Tailoring the Molecular Weight of APEG-Based Polycarboxylate Superplasticizers: Mechanistic Insights into the Workability and Compressive Strength of Alkali-Activated Circulating Fluidized Bed Fly Ash Materials
Abstract
1. Introduction
2. Materials and Experimental Methods
2.1. Raw Materials
2.2. Preparation of Samples
2.2.1. Preparation of Polycarboxylate Superplasticizers
2.2.2. Preparation of Alkali-Activated Materials
- Preparation of the blank sample: A certain amount of CFBFA and aged activators were added to a mixing bowl and stirred for 2 min to obtain a paste. The paste was then poured into a cylindrical mold (Φ25 × 50 mm) and placed on a vibrating table to eliminate air bubbles. After smoothing the top surface, the mold was transferred to a constant temperature and humidity chamber maintained at 20 ± 2 °C with a relative humidity greater than 90% for curing. After 24 h, the samples were demolded and returned to the curing chamber until the desired age (1 d, 3 d, 7 d, and 28 d) for compressive strength testing.
- Preparation of samples with PCE: The alkali activator and 2% PCE (by solid content) were thoroughly mixed and added to the mixing bowl along with CFBFA. The mixture was stirred for 2 min to achieve a homogeneous paste. Subsequent steps followed the procedure outlined in step (1).
2.3. Analytical Methods
2.4. Statistical Analysis
3. Results and Discussion
3.1. The Characteristics of Synthesized PCEs
3.2. Effect of PCEs on the Workability of Materials
3.3. Effect of PCEs on the Compressive Strength of Materials
3.3.1. Molecular Structural Changes
3.3.2. Mineralogical Structural Changes
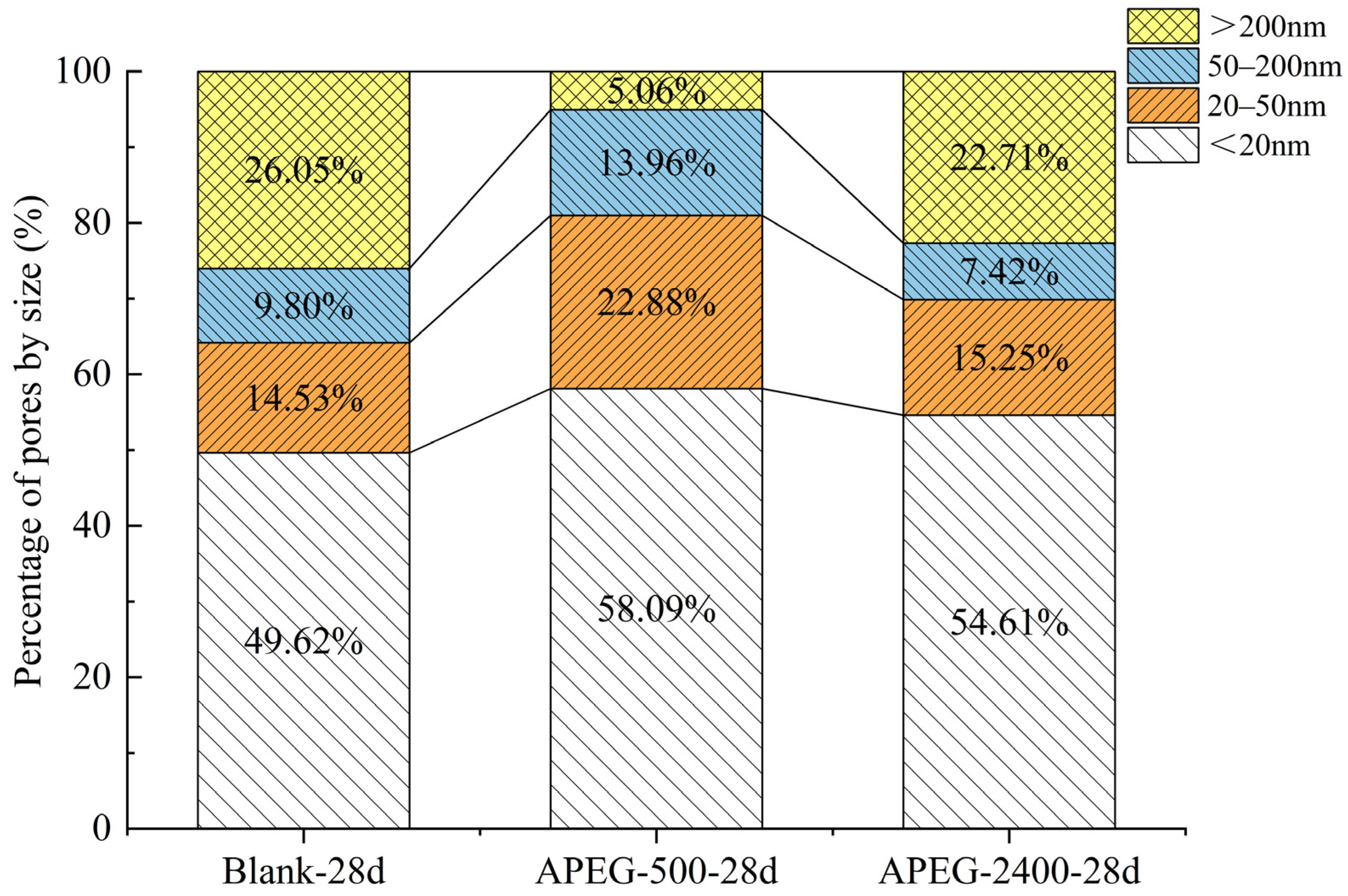
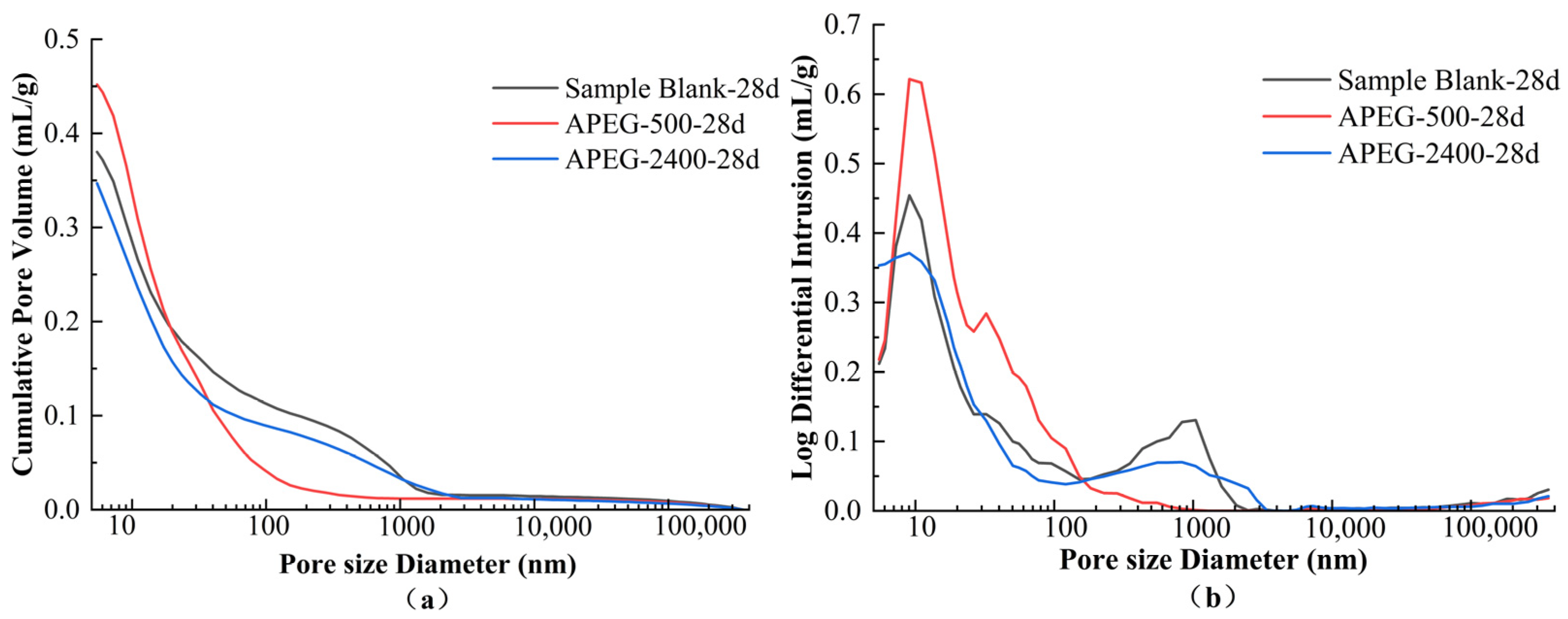
3.3.3. Pore Structural Changes
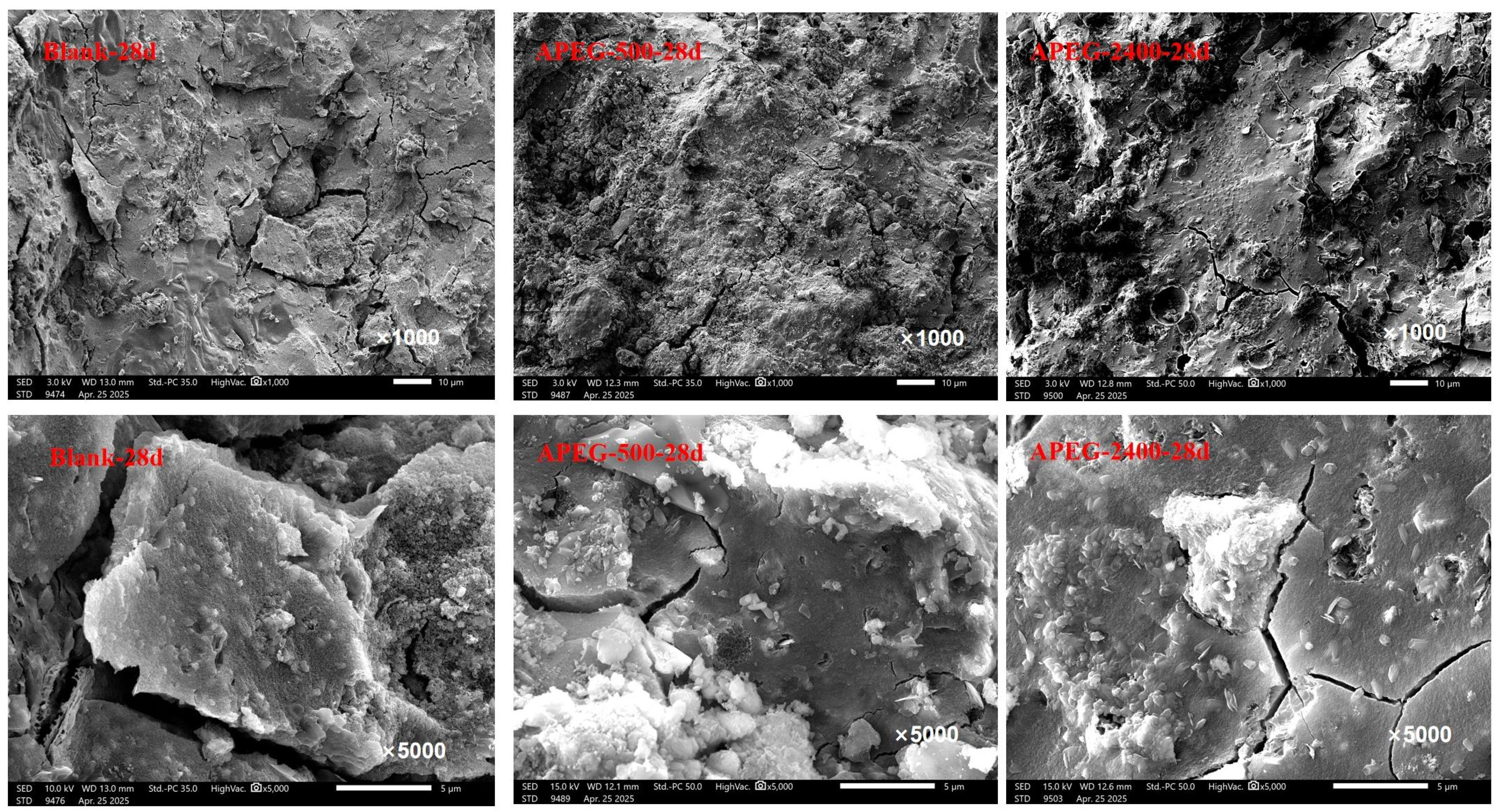
3.3.4. Microscopic Morphological Changes
3.4. Application of PCEs in CFBFA-Based Cementitious Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IPCC. Sections. Climate Change 2023: Synthesis Report. In Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023; pp. 35–115. [Google Scholar] [CrossRef]
- Global Status Report for Buildings and Construction 2024/2025: Not just Another Brick in the Wall—The Solutions Exist. Scaling Them Will Build on Progress and Cut Emissions Fast, United Nations Environment Programme. 2025. Available online: https://wedocs.unep.org/20.500.11822/47214 (accessed on 28 April 2025).
- Wang, C.M.; Kayali, O.; Liow, J.L.; Troitzsch, U. Participation and disturbance of superplasticisers in early-stage reaction of class F fly ash-based geopolymer. Constr. Build. Mater. 2023, 403, 133176. [Google Scholar] [CrossRef]
- Melichar, J.; Cerny, V.; Meszarosova, L.; Figala, P.; Dufka, A.; Baranek, S.; Drochytka, R. Study of synergistic effect of fly ash and superabsorbent polymers on properties of cement pastes. J. Build. Eng. 2023, 79, 107897. [Google Scholar] [CrossRef]
- Li, J.; Ma, Z.B.; Gao, J.M.; Guo, Y.X.; Cheng, F.Q. Synthesis and characterization of geopolymer prepared from circulating fluidized bed-derived fly ash. Ceram. Int. 2022, 48, 11820–11829. [Google Scholar] [CrossRef]
- Rakngan, W.; Williamson, T.; Ferron, R.D.; Sant, G.; Juenger, M.C.G. Controlling workability in alkali-activated Class C fly ash. Constr. Build. Mater. 2018, 183, 226–233. [Google Scholar] [CrossRef]
- Puertas, F.; Varga, C.; Alonso, M.M. Rheology of alkali-activated slag pastes. Effect of the nature and concentration of the activating solution. Cem. Concr. Compos. 2014, 53, 279–288. [Google Scholar] [CrossRef]
- Ma, R.; Wang, Y.B.; Li, H.; Bai, Y. Progress in the polycarboxylate superplasticizer and their structure-activity relationship—A review. Mater. Today Commun. 2023, 35, 105838. [Google Scholar] [CrossRef]
- Sha, S.N.; Wang, M.; Shi, C.J.; Xiao, Y.C. Influence of the structures of polycarboxylate superplasticizer on its performance in cement-based materials-A review. Constr. Build. Mater. 2020, 233, 117257. [Google Scholar] [CrossRef]
- Winnefeld, F.; Becker, S.; Pakusch, J.; Götz, T. Effects of the molecular architecture of comb-shaped superplasticizers on their performance in cementitious systems. Cem. Concr. Compos. 2007, 29, 251–262. [Google Scholar] [CrossRef]
- Lei, L.; Chan, H.K. Investigation into the molecular design and plasticizing effectiveness of HPEG-based polycarboxylate superplasticizers in alkali-activated slag. Cem. Concr. Res. 2020, 136, 106150. [Google Scholar] [CrossRef]
- Xie, J.T.; Kayali, O. Effect of superplasticiser on workability enhancement of Class F and Class C fly ash-based geopolymers. Constr. Build. Mater. 2016, 122, 36–42. [Google Scholar] [CrossRef]
- Fan, Z.R.; Kong, L.J.; Lu, J.T.; Wang, X.B. Mechanism study of effect of superplasticizers on the fluidity of alkali-activated materials. Mater. Struct. 2023, 56, 29. [Google Scholar] [CrossRef]
- Conte, T.; Plank, J. Impact of molecular structure and composition of polycarboxylate comb polymers on the flow properties of alkali-activated slag. Cem. Concr. Res. 2019, 116, 95–101. [Google Scholar] [CrossRef]
- Jang, J.G.; Lee, N.K.; Lee, H.K. Fresh and hardened properties of alkali-activated fly ash/slag pastes with superplasticizers. Constr. Build. Mater. 2014, 50, 169–176. [Google Scholar] [CrossRef]
- Alrefaei, Y.; Wang, Y.S.; Dai, J.G. The effectiveness of different superplasticizers in ambient cured one-part alkali activated pastes. Cem. Concr. Compos. 2019, 97, 166–174. [Google Scholar] [CrossRef]
- Nematollahi, B.; Sanjayan, J. Effect of different superplasticizers and activator combinations on workability and strength of fly ash based geopolymer. Mater. Des. 2014, 57, 667–672. [Google Scholar] [CrossRef]
- Carabba, L.; Manzi, S.; Bignozzi, M.C. Superplasticizer Addition to Carbon Fly Ash Geopolymers Activated at Room Temperature. Materials 2016, 9, 586. [Google Scholar] [CrossRef]
- Keulen, A.; Yu, Q.L.; Zhang, S.; Grünewald, S. Effect of admixture on the pore structure refinement and enhanced performance of alkali-activated fly ash-slag concrete. Constr. Build. Mater. 2018, 162, 27–36. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, Y.; Du, W.; Li, M.; Wang, Y.; Zhang, C.; Shi, M.; Xue, B. Influence of Polycarboxylate Superplasticizer on the Properties of Cement-Fly Ash Cementitious Materials and Concrete. Sustainability 2022, 14, 13440. [Google Scholar] [CrossRef]
- Magazzù, A.; Marcuello, C. Investigation of Soft Matter Nanomechanics by Atomic Force Microscopy and Optical Tweezers: A Comprehensive Review. Nanomaterials 2023, 13, 963. [Google Scholar] [CrossRef]
- GB/T 176-2017; Methods for Chemical Analysis of Cement. Chinese Standard: Beijing, China, 2017.
- GB/T 8077-2023; Methods for Testing Uniformity of Concrete Admixtures. Chinese Standard: Beijing, China, 2012.
- GB/T 17671-2021; Test Method of Cement Mortar Strength (ISO Method). Chinese Standard: Beijing, China, 2021.
- Li, S.; Yu, Q.J.; Wei, J.X.; Ji, Y.J. Effects of Molecular Mass and Its Distribution on Adsorption Behavior of Polycarboxylate Water Reducers. J. Chin. Ceram. Soc. 2011, 39, 7. [Google Scholar] [CrossRef]
- Ng, P.G.; Cheah, C.B.; Ng, E.P.; Oo, C.W.; Leow, K.H. The influence of main and side chain densities of PCE superplasticizer on engineering properties and microstructure development of slag and fly ash ternary blended cement concrete. Constr. Build. Mater. 2020, 242, 118103. [Google Scholar] [CrossRef]
- Moeinian, M.; Ardjmand, M.; Nosratinia, F. Evaluating the operational properties of concrete admixtures containing molecularly modified polycarboxylate superplasticizers. Sci. Rep. 2024, 14, 20170. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.C.; Shi, W.; Xiang, S.C.; Yang, X.; Yuan, M.; Zhou, H.; Yu, H.T.; Zheng, T.X.; Zhang, J.K.; Jiang, Z.; et al. Synthesis and Modification of Polycarboxylate Superplasticizers-A Review. Materials 2024, 17, 1092. [Google Scholar] [CrossRef] [PubMed]
- Janardhan, R.; Gedam, P.H.; Sampathkumaran, P.S. The effect of polymer molecular weight in the adsorption process. J. Colloid Interface Sci. 1990, 140, 391–400. [Google Scholar] [CrossRef]
- Li, R.; Chen, W.C.; Lei, L.; Plank, J. Dispersing Efficacy of Tailored IPEG PCEs in AAS Binders: Elucidating the Impact of PCE Molecular Weight. Ind. Eng. Chem. Res. 2023, 62, 1776–1787. [Google Scholar] [CrossRef]
- Palacios, M.; Puertas, F. Effect of superplasticizer and shrinkage-reducing admixtures on alkali-activated slag pastes and mortars. Cem. Concr. Res. 2005, 35, 1358–1367. [Google Scholar] [CrossRef]
- Palacios, M.; Houst, Y.F.; Bowen, P.; Puertas, F. Adsorption of superplasticizer admixtures on alkali-activated slag pastes. Cem. Concr. Res. 2009, 39, 670–677. [Google Scholar] [CrossRef]
- Liang, D.C.; Yang, G.M.; Zhang, X.L.; Liu, D.Q.; Liu, J.C.; Xie, Q. Synthesis of geopolymer from fly ash and optimization of process parameters by neural network. J. China Coal Soc. 2021, 46, 1194–1202. [Google Scholar] [CrossRef]
- Hamidi, R.M.; Man, Z.; Azizli, K.A. Concentration of NaOH and the Effect on the Properties of Fly Ash Based Geopolymer. Procedia Eng. 2016, 148, 189–193. [Google Scholar] [CrossRef]
- Koshy, N.; Dondrob, K.; Hu, L.; Wen, Q.; Meegoda, J.N. Synthesis and characterization of geopolymers derived from coal gangue, fly ash and red mud. Constr. Build. Mater. 2019, 206, 287–296. [Google Scholar] [CrossRef]
- Idrees, M.; Ameen, A.; Shi, J.; Saeed, F.; Gencel, O. Preparation and performance optimization of eco-friendly geopolymers prepared from coarser lignite-based waste fly ash. Constr. Build. Mater. 2023, 391, 131804. [Google Scholar] [CrossRef]
- Sha, S.N.; Shi, C.J.; Xiang, S.C.; Jiao, D.W. The State-of-the-art Synthesis Techniques of Polycarboxylate Superplasticizer. Mater. Rep. 2019, 33, 558–568. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Y. Microstructure Evaluation of Fly Ash Geopolymers Alkali-Activated by Binary Composite Activators. Minerals 2023, 13, 910. [Google Scholar] [CrossRef]
- Wu, Z. Discussion on recent development directions of concrete science and technology. J. Chin. Ceram. Soc. 1979, 7, 262–270. [Google Scholar]
- Zhang, R.; Li, F.; Zhou, S.; Hou, Y. Carbon nanofiber dispersion in alkali solution and its reinforcement of alkali-activated volcanic ash-based geopolymers. J. Clean. Prod. 2023, 405, 137021. [Google Scholar] [CrossRef]
- Zhang, Y.; Chan, H.-K.; Han, Z.; Lei, L. Why do conventional MAA-MPEG PCEs not work in alkali-activated slag systems? Cem. Concr. Res. 2024, 184, 107599. [Google Scholar] [CrossRef]
- Partschefeld, S.; Tutal, A.; Halmanseder, T.; Schneider, J.; Osburg, A. Investigations on Stability of Polycarboxylate Superplasticizers in Alkaline Activators for Geopolymer Binders. Materials 2023, 16, 5369. [Google Scholar] [CrossRef]
- Lei, L.; Hirata, T.; Plank, J. 40 years of PCE superplasticizers—History, current state-of-the-art and an outlook. Cem. Concr. Res. 2022, 157, 106826. [Google Scholar] [CrossRef]
- Wang, X.; Li, D.; Bai, R.; Liu, S.; Yan, C.; Zhang, J. Evolution of the pore structure of pumice aggregate concrete and the effect on compressive strength. Rev. Adv. Mater. Sci. 2023, 62, 20230112. [Google Scholar] [CrossRef]
- Sun, J.; Hou, S.; Guo, Y.; He, W.; Bao, J.; Cui, Y.; Zhang, P. Sustainable utilization of alkali-activated steel slag material: Effects of silicate modulus and GBFS on fresh, mechanical and pore structure properties. Dev. Built Environ. 2024, 18, 100410. [Google Scholar] [CrossRef]
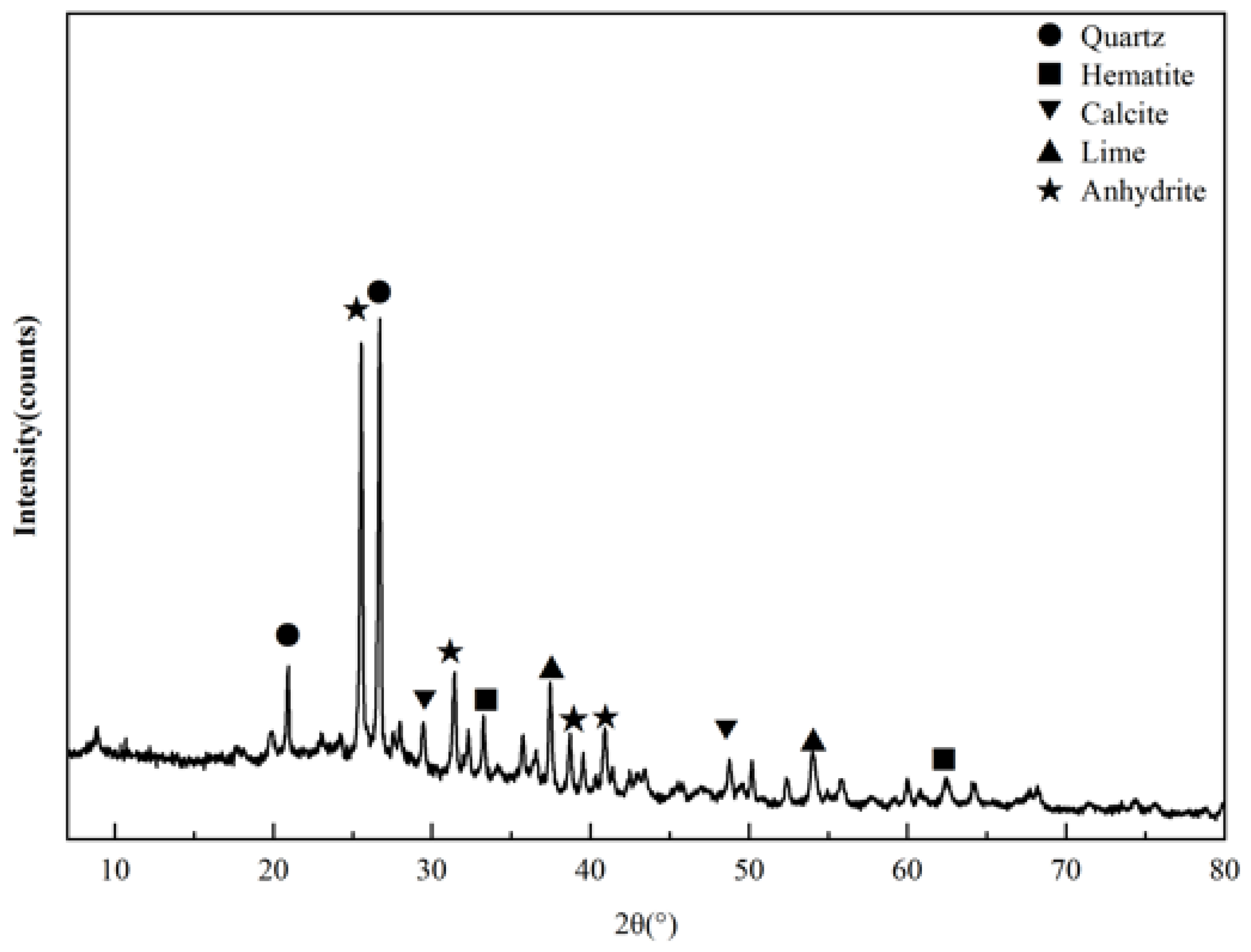


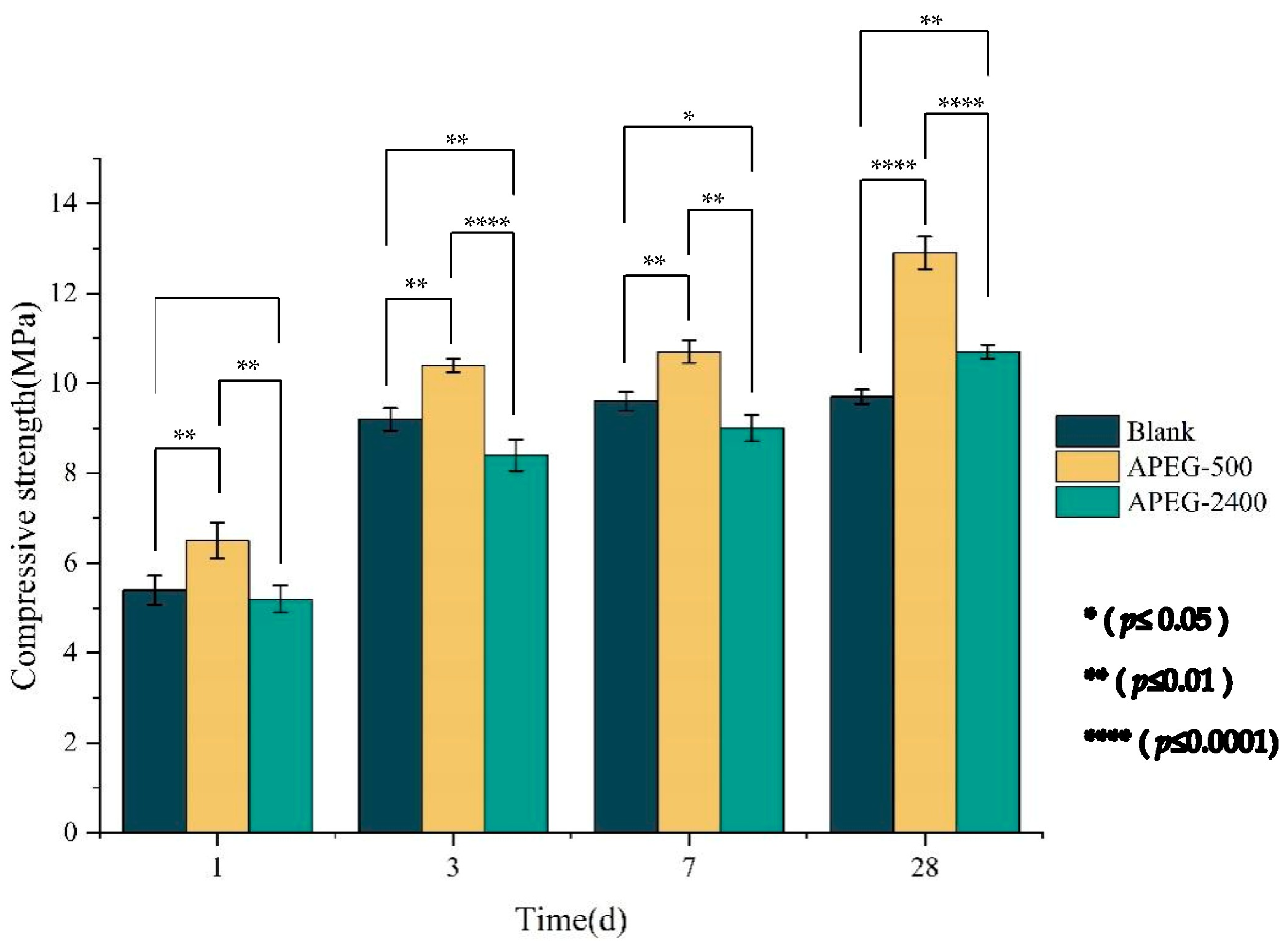
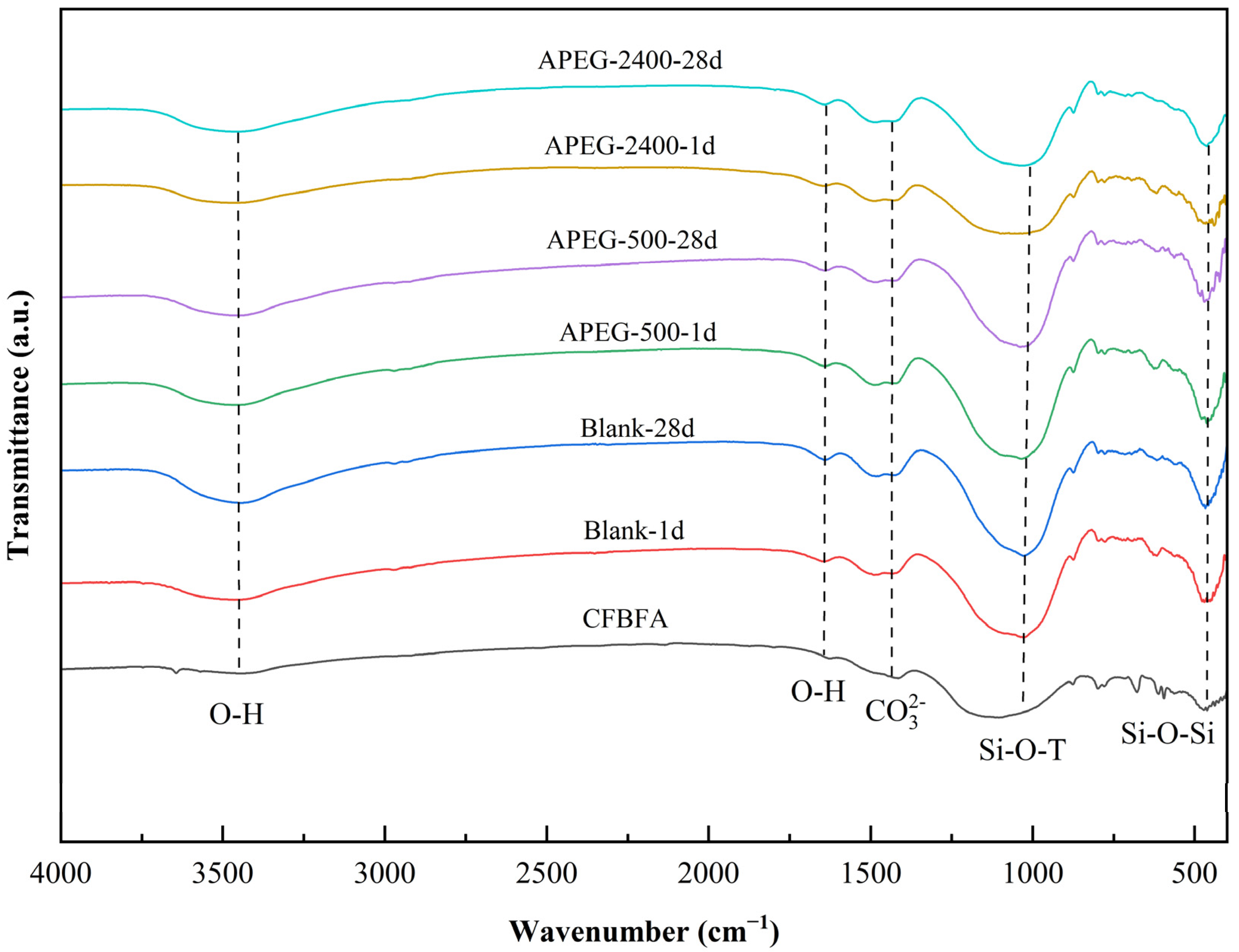
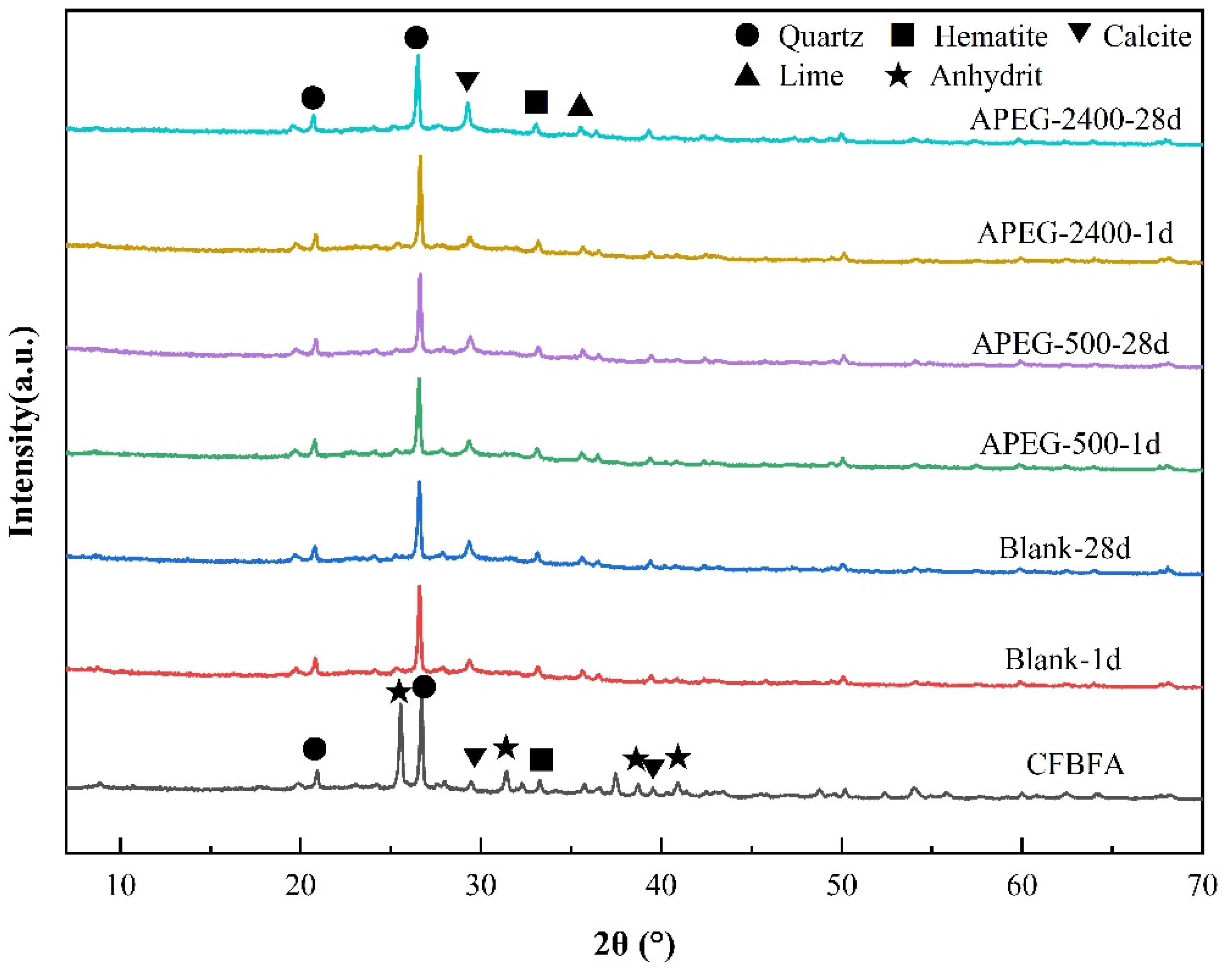
| Chemical | SiO2 | Al2O3 | CaO | Fe2O3 | SO3 | MgO | K2O | Na2O | LOI |
|---|---|---|---|---|---|---|---|---|---|
| Mass fraction/% | 38.85 | 23.11 | 15.82 | 4.94 | 6.44 | 1.39 | 0.31 | 0.44 | 3.92 |
| Tests | Fluidity of Paste (mm) |
|---|---|
| CFBFA + water | 72 |
| CFBFA + water + APEG-500 | 205 |
| CFBFA + water + APEG-2400 | 262 |
| CFBFA + water +APEG-500 + composite alkali activator | Set after 72 s |
| CFBFA + water + APEG-2400 + composite alkali activator | Set after 70 s |
| Sample | Porosity/% | Average Pore Size/nm | Pore Size Distribution/% | |||
|---|---|---|---|---|---|---|
| <20 nm | 20–50 nm | 50–200 nm | >200 nm | |||
| Blank-28d | 44.23 | 17.36 | 49.62 | 14.53 | 9.80 | 26.05 |
| APEG-500-28d | 48.75 | 15.09 | 58.09 | 22.88 | 13.96 | 5.06 |
| APEG-2400-28d | 42.58 | 15.67 | 54.61 | 15.25 | 7.42 | 22.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Yan, T.; Chen, C.; Qiao, X.; Yuan, J. Tailoring the Molecular Weight of APEG-Based Polycarboxylate Superplasticizers: Mechanistic Insights into the Workability and Compressive Strength of Alkali-Activated Circulating Fluidized Bed Fly Ash Materials. Materials 2025, 18, 2239. https://doi.org/10.3390/ma18102239
Li X, Yan T, Chen C, Qiao X, Yuan J. Tailoring the Molecular Weight of APEG-Based Polycarboxylate Superplasticizers: Mechanistic Insights into the Workability and Compressive Strength of Alkali-Activated Circulating Fluidized Bed Fly Ash Materials. Materials. 2025; 18(10):2239. https://doi.org/10.3390/ma18102239
Chicago/Turabian StyleLi, Xiaojiao, Tong Yan, Chuanlong Chen, Xiuchen Qiao, and Jin Yuan. 2025. "Tailoring the Molecular Weight of APEG-Based Polycarboxylate Superplasticizers: Mechanistic Insights into the Workability and Compressive Strength of Alkali-Activated Circulating Fluidized Bed Fly Ash Materials" Materials 18, no. 10: 2239. https://doi.org/10.3390/ma18102239
APA StyleLi, X., Yan, T., Chen, C., Qiao, X., & Yuan, J. (2025). Tailoring the Molecular Weight of APEG-Based Polycarboxylate Superplasticizers: Mechanistic Insights into the Workability and Compressive Strength of Alkali-Activated Circulating Fluidized Bed Fly Ash Materials. Materials, 18(10), 2239. https://doi.org/10.3390/ma18102239







