Effect of Cr:Al Ratio on Corrosion Mechanism of Ni-Cr-Mo-Al Alloys in 3.5 wt.% NaCl Solution: Microstructure and Electrochemical and Passive Characteristics
Abstract
1. Introduction
2. Experimental Procedures
2.1. Sample Preparation
2.2. Electrochemical Measurements
2.3. Characterization
3. Results and Discussion
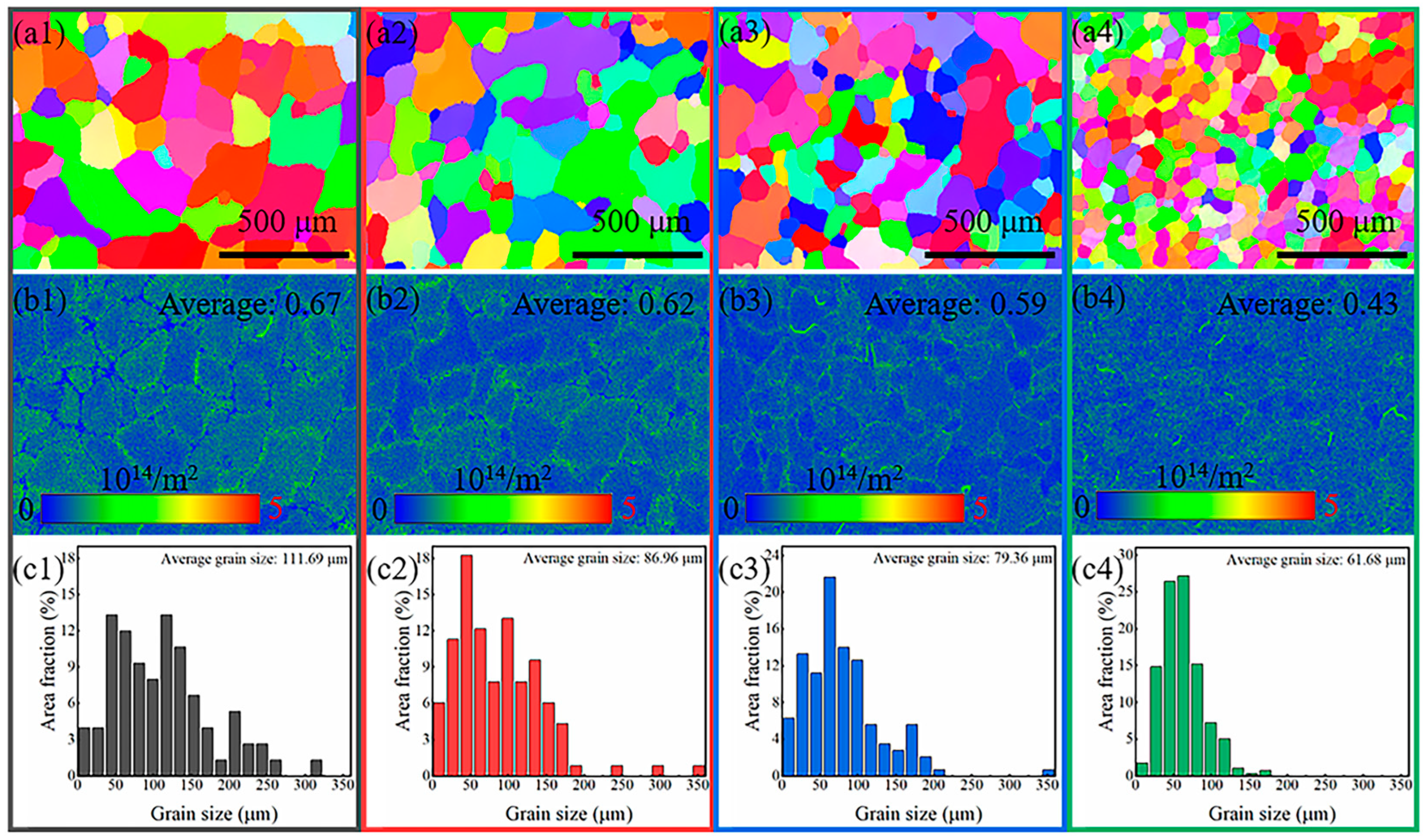
3.1. Microstructure Analysis
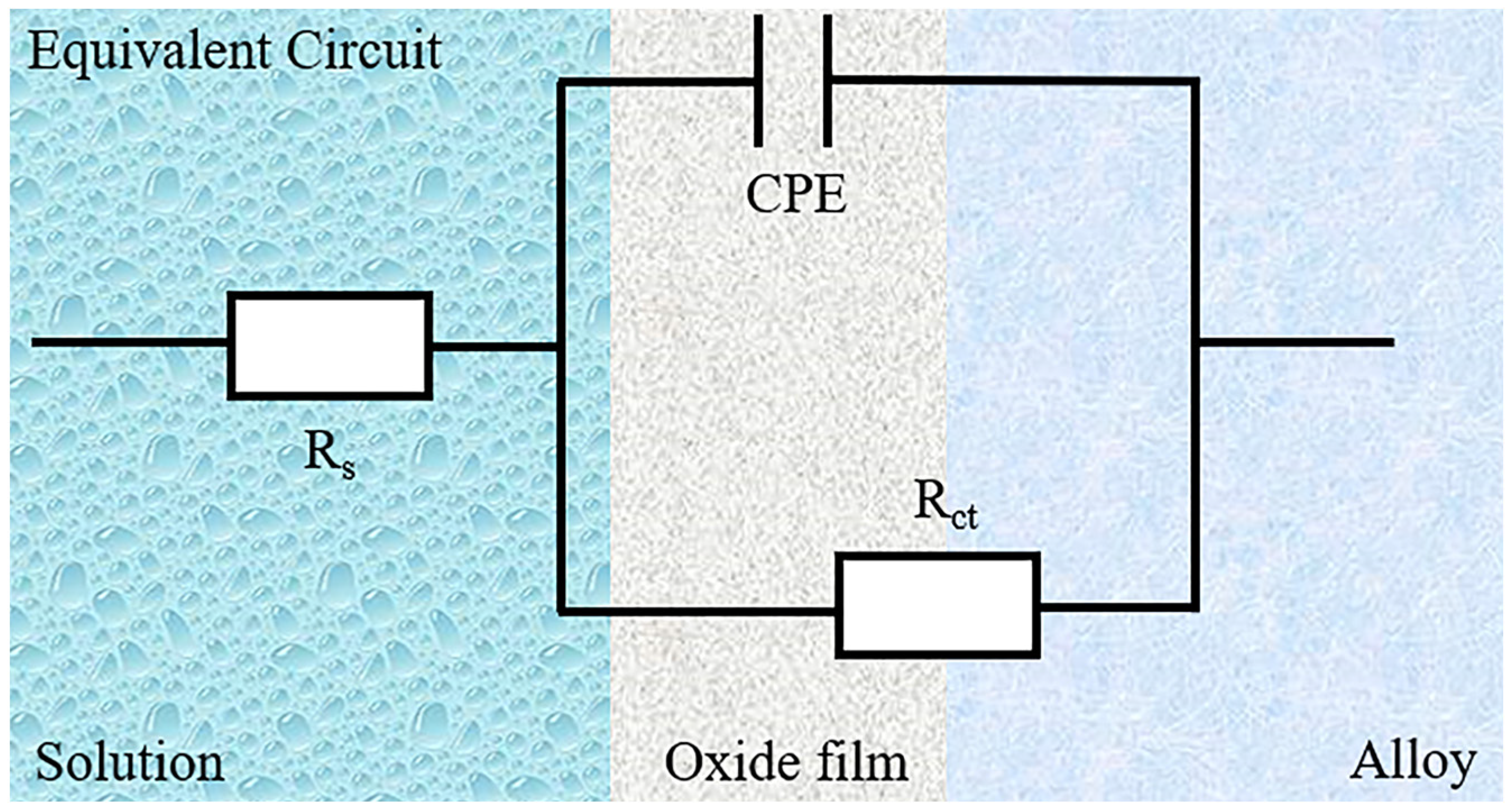
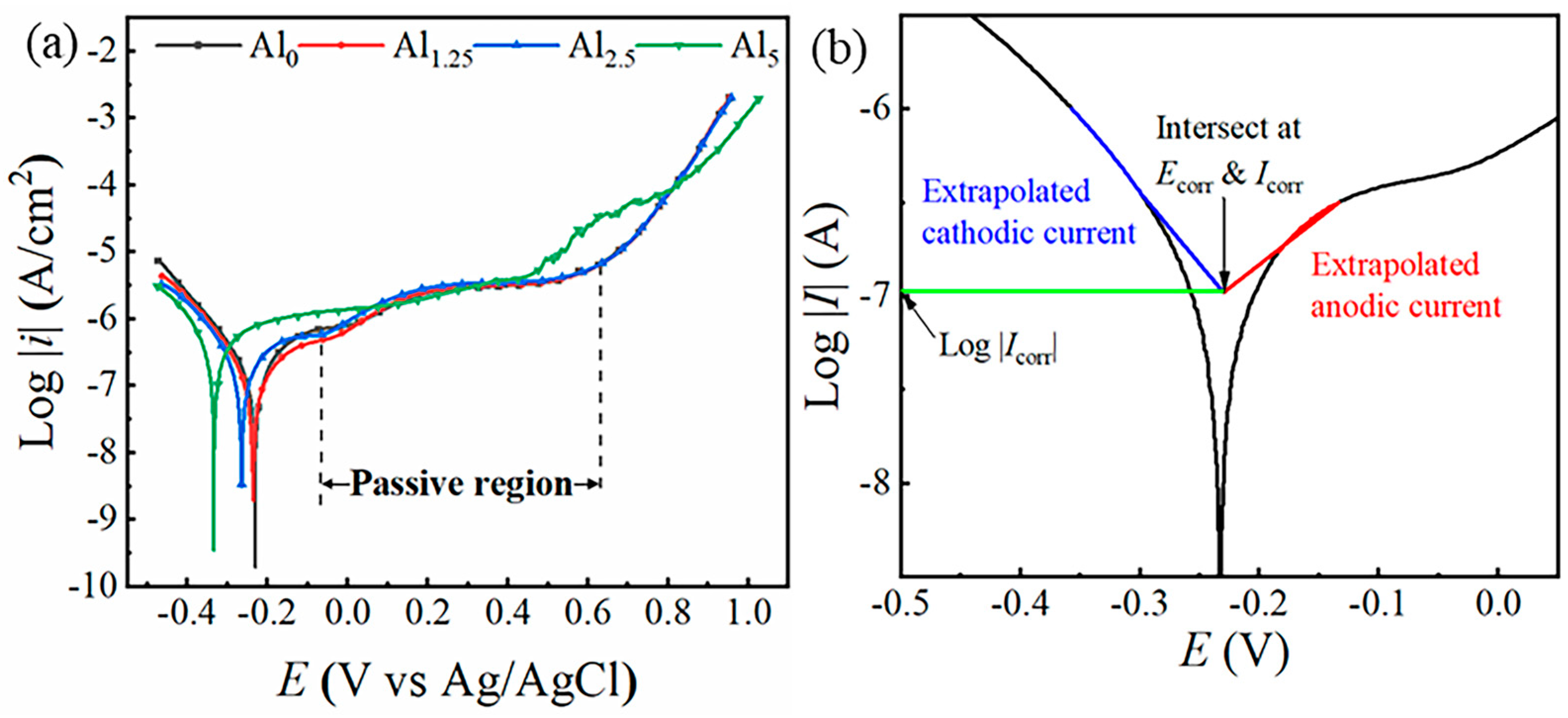
3.2. Electrochemical Characteristic Analysis
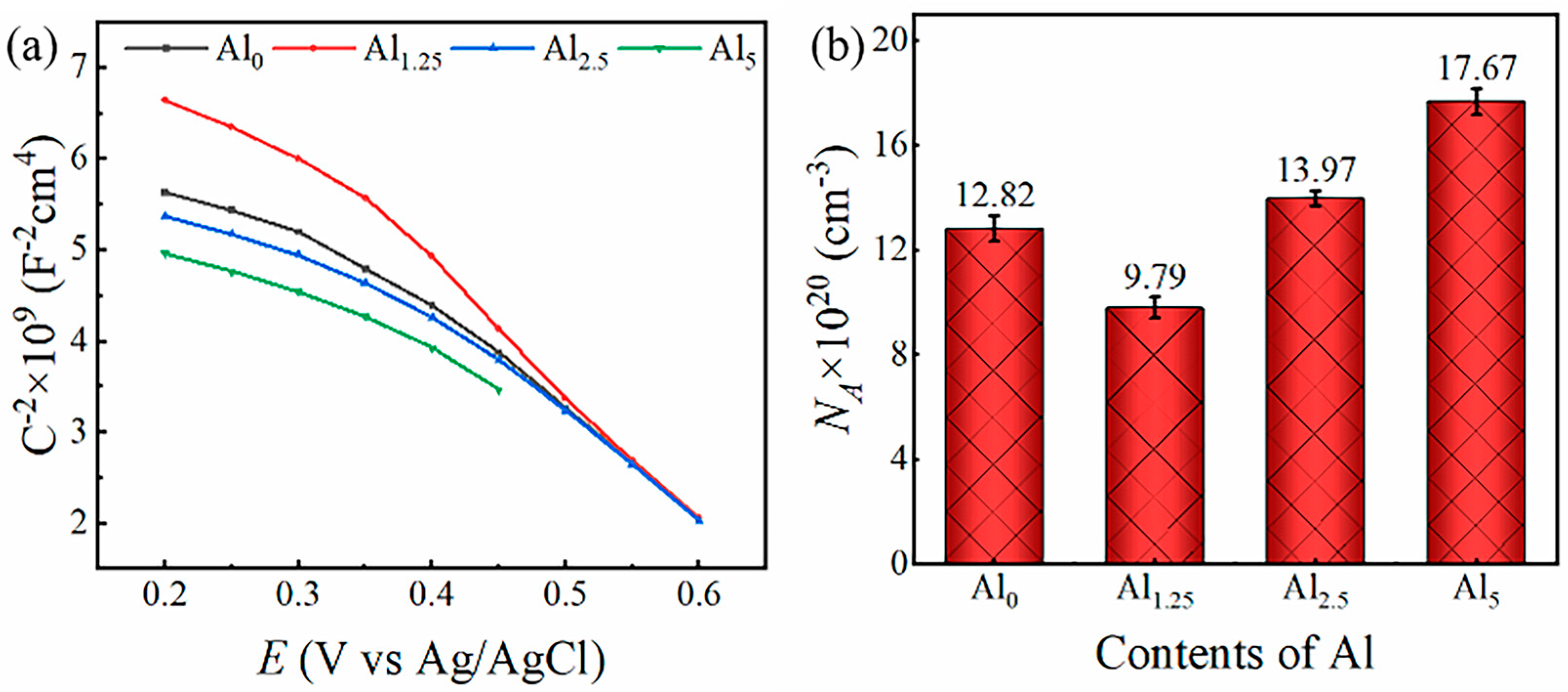
3.3. Passivation Characteristic Analysis
4. Conclusions
- (1)
- The Ni-Cr-Mo-Al alloys with varying Cr:Al ratio both exhibited a single face-centered cubic (FCC) phase without any second-phase precipitates. In addition, uniform equiaxed structures were observed in all specimens; however, the results of EBSD showed a decreased dislocation density and grain size as Al content in NiCrMoAl alloys increased. The decrease in grain size was beneficial to the corrosion resistance of the alloy, while a decreasing dislocation density affected the nucleation and growth of passivation film, thereby decreasing anti-corrosion performance.
- (2)
- The corrosion resistance of the alloy samples with different Cr:Al ratio was compared by electrochemical methods, including EIS and PDP. A maximum radius of a semicircle was found in the Al1.25 specimen on EIS test, whereas the highest Ecorr was found in the Al0 specimen, and as Al content increased, Ecorr gradually decreased. However, the Al1.25 specimen unexpectedly showed the lowest Icorr. The results of EIS and PDP both indicated that the Al1.25 sample exhibited the best electrochemical performance (Rct: 8.08 ± 0.368 × 105 Ω cm2, Icorr: 1.05 ± 0.003 × 10−7 A/cm2).
- (3)
- The density of passivation film has an essential influence on the corrosion resistance of alloys. In the potentiostatic polarization test, steady-state current densities were observed to follow the order 5 nA (Al1.25) <12 nA (Al0) <23 nA (Al2.5) < 31 nA (Al5), indicating that the passive film with a denser and more stable microstructure was formed in Al1.25 specimen. The similar conclusion was demonstrated again in Mott–Schottky experiments. When the Al content was 1.25 mol.%, the amount of NA was 9.79 × 1020 cm−3, which was lowest among these alloys.
- (4)
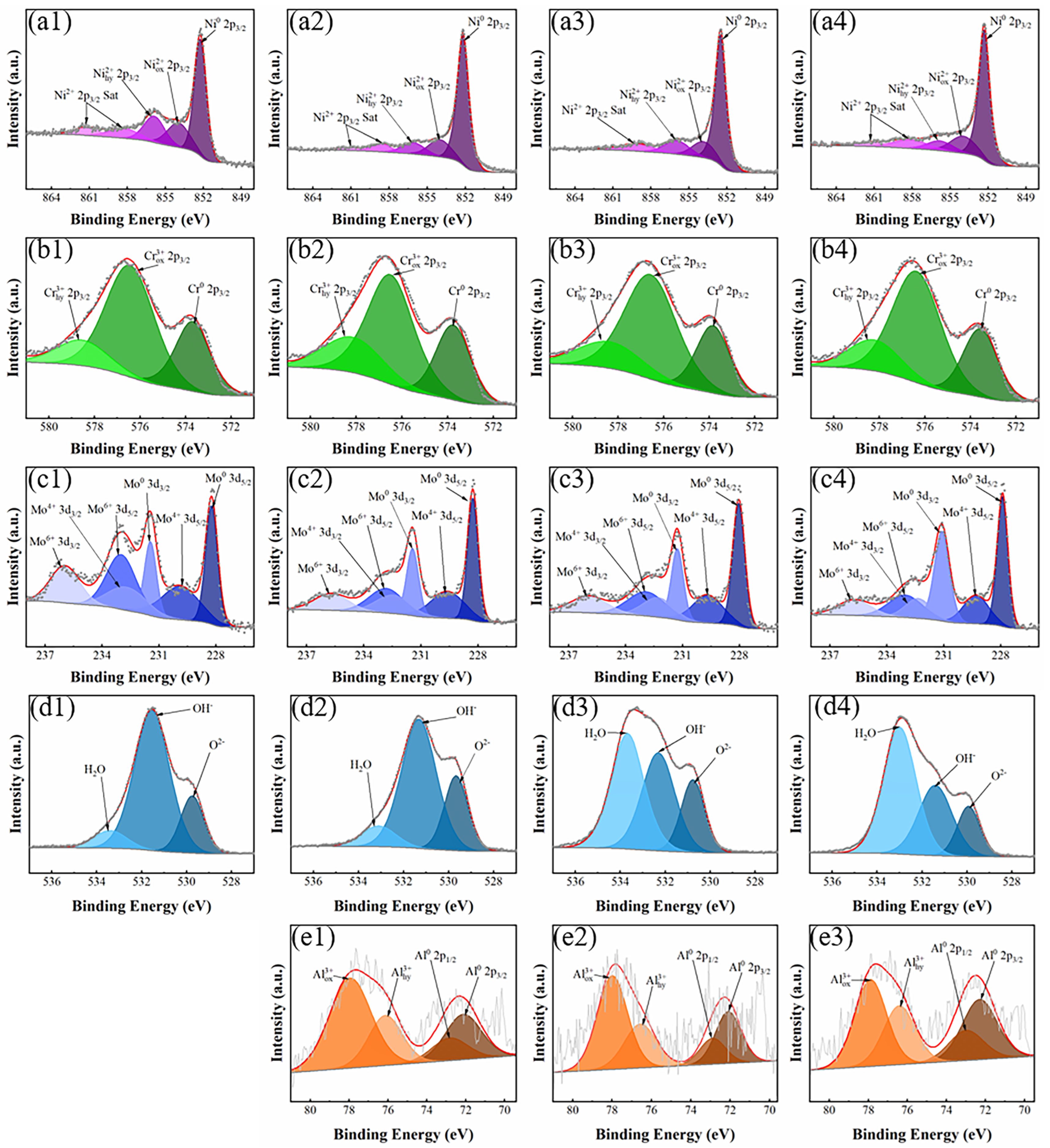
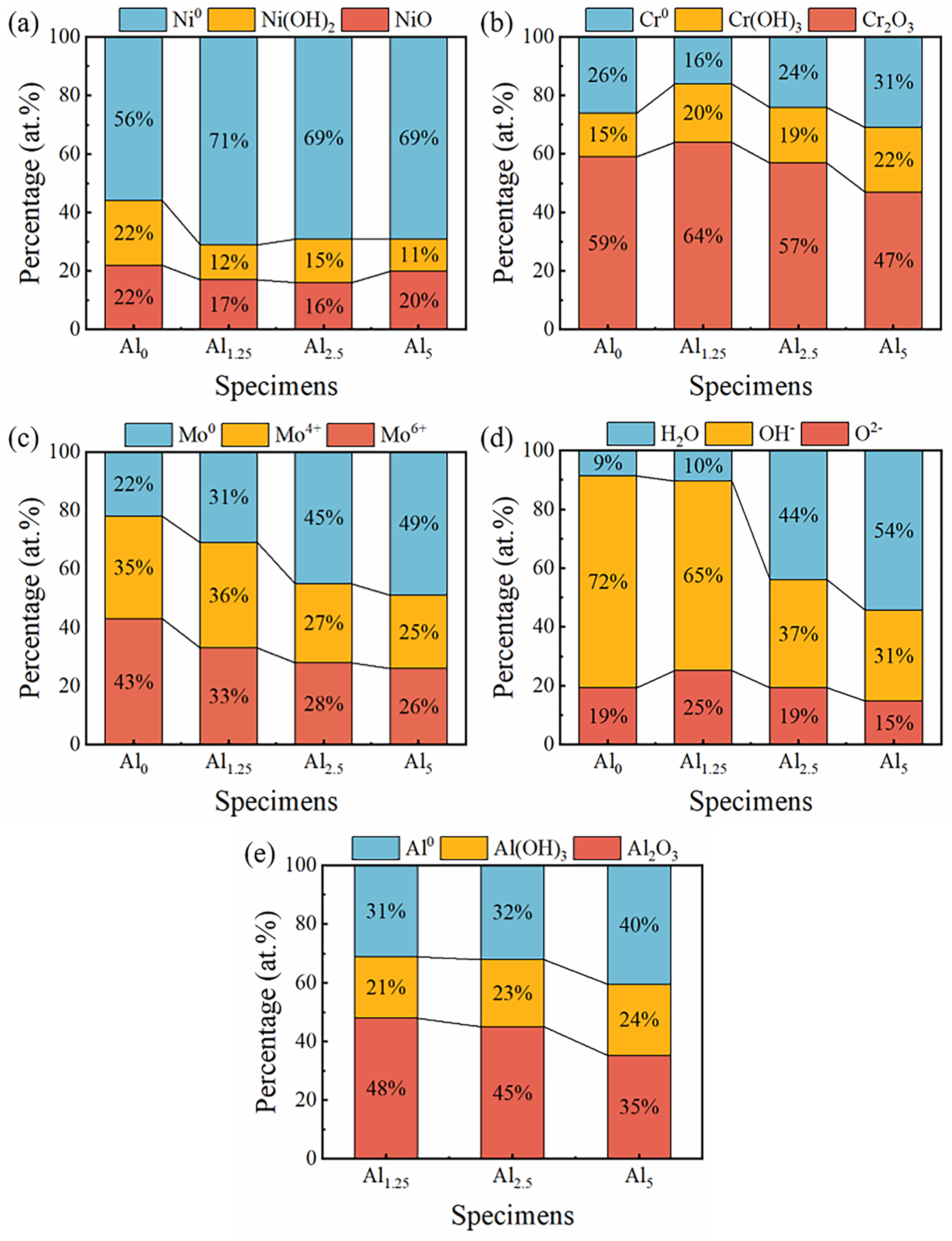
- The composition of a passive film was another important factor affecting its protective performance. It can be found from XPS results that the content of Cr2O3 in the passive film, the main component of the passive film, first increased and then decreased. Among these Ni-Cr-Mo-Al alloys, the Al1.25 specimen had a highest Cr2O3 content, implying an enhanced protective effect of the passive film.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Wharton, J.A.; Shenoi, R.A. Ultimate strength analysis of aged steel-plated structures exposed to marine corrosion damage: A review. Corros. Sci. 2014, 86, 42–60. [Google Scholar] [CrossRef]
- Hou, B.; Li, X.; Ma, X.; Du, C.; Zhang, D.; Zheng, M.; Xu, W.; Lu, D.; Ma, F. The cost of corrosion in China. NPJ Mater. Degrad. 2017, 1, 4. [Google Scholar] [CrossRef]
- Wang, B.; Liu, L.; Cheng, X.; Wu, W.; Liu, C.; Zhang, D.; Li, X. Advanced multi-image segmentation-based machine learning modeling strategy for corrosion prediction and rust layer performance evaluation of weathering steel. Corros. Sci. 2024, 237, 112334. [Google Scholar] [CrossRef]
- He, Z.; Chen, B.; Zhou, B.; Liu, F.; Hu, Q.; Qin, Z.; Gao, Z.; Hu, W.; Wu, Z. Effect of TiC precipitation on the corrosion behavior of Monel K500 alloy in 3.5 wt.% NaCl solution. Corros. Sci. 2023, 211, 110886. [Google Scholar] [CrossRef]
- Xue, Z.; Wang, H.; He, N.; Sun, Y.; Li, H.; Wang, Z.; Zhang, C.; Fang, Y.; Li, D.; Bao, K.; et al. Study on intergranular corrosion mechanism of low dilution rate Inconel 625 weld overlay. Corros. Sci. 2025, 244, 112659. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, L.; Zhang, N. Improved passivation performance of selective laser melted Inconel 718 alloy via tempering treatment. Corros. Sci. 2024, 238, 112374. [Google Scholar] [CrossRef]
- He, X.; Wang, L.; Kong, D.; Zhang, W.; Dai, K.; Ni, X.; Zhang, L.; Zhou, Y.; Dong, C. The kinetics of pitting corrosion in a defect-contained Hastelloy X alloy fabricated by laser powder bed fusion. Corros. Sci. 2024, 237, 112321. [Google Scholar] [CrossRef]
- An, H.; Yu, Z.; Wang, R.; An, H.; Kang, Y.; Qin, J.; Zhao, Y.; An, H.; Hu, Y.; Tuo, L.; et al. Microstructure evolution and corrosion behavior of the Incoloy 028 alloy upon aging. Corros. Sci. 2025, 244, 112634. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Cheng, K.; Kong, Y. Chlorine-induced high-temperature corrosion characteristics of Ni-Cr alloy cladding layer and Ni-Cr-Mo alloy cladding layer. Corros. Sci. 2023, 216, 111102. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, Y.; Shi, Y.; Su, G.; Gu, Y.; Volodymyr, K. Intergranular corrosion characteristics of high-efficiency wire laser additive manufactured Inconel 625 alloys. Corros. Sci. 2022, 205, 110422. [Google Scholar] [CrossRef]
- Li, S.; Wei, Q.; Shi, Y.; Zhu, Z.; Zhang, D. Microstructure Characteristics of Inconel 625 Superalloy Manufactured by Selective Laser Melting. J. Mater. Sci. Technol. 2015, 31, 946–952. [Google Scholar] [CrossRef]
- Dai, H.; Shi, S.; Yang, L.; Hu, J.; Liu, C.; Guo, C.; Chen, X. Effects of elemental composition and microstructure inhomogeneity on the corrosion behavior of nickel-based alloys in hydrofluoric acid solution. Corros. Sci. 2020, 176, 108917. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Z.; Liu, W.; Yin, H.; Tang, Z.; Qian, Y. Ni-Mo-Cr alloy corrosion in molten NaCl-KCl-MgCl2 salt and vapour. Corros. Sci. 2021, 180, 109183. [Google Scholar] [CrossRef]
- D’Souza, B.; Leong, A.; Yang, Q.; Zhang, J. Corrosion behavior of boronized nickel-based alloys in the molten chloride Salt. Corros. Sci. 2021, 182, 109285. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, J.; Lin, X.; Guo, P.; Yu, X.; Zhang, S.; Liu, J.; Huang, W. Passive behavior of laser directed energy deposited Inconel 718 after homogenization and aging heat treatment. Corros. Sci. 2022, 205, 110439. [Google Scholar] [CrossRef]
- Morón, R.C.; Contla-Pacheco, A.D.; Castrejón-Sánchez, V.H.; Melo-Máximo, L.; Campos-Silva, I. Estimation of the wear and corrosion synergism of borided Inconel 718 alloy immersed in a neutral aqueous solution. Ceram. Int. 2023, 49, 2495–2505. [Google Scholar] [CrossRef]
- Valle, L.C.M.; Santana, A.I.C.; Rezende, M.C.; Dille, J.; Mattos, O.R.; de Almeida, L.H. The influence of heat treatments on the corrosion behaviour of nickel-based alloy 718. J. Alloys Compd. 2019, 809, 151781. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, J.; Lin, X.; Guo, P.; Yan, Q.; Liu, J.; Huang, W. Effects of the electrolytes on the electrochemical behavior of nickel-base superalloys prepared by laser directed energy deposition. Corros. Sci. 2024, 228, 111785. [Google Scholar] [CrossRef]
- Czerski, J.; Mitoraj-Królikowska, M.; Godlewska, E.; Wetzel, A.; Witt, J.; Ozcan, O.; Marzec, M.; Goły, M. Corrosion and passivation of AlCrFe2Ni2Mox high-entropy alloys in sulphuric acid. Corros. Sci. 2024, 229, 111855. [Google Scholar] [CrossRef]
- Pan, Z.; Luo, H.; Zhao, Q.; Cheng, H.; Wang, X.; Ma, Y.; Li, X. Novel Mo-modified medium entropy alloys achieving enhanced corrosion resistance in acidic solution. Corros. Sci. 2023, 216, 111094. [Google Scholar] [CrossRef]
- Hou, D.; Luo, H.; Pan, Z.; Zhao, Q.; Cheng, H.; Wang, X. Effect of Cr on the microstructure and corrosion behavior of nickel-based alloys in hydrochloric acid. J. Mater. Res. Technol. 2024, 32, 2867–2881. [Google Scholar] [CrossRef]
- Pan, Z.; Luo, H.; Zhao, Q.; Cheng, H.; Wei, Y.; Wang, X.; Zhang, B.; Li, X. Tailoring microstructure and corrosion behavior of CoNiVAlx medium entropy alloys via Al addition. Corros. Sci. 2022, 207, 110570. [Google Scholar] [CrossRef]
- Zhao, Q.; Pan, Z.; Wang, X.; Luo, H.; Liu, Y.; Li, X. Corrosion and passive behavior of AlxCrFeNi3−x (x = 0.6, 0.8, 1.0) eutectic high entropy alloys in chloride environment. Corros. Sci. 2022, 208, 110666. [Google Scholar] [CrossRef]
- Feng, X.; Wang, H.; Liu, X.; Wang, C.; Cui, H.; Song, Q.; Huang, K.; Li, N.; Jiang, X. Effect of Al content on wear and corrosion resistance of Ni-based alloy coatings by laser cladding. Surf. Coat. Technol. 2021, 412, 126976. [Google Scholar] [CrossRef]
- Wang, J.; Yan, K.; Huang, W.; Lu, Z. Mechanisms of Al2O3 and Cr2O3 formation during FeCrAl alloy Oxidation: A First-Principles study. Appl. Surf. Sci. 2024, 644, 158782. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Cheng, K.; Shen, Y.; Li, J. Corrosion characteristics of Inconel 625 cladding layer and NiCrMoAl cladding layer in molten NaCl-KCl and NaCl-KCl-Na2SO4. Corros. Sci. 2023, 221, 111308. [Google Scholar] [CrossRef]
- Che, Z.; Xue, H.; Liu, J.; Zhou, X.; Liu, W.; Yang, S.; Du, Y.; Cheng, X.; Li, X.; Liu, C. A novel understanding of dislocation density effect on the corrosion resistance of 316L stainless steel with passive film nucleation growth kinetic calculation. Corros. Sci. 2025, 248, 112810. [Google Scholar] [CrossRef]
- Fu, Y.; Dai, C.; Luo, H.; Li, D.; Du, C.; Li, X. The corrosion behavior and film properties of Al-containing high-entropy alloys in acidic solutions. Appl. Surf. Sci. 2021, 560, 149854. [Google Scholar] [CrossRef]
- Pan, Z.; Luo, H.; Zhao, Q.; Cheng, H.; Li, X. Effect of Hf addition on microstructural evolution and corrosion behavior of nickel-based alloys in hydrochloric acid. Corros. Sci. 2023, 224, 111507. [Google Scholar] [CrossRef]
- Fu, Y.; Luo, H.; Chen, X.; Prabhakar, J.M.; Wang, X.; Cheng, H.; Du, C.; Hu, S.; Li, X. The corrosion behavior and passive film properties of the cast and annealed AlCoCrFeNi2.1 eutectic high-entropy alloy in sulfuric acid solution. Corros. Sci. 2024, 240, 112456. [Google Scholar] [CrossRef]
- Che, Z.; Revilla, R.I.; Li, C.; Liu, J.; Liu, W.; Wouters, B.; Marcoen, K.; Cheng, X.; Liu, C.; Li, X. Role of Te-RE alloying on the passive film and pitting corrosion behavior of 316L stainless steel. Corros. Sci. 2024, 240, 112457. [Google Scholar] [CrossRef]
- Bi, D.; Chang, Y.; Luo, H.; Pan, Z.; Zhao, Q.; Cheng, H.; Wang, X.; Qiao, C.; Ni, Z.; Liu, A.; et al. Corrosion behavior and passive film characteristics of AlNbTiZrSix high-entropy alloys in simulated seawater environment. Corros. Sci. 2023, 224, 111530. [Google Scholar] [CrossRef]
- McCafferty, E. Validation of corrosion rates measured by the Tafel extrapolation method. Corros. Sci. 2005, 47, 3202–3215. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, M.; Han, C.; Zhao, Z.; Jia, X.; Zhao, M.; Li, S.; Liu, J. Evolution and corrosion resistance of passive film with polarization potential on Ti-5Al-5Mo-5V-1Fe-1Cr alloy in simulated marine environments. Corros. Sci. 2023, 221, 111334. [Google Scholar] [CrossRef]
- Araneda, A.A.B.; Kappes, M.A.; Rodríguez, M.A.; Carranza, R.M. Pitting corrosion of Ni-Cr-Fe alloys at open circuit potential in chloride plus thiosulfate solutions. Corros. Sci. 2022, 198, 110121. [Google Scholar] [CrossRef]
- Wei, Y.; Pan, Z.; Fu, Y.; Yu, W.; He, S.; Yuan, Q.; Luo, H.; Li, X. Effect of annealing temperatures on microstructural evolution and corrosion behavior of Ti-Mo titanium alloy in hydrochloric acid. Corros. Sci. 2022, 197, 110079. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, X.; Qi, M.; Zhang, Q.; Qi, Y.; Jin, G. Corrosion and passivation behavior of in-situ TiC reinforced Al0.1CrNbSi0.1TaTiV refractory high entropy alloy coatings via doping C. Corros. Sci. 2024, 227, 111736. [Google Scholar] [CrossRef]
- Morshed-Behbahani, K.; Najafisayar, P.; Pakshir, M.; Shahsavari, M. An electrochemical study on the effect of stabilization and sensitization heat treatments on the intergranular corrosion behaviour of AISI 321H austenitic stainless steel. Corros. Sci. 2018, 138, 28–41. [Google Scholar] [CrossRef]
- Macdonald, D.D. The history of the Point Defect Model for the passive state: A brief review of film growth aspects. Electrochim. Acta 2011, 56, 1761–1772. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, W.; Fan, Y.; Fan, E.; Dong, B.; Zhang, T.; Li, X. Effect of Cr content on the passivation behavior of Cr alloy steel in a CO2 aqueous environment containing silty sand. Corros. Sci. 2020, 168, 108591. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, W.; Huo, W.; Zhao, Y.; Cui, W.; Zhang, Y. Electrochemical Corrosion Behavior and Mechanical Properties of Nanocrystalline Ti–6Al–4V Alloy Induced by Sliding Friction Treatment. Metals 2019, 12, 760. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Qin, W.-M.; Chen, J.-Y.; Lei, W.-X.; Xi, J.-Y. L21-strengthened face-centered cubic high-entropy alloy with well pitting resistance. Corros. Sci. 2023, 215, 111043. [Google Scholar] [CrossRef]
- Zhao, T.; Chen, S.; Qiu, J.; Sun, L.; Macdonald, D.D. Study on the passivation properties of austenitic stainless steel 316LN based on the point defect model. Corros. Sci. 2024, 237, 112293. [Google Scholar] [CrossRef]
- Hsu, K.-M.; Chen, S.-H.; Lin, C.-S. Microstructure and corrosion behavior of FeCrNiCoMnx (x = 1.0, 0.6, 0.3, 0) high entropy alloys in 0.5 M H2SO4. Corros. Sci. 2021, 190, 109694. [Google Scholar] [CrossRef]
- Montemor, M.F.; Simões, A.M.P.; Ferreira, M.G.S.; Belo, M.D.C. The role of Mo in the chemical composition and semiconductive behaviour of oxide films formed on stainless steels. Corros. Sci. 1999, 41, 17–34. [Google Scholar] [CrossRef]
- Olsson, C.O.A.; Landolt, D. Passive films on stainless steels—Chemistry, structure and growth. Electrochim. Acta 2003, 48, 1093–1104. [Google Scholar] [CrossRef]
- Luo, H.; Wang, X.; Dong, C.; Xiao, K.; Li, X. Effect of cold deformation on the corrosion behaviour of UNS S31803 duplex stainless steel in simulated concrete pore solution. Corros. Sci. 2017, 124, 178–192. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, B.; Rack, P.D.; Guo, S.; Liaw, P.K.; Zhao, Y. High-throughput synthesis and corrosion behavior of sputter-deposited nanocrystalline Alx(CoCrFeNi)100-x combinatorial high-entropy alloys. Mater. Des. 2020, 195, 109018. [Google Scholar] [CrossRef]



| Alloy | Elements | ||||
|---|---|---|---|---|---|
| Ni | Cr | Mo | Al | ||
| Al0 | Nominal (at.%) | 71.7 | 25.2 | 3.1 | - |
| Weight (wt.%) | 72.4 | 22.5 | 5.1 | - | |
| Al1.25 | Nominal (at.%) | 71.7 | 23.95 | 3.1 | 1.25 |
| Weight (wt.%) | 72.8 | 21.5 | 5.1 | 0.6 | |
| Al2.5 | Nominal (at.%) | 71.7 | 22.7 | 3.1 | 2.5 |
| Weight (wt.%) | 73.1 | 20.5 | 5.2 | 1.2 | |
| Al5 | Nominal (at.%) | 71.7 | 20.2 | 3.1 | 5 |
| Weight (wt.%) | 73.9 | 18.5 | 5.2 | 2.4 | |
| Alloy | Rs (Ω·cm2) | CPE × 10−5 (Ω−1 cm−2 sn) | n | Rct × 10−5 (Ω·cm2) |
|---|---|---|---|---|
| Al0 | 6.788 ± 0.04 | 2.23 ± 0.02 | 0.9417 ± 0.002 | 7.66 ± 0.362 |
| Al1.25 | 6.276 ± 0.04 | 2.22 ± 0.01 | 0.9461 ± 0.001 | 8.08 ± 0.368 |
| Al2.5 | 6.807 ± 0.03 | 2.53 ± 0.02 | 0.9381 ± 0.002 | 6.95 ± 0.356 |
| Al5 | 6.614 ± 0.05 | 3.52 ± 0.03 | 0.8961 ± 0.002 | 6.26 ± 0.522 |
| Alloy | Icorr × 10−7 (A) | Ecorr (V) | Ip × 10−6 (A) | Eb (V) |
|---|---|---|---|---|
| Al0 | 1.55 ± 0.004 | −0.23 ± 0.005 | 3.14 ± 0.002 | 0.61 ± 0.02 |
| Al1.25 | 1.05 ± 0.003 | −0.24 ± 0.003 | 3.01 ± 0.002 | 0.61 ± 0.01 |
| Al2.5 | 1.58 ± 0.005 | −0.26 ± 0.004 | 3.50 ± 0.004 | 0.61 ± 0.01 |
| Al5 | 2.21 ± 0.002 | −0.33 ± 0.002 | 6.17 ± 0.003 | 0.46 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lian, C.; Xie, W.; Fang, H.; Wang, W.; Yu, J.; Li, J.; He, X. Effect of Cr:Al Ratio on Corrosion Mechanism of Ni-Cr-Mo-Al Alloys in 3.5 wt.% NaCl Solution: Microstructure and Electrochemical and Passive Characteristics. Materials 2025, 18, 2177. https://doi.org/10.3390/ma18102177
Lian C, Xie W, Fang H, Wang W, Yu J, Li J, He X. Effect of Cr:Al Ratio on Corrosion Mechanism of Ni-Cr-Mo-Al Alloys in 3.5 wt.% NaCl Solution: Microstructure and Electrochemical and Passive Characteristics. Materials. 2025; 18(10):2177. https://doi.org/10.3390/ma18102177
Chicago/Turabian StyleLian, Chenggang, Wei Xie, Huanjie Fang, Wenqian Wang, Jianhao Yu, Jicheng Li, and Xiaodong He. 2025. "Effect of Cr:Al Ratio on Corrosion Mechanism of Ni-Cr-Mo-Al Alloys in 3.5 wt.% NaCl Solution: Microstructure and Electrochemical and Passive Characteristics" Materials 18, no. 10: 2177. https://doi.org/10.3390/ma18102177
APA StyleLian, C., Xie, W., Fang, H., Wang, W., Yu, J., Li, J., & He, X. (2025). Effect of Cr:Al Ratio on Corrosion Mechanism of Ni-Cr-Mo-Al Alloys in 3.5 wt.% NaCl Solution: Microstructure and Electrochemical and Passive Characteristics. Materials, 18(10), 2177. https://doi.org/10.3390/ma18102177





