Tribological Properties of Selected Ionic Liquids in Lubricated Friction Nodes
Abstract
1. Introduction
2. Materials and Methods
- Load (P) = 10 N;
- Sliding velocity (v) = 0.1 m/s;
- Sliding distance (s) = 1000 m;
- Friction node: a ball of 100Cr6 steel—a disc of 100Cr6 steel;
- Radius: 8 mm;
- Lubricant—ionic liquid:
- ◦
- Tributyl(methyl)phosphonium dimethyl phosphate 97%—MFCD;
- ◦
- 1-Butyl-3-methylimidazolium hexafluorophosphate 97%—BMIMPF6;
- ◦
- 1-Butyl-3-methylimidazolium tetrafluoroborate 98%—BMIMBF4;
- The amount of the ionic liquids during tests at ambient temperature and 40 °C: about 3 mL and 40 mL, respectively;
- Test execution temperatures: ambient (25 ± 1.5 °C) and 40 °C;
- Humidity: 40 ± 0.5%.
3. Results
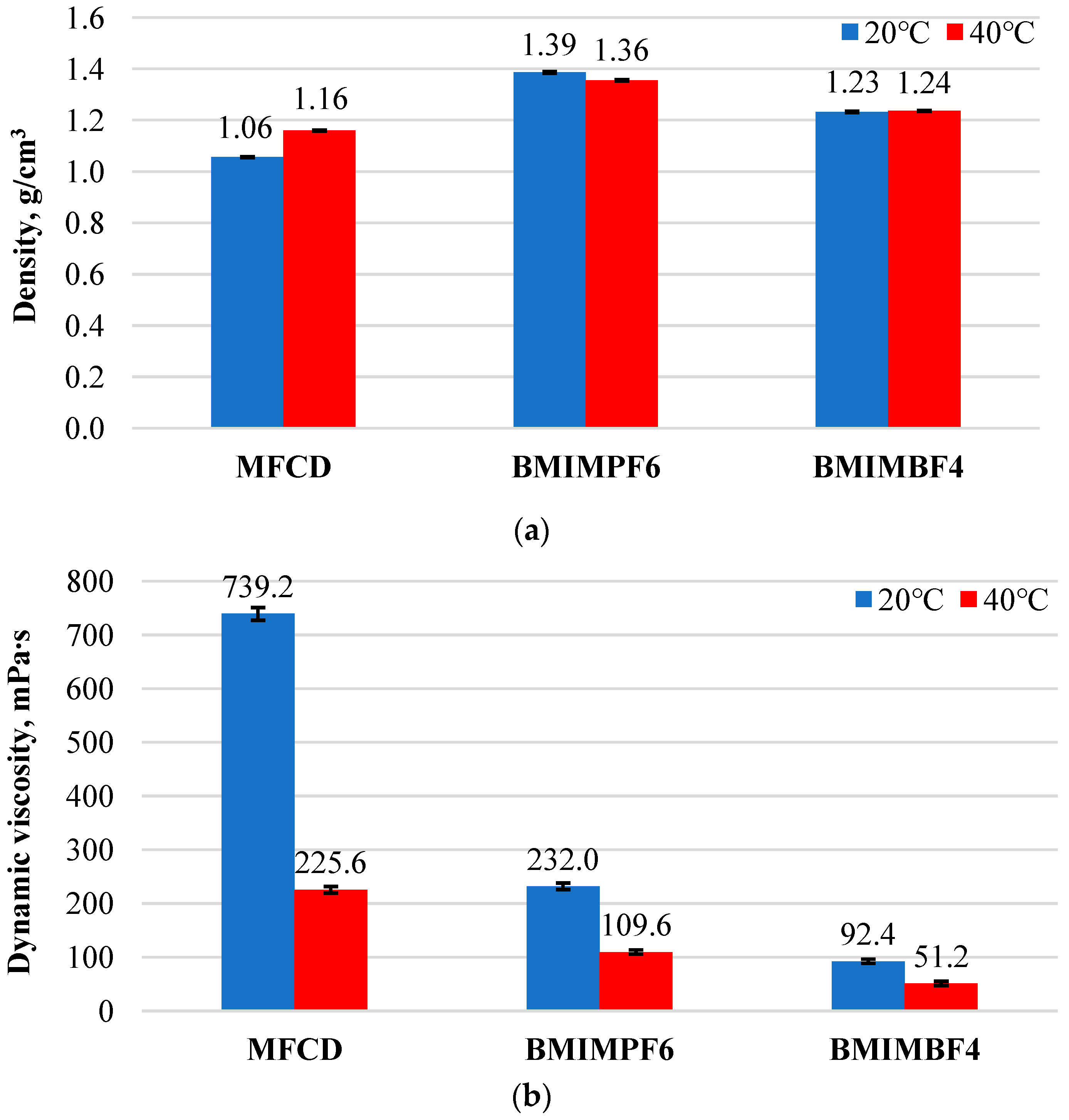
3.1. Density and Dynamic Viscosity Results
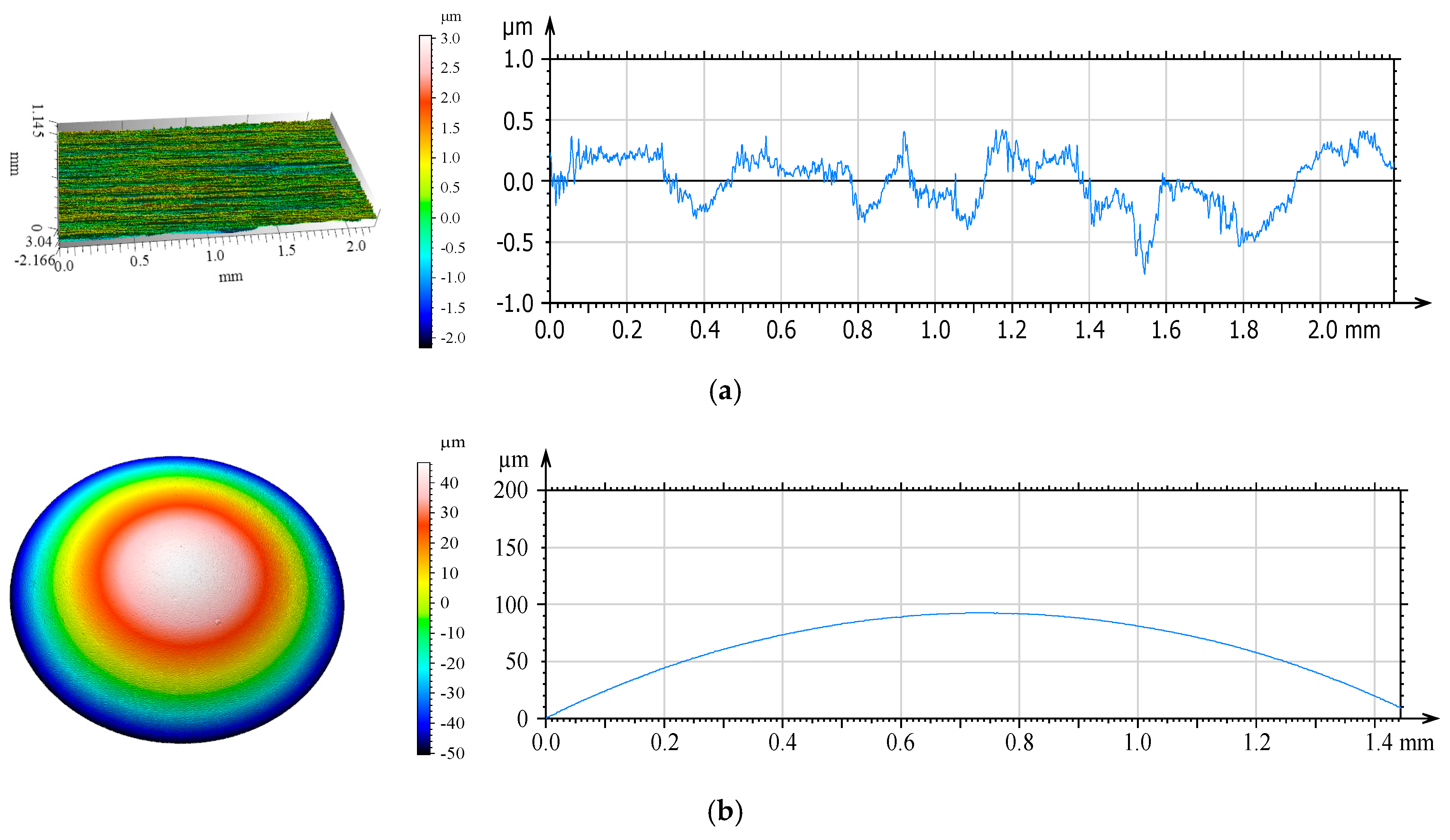
3.2. Confocal Microscopy Results
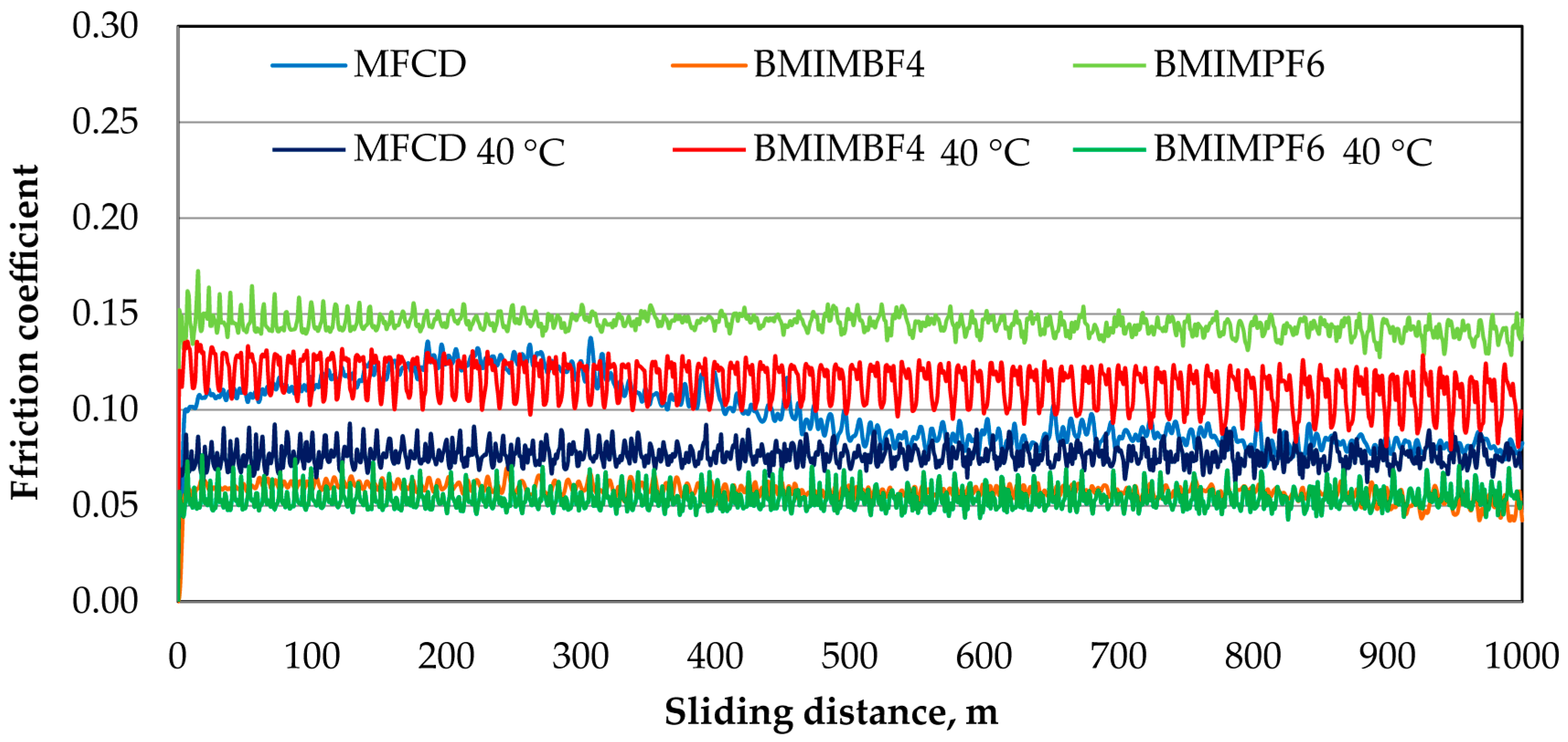
3.3. Tribological Tests
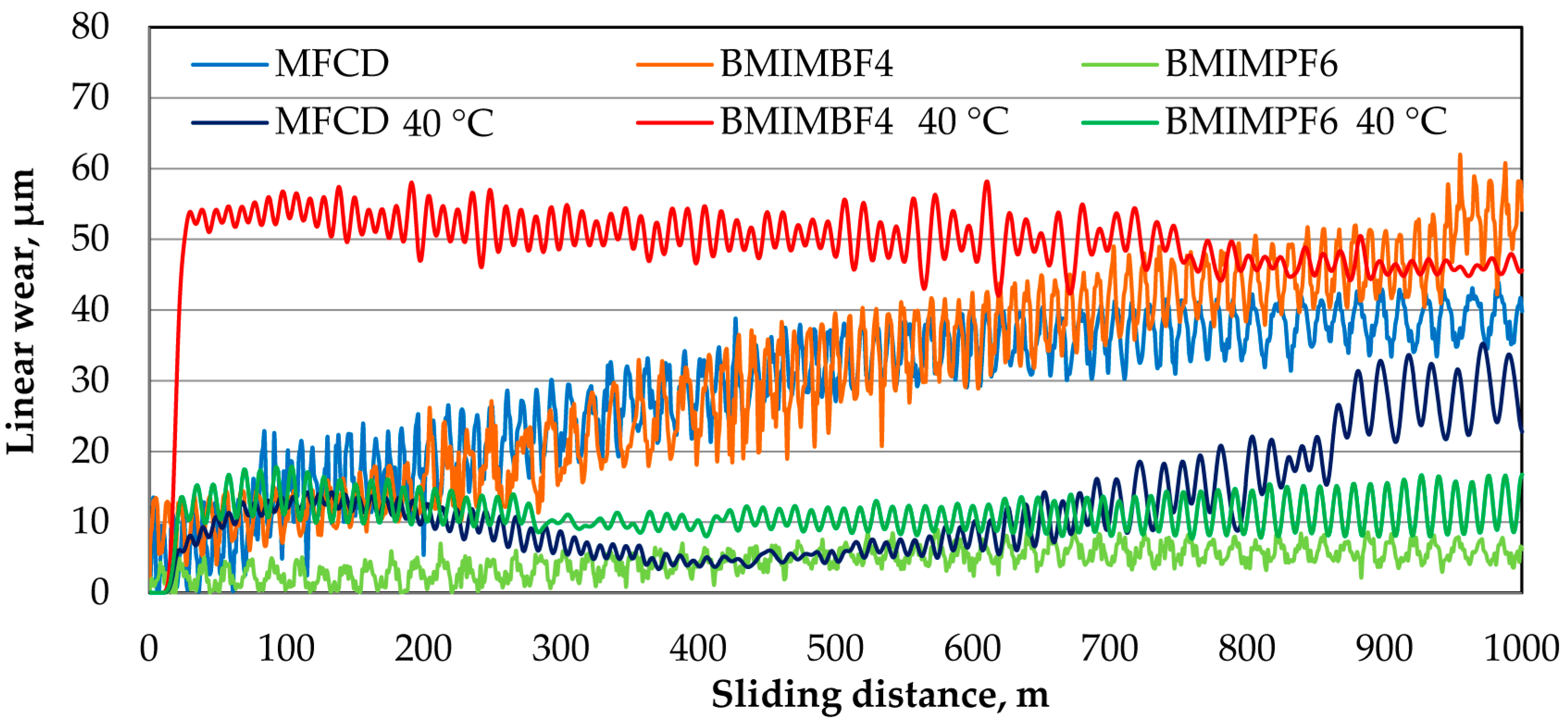
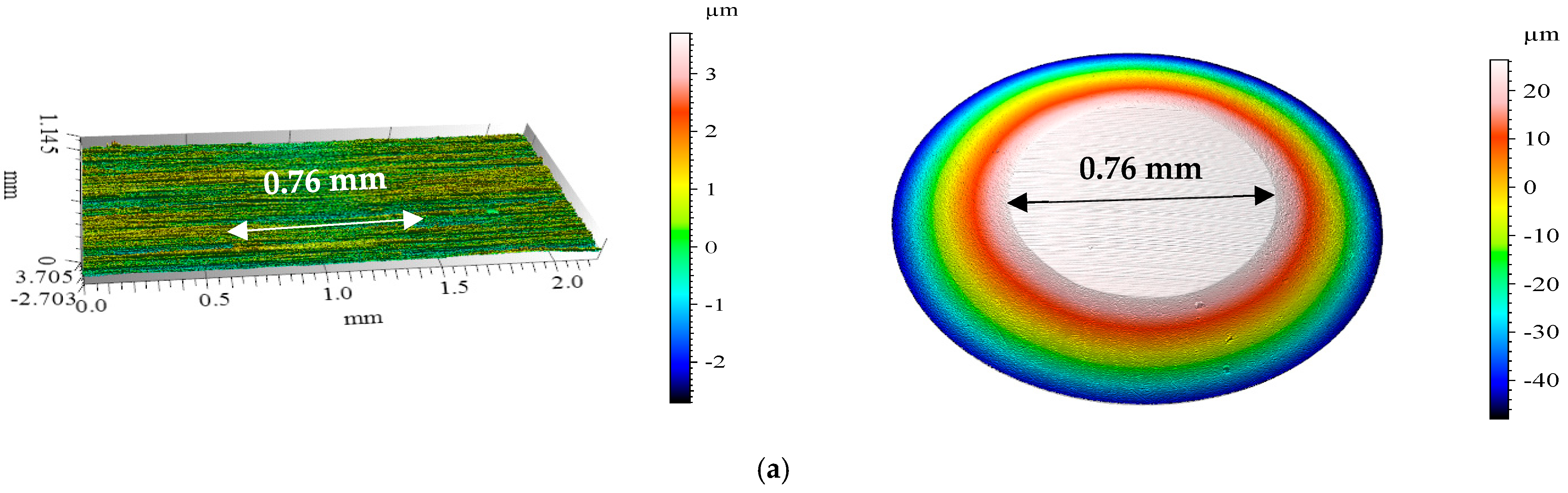
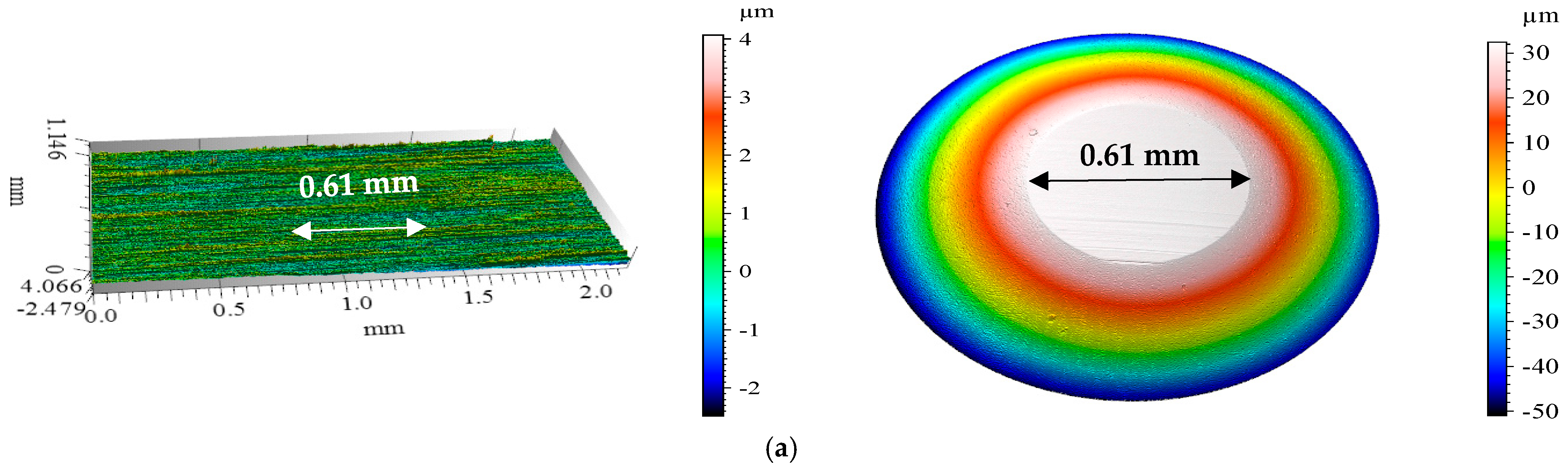
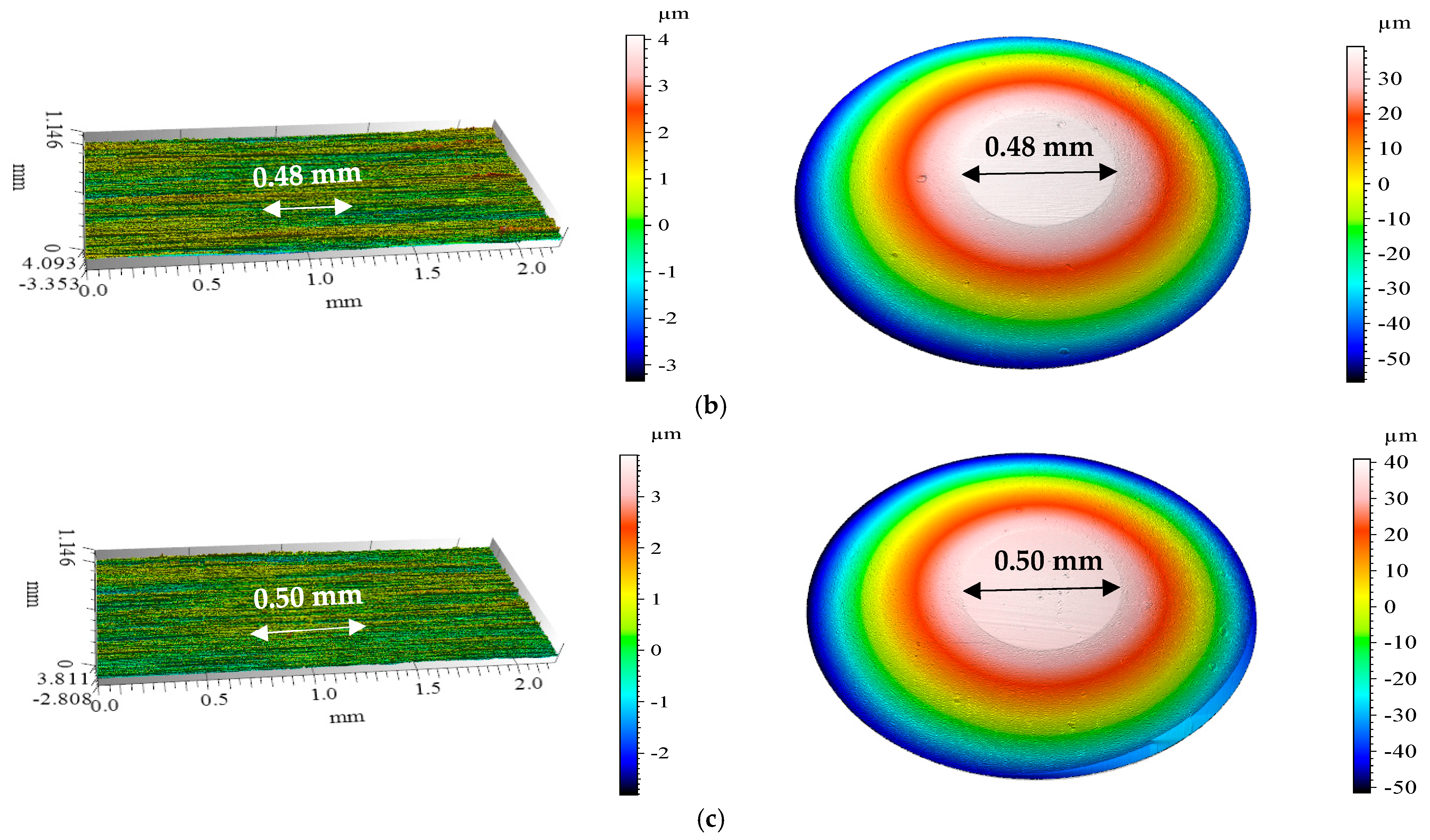
3.4. Confocal Microscopy Results After Tribological Tests
- r—the radius of the wear track (mm);
- h—the height of the worn surface (mm);
- R—the radius of the sphere (mm).
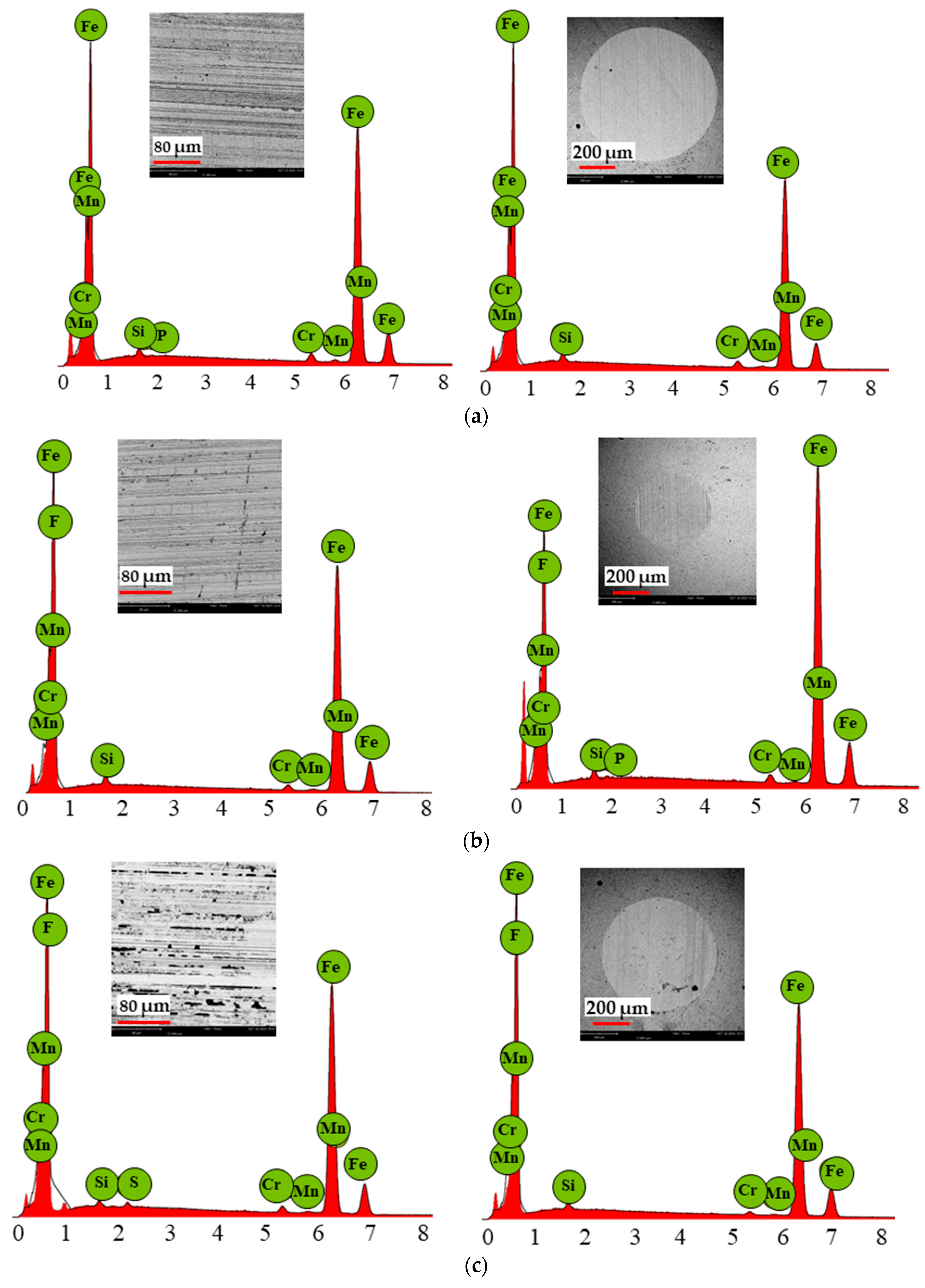
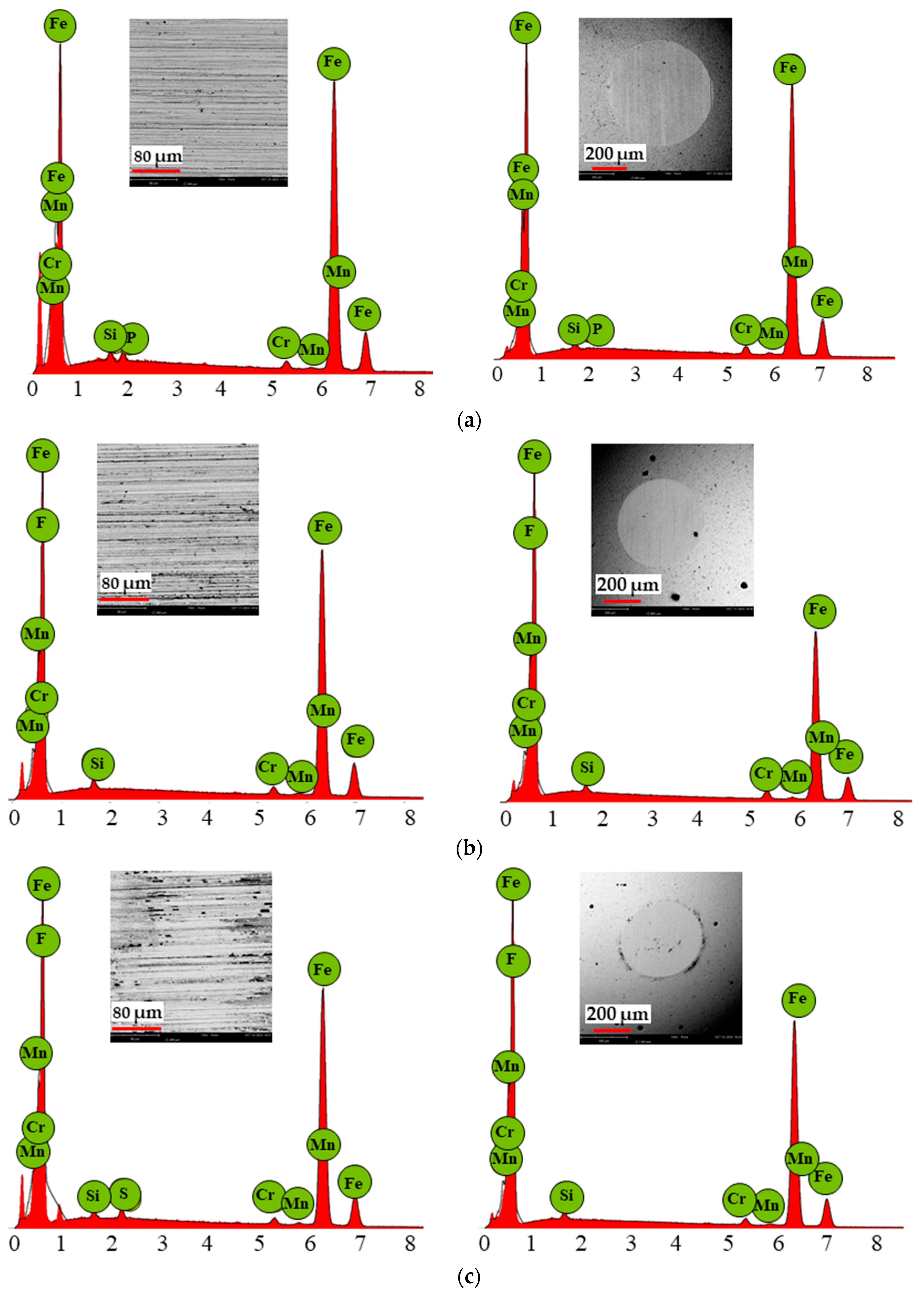
3.5. Scanning Electron Microscopy Results
4. Conclusions
- Temperature was found to influence the density and viscosity of the ionic liquids. The density behavior varied, while the dynamic viscosity of all ionic liquids decreased with increasing temperature. BMIMPF6 showed a decrease in density, whereas MFCD and BMIMBF4 exhibited an increase in density as temperature increased.
- After conducting tribological tests under ambient conditions, the BMIMBF4 ionic liquid exhibited the lowest coefficient of friction. On the other hand, the BMIMPF6 ionic liquid achieved the lowest linear wear, which is supported by the minimal wear traces observed on the disc and ball.
- Tests of friction at 40 °C showed that the BMIMPF6 ionic liquid gave the least friction and wear. This is because the fluorine in the liquid helped create a protective layer that reduced wear, and the liquid itself was dense and viscous enough to fill in gaps on the surfaces, providing good lubrication.
- The increased temperature contributed to a higher phosphorus concentration on both the disc and ball surfaces during lubrication with the MFCD ionic liquid. A tribofilm formed, acting as an anti-wear surface layer, which reduced wear on both the sample and counter-sample.
- A fluorine layer formed on the discs and balls during the tribological tests with BMIMBF4 and BMIMPF6 ionic liquids and caused corrosion at room temperature and 40 °C. Corrosion was less severe when both fluorine and phosphorus (in BMIMPF6) were present. Conversely, corrosion was more pronounced when only BF4- anions (from BMIMBF4) were present. Pitting was observed on the surfaces of the discs and balls.
Author Contributions
Funding
Institutional Review Boards Statement
Informer Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alqahtani, B.; Hoziefa, W.; Abdel Moneam, H.M.; Hamoud, M.; Salunkhe, S.; Elshalakany, A.B.; Abdel-Mottaleb, M.; Davim, J.P. Tribological Performance and Rheological Properties of Engine Oil with Graphene Nano-Additives. Lubricants 2022, 10, 137. [Google Scholar] [CrossRef]
- Dzierwa, A. Effects of Surface Preparation on Friction and Wear in Dry Sliding Conditions. Tribologia 2017, 272, 25–31. [Google Scholar] [CrossRef]
- Tian, J.; Qi, X.; Xian, G. Effect of Hygrothermal Aging on the Friction Behavior and Wear Mechanism of the Multi-Filler Reinforced Epoxy Composites for Coated Steel. J. Mater. Res. Technol. 2024, 32, 140–151. [Google Scholar] [CrossRef]
- Madej, M.; Styp-Rekowski, M.; Wieciński, P.; Płociński, T.; Ozimina, D.; Kurzydłowski, K.; Matuszewski, M. Properties of Diamond-like Carbon Coatings Deposited on Cocrmo Alloys. Trans. FAMENA 2015, 39, 79–88. [Google Scholar]
- Sovelto, O. Principles of Lasers; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Radek, N.; Szczotok, A.; Gądek-Moszczak, A.; Dwornicka, R.; Bronček, J.; Pietraszek, J. The Impact of Laser Processing Parameters on the Properties of Electro-Spark Deposited Coatings. Arch. Metall. Mater. 2018, 63, 809–816. [Google Scholar] [CrossRef]
- Madej, M.; Ozimina, D. Electroless Ni-P-Al2O3 Composite Coatings. Kov. Mater. 2006, 44, 291–296. [Google Scholar]
- Li, C.; Li, X.; Huang, S.; Li, L.; Zhang, F. Ultra-Precision Grinding of Gd3Ga5O12 Crystals with Graphene Oxide Coolant: Material Deformation Mechanism and Performance Evaluation. J. Manuf. Process. 2021, 61, 417–427. [Google Scholar] [CrossRef]
- Kowalczyk, J.; Madej, M.; Ozimina, D. Evaluation of Performance Characteristics of the Environmentally Friendly Cutting Fluid with Zinc Aspartate. Eksploat. I Niezawodn. Maint. Reliab. 2020, 22, 465–471. [Google Scholar] [CrossRef]
- Dobrovolný, K.; Ulbrich, P.; Bartůněk, V. Synthesis of Ultrafine Metallic Copper Nanocubes Using Ethanol-Ionic Liquid Approach. J. Clust. Sci. 2016, 27, 1843–1847. [Google Scholar] [CrossRef]
- Ling, D.; Tian-Xi, H.E.; Yun, X.; Jia-Feng, W.U.; Li-Gong, C.; Guo-Nuo, C. Ionic Liquids as Novel Lubricants. Prog. Chem. 2010, 22, 298–308. [Google Scholar]
- Sasaki, S. Environmentally Friendly Tribology (Eco-Tribology). J. Mech. Sci. Technol. 2010, 24, 67–71. [Google Scholar] [CrossRef]
- Sharma, V.; Doerr, N.; Aswath, P.B. Chemical–Mechanical Properties of Tribofilms and Their Relationship to Ionic Liquid Chemistry. RSC Adv. 2016, 6, 22341–22356. [Google Scholar] [CrossRef]
- Barnhill, W. Tribological Testing and Analysis of Ionic Liquids as Candidate Anti-Wear Additives for Next- Generation Engine Lubricants. Master’s Thesis, University of Tennessee, Knoxville, TN, USA, 2016. [Google Scholar]
- Avilés, M.-D.; Saurín, N.; Sanes, J.; Carrión, F.-J.; Bermúdez, M.-D. Ionanocarbon Lubricants. The Combination of Ionic Liquids and Carbon Nanophases in Tribology. Lubricants 2017, 5, 14. [Google Scholar] [CrossRef]
- Li, C.; Wang, G.; Han, Q.; Feng, G.; Wang, L.; Wang, S. Effects of Current-Carrying Conditions on Lubrication and Tribological Performance of Ionic Liquid. J. Mol. Liq. 2022, 367, 120471. [Google Scholar] [CrossRef]
- Shaikh, S.; Sadeghi, M.; Cruz, S.; Ferreira, F. Recent Progress on the Tribology of Pure/Doped Diamond-like Carbon Coatings and Ionic Liquids. Coatings 2024, 14, 71. [Google Scholar] [CrossRef]
- Molaei Yielzoleh, F.; Nikoofar, K. Nano Silicated-FeAl2O4 Functionalized by DL-Alaninium Nitrate Ionic Liquid (FeAl2O4-SiO2@[DL-Ala][NO3]) as Versatile Promotor for Aqua-Mediated Synthesis of Spiro[Chromenopyrazole-Indene-Triones and Spiro[Chromenopyrazole-Indoline-Diones. Sci. Rep. 2024, 14, 16296. [Google Scholar] [CrossRef]
- Liu, W.; Ye, C.; Gong, Q.; Wang, H.; Wang, P. Tribological Performance of Room-Temperature Ionic Liquids as Lubricant. Tribol. Lett. 2002, 13, 81–85. [Google Scholar] [CrossRef]
- Kaisy, G.M.J.A.; Mutalib, M.I.A.; Rao, T.V.V.L.N.; Senatore, A. Tribological Performance of Low Viscosity Halogen-Free Ammonium Based Protic Ionic Liquids with Carboxylate Anions as Neat Lubricants. Tribol. Int. 2021, 160, 107058. [Google Scholar] [CrossRef]
- He, Y.; Li, H.; Qu, C.; Cao, W.; Ma, M. Recent Understanding of Solid-Liquid Friction in Ionic Liquids. Green Chem. Eng. 2021, 2, 145–157. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Gao, X.; Liao, H. The Preparation of Crumpled Graphene Oxide Balls and Research in Tribological Properties. Materials 2024, 17, 2383. [Google Scholar] [CrossRef]
- Karpierz, E.; Niedzicki, L.; Trzeciak, T.; Zawadzki, M.; Dranka, M.; Zachara, J.; Żukowska, G.Z.; Bitner-Michalska, A.; Wieczorek, W. Ternary Mixtures of Ionic Liquids for Better Salt Solubility, Conductivity and Cation Transference Number Improvement. Sci. Rep. 2016, 6, 35587. [Google Scholar] [CrossRef] [PubMed]
- Vyavhare, K.; Aswath, P.B. Tribological Properties of Novel Multi-Walled Carbon Nanotubes and Phosphorus Containing Ionic Liquid Hybrids in Grease. Front. Mech. Eng. 2019, 5, 15. [Google Scholar] [CrossRef]
- Nooruddin, N.S.; Wahlbeck, P.G.; Carper, W.R. Molecular Modeling of Ionic Liquid Tribology: Semi-Empirical Bonding and Molecular Structure. J. Mol. Struct. Theochem 2007, 822, 1–7. [Google Scholar] [CrossRef]
- Silva, D.M.e.; Ribeiro, T.; Branco, L.C.; Colaço, R.; da Silva, A.G.; Saramago, B. Hydrophobic Ionic Liquids at Liquid and Solid Interfaces. Tribol. Int. 2019, 129, 459–467. [Google Scholar] [CrossRef]
- Liu, M.; Ni, J.; Zhang, C.; Wang, R.; Cheng, Q.; Liang, W.; Liu, Z. The Application of Ionic Liquids in the Lubrication Field: Their Design, Mechanisms, and Behaviors. Lubricants 2024, 12, 24. [Google Scholar] [CrossRef]
- Jiménez, A.E.; Bermúdez, M.D.; Iglesias, P.; Carrión, F.J.; Martínez-Nicolás, G. 1-N-Alkyl-3-Methylimidazolium Ionic Liquids as Neat Lubricants and Lubricant Additives in Steel–Aluminium Contacts. Wear 2006, 260, 766–782. [Google Scholar] [CrossRef]
- Jiménez, A.-E.; Bermúdez, M.-D. Ionic Liquids as Lubricants for Steel–Aluminum Contacts at Low and Elevated Temperatures. Tribol. Lett. 2007, 26, 53–60. [Google Scholar] [CrossRef]
- Madej, M.; Ozimina, D.; Marczewska-Boczkowska, K. Effect of Tungsten on the Durability of Diamond-like Carbon Coatings in the Chemical Industry. Przemysł Chem. 2014, 93, 500–505. [Google Scholar]
- Totolin, V.; Minami, I.; Gabler, C.; Brenner, J.; Dörr, N. Lubrication Mechanism of Phosphonium Phosphate Ionic Liquid Additive in Alkylborane–Imidazole Complexes. Tribol. Lett. 2014, 53, 421–432. [Google Scholar] [CrossRef]
- Wang, H.; Lu, Q.; Ye, C.; Liu, W.; Cui, Z. Friction and Wear Behaviors of Ionic Liquid of Alkylimidazolium Hexafluorophosphates as Lubricants for Steel/Steel Contact. Wear 2004, 256, 44–48. [Google Scholar] [CrossRef]
- Safety Data Sheet. Available online: https://www.chemsrc.com/en/cas/20445-88-9_1188873.html (accessed on 1 December 2024).
- Safety Data Sheet. Available online: https://www.sigmaaldrich.com/PL/pl/search/1-butyl-3-methylimidazolium-tetrafluoroborate?focus=products&page=1&perpage=30&sort=relevance&term=1-Butyl-3-methylimidazolium%20tetrafluoroborate&type=product (accessed on 1 December 2024).
- Cverna, F.; Conti, P.; ASM International Materials Properties Database Committee. Worldwide Guide to Equivalent Irons and Steels, 5th ed.; Materials Data Series; ASM International Materials Park: Russell Township, OH, USA, 2006; ISBN 978-0-87170-822-9. [Google Scholar]
- Minami, I.; Inada, T.; Sasaki, R.; Nanao, H. Tribo-Chemistry of Phosphonium-Derived Ionic Liquids. Tribol. Lett. 2010, 40, 225–235. [Google Scholar] [CrossRef]
- Battez, A.H.; Bartolomé, M.; Blanco, D.; Viesca, J.L.; Fernández-González, A.; González, R. Phosphonium Cation-Based Ionic Liquids as Neat Lubricants: Physicochemical and Tribological Performance. Tribol. Int. 2016, 95, 118–131. [Google Scholar] [CrossRef]
- Al-Sallami, W.; Parsaeian, P.; Neville, A. An Appraisal of the Thermal Decomposition Mechanisms of ILs as Potential Lubricants. Lubr. Sci. 2019, 31, 229–238. [Google Scholar] [CrossRef]
- Forbes, E.S.; Battersby, J. The Effect of Chemical Structure on the Load-Carrying and Adsorption Properties of Dialkyl Phosphites. ASLE Trans. 1974, 17, 263–270. [Google Scholar] [CrossRef]
- Forbes, E.S.; Neustadter, E.L.; Silver, H.B.; Upsdell, N.T. The Effect of Chemical Structure on the Load-Carrying Properties of Amine Phosphates. Wear 1971, 18, 269–278. [Google Scholar] [CrossRef]
- Sakurai, T.; Sato, K. Chemical Reactivity and Load Carrying Capacity of Lubricating Oils Containing Organic Phosphorus Compounds. ASLE Trans. 1970, 13, 252–261. [Google Scholar] [CrossRef]
- Bertrand, P.A. Reactions of Tricresyl Phosphate with Bearingmaterials. Tribol. Lett. 1997, 3, 367–377. [Google Scholar] [CrossRef]
- Minami, I. Ionic Liquids in Tribology. Molecules 2009, 14, 2286–2305. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Lee, T.-C.; Lin, J.-Y.; Chang, J.-K.; Tseng, C.-M. Corrosion Properties of Metals in Dicyanamide-Based Ionic Liquids. Corros. Sci. 2014, 78, 81–88. [Google Scholar] [CrossRef]
- Skonieczny, M.; Izdebska, N.; Królikowska, M.; Marczewski, M. Corrosion Behaviour of Aluminum and Copper in Dimethyl- and Diethyl Phosphate Ionic Liquids. Electrochim. Acta 2024, 500, 144770. [Google Scholar] [CrossRef]
- Kinoshita, H.; Kondo, M.; Nisina, Y.; Fujii, M. Anti-Wear Effect of Graphene Oxide in Lubrication by Fluorine-Containing Ionic Liquid for Steel. Tribol. Online 2015, 10, 91–95. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, D.; Xu, B. Tribological Characteristics of Alkylimidazolium Diethyl Phosphates Ionic Liquids as Lubricants for Steel–Steel Contact. Tribol. Lett. 2009, 34, 95–101. [Google Scholar] [CrossRef]


| Short Name | Chemical Formula | Chemical Structure | Molecular Weight, g/mol |
|---|---|---|---|
| MFCD | C15H36O4P2 | 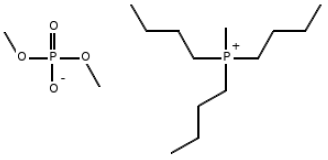 | 342.39 |
| BMIMPF6 | C8H15F6N2P | 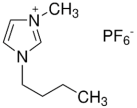 | 284.18 |
| BMIMBF4 | C8H15BF4N2 | 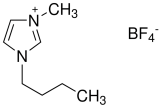 | 226.02 |
| Short Name | Purity, % | Amount of Water, % | Color | State of Concentration |
|---|---|---|---|---|
| MFCD | 97 | X | colorless to light yellow | liquid |
| BMIMPF6 | ≥97.0 (HPLC) | X | light yellow | liquid |
| BMIMBF4 | ≥98 | ≤0.5% | yellow | liquid |
| Element | C | Cr | Mn | Mo | Ni | P | S | Si | Al | Cu |
|---|---|---|---|---|---|---|---|---|---|---|
| Weight, % | 0.95–1.10 | 1.35–1.60 | 0.25–0.45 | max 0.10 | max 0.30 | max 0.03 | max 0.02 | 0.15–0.35 | max 0.05 | max 0.35 |
| Temperature | Ionic Liquid | Max. Depth, µm | Wear Track Area, µm2 | Max. Height, µm | Peak Area, µm2 |
|---|---|---|---|---|---|
| Ambient temperature | MFCD | 0.40 | 87.59 | 0.37 | 47.6 |
| BMIMPF6 | 0.18 | 3.65 | 0.41 | 54.94 | |
| BMIMBF4 | 0.60 | 60.12 | 0.50 | 69.39 | |
| 40 °C | MFCD | 0.37 | 52.03 | 0.37 | 55.21 |
| BMIMPF6 | 0.71 | 125.40 | 0.33 | 9.18 | |
| BMIMBF4 | 0.30 | 19.76 | 0.54 | 68.00 |
| Surface Roughness Parameters | Reference | MFCD | BMIMPF6 | BMIMPF4 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Disc | Ball | Disc | Ball | Disc | Ball | Disc | Ball | ||
| Sq | µm | 0.42 | 0.23 | 0.78 | 3.83 | 0.46 | 1.34 | 0.89 | 3.01 |
| Ssk | - | −0.31 | 0.70 | −0.11 | −0.57 | −0.66 | −2.22 | −0.12 | −1.10 |
| Sku | - | 3.16 | 3.86 | 2.63 | 2.33 | 3.72 | 7.79 | 3.11 | 3.45 |
| Sp | µm | 2.54 | 3.38 | 2.34 | 6.78 | 2.19 | 2.96 | 2.60 | 4.58 |
| Sv | µm | 1.81 | 1.29 | 2.89 | 8.74 | 2.16 | 5.54 | 2.99 | 8.53 |
| Sa | µm | 0.33 | 0.18 | 0.63 | 3.15 | 0.36 | 0.87 | 0.70 | 2.32 |
| Surface Roughness Parameters | Reference | MFCD | BMIMPF6 | BMIMPF4 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Disc | Ball | Disc | Ball | Disc | Ball | Disc | Ball | ||
| Sq | µm | 0.42 | 0.23 | 0.79 | 3.08 | 0.76 | 1.79 | 0.51 | 1.78 |
| Ssk | - | −0.31 | 0.70 | −0.31 | −1.07 | 0.00 | −1.82 | −1.03 | −1.62 |
| Sku | - | 3.16 | 3.86 | 2.79 | 3.39 | 2.93 | 5.85 | 4.75 | 5.11 |
| Sp | µm | 2.54 | 3.38 | 2.51 | 6.09 | 2.82 | 4.37 | 3.53 | 4.09 |
| Sv | µm | 1.81 | 1.29 | 3.04 | 8.60 | 2.51 | 6.41 | 2.82 | 6.99 |
| Sa | µm | 0.33 | 0.18 | 0.64 | 2.39 | 0.61 | 1.25 | 0.39 | 1.28 |
| Temperature |
Ionic Liquid | Wear of the Balls | ||
|---|---|---|---|---|
| Diameter, mm | High h, mm | Volume V, mm3 | ||
| Ambient temperature | MFCD | 0.76 | 0.048 | 0.044 |
| BMIMPF6 | 0.41 | 0.014 | 0.004 | |
| BMIMBF4 | 0.62 | 0.032 | 0.019 | |
| 40 °C | MFCD | 0.61 | 0.031 | 0.018 |
| BMIMPF6 | 0.48 | 0.019 | 0.007 | |
| BMIMBF4 | 0.50 | 0.021 | 0.008 | |
| Ionic Liquid | MFCD | BMIMPF6 | BMIMBF4 | |||
|---|---|---|---|---|---|---|
| Element | Disc | Ball | Disc | Ball | Disc | Ball |
| Fe | 97.51 | 97.51 | 85.74 | 87.60 | 85.09 | 85.74 |
| F | - | - | 12.54 | 10.62 | 13.34 | 12.54 |
| Cr | 1.27 | 1.42 | 1.05 | 0.79 | 1.02 | 1.05 |
| Si | 0.88 | 0.85 | 0.52 | 0.51 | 0.36 | 0.52 |
| Mn | 0.25 | 0.17 | 0.15 | 0.26 | 0.19 | 0.15 |
| P | - | - | - | 0.23 | - | - |
| S | - | - | - | - | - | - |
| Ionic Liquid | MFCD | BMIMPF6 | BMIMBF4 | |||
|---|---|---|---|---|---|---|
| Element | Disc | Ball | Disc | Ball | Disc | Ball |
| Fe | 97.76 | 97.83 | 85.61 | 81.76 | 86.08 | 84.09 |
| F | - | - | 12.28 | 16.25 | 11.23 | 13.75 |
| Cr | 1.23 | 1.20 | 1.14 | 1.32 | 0.94 | 1.16 |
| Si | 0.73 | 0.63 | 0.62 | 0.48 | 0.54 | 0.49 |
| Mn | 0.26 | 0.22 | 0.21 | 0.19 | 0.22 | 0.25 |
| P | - | - | 0.15 | - | - | - |
| S | - | - | - | - | 0.99 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madej, M.; Kowalczyk, J.; Kowalski, M.; Grabowski, P.; Wernik, J. Tribological Properties of Selected Ionic Liquids in Lubricated Friction Nodes. Materials 2025, 18, 18. https://doi.org/10.3390/ma18010018
Madej M, Kowalczyk J, Kowalski M, Grabowski P, Wernik J. Tribological Properties of Selected Ionic Liquids in Lubricated Friction Nodes. Materials. 2025; 18(1):18. https://doi.org/10.3390/ma18010018
Chicago/Turabian StyleMadej, Monika, Joanna Kowalczyk, Marcin Kowalski, Paweł Grabowski, and Jacek Wernik. 2025. "Tribological Properties of Selected Ionic Liquids in Lubricated Friction Nodes" Materials 18, no. 1: 18. https://doi.org/10.3390/ma18010018
APA StyleMadej, M., Kowalczyk, J., Kowalski, M., Grabowski, P., & Wernik, J. (2025). Tribological Properties of Selected Ionic Liquids in Lubricated Friction Nodes. Materials, 18(1), 18. https://doi.org/10.3390/ma18010018








