Size-Controllable Synthesis of Cu Nanoparticles via Hydrogen Reduction at Low Temperatures
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of CuNPs
3. Results and Discussion
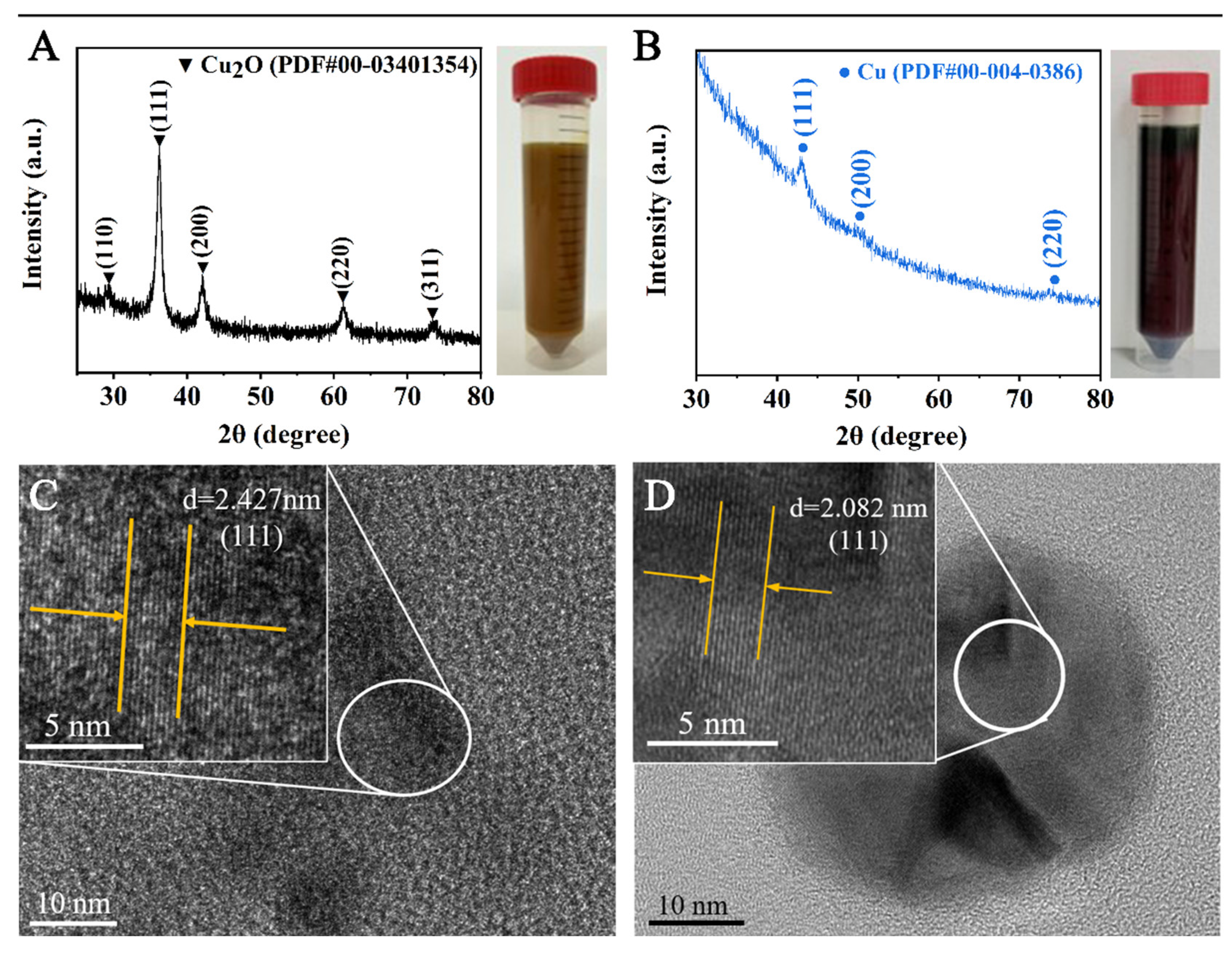
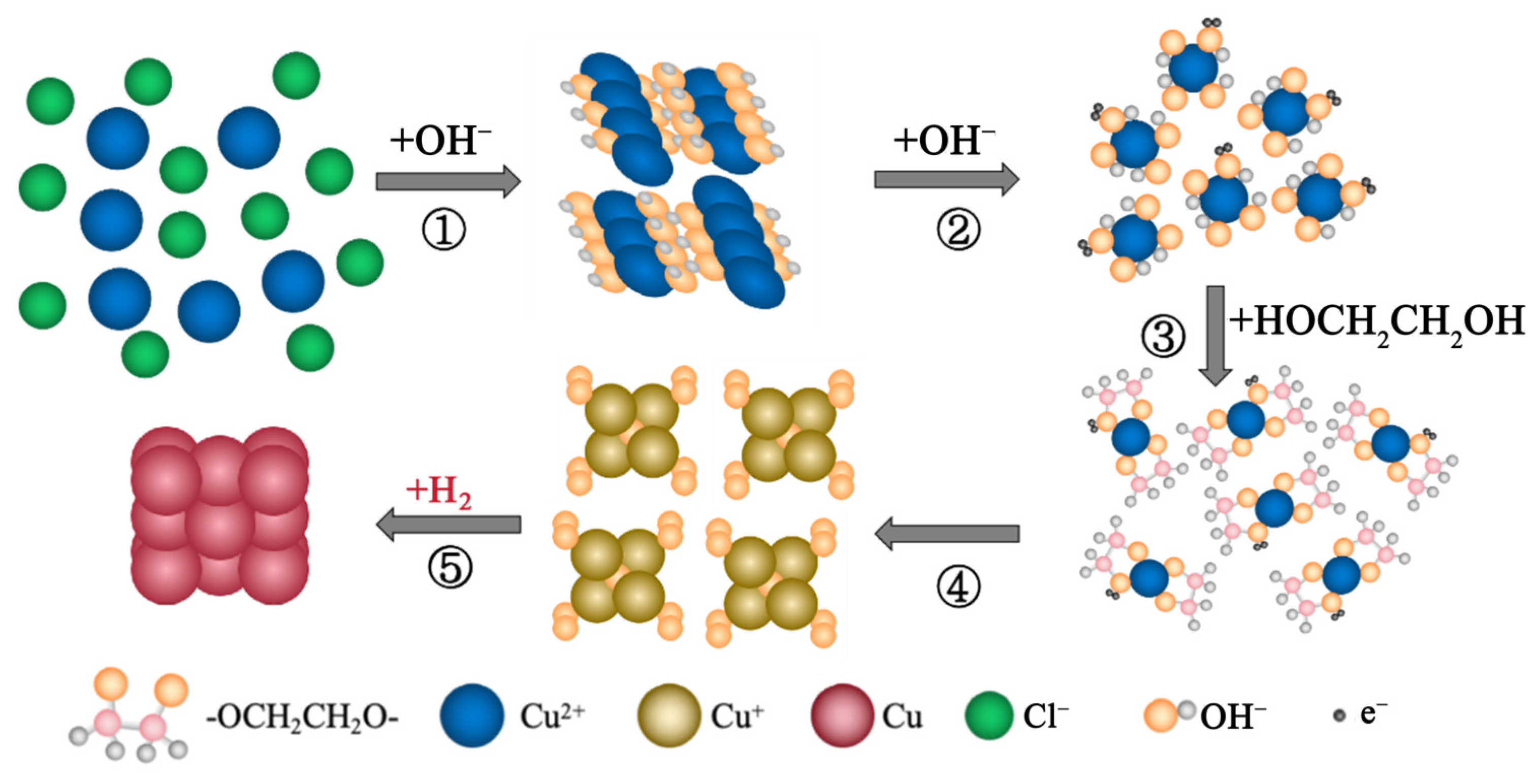
3.1. Verification of H2 as the Reductant
3.2. Control of the CuNP Size
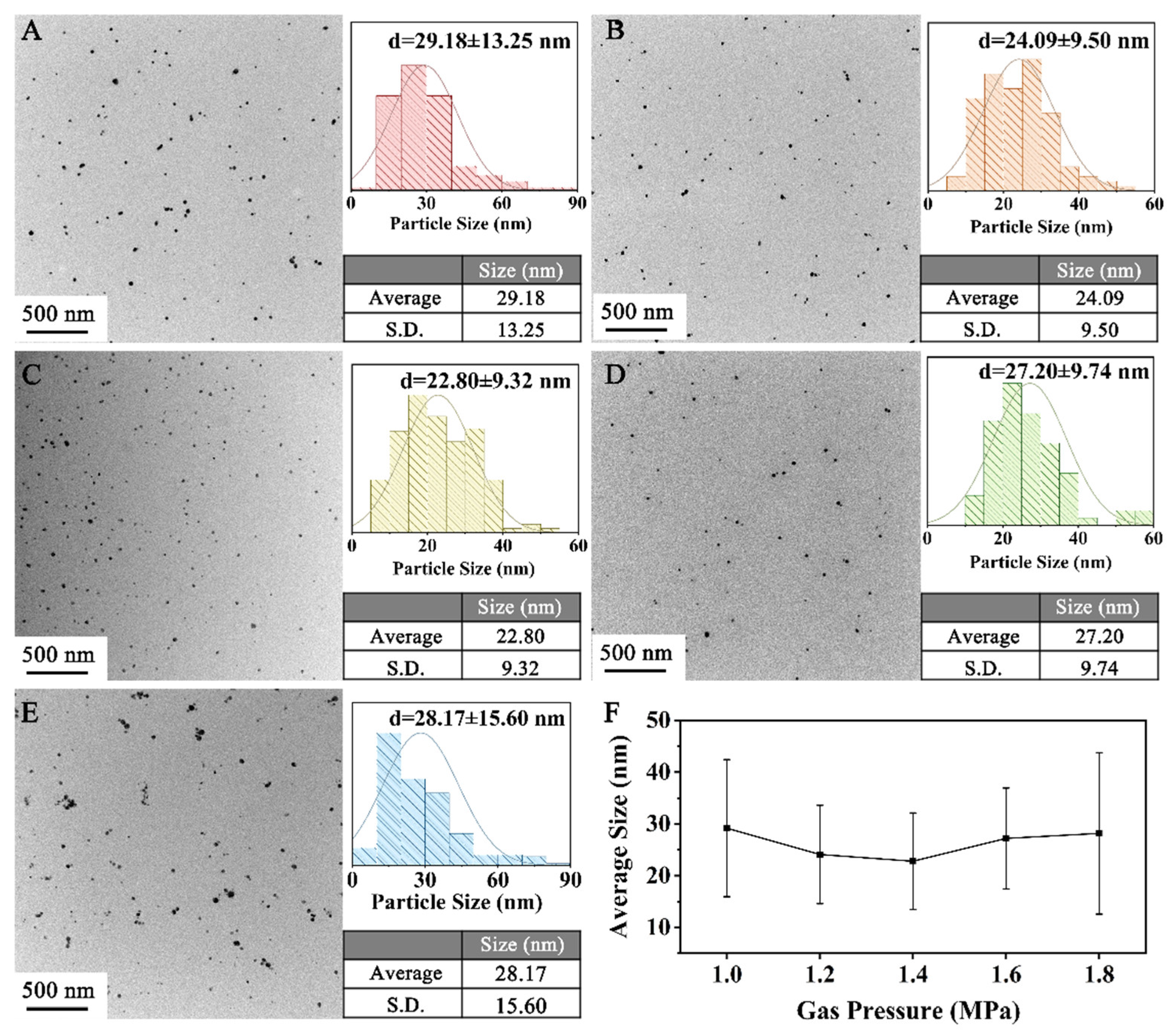
3.2.1. H2 Pressure
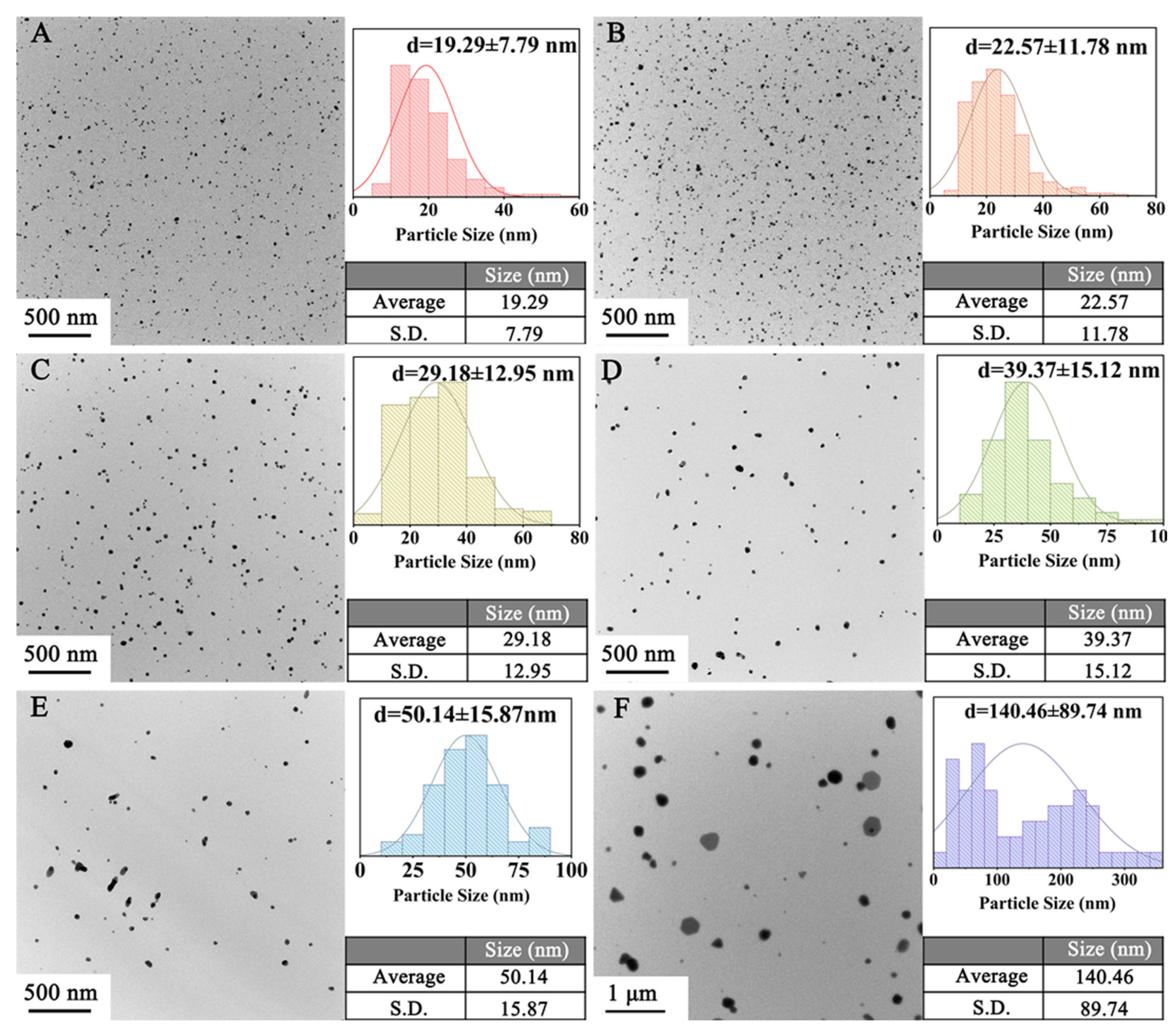
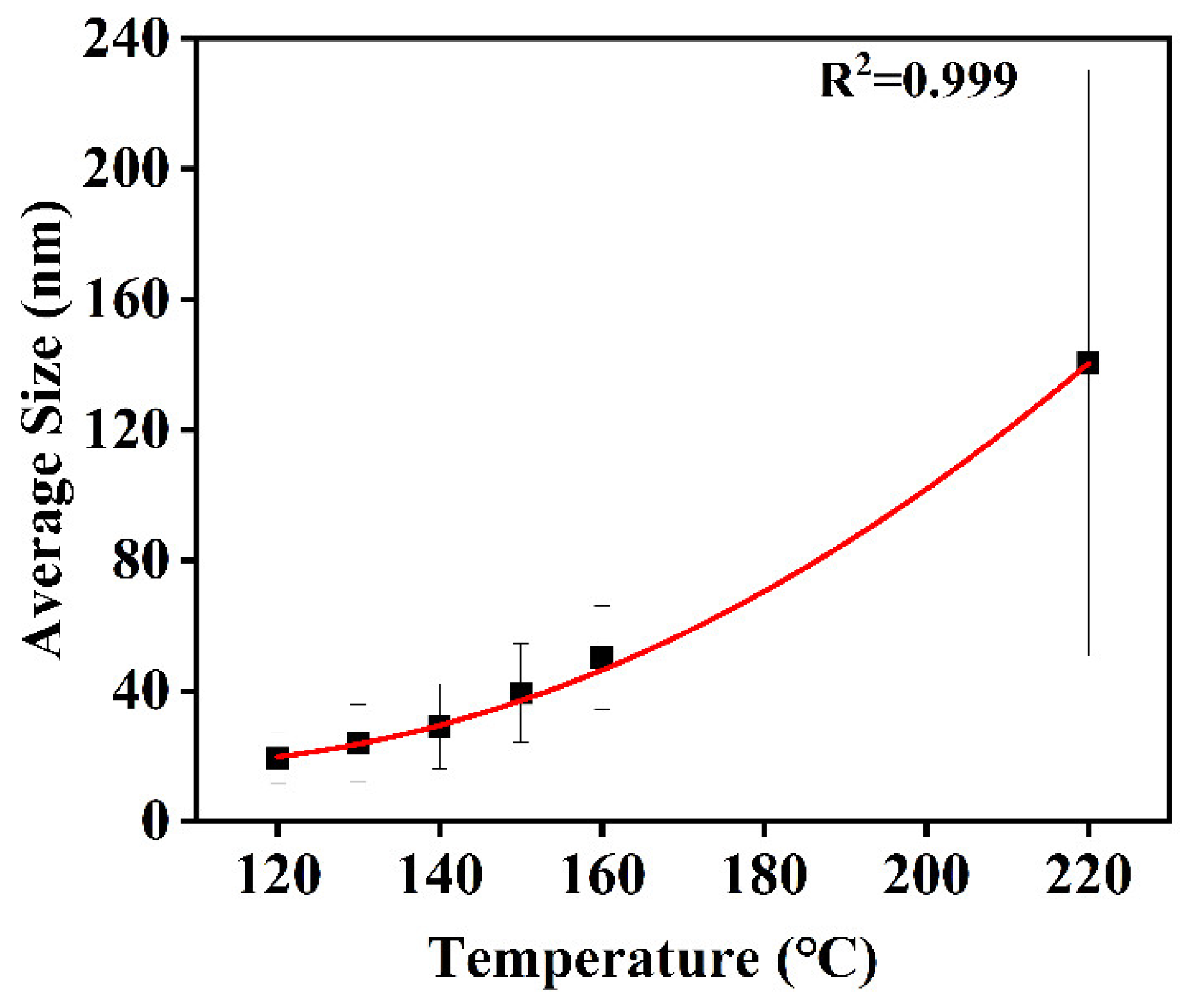
3.2.2. Reaction Temperature
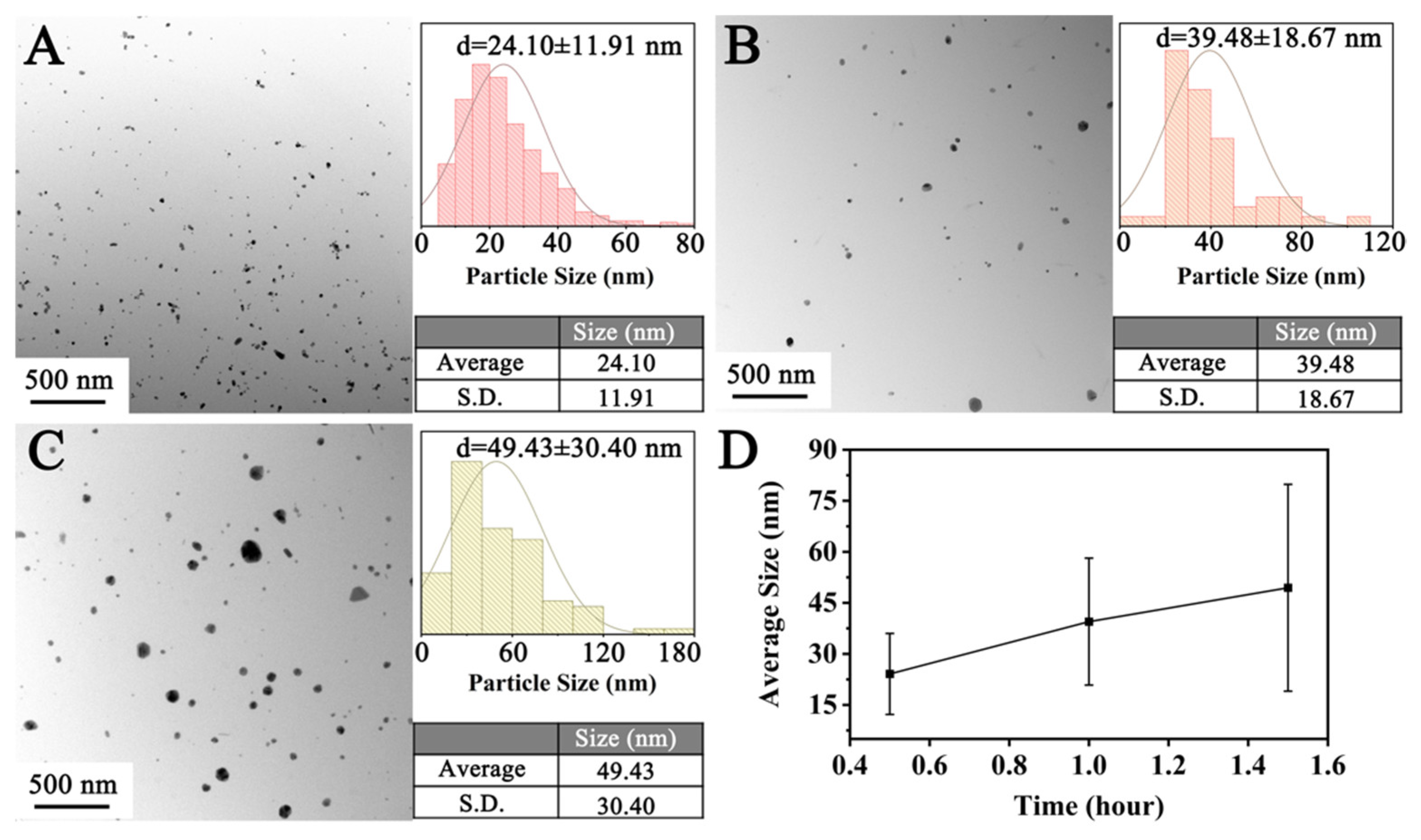
3.2.3. Reaction Duration
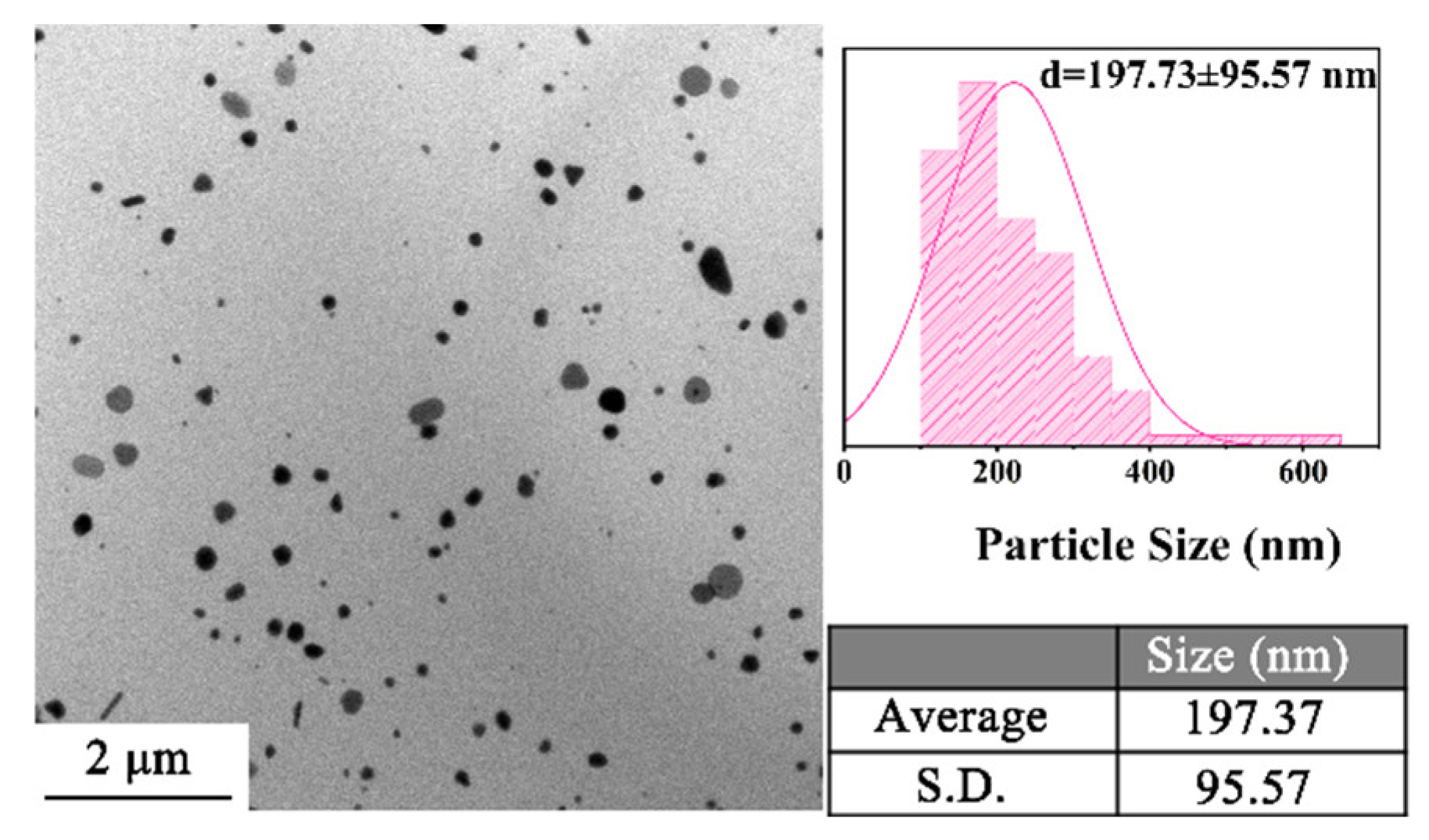
3.3. Outlook of the CuNPs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CuNPs | Copper nanoparticles |
| EG | Ethylene glycol |
| PVP | Polyvinylpyrrolidone |
| XRD | X-ray diffraction |
| TEM | Transmission electron microscope |
| S.D. | Standard deviation |
References
- Xia, S.Y.; Li, X.J.; Guo, Y.J.; Yuan, J.J.; Sun, Z.F.; Cao, H.J.; Zhang, S.Y.; Cai, W.Z.; Li, J.T.; Zhang, Z.H. High-performance Cu-Cu interconnects attained through air sintering of oleylamine-capped Cu nanoparticles for power electronics packaging. Rare Met. 2025, 44, 3291–3298. [Google Scholar] [CrossRef]
- Li, Y.; Qi, T.K.; Chen, M.; Xiao, F. Mixed ink of copper nanoparticles and copper formate complex with low sintering temperatures. J. Mater. Sci.-Mater. Electron. 2016, 27, 11432–11438. [Google Scholar] [CrossRef]
- Lu, R.H.; Hao, W.C.; Kong, L.; Zhao, K.L.; Bai, H.; Lei, L.J.; Liu, Z.G. Self-reducing copper paste with high conductivity and oxidation resistance for flexible substrate by intensive pulsed light sintering. J. Mater. Sci.-Mater. Electron. 2023, 34, 510. [Google Scholar] [CrossRef]
- Jun, H.Y.; Lee, E.J.; Ryu, S.O. Synthesis and characterization of copper ink and direct printing of copper patterns by inkjet printing for electronic devices. Curr. Appl. Phys. 2020, 20, 853–861. [Google Scholar] [CrossRef]
- Peng, Y.; Mou, Y.; Liu, J.X.; Chen, M.X. Fabrication of high-strength Cu–Cu joint by low-temperature sintering micron–nano Cu composite paste. J. Mater. Sci. Mater. Electron. 2020, 31, 8456–8463. [Google Scholar] [CrossRef]
- Gutiérrez, V.; Nador, F.; Radivoy, G.; Volpe, M.A. Highly selective copper nanoparticles for the hydrogenation of α,β-unsaturated aldehydes in liquid phase. Appl. Catal. A Gen. 2013, 464–465, 109–115. [Google Scholar] [CrossRef]
- Helgadottir, I.S.; Arquillière, P.P.; Bréa, P.; Santini, C.C.; Haumesser, P.H.; Richter, K.; Mudring, A.V.; Aouine, M. Synthesis of bimetallic nanoparticles in ionic liquids: Chemical routes vs physical vapor deposition. Microelectron. Eng. 2013, 107, 229–232. [Google Scholar] [CrossRef]
- Sadrolhosseini, A.R.; Noor, A.S.B.M.; Shameli, K.; Mamdoohi, G.; Mahdi, M.A. Laser ablation synthesis and optical properties of copper nanoparticles. J. Mater. Res. 2013, 28, 2629–2636. [Google Scholar] [CrossRef]
- Din, M.I.; Rani, A. Recent Advances in the Synthesis and Stabilization of Nickel and Nickel Oxide Nanoparticles: A Green Adeptness. Int. J. Anal. Chem. 2016, 2016, 3512145. [Google Scholar]
- Hernández-Acosta, M.A.; Trejo-Valdez, M.; Castro-Chacón, J.H.; Torres-San Miguel, C.R.; Martínez-Gutiérrez, H.; Torres-Torres, C. Chaotic signatures of photoconductive Cu2ZnSnS4 nanostructures explored by Lorenz attractors. New J. Phys. 2018, 20, 023048. [Google Scholar] [CrossRef]
- Hikmah, N.; Idrus, N.F.; Jai, J.; Hadi, A. Synthesis and characterization of silver-copper core-shell nanoparticles using polyol method for antimicrobial agent. IOP Conf. Ser. Earth Environ. Sci. 2016, 36, 012050. [Google Scholar] [CrossRef]
- Fiévet, F.; Merah, S.A.; Brayner, R.; Chau, F.; Giraud, M.; Mammeri, F.; Peron, J.; Piquemal, J.-Y.; Sicarda, L.; Viaub, G. The polyol process: A unique method for easy access to metal nanoparticles with tailored sizes, shapes and compositions. Chem. Soc. Rev. 2018, 47, 5187–5233. [Google Scholar] [CrossRef] [PubMed]
- Orel, Z.C.; Matijevic, E.; Goia, D.V. Conversion of uniform colloidal Cu2O spheres to copper in polyols. J. Mater. Res. 2003, 18, 1017–1022. [Google Scholar] [CrossRef]
- Oh, S.H.; Legros, M.; Kiener, D.; Dehm, G. Observation of dislocation nucleation and escape in a submicrometre aluminium single crystal. Nat. Mater. 2009, 8, 95–100. [Google Scholar] [CrossRef]
- Dahal, R.; Ray, S.K.; Pathiraja, G.; Bastakoti, B.P. Low-temperature fabrication of morphology-controllable Cu2O for electrochemical CO2 reduction. J. Mater. Sci. 2024, 59, 13896–13907. [Google Scholar] [CrossRef]
- Gattinoni, C.; Michaelides, A. Atomistic details of oxide surfaces and surface oxidation: The example of copper and its oxides. Surf. Sci. Rep. 2015, 70, 424–447. [Google Scholar] [CrossRef]
- Peng, J.; Chen, B.L.; Wang, Z.C.; Guo, J.; Wu, B.H.; Hao, S.Q.; Zhang, Q.H.; Gu, L.; Zhou, Q.; Liu, Z.; et al. Surface coordination layer passivates oxidation of copper. Nature 2020, 586, 390. [Google Scholar] [CrossRef]
- Sun, J.H.; Jing, Y.; Jia, Y.Z.; Tillard, M.; Belin, C. Mechanism of preparing ultrafine copper powder by polyol process. Mater. Lett. 2005, 59, 3933–3936. [Google Scholar] [CrossRef]
- Rosenberg, R.M.; Peticolas, W.L. Henry’s law: A retrospective. J. Chem. Educ. 2004, 81, 1647–1652. [Google Scholar] [CrossRef]
- Johannes, T.; Thomas, D.; Michael, R. Mechanisms of the polyol reduction of copper(ii) salts depending on the anion type and diol chain length. Dalton Trans. 2018, 47, 14085–14093. [Google Scholar]
- Ben Aissa, M.A.; Tremblay, B.; Andrieux-Ledier, A.; Maisonhaute, E.; Raouafi, N.; Courty, A. Copper nanoparticles of well-controlled size and shape: A new advance in synthesis and self-organization. Nanoscale 2015, 7, 3189–3195. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, S.; Gao, A.; Ma, Z.; Xu, H.; Xue, Z. Size-Controllable Synthesis of Cu Nanoparticles via Hydrogen Reduction at Low Temperatures. Materials 2025, 18, 2315. https://doi.org/10.3390/ma18102315
Feng S, Gao A, Ma Z, Xu H, Xue Z. Size-Controllable Synthesis of Cu Nanoparticles via Hydrogen Reduction at Low Temperatures. Materials. 2025; 18(10):2315. https://doi.org/10.3390/ma18102315
Chicago/Turabian StyleFeng, Songya, Anxin Gao, Zihang Ma, Hongjie Xu, and Zhiyong Xue. 2025. "Size-Controllable Synthesis of Cu Nanoparticles via Hydrogen Reduction at Low Temperatures" Materials 18, no. 10: 2315. https://doi.org/10.3390/ma18102315
APA StyleFeng, S., Gao, A., Ma, Z., Xu, H., & Xue, Z. (2025). Size-Controllable Synthesis of Cu Nanoparticles via Hydrogen Reduction at Low Temperatures. Materials, 18(10), 2315. https://doi.org/10.3390/ma18102315






