Catalytic Degradation of Organic Dyes Induced by Tribo-Electrification Between Insulating Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
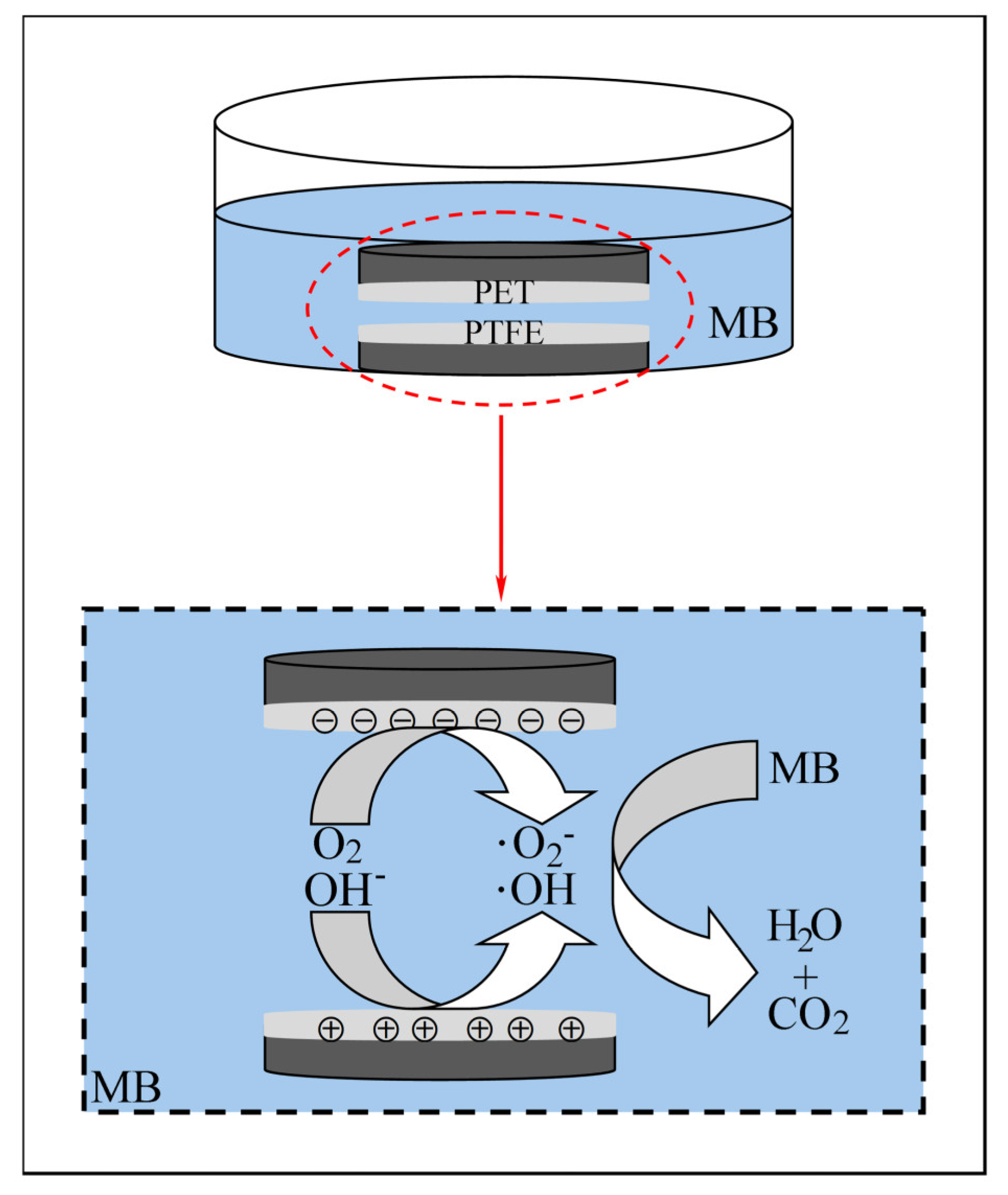
2.2. Experimental Setup
2.3. Experimental Methods
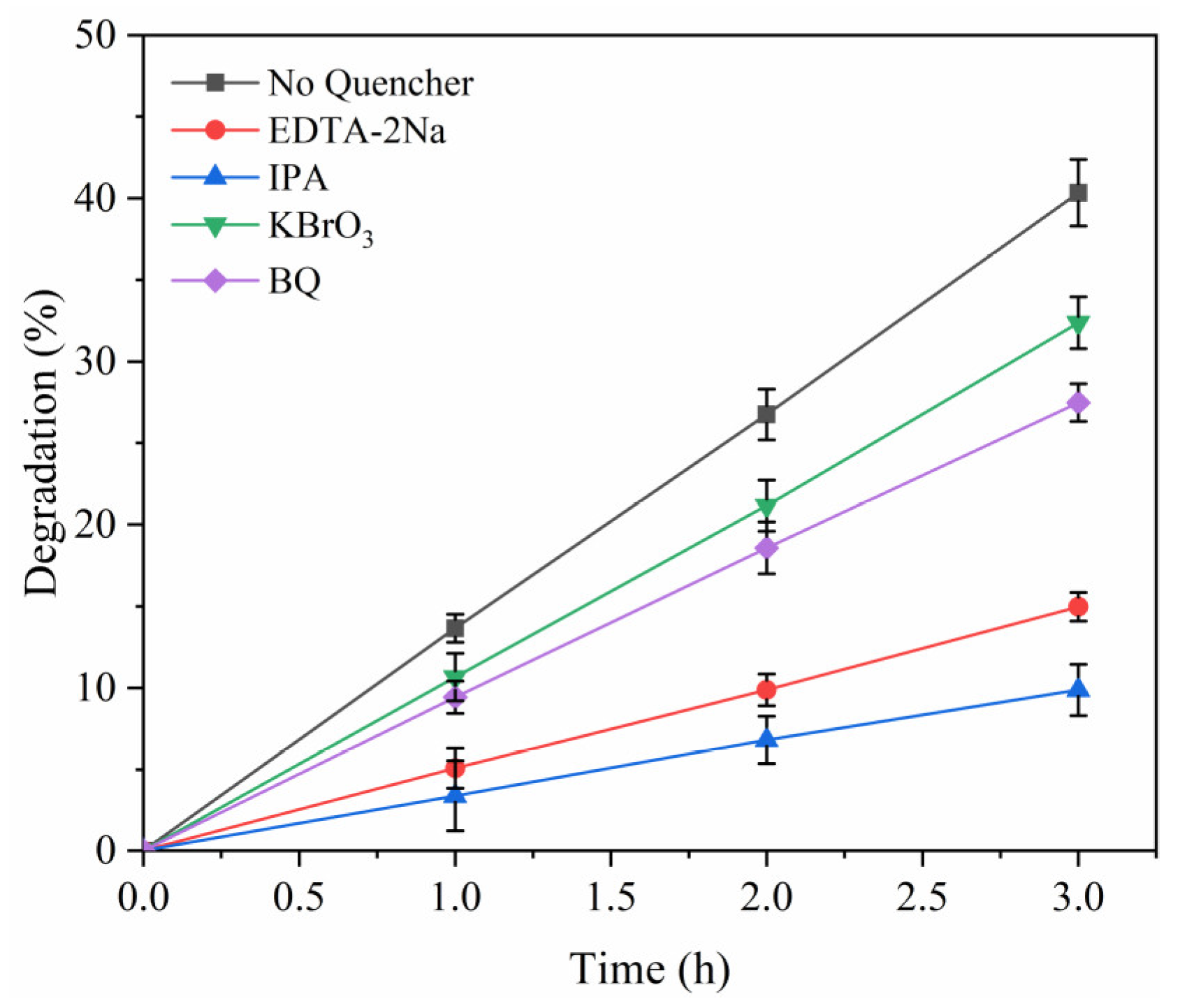
2.4. Quenching Experiment of Active Substances
3. Results and Discussion
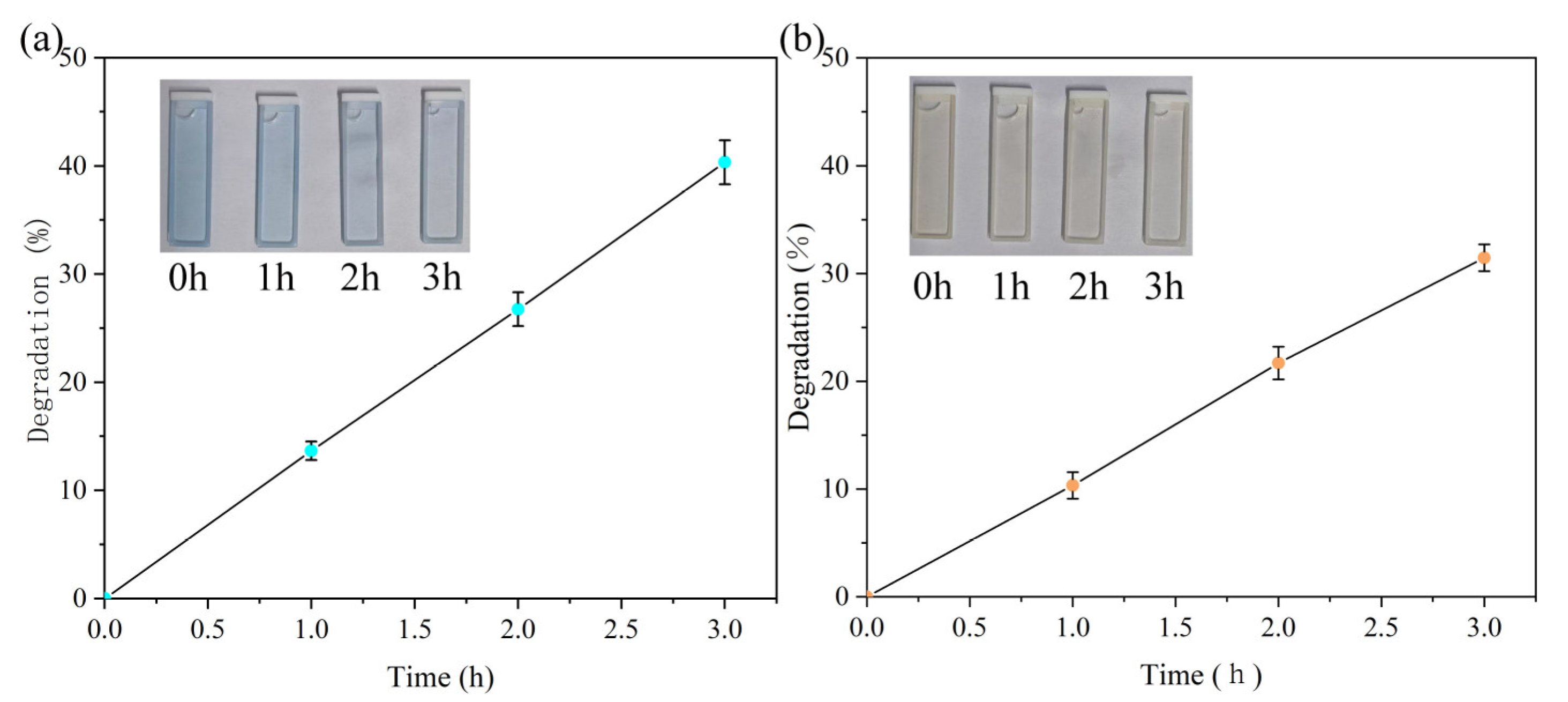
3.1. Degradation Effect of PET and PTFE for Organic Dyes
3.2. Degradation of Free Radicals
3.3. Contact Separation Tribocatalytic Mechanism
- (1)
- Tribo-electric energizing:
- (2)
- Free radical generation:
- (3)
- Dye degradation:
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Chen, M.; Hu, C.; Wang, J. A Study of Dye Degradation in the Combination Process of Photocatalytic Oxidation with Biochemical Oxidation. Acta Sci. Circumstantiae 2000, 20, 772–775. [Google Scholar]
- Hugine, J.; Bader, H. Rate Constants of Reactions of Ozone with Organic and Inorganic Compounds in Water—II. Water Res. 1983, 17, 185–194. [Google Scholar] [CrossRef]
- Jin, X.; Liu, M.; Zong, Y.; Hu, S.; Li, Y.; Xu, L.; Bai, X.; Shi, X.; Jin, P.; Song, J.; et al. Unraveling the Over-Oxidation Inhibition Mechanism During the Hybrid Ozonation-Coagulation Process: Immediate Entrapment and Complexation Between Intermediate Organic Matter and Coagulants. Water Res. 2023, 232, 119692. [Google Scholar] [CrossRef]
- Sobczak, M.; Bujnowicz, S.; Bilinska, L. Fenton and Electro-Fenton Treatment for Industrial Textile Wastewater Recycling. Comparison of By-Products Removal, Biodegradability, Toxicity, and Re-Dyeing. Water Resour. Ind. 2024, 31, 100256. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, B. Recent Advances in Porous Pt-based Nanostructures: Synthesis and Electrochemical Applications. Chem. Soc. Rev. 2014, 43, 2439–2450. [Google Scholar] [CrossRef]
- Pilipavicius, J.; Traskina, N.; Juodkazyte, J.; Vilciauskas, L. The Mechanism of NaTi2(PO4)3 Aqueous Electrochemical Degradation Revisited. Electrochim. Acta 2023, 465, 142993. [Google Scholar] [CrossRef]
- Li, H.; Sun, B.; Gao, T.; Li, H.; Ren, Y.; Zhou, G. Ti3C2 MXene Co-Catalyst Assembled with Mesoporous TiO2 for Boosting Photocatalytic Activity of Methyl Orange Degradation and Hydrogen Production. Chin. J. Catal. 2022, 43, 461–471. [Google Scholar] [CrossRef]
- Zeng, Y.; Lu, D.; Kondamareddy, K.K.; Wang, H.; Wu, Q.; Fan, H.; Wang, Q.; Zhang, B.; Xie, L.; Zhang, Y.; et al. Enhanced Visible Light Photocatalysis and Mechanism Insight for Novel Z-Scheme MoS2/Ag2S/AgVOx Ternary Heterostructure with Fast Interfacial Charges Transfer. J. Alloys Compd. 2022, 908, 164642. [Google Scholar] [CrossRef]
- You, H.; Ma, X.; Wu, Z.; Fei, L.; Chen, X.; Yang, J.; Liu, Y.; Jia, Y.; Li, H.; Wang, F.; et al. Piezoelectrically/pyroelectrically-driven Vibration/cold-Hot Energy Harvesting for Mechano-/Pyro- Bi-Catalytic Dye Decomposition of NaNbO3 Nanofibers. Nano Energy 2018, 52, 351–359. [Google Scholar] [CrossRef]
- Zhu, M.; Li, S.; Zhang, H.; Gao, J.; Kwok, K.W.; Jia, Y.; Kong, L.; Zhou, W.; Peng, B. Diffused Phase Transition Boosted Dye Degradation with Ba (Zrxti1-x)O3 Solid Solutions Through Piezoelectric Effect. Nano Energy 2021, 89, 106474. [Google Scholar] [CrossRef]
- Liu, Z.; Su, R.; Xu, F.; Xu, X.; Gao, B.; Li, Q. The Unique Fe3Mo3N Structure Bestowed Efficient Fenton-Like Performance of the Iron-Based Catalysts: The Double Enhancement of Radicals and Nonradicals. Adv. Mater. 2024, 36, 2311869. [Google Scholar] [CrossRef] [PubMed]
- Kibsgaard, J.; Gorlin, Y.; Chen, Z.; Jaramillo, T.F. Meso-Structured Platinum Thin Films: Active and Stable Electrocatalysts for the Oxygen Reduction Reaction. J. Am. Chem. Soc. 2012, 134, 7758–7765. [Google Scholar] [CrossRef]
- Li, X.; Fang, G.; Tian, Q.; Wu, T. Crystal Regulation of BiVO4 for Efficient Photocatalytic Degradation in G-C3N4/BiVO4 Heterojunction. Appl. Surf. Sci. 2022, 584, 152642. [Google Scholar] [CrossRef]
- Zhuang, W.; Zhang, Y.; Luo, Q.; Du, S.; Wu, B.; Sui, M. Piezoelectric Catalytic Process: A Genuinely Energy-Saving Approach for Water Treatment? A Critical Review. Chem. Eng. J. 2024, 499, 155956. [Google Scholar] [CrossRef]
- Lowell, J.; Rose-Innes, A.C. Contact electrification. Adv. Phys. 1980, 29, 947–1023. [Google Scholar] [CrossRef]
- Horn, R.G.; Smith, D.T.; Grabbe, A. Contact electrification induced by monolayer modification of a surface and relation to acid–base interactions. Nature 1993, 366, 442–443. [Google Scholar] [CrossRef]
- Liang, X.; Jiang, T.; Liu, G.; Xiao, T.; Xu, L.; Li, W.; Xi, F.; Zhang, C.; Wang, Z.L. Triboelectric nanogenerator networks integrated with power management module for water wave energy harvesting. Adv. Funct. Mater. 2019, 29, 1807241. [Google Scholar] [CrossRef]
- Shan, C.; Liu, W.; Wang, Z.; Pu, X.; He, W.; Tang, Q.; Fu, S.; Li, G.; Long, L.; Guo, H.; et al. An Inverting TENG to Realize the AC Mode Based on the Coupling of Triboelectrification and Air-Breakdown. Energy Environ. Sci. 2021, 14, 5395–5405. [Google Scholar] [CrossRef]
- Sayyah, A.; Crowell, D.R.; Raychowdhury, A.; Horenstein, M.N.; Mazumder, M.K. An Experimental Study on the Characterization of Electric Charge in Electrostatic Dust Removal. J. Electrost. 2017, 87, 173–179. [Google Scholar] [CrossRef]
- Panat, S.; Varanasi, K.K. Electrostatic dust removal using adsorbed moisture–assisted charge induction for sustainable operation of solar panels. Sci. Adv. 2022, 8, eabm0078. [Google Scholar] [CrossRef]
- Yang, J.; Chen, J.; Su, Y.; Jing, Q.; Li, Z.; Yi, F.; Wen, X.; Wang, Z.; Wang, Z.L. Eardrum-Inspired Active Sensors for Self-Powered Cardiovascular System Characterization and Throat-Attached Anti-Interference Voice Recognition. Adv. Mater. 2015, 27, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Pu, X.; Chen, J.; Meng, Y.; Yeh, M.; Liu, G.; Tang, Q.; Chen, B.; Liu, D.; Qi, S.; et al. A Highly Sensitive, Self-Powered Triboelectric Auditory Sensor for Social Robotics and Hearing Aids. Sci. Robot. 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Kajdas, C.; Hiratsuka, K. Tribochemistry, Tribocatalysis, and the Negative-Ion-radical Action Mechanism. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2009, 223, 827–848. [Google Scholar] [CrossRef]
- Li, P.; Wu, J.; Wu, Z.; Jia, Y.; Ma, J.; Chen, W.; Zhang, L.; Yang, J.; Liu, Y. Strong Tribocatalytic Dye Decomposition Through Utilizing Triboelectric Energy of Barium Strontium Titanate Nanoparticles. Nano Energy 2019, 63, 103832. [Google Scholar] [CrossRef]
- Lei, H.; Wu, M.; Mo, F.; Ji, S.; Dong, X.; Wu, Z.; Gao, J.; Yang, Y.; Jia, Y. Tribo-catalytic Degradation of Organic Pollutants Through Bismuth Oxyiodate Triboelectrically Harvesting Mechanical Energy. Nano Energy 2020, 78, 105290. [Google Scholar] [CrossRef]
- Sun, C.; Guo, X.; Ji, R.; Hu, C.; Liu, L.; Fang, L.; Cheng, Z.; Luo, N. Strong Tribocatalytic Dye Degradation by Tungsten Bronze Ba4Nd2Fe2Nb8O30. Ceram. Int. 2020, 47, 5038–5043. [Google Scholar] [CrossRef]
- Cui, X.; Li, P.; Lei, H.; Tu, C.; Wang, D.; Wang, Z.; Chen, W. Greatly enhanced tribocatalytic degradation of organic pollutants by TiO2 nanoparticles through efficiently harvesting mechanical energy. Sep. Purif. Technol. 2022, 289, 120814. [Google Scholar] [CrossRef]
- Wu, M.; Xu, Y.; He, Q.; Sun, P.; Weng, X.; Dong, X. Tribocatalysis of Homogeneous Material with Multi-Size Granular Distribution for Degradation of Organic Pollutants. J. Colloid Interface Sci. 2022, 622, 602–611. [Google Scholar] [CrossRef]
- Wang, Z.; Berbille, A.; Feng, Y.; Li, S.; Zhu, L.; Tang, W.; Wang, Z.L. Contact-electro-catalysis for the Degradation of Organic Pollutants Using Pristine Dielectric Powders. Nat. Commun. 2022, 13, 130. [Google Scholar] [CrossRef]
- Zevatskii, Y.E.; Lysova, S.S.; Skripnikova, T.A.; Vorona, S.V.; Myznikov, L.V. Bouguer–Lambert–Beer Law of Absorption: Spectrophotometry in Electrolyte Solutions. Russ. J. Phys. Chem. A 2024, 98, 242–247. [Google Scholar] [CrossRef]
- Rtimi, S.; Pulgarin, C.; Sanjines, R.; Kiwi, J. Kinetics and mechanism for transparent polyethylene-TiO2 films mediated self-cleaning leading to MB dye discoloration under sunlight irradiation. Appl. Catal. B Environ. 2015, 162, 236–244. [Google Scholar] [CrossRef]
- Lu, P.; Jing, Y.; Zhang, Q.; Ying, H.; Li, L. Photocatalytic Degradation Of Methyl Orange By Chitosan Modified TiO2. Conf. Environ. Pollut. Public Health 2010, 114, 105088. [Google Scholar]
- Almulhem, N.K.; Awada, C.; Alnaim, N.M.; Al Taisan, N.; Alshoaibi, A.A.; Shaalan, N.M. Synergistic Effect of the KBrO3 Electron Acceptor on the Photocatalytic Performance of the Nb-TiO2 Nanocomposite for Polluted Phenol Red Wastewater Treatment. Crystals 2022, 12, 1758. [Google Scholar] [CrossRef]
- Gao, E.; Wang, W. Role of graphene on the surface chemical reactions of BiPO4–rGO with low OH-related defects. Nanoscale 2013, 5, 11248. [Google Scholar] [CrossRef]
- Luque, P.A.; Nava, O.; Soto-Robles, C.A.; Chinchillas-Chinchillas, M.J.; Garrafa-Galvez, H.E.; Baez-Lopez, Y.A.; Valdez-Núñez, K.P.; Vilchis-Nestor, A.R.; Castro-Beltrán, A. Improved photocatalytic efficiency of SnO2 nanoparticles through green synthesis. Optik 2020, 206, 164299. [Google Scholar] [CrossRef]
- Su, S.; Liu, Y.; Liu, X.; Jin, W.; Zhao, Y. Transformation pathway and degradation mechanism of methylene blue through β-FeOOH@ GO catalyzed photo-Fenton-like system. Chemosphere 2019, 218, 83–92. [Google Scholar] [CrossRef]
- Ikhlaq, A.; Parveen, S.; Raashid, M.; Masood, Z.; Rizvi, O.S.; Al Johani, T.A.; Ahsan, M.; Amjad, H.; Qi, F. Methylene blue (MB) removal from aqueous solution by alum; catalytic ozonation process. Discov. Chem. Eng. 2024, 4, 8. [Google Scholar] [CrossRef]
- Wang, Q.; Tian, S.; Ning, P. Degradation Mechanism of Methylene Blue in a Heterogeneous Fenton-like Reaction Catalyzed by Ferrocene. Ind. Eng. Chem. Res. 2014, 53, 643–649. [Google Scholar] [CrossRef]
- Sun, Y.; Xia, R.; Zhang, J.; Xu, J. Preparation of Ferric Oxide for Efficient Electrocatalytic Oxidation of Methylene Blue. Inorg. Chem. Commun. 2022, 140, 109364. [Google Scholar] [CrossRef]
- Sun, P.; Yu, H.; Liu, T.; Li, Y.; Wang, Z.; Xiao, Y.; Dong, X. Efficiently Photothermal Conversion in a MnOx-based Monolithic Photothermocatalyst for Gaseous Formaldehyde Elimination. Chin. Chem. Lett. 2022, 33, 2564–2568. [Google Scholar] [CrossRef]
- Li, X.; Tong, W.; Shi, J.; Chen, Y.; Zhang, Y.; An, Q. Tribocatalysis Mechanisms: Electron Transfer and Transition. J. Mater. Chem. A 2023, 11, 4458–4472. [Google Scholar] [CrossRef]
- Chen, Z.; Pan, M.; Cheng, C.; Luo, J.; Deng, X. Water Disinfection: Advances in Photocatalysis and Piezo/triboelectric Catalysis with Progressively Enhanced Energy Utilization. SusMat 2024, 5, e232. [Google Scholar] [CrossRef]
- Che, J.; Gao, Y.; Wu, Z.; Ma, J.; Wang, Z.; Liu, C.; Jia, Y.; Wang, X. Review on Tribocatalysis Through Harvesting Friction Energy for Mechanically-Driven Dye Decomposition. J. Alloys Compd. 2024, 1002, 175413. [Google Scholar] [CrossRef]


| Degradation Method | Degradation (%) | Experimental Time (min) | Reference |
|---|---|---|---|
| photodegradation | 94.4 | 120 | [35] |
| Fenton and photo-Fenton | 99.7 | 60 | [36] |
| catalytic ozonation | 84 | 20 | [37] |
| heterogeneous catalytic degradation | nearly 100 | 120 | [38] |
| electrocatalytic degradation | 95.56 | 90 | [39] |
| tribo-catalysis | 40.34 | 180 | this study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Xu, X. Catalytic Degradation of Organic Dyes Induced by Tribo-Electrification Between Insulating Films. Materials 2025, 18, 2327. https://doi.org/10.3390/ma18102327
Li J, Xu X. Catalytic Degradation of Organic Dyes Induced by Tribo-Electrification Between Insulating Films. Materials. 2025; 18(10):2327. https://doi.org/10.3390/ma18102327
Chicago/Turabian StyleLi, Junhao, and Xuefeng Xu. 2025. "Catalytic Degradation of Organic Dyes Induced by Tribo-Electrification Between Insulating Films" Materials 18, no. 10: 2327. https://doi.org/10.3390/ma18102327
APA StyleLi, J., & Xu, X. (2025). Catalytic Degradation of Organic Dyes Induced by Tribo-Electrification Between Insulating Films. Materials, 18(10), 2327. https://doi.org/10.3390/ma18102327





