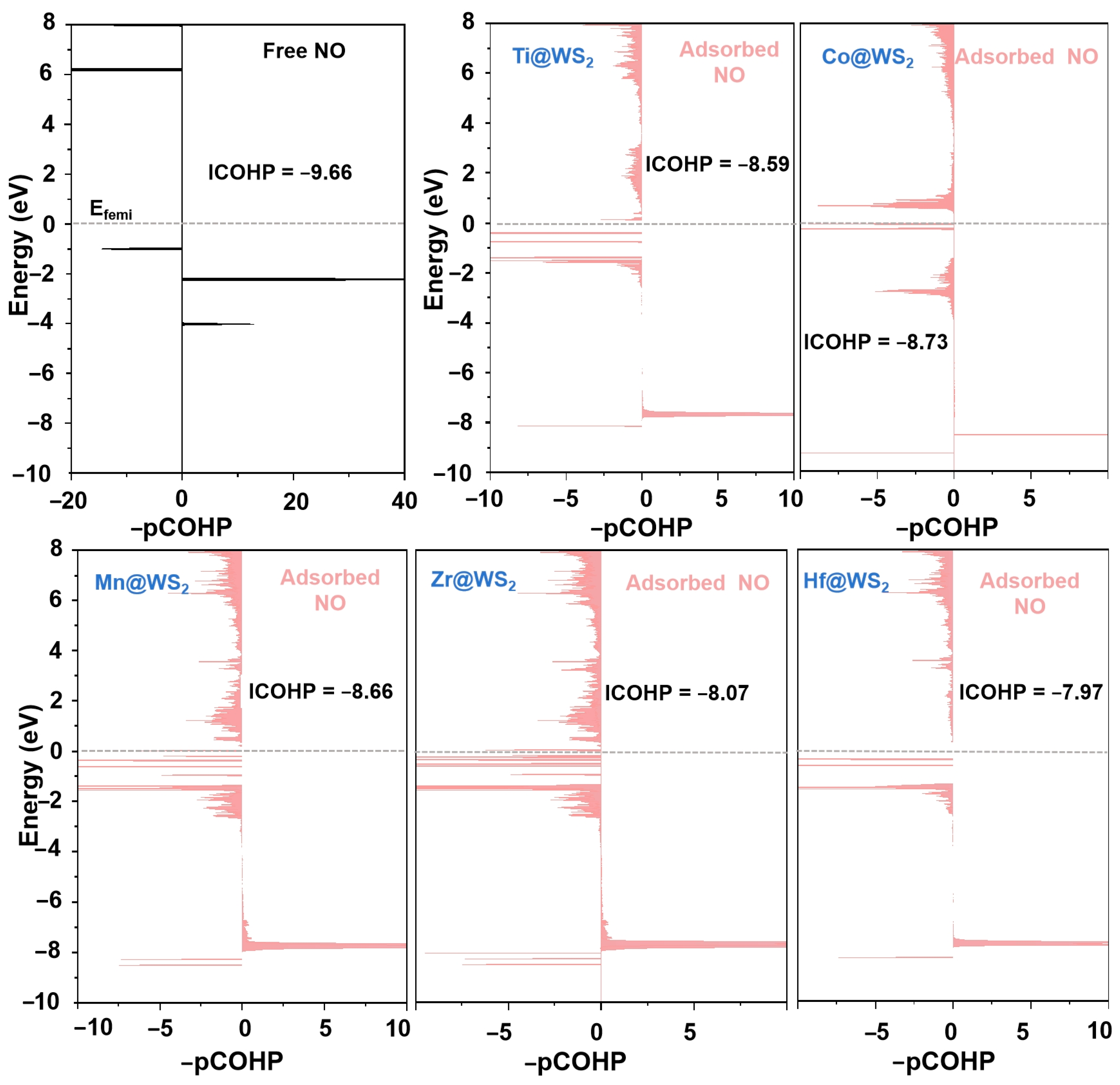
The analysis of the crystal orbital Hamiltonian population (COHP) and the integrated crystal orbital Hamiltonian population (ICOHP) further supports the above findings. These metrics have been established as effective indicators for characterizing the level of NO activation [25,60]. The quantitative determination of ICOHP values is achieved by integrating the energy up to the Fermi level. Typically, a more negative ICOHP value signifies a stronger binding interaction.
As demonstrated in Figure 11, upon NO adsorption, the COHP of the adsorbed *NO exhibits a greater number of antibonding states compared to that of the isolated NO molecule. Additionally, the ICOHP (N-O) values for the N-O bond of *NO adsorbed on Ti, Mn, Co, Zr, and Hf@WS2 catalysts are −8.59, −8.66, −8.73, −8.07, and −7.97, respectively. It is evident that this is less pronounced in negativity compared to that of the isolated NO molecule (−9.66), indicating a substantial weakening of the N-O bond upon adsorption.
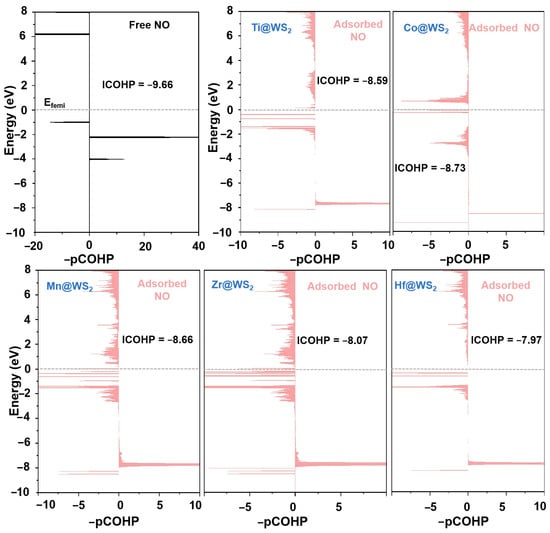
Figure 11.
Projected COHP (-pCOHP) analysis of the N-O bond in free NO molecule (dark) versus that in the *NO species (light red) on five TM@WS2 catalysts.
4. Conclusions
In summary, a range of single-atom catalysts comprising 27 transition metal (TM) atoms deposited on single sulfur vacancy sites of WS2 nanosheets have been successfully designed and their performance in eNORR evaluated using DFT calculations. The results indicate that 19 TM atoms exhibit potential stability. In terms of eNORR activity for NH3 production, the five TM@WS2 catalysts—specifically Ti@WS2, Mn@WS2, Co@WS2, Zr@WS2, and Hf@WS2—show remarkable potential, with their UL’s being 0.00, −0.19, −0.26, 0.00, and −0.15 V, respectively. Moreover, by comparing the differences in the limiting potentials for NO reduction to NH3 and N2O/N2, as well as the HER, we reveal their exceptional selectivity for NH3 production.
This study is significant for both environmental and energy applications, as it provides a sustainable route for ammonia synthesis and reduces NO emissions through electrocatalytic NO reduction. The identified TM@WS2 catalysts, with high activity, selectivity, and stability, are promising for experimental validation. However, limitations exist due to the idealized models and approximations used in DFT calculations, which may not fully reflect real-world complexities such as impurities and solvent conditions. Therefore, close collaboration between theoretical and experimental researchers is essential to validate and refine these predictions.
Overall, this work highlights the potential of TM@WS2 catalysts for efficient NO removal and sustainable NH3 synthesis, emphasizing the need for further experimental research to realize their practical application.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ma18102341/s1. Figure S1: Ab initio molecular dynamics (AIMD) simulations at 500 K forZn@WS2 catalysts; Figure S2: Free energy diagrams of eNORR over undoped defective WS2 catalyst; Table S1: The Gibbs energies of NO adsorptipon on TM@WS2 catalysts via different configurations (eV); Table S2: Limiting potentials of eNORR toward NH3 over different single atom catalysts. References [28,30,32,34,37,38,40,42,44,45,46,47,48,51,66,67,68] are cited in the Supplementary Materials.
Author Contributions
M.T.: conceptualization, data curation, formal analysis, writing—original draft. C.W.: conceptualization, software, writing—review and editing. A.A.: data curation, formal analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the “Fundamental Research Grants for Universities in the Autonomous Region (Grant No. XJEDU2024P114)”, Tianshan Innovation Team Plan of Xinjiang Uygur Autonomous Region (2023D14002), and the “Research Initiation Fund for High-level Talents at Kashi University (Grant No. 022024001)”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| eNORR | Electrocatalytic nitric oxide reduction |
| TM | Transition metal |
| HER | Hydrogen evolution reaction |
| eNO3RR | Electrocatalytic nitrate reduction |
| eNRR | Electrocatalytic N2 reduction reaction |
| SACs | Single-atom catalysts |
| Eb | Binding energies |
References
- Christensen, C.H.; Johannessen, T.; Sørensen, R.Z.; Nørskov, J.K. Towards an ammonia-mediated hydrogen economy? Catal. Today 2006, 111, 140–144. [Google Scholar] [CrossRef]
- Soloveichik, G. Electrochemical synthesis of ammonia as a potential alternative to the Haber–Bosch process. Nat. Catal. 2019, 2, 377–380. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Cherepanov, P.V.; Choi, J.; Suryanto, B.H.R.; Hodgetts, R.Y.; Bakker, J.M.; Ferrero Vallana, F.M.; Simonov, A.N. A Roadmap to the Ammonia Economy. Joule 2020, 4, 1186–1205. [Google Scholar] [CrossRef]
- Lin, Q.F.; Jiang, Y.M.; Liu, C.Z.; Chen, L.W.; Zhang, W.J.; Ding, J.; Li, J.G. Instantaneous hydrogen production from ammonia by non-thermal arc plasma combining with catalyst. Energy Rep. 2021, 7, 4064–4070. [Google Scholar] [CrossRef]
- Wang, M.; Khan, M.A.; Mohsin, I.; Wicks, J.; Ip, A.H.; Sumon, K.Z.; Dinh, C.-T.; Sargent, E.H.; Gates, I.D.; Kibria, M.G. Can sustainable ammonia synthesis pathways compete with fossil-fuel based Haber–Bosch processes? Energy Environ. Sci. 2021, 14, 2535–2548. [Google Scholar] [CrossRef]
- Mohan, N.G.; Ramanujam, K. Electrocatalysts for ammonia synthesis: How close are we to the Haber-Bosch process? Curr. Opin. Electrochem. 2024, 45, 101520. [Google Scholar] [CrossRef]
- Liu, H.-M.; Han, S.-H.; Zhao, Y.; Zhu, Y.-Y.; Tian, X.-L.; Zeng, J.-H.; Jiang, J.-X.; Xia, B.Y.; Chen, Y. Surfactant-free atomically ultrathin rhodium nanosheet nanoassemblies for efficient nitrogen electroreduction. J. Mater. Chem. A 2018, 6, 3211–3217. [Google Scholar] [CrossRef]
- Zeng, Y.; Priest, C.; Wang, G.; Wu, G. Restoring the Nitrogen Cycle by Electrochemical Reduction of Nitrate: Progress and Prospects. Small Methods 2020, 4, 2000672. [Google Scholar] [CrossRef]
- Liu, D.; Qiao, L.; Peng, S.; Bai, H.; Liu, C.; Ip, W.F.; Lo, K.H.; Liu, H.; Ng, K.W.; Wang, S.; et al. Recent Advances in Electrocatalysts for Efficient Nitrate Reduction to Ammonia. Adv. Funct. Mater. 2023, 33, 2303480. [Google Scholar] [CrossRef]
- Liang, X.; Zhu, H.; Yang, X.; Xue, S.; Liang, Z.; Ren, X.; Liu, A.; Wu, G. Recent Advances in Designing Efficient Electrocatalysts for Electrochemical Nitrate Reduction to Ammonia. Small Struct. 2022, 4, 2200202. [Google Scholar] [CrossRef]
- Song, Z.; Qin, L.; Liu, Y.; Zhong, Y.; Guo, Q.; Geng, Z.; Zeng, J. Efficient Electroreduction of Nitrate to Ammonia with CuPd Nanoalloy Catalysts. ChemSusChem 2023, 16, e202300202. [Google Scholar] [CrossRef]
- Qing, G.; Ghazfar, R.; Jackowski, S.T.; Habibzadeh, F.; Ashtiani, M.M.; Chen, C.P.; Smith, M.R., III; Hamann, T.W. Recent Advances and Challenges of Electrocatalytic N2 Reduction to Ammonia. Chem. Rev. 2020, 120, 5437–5516. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Mi, Y.; Bao, H.; Liu, Y.; Qi, D.; Qiu, Y.; Zhuo, L.; Zhao, S.; Sun, J.; Tang, X.; et al. Ambient electrosynthesis of ammonia with efficient denitration. Nano Energy 2020, 78, 105321. [Google Scholar] [CrossRef]
- Miao, R.; Chen, D.; Guo, Z.; Zhou, Y.; Chen, C.; Wang, S. Recent advances in electrocatalytic upgrading of nitric oxide and beyond. Appl. Catal. B-Environ. 2024, 344, 123662. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Karamad, M.; Yong, X.; Huang, Q.; Cullen, D.A.; Zhu, P.; Xia, C.; Xiao, Q.; Shakouri, M.; Chen, F.Y.; et al. Electrochemical ammonia synthesis via nitrate reduction on Fe single atom catalyst. Nat. Commun. 2021, 12, 2870. [Google Scholar] [CrossRef]
- Chen, D.; Yin, D.; Zhang, S.; Yip, S.; Ho, J.C. Nitrate electroreduction: Recent development in mechanistic understanding and electrocatalyst design. Mater. Today Energy 2024, 44, 101610. [Google Scholar] [CrossRef]
- Jiang, H.; Chen, G.F.; Savateev, O.; Xue, J.; Ding, L.X.; Liang, Z.; Antonietti, M.; Wang, H. Enabled Efficient Ammonia Synthesis and Energy Supply in a Zinc-Nitrate Battery System by Separating Nitrate Reduction Process into Two Stages. Angew. Chem. Int. Ed. 2023, 62, e202218717. [Google Scholar] [CrossRef]
- Liu, H.; Xiang, K.; Yang, B.; Xie, X.; Wang, D.; Zhang, C.; Liu, Z.; Yang, S.; Liu, C.; Zou, J.; et al. The electrochemical selective reduction of NO using CoSe2@CNTs hybrid. Environ. Sci. Pollut. Res. Int. 2017, 24, 14249–14258. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; Haller, G.; Li, Y. Recent advances in the selective catalytic reduction of NOx with NH3 on Cu-Chabazite catalysts. Appl. Catal. B Environ. 2017, 202, 346–354. [Google Scholar] [CrossRef]
- Tursun, M.; Wu, C. Vacancy-triggered and dopant-assisted NO electrocatalytic reduction over MoS2. Phys. Chem. Chem. Phys. 2021, 23, 19872–19883. [Google Scholar] [CrossRef]
- Katsounaros, I.; Figueiredo, M.C.; Chen, X.; Calle-Vallejo, F.; Koper, M.T.M. Structure- and Coverage-Sensitive Mechanism of NO Reduction on Platinum Electrodes. ACS Catal. 2017, 7, 4660–4667. [Google Scholar] [CrossRef]
- Shi, J.; Wang, C.; Yang, R.; Chen, F.; Meng, N.; Yu, Y.; Zhang, B. Promoting nitric oxide electroreduction to ammonia over electron-rich Cu modulated by Ru doping. Sci. China Chem. 2021, 64, 1493–1497. [Google Scholar] [CrossRef]
- Tursun, M.; Wu, C. NO Electroreduction by Transition Metal Dichalcogenides with Chalcogen Vacancies. ChemElectroChem 2021, 8, 3113–3122. [Google Scholar] [CrossRef]
- Ko, B.H.; Hasa, B.; Shin, H.; Zhao, Y.; Jiao, F. Electrochemical Reduction of Gaseous Nitrogen Oxides on Transition Metals at Ambient Conditions. J. Am. Chem. Soc. 2022, 144, 1258–1266. [Google Scholar] [CrossRef]
- Tursun, M.; Wu, C. Defective 1T’-MoX2 (X = S, Se, Te) monolayers for electrocatalytic ammonia synthesis: Steric and electronic effects on the catalytic activity. Fuel 2023, 342, 127779. [Google Scholar] [CrossRef]
- Clayborne, A.; Chun, H.-J.; Rankin, R.B.; Greeley, J. Elucidation of Pathways for NO Electroreduction on Pt(111) from First Principles. Angew. Chem. Int. Ed. 2015, 54, 8255–8258. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Jiang, Y.-X.; Tian, N.; Zhou, Z.-Y.; Sun, S.-G. Electrocatalytic reduction of nitric oxide on Pt nanocrystals of different shape in sulfuric acid solutions. Electrochim. Acta 2010, 55, 8273–8279. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, J.; Wang, J.; Cabrera, C.R.; Chen, Z. A Co–N4 moiety embedded into graphene as an efficient single-atom-catalyst for NO electrochemical reduction: A computational study. J. Mater. Chem. A 2018, 6, 7547–7556. [Google Scholar] [CrossRef]
- Li, H.; Wu, D.; Wu, J.; Lv, W.; Duan, Z.; Ma, D. Graphene-based iron single-atom catalysts for electrocatalytic nitric oxide reduction: A first-principles study. Nanoscale 2024, 16, 7058–7067. [Google Scholar] [CrossRef]
- Wang, J.; Li, K.; Hao, Q.; Liu, D.; Zhang, X. Electroreduction NO to NH3 over single metal atom anchored on pyrrole type defective graphene: A DFT study. Chin. Chem. Lett. 2023, 34, 107567. [Google Scholar] [CrossRef]
- Wu, Q.; Huang, B.; Dai, Y.; Heine, T.; Ma, Y. Main-group metal elements as promising active centers for single-atom catalyst toward nitric oxide reduction reaction. Npj 2d Mater. Appl. 2022, 6, 52. [Google Scholar] [CrossRef]
- Tursun, M.; Wu, C. Single Transition Metal Atoms Anchored on Defective MoS2 Monolayers for the Electrocatalytic Reduction of Nitric Oxide into Ammonia and Hydroxylamine. Inorg. Chem. 2022, 61, 17448–17458. [Google Scholar] [CrossRef] [PubMed]
- Ruan, W.; Yang, C.; Hu, J.; Lin, W.; Guo, X.; Ding, K. Investigation of a Single Atom Iron Catalyst for the Electrocatalytic Reduction of Nitric Oxide to Hydroxylamine: A DFT Study. Langmuir 2024, 40, 24062–24073. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zheng, J.; Yao, Z.; Deng, S.; Pan, Z.; Wang, S.; Wang, J. DFT Investigation of Single Metal Atom-Doped 2D MA2Z4 Materials for NO Electrocatalytic Reduction to NH3. J. Phys. Chem. C 2022, 126, 17598–17607. [Google Scholar] [CrossRef]
- Xiao, X.; Cao, Y.; Hu, L. First-principles study on single-layer electronic structure of Fe-doped MoS2 and the reduction of NO on the doped surface. Comput. Theor. Chem. 2025, 1248, 115172. [Google Scholar] [CrossRef]
- Wu, Y.-W.; Wang, H.-W.; Wu, Z.-L.; Zhang, X.; Dong, Y.; Hu, Z.; Lv, Y.; Zhou, X.-Y.; Zhao, L.; Zhang, B.; et al. Mechanism of NO electrocatalytic reduction over the MoS2-based single atom catalyst: A DFT investigation. Sep. Purif. Technol. 2025, 366, 132813. [Google Scholar] [CrossRef]
- Lin, L.; Pang, D.; Shi, P.; Xie, K.; Su, L.; Zhang, Z. First-principles study of TM supported SnSe2 monolayer as an efficient electrocatalyst for NOER. Mol. Catal. 2022, 533, 112789. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, N.; Wang, F.; Kang, J.; Chu, K. Main-group indium single-atom catalysts for electrocatalytic NO reduction to NH3. J. Mater. Chem. A 2023, 11, 6814–6819. [Google Scholar] [CrossRef]
- Venkateswara Rao Nulakani, N.; Surya Kumar Choutipalli, V.; Akbar Ali, M. Efficient electrocatalytic reduction of nitric oxide (NO) to ammonia (NH3) on metal-free B4@g-C3N4 nanosheet. Appl. Surf. Sci. 2025, 680, 161470. [Google Scholar] [CrossRef]
- Guo, W.; Tang, X.; Liao, H.; Peng, J.; Lian, X. Theoretical screening of single-metal atom deposited on 2D BC3N2 monolayers for NO electrocatalytic reduction to NH3. Appl. Surf. Sci. 2025, 690, 162605. [Google Scholar] [CrossRef]
- Xiao, Y.; Shen, C.; Zhang, W.B. Screening and prediction of metal-doped α-borophene monolayer for nitric oxide elimination. Mater. Today Chem. 2022, 25, 100958. [Google Scholar] [CrossRef]
- Yang, L.; Fan, J.; Zhu, W. Single atom decorated wavy antimony nitride for nitric oxide degradation: A first-principles and machine learning study. Fuel 2025, 380, 133219. [Google Scholar] [CrossRef]
- Wang, J.; Sun, C.; Sheng, L.; Zhuo, Z.; Li, S.; Wang, J.; Wang, W.; Sun, J.; Yang, J.; Xu, K.; et al. Unveiling the electrocatalytic potential of main-group metal-embedded BC3 monolayer for highly efficient NO reduction to NH3. Chin. Chem. Lett. 2025, in press. [CrossRef]
- Niu, H.; Zhang, Z.; Wang, X.; Wan, X.; Kuai, C.; Guo, Y. A Feasible Strategy for Identifying Single-Atom Catalysts Toward Electrochemical NO-to-NH3 Conversion. Small 2021, 17, e2102396. [Google Scholar] [CrossRef]
- He, C.-Z.; Zhang, Y.-X.; Wang, J.; Fu, L. Anchor single atom in h-BN assist NO synthesis NH3: A computational view. Rare Met. 2022, 41, 3456–3465. [Google Scholar] [CrossRef]
- Sun, P.F.; Wang, W.L.; Zhao, X.; Dang, J.S. Defective h-BN sheet embedded atomic metals as highly active and selective electrocatalysts for NH3 fabrication via NO reduction. Phys. Chem. Chem. Phys. 2020, 22, 22627–22634. [Google Scholar] [CrossRef]
- Fan, J.; Yang, L.; Zhu, W. Transition metal-anchored BN tubes as single-atom catalysts for NO reduction reaction: A study of DFT and deep learning. Fuel 2025, 386, 134302. [Google Scholar] [CrossRef]
- Wu, J.; Yu, Y.X. A theoretical descriptor for screening efficient NO reduction electrocatalysts from transition-metal atoms on N-doped BP monolayer. J. Colloid. Interface Sci. 2022, 623, 432–444. [Google Scholar] [CrossRef]
- Zang, Y.; Wu, Q.; Wang, S.; Huang, B.; Dai, Y.; Ma, Y. Activating dual atomic electrocatalysts for the nitric oxide reduction reaction through the P/S element. Mater. Horiz. 2023, 10, 2160–2168. [Google Scholar] [CrossRef]
- Liu, S.; Xing, G.; Liu, J.-Y. Computational screening of single-atom catalysts for direct electrochemical NH3 synthesis from NO on defective boron phosphide monolayer. Appl. Surf. Sci. 2023, 611, 155764. [Google Scholar] [CrossRef]
- Tong, T.; Linghu, Y.; Wu, G.; Wang, C.; Wu, C. Nitric oxide electrochemical reduction reaction on transition metal-doped MoSi2N4 monolayers. Phys. Chem. Chem. Phys. 2022, 24, 18943–18951. [Google Scholar] [CrossRef]
- Wang, X.; Wu, J.; Zhang, Y.; Sun, Y.; Ma, K.; Xie, Y.; Zheng, W.; Tian, Z.; Kang, Z.; Zhang, Y. Vacancy Defects in 2D Transition Metal Dichalcogenide Electrocatalysts: From Aggregated to Atomic Configuration. Adv. Mater. 2023, 35, e2206576. [Google Scholar] [CrossRef]
- Zheng, J.; Lebedev, K.; Wu, S.; Huang, C.; Ayvali, T.; Wu, T.S.; Li, Y.; Ho, P.L.; Soo, Y.L.; Kirkland, A.; et al. High Loading of Transition Metal Single Atoms on Chalcogenide Catalysts. J. Am. Chem. Soc. 2021, 143, 7979–7990. [Google Scholar] [CrossRef] [PubMed]
- Tursun, M.; Abdukayum, A.; Wu, C.; Wang, C. Screening WS2−based single−atom catalysts for electrocatalytic nitrate reduction to ammonia. Int. J. Hydrogen Energy 2024, 73, 183–190. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Blochl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Norskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Farberow, C.A.; Dumesic, J.A.; Mavrikakis, M. Density Functional Theory Calculations and Analysis of Reaction Pathways for Reduction of Nitric Oxide by Hydrogen on Pt(111). ACS Catal. 2014, 4, 3307–3319. [Google Scholar] [CrossRef]
- Wang, V.; Xu, N.; Liu, J.-C.; Tang, G.; Geng, W.-T. VASPKIT: A user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comput. Phys. Commun. 2021, 267, 108033. [Google Scholar] [CrossRef]
- Mathew, K.; Sundararaman, R.; Letchworth-Weaver, K.; Arias, T.A.; Hennig, R.G. Implicit solvation model for density-functional study of nanocrystal surfaces and reaction pathways. J. Chem. Phys. 2014, 140, 084106. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhu, Z.; Chen, J.; Xia, L.; Gan, L.; Zhou, Y. Evaluating the efficiency of single-double atom catalysts in electrochemical NH3 production from NO based on CN monolayers. J. Mater. Chem. A 2024, 12, 14035–14044. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, Y.; Liu, W.; Yang, D.; Zhou, G.; Yang, Z. Exploring the Catalytic Performance of Oxygen-Coordinated Single-Atom Catalysts for Nitric Oxide Electroreduction. J. Phys. Chem. C 2025, 129, 3522–3530. [Google Scholar] [CrossRef]
- Guo, X.; Lin, S.; Gu, J.; Zhang, S.; Chen, Z.; Huang, S. Establishing a Theoretical Landscape for Identifying Basal Plane Active 2D Metal Borides (MBenes) toward Nitrogen Electroreduction. Adv. Funct. Mater. 2020, 31, 2008056. [Google Scholar] [CrossRef]
- Wu, Q.; Wei, W.; Lv, X.; Wang, Y.; Huang, B.; Dai, Y. Cu@g-C3N4: An Efficient Single-Atom Electrocatalyst for NO Electrochemical Reduction with Suppressed Hydrogen Evolution. J. Phys. Chem. C 2019, 123, 31043–31049. [Google Scholar] [CrossRef]
- Lin, L.; Yan, L.; Fu, L.; He, C.; Xie, K.; Zhu, L.; Sun, J.; Zhang, Z. First principle investigation of W/P3C sheet as an efficient single atom electrocatalyst for N2 and NO electrochemical reaction with suppressed hydrogen evolution. Fuel 2022, 308, 122068. [Google Scholar] [CrossRef]
- Liu, L.; Zuo, Z.J.; Du, Y.; Wu, T.; Wu, J.; Gao, J.; Mu, T.; Zhang, Y.C.; Zhu, X.D. Role of synergies of Cu/Fe3O4 electrocatalyst for nitric oxide reduction to ammonia. J. Colloid Interface Sci. 2025, 691, 137376. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).