Effect of Magnesium and Temperature on the Accelerated Carbonation Progress of β-Dicalcium Silicate
Abstract
1. Introduction
2. Accelerated Carbonation of β-C2S
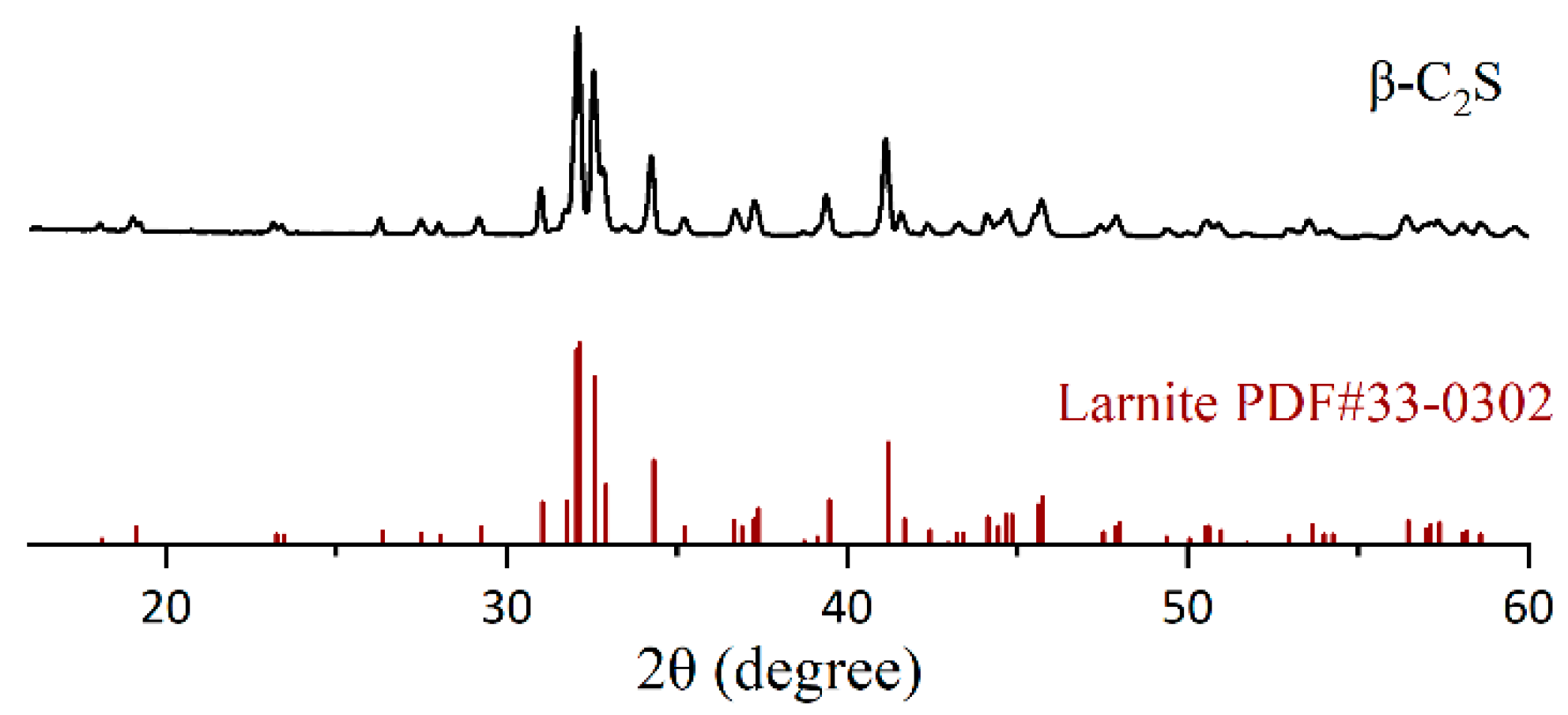
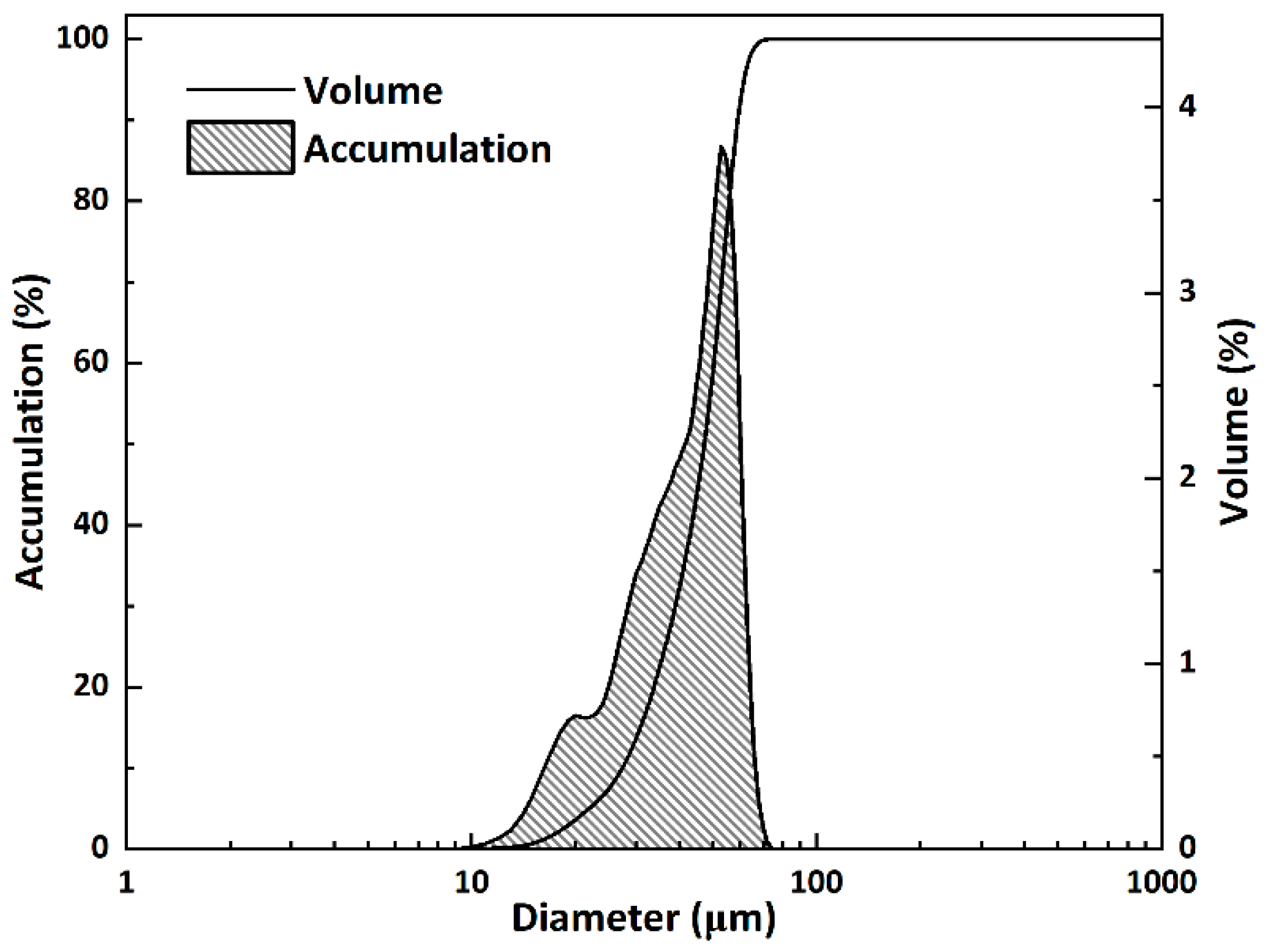
2.1. Synthesis of β-C2S
2.2. Characterization Method
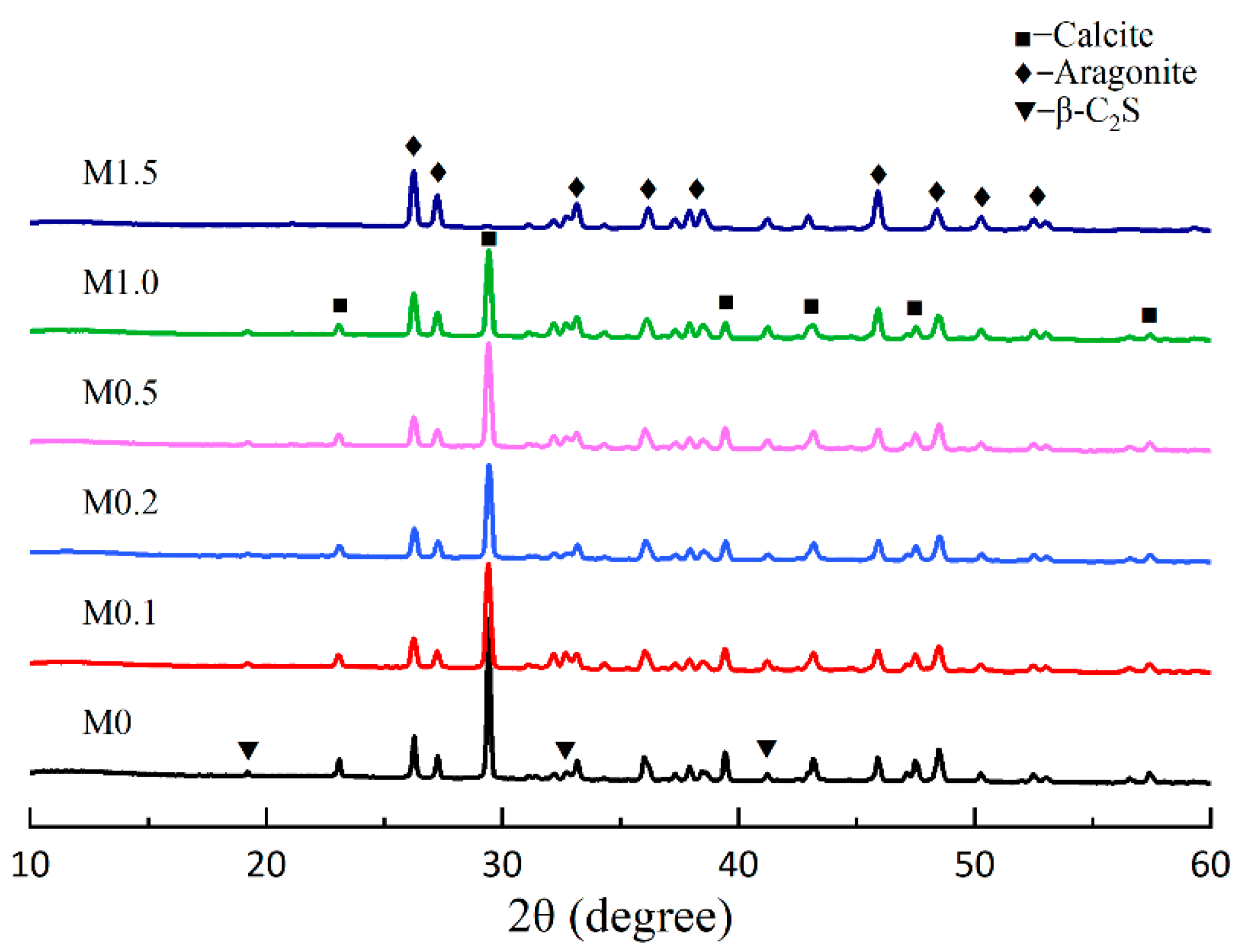
2.2.1. X-Ray Diffraction (XRD)
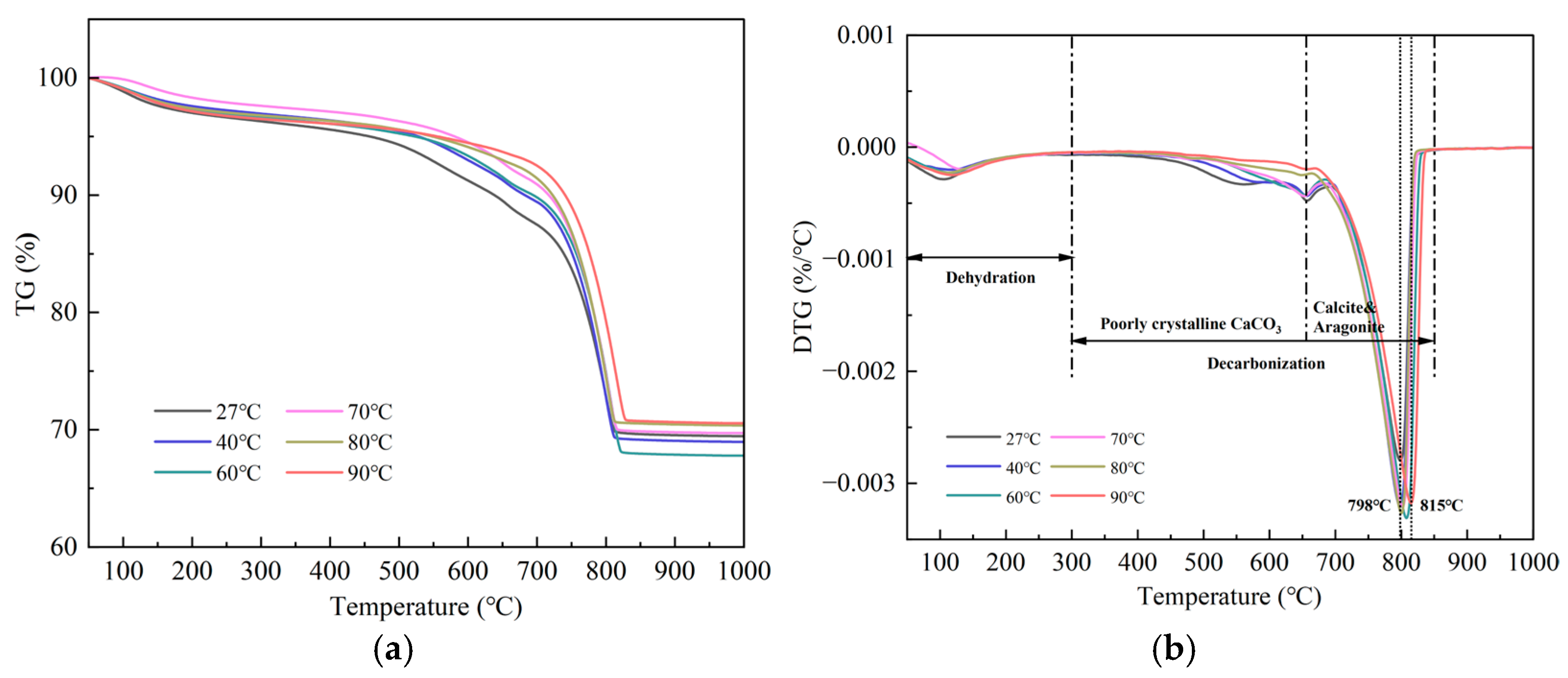
2.2.2. Thermogravimetric Analyzer (TGA)
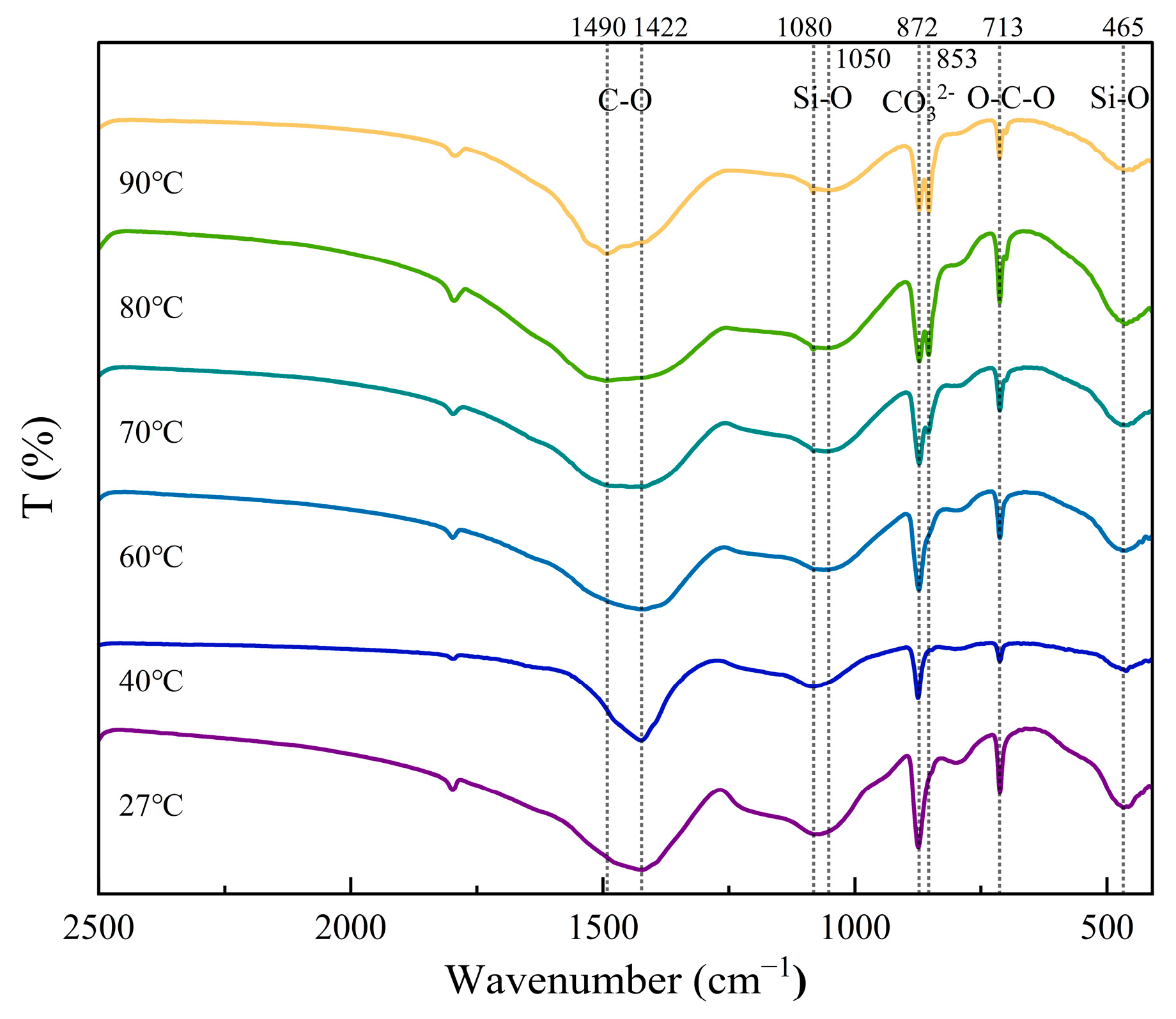
2.2.3. Fourier Transform Infrared Spectroscopy (FT-IR)
2.2.4. Field Emission Scanning Electron Microscopy (FE-SEM)
2.2.5. Inductively Coupled Plasma–Optical Emission Spectrometry (ICP-OES)
3. Results and Discussion
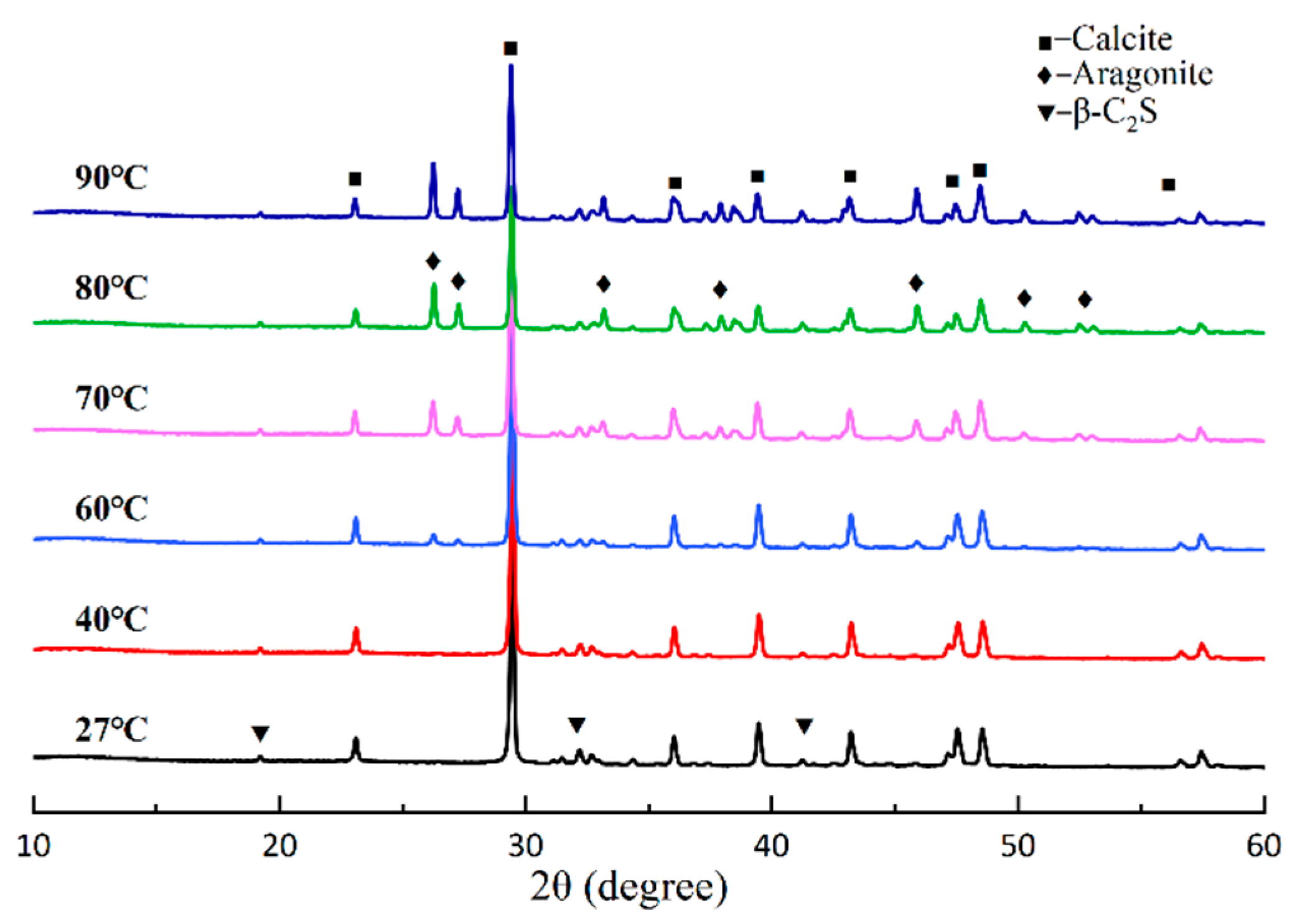
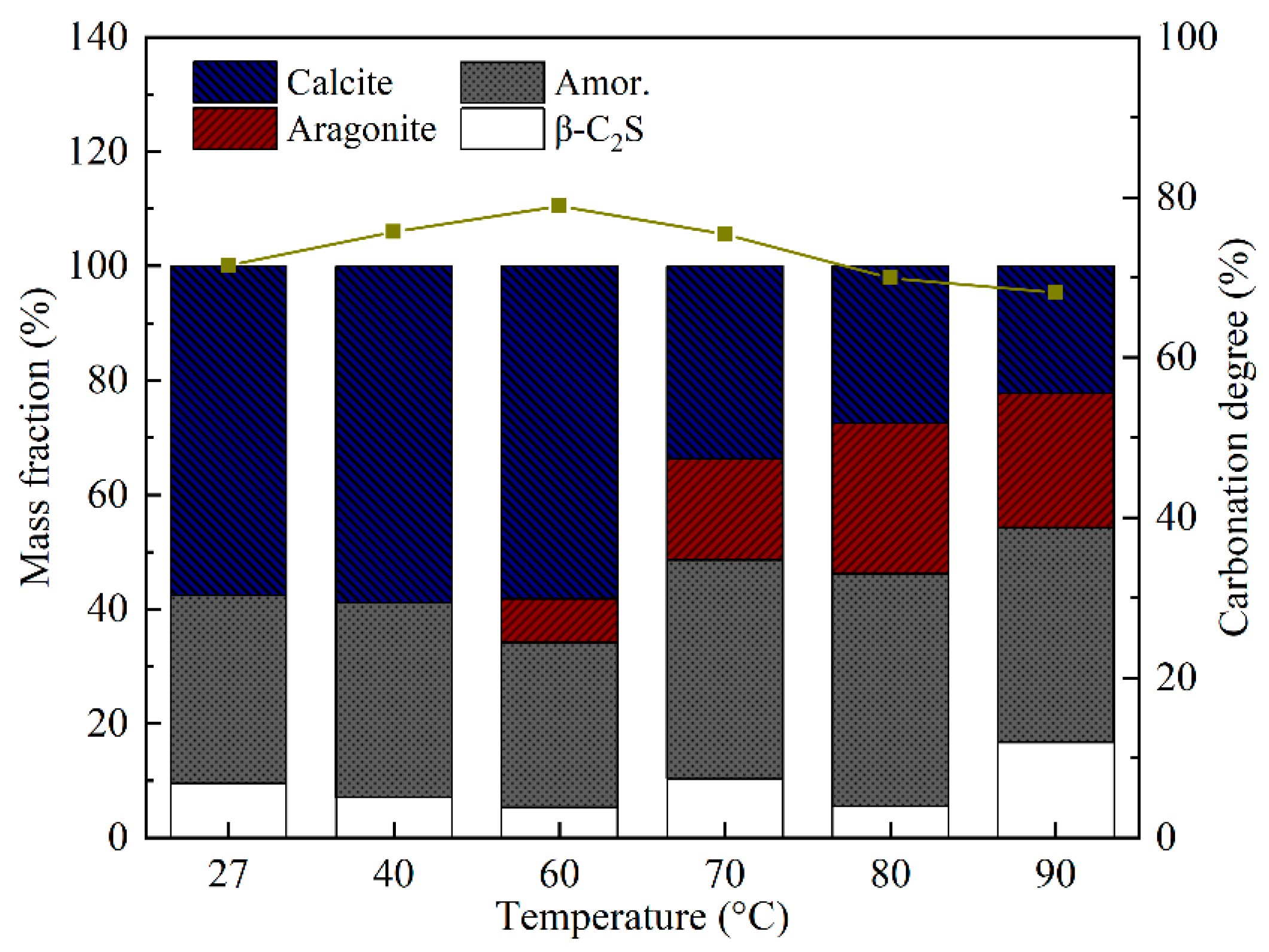
3.1. Effect of Temperature on the Carbonation Process of β-C2S
3.1.1. Development of Carbonation Products
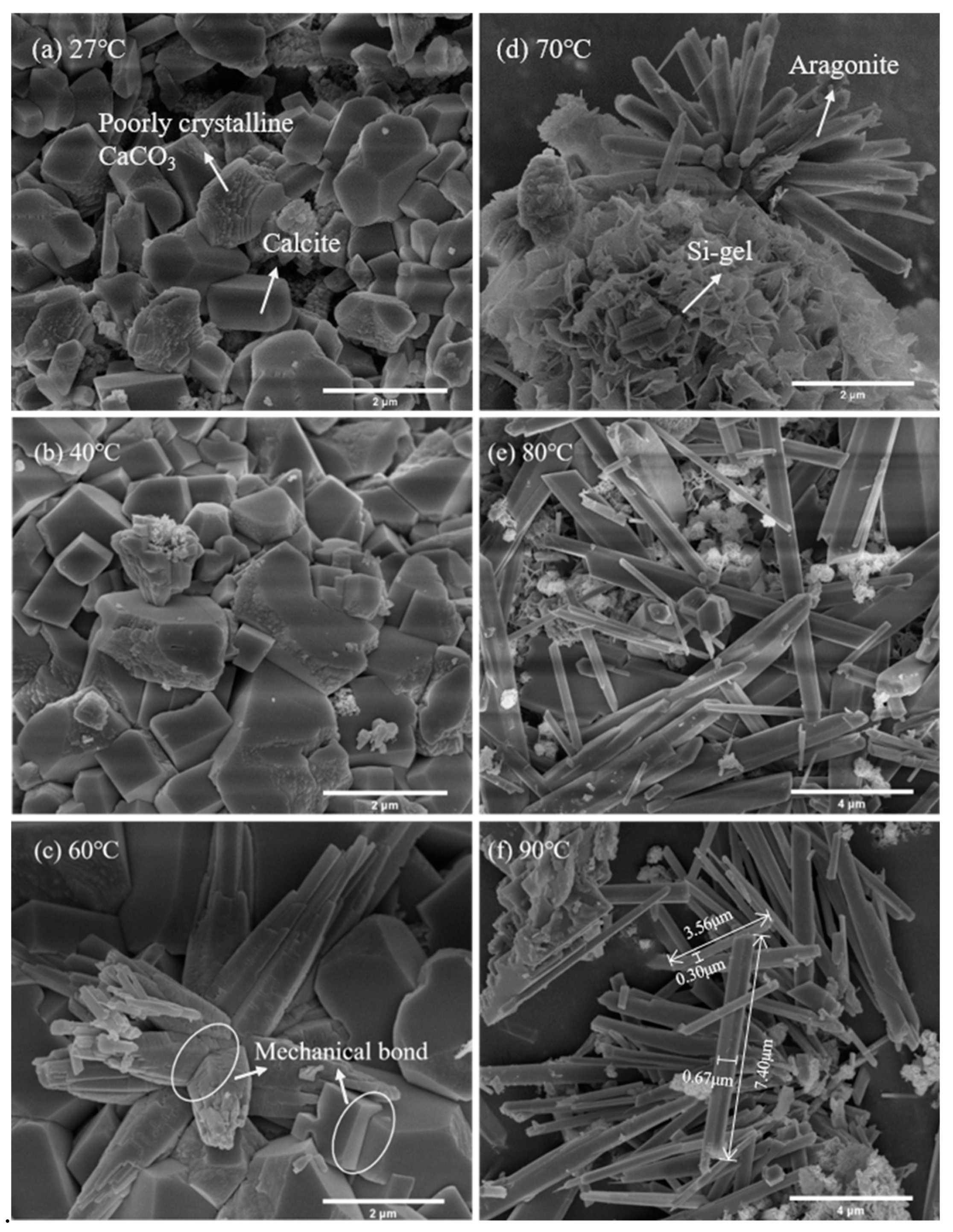
3.1.2. Microstructural Analysis
3.2. Effect of Mg2+/Ca2+ on the Carbonation Products of β-C2S
3.2.1. Effect of Mg2+/Ca2+ on the Aragonite Formation
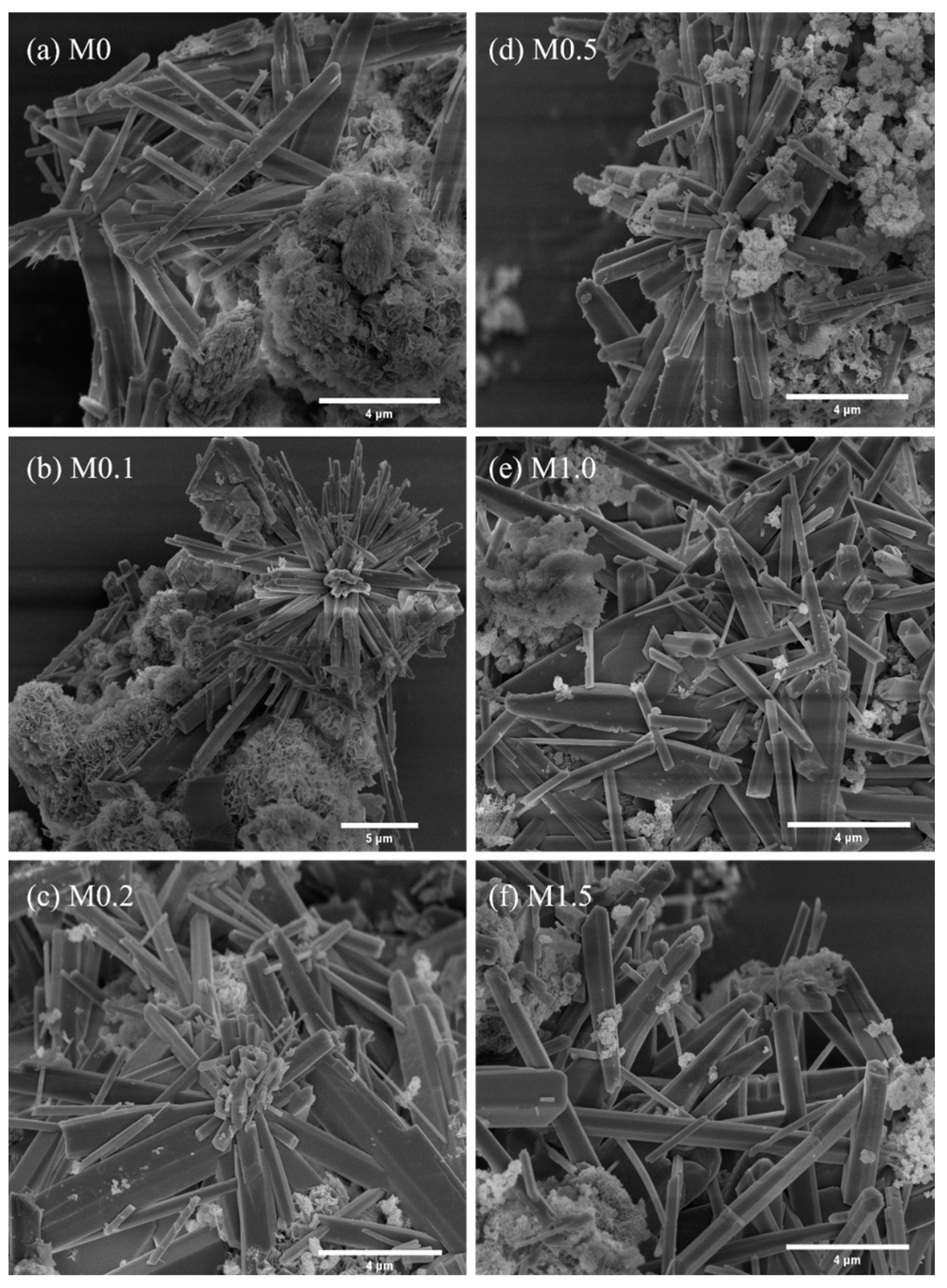
3.2.2. Microstructure Analysis
4. Conclusions
- (1)
- The needle-like aragonite, having an average length of 1–6 μm and an average aspect ratio of 6–13, can be quickly synthesized when the temperature of carbonation increases from 60 °C to 90 °C. Although 60 °C was the optimal temperature for enhancing the carbonation degree of β-C2S, the most appropriate temperature for producing aragonite with a high aspect ratio was 80 °C. At this temperature, the carbon sequestration capacity of β-C2S was 357.82 g/kg.
- (2)
- Besides carbonation temperature, the molar ratio of Mg2+/Ca2+ was also a crucial factor influencing the formation of needle-like aragonite. The combined effect of these two factors promoted the formation of aragonite. Mg2+ promoted the formation of aragonite and hindered the transformation of aragonite to calcite at the reaction temperature of 80 °C. Aragonite became the primary crystal form of calcium carbonate when the Mg2+/Ca2+ molar ratio was above 1.0. Under the condition of a Mg2+/Ca2+ molar ratio of 1.5, the content of aragonite was 55.2%, while calcite was almost non-existent, and calcium carbonate existed as aragonite crystals.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ritchie, H.; Rosado, P.; Roser, M. CO2 and Greenhouse Gas Emissions; OurWorldInData.org: Oxford, UK, 2020. [Google Scholar]
- Zhao, D.; Williams, J.M.; Hou, P.; Moment, A.J.; Kawashima, S. Stabilizing mechanisms of metastable vaterite in cement systems. Cem. Concr. Res. 2024, 178, 107441. [Google Scholar] [CrossRef]
- Chen, T.; Li, L.; Gao, X.; Guo, M.; Qin, L. New insights into the role of early accelerated carbonation on the calcium leaching behavior of cement paste. Cem. Concr. Compos. 2023, 140, 105103. [Google Scholar] [CrossRef]
- Lu, B.; Drissi, S.; Liu, J.; Hu, X.; Song, B.; Shi, C. Effect of temperature on CO2 curing, compressive strength and microstructure of cement paste. Cem. Concr. Res. 2022, 157, 106827. [Google Scholar] [CrossRef]
- Shen, P.; Lu, J.; Zhang, Y.; Jiang, Y.; Zhang, S.; Poon, C.S. Preparation aragonite whisker-rich materials by wet carbonation of cement: Towards yielding micro-fiber reinforced cement and sequestrating CO2. Cem. Concr. Res. 2022, 159, 106891. [Google Scholar] [CrossRef]
- Smit, B.; Reimer, J.; Oldenburg, C.M.; Bourg, I.C. Introduction to Carbon Capture and Sequestration; World Scientific Publishing Co. Pte Ltd.: Singapore, 2014. [Google Scholar]
- Ashraf, W. Carbonation of cement-based materials: Challenges and opportunities. Constr. Build. Mater. 2016, 120, 558–570. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Li, Y.; Tian, J.; You, X.; Mao, Y.; Hu, X.; Shi, C. Carbonated concrete brick capturing carbon dioxide from cement kiln exhaust gas. Case Stud. Constr. Mater. 2022, 17, e01474. [Google Scholar] [CrossRef]
- Owais, M.; Järvinen, M.; Taskinen, P.; Said, A. Experimental study on the extraction of calcium, magnesium, vanadium and silicon from steelmaking slags for improved mineral carbonation of CO2. J. CO2 Util. 2019, 31, 1–7. [Google Scholar] [CrossRef]
- Yang, S.; Mo, L.; Deng, M. Effects of ethylenediamine tetra-acetic acid (EDTA) on the accelerated carbonation and properties of artificial steel slag aggregates. Cem. Concr. Compos. 2021, 138, 103948. [Google Scholar] [CrossRef]
- Chen, Z.; Li, R.; Zheng, X.; Liu, J. Carbon sequestration of steel slag and carbonation for activating RO phase. Cem. Concr. Res. 2021, 139, 106271. [Google Scholar] [CrossRef]
- Li, L.; Chen, T.; Gao, X. Effects of superimposed carbonation synergy on BOFS cement-based materials. Cem. Concr. Compos. 2023, 138, 105008. [Google Scholar] [CrossRef]
- Liu, P.; Mo, L.; Zhang, Z. Effects of carbonation degree on the hydration reactivity of steel slag in cement-based materials. Constr. Build. Mater. 2023, 370, 130653. [Google Scholar] [CrossRef]
- Koumpouri, D.; Angelopoulos, G. Effect of boron waste and boric acid addition on the production of low energy belite cement. Cem. Concr. Compos. 2016, 68, 1–8. [Google Scholar] [CrossRef]
- Wang, D.; Fang, Y.; Zhang, Y.; Chang, J. Changes in mineral composition, growth of calcite crystal, and promotion of physico-chemical properties induced by carbonation of β-C2S. J. CO2 Util. 2019, 34, 149–162. [Google Scholar] [CrossRef]
- Mu, Y.; Liu, Z.; Wang, F. Comparative Study on the Carbonation-Activated Calcium Silicates as Sustainable Binders: Reactivity, Mechanical Performance, and Microstructure. Acs Sustain. Chem. Eng. 2019, 7, 7058–7070. [Google Scholar] [CrossRef]
- Songhui, L.; Junjie, W.; Haibo, Z.; Xuemao, G.; Man, Q.; Zhenzhen, D. Microstructure of β-Dicalcium Silicate after Accelerated Carbonation. J. Wuhan Univ. Technol. 2019, 34, 122–126. [Google Scholar]
- Wang, D.; Chang, J. Comparison on accelerated carbonation of β-C2S, Ca(OH)2, and C4AF: Reaction degree, multi-properties, and products. Constr. Build. Mater. 2019, 224, 336–347. [Google Scholar] [CrossRef]
- Song, X.; Zhang, L.; Cao, Y.; Zhu, J.; Luo, X. Effect of pH and temperatures on the fast precipitation vaterite particle size and polymorph stability without additives by steamed ammonia liquid waste. Powder Technol. 2020, 374, 263–273. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Vikulina, A.S.; Volodkin, D. CaCO3 crystals as versatile carriers for controlled delivery of antimicrobials. J. Control. Release 2020, 328, 470–489. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Dou, Z.; Zhang, S.; Zhang, H.; Guan, X.; Feng, C.; Zhang, J. Effect of sodium hydroxide on the carbonation behavior of β-dicalcium silicate. Constr. Build. Mater. 2017, 150, 591–594. [Google Scholar] [CrossRef]
- Chang, R.; Choi, D.; Kim, M.H.; Park, Y. Tuning Crystal Polymorphisms and Structural Investigation of Precipitated Calcium Carbonates for CO2 Mineralization. ACS Sustain. Chem. Eng. 2017, 5, 1659–1667. [Google Scholar] [CrossRef]
- Oral, Ç.M.; Ercan, B. Influence of pH on morphology, size and polymorph of room temperature synthesized calcium carbonate particles. Powder Technol. 2018, 339, 781–788. [Google Scholar] [CrossRef]
- Yang, T.; Fu, J.; Ma, L.; Du, H.; Yue, X.; Zhao, B.; Wang, C. Biomimetic synthesis of calcium carbonate under phenylalanine: Control of polymorph and morphology. Mater. Sci. Eng. C 2020, 114, 111019. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Zhang, C.; Lv, H.; Xu, L. Characterization of mechanical behavior and mechanism of calcium carbonate whisker-reinforced cement mortar. Constr. Build. Mater. 2014, 66, 89–97. [Google Scholar] [CrossRef]
- Wu, F.; You, X.; Wang, M.; Liu, T.; Lu, B.; Hou, G.; Jiang, R.; Shi, C. Increasing flexural strength of CO2 cured cement paste by CaCO3 polymorph control. Cem. Concr. Compos. 2023, 141, 105128. [Google Scholar] [CrossRef]
- You, X.; Hu, X.; Xiao, Z.; Bairq, Z.A.S.; Chen, W.; Shi, C. Thermodynamic modelling of CaCO3 polymorphs during CO2 sequestration by cement slurry with the addition of MgCl2. J. Clean. Prod. 2023, 410, 137294. [Google Scholar] [CrossRef]
- Han, Y.J.; Aizenberg, J. Effect of magnesium ions on oriented growth of calcite on carboxylic acid functionalized self-assembled monolayer. J. Am. Chem. Soc. 2003, 125, 4032–4033. [Google Scholar] [CrossRef]
- Blue, C.R.; Giuffre, A.; Mergelsberg, S.; Han, N.; De Yoreo, J.J.; Dove, P.M. Chemical and physical controls on the transformation of amorphous calcium carbonate into crystalline CaCO3 polymorphs. Geochim. Cosmochim. Acta 2017, 196, 179–196. [Google Scholar] [CrossRef]
- Németh, P.; Mugnaioli, E.; Gemmi, M.; Czuppon, G.; Demény, A.; Spötl, C. A nanocrystalline monoclinic CaCO3 precursor of metastable aragonite. Sci. Adv. 2018, 4, eaau6178. [Google Scholar] [CrossRef]
- Álvarez-Pinazo, G.; Cuesta, A.; García-Maté, M.; Santacruz, I.; Losilla, E.R.; la Torre, A.G.D.; León-Reina, L.; Aranda, M.A.G. Rietveld quantitative phase analysis of Yeelimite-containing cements. Cem. Concr. Res. 2012, 42, 960–971. [Google Scholar] [CrossRef]
- Chang, J.; Fang, Y.; Shang, X. The role of β-C2S and γ-C2S in carbon capture and strength development. Mater. Struct. 2016, 49, 4417–4424. [Google Scholar] [CrossRef]
- Huijgen, W.J.J.; Witkamp, G.; Comans, R.N.J. Mineral CO2 Sequestration by Steel Slag Carbonation. Environ. Sci. Technol. 2005, 39, 9676–9682. [Google Scholar] [CrossRef] [PubMed]
- Igathinathane, C.; Pordesimo, L.O.; Columbus, E.P.; Batchelor, W.D.; Methuku, S.R. Shape identification and particles size distribution from basic shape parameters using ImageJ. Comput. Electron. Agric. 2008, 63, 168–182. [Google Scholar] [CrossRef]
- Chen, J.; Xiang, L. Controllable synthesis of calcium carbonate polymorphs at different temperatures. Powder Technol. 2009, 189, 64–69. [Google Scholar] [CrossRef]
- Ogino, T.; Suzuki, T.; Sawada, K. The formation and transformation mechanism of calcium carbonate in water. Geochim. Cosmochim. Acta 1987, 51, 2757–2767. [Google Scholar] [CrossRef]
- Ji, L.; Yu, H.; Yu, B.; Zhang, R.; French, D.; Grigore, M.; Wang, X.; Chen, Z.; Zhao, S. Insights into Carbonation Kinetics of Fly Ash from Victorian Lignite for CO2 Sequestration. Energy Fuels 2018, 32, 4569–4578. [Google Scholar] [CrossRef]
- Zhan, B.J.; Xuan, D.X.; Poon, C.S.; Shi, C.J. Effect of curing parameters on CO2 curing of concrete blocks containing recycled aggregates. Cem. Concr. Compos. 2016, 71, 122–130. [Google Scholar] [CrossRef]
- Guan, X.; Liu, S.; Feng, C.; Qiu, M. The hardening behavior of γ-C2S binder using accelerated carbonation. Constr. Build. Mater. 2016, 114, 204–207. [Google Scholar] [CrossRef]
- Ashraf, W.; Olek, J. Elucidating the accelerated carbonation products of calcium silicates using multi-technique approach. J. CO2 Util. 2018, 23, 61–74. [Google Scholar] [CrossRef]
- Del Bosque, I.F.S.; Martínez-Ramírez, S.; Blanco-Varela, M.T. FTIR study of the effect of temperature and nanosilica on the nano structure of C–S–H gel formed by hydrating tricalcium silicate. Constr. Build. Mater. 2014, 52, 314–323. [Google Scholar] [CrossRef]
- Fernández Carrasco, L.; Torrens Martín, D.; Morales, L.M.; Martínez Ramírez, S. Infrared Spectroscopy in the Analysis of Building and Construction Materials, Infrared Spectroscopy—Materials Science, Engineering and Technology; InTech Open: London, UK, 2012. [Google Scholar]
- Khan, R.I.; Ashraf, W.; Olek, J. Amino acids as performance-controlling additives in carbonation-activated cementitious materials. Cem. Concr. Res. 2021, 147, 106501. [Google Scholar] [CrossRef]
- Wang, Y.J.; Li, J.G.; Tao, M.J.; Zhang, X.; Zhang, J.B.; Qin, S.; Liu, S.H.; Peng, L.J.; Zhang, X.P.; Zeng, Y.N. Investigation of the acicular aragonite growth behavior in AOD stainless steel slag during slurry-phase carbonation. Sci. Total Environ. 2023, 904, 166750. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Li, Z.; Zhao, J.; Wang, Y. Synergistic enhancement effect of recycled fine powder (RFP) cement paste and carbonation on recycled aggregates performances and its mechanism. J. Clean. Prod. 2022, 344, 130848. [Google Scholar] [CrossRef]
- Hong, S.; Jiang, R.; Zheng, F.; Fan, S.; Dong, B. Quantitative characterization of carbonation of cement-based materials using X-ray imaging. Cem. Concr. Compos. 2022, 134, 104794. [Google Scholar] [CrossRef]
- Villain, G.; Thiery, M.; Platret, G. Measurement methods of carbonation profiles in concrete: Thermogravimetry, chemical analysis and gammadensimetry. Cem. Concr. Res. 2007, 37, 1182–1192. [Google Scholar] [CrossRef]
- GB/T 17671-2021; Test Method of Cement Mortar Strength (ISO Method). Standardization Administration of the People’s Republic of China: Beijing, China, 2021.
- Cao, M.; Liu, Z.; Xie, C. Effectiveness of calcium carbonate whiskers in mortar for improving the abrasion resistance. Constr. Build. Mater. 2021, 295, 123583. [Google Scholar] [CrossRef]




| Temperature/°C | Length/μm | Diameter/μm | Aspect Ratio |
|---|---|---|---|
| 60 | 1.29 | 0.19 | 6.91 |
| 70 | 3.49 | 0.44 | 7.98 |
| 80 | 4.77 | 0.39 | 12.09 |
| 90 | 5.67 | 0.45 | 12.60 |
| Sample | Length/μm | Diameter/μm | Aspect Ratio |
|---|---|---|---|
| M0 | 7.80 | 0.59 | 13.22 |
| M0.1 | 3.44 | 0.30 | 11.47 |
| M0.2 | 5.15 | 0.44 | 11.70 |
| M0.5 | 4.25 | 0.40 | 10.63 |
| M1.0 | 4.86 | 0.46 | 10.56 |
| M1.5 | 3.96 | 0.43 | 9.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, B.; Wang, C.; Wang, D. Effect of Magnesium and Temperature on the Accelerated Carbonation Progress of β-Dicalcium Silicate. Materials 2025, 18, 2232. https://doi.org/10.3390/ma18102232
Fu B, Wang C, Wang D. Effect of Magnesium and Temperature on the Accelerated Carbonation Progress of β-Dicalcium Silicate. Materials. 2025; 18(10):2232. https://doi.org/10.3390/ma18102232
Chicago/Turabian StyleFu, Binbin, Chaoran Wang, and Dan Wang. 2025. "Effect of Magnesium and Temperature on the Accelerated Carbonation Progress of β-Dicalcium Silicate" Materials 18, no. 10: 2232. https://doi.org/10.3390/ma18102232
APA StyleFu, B., Wang, C., & Wang, D. (2025). Effect of Magnesium and Temperature on the Accelerated Carbonation Progress of β-Dicalcium Silicate. Materials, 18(10), 2232. https://doi.org/10.3390/ma18102232





