Ti-Based Metallic Biomaterials for Antitumor Applications
Abstract
1. Introduction
2. Tumor Hallmarks and the Classification of Antitumor Approaches for Ti-Based MBs
3. Physical Antitumor Approaches
3.1. Photothermal Therapy
3.2. Photodynamic Therapy
3.3. Irreversible Electroporation
4. Chemical Antitumor Approaches
4.1. Diffusion-Controlled LDD Systems
4.2. Internal Stimuli-Responsive LDD Systems
4.3. External Stimuli-Responsive LDD Systems
5. Challenges and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ag | Silver |
| AXL | AXL Receptor Tyrosine Kinase |
| BP | Black Phosphorus |
| CP-Ti | Commercial Pure Ti |
| CT-26 cells | Mouse Colon Cancer cells |
| DAPI | 4’,6-Diamidino-2-Phenylindole |
| DC | Dendritic Cell |
| DISC | Death-Inducing Signaling Complex |
| DOX | Doxorubicin |
| ECM | Extracellular Matrix |
| EPR | Enhanced Permeability and Retention |
| F | Fluorine |
| FADD | Fas-associated Death Domain |
| HeLa cell | Human Cervical Carcinoma cell |
| HIF-1 | Hypoxia-Inducible Factor-1 |
| HMGB1 | High Mobility Group Box Protein B1 |
| H2O2 | Hydrogen Peroxide |
| HSPs | Heat Shock Proteins |
| HSP70 | Heat Shock Protein 70 |
| H-TiO2 | Hydrogenated Black Titanium Dioxide |
| H&E | Hematoxylin and Eosin |
| LDHs | Double Hydroxides |
| ICD | Immunogenic Cell Death |
| IRE | Irreversible Electroporation |
| LDD | Local Drug Delivery |
| MAO | Microarc Oxidation |
| MBs | Metallic Biomaterials |
| MC | Mucosa |
| MCE | Magnetocaloric Effect |
| MCF-7 cell | Human Breast Carcinoma Cell |
| MC3T3 cells | Mouse Embryo Osteoblast Precursor Cells |
| MET | c-Mesenchymal–Epithelial Transition Factor |
| MOFs | Metal–Organic Frameworks |
| MS | Muscular Layer |
| NIR | Near-infrared |
| Ni-Ti | Nickel–Titanium |
| NMPA | National Medical Products Administration |
| O2− | Superoxide Anion |
| 1O2 | Singlet Oxygen |
| ·OH | Hydroxyl Radical |
| OCN, RUNX2 | Osteogenic Marker |
| PDA | Polydopamine |
| PDT | Photodynamic Therapy |
| PEG | Polyethylene Glycol |
| PTX | Paclitaxel |
| PTX-EVA | Paclitaxel-Ethylene-Vinyl Acetate |
| PTX/5-FU stent | Polymer-Coated Esophageal Stent |
| ROS | Reactive Oxygen Species |
| Saos-2 | Osteosarcoma Cell |
| SM | Submucosa |
| TAMs | Tumor-Associated Macrophages |
| TES | Thermo-chemotherapeutic Esophageal Stent |
| Ti | Titanium |
| TNA-PDA-DOX | Polydopamine-Coated TNT Arrays On Pure Ti |
| TNT | TiO2 Nanotube |
| TMZ | Ti-12Mo-10Zr |
| TRAIL | TNF-Related Apoptosis-Inducing Ligand |
| TUNEL | Terminal dUTP Nick End Labeling |
| UV | Under Ultraviolet |
| 5-FU | 5-Fluorouracil |
References
- Huang, X.; Li, S.; Ding, R.; Li, Y.; Li, C.; Gu, R. Antitumor effects of polysaccharides from medicinal lower plants: A review. Int. J. Biol. Macromol. 2023, 252, 126313. [Google Scholar] [CrossRef]
- Brianna; Lee, S.H. Chemotherapy: How to reduce its adverse effects while maintaining the potency? Med. Oncol. 2023, 40, 88. [Google Scholar] [CrossRef]
- Arranja, A.G.; Pathak, V.; Lammers, T.; Shi, Y. Tumor-targeted nanomedicines for cancer theranostics. Pharmacol. Res. 2017, 115, 87–95. [Google Scholar] [CrossRef]
- Ma, X.; Gao, Y.; Zhao, D.; Zhang, W.; Zhao, W.; Wu, M.; Cui, Y.; Li, Q.; Zhang, Z.; Ma, C. Titanium Implants and Local Drug Delivery Systems Become Mutual Promoters in Orthopedic Clinics. Nanomaterials 2021, 12, 47. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, M.J.; Wang, J.; Zhang, T.; Xue, P.; Kang, Y.; Sun, Z.J.; Xu, Z. Emerging Biomaterials Imaging Antitumor Immune Response. Adv. Mater. 2022, 34, e2204034. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Yang, W.; Liang, Z.; Zhou, Z.; Song, Q.; Liu, W.; Deng, X.; Zhu, J.; Xing, X.; Zhong, B.; et al. Amplification of oxidative stress with lycorine and gold-based nanocomposites for synergistic cascade cancer therapy. J. Nanobiotechnol. 2021, 19, 221. [Google Scholar] [CrossRef] [PubMed]
- Nirmala, M.J.; Kizhuveetil, U.; Johnson, A.; G, B.; Nagarajan, R.; Muthuvijayan, V. Cancer nanomedicine: A review of nano-therapeutics and challenges ahead. RSC Adv. 2023, 13, 8606–8629. [Google Scholar] [CrossRef]
- Sun, L.; Sun, J.; Li, C.; Wu, K.; Gu, Z.; Guo, L.; Zhou, Y.; Han, B.; Chang, J. STAT3-specific nanocarrier for shRNA/drug dual delivery and tumor synergistic therapy. Bioact. Mater. 2024, 41, 137–157. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, D.; Wu, Z.; Wang, Q.; Ma, Z.; Zhang, C. Research Progress and Prospect of Nanoplatforms for Treatment of Oral Cancer. Front. Pharmacol. 2020, 11, 616101. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Swiatkowska, I.; Martin, N.; Hart, A.J. Blood titanium level as a biomarker of orthopaedic implant wear. J. Trace Elem. Med. Biol. 2019, 53, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Liu, J.; Dong, J.; Xie, H.; Wang, X.; Yang, X.; Yang, Y. Multifunctional coatings of nickel-titanium implant toward promote osseointegration after operation of bone tumor and clinical application: A review. Front. Bioeng. Biotechnol. 2024, 12, 1325707. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M.; Ahmadi, F.; Farzin, M. Production of Ultrafine-Grained Titanium with Suitable Properties for Dental Implant Applications by RS-ECAP Process. Met. Mater. Int. 2020, 27, 705–716. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, J.; Nie, J.; Chen, D.; Qin, G.; Zhang, E. Enhanced antibacterial activity, corrosion resistance and endothelialization potential of Ti-5Cu alloy by oxygen and nitrogen plasma-based surface modification. J. Mater. Sci. Technol. 2024, 168, 250–264. [Google Scholar] [CrossRef]
- Marin, E.; Lanzutti, A. Biomedical Applications of Titanium Alloys: A Comprehensive Review. Materials 2023, 17, 114. [Google Scholar] [CrossRef] [PubMed]
- Jing, Z.; Ni, R.; Wang, J.; Lin, X.; Fan, D.; Wei, Q.; Zhang, T.; Zheng, Y.; Cai, H.; Liu, Z. Practical strategy to construct anti-osteosarcoma bone substitutes by loading cisplatin into 3D-printed titanium alloy implants using a thermosensitive hydrogel. Bioact. Mater. 2021, 6, 4542–4557. [Google Scholar] [CrossRef]
- Zhang, W.; Gu, J.; Li, K.; Zhao, J.; Ma, H.; Wu, C.; Zhang, C.; Xie, Y.; Yang, F.; Zheng, X. A hydrogenated black TiO2 coating with excellent effects for photothermal therapy of bone tumor and bone regeneration. Mater. Sci. Eng. C 2019, 102, 458–470. [Google Scholar] [CrossRef]
- Hu, H.; Zhao, J.; Ma, K.; Wang, J.; Wang, X.; Mao, T.; Xiang, C.; Luo, H.; Cheng, Y.; Yu, M.; et al. Sonodynamic therapy combined with phototherapy: Novel synergistic strategy with superior efficacy for antitumor and antiinfection therapy. J. Control. Release 2023, 359, 188–205. [Google Scholar] [CrossRef]
- Fan, D.; Zhang, C.; Wang, H.; Wei, Q.; Cai, H.; Wei, F.; Bian, Z.; Liu, W.; Wang, X.; Liu, Z. Fabrication of a composite 3D-printed titanium alloy combined with controlled in situ drug release to prevent osteosarcoma recurrence. Mater. Today Bio 2023, 20, 100683. [Google Scholar] [CrossRef]
- Xiao, Q.; Wan, C.; Zhang, Z.; Liu, H.; Liu, P.; Huang, Q.; Zhao, D. A pH-Responsive Ti-Based Local Drug Delivery System for Osteosarcoma Therapy. J. Funct. Biomater. 2024, 15, 312. [Google Scholar] [CrossRef]
- Kalbacova, M.; Macak, J.M.; Schmidt-Stein, F.; Mierke, C.T.; Schmuki, P. TiO2 nanotubes: Photocatalyst for cancer cell killing. Phys. Status solidi (RRL)–Rapid Res. Lett. 2008, 2, 194–196. [Google Scholar] [CrossRef]
- Heidari Khoee, M.; Khoee, S.; Lotfi, M. Synthesis of titanium dioxide nanotubes with liposomal covers for carrying and extended release of 5-FU as anticancer drug in the treatment of HeLa cells. Anal. Biochem. 2019, 572, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Zandvakili, A.; Moradi, M.; Ashoo, P.; Pournejati, R.; Yosefi, R.; Karbalaei-Heidari, H.R.; Behaein, S. Investigating cytotoxicity effect of Ag- deposited, doped and coated titanium dioxide nanotubes on breast cancer cells. Mater. Today Commun. 2022, 32, 103915. [Google Scholar] [CrossRef]
- Xing, S.; Zhang, H.; Hou, Z.; Peng, F.; Liu, L.; Wang, D.; Ge, N.; Liu, X. NIR-triggered arsenic-loaded layered double hydroxide-based films for localized thermal synergistic chemotherapy. J. Colloid Interface Sci. 2024, 675, 857–869. [Google Scholar] [CrossRef]
- Cai, S.; Jin, Z.; Zeng, P.; Yang, L.; Yan, Y.; Wang, Z.; Shen, Y.; Guo, S. Structural optimization and in vivo evaluation of a colorectal stent with anti-migration and anti-tumor properties. Acta Biomater. 2022, 154, 123–134. [Google Scholar] [CrossRef]
- Kim, S.H.; Kang, J.M.; Park, Y.; Jeong, S.; Na, Y.; Jung, H.-D.; An, J.; Kim, H.-S.; Lee, S.S.; Park, J.-H. Self-Expandable Electrode Based on Chemically Polished Nickel–Titanium Alloy Wire for Treating Endoluminal Tumors Using Bipolar Irreversible Electroporation. ACS Appl. Mater. Interfaces 2023, 15, 34475–34487. [Google Scholar] [CrossRef]
- Xing, S.; Zhang, H.; Liu, L.; Wang, D.; Ge, N.; Liu, X. Selective Tumor Inhibition Effect of Drug-Free Layered Double Hydroxide-Based Films via Responding to Acidic Microenvironment. ACS Biomater. Sci. Eng. 2024, 10, 4927–4937. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Wang, D.-H.; Zhang, H.-F.; Liu, L.-D.; Li, C.-C.; Wei, C.; Liu, J.-Y.; Ge, N.-J.; Liu, X.-Y. Tumor microenvironment-responsive arsenic-loaded layered double hydroxides film with synergistic anticancer and bactericidal activity. Rare Met. 2023, 43, 1207–1221. [Google Scholar] [CrossRef]
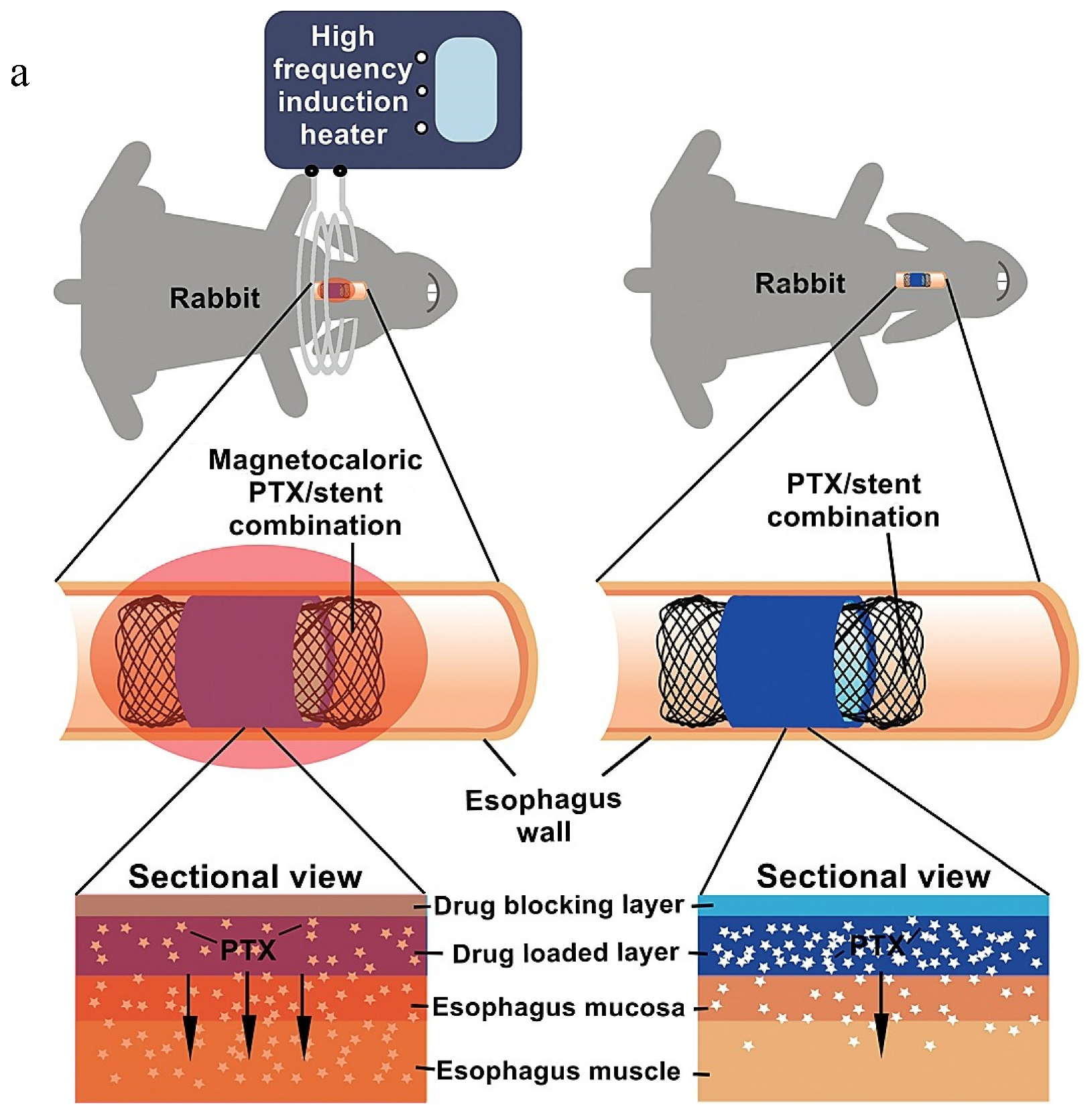
- Jin, Z.; Wu, K.; Hou, J.; Yu, K.; Shen, Y.; Guo, S. A PTX/nitinol stent combination with temperature-responsive phase-change 1-hexadecanol for magnetocaloric drug delivery: Magnetocaloric drug release and esophagus tissue penetration. Biomaterials 2018, 153, 49–58. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, T.; Lv, W.; Yang, K.; Ouyang, C.; Deng, M.; Yi, R.; Chu, H.; Chen, J. Controlled growth of titanium dioxide nanotubes for doxorubicin loading and studies of in vitro antitumor activity. Front. Bioeng. Biotechnol. 2023, 11, 1201320. [Google Scholar] [CrossRef]
- Jafari, B.; Hamzeh-Mivehroud, M.; Morris, M.B.; Dastmalchi, S. Exploitation of phage display for the development of anti-cancer agents targeting fibroblast growth factor signaling pathways: New strategies to tackle an old challenge. Cytokine Growth Factor Rev. 2019, 46, 54–65. [Google Scholar] [CrossRef]
- Farhi, L.; Yusuf, A. Comparison of brain tumor MRI classification methods using probabilistic features. In Proceedings of the 2017 13th IASTED International Conference on Biomedical Engineering (BioMed), Innsbruck, Austria, 20–21 February 2017. [Google Scholar]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Casey, S.C.; Vaccari, M.; Al-Mulla, F.; Al-Temaimi, R.; Amedei, A.; Barcellos-Hoff, M.H.; Brown, D.G.; Chapellier, M.; Christopher, J.; Curran, C.S.; et al. The effect of environmental chemicals on the tumor microenvironment. Carcinogenesis 2015, 36, S160–S183. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.; Lu, Y.; Wang, S.; Zheng, Y.; Xia, D.; Liu, Y. Magnesium alloys in tumor treatment: Current research status, challenges and future prospects. J. Magnes. Alloys 2023, 11, 3399–3426. [Google Scholar] [CrossRef]
- Hirschhaeuser, F.; Sattler, U.G.A.; Mueller-Klieser, W. Lactate: A Metabolic Key Player in Cancer. Cancer Res. 2011, 71, 6921–6925. [Google Scholar] [CrossRef] [PubMed]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Semenza, G.L. Hypoxia-Inducible Factors in Physiology and Medicine. Cell 2012, 148, 399–408. [Google Scholar] [CrossRef]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef]
- Hu, H.; Wang, K.; Jia, R.; Zeng, Z.-X.; Zhu, M.; Deng, Y.-L.; Xiong, Z.-J.; Tang, J.-N.; Xie, H.; Wang, Y.; et al. Current Status in Rechallenge of Immunotherapy. Int. J. Biol. Sci. 2023, 19, 2428–2442. [Google Scholar] [CrossRef]
- Hildebrandt, B.; Wust, P.; Ahlers, O.; Dieing, A.; Sreenivasa, G.; Kerner, T.; Felix, R.; Riess, H. The cellular and molecular basis of hyperthermia. Crit. Rev. Oncol./Hematol. 2002, 43, 33–56. [Google Scholar] [CrossRef]
- Vishnubalaji, R.; Elango, R.; Alajez, N.M. LncRNA-Based Classification of Triple Negative Breast Cancer Revealed Inherent Tumor Heterogeneity and Vulnerabilities. Non-Coding RNA 2022, 8, 44. [Google Scholar] [CrossRef]
- Gensbittel, V.; Kräter, M.; Harlepp, S.; Busnelli, I.; Guck, J.; Goetz, J.G. Mechanical Adaptability of Tumor Cells in Metastasis. Dev. Cell 2021, 56, 164–179. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Shi, Y.; Li, Q.; Luo, L.; Li, X.; Luo, Z.; Lu, Y.; Zhang, J.; Jiang, M.; Qin, B.; et al. Rational administration sequencing of immunochemotherapy elicits powerful anti-tumor effect. J. Control. Release 2022, 341, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Zheng, Y.; Li, B.; Yang, F.; Chen, W.; Li, Y.; Deng, Y.; Bai, D.; Shu, R. A 3D-printed orthopedic implant with dual-effect synergy based on MoS2 and hydroxyapatite nanoparticles for tumor therapy and bone regeneration. Colloids Surf. B Biointerfaces 2023, 228, 113384. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Luo, S.; Cai, Q.; Yao, S. A review on TiO2 nanotube arrays: Fabrication, properties, and sensing applications. Sci. Bull. 2010, 55, 331–333. [Google Scholar] [CrossRef]
- Yang, L.; Li, D.; Deng, H.; Li, T. Preparation of Dental Implant Polydopamine-D-Amino-Titanium Dioxide Nanotube Composite Coating Involves Preparing Anode Oxidized Titanium Dioxide Array, Soaking in Tris(hydroxymethyl)aminomethane Solution of Dopamine, Vibrating, Washing, Soaking in D-Amino Polypeptide Solution, Washing, and Drying; University Capital Medical Beijing Stomatology: Beijing, China, 2023. [Google Scholar]
- Liu, Y.; Rao, X. Infrared-Visible Light Photothermophotodynamic Synergistic Low Temperature Antibacterial Dental Implant Material Useful for Repairing and Fixing Tooth of Human Body, Comprises Pure Titanium Matrix and PDA-Cuprous Oxide/TNT Coating; University Southwest: El Paso, TX, USA, 2022. [Google Scholar]
- Yang, Y.; Tao, B.; Gong, Y.; Chen, R.; Yang, W.; Lin, C.; Chen, M.; Qin, L.; Jia, Y.; Cai, K. Functionalization of Ti substrate with pH-responsive naringin-ZnO nanoparticles for the reconstruction of large bony after osteosarcoma resection. J. Biomed. Mater. Res. Part A 2020, 108, 2190–2205. [Google Scholar] [CrossRef]
- Zhang, M.; Pu, X.; Chen, X.; Yin, G. In vivo performance of plasma-sprayed CaO-MgO-SiO2 based bioactive glass-ceramic coating on Ti-6Al-4V alloy for bone regeneration. Heliyon 2019, 5, e02824. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Y.; Xu, P.; Li, H.; Fang, S. Performing Acetabular Bone Defect Reconstruction Based on Three-Dimensional Printing Involves Preparing, Characterizing and Cytocompatibility Testing of Titanium Metal Implants; Changzhou First Peoples Hospital: Changzhou, China, 2022. [Google Scholar]
- Cai, B.Y.; Huang, L.Z.; Zhou, X.K.; Zhou, X.; Lei, K.; Han, M.; Zhang, Z.L.; Li, X.F.; Li, G.D. Black phosphorus-incorporated novel Ti-12Mo-10Zr implant for multimodal treatment of osteosarcoma. Biometals 2023, 37, 131–142. [Google Scholar] [CrossRef]
- Wu, Z.; Tian, Q.; Wang, J.; Feng, Y.; Li, L.; Xu, C.; Lv, J.; Lv, Z. A bone implant with NIR-responsiveness for eliminating osteosarcoma cells and promoting osteogenic differentiation of BMSCs. Colloids Surf. B Biointerfaces 2021, 211, 112296. [Google Scholar] [CrossRef]
- Lyngstadaas, S.P.; Haugen, H.J. Antimicrobial and/or Antiinflammatory Composition, Useful e.g., for Implant Cleaning and/or Debridement of a Hard Surface in the Oral Cavity, Comprises Nanoparticles of Titanium Dioxide, Hydrogen Peroxide and Solid Microparticles. U.S. Patent 2011073194, 7 January 2011. [Google Scholar]
- Sui, J.; Chen, Z.; Liu, G.; Dong, X.; Yu, W.; Wang, J. Multifunctional Ag@NaGdF4:Yb3+, Er3+ core-shell nanocomposites for dual-mode imaging and photothermal therapy. J. Lumin. 2019, 209, 357–364. [Google Scholar] [CrossRef]
- Bian, K.; Zhang, X.; Liu, K.; Yin, T.; Liu, H.; Niu, K.; Cao, W.; Gao, D. Peptide-Directed Hierarchical Mineralized Silver Nanocages for Anti-Tumor Photothermal Therapy. ACS Sustain. Chem. Eng. 2018, 6, 7574–7588. [Google Scholar] [CrossRef]
- Qi, X.; Xiang, Y.; Cai, E.; Ge, X.; Chen, X.; Zhang, W.; Li, Z.; Shen, J. Inorganic–organic hybrid nanomaterials for photothermal antibacterial therapy. Coord. Chem. Rev. 2023, 496, 215426. [Google Scholar] [CrossRef]
- Li, B.; Liu, F.; Ye, J.; Cai, X.; Qian, R.; Zhang, K.; Zheng, Y.; Wu, S.; Han, Y. Regulation of Macrophage Polarization Through Periodic Photo-Thermal Treatment to Facilitate Osteogenesis. Small 2022, 18, e2202691. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wang, H.; Ying, B.; Mei, X.; Zeng, D.; Liu, S.; Qu, W.; Pan, X.; Pu, S.; Li, R.; et al. Mild photothermal therapy assist in promoting bone repair: Related mechanism and materials. Mater. Today Bio 2023, 23, 100834. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Chai, T.; Nguyen, W.; Liu, J.; Xiao, E.; Ran, X.; Ran, Y.; Du, D.; Chen, W.; Chen, X. Phototherapy in cancer treatment: Strategies and challenges. Signal Transduct. Target. Ther. 2025, 10, 115. [Google Scholar] [CrossRef]
- Xu, P.; Wang, R.; Ouyang, J.; Chen, B. A new strategy for TiO2 whiskers mediated multi-mode cancer treatment. Nanoscale Res. Lett. 2015, 10, 94. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Kobayashi, H.; Narita, T.; Kanehira, K.; Sonezaki, S.; Kubota, Y.; Terasaka, S.; Iwasaki, Y. Novel Photodynamic Therapy Using Water-dispersed TiO2-Polyethylene Glycol Compound: Evaluation of Antitumor Effect on Glioma Cells and Spheroids In Vitro. Photochem. Photobiol. 2010, 86, 964–971. [Google Scholar] [CrossRef]
- Zhang, N.; Li, Z.; Han, X.; Zhu, Z.; Li, Z.; Zhao, Y.; Liu, Z.; Lv, Y. Irreversible Electroporation: An Emerging Immunomodulatory Therapy on Solid Tumors. Front. Immunol. 2022, 12, 811726. [Google Scholar] [CrossRef]
- Jeon, S.-M.; Davaa, E.; Jenjob, R.; Pechyen, C.; Natphopsuk, S.; Jeong, S.; Yoo, H.J.; Yang, S.-G. The Induction of Combined Hyperthermal Ablation Effect of Irreversible Electroporation with Polydopamine Nanoparticle-Coated Electrodes. Int. J. Mol. Sci. 2024, 25, 4317. [Google Scholar] [CrossRef]
- He, C.; Shuxin, S.; Yu, Z.; Fengxiao, X.; Li, S. The role of irreversible electroporation in promoting M1 macrophage polarization via regulating the HMGB1-RAGE-MAPK axis in pancreatic cancer. OncoImmunology 2021, 10, 1897295. [Google Scholar] [CrossRef]
- Kim, S.H.; Kang, J.M.; Park, Y.; Kim, Y.; Lim, B.; Park, J.-H. Effects of bipolar irreversible electroporation with different pulse durations in a prostate cancer mouse model. Sci. Rep. 2024, 14, 9902. [Google Scholar] [CrossRef] [PubMed]
- Wagstaff, P.; Buijs, M.; van den Bos, W.; de Bruin, D.; Zondervan, P.; de la Rosette, J.; Laguna, P. Irreversible electroporation: State of the art. OncoTargets Ther. 2016, 9, 2437–2446. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.-B.; Wang, J.-J.; Jin, L.-J.; Shan, H.-S.; Zhang, X.; Ma, L.; Xue, X.-D.; Zhang, X.; Zhang, Z.-L.; et al. Comparison between high-frequency irreversible electroporation and irreversible electroporation ablation of small swine liver: Follow-up of DCE-MRI and pathological observations. Chin. Med. J. 2021, 134, 2081–2090. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, Y.; Ding, L.; Moser, M.A.J.; Zhang, E.M.; Zhang, W. Tumor Ablation Enhancement by Combining Radiofrequency Ablation and Irreversible Electroporation: An In Vitro 3D Tumor Study. Ann. Biomed. Eng. 2018, 47, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Blum, N.T.; Lin, J.; Qu, J.; Huang, P. Biomaterial scaffold-based local drug delivery systems for cancer immunotherapy. Sci. Bull. 2020, 65, 1489–1504. [Google Scholar] [CrossRef] [PubMed]
- Shaha, S.; Rodrigues, D.; Mitragotri, S. Locoregional drug delivery for cancer therapy: Preclinical progress and clinical translation. J. Control. Release 2024, 367, 737–767. [Google Scholar] [CrossRef]
- Majumder, J.; Taratula, O.; Minko, T. Nanocarrier-based systems for targeted and site specific therapeutic delivery. Adv. Drug Deliv. Rev. 2019, 144, 57–77. [Google Scholar] [CrossRef]
- Park, J.; Choi, Y.; Chang, H.; Um, W.; Ryu, J.H.; Kwon, I.C. Alliance with EPR Effect: Combined Strategies to Improve the EPR Effect in the Tumor Microenvironment. Theranostics 2019, 9, 8073–8090. [Google Scholar] [CrossRef]
- Fan, D.; Cao, Y.; Cao, M.; Wang, Y.; Cao, Y.; Gong, T. Nanomedicine in cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 293. [Google Scholar] [CrossRef]
- Mazidi, Z.; Javanmardi, S.; Naghib, S.M.; Mohammadpour, Z. Smart stimuli-responsive implantable drug delivery systems for programmed and on-demand cancer treatment: An overview on the emerging materials. Chem. Eng. J. 2022, 433, 134569. [Google Scholar] [CrossRef]
- De Souza, R.; Zahedi, P.; Allen, C.J.; Piquette-Miller, M. Polymeric drug delivery systems for localized cancer chemotherapy. Drug Deliv. 2010, 17, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Weiser, J.R.; Saltzman, W.M. Controlled release for local delivery of drugs: Barriers and models. J. Control. Release 2014, 190, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Wolinsky, J.B.; Colson, Y.L.; Grinstaff, M.W. Local drug delivery strategies for cancer treatment: Gels, nanoparticles, polymeric films, rods, and wafers. J. Control. Release 2012, 159, 14–26. [Google Scholar] [CrossRef]
- Wei, H.; Cui, J.; Lin, K.; Xie, J.; Wang, X. Recent advances in smart stimuli-responsive biomaterials for bone therapeutics and regeneration. Bone Res. 2022, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Willsmore, T.; Gulati, K.; Zinonos, I.; Wang, Y.; Kurian, M.; Hay, S.; Losic, D.; Evdokiou, A. Titanium wire implants with nanotube arrays: A study model for localized cancer treatment. Biomaterials 2016, 101, 176–188. [Google Scholar] [CrossRef]
- Quiroz-Reyes, A.G.; Delgado-Gonzalez, P.; Islas, J.F.; Gallegos, J.L.D.; Martínez Garza, J.H.; Garza-Treviño, E.N. Behind the Adaptive and Resistance Mechanisms of Cancer Stem Cells to TRAIL. Pharmaceutics 2021, 13, 1062. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Wu, K.; Li, J.; Chen, W.; Shen, Y.; Guo, S. Paclitaxel or 5-fluorouracil/esophageal stent combinations as a novel approach for the treatment of esophageal cancer. Biomaterials 2015, 53, 592–599. [Google Scholar] [CrossRef]
- Ji, K.; Mayernik, L.; Moin, K.; Sloane, B.F. Acidosis and proteolysis in the tumor microenvironment. Cancer Metastasis Rev. 2019, 38, 103–112. [Google Scholar] [CrossRef]
- Khawar, I.A.; Kim, J.H.; Kuh, H.-J. Improving drug delivery to solid tumors: Priming the tumor microenvironment. J. Control. Release 2015, 201, 78–89. [Google Scholar] [CrossRef]
- Zhang, J.; Zhuang, Y.; Sheng, R.; Tomás, H.; Rodrigues, J.; Yuan, G.; Wang, X.; Lin, K. Smart stimuli-responsive strategies for titanium implant functionalization in bone regeneration and therapeutics. Mater. Horiz. 2024, 11, 12–36. [Google Scholar] [CrossRef]
- Ibrahim-Hashim, A.; Estrella, V. Acidosis and cancer: From mechanism to neutralization. Cancer Metastasis Rev. 2019, 38, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, S.; Sharma, D.; Kalia, K.; Tekade, R.K. Tumor microenvironment targeted nanotherapeutics for cancer therapy and diagnosis: A review. Acta Biomater. 2020, 101, 43–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Sun, Y.H.; Zhao, Y.; Li, C.L.; Zhao, F.L.; Yao, X.H.; Hang, R.Q.; Chu, P.K. Selective inhibition effects on cancer cells and bacteria of Ni-Ti-O nanoporous layers grown on biomedical NiTi alloy by anodization. Rare Met. 2022, 41, 78–85. [Google Scholar] [CrossRef]
- Yan, B.C.; Tan, J.; Chen, L.; Zhang, H.F.; Ma, X.H.; Qiao, Y.Q.; Liu, X.Y. Stimuli-responsive doxorubicin-loading Zr-MOF film for time-ordered tumor therapy and bone regeneration. Compos. Part B-Eng. 2023, 250, 110452. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, L.; Li, X.; Wang, L.; Yang, Z. Chemo-immunotherapy by dual-enzyme responsive peptide self-assembling abolish melanoma. Bioact. Mater. 2024, 31, 549–562. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, X.W.; Wang, M.; Sun, J.; Chen, J.; Guan, Y.; Pang, X. Trypsin-instructed bioactive peptide nanodrugs with cascading transformations to improve chemotherapy against colon cancer. J. Nanobiotechnol. 2025, 23, 66. [Google Scholar] [CrossRef] [PubMed]
- Markezana, A.; Goldberg, S.N.; Kumar, G.; Zorde-Khvalevsky, E.; Gourevtich, S.; Rozenblum, N.; Galun, E.; Ahmed, M. Incomplete thermal ablation of tumors promotes increased tumorigenesis. Int. J. Hyperth. 2021, 38, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Ayav, A.; Germain, A.; Marchal, F.; Tierris, I.; Laurent, V.; Bazin, C.; Yuan, Y.; Robert, L.; Brunaud, L.; Bresler, L. Radiofrequency ablation of unresectable liver tumors: Factors associated with incomplete ablation or local recurrence. Am. J. Surg. 2010, 200, 435–439. [Google Scholar] [CrossRef]
- Wilson, T.R.; Fridlyand, J.; Yan, Y.; Penuel, E.; Burton, L.; Chan, E.; Peng, J.; Lin, E.; Wang, Y.; Sosman, J.; et al. Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature 2012, 487, 505–509. [Google Scholar] [CrossRef]
- Zhang, Z.; Lee, J.C.; Lin, L.; Olivas, V.; Au, V.; LaFramboise, T.; Abdel-Rahman, M.; Wang, X.; Levine, A.D.; Rho, J.K.; et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat. Genet. 2012, 44, 852–860. [Google Scholar] [CrossRef]
- Duan, R.; Milton, P.; Sittplangkoon, C.; Liu, X.; Sui, Z.; Boyce, B.F.; Yao, Z. Chimeric antigen receptor dendritic cells targeted delivery of a single tumoricidal factor for cancer immunotherapy. Cancer Immunol. Immunother. 2024, 73, 203. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Liu, Q.; Xiang, Y.; Gou, X.; Li, W. Role of the tumor immune microenvironment in tumor immunotherapy (Review). Oncol. Lett. 2021, 23, 53. [Google Scholar] [CrossRef]
- Liu, A.; Wang, H.; Hou, X.; Ma, Y.; Yang, G.; Hou, Y.; Ding, Y. Combinatory antitumor therapy by cascade targeting of a single drug. Acta Pharm. Sin. B 2020, 10, 667–679. [Google Scholar] [CrossRef]
- Jia, S.; Wang, S.; Li, S.; Hu, P.; Yu, S.; Shi, J.; Yuan, J. Specific modification and self-transport of porphyrins and their multi-mechanism cooperative antitumor studies. J. Mater. Chem. B 2021, 9, 3180–3191. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, H.; Liu, N.; Feng, D.; Wu, W.; Gu, K.; Wu, A.; Li, C.; Wang, X. Multi-mechanism antitumor/antibacterial effects of Cu-EGCG self-assembling nanocomposite in tumor nanotherapy and drug-resistant bacterial wound infections. J. Colloid Interface Sci. 2024, 671, 751–769. [Google Scholar] [CrossRef] [PubMed]
- Genduso, S.; Freytag, V.; Schetler, D.; Kirchner, L.; Schiecke, A.; Maar, H.; Wicklein, D.; Gebauer, F.; Bröker, K.; Stürken, C.; et al. Tumor cell integrin β4 and tumor stroma E-/P-selectin cooperatively regulate tumor growth in vivo. J. Hematol. Oncol. 2023, 16, 23. [Google Scholar] [CrossRef]
- Tamura, K.; Wakimoto, H.; Agarwal, A.S.; Rabkin, S.D.; Bhere, D.; Martuza, R.L.; Kuroda, T.; Kasmieh, R.; Shah, K. Multimechanistic Tumor Targeted Oncolytic Virus Overcomes Resistance in Brain Tumors. Mol. Ther. 2013, 21, 68–77. [Google Scholar] [CrossRef]
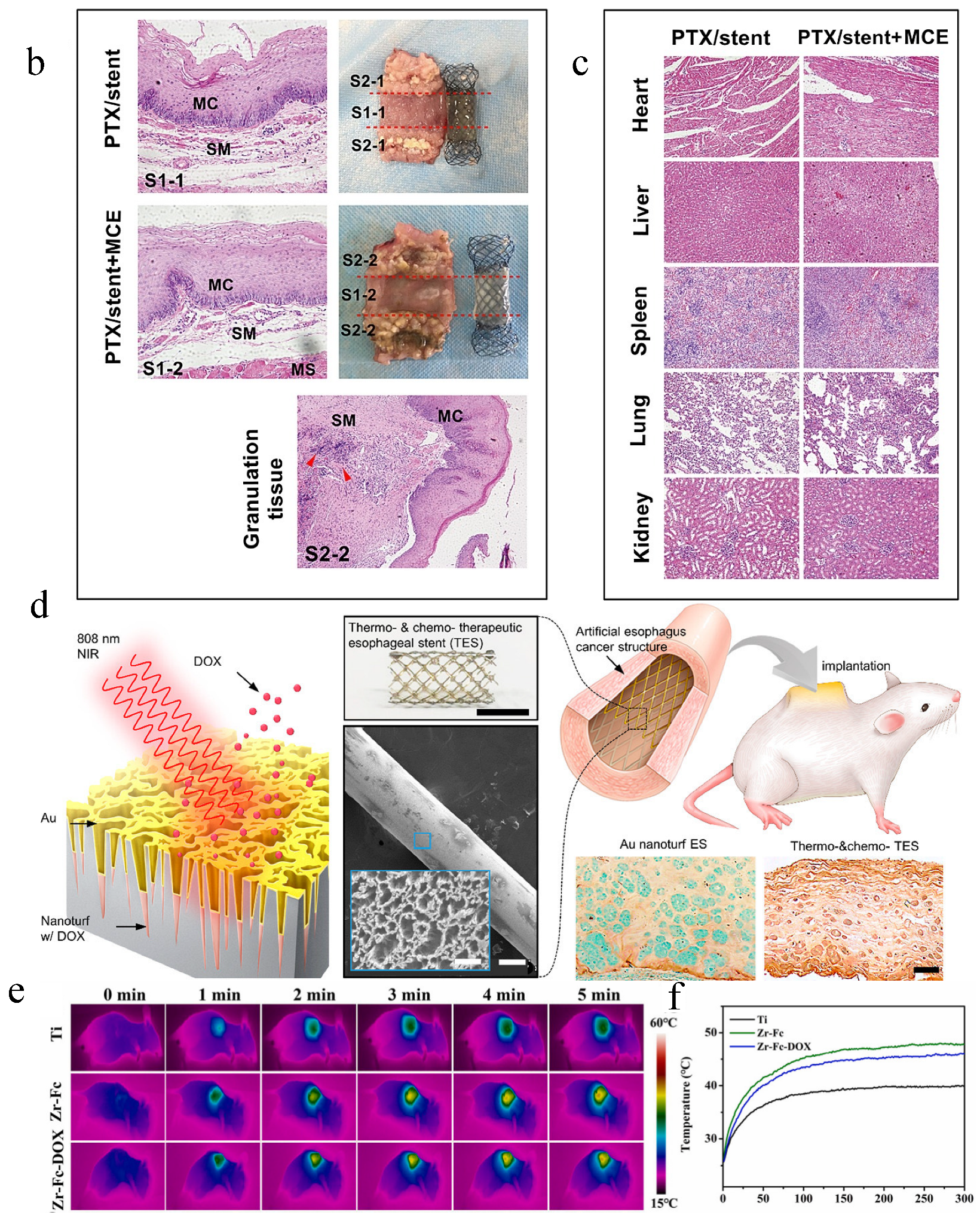
- Lee, S.; Hwang, G.; Kim, T.H.; Kwon, S.J.; Kim, J.U.; Koh, K.; Park, B.; Hong, H.; Yu, K.J.; Chae, H.; et al. On-Demand Drug Release from Gold Nanoturf for a Thermo- and Chemotherapeutic Esophageal Stent. ACS Nano 2018, 12, 6756–6766. [Google Scholar] [CrossRef]
- Klein, M.O.; Bijelic, A.; Ziebart, T.; Koch, F.; Kämmerer, P.W.; Wieland, M.; Konerding, M.A.; Al-Nawas, B. Submicron Scale-Structured Hydrophilic Titanium Surfaces Promote Early Osteogenic Gene Response for Cell Adhesion and Cell Differentiation. Clin. Implant Dent. Relat. Res. 2011, 15, 166–175. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, S.; Jin, W.; Li, Z.; Yang, G.; Li, X.; Li, N.; Wang, Y.; Sheng, F.; Song, Z. An ultrasound-driven PLGA/Zn-KNN hybrid piezoelectric scaffold with direct and immunoregulatory antibacterial activity for bone infection. Bioact. Mater. 2025, 47, 295–312. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, S.; Li, Q.; Yang, M.; Qiu, X.; Yu, B.; Wu, C.; Su, Z.; Du, F.; Zhang, M. Bimetallic infinite coordination nanopolymers via phototherapy and STING activation for eliciting robust antitumor immunity. J. Colloid Interface Sci. 2023, 642, 691–704. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Song, X.; Jiang, Q.; Guo, W.; Liu, J.; Chu, X.; Lei, Z. Transition Metal Oxide Nanomaterials: New Weapons to Boost Anti-Tumor Immunity Cycle. Nanomaterials 2024, 14, 1064. [Google Scholar] [CrossRef] [PubMed]
- Jung, A.C.; Moinard-Butot, F.; Thibaudeau, C.; Gasser, G.; Gaiddon, C. Antitumor Immune Response Triggered by Metal-Based Photosensitizers for Photodynamic Therapy: Where Are We? Pharmaceutics 2021, 13, 1788. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Deng, Z.; Sun, X.; Yuan, K.; Wang, J.; Wu, X.; Zhang, Y.; Yang, K.; Zhang, J.; Yang, G. Petaloid Metal–Organic Frameworks for Resiquimod Delivery To Potentiate Antitumor Immunity. ACS Appl. Mater. Interfaces 2024, 16, 33093–33105. [Google Scholar] [CrossRef]


| The Substrates of Ti-Based MBs | Surface Modification | Properties | Application | Ref. |
|---|---|---|---|---|
| Commercial pure Ti (CP-Ti) | Electrochemical anodization | Excellent biocompatibility, high orientation, and large surface area with tunable pore sizes | Self-organized TiO2 nanotube (TNT) can be used for photo-induced cancer cell killing | [21,46,47] |
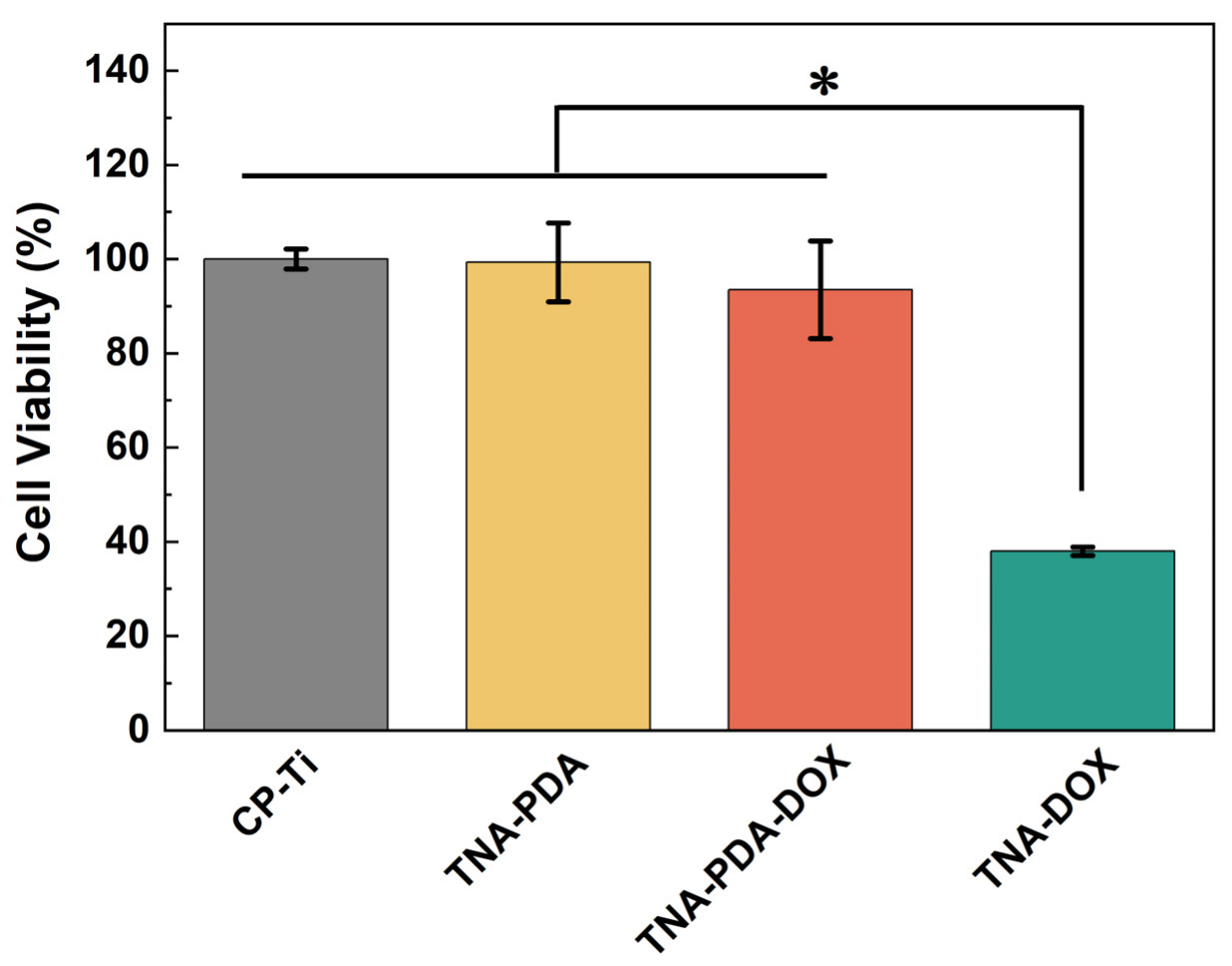
| Polydopamine (PDA)-coated TNT | Good biocompatibility, strong adhesive properties, and sensitive pH-responsiveness | Polydopamine-coated TNT arrays on pure Ti (TNA-PDA-DOX) have a good anti-osteosarcoma function by the pH-responsive release of Doxorubicin (DOX) | [20,48] | |
| Ti substrate with ZnO nanoparticles decorated with naringin | Surface nano-treatment enhances the response of osteoblasts | Ti substrate with pH-responsive naringin ZnO nanoparticle promotes the construction of osteosarcoma resection | [49] | |
| Ti-6Al-4V | Plasma-sprayed CaO-MgO-SiO2 | Excellent biocompatibility, corrosion resistance, and mechanical properties | Plasma-sprayed CaO-MgO-SiO2 bioactive glass–ceramic coatings on Ti-6Al-4V alloy for bone regeneration | [17,50] |
| Hydrogenated black titanium dioxide (H-TiO2) modified 3D printed Ti-6Al-4V | Porous features favor bone ingrowth. | H-TiO2-modified Ti-6Al-4V implants for photothermal therapy of bone tumor and bone regeneration | [17,51] | |
| Ti-12Mo-10Zr (TMZ) | Black phosphorus (BP)-coated TMZ | Low elastic modulus, appropriate compressive yield strength, and high plasticity | BP microarc oxidation (MAO) TMZ implant inhibits osteosarcoma cancer cells under the irradiation of NIR | [52] |
| Nickel-titanium (Ni-Ti) | Chemically polished Ni-Ti wire | Excellent conductivity, biocompatibility, and shape memory properties | Irreversible electroporation (IRE) using chemically polished Ni-Ti wires induces cancer cell death | [26,29] |
| TiO2 nanoparticles | TiO2 nanoparticles doped with fluorine (F)/PDA/collagen | Excellent near-infrared-activated photothermal and photocatalytic properties | TiO2@F/PDA/collagen nanoparticles anchored on Ti surfaces eliminate osteosarcoma cells and promote osteogenic differentiation of BMSCs | [53,54] |
| Therapeutic Strategy | Ti-Based MBs | In Vitro Studies | In Vivo Studies | Ref. |
|---|---|---|---|---|
| Photothermal therapy | BP-coated TMZ implant; H-TiO2 coating on Ti-6Al-4V implant | CCK8 assay test cytocompatibility and bone regeneration; photothermal effects for bone tumor cells | H&E staining; relative tumor volume variation curve | [17,52] |
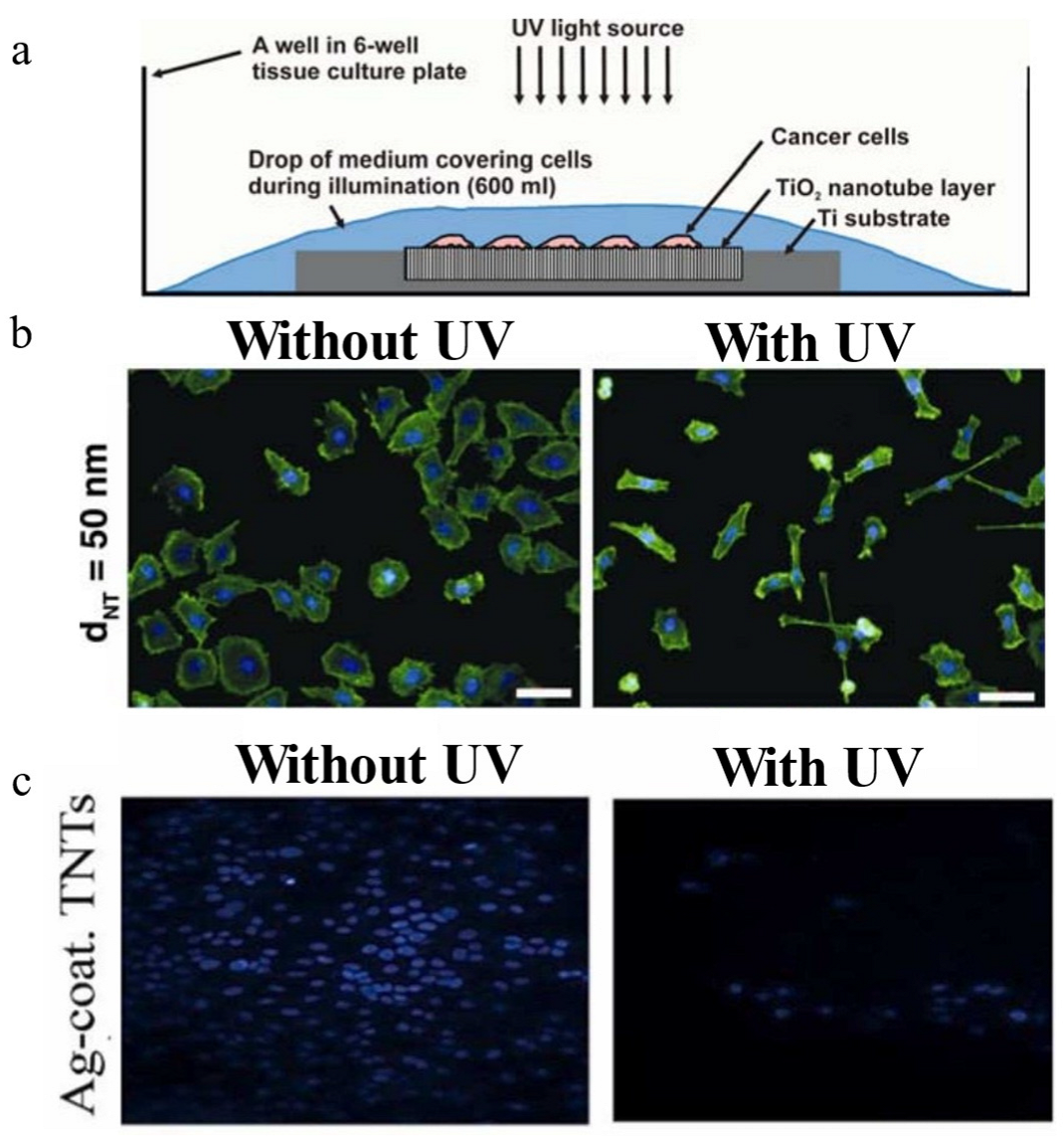
| Photodynamic therapy | Self-organized TNT; Ag-deposited, doped, and coated titanium dioxide nanotubes | Photocatalytic experiment; cytocompatibility and proliferation | - | [21,23] |
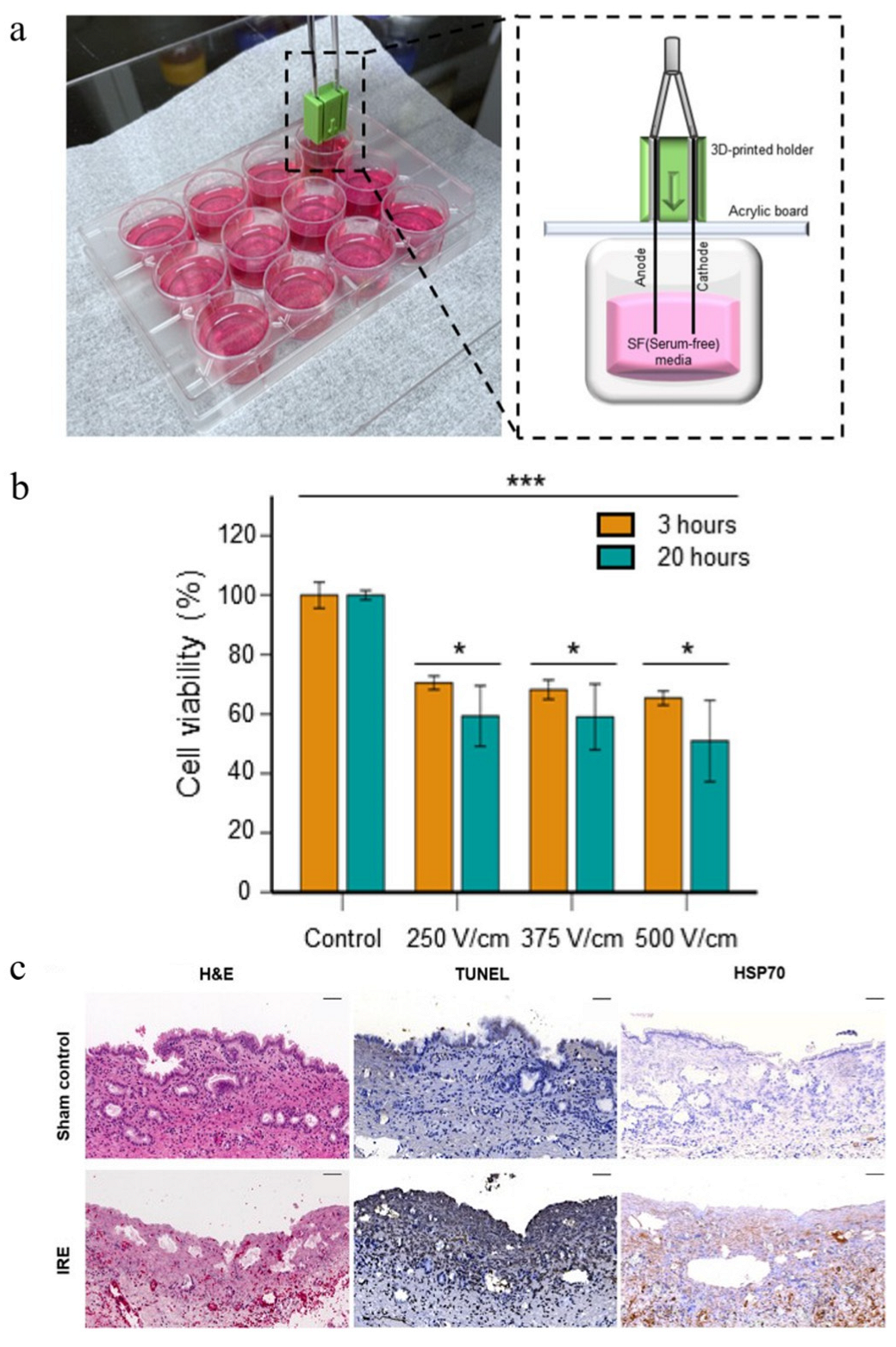
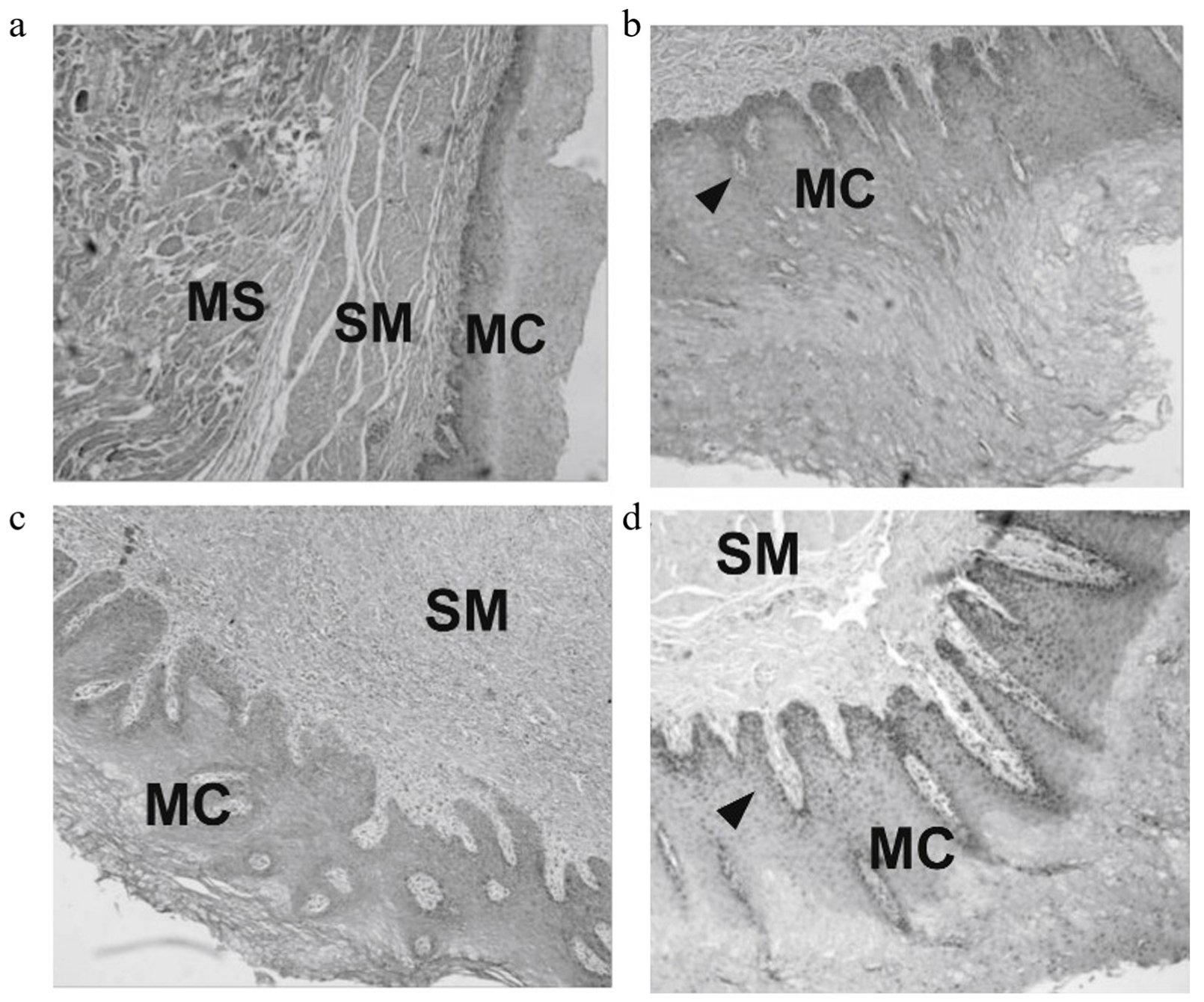
| Irreversible electroporation | Chemically polished Ni-Ti alloy wire | CCK8 assay test cytotoxicity and viability of CT-26 cancer cells | H&E, TUNEL, and HSP70 staining of tumor tissues | [26] |
| Therapeutic Strategy | Ti-Based MBs | In Vitro Studies | In Vivo Studies | Ref. |
|---|---|---|---|---|
| Diffusion-controlled LDD system | PTX or 5FU/nitinol stent | Drug release and permeation | H&E staining; pig body weight changes | [25] |
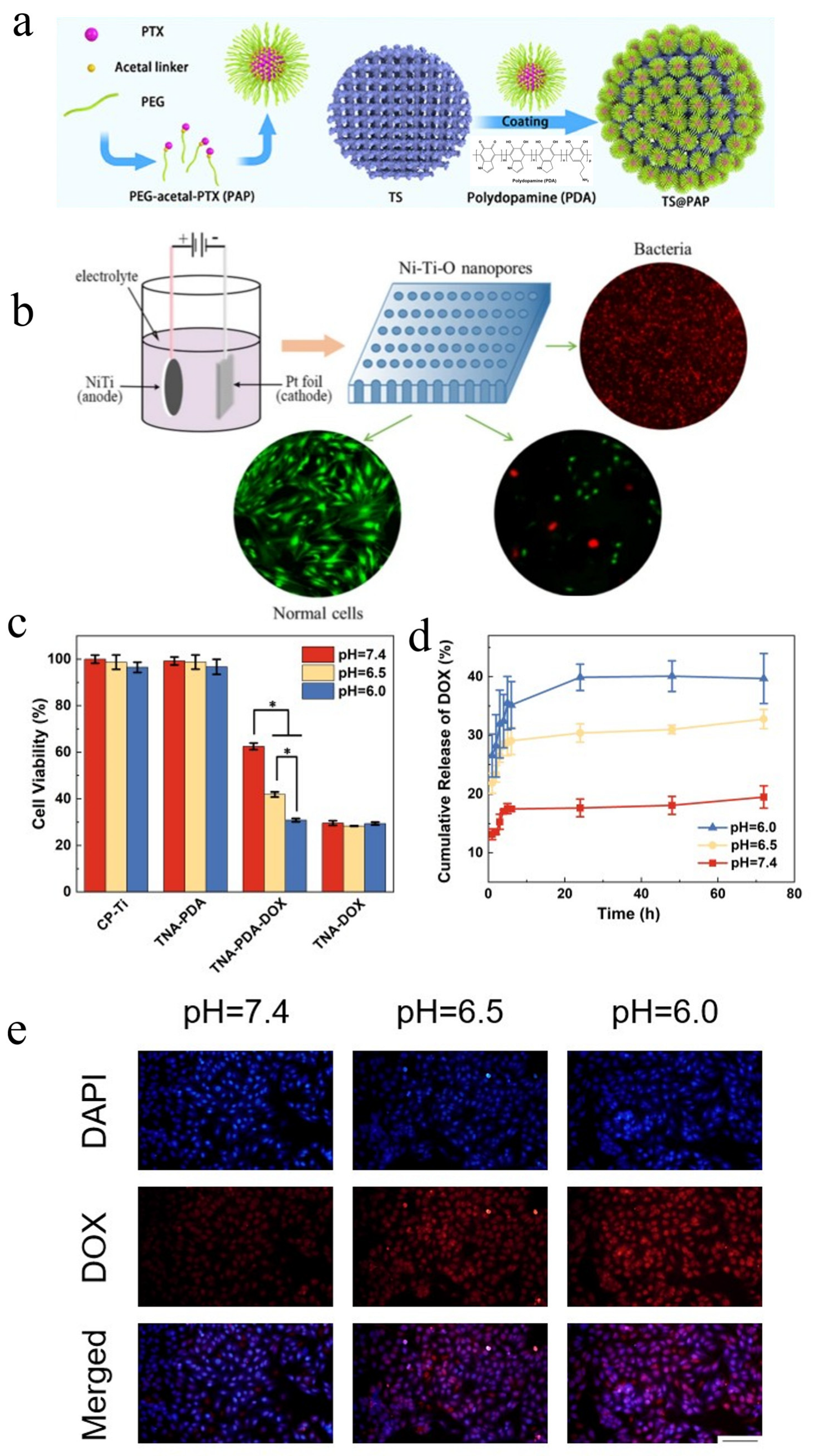
| Internal stimuli-responsive LDD system | 3D-printed TS- and pH-responsive PEGylated paclitaxel prodrugs; Ni-Ti-O nanoporous layers; double hydroxides (LDHs) layer on nitinol; TNA-PDA-DOX | Live/dead staining assay; cell viability assay; pH-dependent drug release profiles | - | [19,20,88] |
| External stimuli-responsive LDD systems | A PTX/nitinol stent combination with temperature-responsive phase-change 1-hexadecanol; DOX/Au-coated nanoturf structures; TiO2 nanoparticles doped with F/PDA)/collagen; a dual-stimuli-responsive Zr-MOF film on Ti substrates. | Osteogenic differentiation of BMSCs; antitumor ability assay; photothermal effect | H&E staining; thermal images of nude mice under NIR; pathological and anatomical images of esophageal tissues | [29,53,89,103] |
| Evaluation Metric | Physical Approaches | Chemical Approaches |
|---|---|---|
| Long-term toxicity | Low risk | Potential risk |
| Therapeutic control | Real-time modulation | Release kinetics depend on coating design |
| Drug resistance | Irrelevant | Possible |
| Immunization | Controllable | Possible immune suppression |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Liu, H.; Zhang, Z.; Deng, X.; Lin, M.; Cai, Z.; Tang, D.; Wang, H.; Liu, W.; Zhao, D. Ti-Based Metallic Biomaterials for Antitumor Applications. Materials 2025, 18, 2262. https://doi.org/10.3390/ma18102262
Yan X, Liu H, Zhang Z, Deng X, Lin M, Cai Z, Tang D, Wang H, Liu W, Zhao D. Ti-Based Metallic Biomaterials for Antitumor Applications. Materials. 2025; 18(10):2262. https://doi.org/10.3390/ma18102262
Chicago/Turabian StyleYan, Xiang, Hui Liu, Zhe Zhang, Xiang Deng, Manfeng Lin, Zongyuan Cai, Dongying Tang, Hang Wang, Wen Liu, and Dapeng Zhao. 2025. "Ti-Based Metallic Biomaterials for Antitumor Applications" Materials 18, no. 10: 2262. https://doi.org/10.3390/ma18102262
APA StyleYan, X., Liu, H., Zhang, Z., Deng, X., Lin, M., Cai, Z., Tang, D., Wang, H., Liu, W., & Zhao, D. (2025). Ti-Based Metallic Biomaterials for Antitumor Applications. Materials, 18(10), 2262. https://doi.org/10.3390/ma18102262










