Influence of Rust Layer on Corrosion-Critical Humidity in Outdoor Environments Based on Corrosion Sensors
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Corrosion Monitoring Technology
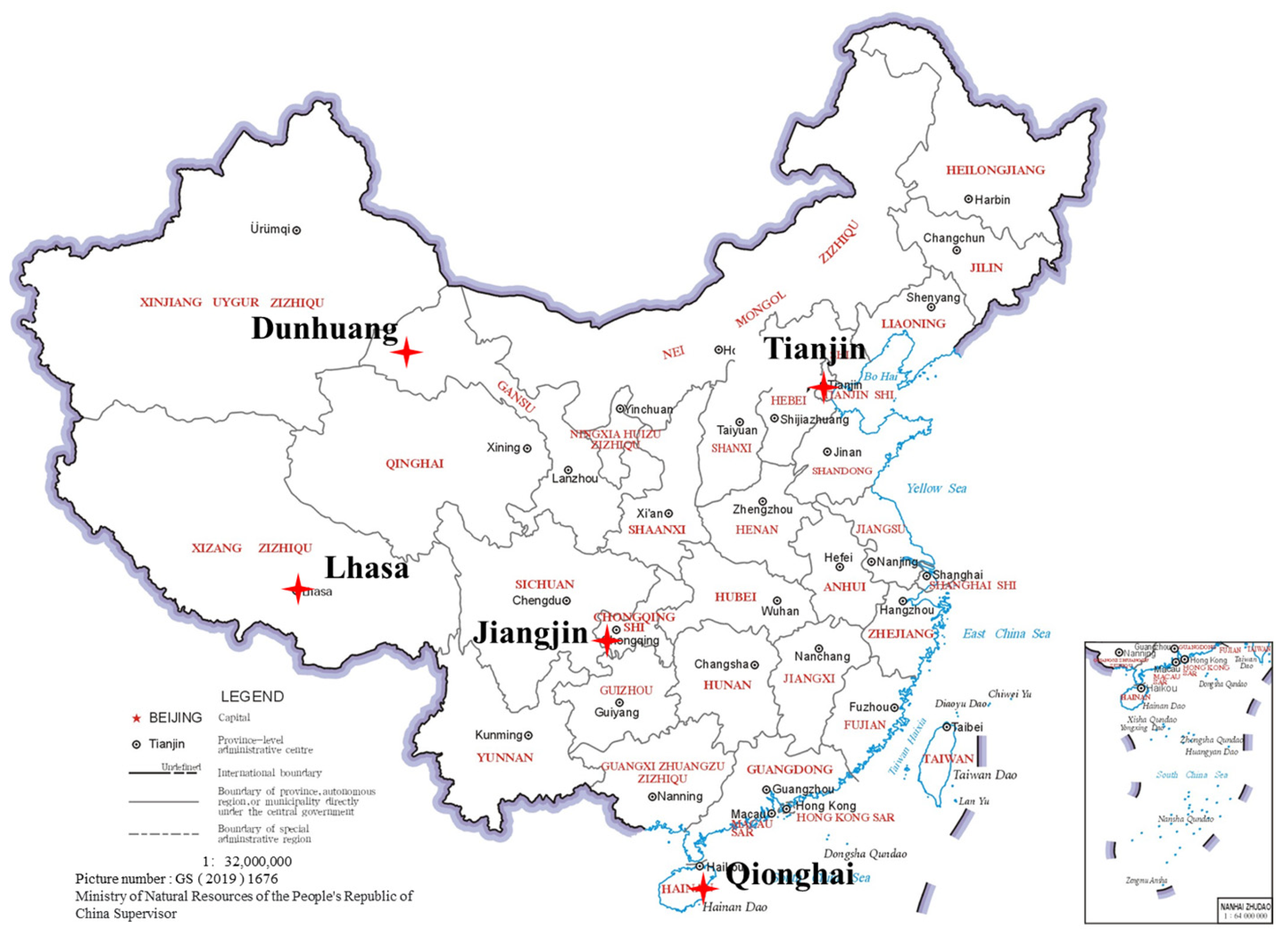
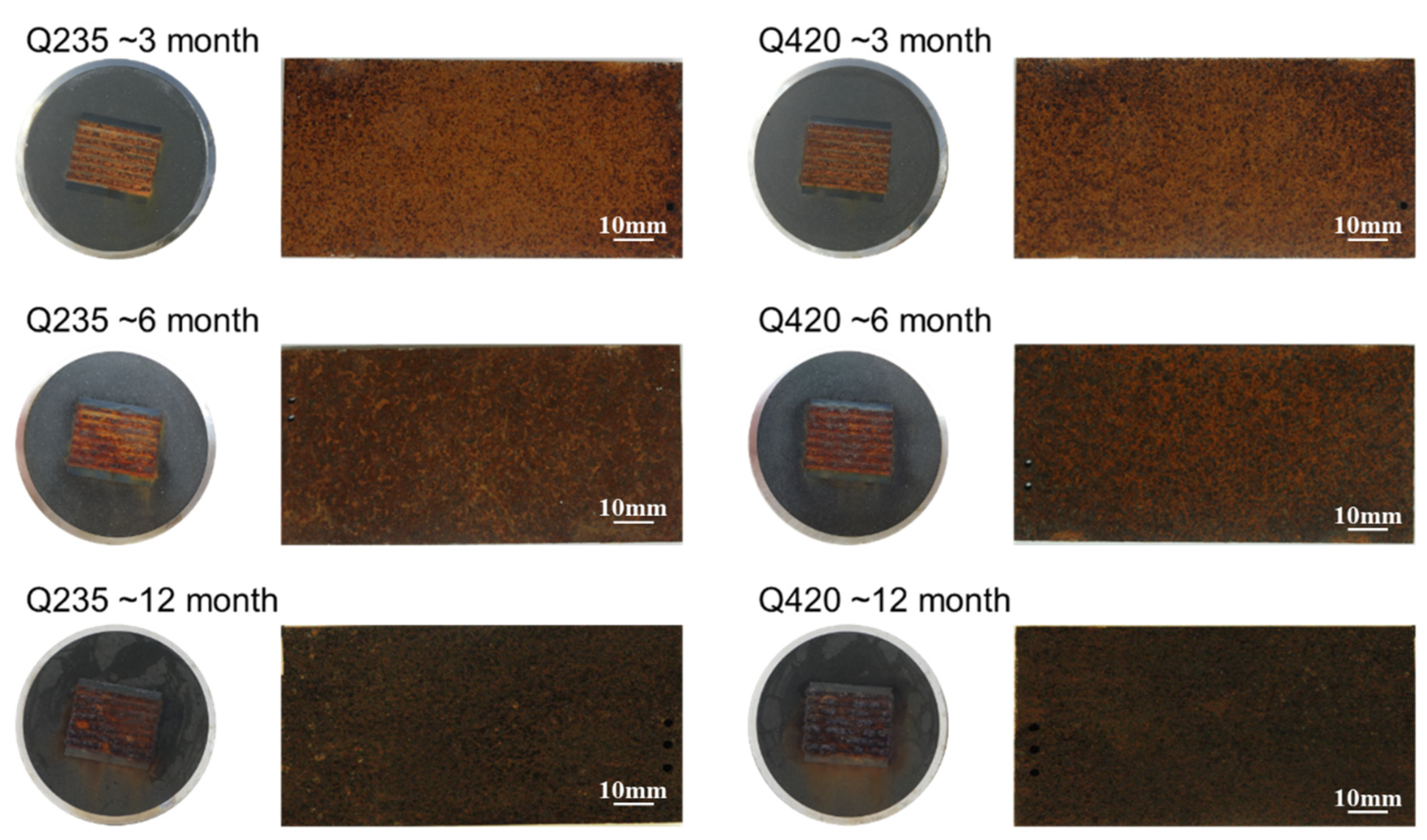
2.3. Exposure Experiments
2.4. Rust Characterization
3. Results
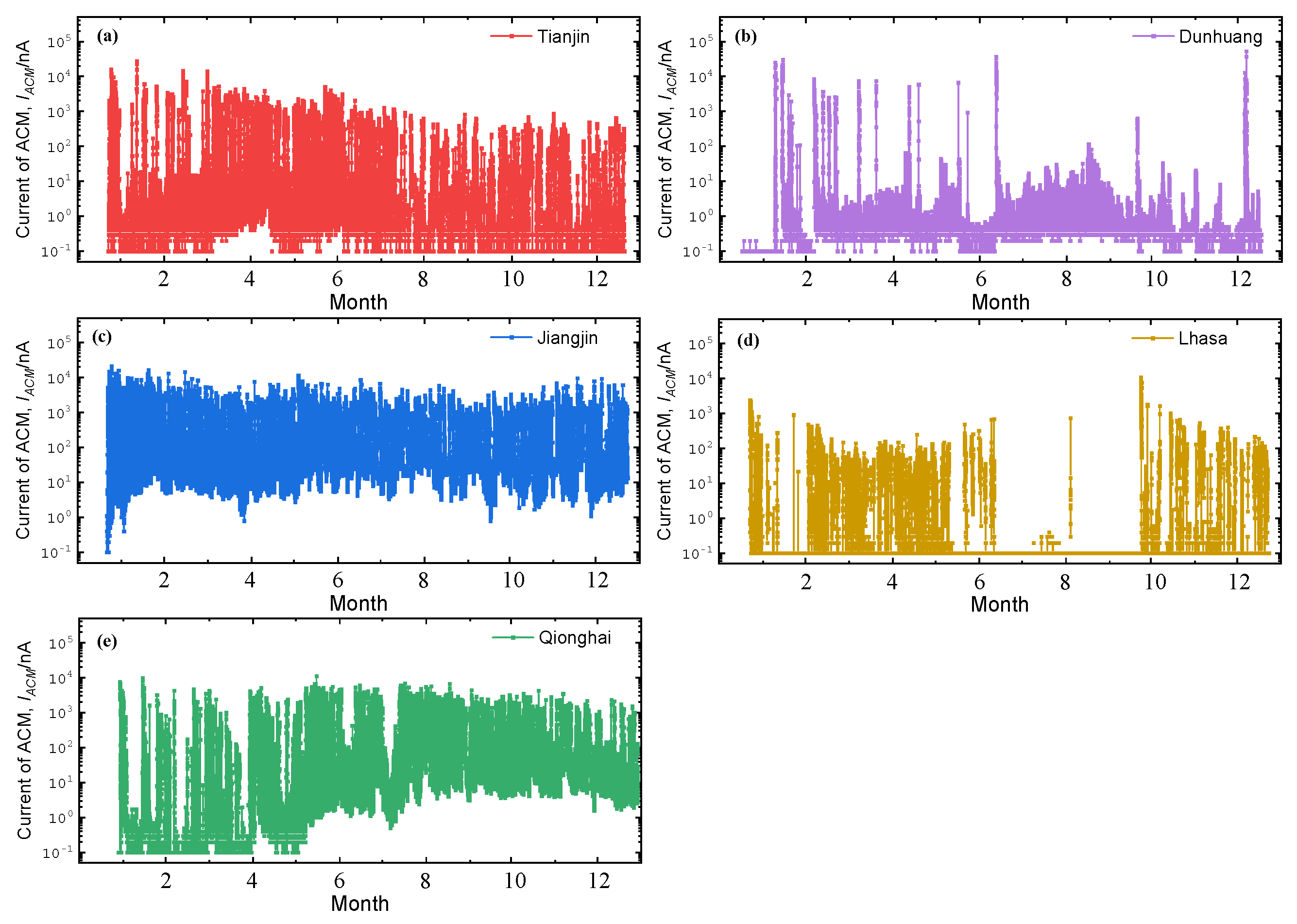
3.1. Corrosion Sensor Results
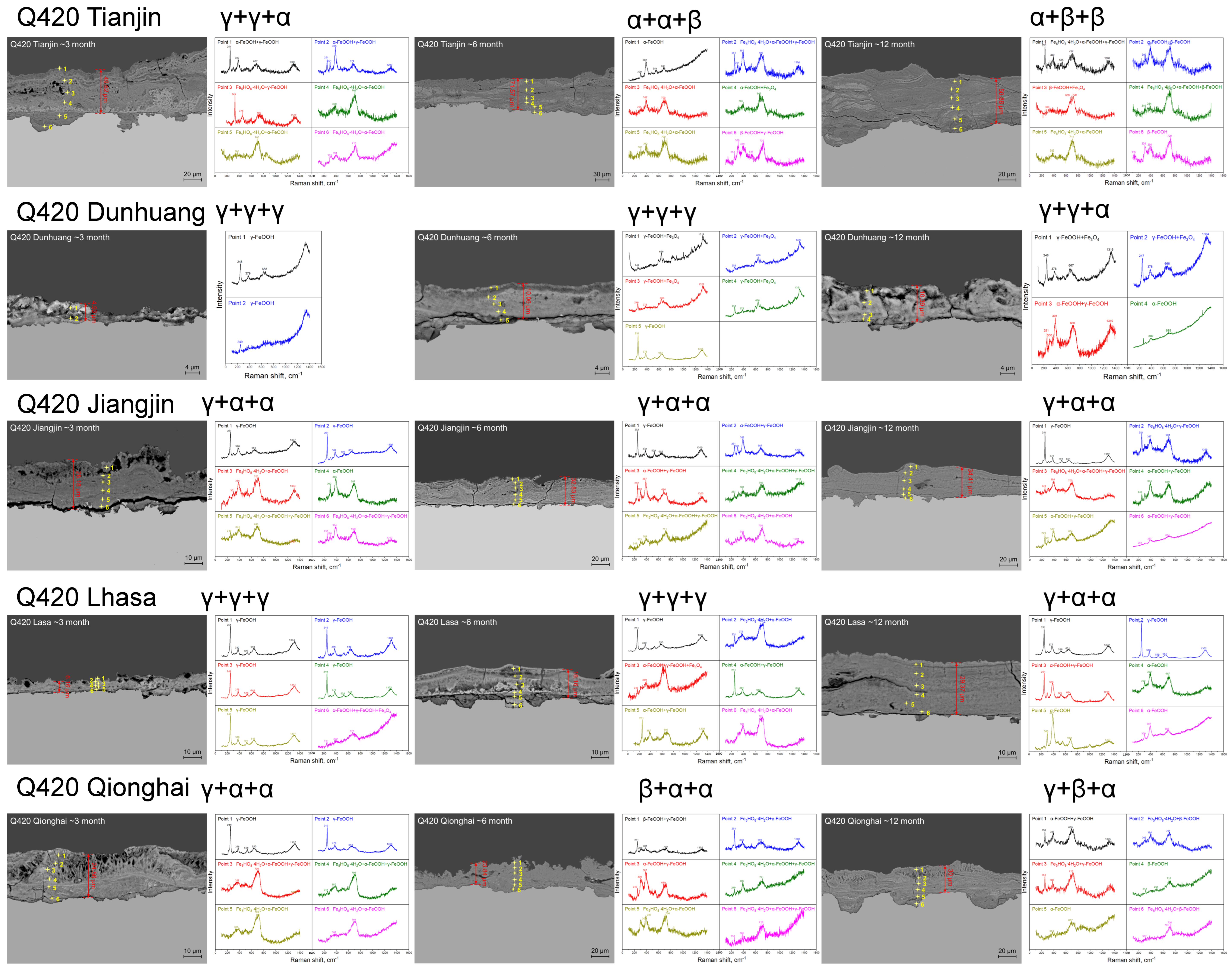
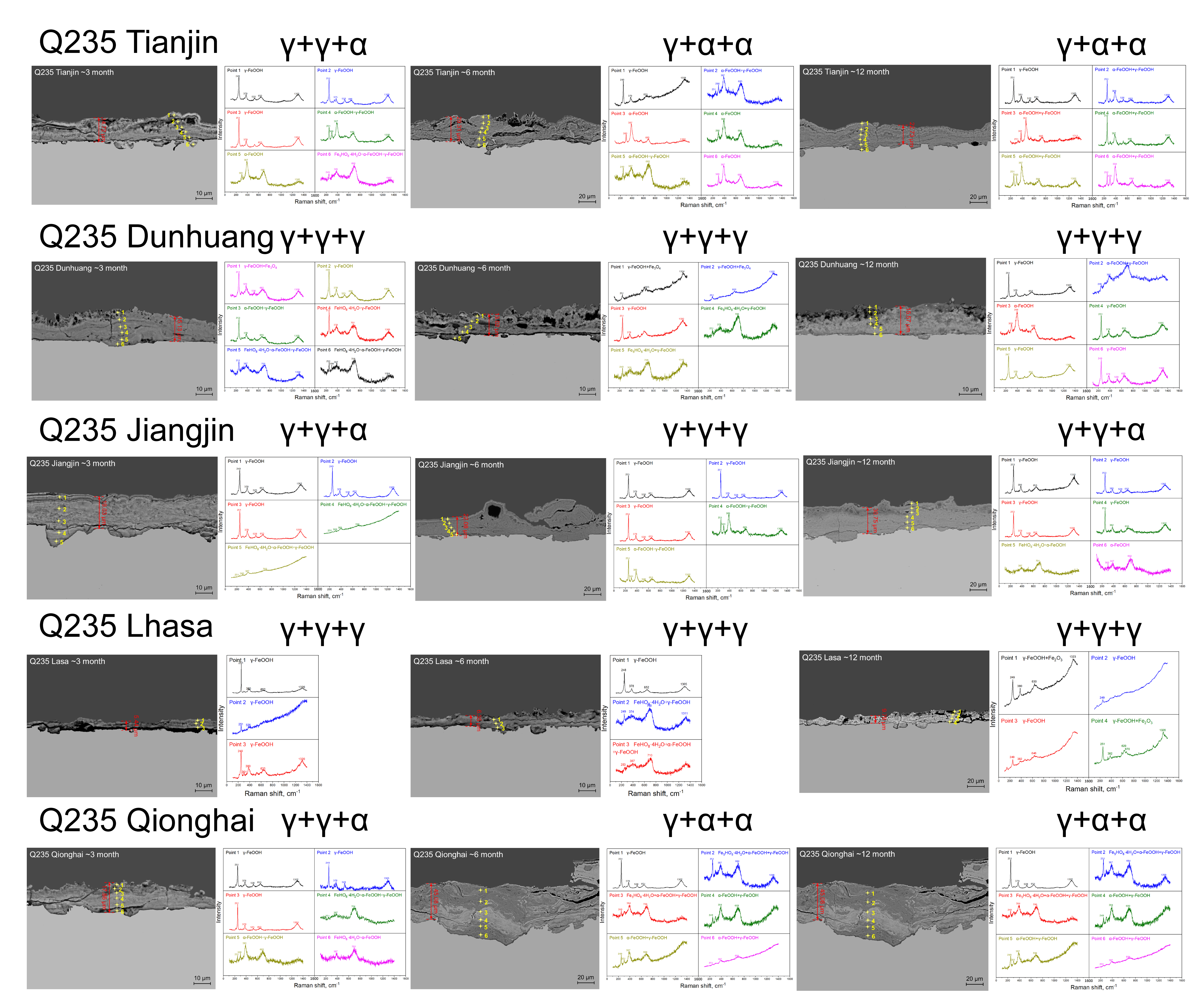
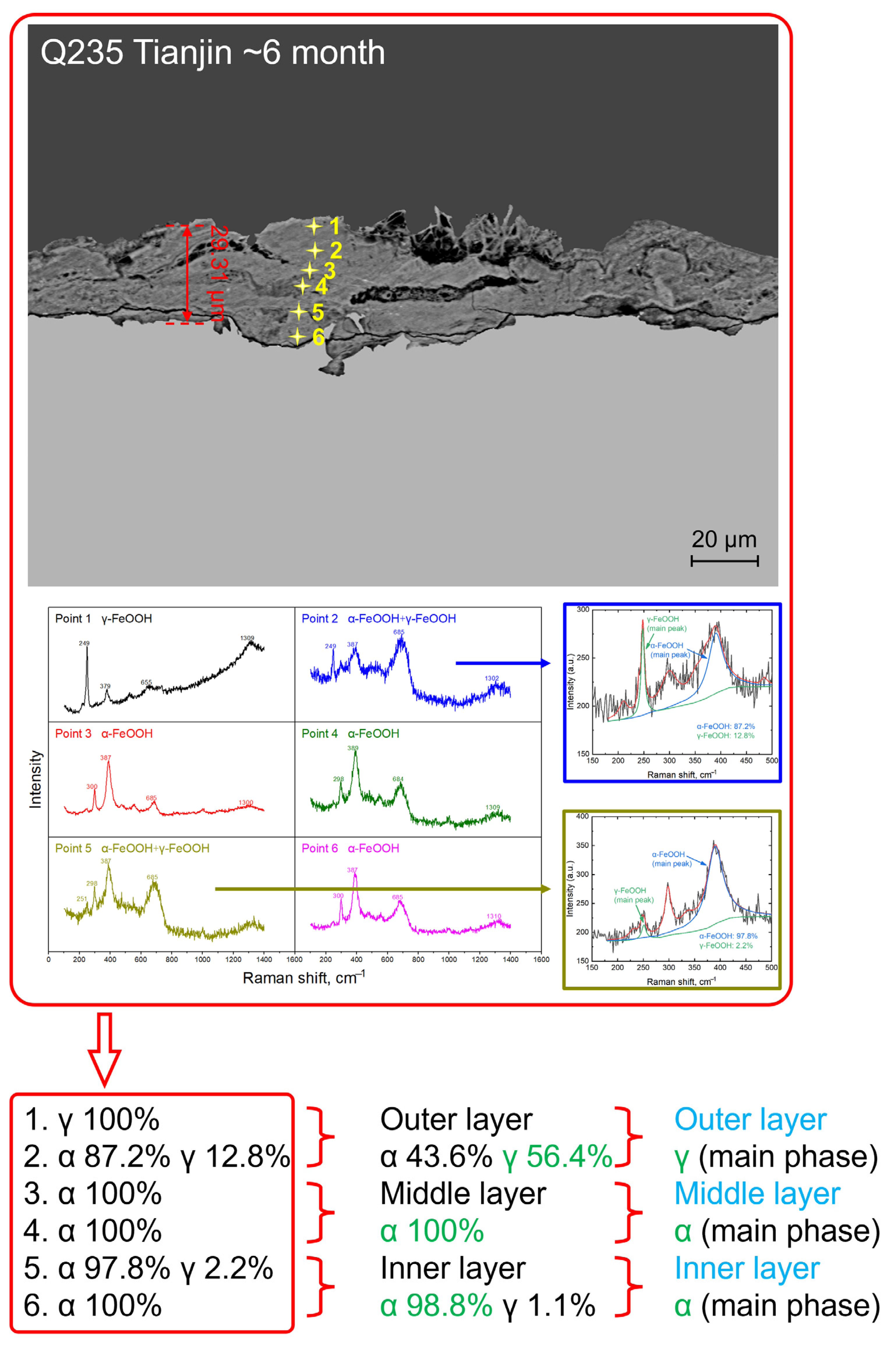
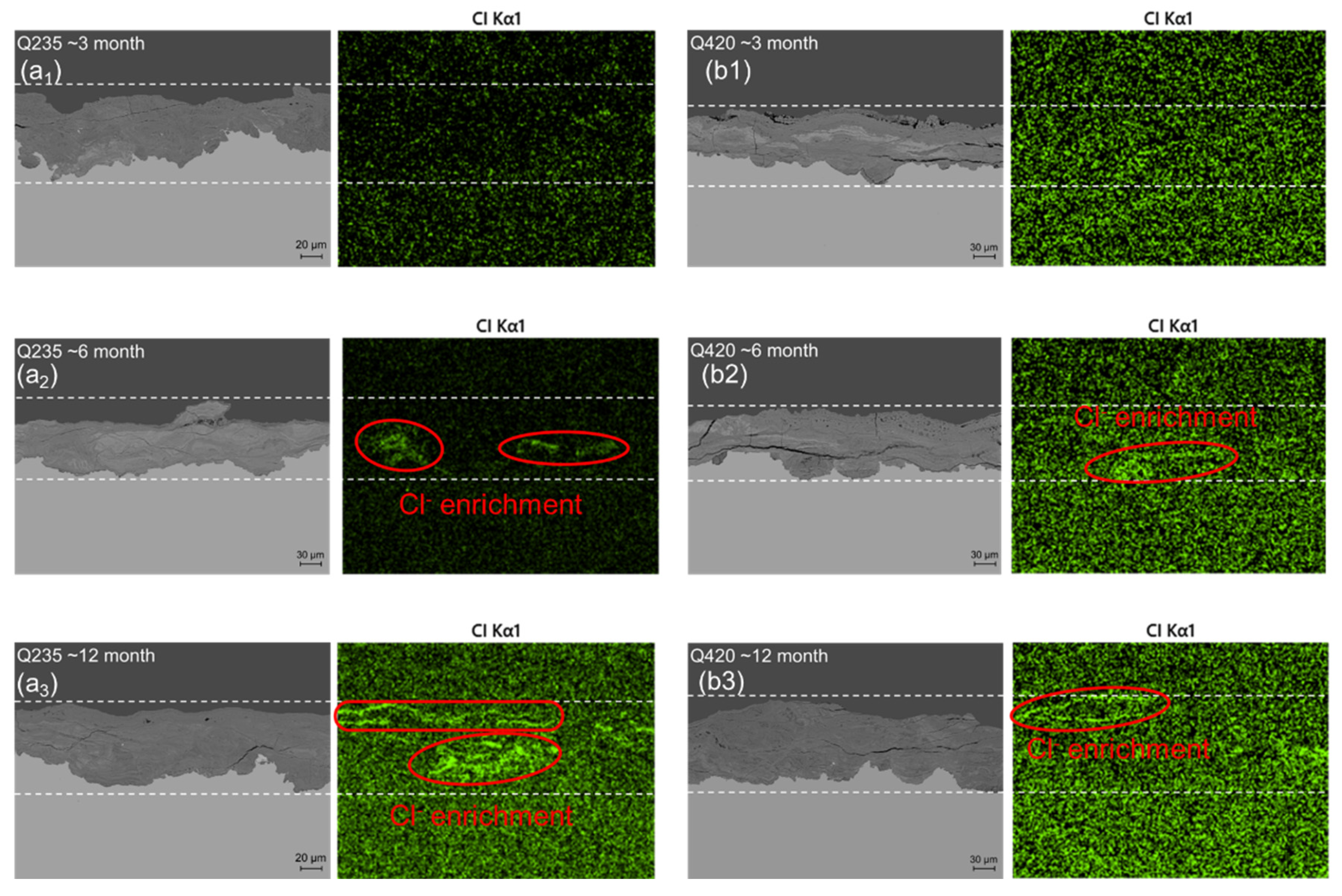
3.2. Rust Layer Characterization
3.3. Corrosion Resistance Analysis
4. Discussion
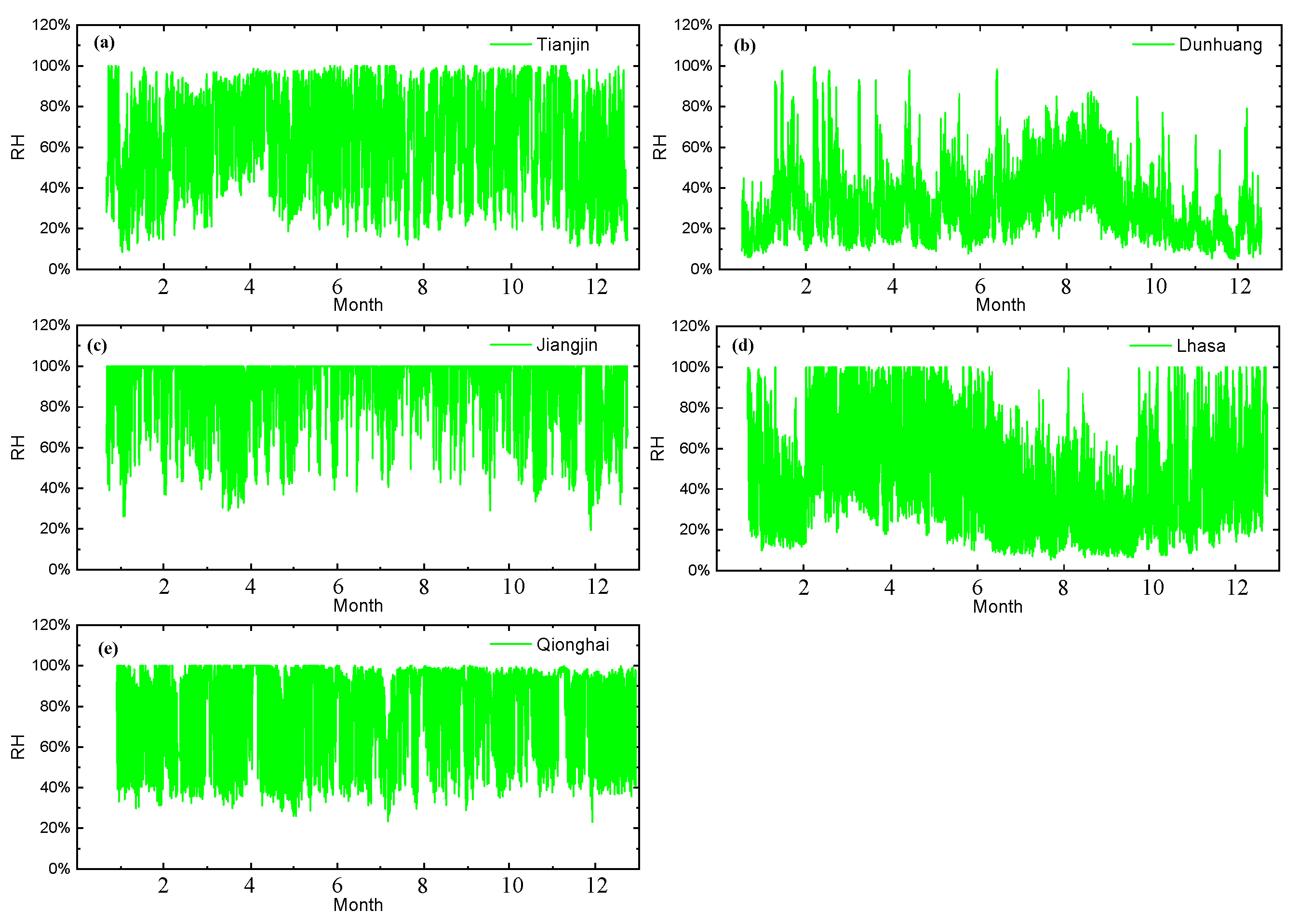
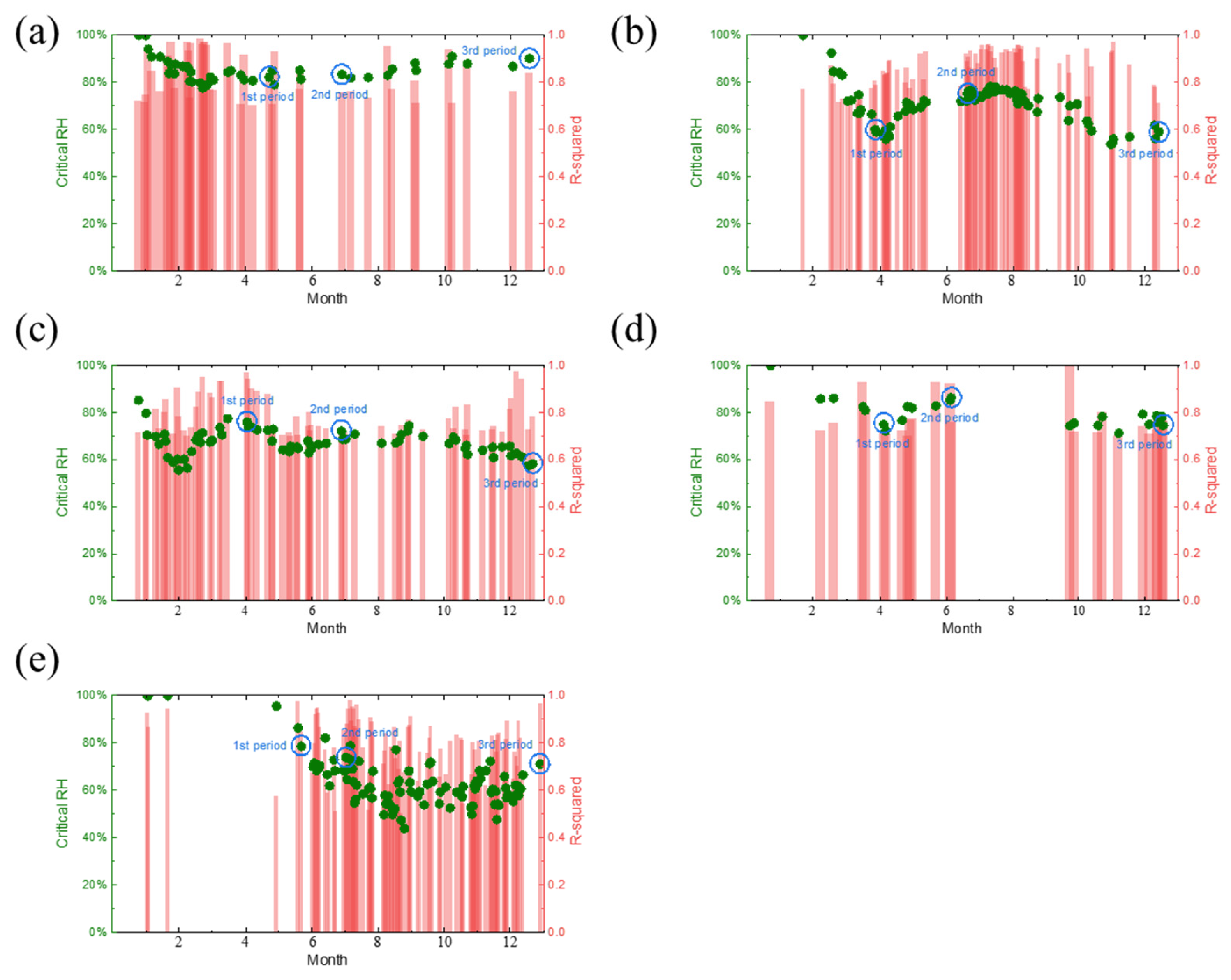
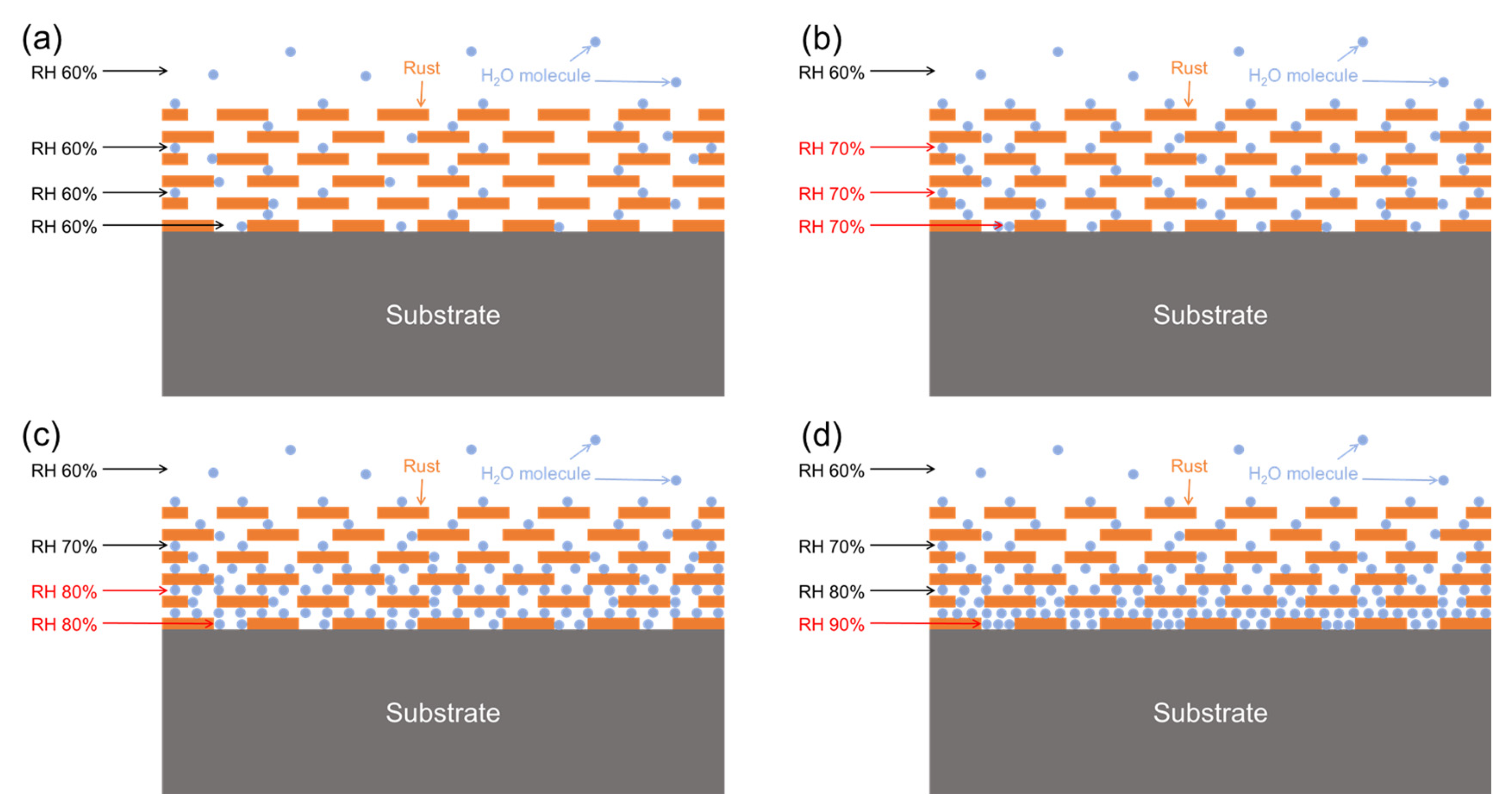
4.1. Critical Humidity Analysis of the Test Sites
4.2. Phase Structure Analysis of the Rust Layer
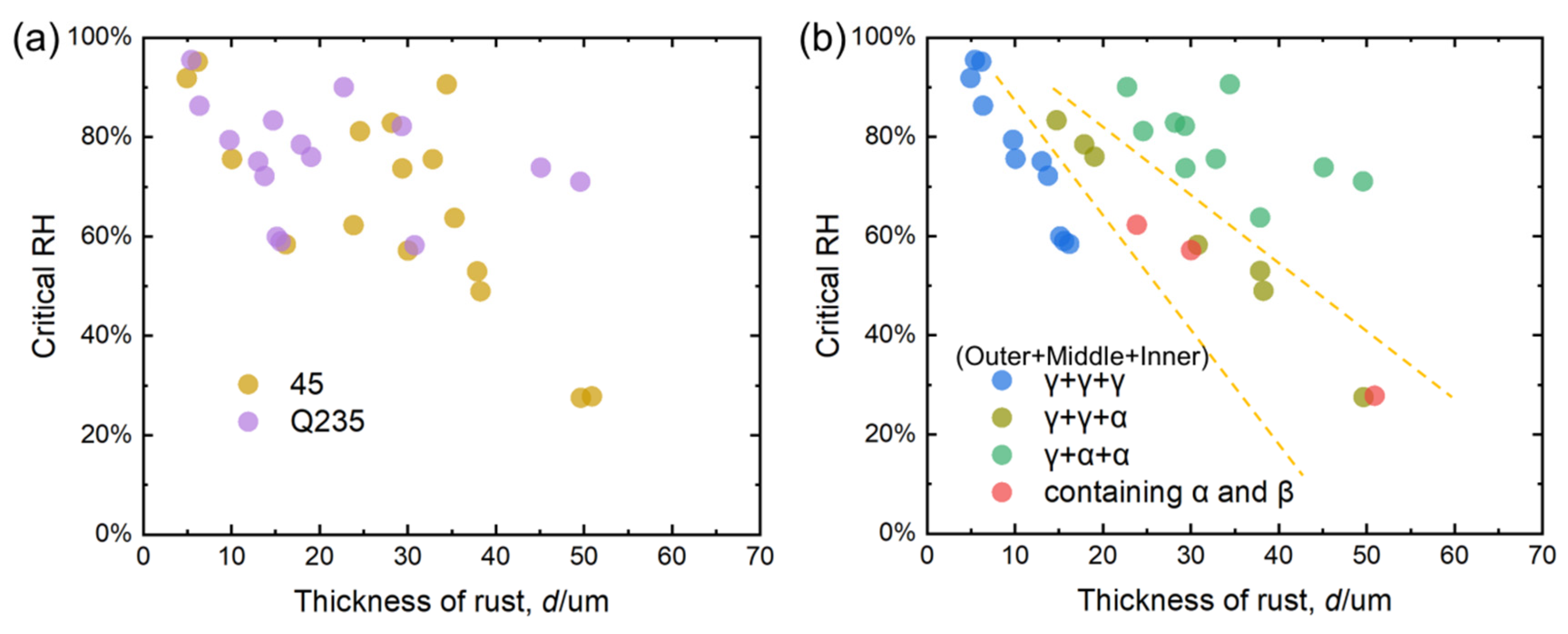
4.3. Relationship Between Thickness of Carbon Steel Rust Layer and Critical Humidity of Corrosion
- (1)
- For γ + γ + γ structure:
- (2)
- For γ + γ + α structure:
- (3)
- For γ + α + α structure:
5. Conclusions
- (1)
- Based on the real-time corrosion monitoring data, a method for calculating the critical humidity of atmospheric corrosion is proposed, and it is found that, in the process of atmospheric corrosion caused by changes in outdoor temperature and humidity, the critical humidity of atmospheric corrosion generally decreases first and then rises. When the thickness of the rust layer is less than 70 μm, the critical humidity of corrosion under each rust layer structure decreases with the increase in thickness, indicating that the rust layer in this thickness range plays a role in promoting atmospheric corrosion in a non-rainfall state.
- (2)
- The rust layer structure with γ-FeOOH as the main phase is easier to observe in the early stages of corrosion, and when the thickness of the rust layer is greater than 20 μm, it tends to evolve into other structures. Compared with α-FeOOH, β-FeOOH has a stronger effect on atmospheric corrosion caused by temperature and humidity changes, and β-FeOOH is not suddenly generated in the outdoor environment but slowly forms in the rust layer over time. The key to improving the corrosion resistance of weathering steel is the transformation of the phase structure of the rust layer, and the difficulty of atmospheric corrosion cannot be evaluated only by the thickness of the rust layer.
- (3)
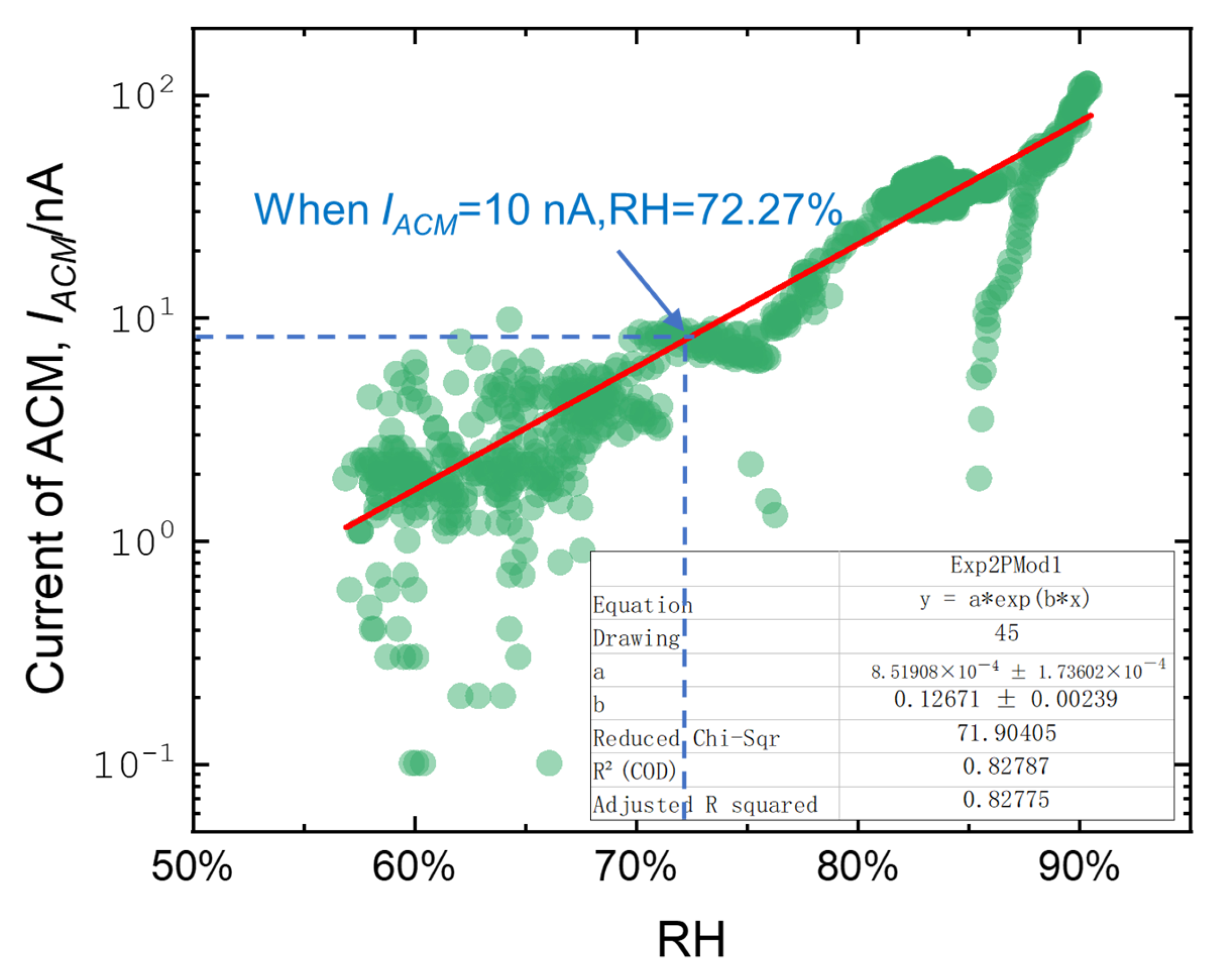
- The corrosion sensor current and corrosion coupons have the following empirical formula conversion relationship: . This conversion relationship is applicable to other low-alloy steels, proving that corrosion-monitoring technology has the potential to replace traditional corrosion coupons for atmospheric corrosion research on low-alloy steels.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Zhang, D.; Liu, Z.; Li, Z.; Du, C.; Dong, C. Materials science: Share corrosion data. Nature 2015, 527, 441–442. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Liu, W.; Li, S.; Chowwanonthapunya, T.; Wongpat, B.; Zhao, Y.; Dong, B.; Zhang, T.; Li, X. Evolution of rust layers on carbon steel and weathering steel in high humidity and heat marine atmospheric corrosion. J. Mater. Sci. Technol. 2020, 39, 190–199. [Google Scholar] [CrossRef]
- Yang, X.; Yang, J.; Yang, Y.; Li, Q.; Xu, D.; Cheng, X.; Li, X. Data-mining and atmospheric corrosion resistance evaluation of Sn- and Sb-additional low alloy steel based on big data technology. Int. J. Miner. Metall. Mater. 2022, 29, 825–835. [Google Scholar] [CrossRef]
- Van den Steen, N.; Simillion, H.; Dolgikh, O.; Terryn, H.; Deconinck, J. An integrated modeling approach for atmospheric corrosion in presence of a varying electrolyte film. Electrochim. Acta 2016, 187, 714–723. [Google Scholar] [CrossRef]
- Hoseinpoor, M.; Prošek, T.; Mallégol, J. Comprehensive assessment of time of wetness on coil-coated steel sheets. Corros. Sci. 2025, 244, 112641. [Google Scholar] [CrossRef]
- ISO 9223:2012; Corrosion of Metals and Alloys—Corrosivity of Atmospheres—Classification, Determination and Estimation. International Organization for Standardization (ISO): Geneva, Switzerland, 2012.
- Corvo, F.; Pérez, T.; Martin, Y.; Reyes, J.; Dzib, L.R.; González-Sánchez, J.; Castañeda, A. Time of wetness in tropical climate: Considerations on the estimation of TOW according to ISO 9223 standard. Corros. Sci. 2008, 50, 206–219. [Google Scholar] [CrossRef]
- Shi, B.; Qin, L.; Xu, D.; Cheng, X.; Liu, C.; Yang, G.; Xu, F.; Li, X. Exploring Corrosion Protection Evolution of Rust Layer on High-Cr-content Weathering Bridge Steel in Simulated Tropical Marine Atmosphere. Int. J. Miner. Metall. Mater. 2024. [Google Scholar] [CrossRef]
- Wan, S.; Liao, B.; Dong, Z.; Guo, X. Comparative investigation on copper atmospheric corrosion by electrochemical impedance and electrical resistance sensors. Trans. Nonferrous Met. Soc. China 2021, 31, 3024–3038. [Google Scholar] [CrossRef]
- Guo, T.; Qin, D.; Zou, Y.; Liu, Z. Research and development of corrosion monitoring sensor for bridge cable using electrical resistance probes technique. Measurement 2024, 237, 115178. [Google Scholar] [CrossRef]
- Rahimi, E.; Zhang, K.; Kosari, A.; Van den Steen, N.; Homborg, A.; Terryn, H.; Mol, A.; Gonzalez-Garcia, Y. Atmospheric corrosion of iron under a single droplet: A new systematic multi-electrochemical approach. Corros. Sci. 2024, 235, 112171. [Google Scholar] [CrossRef]
- Li, C.; Ma, Y.; Li, Y.; Wang, F. EIS monitoring study of atmospheric corrosion under variable relative humidity. Corros. Sci. 2010, 52, 3677–3686. [Google Scholar] [CrossRef]
- Situm, A.; Sabeti, M.; Schmidt, N.; Zagidulin, D.; Behazin, M.; Noël, J.J. Monitoring alterations in a salt layer’s deliquescence properties during the atmospheric corrosion of a metal surface using a quartz crystal microbalance. Corros. Sci. 2024, 229, 111845. [Google Scholar] [CrossRef]
- Mizuno, D.; Suzuki, S.; Fujita, S.; Hara, N. Corrosion monitoring and materials selection for automotive environments by using Atmospheric Corrosion Monitor (ACM) sensor. Corros. Sci. 2014, 83, 217–225. [Google Scholar] [CrossRef]
- Pongsaksawad, W.; Viyanit, E.; Sorachot, S.; Shinohara, T. Corrosion Assessment of Carbon Steel in Thailand by Atmospheric Corrosion Monitoring (ACM) Sensors. J. Met. Mater. Miner. 2020, 20, 2. [Google Scholar]
- Pei, Z.; Zhang, D.; Zhi, Y.; Yang, T.; Jin, L.; Fu, D.; Cheng, X.; Terryn, H.A.; Mol, J.M.C.; Li, X. Towards understanding and prediction of atmospheric corrosion of an Fe/Cu corrosion sensor via machine learning. Corros. Sci. 2020, 170, 108697. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, R.; Han, Q.; Yu, X.; Yu, Z. Experimental investigation on the corrosion and corrosion fatigue behavior of butt weld with G20Mn5QT cast steel and Q355D steel under dry–wet cycle. Eng. Fail. Anal. 2022, 134, 105977. [Google Scholar] [CrossRef]
- Ma, Y.; Li, Y.; Wang, F. Corrosion of low carbon steel in atmospheric environments of different chloride content. Corros. Sci. 2009, 51, 997–1006. [Google Scholar] [CrossRef]
- Ma, Y.; Li, Y.; Wang, F. The atmospheric corrosion kinetics of low carbon steel in a tropical marine environment. Corros. Sci. 2010, 52, 1796–1800. [Google Scholar] [CrossRef]
- Li, Q.; Xia, X.; Pei, Z.; Cheng, X.; Zhang, D.; Xiao, K.; Wu, J.; Li, X. Long-term corrosion monitoring of carbon steels and environmental correlation analysis via the random forest method. Npj Mater. Degrad. 2022, 6, 1. [Google Scholar] [CrossRef]
- Yang, X.; Li, Q.; Liu, S.; Hu, J.; Zhu, R.; Yang, G. Atmospheric corrosion prediction of carbon steel and weathering steel based on big data technology. Corros. Commun. 2025. [Google Scholar] [CrossRef]
- GB/T 41756-2022; Corrosion of Metals and Alloys—Method for Estimating the Atmospheric Corrosion Resistance of Low—Alloy Steels. National Standard of the People’s Republic of China: Beijing, China, 2022.
- Qian, H.; Zhang, D.; Lou, Y.; Li, Z.; Xu, D.; Du, C.; Li, X. Laboratory investigation of microbiologically influenced corrosion of Q235 carbon steel by halophilic archaea Natronorubrum tibetense. Corros. Sci. 2018, 145, 151–161. [Google Scholar] [CrossRef]
- Wu, W.; Zeng, Z.; Cheng, X.; Li, X.; Liu, B. Atmospheric Corrosion Behavior and Mechanism of a Ni-Advanced Weathering Steel in Simulated Tropical Marine Environment. J. Mater. Eng. Perform. 2017, 26, 6075–6086. [Google Scholar] [CrossRef]
- Abbas, M.; Rizvi, S.H.M.; Sarfraz, S.; Raza, A.; Khan, A.; Loya, A.; Najib, A. Evaluation of the influence of dissolved nitrates on corrosion behaviour of ship structural steel exposed to seawater environment. Ocean Eng. 2024, 298, 117268. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Y.; Sun, M.; Jia, J.; Cheng, X.; Pei, Z.; Li, Q.; Xu, D.; Xiao, K.; Li, X. A new understanding of the effect of Cr on the corrosion resistance evolution of weathering steel based on big data technology. J. Mater. Sci. Technol. 2022, 104, 67–80. [Google Scholar] [CrossRef]
- Wu, W.; Dai, Z.; Liu, Z.; Liu, C.; Li, X. Synergy of Cu and Sb to enhance the resistance of 3%Ni weathering steel to marine atmospheric corrosion. Corros. Sci. 2021, 183, 109353. [Google Scholar] [CrossRef]
- Wang, P.; Cheng, X.; Zhang, Z.; Huang, Y.; Ma, L.; Li, X.; Zhang, D. Roles of grain refinement in the rust formation and corrosion resistance of weathering steels. Corros. Sci. 2023, 224, 111561. [Google Scholar] [CrossRef]
- Kimura, M.; Kihira, H.; Ohta, N.; Hashimoto, M.; Senuma, T. Control of Fe(O,OH)6 nano-network structures of rust for high atmospheric-corrosion resistance. Corros. Sci. 2005, 47, 2499–2509. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, W.; Khan, H.I.; Dong, B.; Yang, W.; Sun, Y.; Zhang, B.; Chen, L.; Li, H. Effects of Cu on the corrosion resistance of heat-treated weathering steel in a marine environment. Mater. Today Phys. 2023, 36, 101160. [Google Scholar] [CrossRef]
- Alcántara, J.; Chico, B.; Díaz, I.; de la Fuente, D.; Morcillo, M. Airborne chloride deposit and its effect on marine atmospheric corrosion of mild steel. Corros. Sci. 2015, 97, 74–88. [Google Scholar] [CrossRef]
- Thee, C.; Hao, L.; Dong, J.; Mu, X.; Wei, X.; Li, X.; Ke, W. Atmospheric corrosion monitoring of a weathering steel under an electrolyte film in cyclic wet–dry condition. Corros. Sci. 2014, 78, 130–137. [Google Scholar] [CrossRef]
- Yang, L.; Yang, X.; Xu, F.; Li, Q.; Zhu, R.; Cheng, X.; Yang, G.; Li, X. Investigation of rust layer on low alloy steel with 0.40 wt% Sn in a rural atmospheric environment. Constr. Build. Mater. 2023, 402, 133029. [Google Scholar] [CrossRef]
- Zhang, T.; Hao, L.; Jiang, Z.; Liu, C.; Zhu, L.; Cheng, X.; Liu, Z.; Wang, N.; Li, X. Investigation of rare earth (RE) on improving the corrosion resistance of Zr-Ti deoxidized low alloy steel in the simulated tropic marine atmospheric environment. Corros. Sci. 2023, 221, 111335. [Google Scholar] [CrossRef]
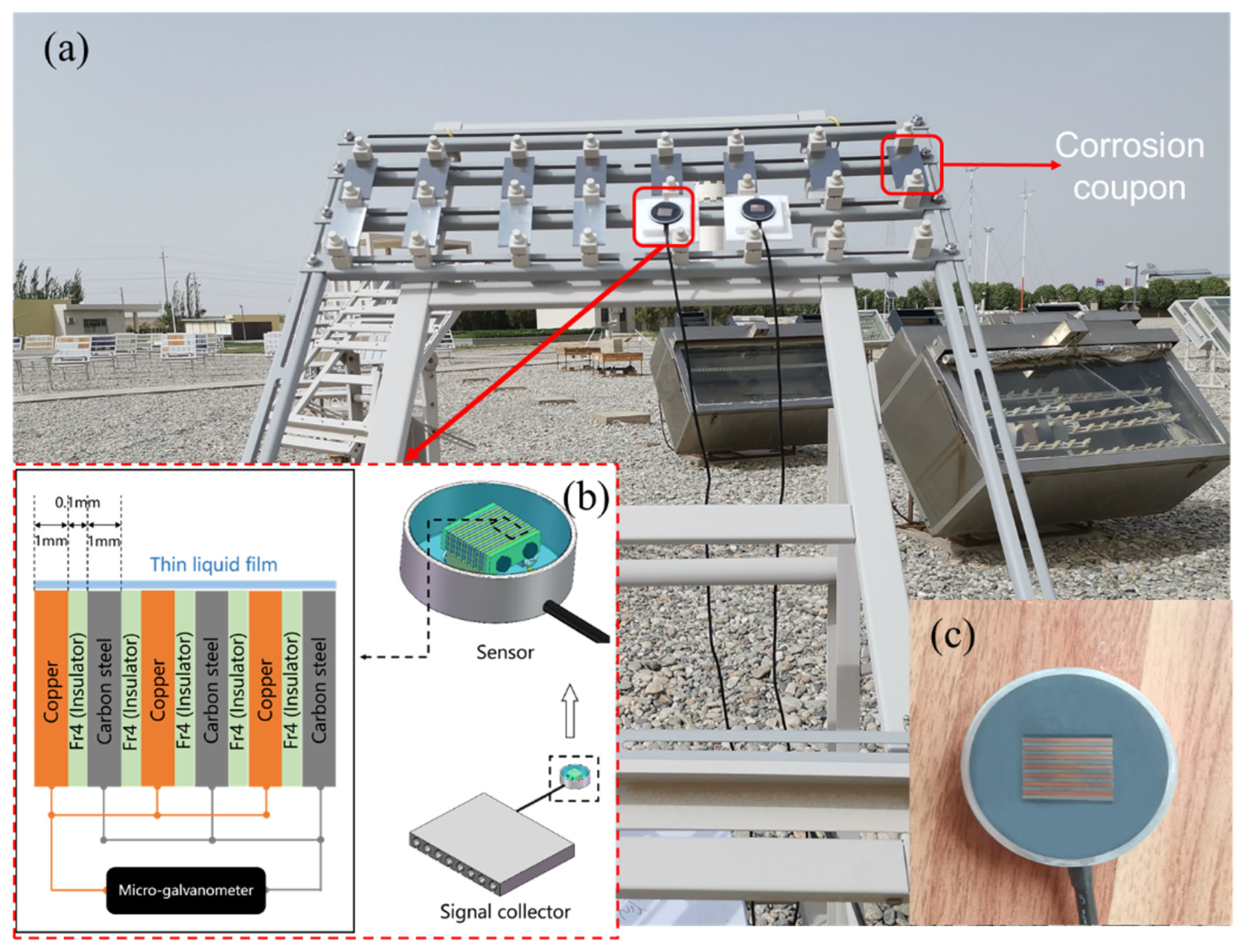



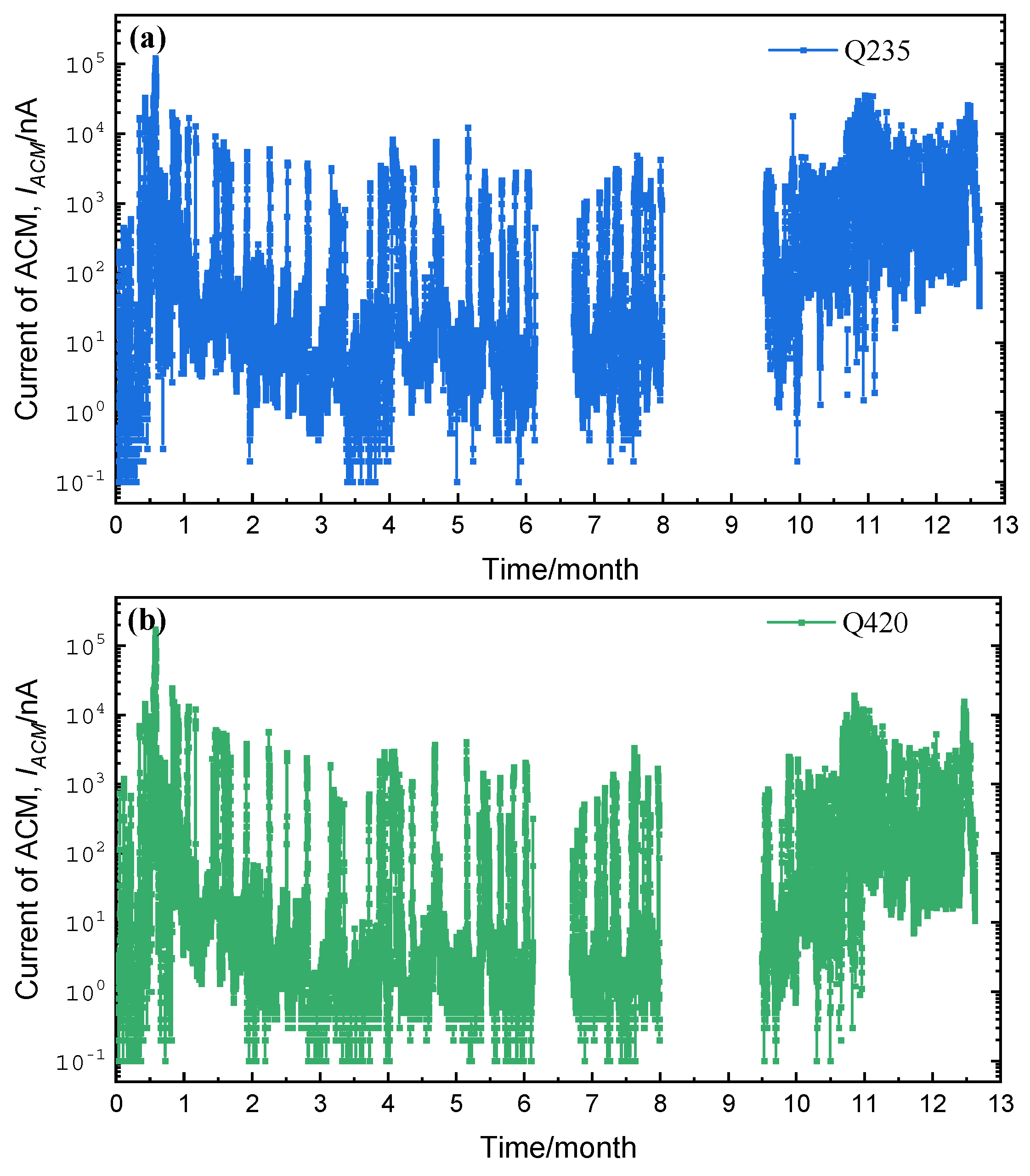
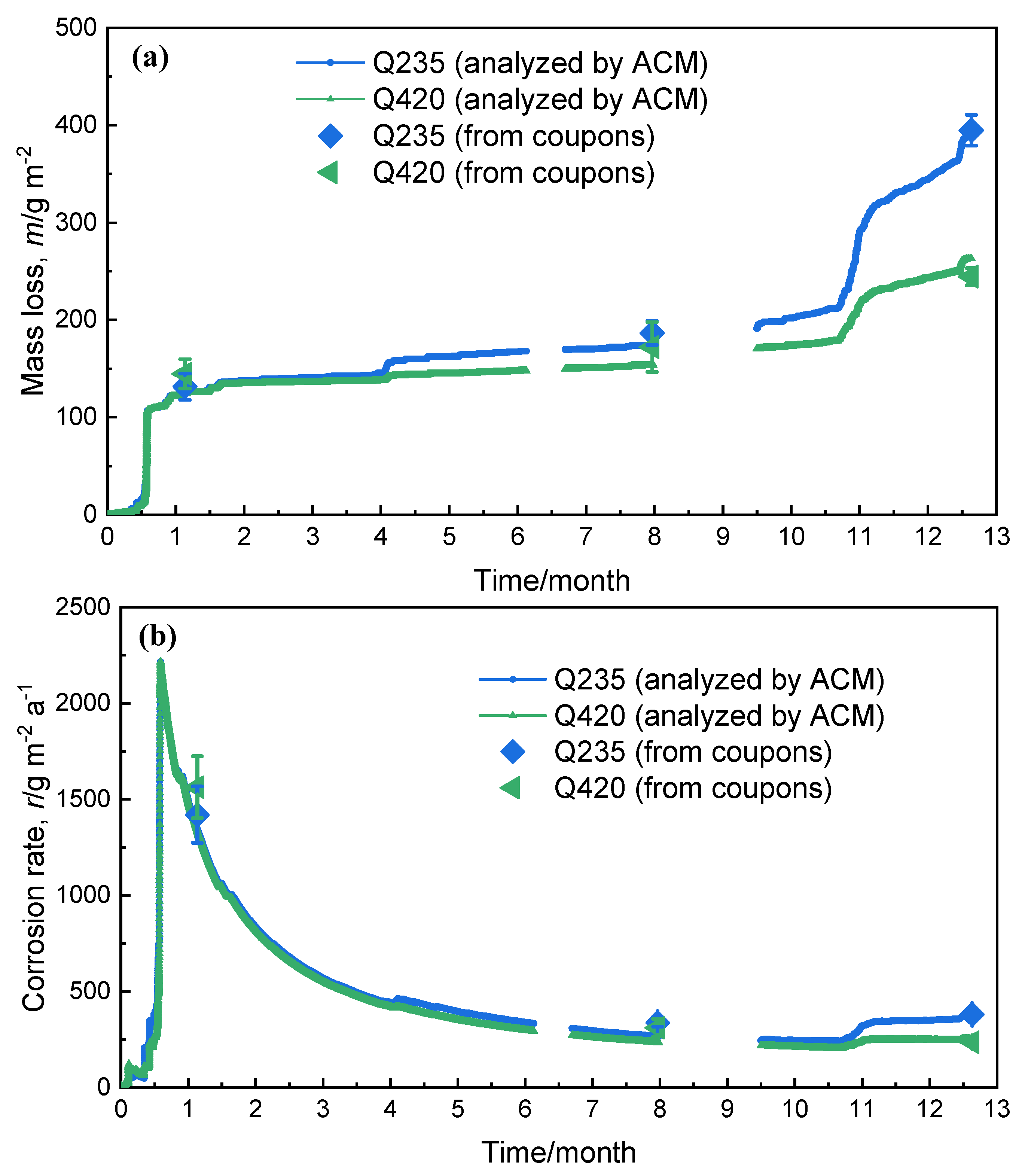
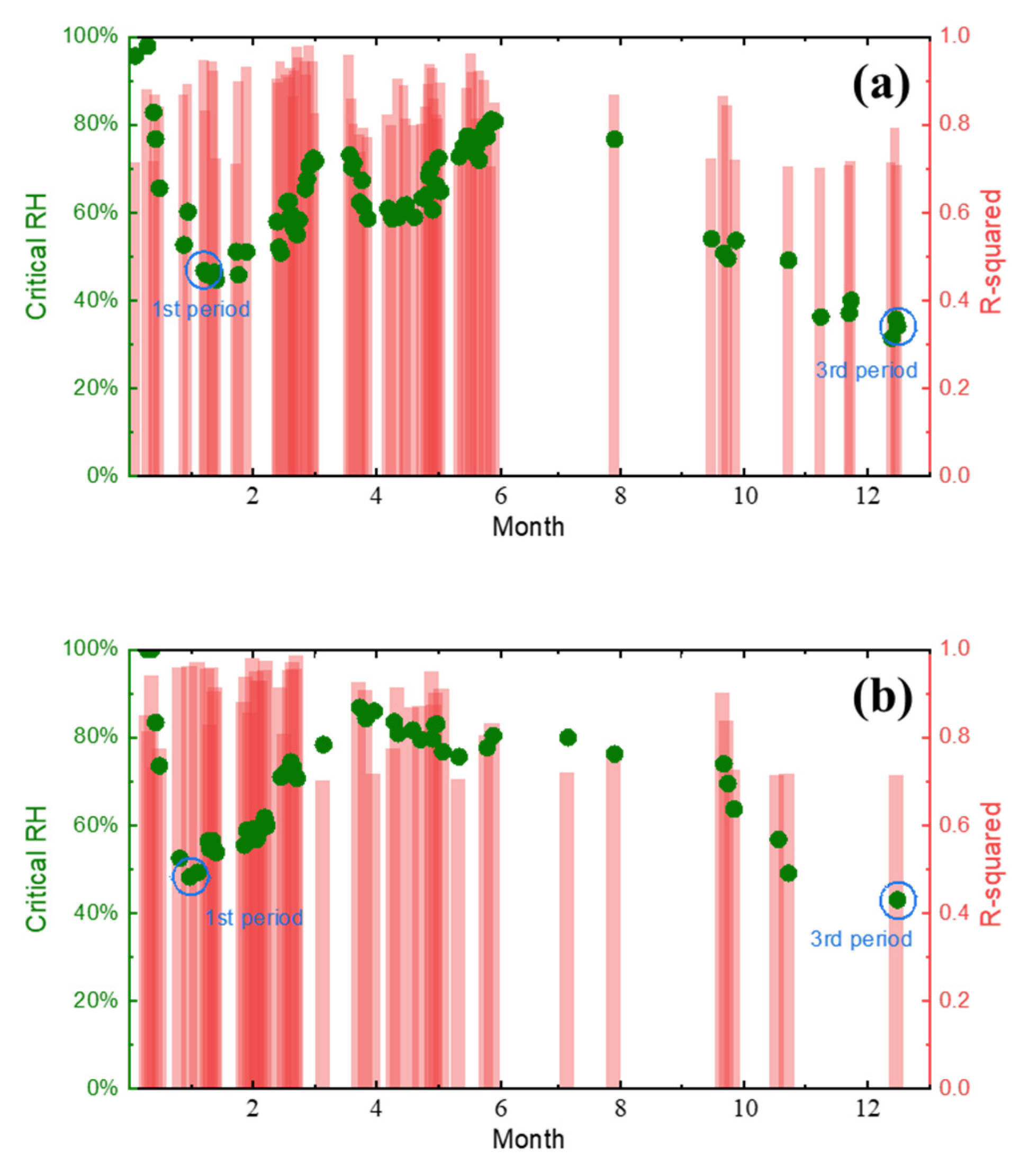
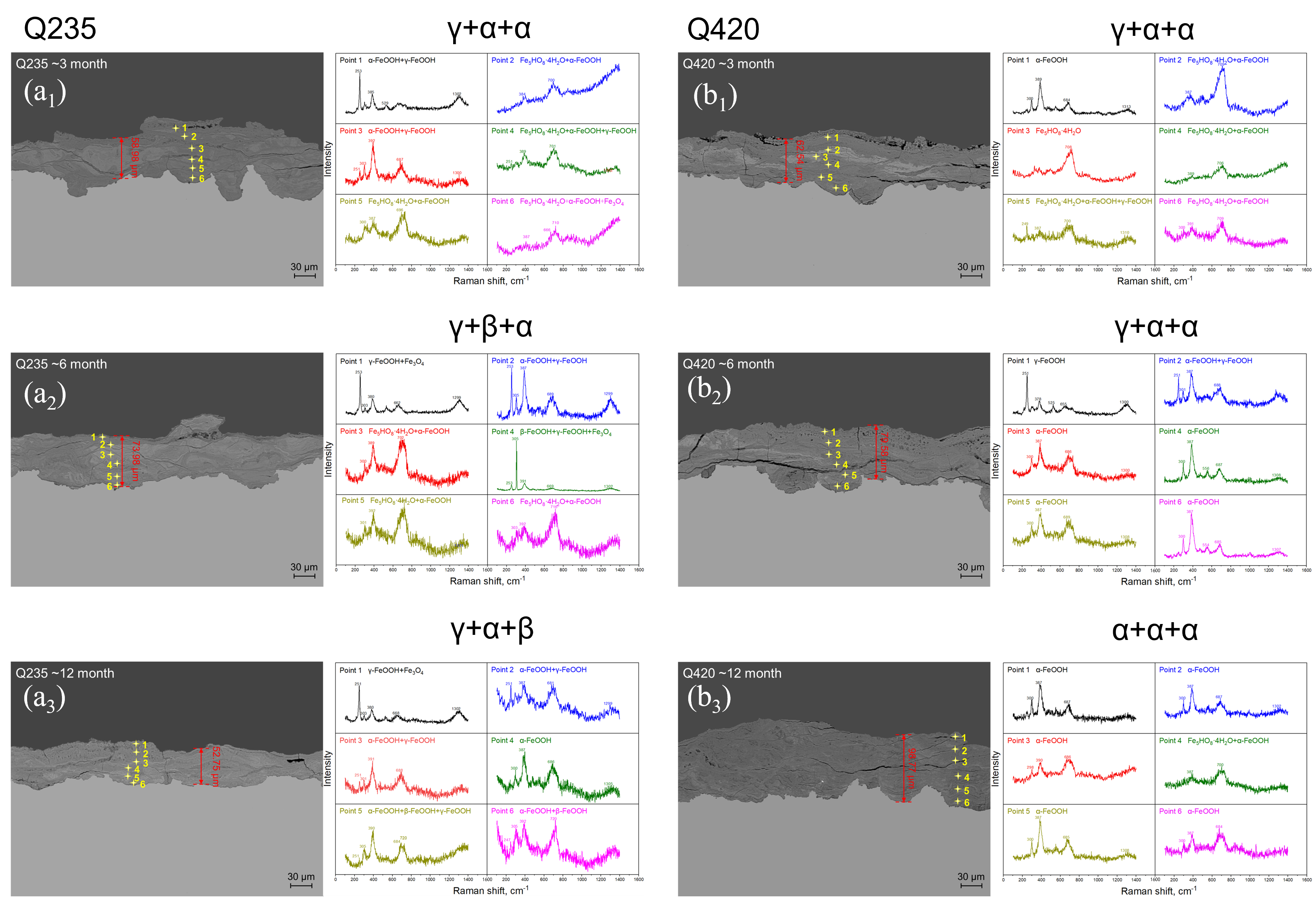

| Steels | C | Si | Mn | S | P | Ni | Cr | Cu | Fe |
|---|---|---|---|---|---|---|---|---|---|
| Q235 | 0.16 | 0.078 | 0.3 | 0.016 | 0.014 | ≤0.01 | ≤0.01 | ≤0.01 | Bal. |
| Q420 | 0.057 | 0.23 | 1.12 | 0.0075 | 0.009 | 0.4 | 0.038 | 0.36 | Bal. |
| Location | Latitude and Longitude | Climate Types | Environmental Corrosive Classification (ISO9223) |
|---|---|---|---|
| Tianjin | 117.40° E, 39.55° N | Rural atmosphere | C2–C3 |
| Dunhuang | 94.55° E, 40.08° N | Desert hot and dry atmosphere | C1–C2 |
| Jiangjin | 106.25° E, 29.32° N | Subtropical humid suburban acid rain environment | C3–C4 |
| Lhasa | 91.15° E, 29.48° N | Highland atmospheric environment | C1–C2 |
| Qionghai | 110.48° E, 19.24° N | Tropical humid rural environment | C3–C4 |
| Phase | Peak/cm−1 |
|---|---|
| γ-FeOOH | 166, 217, 251, 310, 350, 378, 529, 655, 713, 1300 |
| α-FeOOH | 203, 244, 300, 387, 399, 415, 480, 552, 684,1002, 1113, 1304 |
| β-FeOOH | 139, 308, 331, 389, 420, 499, 539, 609, 720, 1410 |
| Fe3O4 | 306, 538, 666 |
| Fe2O3 | 228, 250, 294, 414, 502, 625, 670, 1330 |
| γ-Fe2O3 | Peak width 339~386, 461~512, 671~717, 1430 |
| Fe5HO8·4H2O | Peak width 700~710 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Wang, X.; Pei, Z.; Xiao, K.; Yang, X.; Cheng, X. Influence of Rust Layer on Corrosion-Critical Humidity in Outdoor Environments Based on Corrosion Sensors. Materials 2025, 18, 2299. https://doi.org/10.3390/ma18102299
Li Q, Wang X, Pei Z, Xiao K, Yang X, Cheng X. Influence of Rust Layer on Corrosion-Critical Humidity in Outdoor Environments Based on Corrosion Sensors. Materials. 2025; 18(10):2299. https://doi.org/10.3390/ma18102299
Chicago/Turabian StyleLi, Qing, Xinyu Wang, Zibo Pei, Kui Xiao, Xiaojia Yang, and Xuequn Cheng. 2025. "Influence of Rust Layer on Corrosion-Critical Humidity in Outdoor Environments Based on Corrosion Sensors" Materials 18, no. 10: 2299. https://doi.org/10.3390/ma18102299
APA StyleLi, Q., Wang, X., Pei, Z., Xiao, K., Yang, X., & Cheng, X. (2025). Influence of Rust Layer on Corrosion-Critical Humidity in Outdoor Environments Based on Corrosion Sensors. Materials, 18(10), 2299. https://doi.org/10.3390/ma18102299





