Extraction of Active Compounds from Dioscorea quinqueloba and Their Encapsulation Using Mucin and Chitosan for Application in Cosmetic Formulations
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Equipment
2.2. Synthesis of Chitosan
2.3. Extraction of Active Compounds from Dioscorea quinqueloba Using Ethanol
2.4. Extraction of Mucin
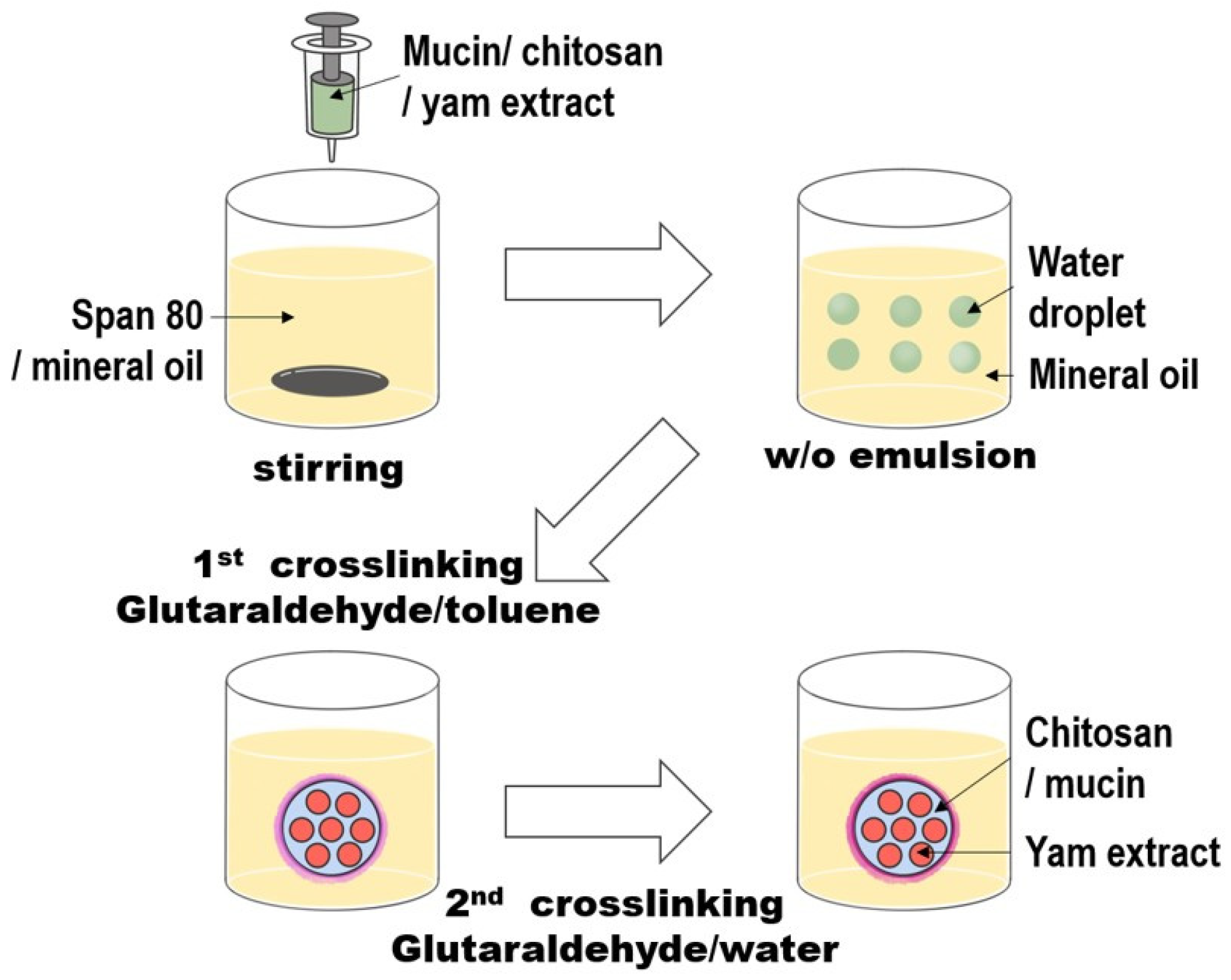
2.5. Preparation of Microcapsules
2.6. Toxicity Test Using MTT Assay
2.7. Antioxidant Effect
2.8. Anti-Aging Experiment Using Real-Time Polymerase Chain Reaction (RT-PCR) [46]
2.9. Controlled-Release Experiment [47]
3. Results and Discussion
3.1. Chitosan Preparation
3.2. Extraction of Mucin from Dioscorea quinqueloba
3.3. Extraction of Active Compounds from Dioscorea quinqueloba
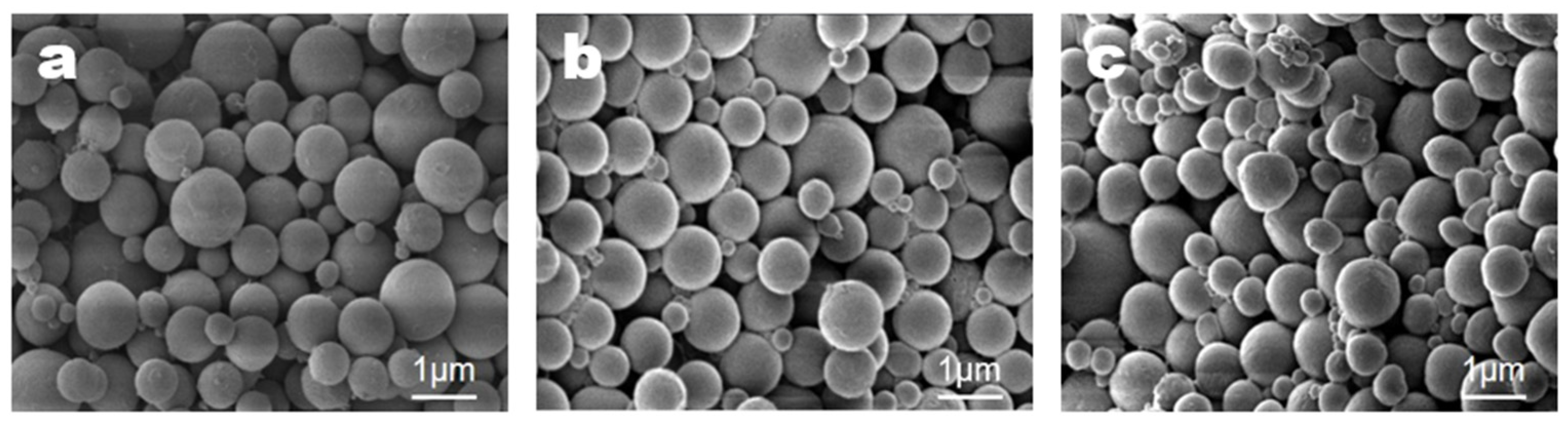
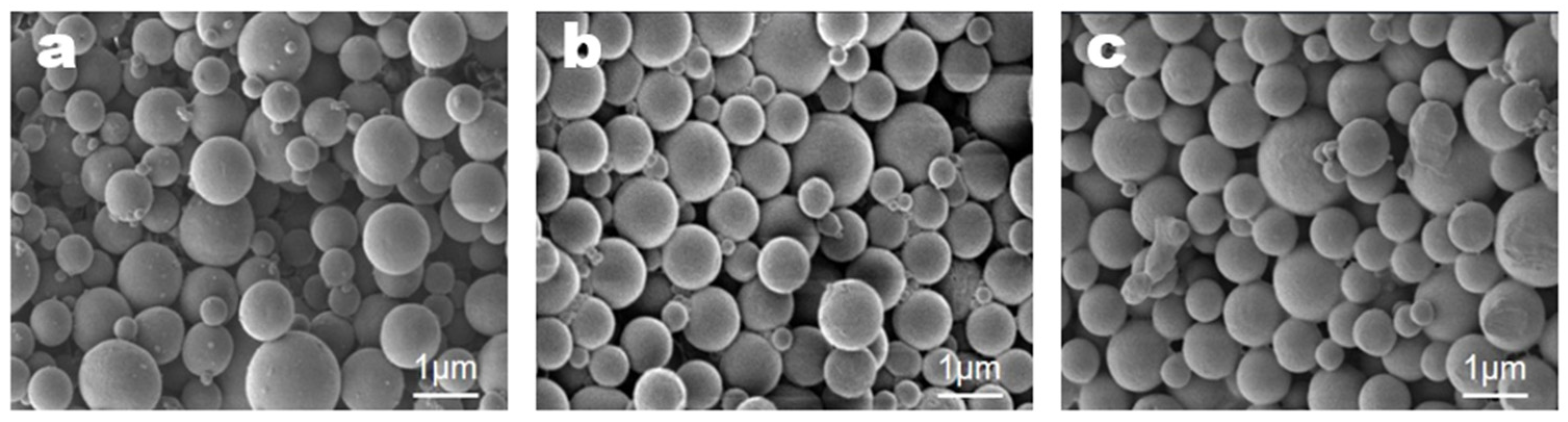
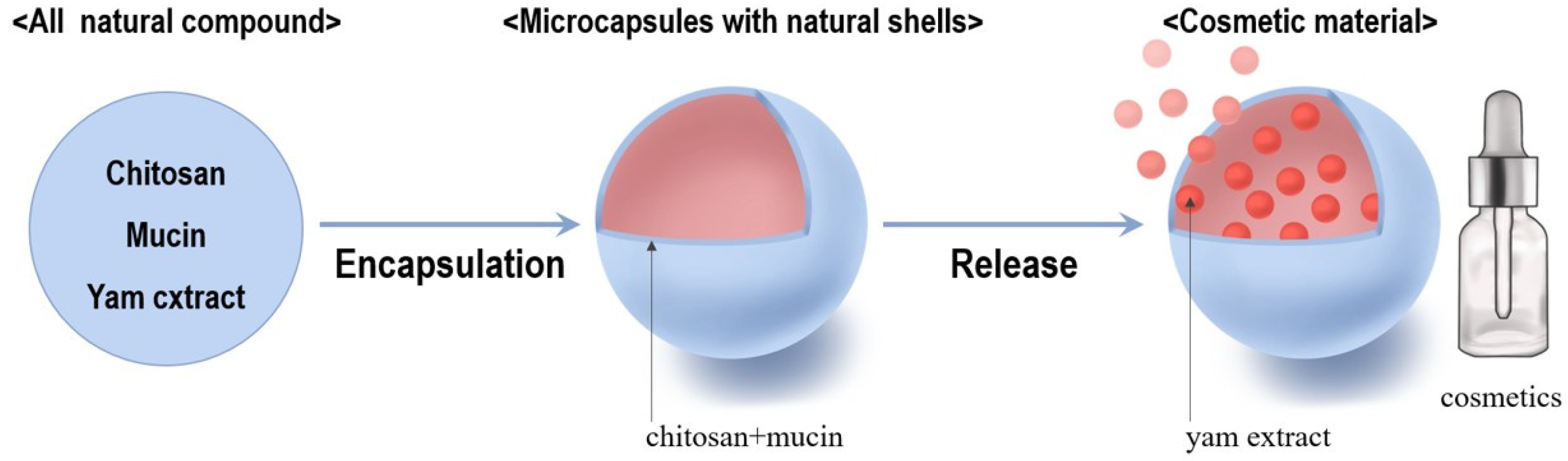
3.4. Microencapsulation
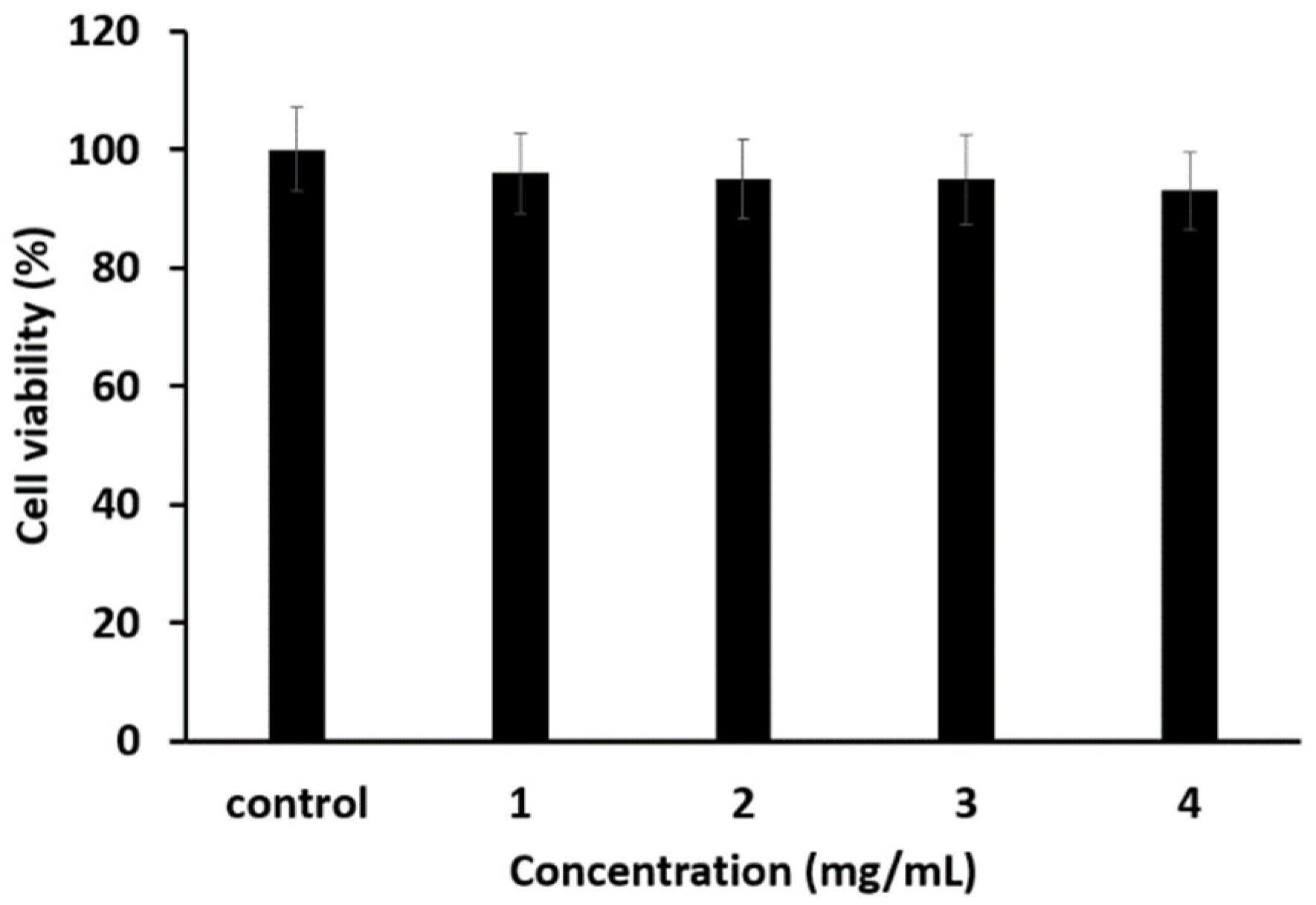
3.5. Toxicity Test
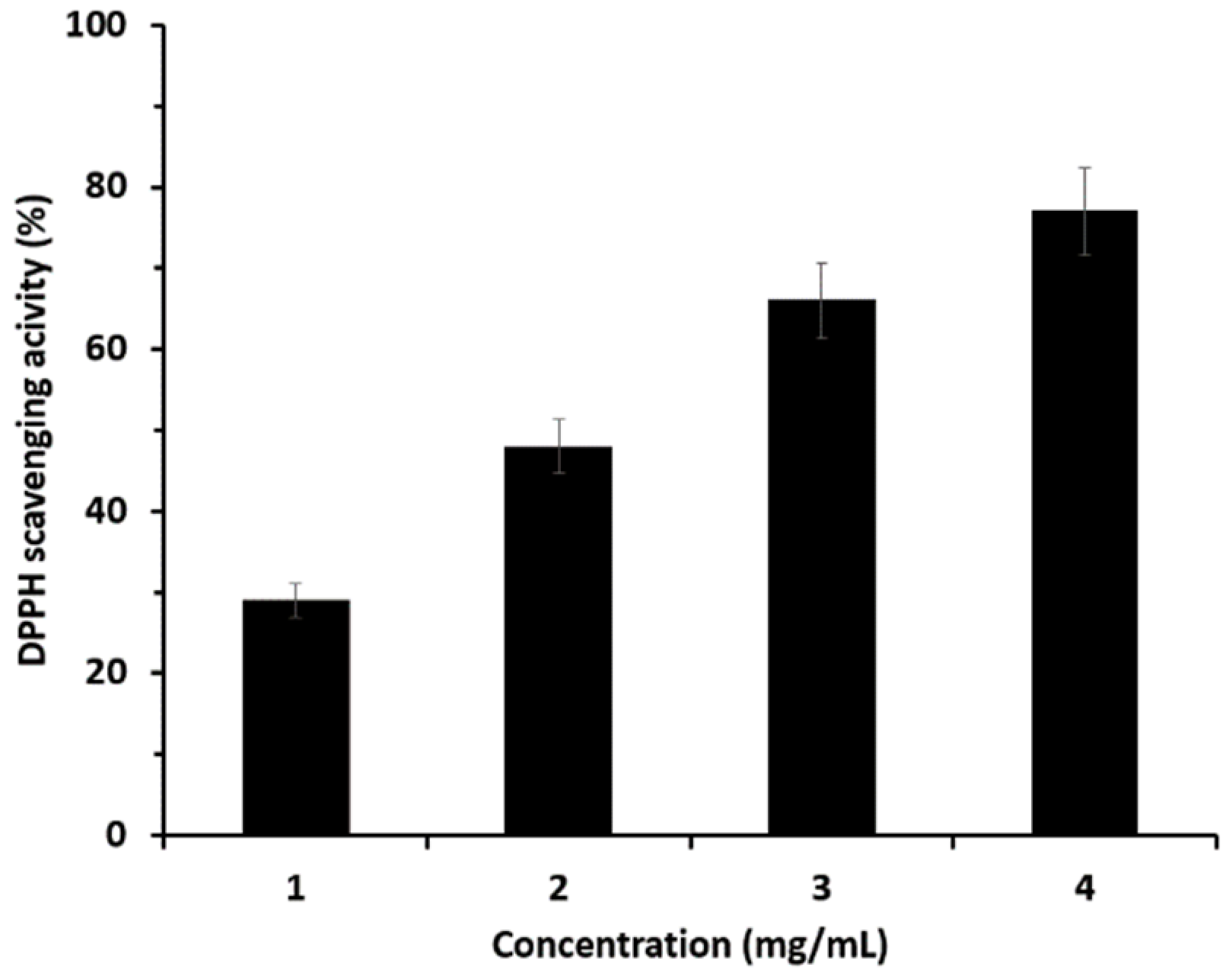
3.6. Antioxidant Effect
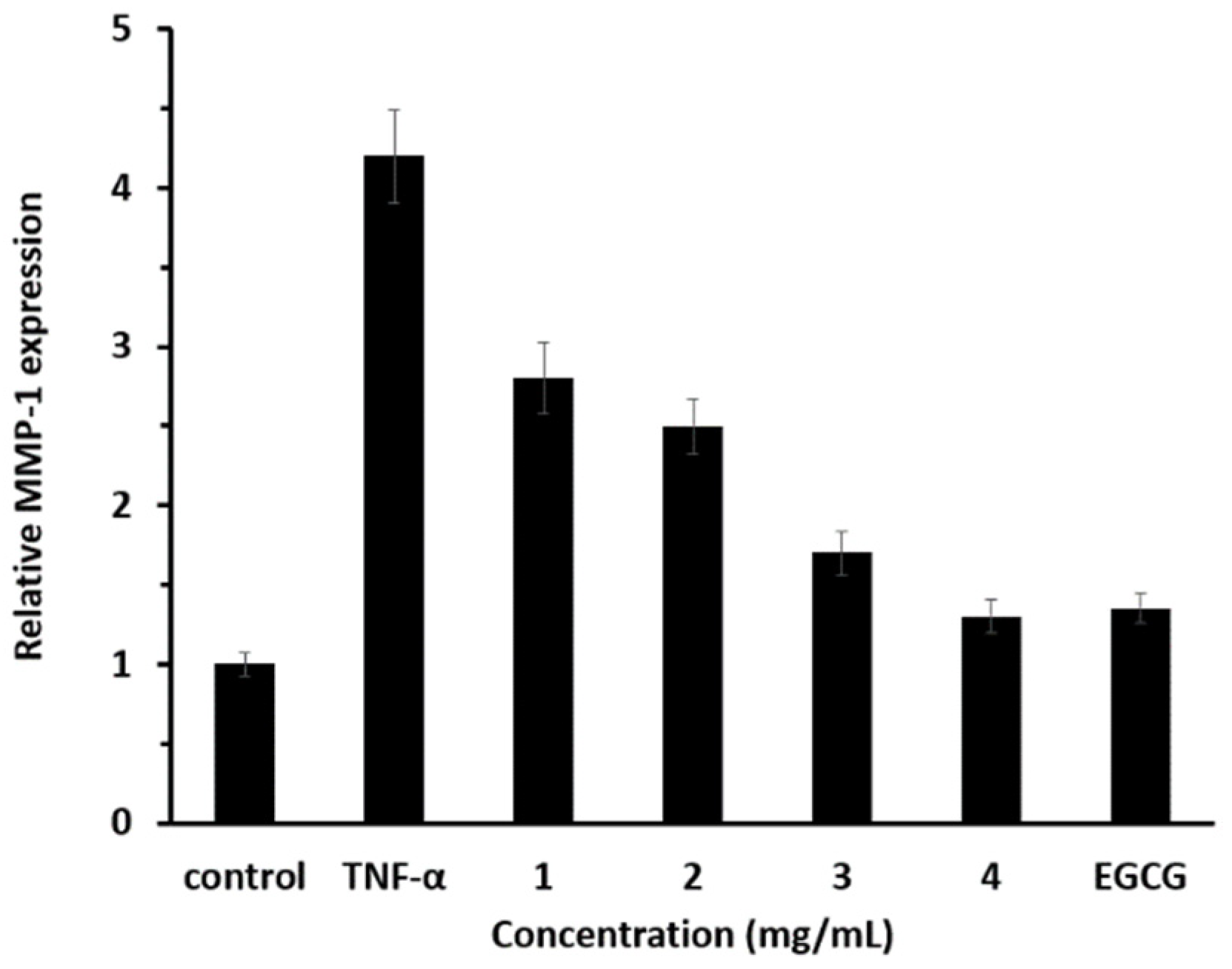
3.7. Anti-Aging Effect
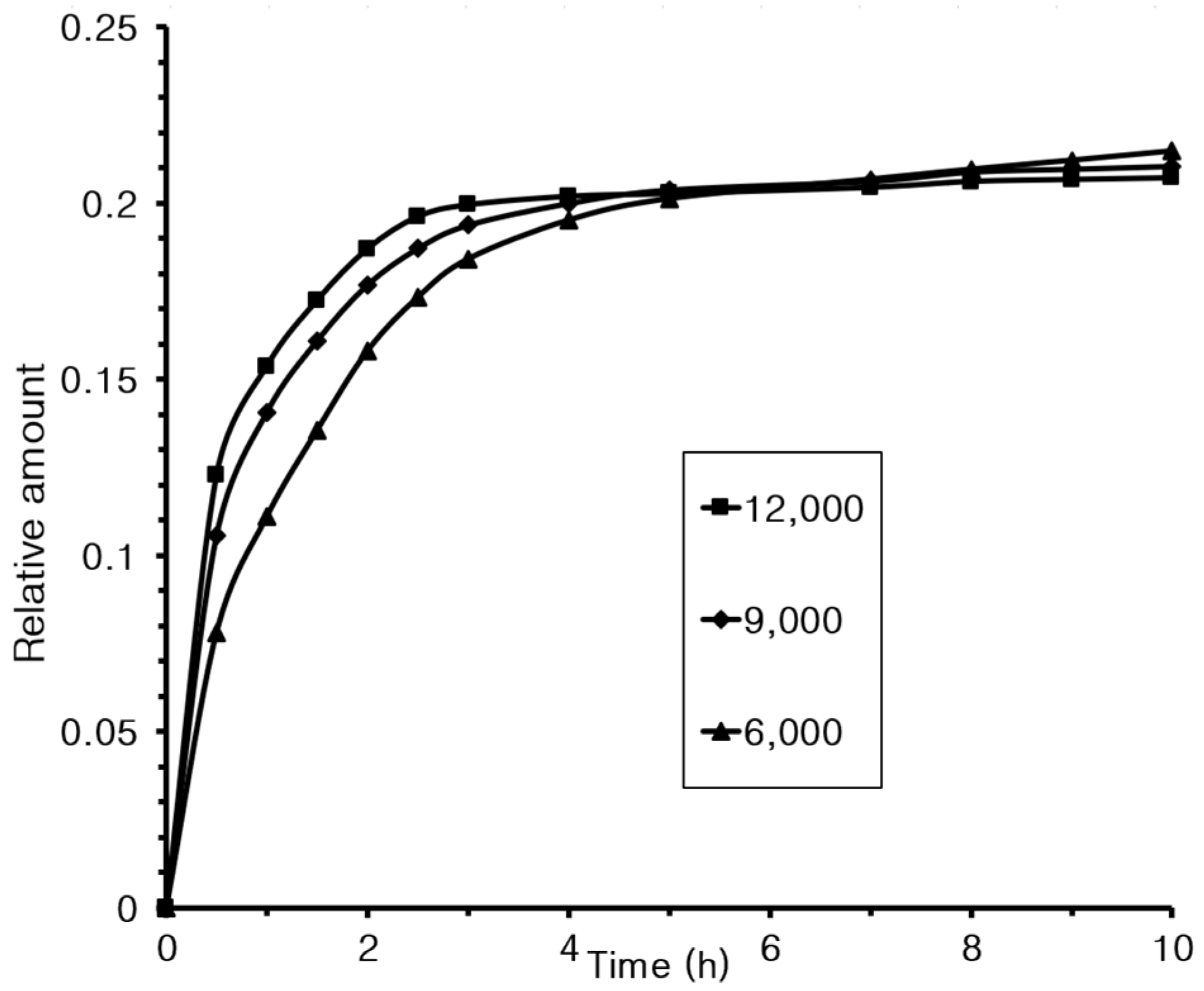
3.8. Controlled Release
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, C.M.; Son, K.H.; Kim, S.H.; Kim, H.P. Steroidal sapogenin contents in some domestic plants. Arch. Pharm. Res. 1991, 14, 305–310. [Google Scholar] [CrossRef]
- Komesaroff, P.A.; Black, C.V.S.; Cable, V.; Sudhir, K. Effects of wild yam extract on menopausal symptoms, lipids and sex hormones in healthy menopausal women. Climacteric 2001, 4, 144–150. [Google Scholar] [CrossRef]
- Bhandari, M.R.; Kawabata, J. Organic acid, phenolic content and antioxidant activity of wild yam (Dioscorea spp.) tubers of Nepal. Food Chem. 2004, 88, 163–168. [Google Scholar] [CrossRef]
- Bhandari, M.R.; Kawabata, J. Bitterness and toxicity in wild yam (Dioscorea spp.) tubers of Nepal. Plant Food Hum. Nutr. 2005, 60, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.H.; Nikolic, D.; Simmler, C.; Qiu, F.; Breemen, R.B.; Soejarto, D.D.; Pauli, G.F.; Chen, S.N. Diarylheptanoids from Dioscorea villosa (wild yam). J. Nat. Prod. 2012, 75, 2168–2177. [Google Scholar] [CrossRef] [PubMed]
- Okigbo, R.N.; Opara, P.U.; Anuagasi, C.L. Efficacy of extracts of water yam (Dioscorea alata) and aerial yam (Dioscorea bulbifera) peels in the control of white yam (Dioscorea rotundata) rot. J. Agr. Technol. 2015, 11, 1823–1842. [Google Scholar]
- Oyovwi, M.O.; Atere, A.D. Exploring the medicinal significance of l-arginine mediated nitric oxide in preventing health disorders. Eur. J. Med. Chem. Rep. 2024, 12, 100175. [Google Scholar] [CrossRef]
- Wolf, A.; Zalpour, C.; Theilmeier, G.; Wang, B.; Ma, A.; Anderson, B.; Tsao, P.S.; Cooke, J.P. Dietary l-arginine supplementation normalizes platelet aggregation in hypercholesterolemic humans. J. Am. Coll. Cardiol. 1997, 29, 479–485. [Google Scholar] [CrossRef]
- Man, S.; Gao, W.; Zhang, Y.; Huang, L.; Liu, C. Chemical study and medical application of saponins as anti-cancer agents. Fitoterapia 2010, 81, 703–714. [Google Scholar] [CrossRef]
- Liu, W.; Deng, S.; Zhou, D.; Huang, Y.; Li, C.; Hao, L.; Zhang, G.; Su, S.; Xu, X.; Yang, R.; et al. 3,4-seco-Dammarane triterpenoid saponins with anti-inflammatory activity isolated from the leaves of Cyclocarya paliurus. J. Agric. Food Chem. 2020, 68, 2041–2053. [Google Scholar] [CrossRef]
- Grondin, J.A.; Kwon, Y.H.; Far, P.M.; Haq, S.; Khan, W.I. Mucins in intestinal mucosal defense and inflammation: Learning from clinical and experimental studies. Front. Immunol. 2020, 11, 2054. [Google Scholar] [CrossRef] [PubMed]
- Arai, J.; Hayakawa, Y.; Tateno, H.; Fujiwara, H.; Kasuga, M.; Fujishiro, M. The role of gastric mucins and mucin-related glycans in gastric cancers. Cancer Sci. 2024, 115, 2853–2861. [Google Scholar] [CrossRef]
- Breugelmans, T.; Oosterlinck, B.; Arras, W.; Ceuleers, H.; Man, J.; Hold, G.L.; Winter, B.Y.; Smet, A. The role of mucins in gastrointestinal barrier function during health and disease. Lancet 2022, 7, 455–471. [Google Scholar] [CrossRef]
- Belzer, C. Nutritional strategies for mucosal health: The interplay between microbes and mucin glycans. Trends Microbiol. 2022, 30, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jeong, E.J.; Park, C.; Lee, J.S.; Kim, W.J.; Yu, K.W.; Suh, H.J.; Ahn, Y.; Moon, S.K. Modulation of gut microbiota ecosystem by a glucan-rich snail mucin heteropolysaccharide attenuates loperamide-induced constipation. Int. J. Biol. Macromol. 2023, 253, 126560. [Google Scholar] [CrossRef]
- Wu, X.; Yang, H.J.; Ryu, M.S.; Jung, S.J.; Ha, K.; Jeong, D.Y.; Park, S. Association of mucin-degrading gut microbiota and dietary patterns with colonic transit time in constipation: A secondary analysis of a randomized clinical trial. Nutrients 2025, 17, 138. [Google Scholar] [CrossRef]
- Kim, Y.; Sim, W.J.; Lee, J.S.; Lim, T.G. Snail mucin is a functional food ingredient for skin. J. Funct. Foods 2022, 92, 105053. [Google Scholar] [CrossRef]
- Singh, N.; Brown, A.N.; Gold, M.H. Snail extract for skin: A review of uses, projections, and limitations. J. Cosmet. Dermatol. 2024, 23, 1113–1121. [Google Scholar] [CrossRef]
- Stasse, M.; Ribaut, T.; Schmitt, V.; Heroguez, V. Encapsulation of lipophilic fragrance by polymerization of the intermediate aqueous phase of an oil-in-water-in-oil (O/W/O) double emulsion. Polym. Chem. 2019, 10, 4154–4162. [Google Scholar] [CrossRef]
- Dang, Y.T.; Tran, H.; Kha, T.C. Encapsulation of W/O/W acerola emulsion by spray drying: Optimization, release kinetics, and storage stability. Foods 2024, 13, 1463. [Google Scholar] [CrossRef]
- Fraj, J.; Petrovic, L.; Dekic, L.; Budincic, J.M.; Bucko, S.; Katona, J. Encapsulation and release of vitamin C in double W/O/W emulsions followed by complex coacervation in gelatin-sodium caseinate system. J. Food Eng. 2021, 292, 110353. [Google Scholar] [CrossRef]
- Wan, C.; He, S.; Cheng, Q.; Du, K.; Song, Y.; Yu, X.; Jiang, H.; Huang, C.; Xu, J.; Ma, C.; et al. Bridged emulsion gels from polymer–nanoparticle enabling large-amount biomedical encapsulation and functionalization. Nature Commun. 2024, 15, 10789. [Google Scholar] [CrossRef] [PubMed]
- Tessar, L.; Martelli-Tosi, M.; Sobral, P.J. Development of W/O emulsion for encapsulation of “Pitanga” (Eugenia uniflora L.) leaf hydroethanolic extract: Droplet size, physical stability and rheology. Food Sci. Technol. 2022, 42, e65320. [Google Scholar] [CrossRef]
- Hino, T.; Shimabayashi, S.; Tanaka, M.; Nakano, M.; Okochi, H. Improvement of encapsulation efficiency of water-in-oil-inwater emulsion with hypertonic inner aqueous phase. J. Microencapsul. 2001, 18, 19–28. [Google Scholar] [PubMed]
- Lv, J.; Lv, X.; Ma, M.; Oh, D.H.; Jiang, Z.; Fu, X. Chitin and chitin-based biomaterials: A review of advances in processing and food applications. Carbohydr. Polym. 2023, 299, 120142. [Google Scholar] [CrossRef]
- Sharp, R.G. A Review of the applications of chitin and its derivatives in agriculture to modify plant-microbial interactions and improve crop yields. Agronomy 2013, 3, 757–793. [Google Scholar] [CrossRef]
- Crini, G. Historical review on chitin and chitosan biopolymers. Environ. Chem. Lett. 2019, 17, 1623–1643. [Google Scholar] [CrossRef]
- Che, X.; Zhao, T.; Hu, J.; Yang, K.; Ma, N.; Li, A.; Sun, Q.; Ding, C.; Ding, Q. Application of chitosan-based hydrogel in promoting wound healing. Polymers 2024, 16, 344. [Google Scholar] [CrossRef]
- Pramanik, S.; Aggarwal, A.; Kadi, A.; Alhomrani, M.; Alamri, A.S.; Alsanie, W.F.; Koul, K.; Deepak, A.; Bellucci, S. Chitosan alchemy: Transforming tissue engineering and wound healing. RSC Adv. 2024, 14, 19219–19256. [Google Scholar] [CrossRef]
- Fu, C.; Qi, Z.; Zhao, C.; Kong, W.; Li, H.; Guo, W.; Yang, X. Enhanced wound repair ability of arginine-chitosan nanocomposite membrane through the antimicrobial peptides-loaded polydopamine-modified graphene oxide. J. Biol. Eng. 2021, 15, 17. [Google Scholar] [CrossRef]
- Ahn, S.I.; Cho, S.; Choi, N.J. Effectiveness of chitosan as a dietary supplement in lowering cholesterol in murine models: A meta-analysis. Mar. Drugs 2021, 19, 26. [Google Scholar] [CrossRef] [PubMed]
- Bokura, H.; Kobayashi, S. Chitosan decreases total cholesterol in women: A randomized, double-blind, placebo-controlled trial. Eur. J. Clin. Nutr. 2003, 57, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Moraru, C.; Mincea, M.M.; Frandes, M.; Timar, B.; Ostafe, V. A meta-analysis on randomised controlled clinical trials evaluating the effect of the dietary supplement chitosan on weight loss, lipid parameters and blood pressure. Medicina 2018, 54, 109. [Google Scholar] [CrossRef]
- Tao, W.; Wang, G.; Wei, J. The role of chitosan oligosaccharide in metabolic syndrome: A review of possible mechanisms. Mar. Drugs 2021, 19, 501. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, C.; Davachi, S.M.; Li, P.; Davidowsky, P.; Yan, B. Development of microcapsules using chitosan and alginate via W/O emulsion for the protection of hydrophilic compounds by comparing with hydrogel beads. Int. J. Biol. Macromol. 2021, 177, 92–99. [Google Scholar] [CrossRef]
- Nemickaite, E.; Zlabiene, U.; Mazurkeviciute, A.; Marksa, M.; Bernatoniene, J. Formulation of W/O/W emulsion-based chitosan-alginate microcapsules for encapsulation of cannabidiol and A. annua L. extract containing luteolin and apigenin: A response surface optimization approach. Pharmaceutics 2025, 17, 309. [Google Scholar] [CrossRef]
- Park, J.K.; Lee, D.H.; Lee, C.I.; Kang, K.C.; Pyo, H.B.; Shin, J.S. Preparation of chitosan microcapsules containing rosmarinic acid. J. Adhes. Interface 2009, 10, 11–16. [Google Scholar]
- Hwang, S.W.; Shin, J.S. Pectin-coated curcumin-chitosan microparticles crosslinked with Mg2+ for delayed drug release in the digestive system. Int. J. Polym. Sci. 2018, 2018, 2071071. [Google Scholar] [CrossRef]
- Liu, Y.F.; Huang, K.L.; Peng, D.M.; Ding, P.; Li, G.Y. Preparation and characterization of glutaraldehyde cross-linked O-carboxymethylchitosan microspheres for controlled delivery of pazufloxacin mesylate. Int. J. Biol. Macromol. 2007, 41, 87–93. [Google Scholar] [CrossRef]
- Qin, S.; Li, H.; Hu, C. Thermal properties and morphology of chitosan/gelatin composite shell microcapsule via multi-emulsion. Mater. Lett. 2021, 291, 129475. [Google Scholar] [CrossRef]
- Back, S.H.; Kim, S.H.; Son, K.H.; Chung, K.C.; Chang, H.W. Inactivation of human pleural fluid phospholipse A2 by dioscin. Arch. Pharm. Res. 1994, 17, 218–222. [Google Scholar] [CrossRef]
- Mazzio, E.; Almalki, A.; Darling-Reed, S.F.; Soliman, K.F.A. Effects of wild yam root (Dioscorea villosa) extract on the gene expression profile of triple-negative breast cancer cells. Cancer Genom. Proteom. 2021, 18, 735–755. [Google Scholar] [CrossRef] [PubMed]
- Raj, P.S.; Bergfeld, W.F.; Belsito, D.V.; Cohen, D.E.; Klaassen, C.D.; Rettie, A.E.; Ross, D.; Slaga, T.J.; Snyder, P.W.; Tilton, S.; et al. Dioscorea villosa (wild yam) root extract. Int. J. Toxicol. 2023, 42, 29S–31S. [Google Scholar] [CrossRef]
- Ma, L.; Liu, M.; Shi, X. pH- and temperature-sensitive self-assembly microcapsules/microparticles: Synthesis, characterization, in vitro cytotoxicity, and drug release properties. J. Biomed. Mater. Res. B 2012, 100B, 305–313. [Google Scholar] [CrossRef]
- Kwon, J.B.; Kim, M.S.; Sohn, H.Y. Evaluation of antimicrobial, antioxidant, and antithrombin activities of the rhizome of various Dioscorea species. Korean J. Food Presev. 2010, 17, 391–397. [Google Scholar]
- Shim, J.H. Anti-aging effect of Agarum cribrosum in UV-irradiated normal human epidermal keratinocytes. Kor. J. Pharmacogn. 2021, 52, 228–233. [Google Scholar]
- Shin, M.J.; Kim, J.G.; Shin, J.S. Microencapsulation of imidazole curing agents by spray-drying method using W/O emulsion. J. Appl. Polym. Sci. 2012, 126 (Suppl. S2), E108–E115. [Google Scholar] [CrossRef]
- Dimzon, K.D.; Knepper, T.P. Degree of deacetylation of chitosan by infrared spectroscopy and partial least squares. Int. J. Biol. Macromol. 2015, 72, 939–945. [Google Scholar] [CrossRef]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.M.; Arguelles-Monal, W.; Desbrieres, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polymer 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Jung, H.S.; Kim, M.H.; Park, W.H. Preparation and structural investigation of novel β-chitin nanocrystals from cuttlefish bone. ACS Biomater. Sci. Eng. 2019, 5, 1744–1752. [Google Scholar] [CrossRef]
- Adomeniene, A.; Venskutonis, P.R. Dioscorea spp.: Comprehensive review of antioxidant properties and their relation to phytochemicals and health benefits. Molecules 2022, 27, 2530. [Google Scholar] [CrossRef] [PubMed]
- Tabopda, T.K.; Mitaine-Offer, A.C.; Tanaka, C.; Miyamoto, T.; Mirjolet, J.F.; Duchamp, O.; Ngadjui, B.T.; Lacaille-Dubois, M.A. Steroidal saponins from Dioscorea preussii. Fitoterapia 2014, 97, 198–203. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, M.J. Extraction of Active Compounds from Dioscorea quinqueloba and Their Encapsulation Using Mucin and Chitosan for Application in Cosmetic Formulations. Materials 2025, 18, 2178. https://doi.org/10.3390/ma18102178
Shin MJ. Extraction of Active Compounds from Dioscorea quinqueloba and Their Encapsulation Using Mucin and Chitosan for Application in Cosmetic Formulations. Materials. 2025; 18(10):2178. https://doi.org/10.3390/ma18102178
Chicago/Turabian StyleShin, Min Jae. 2025. "Extraction of Active Compounds from Dioscorea quinqueloba and Their Encapsulation Using Mucin and Chitosan for Application in Cosmetic Formulations" Materials 18, no. 10: 2178. https://doi.org/10.3390/ma18102178
APA StyleShin, M. J. (2025). Extraction of Active Compounds from Dioscorea quinqueloba and Their Encapsulation Using Mucin and Chitosan for Application in Cosmetic Formulations. Materials, 18(10), 2178. https://doi.org/10.3390/ma18102178



