Enhancement of Bacterial Survival and Self-Healing Performance in Mortars After Exposure to Negative Temperature Using Alumina Hollow Spheres as Bacterial Carriers
Abstract
:1. Introduction
2. Materials and Methods
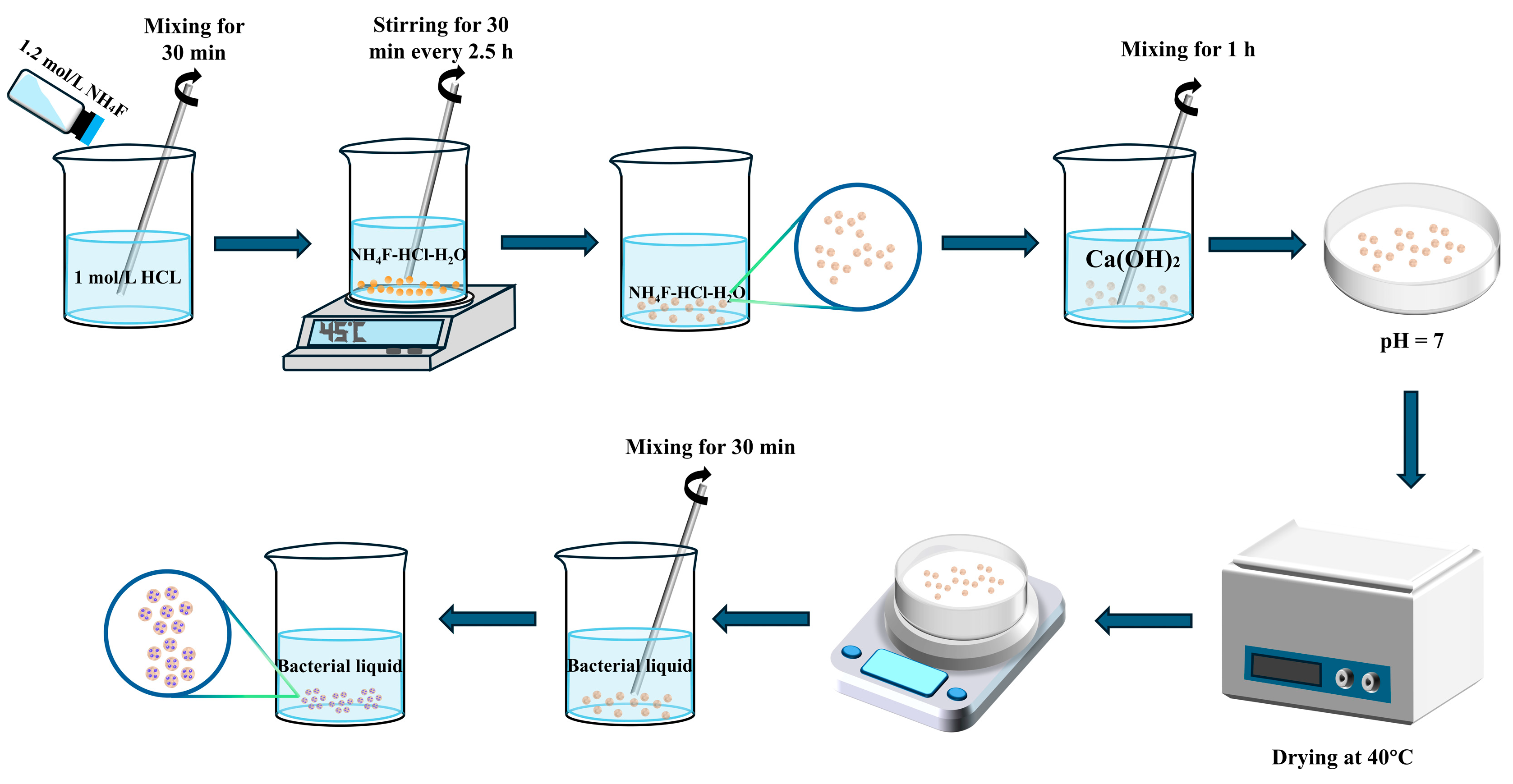
2.1. Acid-Modified Alumina Hollow Spheres
2.2. Self-Healing Agent Preparation
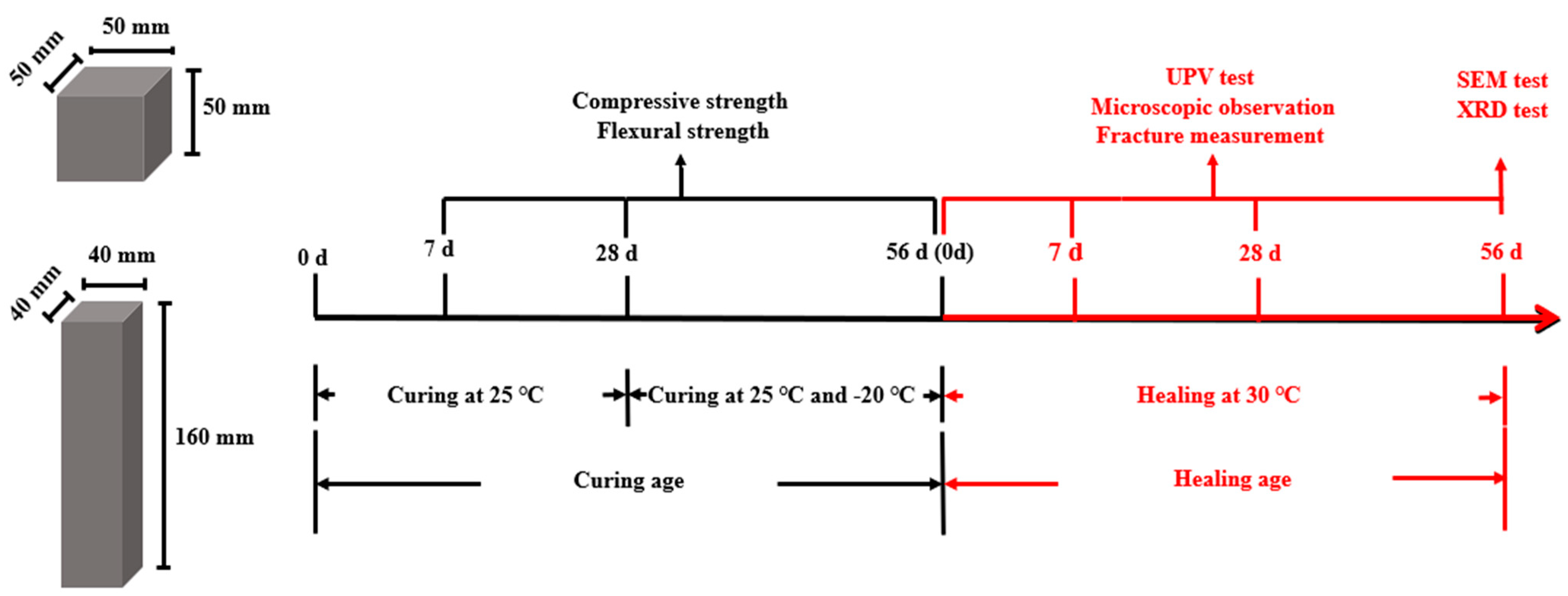
2.3. Mortar Specimen Ratios and Preparation
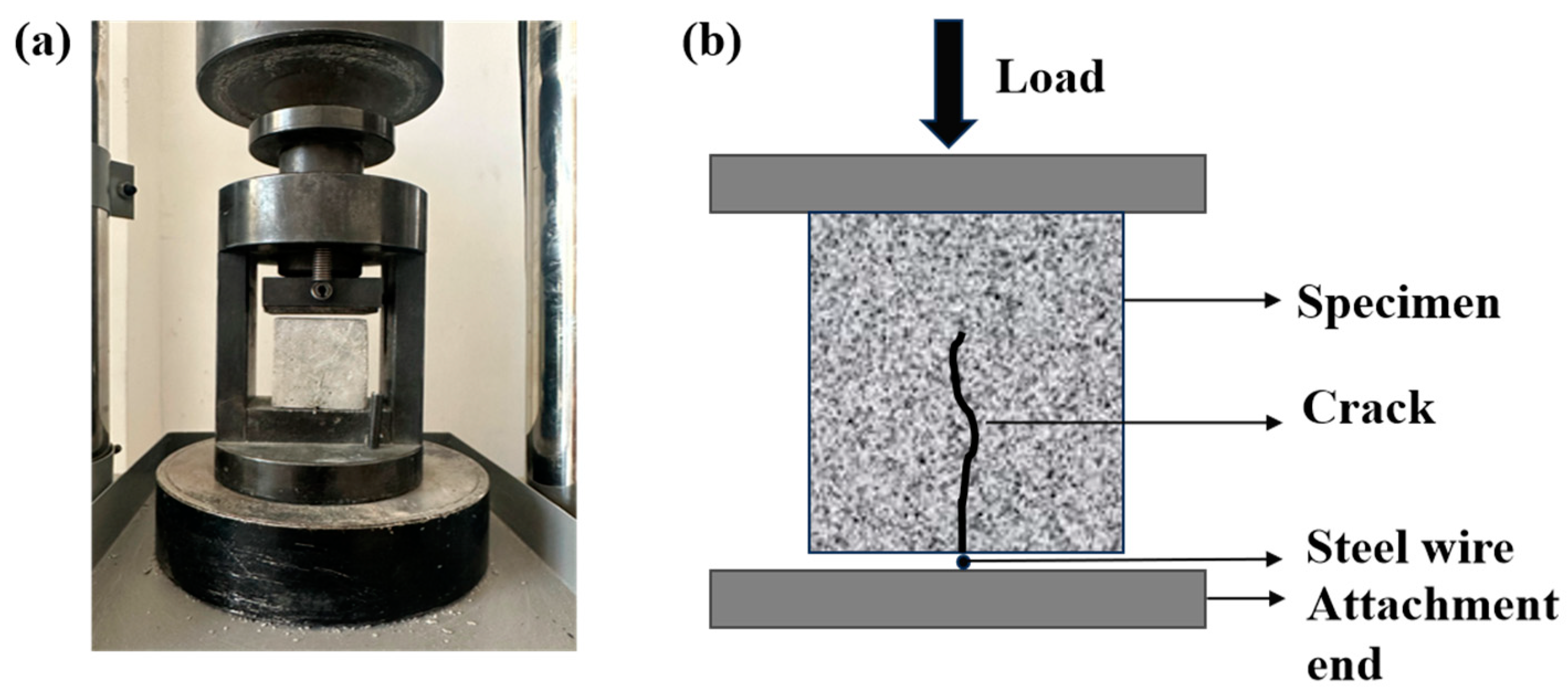
2.4. Crack Preparation and Healing Method
2.5. Test Procedure
2.5.1. Thermal Conductivity
2.5.2. Mechanical Properties Test
2.5.3. Measurement of Crack Filling
2.5.4. Scanning Electron Microscopy (SEM) Test
2.5.5. X-Ray Diffraction (XRD) Test
2.5.6. Ultrasonic Pulse Velocity (UPV) Test
3. Results and Discussion
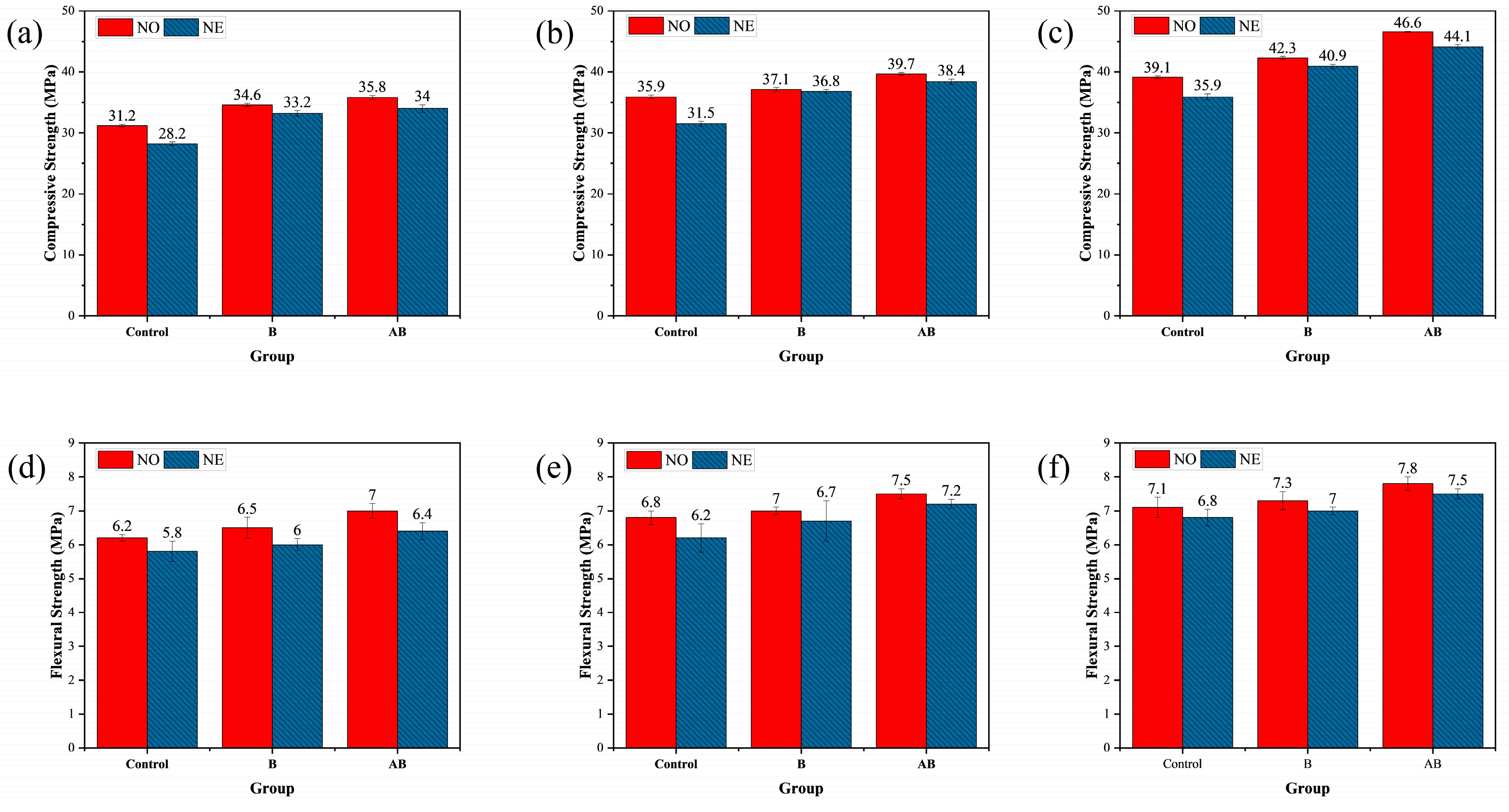
3.1. Mechanical Properties
3.2. Thermal Conductivity of the Mortars
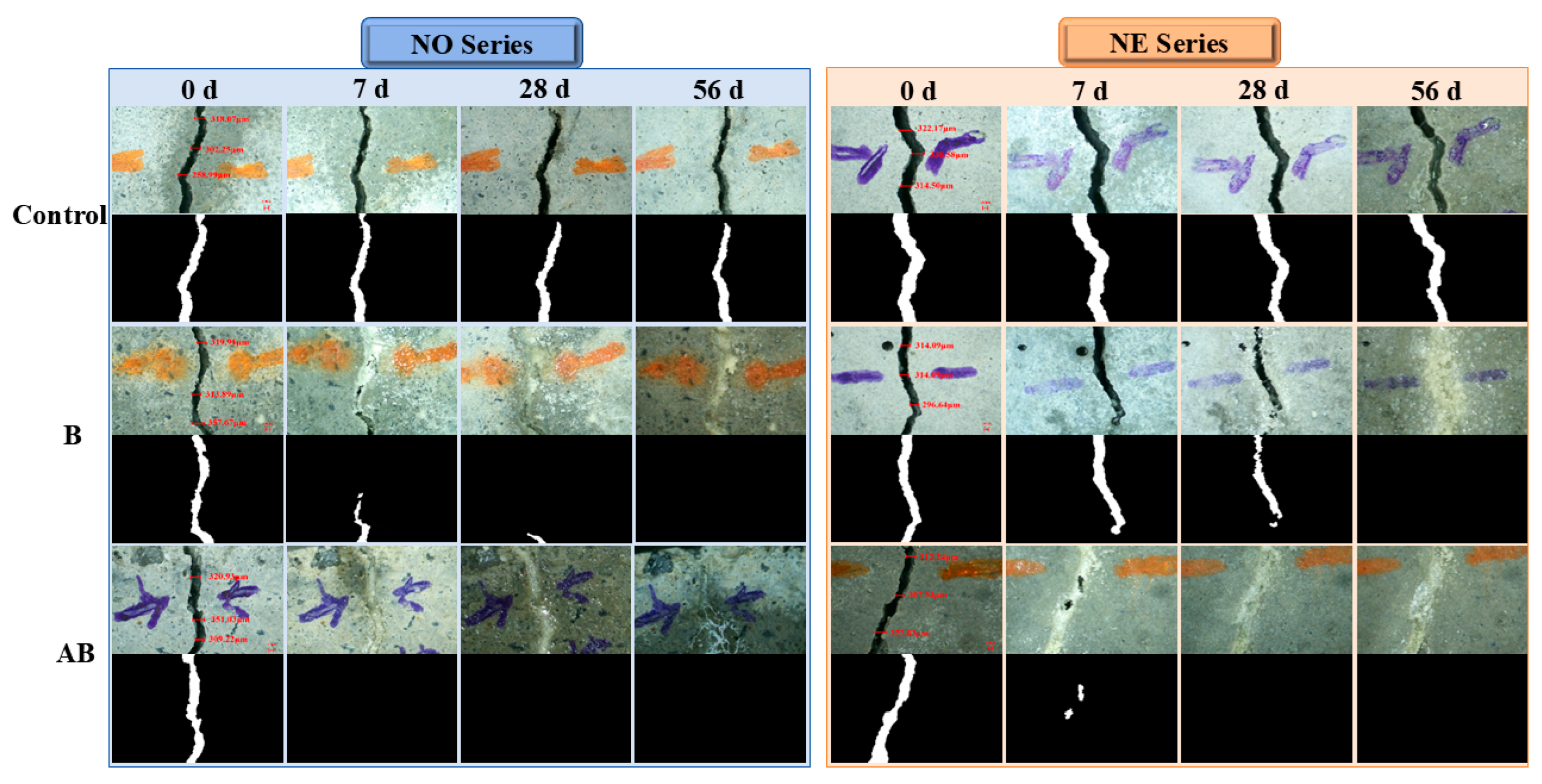
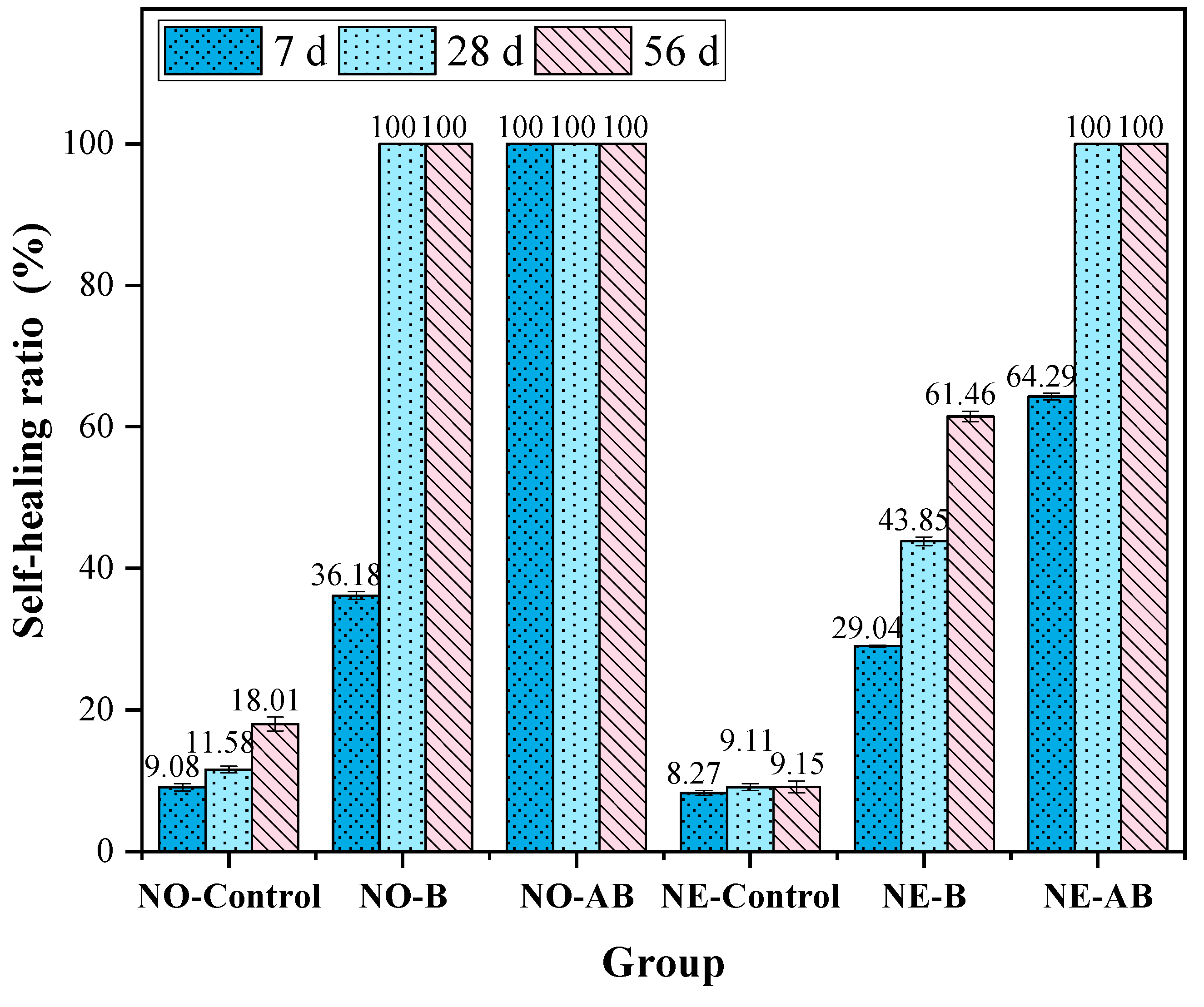
3.3. Self-Healing Properties
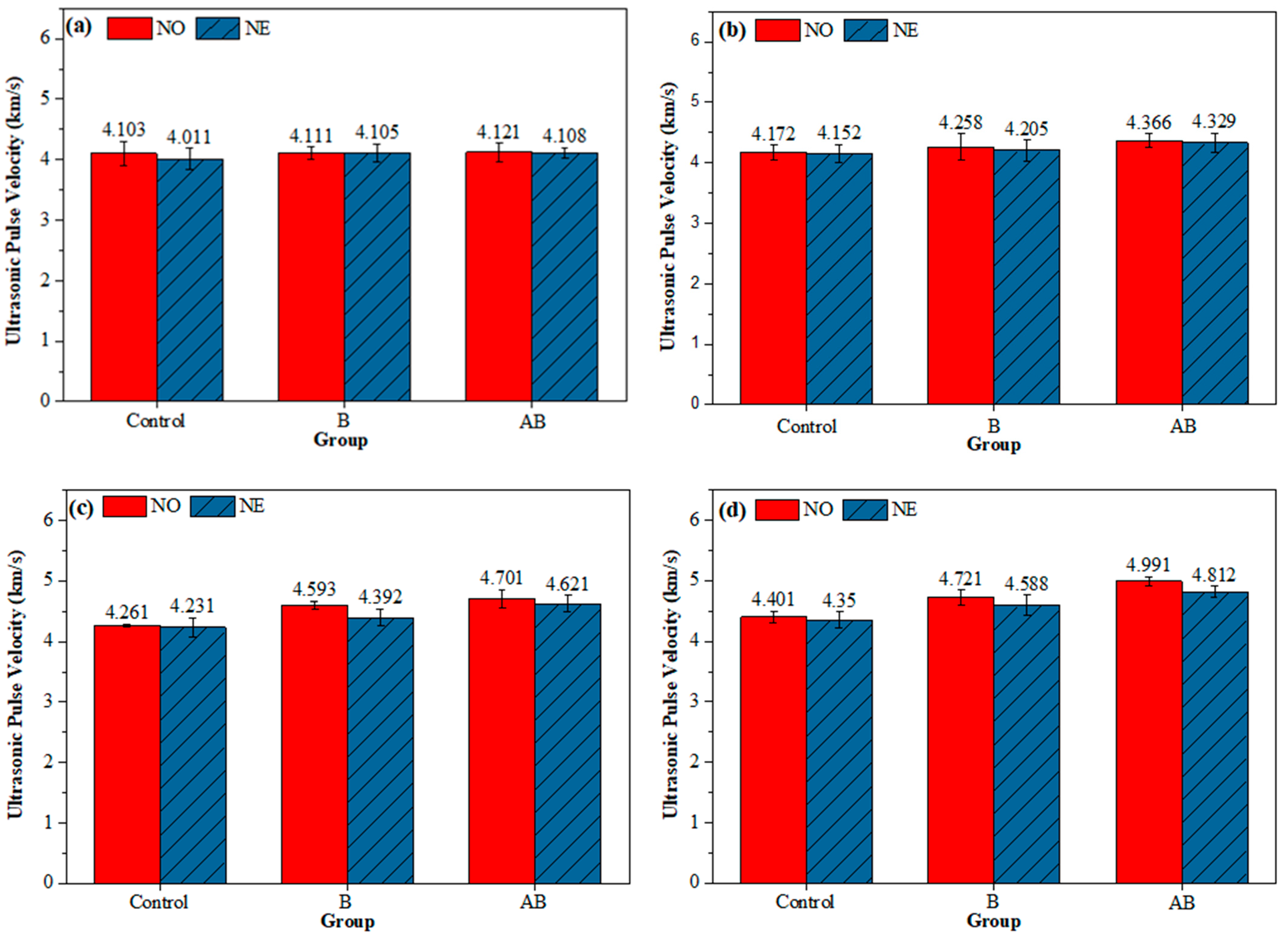
3.4. UPV Test
3.5. Microscopic Analysis
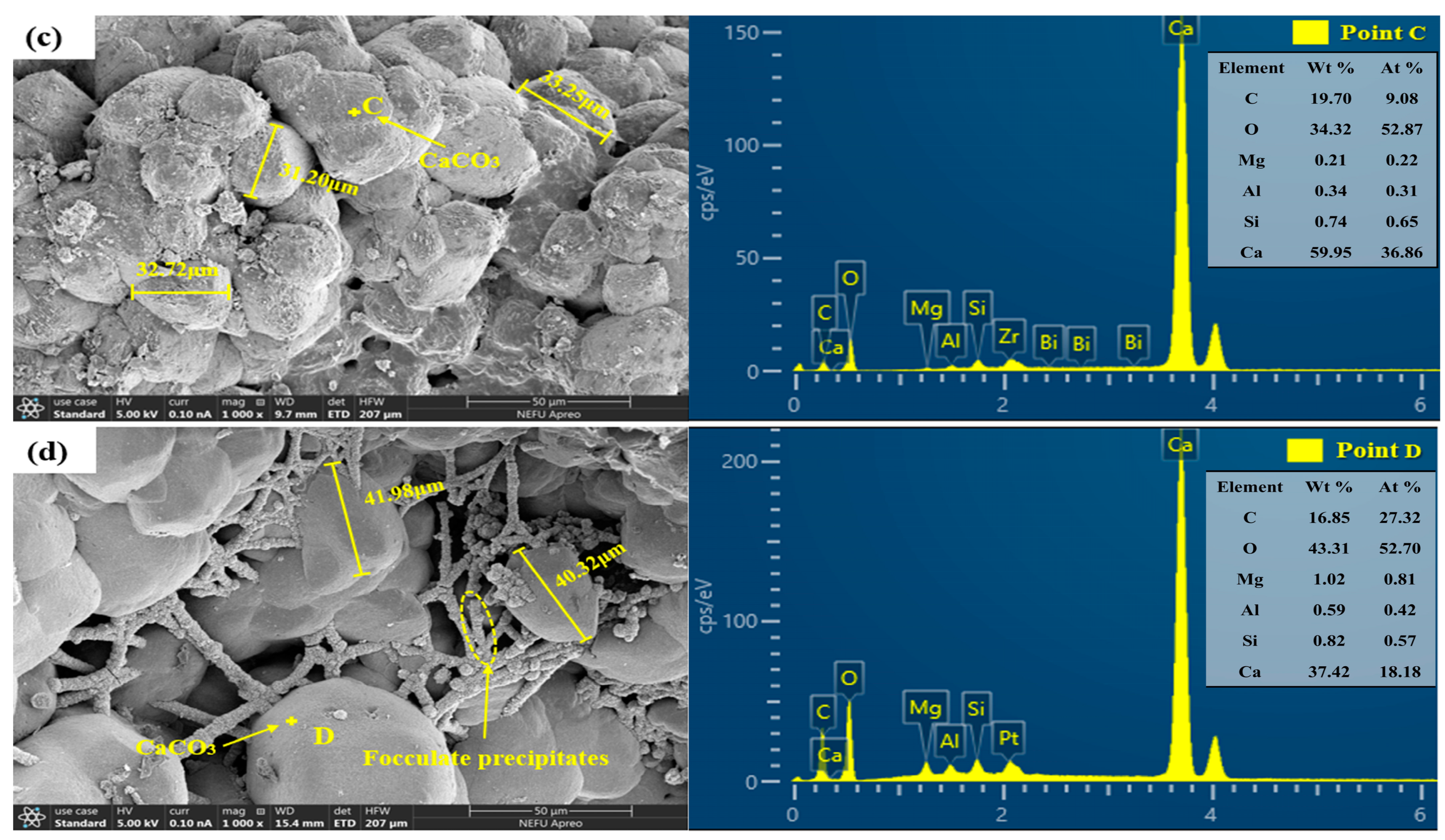
3.5.1. SEM Test
3.5.2. XRD Test
4. Conclusions
- Alumina hollow spheres as bacterial carriers enhanced mortar strength by generating calcium aluminate via hydration and protecting bacteria under a negative temperature through low thermal conductivity, with flexural strength trending similarly to compressive strength.
- Thermal conductivity tests evaluated the thermal properties of the Control specimens and those incorporating the alumina hollow spheres at −20 °C and 25 °C. The results showed that the addition of the alumina hollow spheres reduced the thermal conductivity of the specimens by 14.8% at −20 °C and 11.9% at 25 °C, respectively. This confirmed that the complex crystal structure of the hollow spheres disrupted heat conduction pathways, while their hollow architecture decreased the heat transfer rates within the matrix. These combined mechanisms mitigated the temperature sensitivity of the concrete.
- Crack healing tests showed that the alumina hollow spheres enhanced bacterial self-healing. The specimens with the self-healing agent cured at 25 °C achieved full crack closure within 7 days. At −20 °C, the healing efficiency of the bacteria-only specimens dropped to 61.46%, but full healing occurred with the alumina hollow sphere carriers. Note: cracks > 500 μm could not fully close, as the negative temperature-induced microbial cell damage overwhelmed the carrier’s protective effect.
- SEM revealed numerous small cubic CaCO3 crystals on deposition surfaces, attributed to aluminum ion hydrolysis from the alumina hollow spheres, which generated aluminum hydroxide flocs that induced free CaCO3 precipitation. The carriers also supported bacterial survival, promoting synergistic CaCO3 crystal growth.
- XRD confirmed that the alumina hollow spheres enhanced the intensity of the calcite peaks observed for the NO-AB and NE-AB specimens. Aluminum ion hydrolysis produced aluminum hydroxide flocs that adsorbed free calcium carbonate, promoting crystal formation. The higher calcite peak intensity in the NO-series specimens (NO-B/NO-AB) was linked to temperature-dependent bacterial survival affecting CaCO3 yield.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MICP | Microbially induced carbonate precipitation |
| SEM-EDS | Scanning electron microscopy and energy-dispersive X-ray spectroscopy |
| XRD | X-ray diffraction |
| UPV | Ultrasonic pulse velocity |
| NO | Normal temperature |
| NE | Negative temperature |
| NO-Control | Control specimens cured at normal temperature |
| NO-B | Specimens incorporating only bacteria and cured at normal temperature |
| NO-AB | Specimens incorporating both alumina hollow spheres and bacteria and cured atnormal temperature |
| NE-Control | Control specimens cured at negative temperature |
| NE-B | Specimens incorporating only bacteria and cured at negative temperature |
| NE-AB | Specimens incorporating both alumina hollow spheres and bacteria and cured atnegative temperature |
References
- Kua, H.W.; Gupta, S.; Aday, A.N.; Srubar, W.V. Biochar-immobilized bacteria and superabsorbent polymers enable self-healing of fiber-reinforced concrete after multiple damage cycles. Cem. Concr. Compos. 2019, 100, 35–52. [Google Scholar] [CrossRef]
- Wong, P.Y.; Mal, J.; Sandak, A.; Luo, L.; Jian, J.; Pradhan, N. Advances in microbial self-healing concrete: A critical review of mechanisms, developments, and future directions. Sci. Total Environ. 2024, 947, 174553. [Google Scholar] [CrossRef] [PubMed]
- Khattab, I.M.; Shekha, H.; Abdi, M. Study on Self-healing Concrete types—A review. Sustain. Struct. Mater. 2019, 2, 76–87. [Google Scholar]
- Kannan, U.; Gafoor, S.A.; Srivastava, S.; Nithyadharan, M.; Gupta, S.S.; Maliyekkal, S.M. A waste-derived nanocomposite sealant for repairing micro-cracks in concrete. J. Build. Eng. 2022, 48, 103965. [Google Scholar] [CrossRef]
- Stuckrath, C.; Serpell, R.; Valenzuela, L.M.; Lopez, M. Quantification of chemical and biological calcium carbonate precipitation: Performance of self-healing in reinforced mortar containing chemical admixtures. Cem. Concr. Compos. 2014, 50, 10–15. [Google Scholar] [CrossRef]
- Luo, M.; Bai, J.; Jing, K.; Ding, Z.; Yang, D.; Qian, C. Self-healing of early-age cracks in cement mortars with artificial functional aggregates. Constr. Build. Mater. 2021, 272, 121846. [Google Scholar] [CrossRef]
- Jiang, Z.; Xing, F.; Sun, Z.; Wang, P. Healing effectiveness of cracks rehabilitation in reinforced concrete using electrodeposition method. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2008, 23, 917–922. [Google Scholar] [CrossRef]
- Gupta, S. Comparison of improved autogenous and bio-based self-healing techniques in fiber-reinforced mortar: Effect of bacteria incorporation strategy and fiber hybridization. J. Build. Eng. 2022, 45, 103607. [Google Scholar] [CrossRef]
- Ali, M.; Mukhtar, H.; Dufossé, L. Microbial calcite induction: A magic that fortifies and heals concrete. Int. J. Environ. Sci. Technol. 2022, 20, 1113–1134. [Google Scholar] [CrossRef]
- Al-Salloum, Y.; Hadi, S.; Abbas, H.; Almusallam, T.; Moslem, M.A. Bio-induction and bioremediation of cementitious composites using microbial mineral precipitation—A review. Constr. Build. Mater. 2017, 154, 857–876. [Google Scholar] [CrossRef]
- Amidi, S.; Wang, J. Surface treatment of concrete bricks using calcium carbonate precipitation. Constr. Build. Mater. 2015, 80, 273–278. [Google Scholar] [CrossRef]
- De Muynck, W.; De Belie, N.; Verstraete, W. Microbial carbonate precipitation in construction materials: A review. Ecol. Eng. 2010, 36, 118–136. [Google Scholar] [CrossRef]
- Galano, S.; Calabrese, A.; Asvapathanagul, P.; Shadravan, S.; Ly, M.; Flores, D.; Hernandez, M.; Rahmani, M. Innovative Approaches to Enhancing Concrete Compressive Strength: An Extensive Investigation of Biochar-Embedded and Self-Repairing Techniques. J. Mater. Civ. Eng. 2025, 37, 04025112. [Google Scholar] [CrossRef]
- Intarasoontron, J.; Pungrasmi, W.; Nuaklong, P.; Jongvivatsakul, P.; Likitlersuang, S. Comparing performances of MICP bacterial vegetative cell and microencapsulated bacterial spore methods on concrete crack healing. Constr. Build. Mater. 2021, 302, 124227. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, L.; Wang, J.; Meng, Q.; Zhong, S.; Gao, Y.; Cui, X. Recent advances in polysaccharide-based self-healing hydrogels for biomedical applications. Carbohydr. Polym. 2022, 283, 119161. [Google Scholar] [CrossRef] [PubMed]
- Achal, V.; Mukherjee, A.; Reddy, M.S. Microbial Concrete: Way to Enhance the Durability of Building Structures. J. Mater. Civ. Eng. 2011, 23, 730–734. [Google Scholar] [CrossRef]
- Siddique, R.; Chahal, N.K. Effect of ureolytic bacteria on concrete properties. Constr. Build. Mater. 2011, 25, 3791–3801. [Google Scholar] [CrossRef]
- Šovljanski, O.; Tomić, A.; Markov, S. Relationship between Bacterial Contribution and Self-Healing Effect of Cement-Based Materials. Microorganisms 2022, 10, 1399. [Google Scholar] [CrossRef]
- Wang, J.Y.; Soens, H.; Verstraete, W.; De Belie, N. Self-healing concrete by use of microencapsulated bacterial spores. Cem. Concr. Res. 2014, 56, 139–152. [Google Scholar] [CrossRef]
- Ul Islam, S.; Waseem, S.A. Strength retrieval and microstructural characterization of Bacillus subtilis and Bacillus megaterium incorporated plain and reinforced concrete. Constr. Build. Mater. 2023, 404, 133331. [Google Scholar] [CrossRef]
- Šovljanski, O.; Pezo, L.; Grahovac, J.; Tomić, A.; Ranitović, A.; Cvetković, D.; Markov, S. Best-performing Bacillus strains for microbiologically induced CaCO3 precipitation: Screening of relative influence of operational and environmental factors. J. Biotechnol. 2022, 350, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Erşan, Y.Ç.; Belie, N.d.; Boon, N. Microbially induced CaCO3 precipitation through denitrification: An optimization study in minimal nutrient environment. Biochem. Eng. J. 2015, 101, 108–118. [Google Scholar] [CrossRef]
- Prajapati, P.; Rama Raju, P.J.; Garg, N.; Kumar, S.; Rai, N.; Das, S.K. An investigation the effect of bacterial healing on cement-based mortars. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Kliková, K.; Holeček, P.; Koňáková, D.; Stiborová, H.; Nežerka, V. Exploiting Bacillus pseudofirmus and Bacillus cohnii to promote CaCO3 and AFt phase formation for stabilizing waste concrete fines. Cem. Concr. Compos. 2025, 155, 105839. [Google Scholar] [CrossRef]
- Khaliq, W.; Ehsan, M.B. Crack healing in concrete using various bio influenced self-healing techniques. Constr. Build. Mater. 2016, 102, 349–357. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, Z.; Li, M.; Qian, C. Effects of carrier on the performance of bacteria-based self-healing concrete. Constr. Build. Mater. 2021, 305, 124771. [Google Scholar] [CrossRef]
- Luo, M.; Qian, C.; Li, R. Factors affecting crack repairing capacity of bacteria-based self-healing concrete. Constr. Build. Mater. 2015, 87, 1–7. [Google Scholar] [CrossRef]
- Wang, J.; Mignon, A.; Trenson, G.; Van Vlierberghe, S.; Boon, N.; De Belie, N. A chitosan based pH-responsive hydrogel for encapsulation of bacteria for self-sealing concrete. Cem. Concr. Compos. 2018, 93, 309–322. [Google Scholar] [CrossRef]
- Rauf, M.; Khaliq, W.; Khushnood, R.A.; Ahmed, I. Comparative performance of different bacteria immobilized in natural fibers for self-healing in concrete. Constr. Build. Mater. 2020, 258, 119578. [Google Scholar] [CrossRef]
- Wiktor, V.; Jonkers, H.M. Quantification of crack-healing in novel bacteria-based self-healing concrete. Cem. Concr. Compos. 2011, 33, 763–770. [Google Scholar] [CrossRef]
- Bhaskar, S.; Anwar Hossain, K.M.; Lachemi, M.; Wolfaardt, G.; Otini Kroukamp, M. Effect of self-healing on strength and durability of zeolite-immobilized bacterial cementitious mortar composites. Cem. Concr. Compos. 2017, 82, 23–33. [Google Scholar] [CrossRef]
- Wang, J.Y.; Belie, N.D.; Verstraete, W. Diatomaceous earth as a protective vehicle for bacteria applied for self-healing concrete. J. Ind. Microbiol. Biotechnol. 2012, 39, 567–577. [Google Scholar] [CrossRef]
- Alazhari, M.; Sharma, T.; Heath, A.; Cooper, R.; Paine, K. Application of expanded perlite encapsulated bacteria and growth media for self-healing concrete. Constr. Build. Mater. 2018, 160, 610–619. [Google Scholar] [CrossRef]
- Yan, Y.; Tian, H.; Liu, W.; Jia, G.; Li, Z.; Gao, Y.; Zhang, Y.; Ma, G. Study on frost resistance and life prediction of microbial self-healing concrete based on expanded perlite as carrier. J. Build. Eng. 2024, 91, 109693. [Google Scholar] [CrossRef]
- Cacchio, P.; Ercole, C.; Cappuccio, G.; Lepidi, A. Calcium Carbonate Precipitation by Bacterial Strains Isolated from a Limestone Cave and from a Loamy Soil. Geomicrobiol. J. 2003, 20, 85–98. [Google Scholar] [CrossRef]
- Novitsky, J.A. Calcium carbonate precipitation by marine bacteria. Geomicrobiol. J. 1981, 2, 375–388. [Google Scholar] [CrossRef]
- Liu, F.; Wang, J.; Qian, X.; Hollingsworth, J. Internal curing of high performance concrete using cenospheres. Cem. Concr. Res. 2017, 95, 39–46. [Google Scholar] [CrossRef]
- ASTM C128-22; Standard Test Method for Relative Density (Specific Gravity) and Absorption of Fine Aggregate. ASTM International: West Conshohocken, PA, USA, 2015.
- Wang, X.; Wang, W.; Li, Y.; Wang, L.; Duan, P.; Liu, Y. Relative humidity evolution in recycled aggregate concrete with glazed hollow beads internal curing: Self-desiccation and moisture diffusion. Constr. Build. Mater. 2025, 474, 141162. [Google Scholar] [CrossRef]
- Zheng, T.; Su, Y.; Qian, C.; Zhou, H. Low alkali sulpho-aluminate cement encapsulated microbial spores for self-healing cement-based materials. Biochem. Eng. J. 2020, 163, 107756. [Google Scholar] [CrossRef]
- Zhang, G.-Z.; Liu, C.; Cheng, P.-F.; Li, Z.; Han, Y.; Wang, X.-Y. Enhancing the interfacial compatibility and self-healing performance of microbial mortars by nano-SiO2-modified basalt fibers. Cem. Concr. Compos. 2024, 152, 105650. [Google Scholar] [CrossRef]
- Chindasiriphan, P.; Subwilai, N.; Intarasoontron, J.; Nuaklong, P.; Jongvivatsakul, P.; Chompoorat, T.; Pungrasmi, W.; Likitlersuang, S. Synergistic effects of microencapsulated bacterial spores and superabsorbent polymer on self-healing performance in mortar. Constr. Build. Mater. 2024, 414, 135005. [Google Scholar] [CrossRef]
- Zhang, G.-Z.; Tang, Q.; Liu, J. Paraffin encapsulation enhances self-healing ability and frost resistance of bacterial in mortars under extreme low-temperature conditions. Constr. Build. Mater. 2025, 470, 140625. [Google Scholar] [CrossRef]
- Vaezi, M.; Zareei, S.A.; Jahadi, M. Recycled microbial mortar: Effects of bacterial concentration and calcium lactate content. Constr. Build. Mater. 2020, 234, 117349. [Google Scholar] [CrossRef]
- ASTM C109/C109M-23; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50 mm] Cube Specimens). ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM C293/C293M-16; Standard Test Method for Flexual Strength of Concrete (Using Simple Beam with Center-Point Loading) 1. ASTM International: West Conshohocken, PA, USA, 2016.
- Chu, H.; Wang, Q.; Zhang, W. Optimizing ecological ultra-high performance concrete prepared with incineration bottom ash: Utilization of Al2O3 micro powder for improved mechanical properties and durability. Constr. Build. Mater. 2024, 426, 136152. [Google Scholar] [CrossRef]
- Mohammadyan-Yasouj, S.E.; Heidari, N.; Shokravi, H. Influence of waste alumina powder on self-compacting concrete resistance under elevated temperature. J. Build. Eng. 2021, 41, 102360. [Google Scholar] [CrossRef]
- Ji, X.; Liu, M.; Yang, J.; Feng, F. Meta-analysis of the impact of freeze–thaw cycles on soil microbial diversity and C and N dynamics. Soil Biol. Biochem. 2022, 168, 108608. [Google Scholar] [CrossRef]
- Yang, Z.; Miao, C.; Deng, W.; Liu, D.; Zhang, J.; Gu, J. Multi-scale characterization of SAP impact on self-healing behavior in UHPC under varied crack widths and environments. Constr. Build. Mater. 2024, 421, 135649. [Google Scholar] [CrossRef]
- Zhao, J.; Fan, C.; Huang, G.; Guo, Y.; Arachchilage, C.B.; Gupta, R.; Liu, W.V. Machine learning-assisted characterization of the thermal conductivity of cement-based grouts for borehole heat exchangers. Constr. Build. Mater. 2024, 449, 138506. [Google Scholar] [CrossRef]
- Li, L.; Liu, Z.; Liu, W.; Wang, Z.; Zhou, C.; Zeng, Q. Microstructure and transport properties of cement mortar made with recycled fine ceramic aggregates. Dev. Built Environ. 2025, 22, 100643. [Google Scholar] [CrossRef]
- Bohner, M.; Gasser, B.; Baroud, G.; Heini, P. Theoretical and experimental model to describe the injection of a polymethylmethacrylate cement into a porous structure. Biomaterials 2003, 24, 2721–2730. [Google Scholar] [CrossRef]
- Panditharadhya, B.J.; Mulangi, R.H.; Ravi Shankar, A.U. Mechanical properties of pavement quality concrete with aluminium industry waste as a binder. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Kawasaki, N.; Ogata, F.; Tominaga, H. Selective adsorption behavior of phosphate onto aluminum hydroxide gel. J. Hazard. Mater. 2010, 181, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Su, Y.; Liu, S.; Zhang, X. Roles of bacteria and extracellular polymeric substance in calcium carbonate formation: Insights from the effects of calcium source and deposition rate on nucleation. Biochem. Eng. J. 2024, 202, 109160. [Google Scholar] [CrossRef]





| Time (min) | 1 | 5 | 10 | 20 | 30 | 60 | 120 | 720 | 1440 | |
| Water absorption rate (%) | Modified | 2 | 5.1 | 13.4 | 22.1 | 35.0 | 42.6 | 47.3 | 47.3 | 47.3 |
| Unmodified | 4 | 8.2 | 20.5 | 46.7 | 56.6 | 68.7 | 70.2 | 70.2 | 70.2 | |
| Component | Peptone (Biochemistry) | Beef Extract | NaCl | Deionized Water | MnSO4 |
|---|---|---|---|---|---|
| Content | 3 g/L | 3 g/L | 15 g/L | 1 L | 0.005 g/L |
| Material | Composition (wt%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CaO | SiO2 | Al2O3 | SO3 | Fe2O3 | MgO | K2O | Others | Loss on Ignition | |
| Cement | 65.10 | 19.52 | 4.37 | 3.21 | 3.22 | 1.01 | 0.86 | 0.80 | 1.91 |
| Group | Cement/g | Fine Aggregates | Water/g | Bacteria/mL | Calcium Lactate/g | |
|---|---|---|---|---|---|---|
| Standard Sand/g | Modified Alumina Hollow Spheres/g | |||||
| Control | 1000 | 2000 | - | 500 | - | - |
| B | 2000 | - | 460 | 40 | 10 | |
| AB | 1940 | 60 | 460 | 40 | 10 | |
| Test Temperature °C | Flash Points | Control Group | AB Group | ||||
|---|---|---|---|---|---|---|---|
| Thermal Diffusion Coefficient mm2/s | Thermal Conductivity W/(m·K) | Specific Heat Capacity Cp.J/(g·K) | Thermal Diffusion Coefficient mm2/s | Thermal Conductivity W/(m·K) | Specific Heat Capacity Cp.J/(g·K) | ||
| −20 °C | Point 1 | 1.841 | 2.816 | 0.764 | 1.592 | 2.407 | 0.757 |
| Point 2 | 1.851 | 2.829 | 0.769 | 1.582 | 2.392 | 0.753 | |
| Point 3 | 1.834 | 2.805 | 0.760 | 1.588 | 2.401 | 0.758 | |
| Average value | 1.842 | 2.817 | 0.764 | 1.587 | 2.400 | 0.756 | |
| 25 °C | Point 1 | 1.448 | 2.607 | 0.914 | 1.278 | 2.293 | 0.905 |
| Point 2 | 1.458 | 2.625 | 0.901 | 1.288 | 2.311 | 0.899 | |
| Point 3 | 1.456 | 2.622 | 0.885 | 1.292 | 2.318 | 0.888 | |
| Average value | 1.454 | 2.618 | 0.900 | 1.286 | 2.307 | 0.897 | |
| Groups | Healing Age | |||
|---|---|---|---|---|
| 0d | 7d | 28d | 56d | |
| NO-Control | 1.244 | 1.131 | 1.100 | 1.02 |
| NO-B | 1.921 | 1.226 | 0 | 0 |
| NO-AB | 1.465 | 0 | 0 | 0 |
| NE-Control | 2.152 | 1.974 | 1.956 | 1.955 |
| NE-B | 1.243 | 0.882 | 0.698 | 0.479 |
| NE-AB | 1.398 | 0.798 | 0 | 0 |
| Crack Width/(μm) | NO-Control | NO-B | NO-AB | NE-Control | NE-B | NE-AB | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0d | 90d | 0d | 90d | 0d | 90d | 0d | 90d | 0d | 90d | 0d | 90d | |
| <400 | 386.23 | 314.78 | 371.38 | 0 | 366.12 | 0 | 389.10 | 335.21 | 398.91 | 184.93 | 368.05 | 0 |
| 400–450 | 420.28 | 371.32 | 431.04 | 0 | 429.33 | 0 | 441.01 | 405.73 | 410.72 | 228.69 | 435.28 | 0 |
| 450–500 | 472.98 | 442.05 | 459.66 | 0 | 471.87 | 0 | 462.93 | 448.07 | 482.05 | 324.66 | 490.17 | 33.14 |
| >500 | 535.68 | 524.97 | 563.17 | 65.79 | 522.06 | 9.76 | 584.28 | 581.12 | 598.97 | 447.25 | 576.25 | 61.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-S.; Zhou, Y.-Z.; Wang, X.-D.; Zhang, G.-Z. Enhancement of Bacterial Survival and Self-Healing Performance in Mortars After Exposure to Negative Temperature Using Alumina Hollow Spheres as Bacterial Carriers. Materials 2025, 18, 2245. https://doi.org/10.3390/ma18102245
Wang Y-S, Zhou Y-Z, Wang X-D, Zhang G-Z. Enhancement of Bacterial Survival and Self-Healing Performance in Mortars After Exposure to Negative Temperature Using Alumina Hollow Spheres as Bacterial Carriers. Materials. 2025; 18(10):2245. https://doi.org/10.3390/ma18102245
Chicago/Turabian StyleWang, Yan-Sheng, Yi-Ze Zhou, Xu-Dong Wang, and Guang-Zhu Zhang. 2025. "Enhancement of Bacterial Survival and Self-Healing Performance in Mortars After Exposure to Negative Temperature Using Alumina Hollow Spheres as Bacterial Carriers" Materials 18, no. 10: 2245. https://doi.org/10.3390/ma18102245
APA StyleWang, Y.-S., Zhou, Y.-Z., Wang, X.-D., & Zhang, G.-Z. (2025). Enhancement of Bacterial Survival and Self-Healing Performance in Mortars After Exposure to Negative Temperature Using Alumina Hollow Spheres as Bacterial Carriers. Materials, 18(10), 2245. https://doi.org/10.3390/ma18102245






