Kinetics and Thermodynamics of Pb(II), Zn(II), and Cd(II) Adsorption from Aqueous Solutions onto Activated Biochar Obtained from Tobacco Waste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Measurements
2.2.2. Physicochemical Analyses of Activated Carbon
2.2.3. Point of Zero Charge pH
2.2.4. Sorption Studies
2.2.5. Optimization of Adsorption Parameters
3. Results and Discussion
3.1. Physicochemical Properties of the Adsorbent
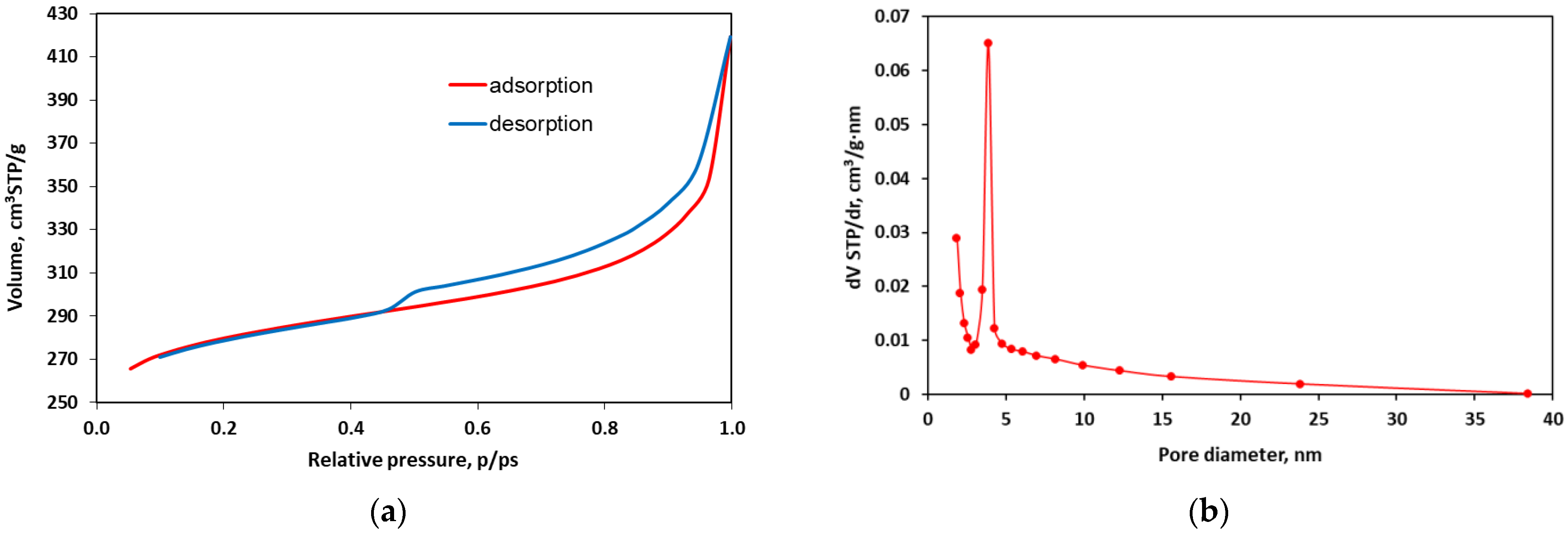
3.2. Structural Characteristics
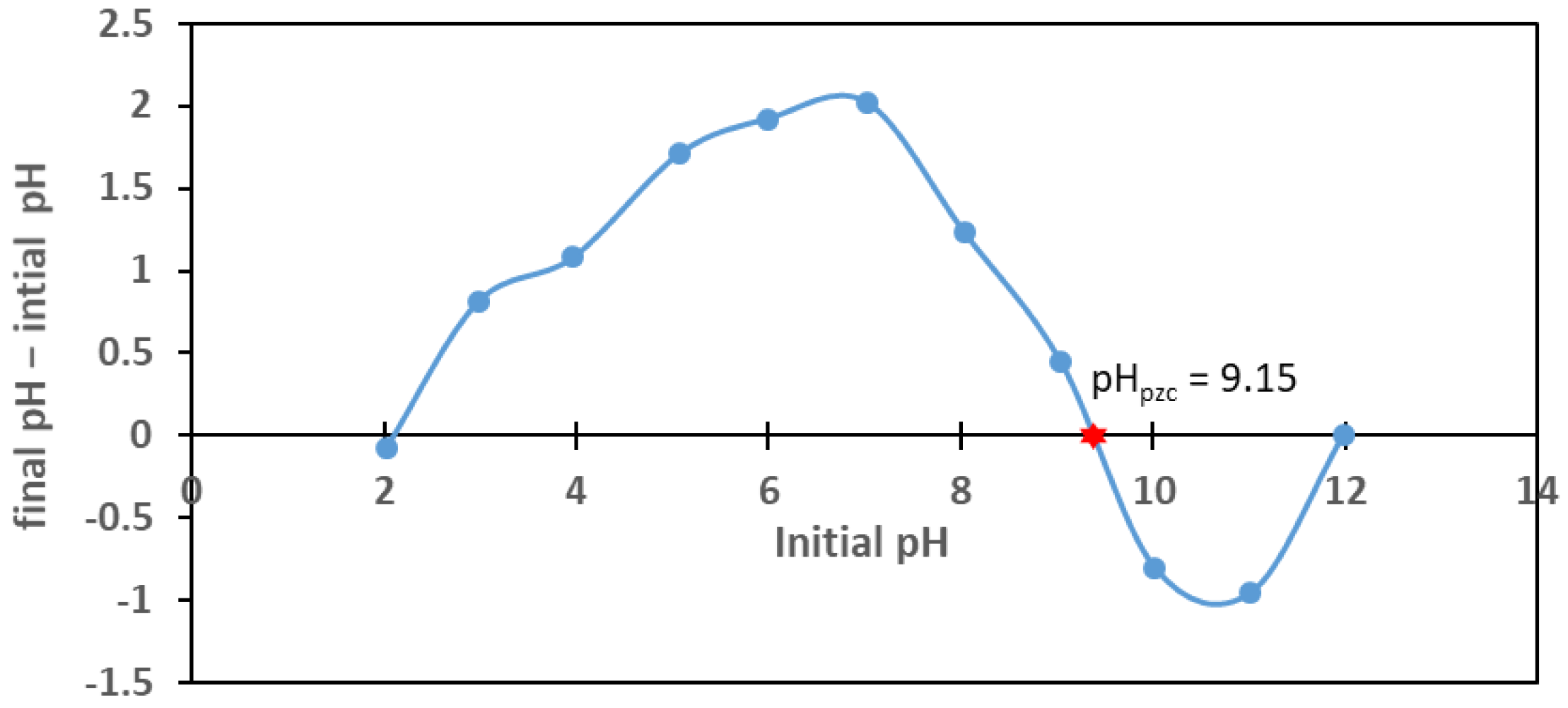
3.3. Point of Zero Charge pH
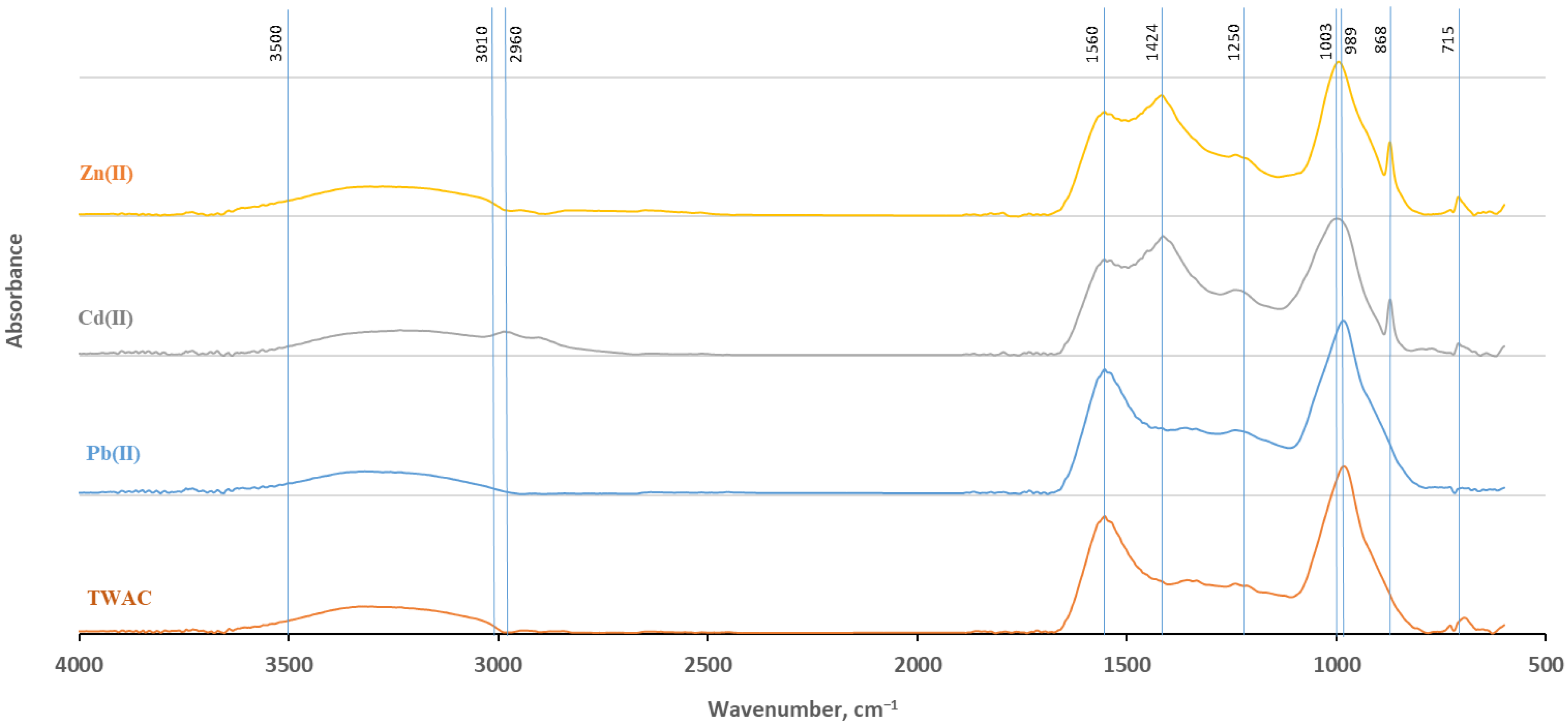
3.4. FTIR Analysis
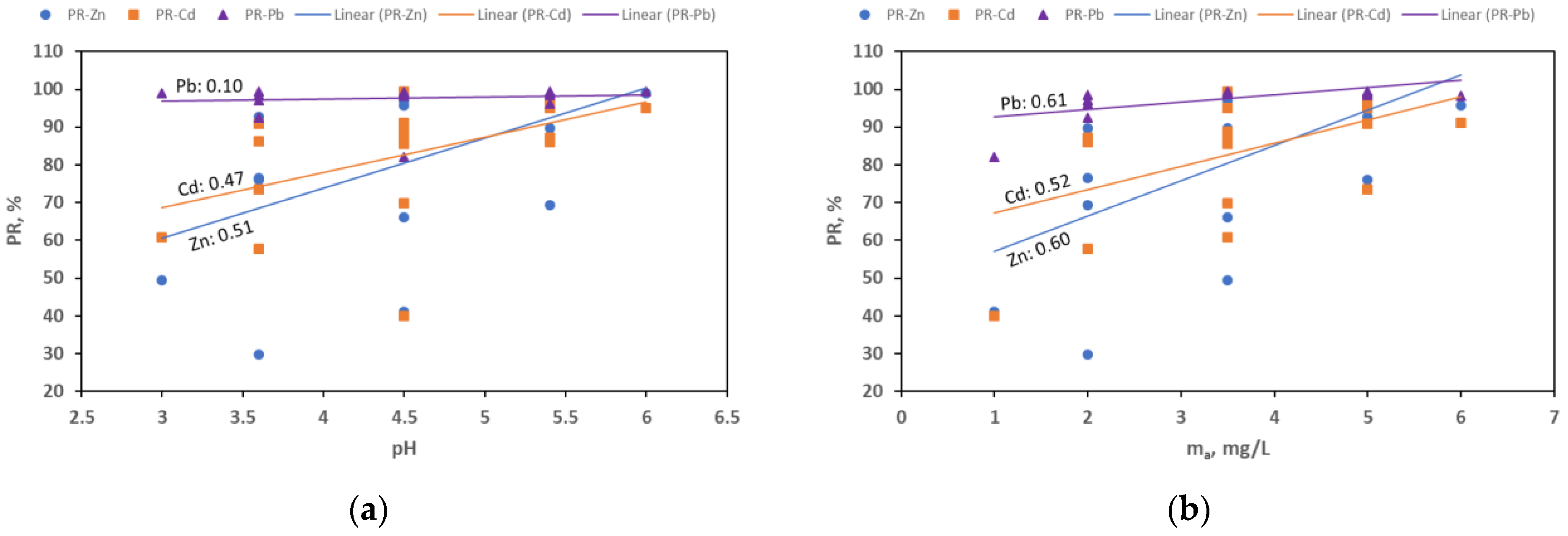
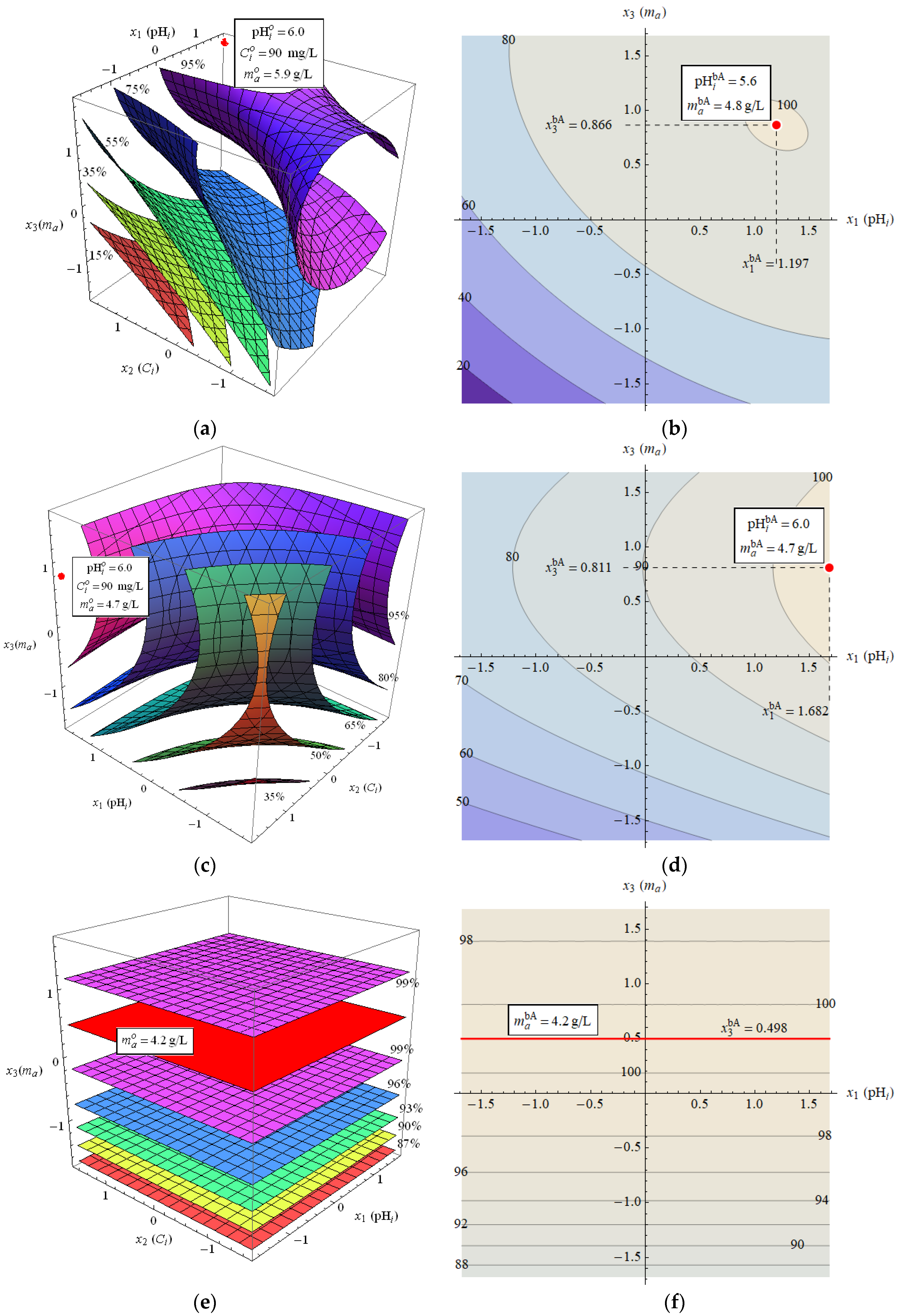
3.5. Optimization of Sorption Conditions
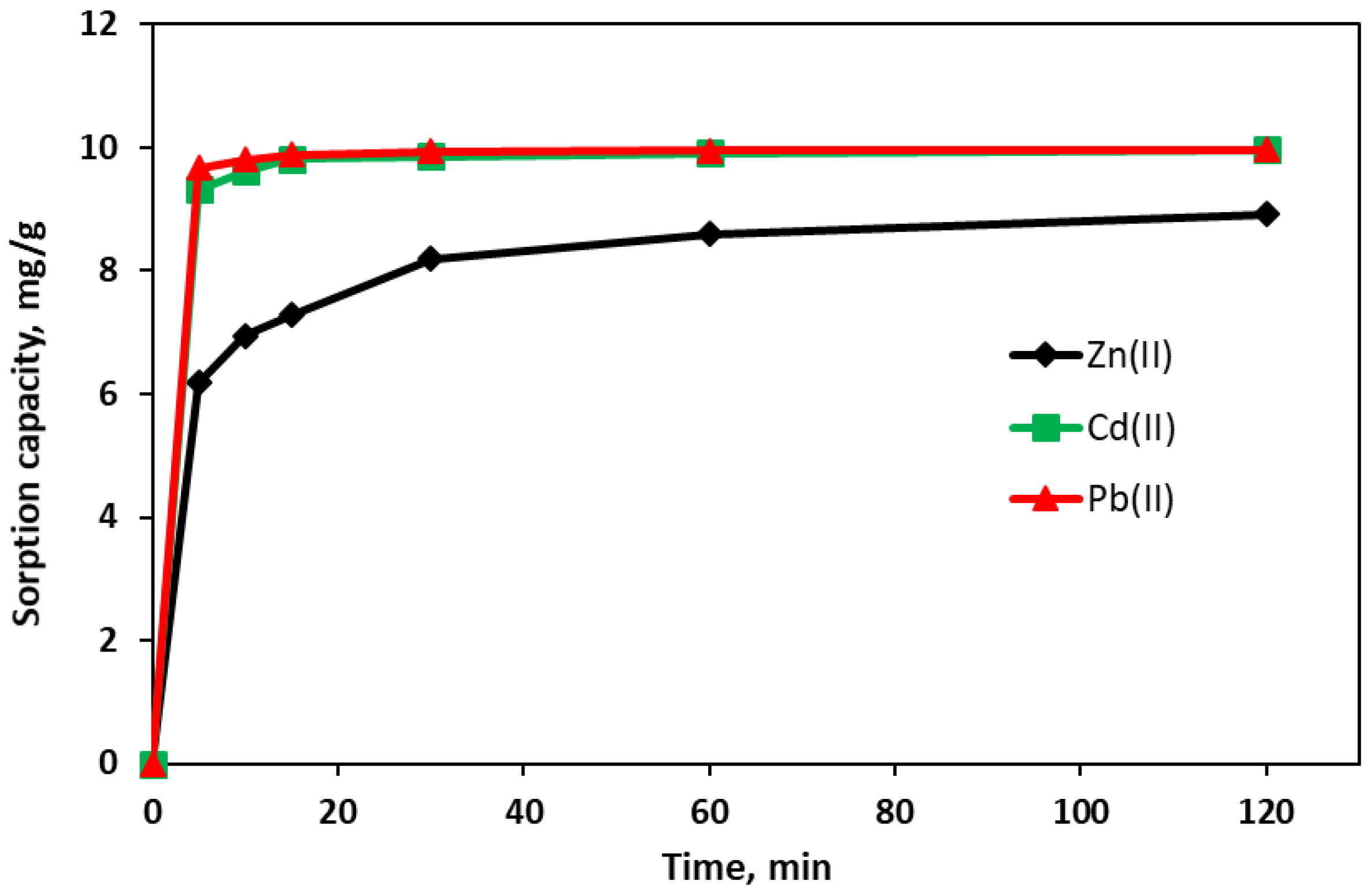
3.6. Sorption Kinetics—The Effect of Contact Time
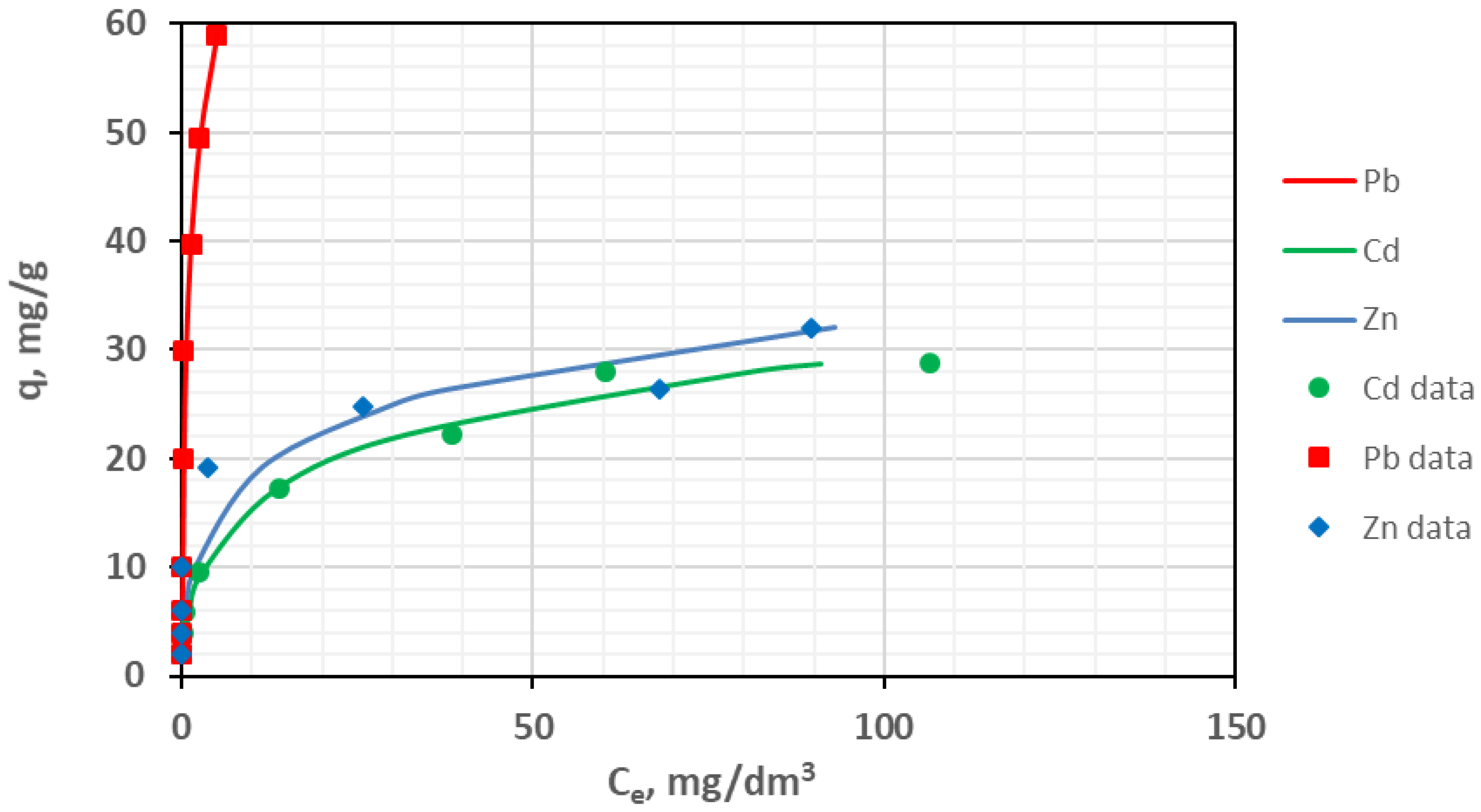
3.7. Adsorption Isotherms
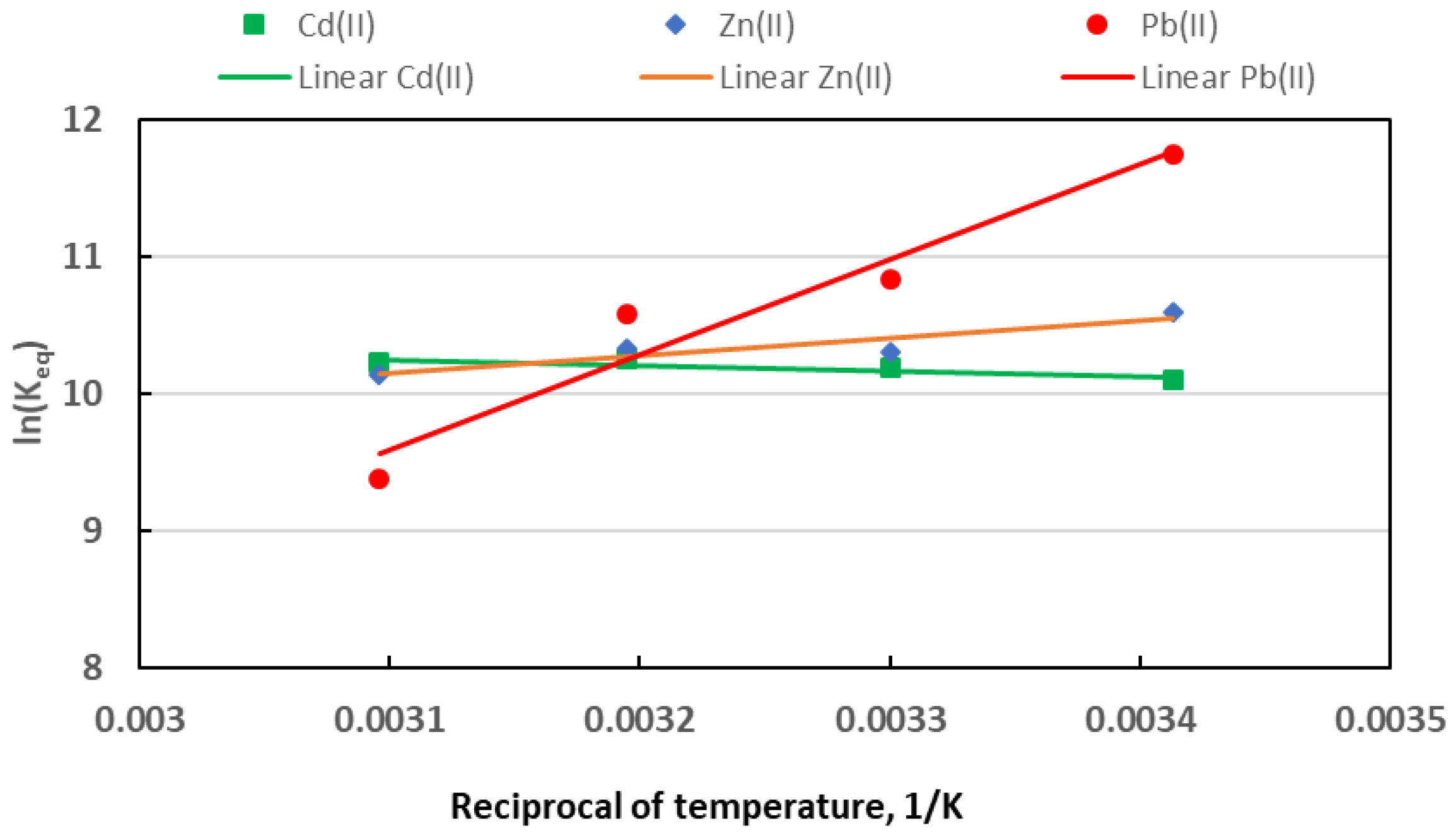
3.8. Sorption Thermodynamics
3.9. Comparative Studies
4. Conclusions
- The research conducted on the selection of optimal parameters for the adsorption of Zn(II), Cd(II), and Pb(II) indicate that the mass of the adsorbent and the initial pH should be 4–5 g/L and pH = 5–6, respectively. To avoid possible ion precipitation, the suggested pH value is 5.0.
- The amount of adsorbed compounds depends on the type of metal and satisfies the approximate relationship Pb(II) > Zn(II) > Cd(II).
- The results of the adsorption kinetics indicate that the adsorption process of Pb(II) and Cd(II) is very fast—after a few minutes, the adsorbent is practically saturated. In the case of Zn(II), the adsorption process is much slower and takes about two hours. The kinetics seem best described by a pseudo-second-order model, which indicates that in addition to physical sorption, adsorption includes chemical interactions leading to the binding of metal ions on the adsorbent surface via mechanisms such as ion exchange or complexation.
- Pb(II) adsorption occurs most strongly, mainly through chemisorption, while Zn(II) and Cd(II) sorption shows a more complex mechanism including both chemisorption and physical adsorption.
- The adsorption occurs spontaneously for all the tested metals; the process is exothermic for Zn(II) and Pb(II), whereas it is endothermic for Cd(II).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APR | Average percentage removal |
| CCD | Central Composite Design (Circumscribed) |
| FTIR | Fourier-transform infrared |
| IPD | Intraparticle diffusion |
| IPD1 | Intraparticle diffusion with one term |
| MPR | Modelled percentage removal |
| PFO | Pseudo-first order |
| PR | Percentage removal |
| PSO | Pseudo-second order |
| SE | Standard error of regression |
| TWAC | Tobacco waste activated carbon |
| VM | Volatile matter |
References
- Global Commission on the Economics of Water. The What, Why and How of the World Water Crisis. Phase 1 Review and Findings, March 2023. Available online: https://watercommission.org/wp-content/uploads/2023/03/Why-What-How-of-Water-Crisis-Web.pdf (accessed on 24 April 2025).
- European Investment Bank. Wastewater as a Resource, May 2022. Available online: https://www.eib.org/en/publications/wastewater-as-a-resource (accessed on 23 April 2025).
- Bislimi, K.; Halili, J.; Sahiti, H.; Bici, M.; Mazreku, I. Effect of Mining Activity in Accumulation of Heavy Metals in Soil and Plant (Urtica dioica L). J. Ecol. Eng. 2021, 22, 1–7. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Brbooti, M.M.; Abid, B.A.; Al-Shuwaiki, N.M. Removal of Heavy Metals Using Chemicals Precipitation. Eng. Technol. J. 2011, 29, 595–612. [Google Scholar] [CrossRef]
- Jaradat, A.Q.; Telfah, D.B.; Ismail, R. Heavy metals removal from landfill leachate by coagulation/flocculation process combined with continuous adsorption using eggshell waste materials. Water Sci. Technol. 2021, 84, 3817–3832. [Google Scholar] [CrossRef]
- Saleh, T.A.; Mustaqeem, M.; Khaled, M. Water treatment technologies in removing heavy metal ions from wastewater: A review. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100617. [Google Scholar] [CrossRef]
- Samavati, Z.; Samavati, A.; Goh, P.S.; Ismail, A.F.; Abdullah, M.S. A comprehensive review of recent advances in nanofiltration membranes for heavy metal removal from wastewater. Chem. Eng. Res. Des. 2023, 189, 530–571. [Google Scholar] [CrossRef]
- Jabłońska, B. Removing of Cr(III) and Cr(VI) compounds from aqueous solutions by shale waste rocks. Desalination Water Treat. 2020, 186, 234–246. [Google Scholar] [CrossRef]
- Maciel, G.P.S.; Lazzari, E.; Bjerk, T.; Ahmad, S.M.; Carvalho, A.P.; Nogueira, J.M.F.; Caramão, E.B. Trace analysis of carbazole in commercial diesel by using adsorption on activated biochar from rice husk pyrolysis. Int. J. Eng. Res. Sci. 2017, 3, 46–57. [Google Scholar] [CrossRef]
- Jabłońska, B.; Gliniak, M. Optimization of methylene blue and povidone-iodine adsorption conditions on activated biochar from sunflower husk pyrolysis. Desalination Water Treat. 2023, 301, 228–241. [Google Scholar] [CrossRef]
- Joshi, S.; Shrestha, R.G.; Pradhananga, R.R.; Ariga, K.; Shrestha, L.K. High Surface Area Nanoporous Activated Carbons Materials from Areca catechu Nut with Excellent Iodine and Methylene Blue Adsorption. C 2022, 8, 2. [Google Scholar] [CrossRef]
- Xiang, H.; Ren, G.; Yang, X.; Xu, D.; Zhang, Z.; Wang, X. A low-cost solvent-free method to synthesize α Fe2O3 nanoparticles with applications to degrade methyl orange in photo-fenton system. Ecotoxicol. Environ. Saf. 2020, 200, 110744. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.; Xu, T.; Jiang, H.; Chen, M.; Lai, Z.; Zhao, W.; Leng, L.; Zeng, Z.; Wang, X. Thermochemical valorization of alcohol industry residues into biocarbon for energy and environmental applications: A review. Green Chem. Eng. 2024, in press. [Google Scholar] [CrossRef]
- Report Linker. Global Tobacco Trends in 2024. Available online: https://www.reportlinker.com/clp/global/6088 (accessed on 23 April 2025).
- Tian, W.W.; Xu, F.; Xing, S.J.; Wu, R.; Yuan, Z.Y. Comprehensive study on the thermal decomposition process of waste tobacco leaves and stems to investigate their bioenergy potential: Kinetic, thermodynamic, and biochar analysis. Thermochim. Acta 2023, 723, 179473. [Google Scholar] [CrossRef]
- Cai, J.; Li, B.; Chen, C.; Wang, J.; Zhao, M.; Zhang, K. Hydrothermal carbonization of tobacco stalk for fuel application. Bioresour. Technol. 2016, 220, 305–311. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Wang, M.; Wu, S.; Wang, H.; Niazi, N.K.; Man, J.B.; Christie, P.; Shan, S.; Wong, M.H. Effect of tobacco stem-derived biochar on soil metal immobilization and the cultivation of tobacco plant. J. Soils Sediments 2019, 19, 2313–2321. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, Y.; Singh, N.K.; Singla, A.; Ong, H.C.; Chen, W.H. Effective utilization of tobacco (Nicotiana Tabaccum) for biodiesel production and its application on diesel engine using response surface methodology approach. Fuel 2020, 273, 117793. [Google Scholar] [CrossRef]
- Sophanodorn, K.; Unpaprom, Y.; Whangchai, K.; Homdoung, N.; Dussadee, N.; Ramaraj, R. Environmental management and valorization of cultivated tobacco stalks by combined pretreatment for potential bioethanol production. Biomass Convers. Biorefinery 2020, 12, 1627–1637. [Google Scholar] [CrossRef]
- Ravindiran, G.; Rajamanickam, S.; Janardhan, G.; Hayder, G.; Alagumalai, A.; Mahian, O.; Lam, S.S.; Sonne, C. Production and modifications of biochar to engineered materials and its application for environmental sustainability: A review. Biochar 2024, 6, 62. [Google Scholar] [CrossRef]
- Yan, S.; Wang, P.; Cai, X.; Wang, C.; Zwieten, L.V.; Wang, H.; Yin, Q.; Liu, G.; Ren, T. Biochar-based fertilizer enhanced tobacco yield and quality by improving soil quality and soil microbial community. Environ. Technol. Innov. 2025, 37, 103964. [Google Scholar] [CrossRef]
- Chen, D.; Wang, M.; Wang, G.; Zhou, Y.; Yang, X.; Li, J.; Zhang, C.; Dai, K. Functional organic fertilizers can alleviate tobacco (Nicotiana tabacum L.) continuous cropping obstacle via ameliorating soil physicochemical properties and bacterial community structure. Front. Bioeng. Biotechnol. 2022, 10, 1023693. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Hameed, B.H. Recent progress on tobacco wastes–derived adsorbents for the remediation of aquatic pollutants: A review. Environ. Res. 2024, 247, 118203. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Sun, J.; Yang, J.; Yuetikuer, A.; Zhang, S.; Leng, L.; Zha, H. Thermochemical valorization of tobacco wastes into biofuels and carbon materials: A comprehensive review. Chem. Eng. J. 2025, 505, 159544. [Google Scholar] [CrossRef]
- Jokić, S.; Gagić, T.; Knez, Ž.; Banožić, M.; Škerget, M. Separation of active compounds from tobacco waste using subcritical water extraction. J. Supercrit. Fluids 2019, 153, 104593. [Google Scholar] [CrossRef]
- Banožić, M.; Babić, J.; Jokić, S. Recent advances in extraction of bioactive compounds from tobacco industrial waste—A review. Ind. Crops Prod. 2020, 144, 112009. [Google Scholar] [CrossRef]
- Barla, F.G.; Kumar, S. Tobacco biomass as a source of advanced biofuels. Biofuels 2016, 10, 335–346. [Google Scholar] [CrossRef]
- Yang, J.; Dai, X.; Zhang, Y.; Xiao, Q.; Yang, C.; Qin, P.; Song, C. Biochar improves root growth and nitrogen metabolism in tobacco through physiological pathways modulation. Rhizosphere 2025, 33, 101011. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Z.; Hu, Z.; Zhang, J. Recent advances in biochar application for water and wastewater treatment: A review. PeerJ 2020, 8, e9164. [Google Scholar] [CrossRef]
- Jung, K.W.; Choi, B.H.; Hwang, M.J.; Choi, J.W.; Lee, S.H.; Chang, J.S.; Ahn, K.H. Adsorptive removal of anionic azo dye from aqueous solution using activated carbon derived from extracted coffee residues. J. Clean. Prod. 2017, 166, 360–368. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, M.; Lan, J.; Huang, Y.; Zhang, J.; Huang, S.; Yang, Y.; Ru, J. Water Quality Degradation Due to Heavy Metal Contamination: Health Impacts and Eco-Friendly Approaches for Heavy Metal Remediation. Toxics 2023, 11, 828. [Google Scholar] [CrossRef]
- Ullah, Z.; Rashid, A.; Ghani, J.; Nawab, J.; Zeng, X.C.; Shah, M.; Alrefaei, A.F.; Kamel, M.; Aleya, L.; Abdel-Daim, M.M.; et al. Groundwater contamination through potentially harmful metals and its implications in groundwater management. Front. Environ. Sci. 2022, 10, 1021596. [Google Scholar] [CrossRef]
- Feng, J.; Burke, I.T.; Chen, X.; Stewart, D.I. Assessing metal contamination and speciation in sewage sludge: Implications for soil application and environmental risk. Rev. Environ. Sci. Biotechnol. 2023, 22, 1037–1058. [Google Scholar] [CrossRef]
- Radfard, M.; Hashemi, H.; Baghapour, M.A.; Samaei, M.R.; Yunesian, M.; Soleimani, H.; Azhdarpoor, A. Prediction of human health risk and disability-adjusted life years induced by heavy metals exposure through drinking water in Fars Province, Iran. Sci. Rep. 2023, 13, 19080. [Google Scholar] [CrossRef] [PubMed]
- PN-EN ISO 18134-2:2024; Solid Biofuels–Determination of Moisture Content–Oven Dry Method–Part 2: Total Moisture–Simplified Method. The Polish Committee for Standardization: Warszawa, Poland, 2024.
- PN-ISO 1171:2002; Solid Mineral Fuels. Determination of Ash; The Polish Committee for Standardization: Warszawa, Poland, 2002.
- PN-EN 12697-6:2020-0; Mieszanki Mineralno-Asfaltowe, Metody Badań, Część 6: Oznaczanie Gęstości Objętościowej Próbek Mieszanki Mineralno-Asfaltowej. The Polish Committee for Standardization: Warszawa, Poland, 2020.
- PN-EN ISO 18123:2023-10; Solid Biofuels. Determination of The Contnet of Volatile Matter; The Polish Committee for Standardization: Warszawa, Poland, 2023.
- ASTM D4607-14 (2021); Activated Carbons, Methods of Tests, Determination of Adsorption Value of Iodine. The Polish Committee for Standardization: Warszawa, Poland, 2013.
- ASTM D2330-20; Standard Test Method for Methylene Blue Active Substances. ASTM International: West Conshokocken, PA, USA, 2020.
- Tripathy, S.S.; Kanungo, S.B. Adsorption of Co2+, Ni2+, Cu2+ and Zn2+ from 0.5 M NaCl and major ion sea water on a mixture of δ-MnO2 and amorphous FeOOH. J. Colloid. Interf. Sci. 2005, 284, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska, B. Optimization of Ni(II), Pb(II), and Zn(II) Ion Adsorption Conditions on Pliocene Clays from Post-Mining Waste. Minerals 2021, 6, 568. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Y.; Zhao, X.; Wang, S.; Xing, G. Comparisons of biochar properties from wood material and crop residues at different temperatures and residence times. Energy Fuels 2013, 27, 5890–5899. [Google Scholar] [CrossRef]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef]
- Li, X.; Shen, Q.; Zhang, D.; Mei, X.; Ran, W.; Xu, Y.; Yu, G.; Motta, A. Functional groups determine biochar properties (pH and EC) as studied by two-dimensional 13C NMR correlation spectroscopy. PLoS ONE 2013, 8, e65949. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic molecular structure of plant derived black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef]
- Dębowski, Z.; Nowak, B. Parametry regeneracji węgli aktywnych stosowanych do uzdatniania wody w ZPW “Dziećkowice”. Ochr. Sr. 1999, 4, 37–40. [Google Scholar]
- Saleh, M.E.; El Refaey, A.A.; Mahmoud, A.H. Effectiveness of Sunflower Seed Husk Biochar for Removing Copper Ions from Wastewater: A Comparative Study. Soil Water Res. 2016, 11, 53–63. [Google Scholar] [CrossRef]
- Siipola, V.; Pflugmacher, S.; Romar, H.; Wendling, L.; Koukkari, P. Low-Cost Biochar Adsorbents for Water Purification Including Microplastics Removal. Appl. Sci. 2020, 10, 788. [Google Scholar] [CrossRef]
- Tan, X.; Liu, Y.; Zeng, G.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Al-Wabel, M.I.; Al-Omran, A.; El-Naggar, A.H.; Nadeem, M.; Usman, A.R.A. Pyrolysis temperature induced changes in characteristics and chemicalcomposition of biochar produced from conocarpus wastes. Bioresour. Technol. 2013, 131, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, F.S.; Wu, J. Characterization and application of chars produced from pinewood pyrolysis and hydrothermal treatment. Fuel 2010, 89, 510–514. [Google Scholar] [CrossRef]
- Chen, X.; Chen, G.; Chen, L.; Chen, Y.; Lehmann, J.; McBride, M.B.; Hay, A.G. Adsorption of copper and zinc by biochars produced from pyrolysis of hardwood and corn straw in aqueous solution. Bioresour. Technol. 2011, 102, 8877–8884. [Google Scholar] [CrossRef]
- Pasieczna-Patkowska, S. Zastosowanie Spektroskopii IR w Inżynierii Środowiska; Komitet Inżynierii Środowiska PAN: Lublin, Poland, 2019; Volume 149. [Google Scholar]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Williams, R.T. Physisorption Hysteresis Loops and the Characterization of Nanoporous Materials. Adsorpt. Sci. Technol. 2004, 22, 773–782. [Google Scholar] [CrossRef]
- Archin, S.; Sharifi, S.H.; Asadpour, G. Optimization and modeling of simultaneous ultrasound-assisted adsorption of binary dyes using activated carbon from tobacco residues: Response surface methodology. J. Clean. Prod. 2019, 239, 118136. [Google Scholar] [CrossRef]
- de Boer, J.H. Studies on pore systems in catalysts II. The shapes of pores in aluminum oxide systems. J. Catal. 1964, 3, 38–43. [Google Scholar] [CrossRef]
- Onorevoli, B.; Maciel, G.P.S.; Machado, M.E.; Corbelini, V.; Caramão, E.B.; Jacques, R.A. Characterization of feedstock and biochar from energetic tobacco seed waste pyrolysis and potential application of biochar as an adsorbent. J. Environ. Chem. Eng. 2018, 6, 1279–1287. [Google Scholar] [CrossRef]
- He, Q.; Luo, Y.; Feng, Y.; Xie, K.; Zhang, K.; Shen, S.; Luo, Y.; Wang, F. Biochar produced from tobacco stalks, eggshells, and Mg for phosphate adsorption from a wide range of pH aqueous solutions. Mater. Res. Express 2020, 7, 115603. [Google Scholar] [CrossRef]
- Manfrin, J.; Gonçalves, A.C., Jr.; Schwantes, D.; Conradi, E., Jr.; Zimmermann, J.; Ziemer, G.L. Development of biochar and activated carbon from cigarettes wastes and their applications in Pb2+ adsorption. J. Environ. Chem. Eng. 2021, 9, 104980. [Google Scholar] [CrossRef]
- Semmens, M.J.; Seyfarth, M. The selectivity of clinoptilolite for certain heavy metals. In Natural Zeolites, Occurrence, Properties, Use; San, L.B., Mumpton, F.A., Eds.; Pergamon: Oxford, UK, 1978; pp. 517–526. [Google Scholar]
- Ouki, S.K.; Kavannagh, M. Performance of Natural Zeolites for the Treatment of Mixed Metal-Contaminated Effluents. Waste Manag. Res. 1997, 15, 383–394. [Google Scholar] [CrossRef]
- Cristiano, M.; Hu, Y.J.; Siegryd, M.; Kaplan, D.; Nitsche, H. A comparison of point of zero charge measurement methodology. Clays Clay Miner. 2011, 59, 107–115. [Google Scholar] [CrossRef]
- Bohli, T.; Ouederni, A.; Fiol, N.; Villaescusa, I. Evaluation of an activated carbon from olive stones used as an adsorbent for heavy metal removal from aqueous phases. Comptes Rendus Chim. 2015, 18, 88–99. [Google Scholar] [CrossRef]
- Li, H.; Budarin, V.L.; Clark, J.H.; North, M.; Wu, X. Rapid and efficient adsorption of methylene blue dye from aqueous solution by hierarchically porous, activated starbons: Mechanism and porosity dependence. J. Hazard. Mater. 2022, 436, 129174. [Google Scholar] [CrossRef]
- Jedynak, K.; Charmas, B. Adsorption properties of biochars obtained by KOH activation. Adsorption 2024, 30, 167–183. [Google Scholar] [CrossRef]
- Antón-Herrero, R.; García-Delgado, C.; Alonso-Izquierdo, M.; García-Rodríguez, G.; Cuevas, J.; Eymar, E. Comparative adsorption of tetracyclines on biochars and stevensite: Looking for the most effective adsorbent. Appl. Clay Sci. 2018, 160, 162–172. [Google Scholar] [CrossRef]
- Qambrani, N.A.; Rahman, M.M.; Won, S.; Shim, S.; Ra, C. Biochar properties and eco-friendly applications for climate change mitigation, waste management, and wastewater treatment: A review. Renew. Sustain. Energy Rev. 2017, 79, 255–273. [Google Scholar] [CrossRef]
- Kumar, S.; Loganathan, V.A.; Gupta, R.B.; Barnett, M.O. An assessment of U(VI) removal from groundwater using biochar produced from hydrothermal carbonization. J. Environ. Manag. 2011, 92, 2504–2512. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.Y.; Deng, J.H.; Huang, G.F.; Li, K.; Cai, K.Z.; Liu, Y.; Huang, F. Relative distribution of Cd2+ adsorption mechanisms on biochars derived from rice straw and sewage sludge. Bioresour. Technol. 2019, 272, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Emeka, E.E.; Ojiefoh, O.C.; Aleruchi, C.; Hassan, L.A.; Christiana, Q.M.; Rebecca, M.; Dare, E.O.; Temitope, A.E. Evaluation of antibacterial activities of silver nanoparticles green-synthesized using pineapple leaf (Ananas comosus). Micron 2014, 57, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, S.H.; Shoja, H. Optimization of process variables by response surface method ogy for methylene blue dye removal using Spruce sawdust/MgO nanobiocomposite. J. Water Environ. Nanotechnol. 2018, 3, 157–172. [Google Scholar] [CrossRef]
- Morterra, C.; O’Shea., M.L.; Low, M.J.D. Infrared studies of carbons—IX. The vacuum pyrolysis of non-oxygen-containing materials: PVC. Mater. Chem. Phys. 1988, 20, 123–144. [Google Scholar] [CrossRef]
- Zhao, M.; Dai, Y.; Zhang, M.; Feng, C.; Qina, B.; Zhang, W.; Zhao, N.; Li, Y.; Ni, Z.; Xuc, Z.; et al. Mechanisms of Pb and/or Zn adsorption by different biochars: Biochar characteristics, stability, and binding energies. Sci. Total Environ. 2020, 717, 136894. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H. Carboxyl functionalized Cinnamomum camphora for removal of heavy metals from synthetic wastewater-contribution to sustainability in agroforestry. J. Clean. Prod. 2018, 184, 921–928. [Google Scholar] [CrossRef]
- Uchimiya, M.; Orlov, A.; Ramakrishnan, G.; Sistani, K. In situ and ex situ spectroscopic monitoring of biochar’s surface functional groups. J. Anal. Appl. Pyrolysis 2013, 102, 53–59. [Google Scholar] [CrossRef]
- de Caprariis, B.; de Filippis, P.; Hernandez, A.D.; Petrucci, E.; Petrullo, A.; Scarsella, M.; Turchi, M. Pyrolysis wastewater treatment by adsorption on biochars produced by poplar biomass. J. Environ. Manag. 2017, 197, 231–238. [Google Scholar] [CrossRef]
- Fito, J.; Abewaa, M.; Mengistu, A.; Angassa, K.; Ambaye, A.D.; Moyo, W.; Nkambule, T. Adsorption of methylene blue from textile industrial wastewater using activated carbon developed from Rumex abyssinicus plant. Sci. Rep. 2023, 13, 5427. [Google Scholar] [CrossRef]
- Vadivelan, V.; Kumar, K.V. Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk. J. Colloid Interface Sci. 2005, 286, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Marungrueng, K.; Pavasant, P. High performance biosorbent (Caulerpa lentillifera) for basic dye removal. Bioresour. Technol. 2007, 98, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Soria, R.I.; Rolfe, S.A.; Betancourth, M.P.; Thornton, S.F. The relationship between properties of plant-based biochars and sorption of Cd(II), Pb(II) and Zn(II) in soil model systems. Heliyon 2020, 6, e05388. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Zu, J.; Feng, Q.; Chen, H.; Li, J.; Zhong, M.; Zhou, Z.; Zhuang, S. Effect of pyrolysis condition on the adsorption mechanism of heavy metals on tobacco stem biochar in competitive mode. Environ. Sci. Pollut. Res. 2019, 26, 26947–26962. [Google Scholar] [CrossRef]
- Bansal, R.C.; Goyal, M. Adsorpcja na Węglu Aktywnym; Wyd. Naukowo-Techniczne: Warszawa, Poland, 2009. [Google Scholar]
- Deryło-Marczewska, A.; Blachnio, M.; Marczewski, A.W.; Swiatkowski, A.; Tarasiuk, B. Adsorption of selected herbicides from aqueous solution on activated carbon. J. Therm. Anal. Calorim. 2010, 101, 785–794. [Google Scholar] [CrossRef]
- Godwin, O.O.; Ogbeide, O.M.; Ezeh, E.C.; Chukwuekeh, J.I.; Nwankwo, P.; Igwilo, C.N. Kinetic and thermodynamic studies on adsorption of lead (II) ions from aqueous solutions using polymer-modified coconut shell activated carbon (MCSAC). Int. J. Chem. Sci. 2020, 4, 01–08. [Google Scholar]
- Acharya, J.; Kumar, U.; Meikap, B.C. Thermodynamic characterization of adsorption of lead(II) ions on activated carbon developed from tamarind wood from aqueous solution. S. Afr. J. Chem. Eng. 2013, 18, 70–76. [Google Scholar]
- Yu, Y.; He, J.; Sun, J.; Pei, Z.; Wu, Q.; Yu, R. Capacity and Mechanisms of Pb(II) and Cd(II) Sorption on Five Plant-Based Biochars. Sustainability 2023, 15, 7627. [Google Scholar] [CrossRef]
- Alkherraz, A.M.; Ali, A.K.; Elsherif, K.M. Removal of Pb(II), Zn(II), Cu(II) and Cd(II) from aqueous solutions by adsorption onto olive branches activated carbon: Equilibrium and thermodynamic studies. Chem. Int. 2020, 6, 11–20. [Google Scholar] [CrossRef]
- Chen, S.; Zhong, M.; Wang, H.; Zhou, S.; Li, W.; Wang, T.; LI, J. Study on adsorption of Cu2+, Pb2+, Cd2+, and Zn2+ by the KMnO4 modified biochar derived from walnut shell. Int. J. Environ. Sci. Technol. 2023, 20, 1551–1568. [Google Scholar] [CrossRef]
- Deng, J.; Liu, Y.; Liu, S.; Zeng, G.; Tan, X.; Huang, B.; Tang, X.; Wang, S.; Hua, Q.; Yan, Z. Competitive adsorption of Pb(II), Cd(II) and Cu(II) onto chitosan-pyromellitic dianhydride modified biochar. J. Colloid Interface Sci. 2017, 506, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.K.; Hinkley, C.; Kondaveeti, S.; Vo, P.H.N.; Ralph, P.; Kuzhiumparambil, U. Influence of pyrolysis time on removal of heavy metals using biochar derived from macroalgal biomass (Oedogonium sp.). Bioresour. Technol. 2024, 414, 131562. [Google Scholar] [CrossRef]
- Li, B.; Yang, L.; Wang, C.Q.; Zhang, Q.P.; Liu, Q.C.; Li, Y.D.; Xiao, R. Adsorption of Cd(II) from aqueous solutions by rape straw biochar derived from different modification processes. Chemosphere 2017, 175, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Pejic, B.; Vukcevic, M.; Kostic, M.; Skundric, P. Biosorption of heavy metal ions from aqueous solutions by short hemp fibers: Effect of chemical composition. J. Hazard. Mater. 2009, 164, 146–153. [Google Scholar] [CrossRef]
- Li, Y.W.; Zhao, B.W.; Wang, L.; Li, Y.Q.; Wang, T.; Jia, Y.H.; Zhao, M.L. Competitive Adsorption of Cd(II) and Zn(II) on Biochar, Loess, and Biochar loess Mixture. Nat. Environ. Pollut. Technol. 2024, 23, 265–273. [Google Scholar] [CrossRef]

| Quantity | pH | Density | BET Surface | Iodine Value | Methylene Blue Index | |||
| Unit | % | % | % | g/cm3 | m2/g | mg/g | cm3 | |
| Value | 9.05 | 1.94 | 26.8 | 21.5 | 0.713 | 875.38 | 285.6 | 20 |
| Quantity | C (%) | H (%) | N (%) | S (%) | O (%) | H/C | O/C |
| Value | 49 | 2.5 | 1.3 | 0.06 | 20.34 | 0.05 | 0.42 |
| Removal (PR), % | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zn | Cd | Pb | Zn | Cd | Pb | ||||||
| 3.6 | −1 | 26 | −1 | 0.2 | −1 | 6.16 | 3.59 | 1.97 | 76.5 | 86.3 | 92.5 |
| 5.4 | 1 | 26 | −1 | 0.2 | −1 | 2.70 | 3.37 | 0.97 | 89.7 | 87.1 | 96.3 |
| 3.6 | −1 | 74 | 1 | 0.2 | −1 | 51.89 | 31.2 | 2.12 | 29.7 | 57.8 | 97.1 |
| 5.4 | 1 | 74 | 1 | 0.2 | −1 | 22.63 | 10.44 | 1.0 | 69.3 | 85.9 | 98.6 |
| 3.6 | −1 | 26 | −1 | 0.5 | 1 | 1.88 | 2.4 | 0.39 | 92.8 | 90.8 | 98.5 |
| 5.4 | 1 | 26 | −1 | 0.5 | 1 | 0.55 | 0.73 | 0,25 | 97.9 | 97.2 | 99.0 |
| 3.6 | −1 | 74 | 1 | 0.5 | 1 | 17.73 | 19.54 | 0.47 | 76.0 | 73.5 | 99.4 |
| 5.4 | 1 | 74 | 1 | 0.5 | 1 | 1.45 | 3.61 | 0.38 | 98.0 | 95.1 | 99.5 |
| 3.0 | 0 | 50 | 0 | 0.35 | 0 | 25.31 | 19.66 | 0.52 | 49.4 | 60.7 | 99.0 |
| 6.0 | 0 | 50 | 0 | 0.35 | 0 | 0.48 | 2.45 | 0.23 | 99.0 | 95.1 | 99.5 |
| 4.5 | 0 | 10 | 0 | 0.35 | 0 | 0.32 | 0.05 | 0.13 | 96.8 | 99.5 | 98.7 |
| 4.5 | −1.68 | 90 | 0 | 0.35 | 0 | 30.59 | 27.21 | 0.62 | 66.0 | 69.8 | 99.3 |
| 4.5 | 1.68 | 50 | 0 | 0.1 | 0 | 29.44 | 30.02 | 0.99 | 41.1 | 40 | 82 |
| 4.5 | 0 | 50 | −1.68 | 0.6 | 0 | 2.11 | 4.42 | 0.88 | 95.8 | 91.2 | 98.2 |
| 4.5 | 0 | 50 | 1.68 | 0.35 | 0 | 5.09 | 5.60 | 0.39 | 89.8 | 88.8 | 99.2 |
| 4.5 | 0 | 50 | 0 | 0.35 | −1.68 | 6.35 | 7.24 | 0.53 | 87.3 | 85.5 | 98.9 |
| 4.5 | 0 | 50 | 0 | 0.35 | 1.68 | 5.51 | 6.31 | 0.44 | 89.0 | 87.4 | 99.1 |
| 4.5 | 0 | 50 | 0 | 0.35 | 0 | 5.95 | 6.93 | 0.41 | 88.1 | 86.1 | 99.2 |
| 4.5 | 0 | 50 | 0 | 0.35 | 0 | 6.21 | 5.86 | 0.49 | 87.6 | 88.3 | 99.0 |
| 4.5 | 0 | 50 | 0 | 0.35 | 0 | 5.72 | 7.11 | 0.51 | 88.6 | 85.8 | 99.0 |
| Adsorbate | Function or Indicator | Value |
|---|---|---|
| Zn(II) | ||
| Cd(II) | ||
| Pb(II) | ||
| Adsorbate | Optimal Conditions for Sorption | Best Parameters for Given Concentration | Best Parameters on Average |
|---|---|---|---|
| Zn(II) | |||
| Cd(II) | |||
| Pb(II) |
| Parameter or Indicator | Unit | Zn(II) | Cd(II) | Pb(II) |
|---|---|---|---|---|
| Pseudo-first-order (PFO) model: | ||||
| mg/g | 8.22 | 9.84 | 9.91 | |
| min−1 | 0.24 | 0.58 | 0.73 | |
| min | 2.87 | 1.19 | 0.95 | |
| mg/g | 0.61 | 0.11 | 0.06 | |
| Pseudo-second-order (PSO) model: | ||||
| mg/g | 8.83 | 9.98 | 9.97 | |
| min−1 | 0.045 | 0.28 | 0.60 | |
| min | 2.49 | 0.35 | 0.17 | |
| mg/g | 0.32 | 0.02 | 0.01 | |
| Intraparticle diffusion (IPD) model: | ||||
| mg/g min−0.5 | 0.67 | 0.64 | 0.62 | |
| mg/g | 3.33 | 5.26 | 5.42 | |
| mg/g | 2.03 | 3.15 | 3.25 | |
| Intraparticle diffusion model with one term (IPD1): | ||||
| mg/g | 8.02 | 9.54 | 9.62 | |
| min−1 | 0.17 | 0.67 | 2.73 | |
| min | 7.19 | 1.78 | 0.44 | |
| mg/g | 1.52 | 1.70 | 1.71 | |
| Parameter | Unit | Zn(II) | Cd(II) | Pb(II) |
|---|---|---|---|---|
| KF | mg/g (L/mg)1/nF | 12.28 | 7.31 | 64.41 |
| nF | – | 4.69 | 3.26 | 1.58 |
| SE | mg/g | 2.98 | 1.323 | 10.63 |
| R2 | – | 0.945 | 0.987 | 0.906 |
| KL | L/mg | 3.67 | 0.16 | 1.51 |
| QL | mg/g | 26.82 | 28.72 | 64.23 |
| SE | mg/g | 4.62 | 2.689 | 5.69 |
| R2 | – | 0.868 | 0.947 | 0.93 |
| KLF | L/mg | 0.034 | 0.034 | 2.362 |
| QLF | mg/g | 26.06 | 21.13 | 107.95 |
| nLF | – | 34.16 | 34.16 | 1.389 |
| R2 | – | 0.748 | 0.621 | 0.943 |
| KE | L/mg | 2.23 | 1.228 | 2.88 |
| QE | mg/g | 8.11 | 8.27 | 68.35 |
| R2 | – | 0.982 | 0.936 | 0.974 |
| KT | mg/g | 3.05 | 3.57 | 13.69 |
| AT | L/mg | 179.17 | 15.96 | 15.54 |
| R2 | – | 0.921 | 0.927 | 0.957 |
| AT | mg/g | 148.02 | 165.86 | 65.11 |
| BT | mg/g | 0.301 | 0.637 | 0.666 |
| mT | – | 0.109 | 0.156 | 0.971 |
| R2 | – | 0.949 | 0.992 | 0.937 |
T, °C | ), kJ/mol | ||
|---|---|---|---|
| Cd(II) | Zn(II) | Pb(II) | |
| 20 | −24.67 | −25.70 | −28.65 |
| 30 | −25.63 | −26.21 | −27.66 |
| 40 | −26.58 | −26.72 | −26.66 |
| 50 | −27.53 | −27.23 | −25.67 |
| , kJ/mol | 3.3 ± 1.3 | −10.69 ± 3.32 | −57.73 ± 10.05 |
| , kJ/(mol K) | 0.095 ± 0.004 | −0.051 ± 0.01 | −0.099 ± 0.03 |
| Biomass Raw Material | Activation Method | Adsorbate | Maximum Sorption Capacity, mg/g | Reference |
|---|---|---|---|---|
| Tobacco stems | Pyrolysis at 800 °C + modification with KOH | Zn Cd Pb | 32 28.7 60 | This study |
| Sorghum straw | Slow pyrolysis at 600 °C | Cd Pb | 29 125 | [90] |
| Olive branches | Pyrolysis at 450 °C + activation with H3PO4 | Zn Cd Pb | 34.97 38.17 41.32 | [91] |
| Walnut shell | Pyrolysis at 400 °C + modification with KMnO4 | Zn Cd Pb | 58.96 44.94 70.37 | [92] |
| Rice straw | Pyrolysis at 450 °C + modification with chitosan and pyromellitic anhydride (PMDA) | Cd Pb | ≈30 ≈9 | [93] |
| Oedogonium biomass | Pyrolysis at 600 °C | Zn Cd | 13.70 9.11 | [94] |
| Rape straw | Pyrolysis at 600 °C | Cd | 81.1 | [95] |
| Hemp fibers | Room temperature + modification with 17.5% NaOH + boiling temperature + modification with 0.7% NaClO2 | Zn Cd Pb | 2.29 3.93 15.32 | [96] |
| Cotton straw (Gossypium sp. L.) | Pyrolysis at 500 °C | Cd Zn | ≈8.7 ≈6 | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabłońska, B.; Jabłoński, P.; Gęga, J. Kinetics and Thermodynamics of Pb(II), Zn(II), and Cd(II) Adsorption from Aqueous Solutions onto Activated Biochar Obtained from Tobacco Waste. Materials 2025, 18, 2324. https://doi.org/10.3390/ma18102324
Jabłońska B, Jabłoński P, Gęga J. Kinetics and Thermodynamics of Pb(II), Zn(II), and Cd(II) Adsorption from Aqueous Solutions onto Activated Biochar Obtained from Tobacco Waste. Materials. 2025; 18(10):2324. https://doi.org/10.3390/ma18102324
Chicago/Turabian StyleJabłońska, Beata, Paweł Jabłoński, and Jerzy Gęga. 2025. "Kinetics and Thermodynamics of Pb(II), Zn(II), and Cd(II) Adsorption from Aqueous Solutions onto Activated Biochar Obtained from Tobacco Waste" Materials 18, no. 10: 2324. https://doi.org/10.3390/ma18102324
APA StyleJabłońska, B., Jabłoński, P., & Gęga, J. (2025). Kinetics and Thermodynamics of Pb(II), Zn(II), and Cd(II) Adsorption from Aqueous Solutions onto Activated Biochar Obtained from Tobacco Waste. Materials, 18(10), 2324. https://doi.org/10.3390/ma18102324










