Abstract
The rational development of single-atom catalysts (SACs) for selective formic acid dehydrogenation (FAD) requires an atomic-scale understanding of metal–support interactions and electronic modulation. In this study, spin-polarized density functional theory (DFT) calculations were performed to systematically examine platinum-group SACs anchored on graphitic carbon nitride (g-C3N4). The findings reveal that Pd and Au SACs exhibit superior selectivity toward the dehydrogenation pathway, lowering the free energy barrier by 1.42 eV and 1.39 eV, respectively, compared to the competing dehydration route. Conversely, Rh SACs demonstrate limited selectivity due to nearly equivalent energy barriers for both reaction pathways. Stability assessments indicate robust metal–support interactions driven by d–p orbital hybridization, while a linear correlation is established between the d-band center position relative to the Fermi level and catalytic selectivity. Additionally, charge transfer (ranging from 0.029 to 0.467 e) substantially modulates the electronic structure of the active sites. These insights define a key electronic descriptor for SAC design and offer a mechanistic framework for optimizing selective hydrogen production.
1. Introduction
Energy is the cornerstone of modern civilization, driving both economic development and technological advancement. However, the extensive reliance on fossil fuels has led to an energy crisis and severe environmental degradation, necessitating the development of sustainable alternatives [1]. Hydrogen, with its high gravimetric energy density, abundance, and environmentally benign combustion byproduct (water), has emerged as a promising energy carrier [2]. Despite these advantages, practical limitations in hydrogen storage and transportation hinder its widespread implementation [3].
Conventional storage methods, including high-pressure and cryogenic systems, pose significant safety risks [4]. In contrast, chemical hydrogen storage via liquid organic hydrogen carriers (LOHCs) offers a safer and more efficient alternative [5], enabling reversible hydrogen release under mild catalytic conditions. Among LOHCs, formic acid is particularly attractive due to its high hydrogen content (4.4 wt%), ambient stability, low toxicity, and non-flammability [6]. Furthermore, formic acid can be synthesized via photocatalytic or electrocatalytic CO2 reduction [7], contributing to a sustainable carbon-neutral cycle.
Formic acid decomposition follows two competing pathways [8]: dehydrogenation (HCOOH → H2 + CO2, ΔG = −32.8 kJ/mol) and dehydration (HCOOH → H2O + CO, ΔG = −14.9 kJ/mol). Selectively promoting the dehydrogenation route is critical for high-purity hydrogen production, as CO from the dehydration pathway poisons Pt-based formic acid dehydrogenation (FAD) catalysts [9]. This challenge has driven extensive research efforts to develop catalysts that achieve near-complete dehydrogenation under mild conditions.
Since Coffey et al. first reported the catalytic dehydrogenation of formic acid in the 1960s [10], a multitude of numerous homogeneous catalysts, particularly those based on Ir and Ru, have demonstrated remarkable activity in this process. However, these systems suffer from drawbacks such as high ligand costs, poor stability, and complex recovery processes, limiting their large-scale application [11]. In contrast, heterogeneous catalysts, particularly those based on noble metals such as Au, Pt, and Pd, offer advantages in recyclability and scalability [12,13]. However, each metal presents challenges: Pd is susceptible to CO poisoning, Au exhibits low intrinsic activity at low temperatures, and non-precious metals, while stable, generally lack selectivity. To enhance catalytic performance, strategies such as alloying (e.g., Ag, Cu, Cr, Mn, Co) [14] and support engineering (e.g., activated carbon, carbon nanotubes, metal–organic frameworks, TiO2) [15] have been explored. These approaches aim to optimize noble metal utilization while balancing activity and stability. However, challenges such as structural degradation and mass transport limitations persist [16]. The scarcity and cost of noble metals have further spurred interest in non-precious metal catalysts, including Co, Ni, and Cu-based systems [17]. While these catalysts demonstrate long-term stability, they often exhibit poor selectivity, sluggish kinetics, and a tendency toward dehydration side reactions [18]. Consequently, the development of efficient formic acid dehydrogenation (FAD) catalysts remains an ongoing challenge.
Recent advancements in single-atom catalysts (SACs) have shown significant promise by maximizing atomic efficiency and enhancing selectivity. Bulusev et al. [19] synthesized single Pd atoms supported on N-doped carbon, achieving excellent catalytic stability and activity at 60 °C. Density functional theory (DFT) calculations indicated that Pd atoms preferentially bind to pyridinic N-edge sites, enhancing catalytic performance. Han et al. [20] developed Pt-modified Te nanowires that achieved 100% selective hydrogen production, while Yang et al. [20] demonstrated that single-atom Pt/TiN catalysts effectively modulate the activity and selectivity of small organic molecules, favoring the direct dehydrogenation pathway in formic acid decomposition. Additionally, Shi et al. [20] reported a non-precious metal Co-based SAC with a hydrogen production rate of 1403.8 mL g⁻1 h⁻1, which is 15 times higher than that of commercial Pd/C catalysts.
Despite these advances, the high surface energy of single atoms and atomic clusters often leads to aggregation, resulting in catalyst deactivation. Thus, the selection of appropriate support material is crucial for stabilizing isolated metal atoms and preventing agglomeration. Among potential supports, graphitic carbon nitride (g-C3N4) has emerged as an excellent candidate due to its structural stability, unique electronic properties, and high porosity, which facilitate strong metal–support interactions [21]. SACs based on Pt-group metals (Pt, Pd, Ru) [22] have demonstrated remarkable catalytic activity and H2 selectivity, attributed to their electronic structure and well-defined metal–support interactions.
In this study, we investigate Pt-group SACs (M = Ru, Rh, Pd, Ag, Ir, Pt, Au) anchored on g-C3N4 using spin-polarized DFT calculations. By correlating d-band center positions, charge redistribution, and reaction energetics, we identify Pd and Au SACs as promising catalysts that favor dehydrogenation over dehydration, thereby suppressing CO formation. This work not only reveals a linear correlation relationship between the d-band center and selectivity but also provides valuable insights for the rational design of efficient SACs for hydrogen production.
2. Methods
Density functional theory (DFT) calculations were carried out using the hybrid Gaussian and plane wave (GPW) method [23] within the QUICKSTEP module of the CP2K 2023.1 package [24]. We utilized the double-zeta valence plus polarization (DZVP-MOLOPT-SR-GTH) basis set along with the Goedecker–Teter–Hutter (GTH) pseudopotential [25,26,27] for calculation. Electron density was expanded in an auxiliary plane wave basis set with a cutoff energy of 500 Ry. Exchange–correlation interactions were described using the spin-polarized Perdew–Burke–Ernzerhof (PBE) functional [28] under the generalized gradient approximation (GGA), with dispersion corrections applied via the DFT-D3 method of Grimme et al. [29].
The Kohn–Sham equations were solved using conventional matrix diagonalization, with Fermi–Dirac smearing (electronic temperature: 300 K) applied to enhance convergence. To improve computational efficiency, diagonalization routines from the ELPA library were employed [29]. Geometry optimizations were performed with energy and force convergence thresholds of 1.0 × 10−6 eV and 3.0 × 10⁻4 eV Å−1, respectively, while static calculations employed a tighter convergence criterion of 1.0 × 10−8 eV.
Brillouin zone sampling was achieved using a 3 × 3 × 1 Monkhorst-Pack k-point mesh for both g-C3N4 and M@g-C3N4 SACs. A 20 Å vacuum layer was introduced to eliminate spurious periodic interactions. Thermodynamic corrections and density of states (DOS) analyses were conducted using the Shermo v.2.6 [30] and Multiwfn v.3.8 [31] software packages.
3. Results and Discussion
3.1. Structure Stability and Metal–Support Interactions
Pt-group metals were chosen for their well-documented catalytic performance in formic acid dehydrogenation (FAD). The g-C3N4 substrate, consisting of 24 carbon and 32 nitrogen atoms, serves as an ideal optimal framework for single-atom anchoring. Previous studies [32] have identified the hollow site as the most thermodynamically favorable position for metal incorporation. Upon adsorption, a stable coordination environment is established between the metal atom and surrounding nitrogen atoms, effectively stabilizing the M@g-C3N4 SACs.
Figure 1 illustrates the atomic structures of pristine g-C3N4 and various M@g-C3N4 SACs. While the undoped substrate retains a planar structure, metal doping induces localized distortions. Notably, Ag, Au, Pd, and Rh occupy nearly central positions within the g-C3N4 cavity, indicating symmetric coordination with the six surrounding nitrogen atoms. In contrast, Ir, Pt, and Ru exhibit off-center displacements, with Ir and Ru preferentially coordinating with two nitrogen atoms, whereas Pt binds to three nitrogen atoms, reflecting distinct metal–support interactions. The most significant factor contributing to this trend in M@g-C3N4 structures is the size of the cationic metal atoms [33]. The ionic radius of early-transition metal atoms (such as Ru in the fifth period, Ir and Pt in the sixth period) is larger than that of late-transition metal atoms (e.g., Rh, Pd, and Ag in the fifth period; Au in the sixth period). To accommodate these larger early-transition metal atoms ions, flexible g-C3N4 undergoes structural distortions.
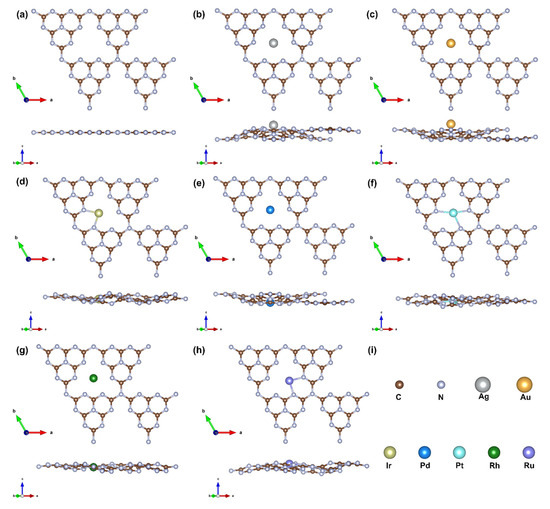
Figure 1.
Atomic structure of the ground state of single-atom catalysts: (a) g-C3N4, (b) Ag@g-C3N4, (c) Au@g-C3N4, (d) Ir@g-C3N4, (e) Pd@g-C3N4, (f) Pt@g-C3N4, (g) Rh@g-C3N4, (h) Ru@g-C3N4. (i) The colors represent various elements.
The stability of SACs is crucial for their catalytic performance. To evaluate their thermodynamic stability, the binding energy (Eb) of each SAC was computed as follows [34]:
where EM@g-C3N4, EM, and Eg-C3N4 represent the total energies of the SACs system, isolated metal atom, and pristine g-C3N4 substrate, respectively. A negative binding energy confirms the thermodynamic stability of M@g-C3N4 SACs, with more negative values, with the magnitude of these energies reflecting the strength of the metal–support interactions, indicating greater structural stability.
As shown in Figure 2, all SACs exhibit negative binding energies, reinforcing their thermodynamic feasibility. The stability trend is as follows: Ag > Ru > Rh > Pt > Ir > Pd > Au. As illustrated in Figure S1, the d orbitals of metal atoms in M@g-C3N4 SACs exhibit strong electronic interactions with the p orbitals of C and N in the g-C3N4 framework [35]. These interactions contribute to the remarkable stability of M@g-C3N4 SACs and serve as a fundamental basis for understanding their catalytic performance in FAD reactions.
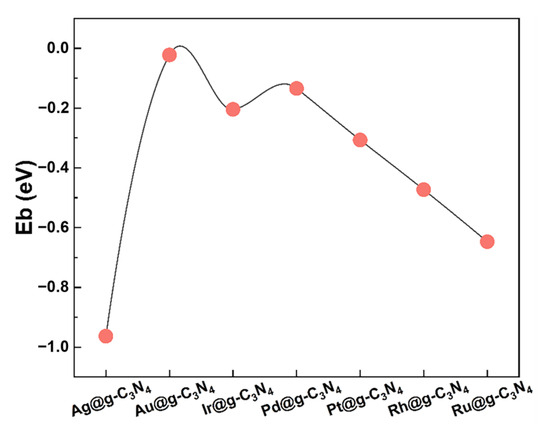
Figure 2.
Binding energy of M@g-C3N4 SACs.
3.2. Adsorption Behavior and Pathways for FAD
The adsorption behavior of formic acid and its key intermediates (HCOO, COOH, and CO) on the M@g-C3N4 SACs surfaces was evaluated to understand the reaction mechanism. The adsorption energy is defined as [36]
where Ead, EM@g-C3N4+x, EM@g-C3N4, and Ex correspond to the adsorption energy, total energy of the SACs with an adsorbate, total energy of pristine SAC, and the energy of gas-phase adsorbates, respectively.
We first computed the adsorption energies (Figure 3a and Table S1) of HCOOH in both cis (HCOOHcis) and trans (HCOOHtrans) configurations, as well as its dehydrogenation and dehydration intermediates. These include the bidentate (HCOObi) and monodentate (HCOOmo) formats of formate, which have been identified through in situ infrared spectroscopy [37], along with COOH (cis and trans) [38], and the final CO product. Our calculations reveal that HCOOH adsorption is exothermic across all SACs, with Ead values ranging from −0.01 to −1.01 eV, suggesting its favorable interaction with the catalyst surface. The bidentate formate (HCOObi) intermediate preferentially adsorbs on Pt and Ru SACs, whereas its monodentate counterpart (HCOOmo) exhibits weaker binding across all SACs, indicating that monodentate HCOO may be a crucial reaction intermediate in formic acid decomposition. Similarly, COOH adsorption is exothermic (−0.20 to −3.23 eV), while CO strongly adsorbs to all SACs, particularly on Pt (−3.08 eV) and Rh (−3.09 eV), where Ead values exceed −3.00 eV. To further refine our thermodynamic analysis, we incorporated zero-point energy and vibrational contributions to compute the adsorption free energy (ΔG), as shown in Figure 3b and Table S2. Notably, while ΔG for CO adsorption remained negative across all SACs, the inclusion of water molecules led to positive ΔG values on Ir and Pt SACs, indicating potential CO desorption in aqueous environments.
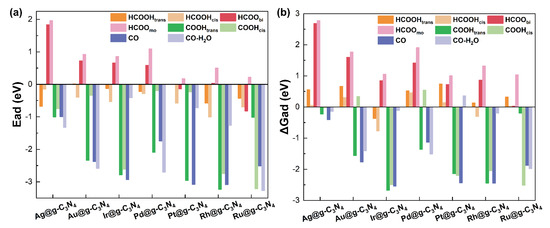
Figure 3.
(a) Adsorption energy and (b) adsorption free energy of reactants and key intermediates on M@g-C3N4 SACs surface.
To elucidate the catalytic mechanism, we investigated two competing pathways for formic acid decomposition: dehydrogenation (Path 1, via the HCOO intermediate) and dehydration (Path 2, via the COOH intermediate), as illustrated in Scheme 1.
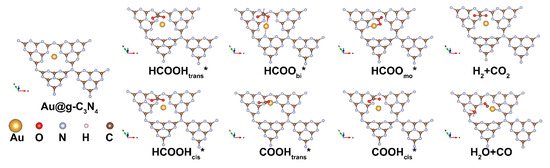
Scheme 1.
Dehydrogenation and dehydration pathways of formic acid on Au@g-C3N4 surface, where the * represents molecules adsorbed on the surface of the catalyst.
Path 1 (dehydrogenation): Formic acid initially adsorbs onto the SAC surface in a trans configuration, undergoes O–H bond cleavage to form bidentate HCOO, and then transitions to a monodentate structure before C–H bond cleavage releases H2 and CO2. Path 2 (dehydration): The reaction proceeds through cis-configured formic acid, followed by C–H bond cleavage yielding trans-configured COOH, which subsequently transitions to a cis configuration before undergoing O–O bond cleavage to generate H2O and CO.
3.3. Catalytic Activity and Selectivity
The free energy profiles (Figure 4) highlight significant differences between these pathways on different SACs. Among the various SACs, the maximum elementary step free energy barriers for dehydrogenation were 1.81 eV (Ag), 0.48 eV (Au), 0.49 eV (Ir), 0.51 eV (Pd), 0.28 eV (Pt), 0.45 eV (Rh), and 1.01 eV (Ru), while the free energy barriers for dehydration were 1.18 eV (Ag), 1.90 eV (Au), 1.24 eV (Ir),1.90 eV (Pd), 1.21 eV (Pt), 0.49 eV (Rh), and 1.98 eV (Ru).
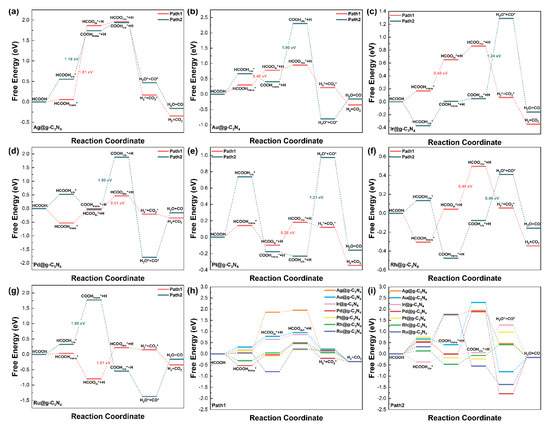
Figure 4.
Free energy diagram for the decomposition of formic acid via dehydrogenation (Path1) and dehydration (Path2) pathways on (a) Ag@g-C3N4 SAC, (b) Au@g-C3N4 SAC, (c) Ir@g-C3N4 SAC, (d) Pd@g-C3N4 SAC, (e) Pt@g-C3N4 SAC, (f) Rh@g-C3N4 SAC, (g) Ru@g-C3N4 SAC, and the free energy for (h) Path1, and (i) Path2 of M@g-C3N4 SACs, where the * represents molecules adsorbed on the surface of the catalyst.
As shown in Figure 4, the rate-determining step (RDS) in the dehydrogenation pathway of formic acid involves O–H bond cleavage in HCOOH for Ag, Au, and Ir SACs, whereas for Pd, Pt, Rh, and Ru SACs, the RDS corresponds to a configurational transformation of the adsorbed HCOO intermediate. In the competing dehydration pathway, the RDS is assigned to C–H bond cleavage in formic acid for Ag and Ru SACs, to an adsorption configuration transition of COOH for Au and Pd SACs, and to O–C bond cleavage in COOH for Ir, Pt, and Rh SACs.
The Au and Pd SACs display notably lower energy barriers for dehydrogenation compared to dehydration, with reductions of approximately 1.42 eV for Au and 1.39 eV for Pd. This energetic preference strongly favors the production of H2 over the formation of CO, which is a critical advantage considering CO’s detrimental effect on catalyst longevity, making them promising candidates for selective H2 generation, as shown in Table S3. In contrast, Rh SACs exhibit similar energy barriers for both pathways, leading to non-selective behavior that may compromise the purity of the hydrogen produced.
To understand the origin of Pd SAC selectivity, we analyzed the density of states (DOS) and d-band center of M@g-C3N4 SACs (Figure 5). The sharp, well-defined d-orbital peaks suggest near-free-electron behavior [39].
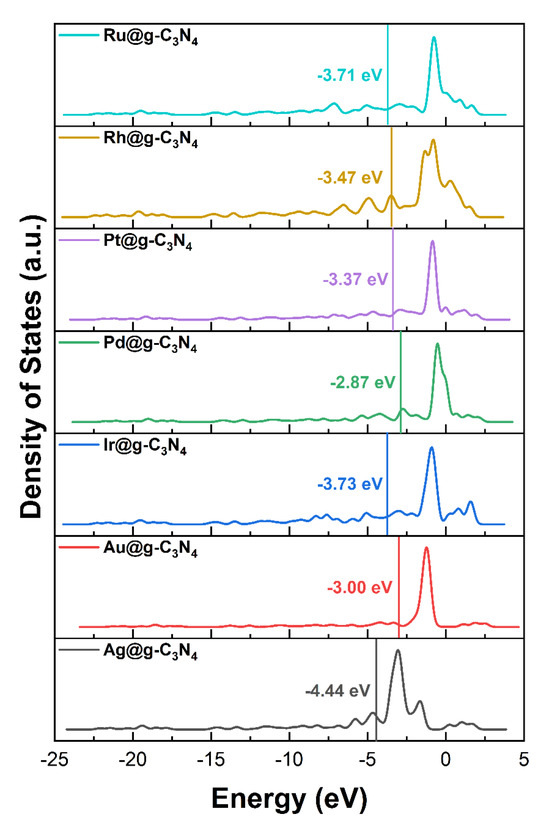
Figure 5.
The d orbital density of state and d-band center of M@g-C3N4.
As shown in Figure 6a, the d-band center exhibits a volcano-type relationship with atomic number for period IV metal SACs, whereas it shifts monotonically positive for period V metals. More importantly, as shown in Figure 6b, d-band center correlates linearly with formic acid decomposition selectivity, which indicates the SACs with a d-band center closer to the Fermi level exhibit higher selectivity toward dehydrogenation. This trend suggests that the electronic structure can be used as a predictive descriptor for catalyst performance. The proximity of the d-band center to the Fermi level directly influences the strength of the intermediate adsorption reaction on the catalyst [40]. Consequently, tuning the electronic structure can effectively alter the reactivity and selectivity of the catalytic material.
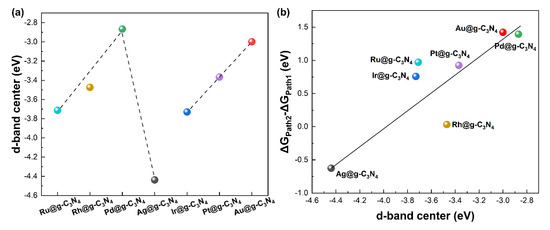
Figure 6.
Relationship between the d band center of (a) atomic number and (b) formic acid decomposition selectivity.
Further, as shown in Figure 7, the Mulliken charge analysis indicates that charge transfer occurs from the metal atom to the g-C3N4 support, with values of 0.467 (Ag), −0.024 (Au), 0.164 (Ir), 0.222 (Pd), 0.073 (Pt), 0.244 (Rh), and 0.029 e (Ru). This redistribution of charge modifies the electronic environment at the active sites, influencing both the adsorption strengths of intermediates and the overall reaction kinetics. The modulation of electronic properties through metal–support interactions provides a clear pathway for tailoring catalyst performance, emphasizing the potential of electronic descriptors in guiding the rational design of next-generation SACs.
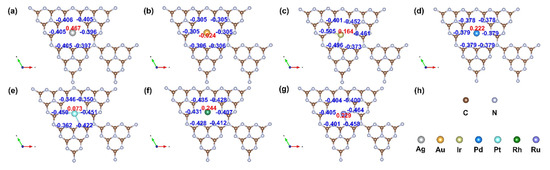
Figure 7.
The Mulliken atomic charge distribution of (a) Ag@g-C3N4, (b) Au@g-C3N4, (c) Ir@g-C3N4, (d) Pd@g-C3N4, (e) Pt@g-C3N4, (f) Rh@g-C3N4, (g) Ru@g-C3N4. (h) The colors represent various elements.
Overall, our results demonstrate that the careful selection of metal species and support material can effectively steer the reaction pathway toward selective dehydrogenation of formic acid. The combined insights from structural, energetic, and electronic analyses pave the way for designing catalysts that optimize hydrogen production while minimizing undesirable side reactions. Future work will focus on experimental validation and the exploration of bimetallic systems to further enhance catalytic performance.
4. Conclusions
This study elucidates the structure–activity relationships governing Pt-group SACs supported on g-C3N4 for formic acid dehydrogenation. Pd and Au SACs emerge as highly selective catalysts, achieving dehydrogenation barriers below 0.51 eV while suppressing CO formation through unfavorable dehydration pathways. Rh SACs, despite high activity, suffer from poor selectivity due to nearly equivalent barriers for both reaction routes. Moreover, Pd and Au emerge as promising catalytic candidates for hydrogen generation from formic acid, owing to their relatively lower cost compared to Rh. The thermodynamic stability of SACs is attributed to strong metal–support interactions, evidenced by negative binding energies and d–p orbital hybridization. A linear correlation between the d-band center position and selectivity highlights the critical role of electronic structure in catalytic performance. Charge redistribution (0.029–0.467 e) further fine-tunes the metal centers’ reactivity. These results underscore the potential of g-C3N4-supported SACs for efficient hydrogen production and provide a roadmap for optimizing catalyst design through electronic modulation and support engineering. Future work should focus on experimental validation and exploring synergistic effects of bimetallic SACs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ma18102328/s1, Figure S1: The density of states of p orbital of CN the d orbital of a metal atom in M@g-C3N4; Table S1: Adsorption energy of reactants and key intermediates on M@g-C3N4 SACs surface; Table S2: Adsorption free energy of reactants and key intermediates on M@g-C3N4 SACs surface, and Table S3: The d-band center, dehydrogenation reaction (Path1) free energy barrier, dehydration reaction (Path2) free energy barrier, formic acid decomposition selectivity and binding energy of M@g-C3N4 SACs.
Author Contributions
Conceptualization, T.J. and K.P.; methodology, T.J., S.L. and J.Z.; software, T.J.; validation, Y.L. and Y.B.; formal analysis, H.W.; investigation, I.R.; data curation, T.J.; writing—original draft preparation, T.J.; data curation and writing—review and editing, F.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Frontier Exploration Projects of Longmen Laboratory (NO. LMQYQN202408), the Frontier Exploration Projects of Longmen Laboratory (LMQYTSKT013), the Key Technologies R & D Program of Henan Province (252102240069), the Henan Province Major Science and Technology Project (241100240400), and the Natural Science Foundation of Henan Province (252300420790).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| DFT | Density Functional Theory |
| SACs | Single Atom Catalysts |
| FAD | Formic Acid Dehydrogenation |
| g-C3N4 | graphitic carbon nitride |
References
- Wang, C.R.; Stansberry, J.M.; Mukundan, R.; Chang, H.-M.J.; Kulkarni, D.; Park, A.M.; Plymill, A.B.; Firas, N.M.; Liu, C.P.; Lang, J.T.; et al. Proton exchange membrane (PEM) water electrolysis: Cell-level considerations for gigawatt-scale deployment. Chem. Rev. 2025, 125, 1257–1302. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Ullah, A.; Samreen, A.; Qasim, M.; Nawaz, K.; Ahmad, W.; Alnaser, A.; Kannan, A.M.; Egilmez, M. Hydrogen production, storage, transportation and utilization for energy sector: A current status review. J. Energy Storage 2024, 101, 113733. [Google Scholar] [CrossRef]
- Squadrito, G.; Maggio, G.; Nicita, A. The green hydrogen revolution. Renew. Energy 2023, 216, 119041. [Google Scholar] [CrossRef]
- Zhu, Q.-L.; Xu, Q. Liquid organic and inorganic chemical hydrides for high-capacity hydrogen storage. Energy Environ. Sci. 2015, 8, 478–512. [Google Scholar] [CrossRef]
- Yadav, M.; Xu, Q. Liquid-phase chemical hydrogen storage materials. Energy Environ. Sci. 2012, 5, 9698. [Google Scholar] [CrossRef]
- Chen, B.W.J.; Mavrikakis, M. Formic acid: A hydrogen-bonding cocatalyst for formate decomposition. ACS Catal. 2020, 10, 10812–10825. [Google Scholar] [CrossRef]
- Martin, C.; Quintanilla, A.; Vega, G.; Casas, J.A. Formic acid-to-hydrogen on pd/AC catalysts: Kinetic study with catalytic deactivation. Appl. Catal. B 2022, 317, 121802. [Google Scholar] [CrossRef]
- Dai, H.; Xia, B.; Wen, L.; Du, C.; Su, J.; Luo, W.; Cheng, G. Synergistic catalysis of AgPd@ZIF-8 on dehydrogenation of formic acid. Appl. Catal. B 2015, 165, 57–62. [Google Scholar] [CrossRef]
- Choi, B.-S.; Song, J.; Song, M.; Goo, B.S.; Lee, Y.W.; Kim, Y.; Yang, H.; Han, S.W. Core–shell engineering of pd–Ag bimetallic catalysts for efficient hydrogen production from formic acid decomposition. ACS Catal. 2019, 9, 819–826. [Google Scholar] [CrossRef]
- Coffey, R.S. A catalyst for the homogeneous hydrogenation of aldehydes under mild conditions. Chem. Commun. 1967, 18, 923a. [Google Scholar] [CrossRef]
- Boddien, A.; Junge, H. Acidic ideas for hydrogen storage. Nat. Nanotechnol. 2011, 6, 265–266. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhou, X.; Yin, M.; Liu, C.; Xing, W. Novel PdAu@Au/C core−shell catalyst: Superior activity and selectivity in formic acid decomposition for hydrogen generation. Chem. Mater. 2010, 22, 5122–5128. [Google Scholar] [CrossRef]
- Yin, R.; Jiang, B.; Guo, H. Mechanism and dynamics of CO2 formation in formic acid decomposition on Pt surfaces. ACS Catal. 2022, 12, 6486–6494. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, N.; Xu, Q.; Yu, J. Nanopore-supported metal nanocatalysts for efficient hydrogen generation from liquid-phase chemical hydrogen storage materials. Adv. Mater. 2020, 32, 2001818. [Google Scholar] [CrossRef]
- Li, Z.; Yang, X.; Tsumori, N.; Liu, Z.; Himeda, Y.; Autrey, T.; Xu, Q. Tandem nitrogen functionalization of porous carbon: Toward immobilizing highly active palladium nanoclusters for dehydrogenation of formic acid. ACS Catal. 2017, 7, 2720–2724. [Google Scholar] [CrossRef]
- Yu, W.-Y.; Mullen, G.M.; Flaherty, D.W.; Mullins, C.B. Selective hydrogen production from formic acid decomposition on pd–Au bimetallic surfaces. J. Am. Chem. Soc. 2014, 136, 11070–11078. [Google Scholar] [CrossRef]
- Onishi, N.; Iguchi, M.; Yang, X.; Kanega, R.; Kawanami, H.; Xu, Q.; Himeda, Y. Development of effective catalysts for hydrogen storage technology using formic acid. Adv. Energy Mater. 2019, 9, 1801275. [Google Scholar] [CrossRef]
- Nie, R.; Tao, Y.; Nie, Y.; Lu, T.; Wang, J.; Zhang, Y.; Lu, X.; Xu, C.C. Recent Advances in Catalytic Transfer Hydrogenation with Formic Acid over Heterogeneous Transition Metal Catalysts. ACS Catal. 2021, 11, 1071–1095. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Zacharska, M.; Shlyakhova, E.V.; Chuvilin, A.L.; Guo, Y.; Beloshapkin, S.; Okotrub, A.V.; Bulusheva, L.G. Single isolated Pd2+ cations supported on N-doped carbon as active sites for hydrogen production from formic acid decomposition. ACS Catal. 2016, 6, 681–691. [Google Scholar] [CrossRef]
- Han, L.; Zhang, L.; Wu, H.; Zu, H.; Cui, P.; Guo, J.; Guo, R.; Ye, J.; Zhu, J.; Zheng, X.; et al. Anchoring Pt single atoms on Te nanowires for plasmon-enhanced dehydrogenation of formic acid at room temperature. Adv. Sci. 2019, 6, 1900006. [Google Scholar] [CrossRef]
- Rocha, G.F.S.R.; da Silva, M.A.R.; Rogolino, A.; Diab, G.A.A.; Noleto, L.F.G.; Antonietti, M.; Teixeira, I.F. Carbon nitride based materials: More than just a support for single-atom catalysis. Chem. Soc. Rev. 2023, 52, 4878–4932. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Su, P.; Chen, Y.; Zhu, B.; Zhang, S.; Huang, W. g-C3N4 supported metal (pd, Ag, Pt) catalysts for hydrogen-production from formic acid. New J. Chem. 2018, 42, 9449–9454. [Google Scholar] [CrossRef]
- Lippert, G.; Hutter, J.; Parrinello, M. A hybrid gaussian and plane wave density functional scheme. Mol. Phys. 1997, 92, 477–487. [Google Scholar] [CrossRef]
- Kühne, T.D.; Iannuzzi, M.; Del Ben, M.; Rybkin, V.V.; Seewald, P.; Stein, F.; Laino, T.; Khaliullin, R.Z.; Schütt, O.; Schiffmann, F.; et al. CP2K: An electronic structure and molecular dynamics software package—Quickstep: Efficient and accurate electronic structure calculations. J. Chem. Phys. 2020, 152, 194103. [Google Scholar] [CrossRef] [PubMed]
- VandeVondele, J.; Hutter, J. Gaussian basis sets for accurate calculations on molecular systems in gas and condensed phases. J. Chem. Phys. 2007, 127, 114105. [Google Scholar] [CrossRef]
- Krack, M. Pseudopotentials for H to Kr optimized for gradient-corrected exchange-correlation functionals. Theor. Chem. Acc. 2005, 114, 145–152. [Google Scholar] [CrossRef]
- Goedecker, S.; Teter, M.; Hutter, J. Separable dual-space gaussian pseudopotentials. Phys. Rev. B 1996, 54, 1703–1710. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Lu, T.; Chen, Q. Shermo: A general code for calculating molecular thermochemistry properties. Comput. Theor. Chem. 2021, 1200, 113249. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Jiao, Y.; Zhu, Y.; Cai, Q.; Vasileff, A.; Li, L.H.; Han, Y.; Chen, Y.; Qiao, S.-Z. Molecule-level g-C3N4 coordinated transition metals as a new class of electrocatalysts for oxygen electrode reactions. J. Am. Chem. Soc. 2017, 139, 3336–3339. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Periyasamy, G.; Pandey, B.; Pati, S.K. Computational studies on magnetism and the optical properties of transition metal embedded graphitic carbon nitride sheets. J. Mater. Chem. C 2014, 2, 7943–7951. [Google Scholar] [CrossRef]
- Jin, T.; Guo, L.; Tang, Q.; Wang, J.; Pan, B.; Wang, C.; Li, Z.; Chen, F. Spontaneous formate oxidation on the 2D surface metal fluoride interface reconstructed from the AgPdF surface. J. Phys. Chem. C 2022, 126, 9683–9695. [Google Scholar] [CrossRef]
- Zhang, N.; Gao, Y.; Ma, L.; Wang, Y.; Huang, L.; Wei, B.; Xue, Y.; Zhu, H.; Jiang, R. Single transition metal atom anchored on g-C3N4 as an electrocatalyst for nitrogen fixation: A computational study. Int. J. Hydrogen Energy 2023, 48, 7621–7631. [Google Scholar] [CrossRef]
- Jin, T.; Chen, F.; Guo, L.; Tang, Q.; Wang, J.; Pan, B.; Wu, Y.; Yu, S. Pd4O3 subsurface oxide on pd(111) formed during oxygen adsorption-induced surface reconstruction and its activity toward formate oxidation reactions. J. Phys. Chem. C 2021, 125, 19497–19508. [Google Scholar] [CrossRef]
- Tang, Y.; Roberts, C.A.; Perkins, R.T.; Wachs, I.E. Revisiting formic acid decomposition on metallic powder catalysts: Exploding the HCOOH decomposition volcano curve. Surf. Sci. 2016, 650, 103–110. [Google Scholar] [CrossRef]
- Fingerhut, J.; Lecroart, L.; Schwarzer, M.; Hörandl, S.; Borodin, D.; Kandratsenka, A.; Kitsopoulos, T.N.; Auerbach, D.J.; Wodtke, A.M. Identification of reaction intermediates in the decomposition of formic acid on pd. Faraday Discuss. 2024, 251, 412–434. [Google Scholar] [CrossRef]
- Greiner, M.T.; Jones, T.E.; Beeg, S.; Zwiener, L.; Scherzer, M.; Girgsdies, F.; Piccinin, S.; Armbrüster, M.; Knop-Gericke, A.; Schlögl, R. Free-atom-like d states in single-atom alloy catalysts. Nature Chem 2018, 10, 1008–1015. [Google Scholar] [CrossRef]
- Yang, Y.; Peltier, C.R.; Zeng, R.; Schimmenti, R.; Li, Q.; Huang, X.; Yan, Z.; Potsi, G.; Selhorst, R.; Lu, X.; et al. Electrocatalysis in alkaline media and alkaline membrane-based energy technologies. Chem. Rev. 2022, 122, 6117–6321. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).