Influence of Ion Chelating Agents with Different Chelating Abilities on the Properties and Microstructure of Cement-Based Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chelating Capacity Test
2.2.1. Preparation of Test Solutions
- (1)
- Ca(OH)2 (1.0 g) was dissolved in 100.0 mL of deionized water, stirred vigorously, and allowed to fully dissolve and then stand. Vacuum filtration was performed to remove undissolved solids and obtain a saturated Ca(OH)2 solution. The pH of the saturated Ca(OH)2 solution was adjusted to 11, 9, and 7 by slowly adding HCl to prepare Ca(OH)2 solutions with different pH levels for studying the chelating ability of ICAs on different Ca2+ ion concentrations.
- (2)
- Solutions of Al3+, Mg2+, Fe3+, and Ca2+ ions were prepared using Al(OH)3, MgCl2·6H2O, FeCl3·6H2O, and CaCl2, respectively.
- (3)
- Next, 0.1 mol/L solutions of A1, A2, A3, and An were prepared, and 10.0 mL of the ICA solution was mixed with 10.0 mL of other related metal ion solutions to prepare different ICA–metal ion mixed solutions.
- (4)
- A quantity of 9.25 g of EDTA was accurately weighed and placed in a 500 mL beaker, and 500 mL of distilled water was added to make a 0.05 mol/L solution. Next, 5.4 g of NH4Cl was weighed and dissolved in water; 36 mL of NH3·H2O was added, and the solution was diluted to 100 mL to obtain an ammonia–ammonium chloride buffer solution for testing Ca2+-chelating capacity.
2.2.2. Calcium Ion-Chelating Capacity Test
2.2.3. Test of Chelating Ability of Ion-Chelating Agents for Different Cations
2.2.4. Saturated Calcium Hydroxide Turbidity Point Test
2.3. Preparation and Curing of Cement Mortar
2.4. Performance Testing and Characterization
2.4.1. Heat of Hydration Test
2.4.2. Mechanical Properties Test
2.4.3. Chloride Ion Diffusion Coefficient Test
2.4.4. Pore Structure Test
2.4.5. Microscopic Morphology Analysis
3. Results and Discussion
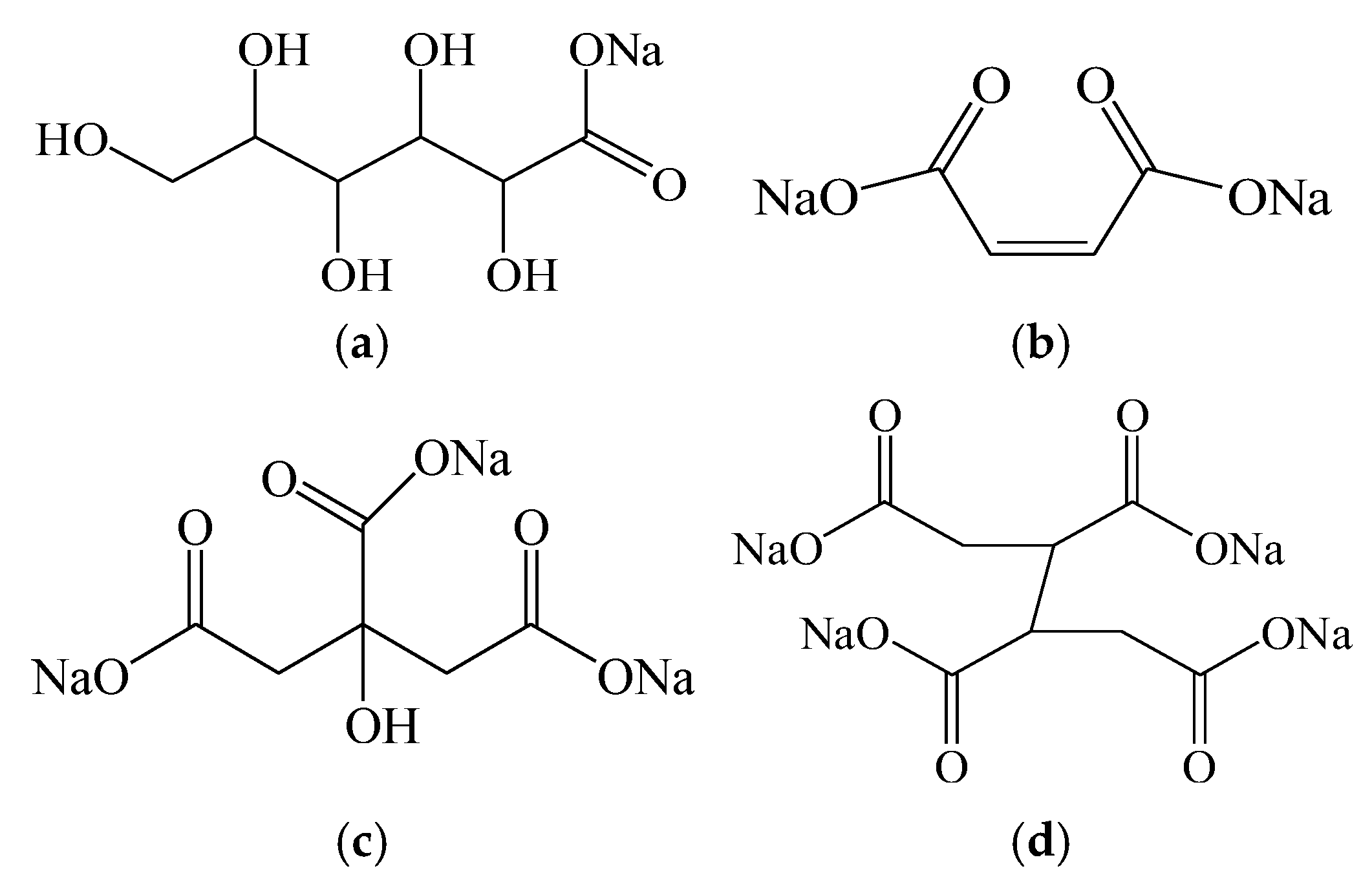
3.1. Chelating Ability Test of Ion-Chelating Agents
3.1.1. Calcium Chelating Capacity of Different Types of Ion-Chelating Agents
3.1.2. Calcium Ion Chelation Rates of Different Types of Ion-Chelating Agents
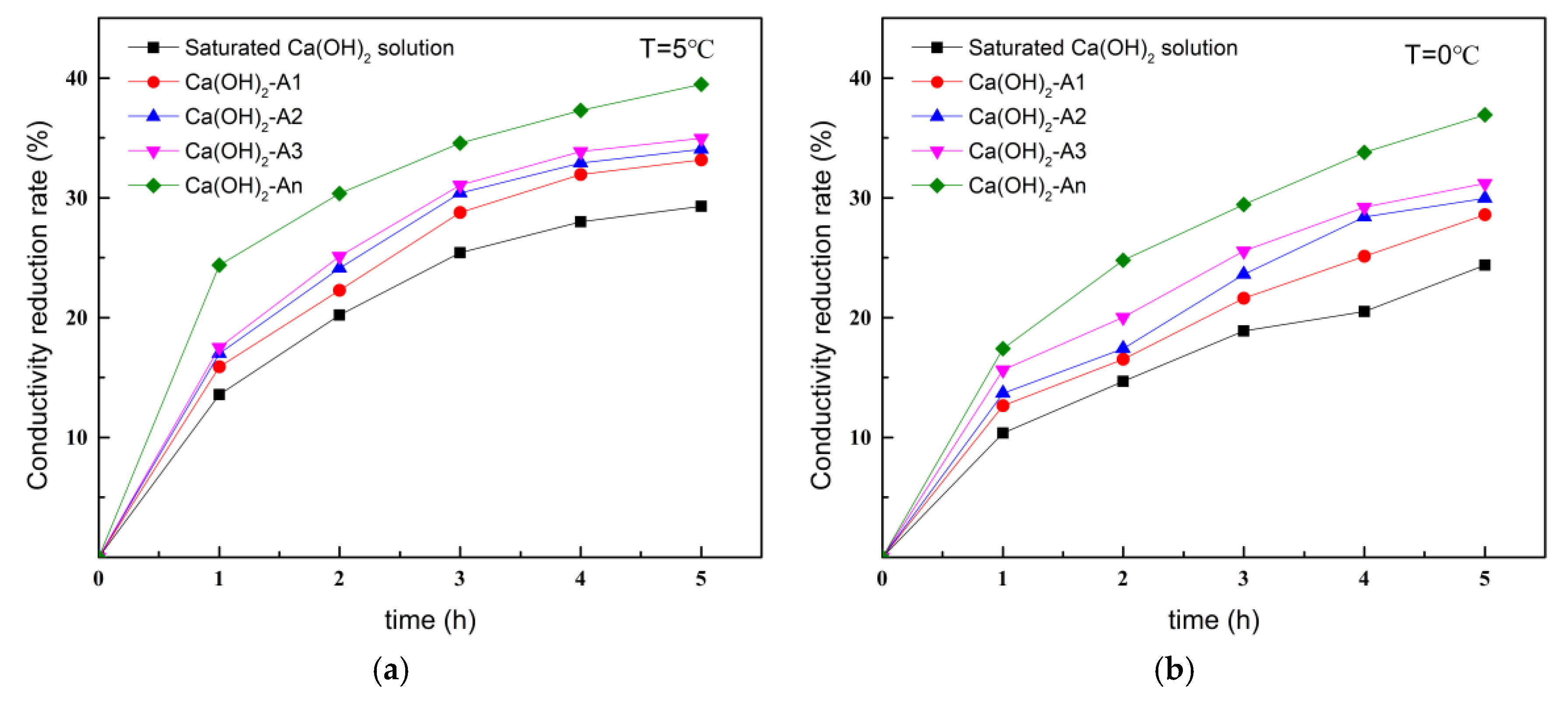
3.1.3. Calcium-Chelating Ability of Ion-Chelating Agents at Different Temperatures
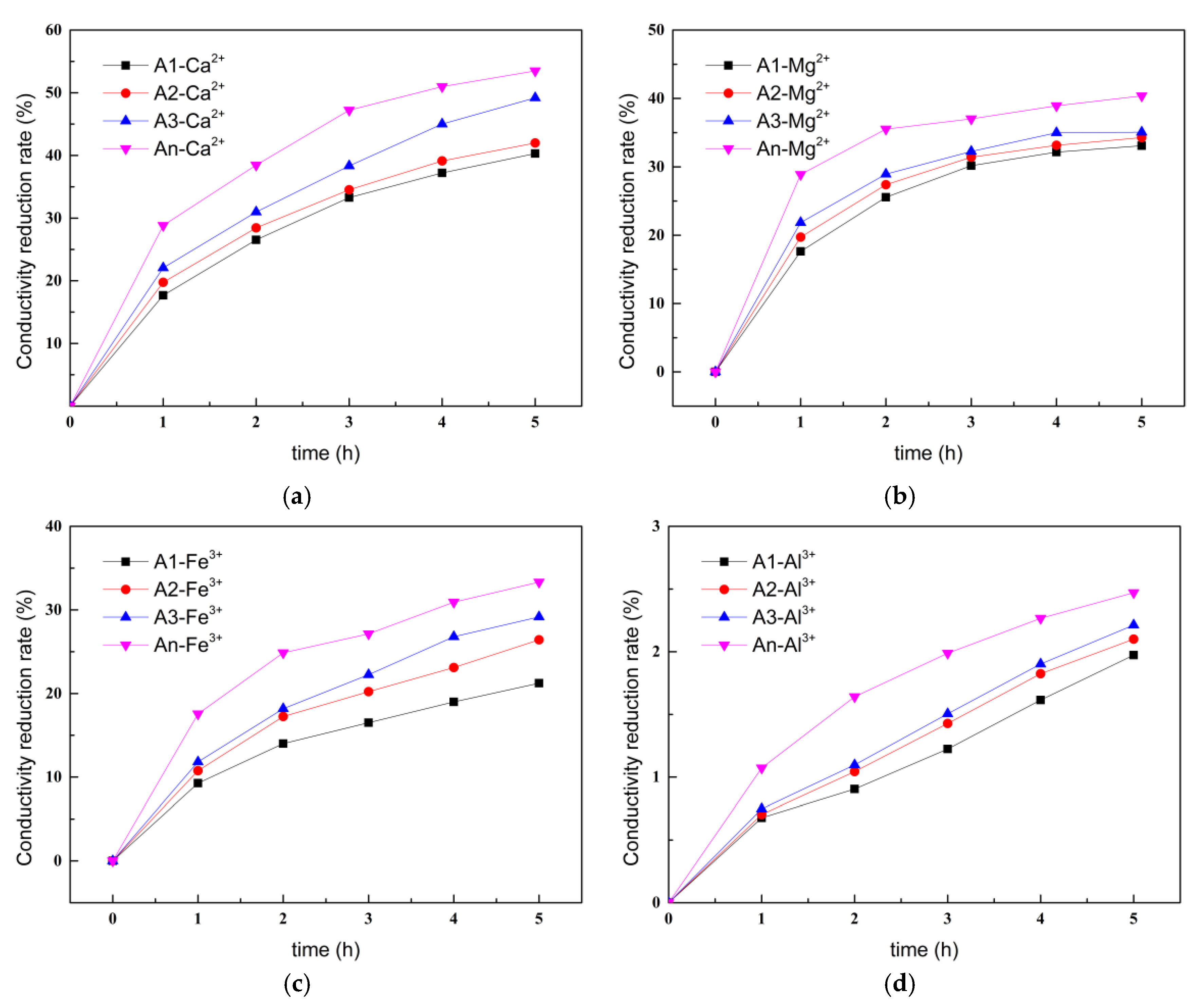
3.1.4. Chelating Ability of Ion-Chelating Agents for Different Metal Ions
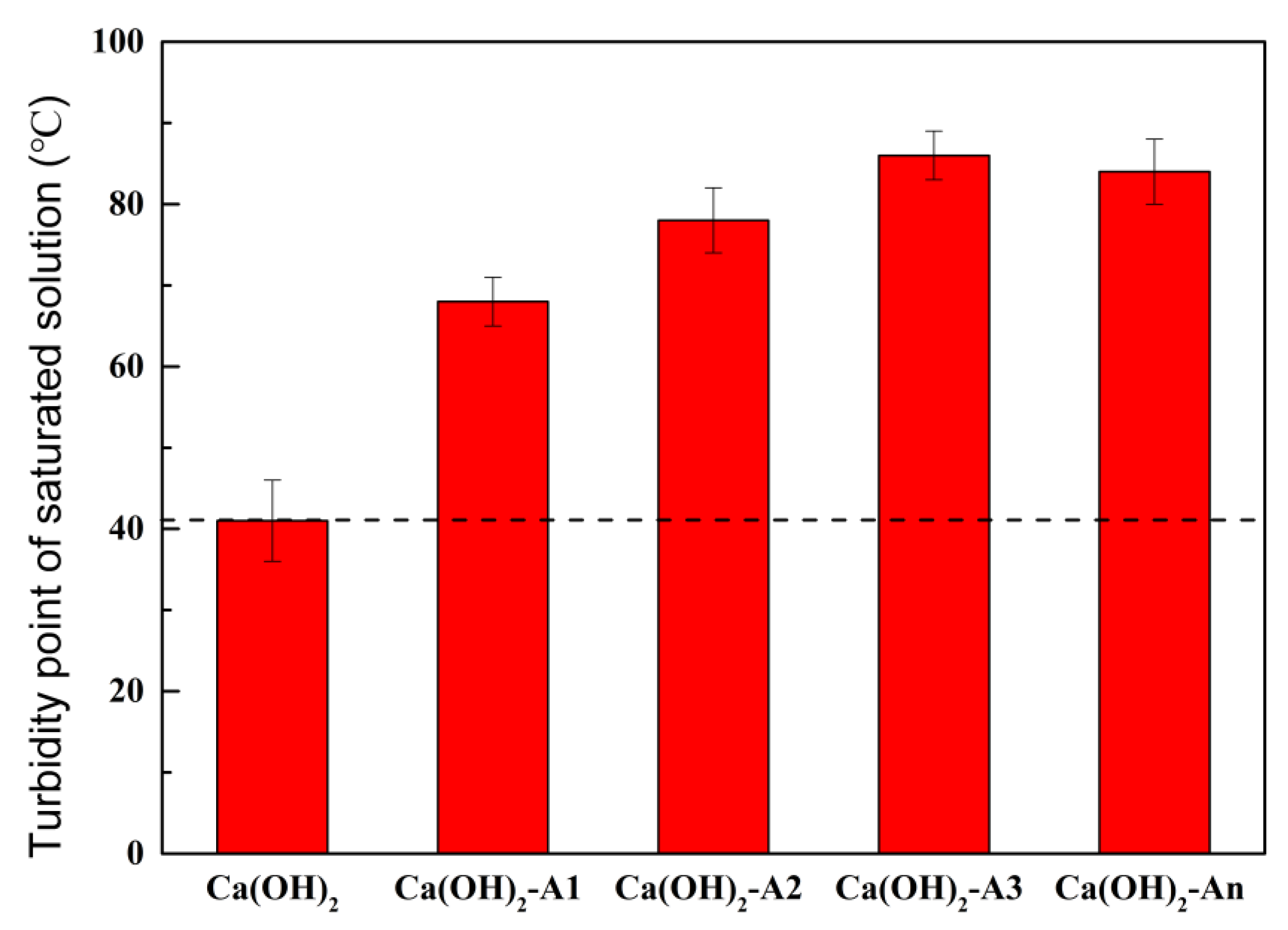
3.1.5. Turbidity Point of Saturated Calcium Hydroxide Solutions Containing Ion-Chelating Agents
3.2. Effect of Ion-Chelating Agents on the Properties and Microstructure of Cement Mortars
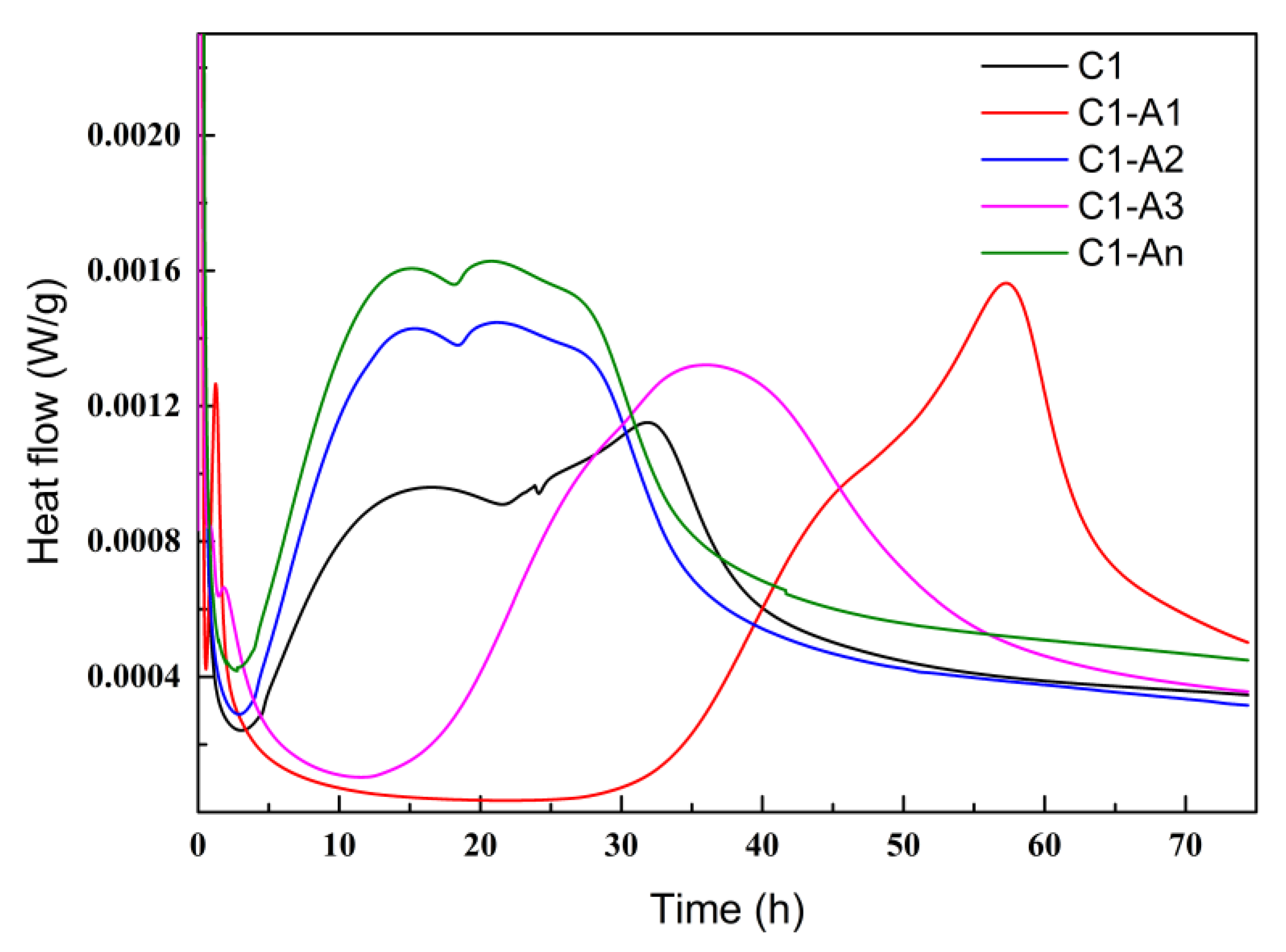
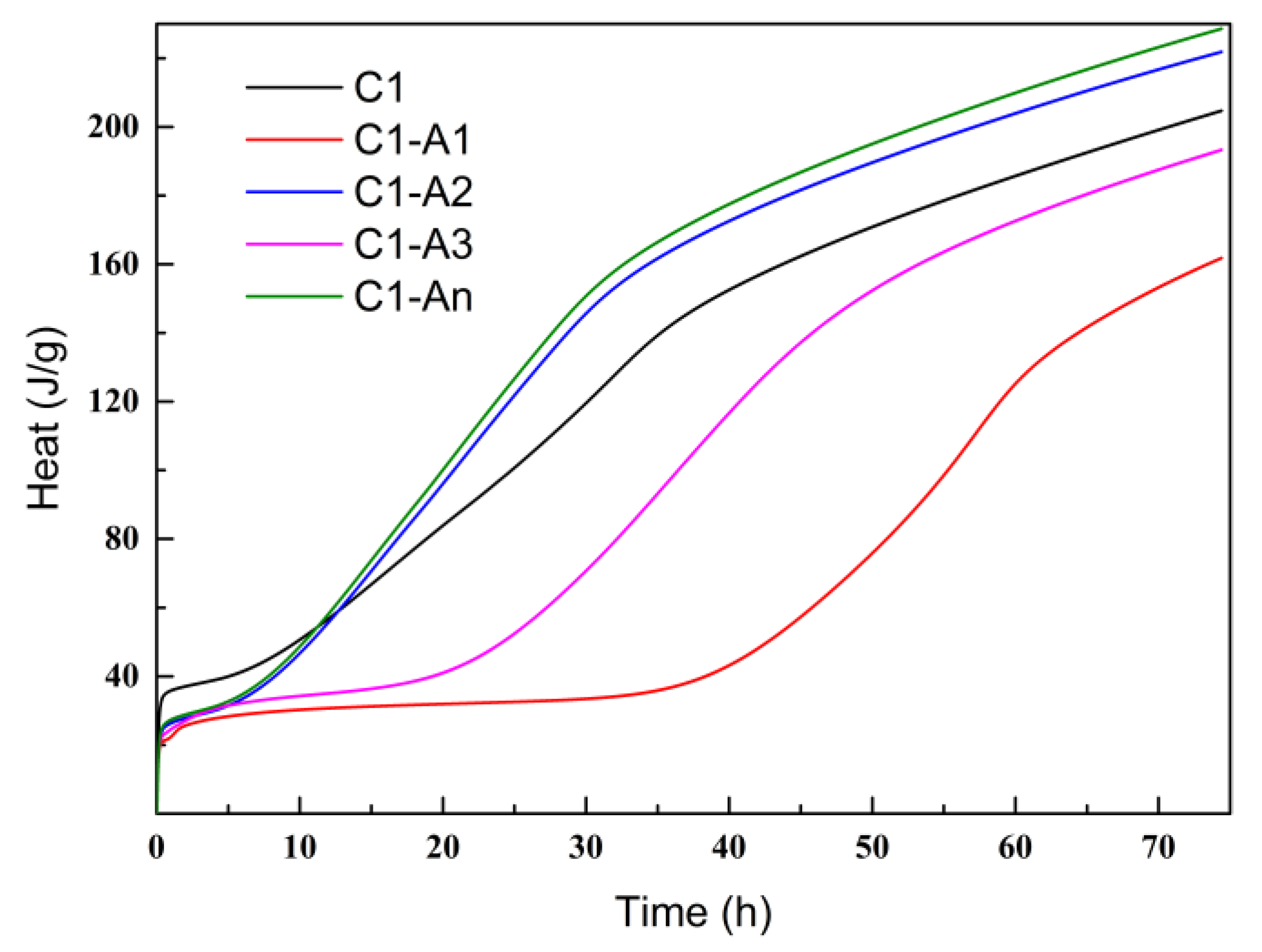
3.2.1. Hydration Heat
3.2.2. Mechanical Strength
3.2.3. Chloride Ion Diffusion Coefficient
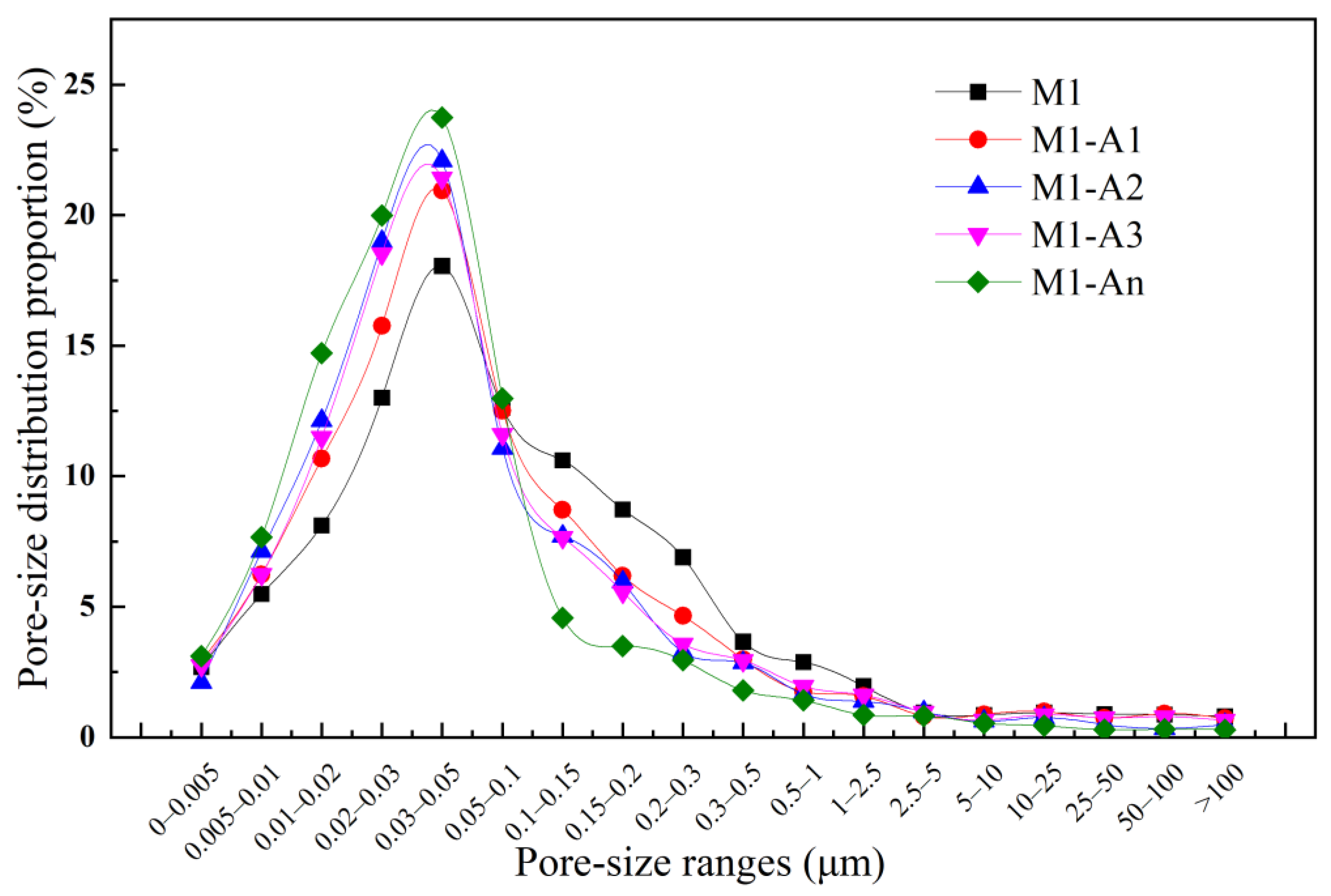
3.2.4. Pore Structure
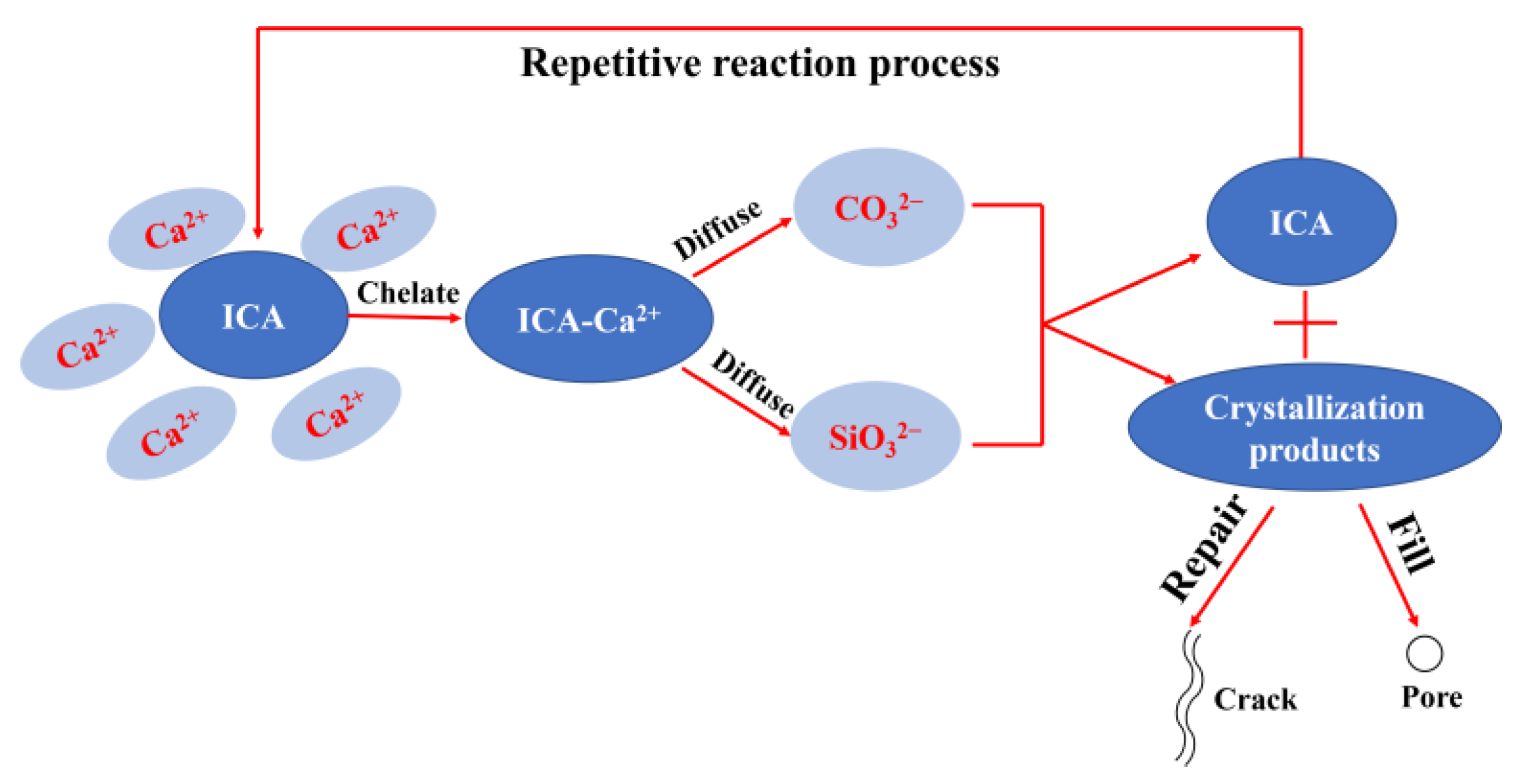
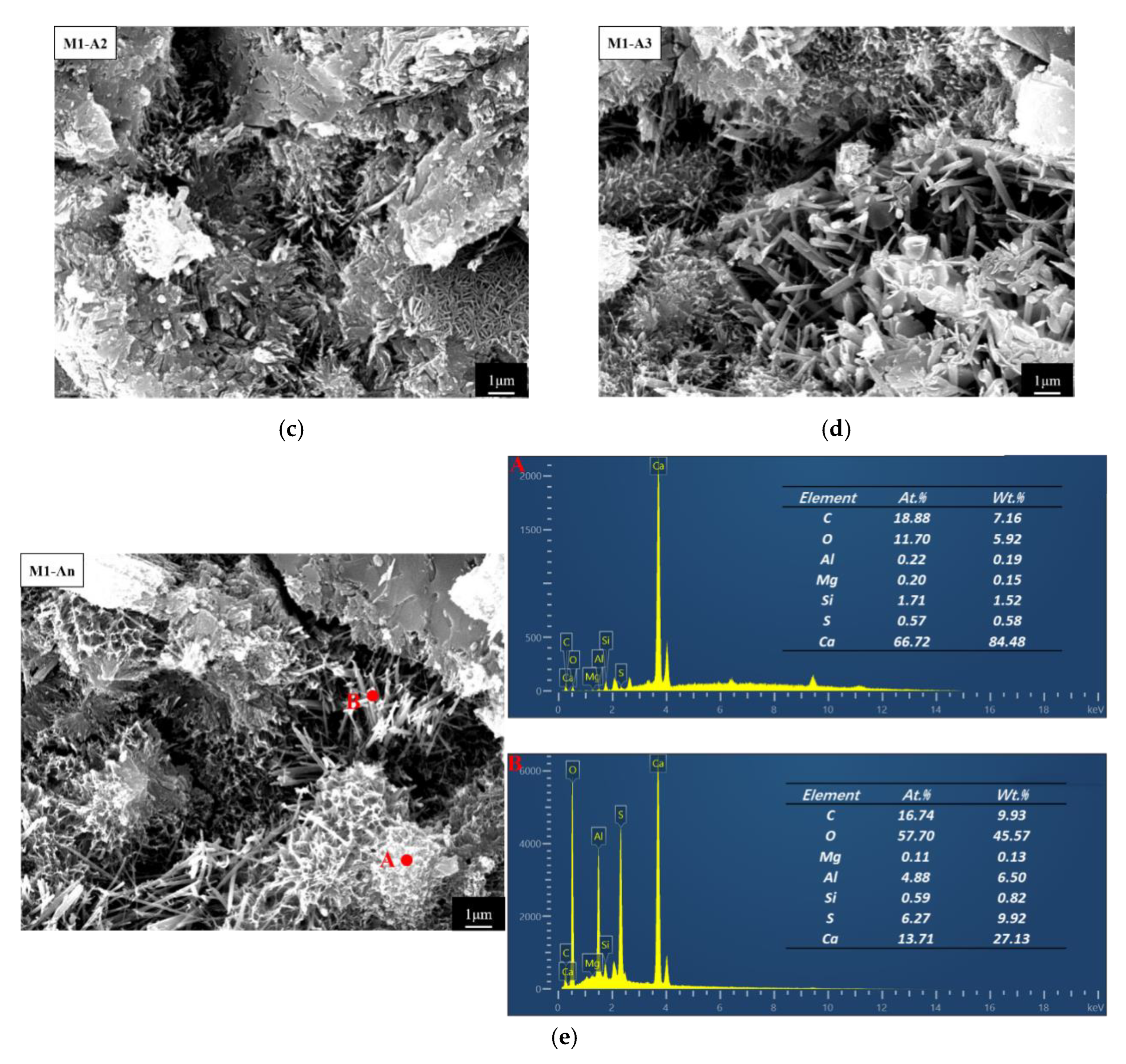
3.2.5. Internal Microscopic Morphology
4. Conclusions
- (1)
- The Ca2+ chelation capacity of the high-efficiency ICA was significantly enhanced compared to sodium gluconate, sodium maleate, and sodium citrate, with a chelation capacity of 813 mg/g. Under various temperature and pH conditions, the high-efficiency ICA effectively chelated Ca2+ and exhibited a chelation rate faster than the other three ICAs.
- (2)
- The ICAs demonstrated varying chelation abilities for different metal ions, with the chelating ability for Ca2+ being the strongest and that for Al3+ being the weakest. The high-efficiency ICA possesses multiple chelation sites, resulting in superior chelation capacity for metal ions compared to other ICAs.
- (3)
- Different ICAs had various impacts on the cement’s hydration process. Sodium gluconate and sodium citrate exhibited a retarding effect on cement, delaying the peak of the hydration heat. In contrast, sodium maleate and the high-efficiency ICA did not show a significant retarding effect, and the high-efficiency ICA facilitated the formation of C-S-H gel, resulting in the highest peak of hydration heat.
- (4)
- The ICAs improved the mechanical strength and impermeability of the mortar, with the mortar containing the high-efficiency ICA exhibiting optimal performance. At 28 d, compared with ordinary mortar, the flexural strength and compressive strength of mortar mixed with high-efficiency ICA increased by 17.1% and 11.6%, respectively, and the chloride ion diffusion coefficient decreased by 37.4%.
- (5)
- The ICAs effectively enhanced the pore size distribution and microstructure of the mortar. Compared to M1, the proportion of pores larger than 0.1 μm in M1-A1, M1-A2, M1-A3, and M1-An decreased by 22.7%, 33.8%, 30.1%, and 55.6%, respectively. SEM-EDS observations revealed that ordinary mortar contained larger and more loosely packed pores, while the mortar with ICAs exhibited a higher quantity of crystalline products within its pores. Notably, the high-efficiency ICA had a pronounced effect on optimizing the microstructure of the mortar, leading to the generation of a number of crystalline products, such as CaSiO3, within its pores.
5. Summary and Discussion of Results
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shao, R.Z.; Wu, C.Q.; Li, J. A comprehensive review on dry concrete: Application, raw material, preparation, mechanical, smart and durability performance. J. Build. Eng. 2022, 55, 104696. [Google Scholar] [CrossRef]
- He, H.J.; Qiao, H.X.; Sun, T.Y.; Yang, H.; He, C. Research progress in mechanisms, influence factors and improvement routes of chloride binding for cement composites. J. Build. Eng. 2024, 86, 108978. [Google Scholar] [CrossRef]
- Wang, X.; Ba, M.F.; Yi, B.; Liu, J. Experimental and numerical investigation on the effect of cracks on chloride diffusion and steel corrosion in concrete. J. Build. Eng. 2024, 86, 108521. [Google Scholar] [CrossRef]
- Zhao, R.Q.; Li, C.F.; Guan, X.M. Advances in modeling surface chloride concentrations in concrete serving in the marine environment: A mini review. Buildings 2024, 14, 1879. [Google Scholar] [CrossRef]
- Belie, N.D.; Gruyaert, E.; Al-Tabbaa, A.; Antonaci, P.; Baera, C.; Bajare, D.; Darquennes, A.; Davies, R.; Ferrara, L.; Jefferson, T.; et al. A review of self-healing concrete for damage management of structures. Adv. Mater. Interfaces 2018, 5, 1800074. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Lei, M.F.; Jia, C.J.; Wu, C.; Yang, Z.; Shi, Y. Influence mechanism of initial mechanical damage on concrete permeability and tunnel lining leakage. Eng. Fract. Mech. 2024, 310, 110531. [Google Scholar] [CrossRef]
- Dousti, A.; Moradian, M.; Taheri, S.R.; Rashetnia, R.; Shekarchi, M. Corrosion assessment of RC deck in a jetty structure damaged by chloride attack. J. Perform. Constr. Facil. 2013, 27, 519–528. [Google Scholar] [CrossRef]
- Zhao, Z.G.; Qu, X.L.; Li, J.H. Application of polymer modified cementitious coatings (PCCs) for impermeability enhancement of concrete. Constr. Build. Mater. 2020, 249, 118769. [Google Scholar] [CrossRef]
- Guo, S.Y.; Luo, H.H.; Tan, Z.; Chen, J.Z.; Zhang, L.; Ren, J. Impermeability and interfacial bonding strength of TiO2-graphene modified epoxy resin coated OPC concrete. Prog. Org. Coat. 2021, 151, 106029. [Google Scholar] [CrossRef]
- Xiao, H.G.; Liu, R.; Zhang, F.L.; Liu, M.; Li, H. Role of nano-SiO2 in improving the microstructure and impermeability of concrete with different aggregate gradations. Constr. Build. Mater. 2018, 188, 537–545. [Google Scholar] [CrossRef]
- Cuenca, E.; Tejedor, A.; Ferrara, L. A methodology to assess crack sealing effectiveness of crystalline admixtures under repeated cracking-healing cycles. Constr. Build. Mater. 2018, 179, 619–632. [Google Scholar] [CrossRef]
- Al-Rashed, R.; Al-Jabari, M. Multi-crystallization enhancer for concrete waterproofing by pore blocking. Constr. Build. Mater. 2021, 272, 121668. [Google Scholar] [CrossRef]
- Oliveira, A.D.S.; Gomes, O.D.F.M.; Ferrara, L.; Fairbairn, E.D.M.R.; Toledo Filho, R.D. An overview of a twofold effect of crystalline admixtures in cement-based materials: From permeability reducers to self-healing stimulators. J. Build. Eng. 2021, 41, 102400. [Google Scholar] [CrossRef]
- Zhong, L.; Zhao, J.Y.; Geng, S.; Qiu, X.; Jiang, P.; Zhou, J.; Jia, S.; Yao, Z. Exploring the effects of cementitious capillary crystalline waterproof material on the properties of cement with/without mineral admixture: A comparison of rheology, hydration process, and self-healing behavior. Constr. Build. Mater. 2025, 470, 140546. [Google Scholar]
- Azarsa, P.; Gupta, R.; Biparva, A. Assessment of self-healing and durability parameters of concretes incorporating crystalline admixtures and Portland limestone cement. Cem. Concr. Compos. 2019, 99, 17–31. [Google Scholar] [CrossRef]
- Park, B.; Choi, Y.C. Self-healing capability of cementitious materials with crystalline admixtures and super absorbent polymers (SAPs). Constr. Build. Mater. 2018, 189, 1054–1066. [Google Scholar] [CrossRef]
- Zhang, C.C.; Lu, R.W.; Li, Y.Z.; Guan, X. Effect of crystalline admixtures on mechanical, self-healing and transport properties of engineered cementitious composite. Cem. Concr. Compos. 2021, 124, 104256. [Google Scholar] [CrossRef]
- Wang, R.Y.; Yu, J.Y.; Gu, S.J.; He, P.; Han, X.; Liu, Q. Investigation of self-healing capability on surface and internal cracks of cement mortar with ion chelator. Constr. Build. Mater. 2020, 236, 117598. [Google Scholar] [CrossRef]
- Oliveira, A.D.S.; Filho, R.D.T.; Fairbairn, E.D.M.R.; de Oliveira, L.F.C.; Gomes, O.D.F.M. Microstructural characterization of self-healing products in cementitious systems containing crystalline admixture in the short- and long-term. Cem. Concr. Compos. 2021, 126, 104369. [Google Scholar] [CrossRef]
- Borg, R.P.; Cuenca, E.; Brac, E.M.G.; Ferrara, L. Crack sealing capacity in chloride-rich environments of mortars containing different cement substitutes and crystalline admixtures. J. Sustain. Cem. Based Mater. 2018, 7, 141–159. [Google Scholar] [CrossRef]
- He, P.; Yu, J.Y.; Xue, L.H.; Han, X. Influence of ion chelator on pore structure, water transport and crack-healing properties of cement pastes incorporating high-volume fly ash and blast-furnace slag. J. Build. Eng. 2022, 55, 104696. [Google Scholar] [CrossRef]
- Wang, R.Y.; Yu, J.Y.; Liu, Q.T. Influence of inorganic compounds on self-repairing capability and frost resistance of concrete incorporating ion chelating agent. J. Build. Eng. 2024, 91, 109754. [Google Scholar] [CrossRef]
- He, C.F.; Ye, J.T.; Gao, Y.; Liu, C.; Pan, X. Comparative analysis of calcium chelation mechanism and chelating ability about sodium tripolyphosphate and citric acid sodium. J. Mol. Sci. 2015, 31, 198–202. [Google Scholar]
- Yu, J.Y.; Zha, Y.G.; Wang, R.Y.; He, P.; Li, Y.; Xie, J.; Wu, S.P. A Concrete Self-Healing Additive and Its Preparation Method. China Patent 201711094897.9, 3 November 2017. [Google Scholar]
- Wang, R.Y.; Yu, J.Y.; Gu, S.J.; He, P.; Han, X.; Liu, Q. Investigation of migration and self-healing ability of ion chelator in cement-based materials by a novel method. Constr. Build. Mater. 2020, 262, 120917. [Google Scholar] [CrossRef]
- Guidone, R.E.; Gaona, X.; Winnefeld, F.; Altmaier, M.; Geckeis, H.; Lothenbach, B. Citrate sorption on cement hydrates. Cem. Concr. Res. 2024, 178, 107404. [Google Scholar] [CrossRef]
- GB/T 17671; Test Method of Cement Mortar Strength (ISO Method). China Building Industry Press: Beijing, China, 2021.
- GB/T 50082-2009; Standard for Test Methods of Long-Term Performance and Durability of Ordinary Concrete. National Standard of the People’s Republic of China: Beijing, China, 2009.
- Rong, Z.D.; Yang, H.L.; Gao, Y.; Chen, H. The Pore Structure Characteristics of Mortar and Its Application in the Study of Chloride Ion Transport Performance. Buildings 2025, 15, 383. [Google Scholar] [CrossRef]
- Xu, X.H.; Qi, C.C.; Aretxabaleta, X.M.; Ma, C.; Spagnoli, D.; Manzano, H. The initial stages of cement hydration at the molecular level. Nat. Commun. 2024, 15, 2731. [Google Scholar] [CrossRef]
- Zhao, Y.; Ning, L.; Bi, J.; Wang, C.; Zhang, Y.; Zhao, Y.; Deng, X.; Shen, M.; Li, Y. Advances in understanding hydration of Portland cement. J. Build. Eng. 2025, 108, 112866. [Google Scholar] [CrossRef]
- Ren, B.; Bai, E.L.; Luo, X.; Wang, T.; Wang, Z. Impact mechanical properties and pore structure of graphene oxide concrete at high temperature. J. Build. Eng. 2024, 85, 108593. [Google Scholar] [CrossRef]
- Zhang, K.; Zhou, J.; Yin, Z.G. Experimental study on mechanical properties and pore structure deterioration of concrete under freeze–thaw cycles. Materials 2021, 14, 6568. [Google Scholar] [CrossRef]








| SiO2 | Al2O3 | CaO | MgO | SO3 | Fe2O3 | LOI | |
|---|---|---|---|---|---|---|---|
| Cement | 22.43 | 5.94 | 61.24 | 1.12 | 2.23 | 3.56 | 1.87 |
| Cement | Water | Sand | A1 | A2 | A3 | An | |
|---|---|---|---|---|---|---|---|
| M1 | 100 | 40 | 200 | 0 | 0 | 0 | 0 |
| M1-A1 | 100 | 40 | 200 | 0.1 | 0 | 0 | 0 |
| M1-A2 | 100 | 40 | 200 | 0 | 0.1 | 0 | 0 |
| M1-A3 | 100 | 40 | 200 | 0 | 0 | 0.1 | 0 |
| M1-An | 100 | 40 | 200 | 0 | 0 | 0 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, K.; Wang, R.; Yu, J.; Liu, Q.; Zha, Y. Influence of Ion Chelating Agents with Different Chelating Abilities on the Properties and Microstructure of Cement-Based Materials. Materials 2025, 18, 2256. https://doi.org/10.3390/ma18102256
Zhao K, Wang R, Yu J, Liu Q, Zha Y. Influence of Ion Chelating Agents with Different Chelating Abilities on the Properties and Microstructure of Cement-Based Materials. Materials. 2025; 18(10):2256. https://doi.org/10.3390/ma18102256
Chicago/Turabian StyleZhao, Ke, Ruiyang Wang, Jianying Yu, Quantao Liu, and Yagang Zha. 2025. "Influence of Ion Chelating Agents with Different Chelating Abilities on the Properties and Microstructure of Cement-Based Materials" Materials 18, no. 10: 2256. https://doi.org/10.3390/ma18102256
APA StyleZhao, K., Wang, R., Yu, J., Liu, Q., & Zha, Y. (2025). Influence of Ion Chelating Agents with Different Chelating Abilities on the Properties and Microstructure of Cement-Based Materials. Materials, 18(10), 2256. https://doi.org/10.3390/ma18102256






