In Vivo Investigation of 3D-Printed Calcium Magnesium Phosphate Wedges in Partial Load Defects
Abstract
1. Introduction
2. Materials and Methods
2.1. Production and Characterization of the Scaffolds
2.1.1. Production of the Scaffolds
2.1.2. Chemical Composition
2.1.3. Determination of Compressive Strength
2.1.4. Porosity
2.1.5. Energy Dispersive X-ray Analysis (EDX) and Scanning Electron Microscopy (SEM) before Implantation
2.2. Animal Model
2.2.1. Implantation of the Wedges
2.2.2. Plate Removal
2.3. Radiological Examination and Semi-Quantitative Evaluation
2.4. In Vivo µCT Examination
2.4.1. Semi-Quantitative Evaluation of the In Vivo µCT Examination
2.4.2. Quantitative Evaluation of the In Vivo µCT Examination
2.5. Histological Examination and Semi-Quantitative Evaluation
Histomorphometric Examination
2.6. Energy Dispersive X-ray Analysis (EDX) and Scanning Electron Microscopy (SEM) of the Implanted Wedges
2.7. Statistics
3. Results
3.1. Chemical and Mechanical Properties of the Scaffolds
3.2. SEM/EDX Analyses before Implantation
3.3. OP and Clinical Examination
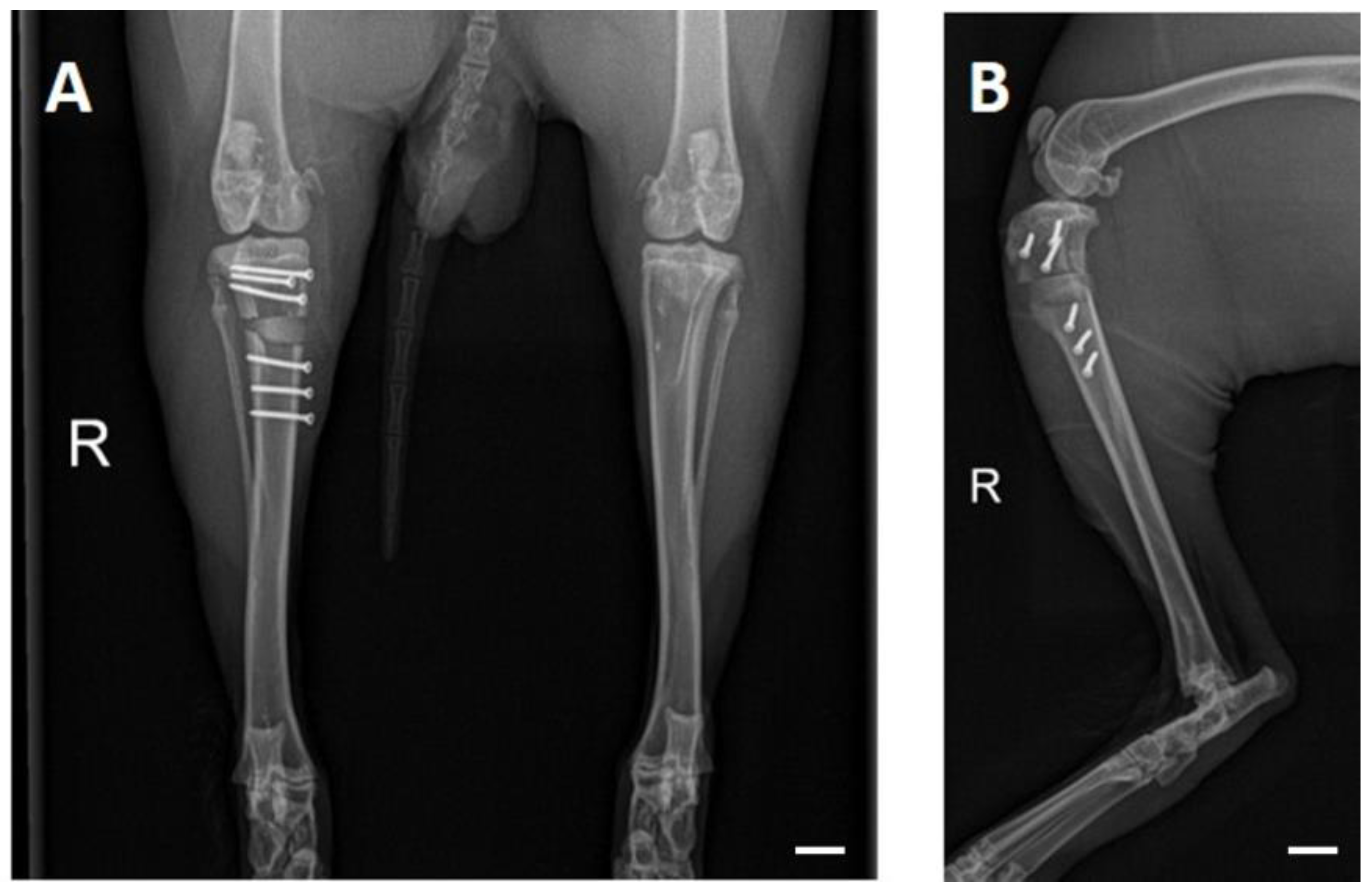
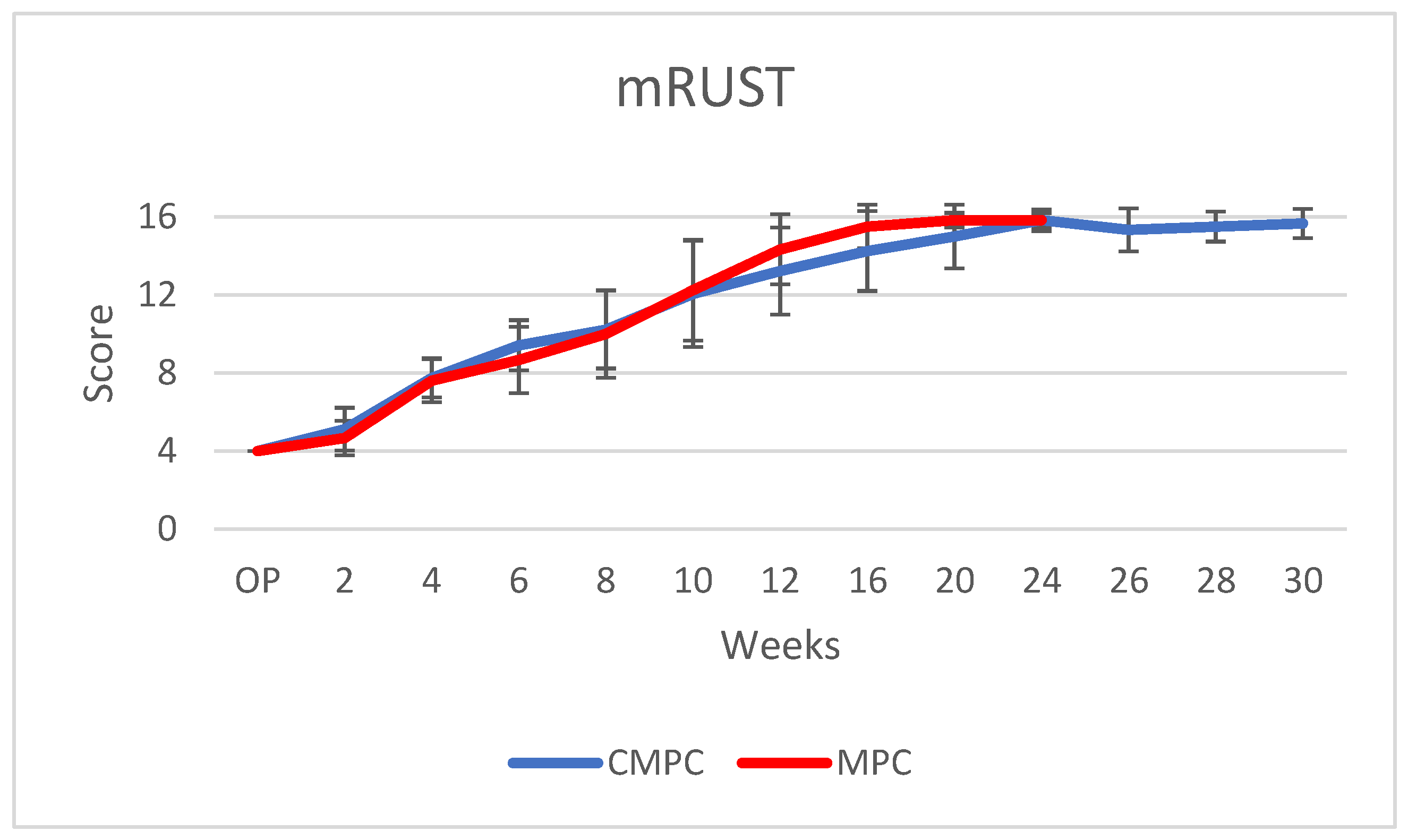
3.4. X-ray Examinations
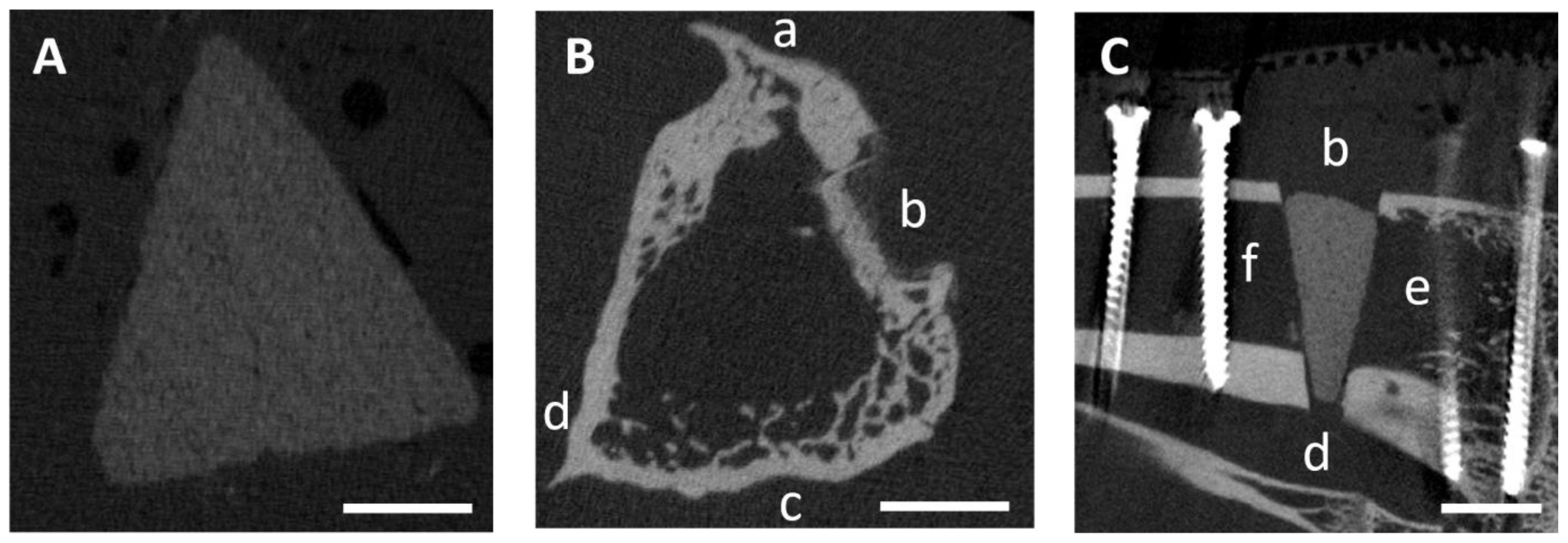
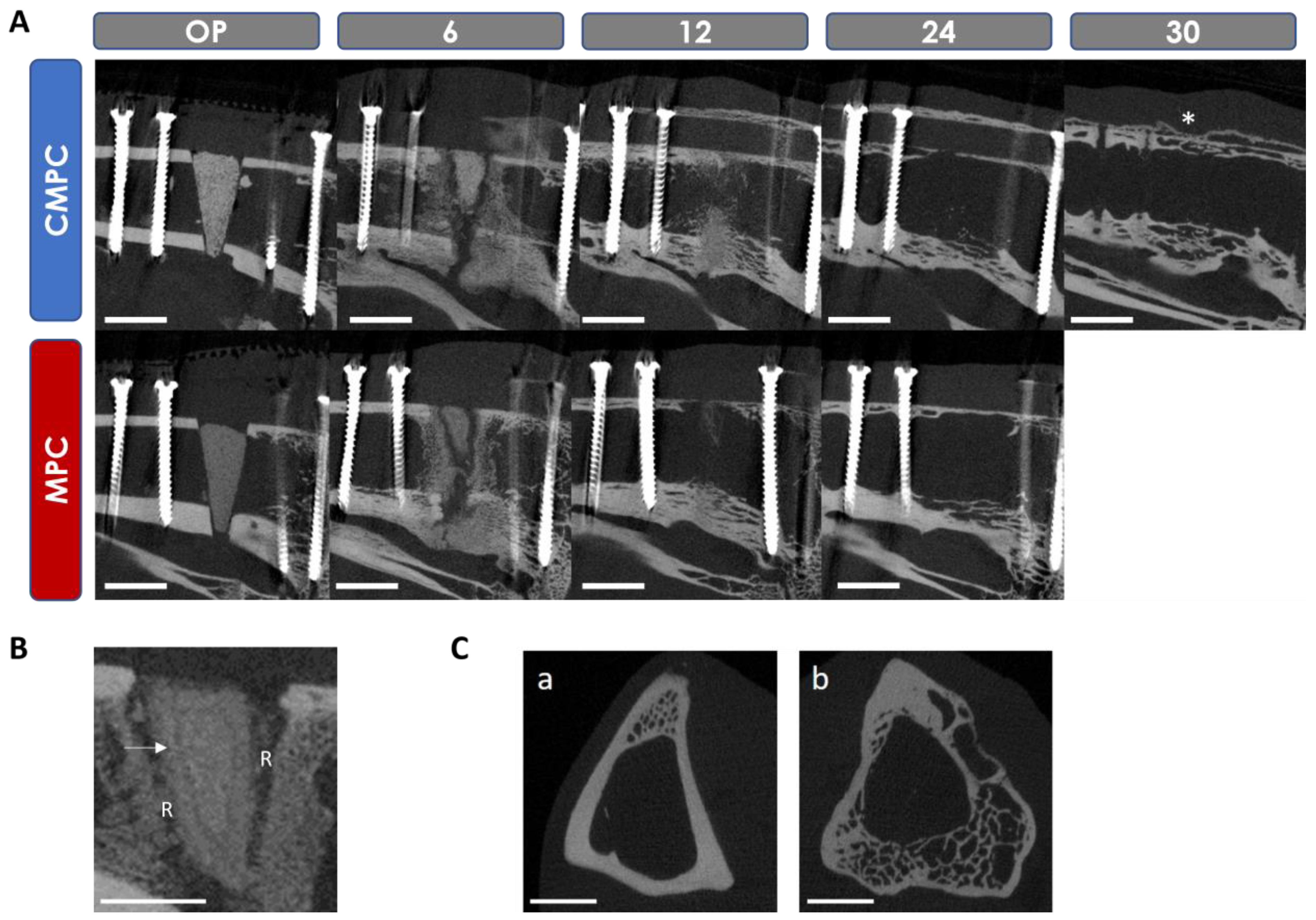
3.5. In Vivo µCT Examinations
3.5.1. Semi-Quantitative Evaluation of the Scans
3.5.2. Quantitative Evaluation of the Scans
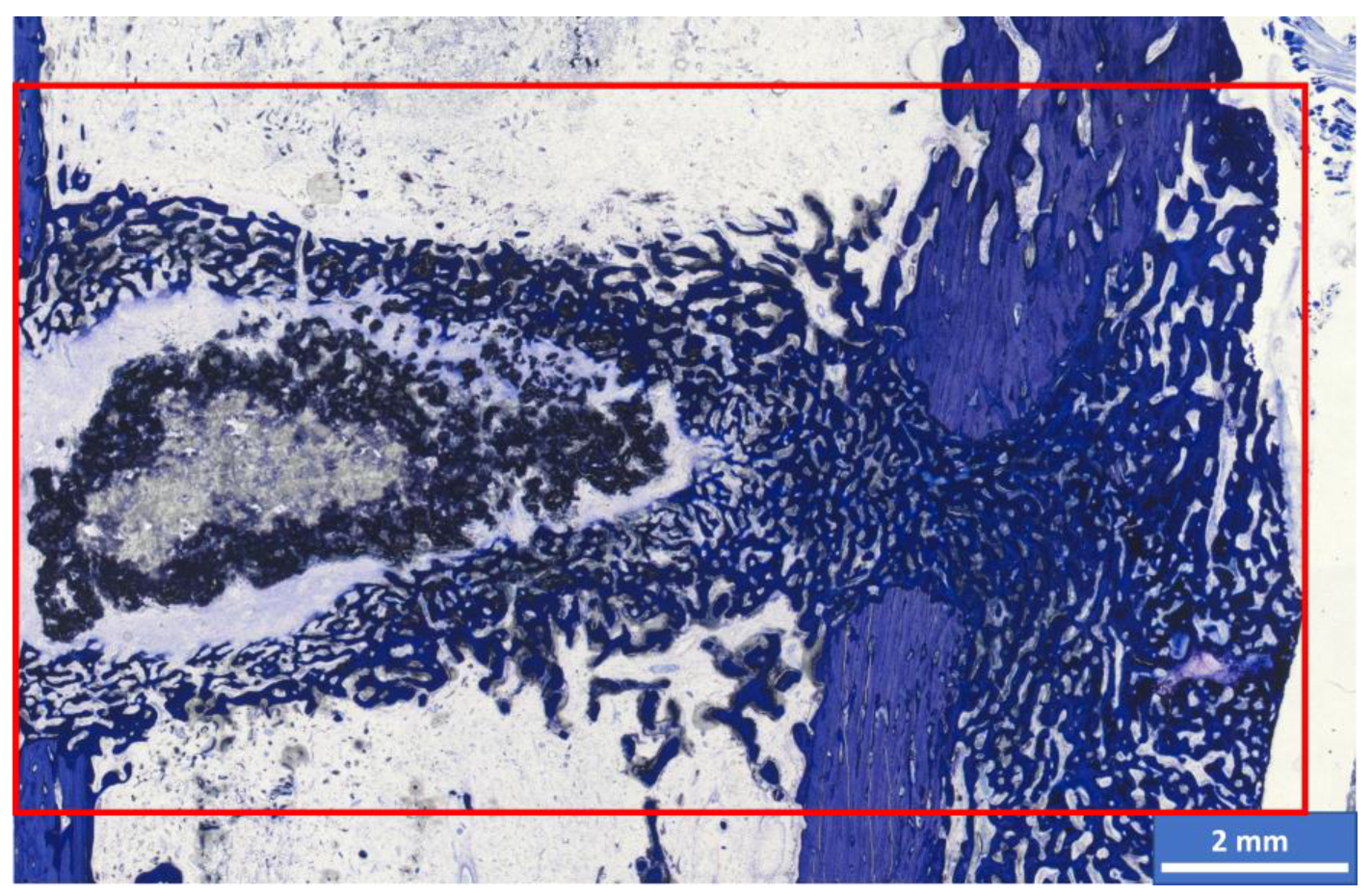
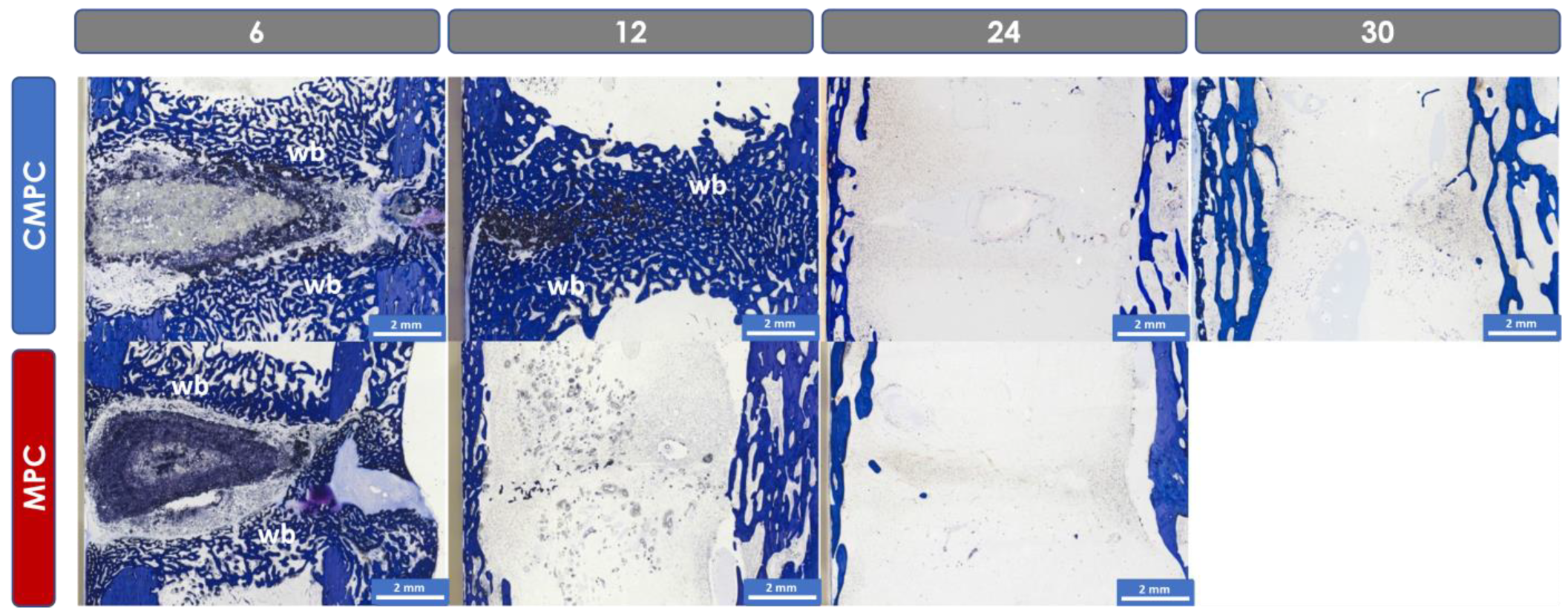
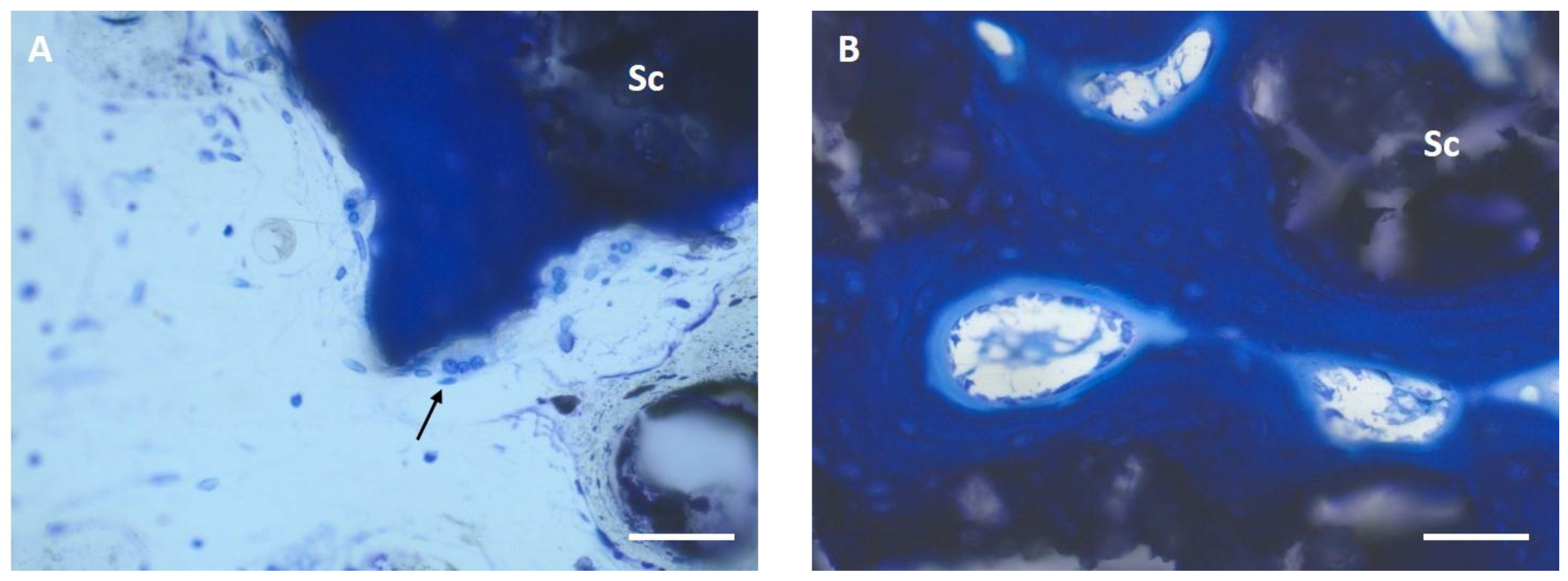
3.6. Histological Examination
3.6.1. Semi-Quantitative Assessment
3.6.2. Histomorphometric Examinations
3.7. SEM/EDX Analyses after Implantation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| µ-CT | µ-computed tomography |
| ATMP | Amorphous trimagnesium phosphate |
| Ca | Calcium |
| CMPC | Ca0.75Mg2.25(PO4)2 post-treated with phosphoric acid |
| CMPCs | Calcium magnesium phosphate cements |
| CPCs | Calcium phosphate cements |
| CTMP | Crystalline trimagnesium phosphate |
| DAHP | Diammonium hydrogen phosphate |
| EDX | Energy dispersive X-ray analysis |
| HA | Hydroxyapatite |
| Mg | Magnesium |
| MPC | Mg3(PO4)2 post-treated with diammonium hydrogen phosphate |
| MPCs | Magnesium phosphate cements |
| mRUST | Modified radiographic union scale for tibial fractures |
| P | Phosphate |
| PA | Phosphoric acid, H3PO4 |
| PEEK | Polyetheretherketone |
| PR | Plate removal |
| RT | Room temperature |
| SD | Scaffold density |
| SEM | Scanning electron microscopy |
| SV | Scaffold volume |
| TCP | Tricalcium phosphate |
| Th | Threshold |
References
- Zimmermann, G.; Moghaddam, A. Allograft bone matrix versus synthetic bone graft substitutes. Injury 2011, 42, S16–S21. [Google Scholar] [CrossRef] [PubMed]
- Valtanen, R.S.; Yang, Y.P.; Gurtner, G.C.; Maloney, W.J.; Lowenberg, D.W. Synthetic and Bone tissue engineering graft substitutes: What is the future? Injury 2021, 52 (Suppl. S2), S72–S77. [Google Scholar] [CrossRef]
- Kheirallah, M.; Almeshaly, H. Bone graft substitutes for bone defect regeneration. A collective review. Int. J. Dent. Oral. Sci. 2016, 3, 247–257. [Google Scholar]
- Brunello, G.; Panda, S.; Schiavon, L.; Sivolella, S.; Biasetto, L.; Del Fabbro, M. The Impact of Bioceramic Scaffolds on Bone Regeneration in Preclinical In Vivo Studies: A Systematic Review. Materials 2020, 13, 1500. [Google Scholar] [CrossRef]
- Linhart, W.; Briem, D.; Peters, A.; Lehmann, W.; Windolf, J.; Rueger, J. Resorbierbare Kalziumphosphatzemente. Trauma Berufskrankh. 2004, 6, 277–284. [Google Scholar] [CrossRef]
- Bohner, M. Resorbable biomaterials as bone graft substitutes. Mater. Today 2010, 13, 24–30. [Google Scholar] [CrossRef]
- Battafarano, G.; Rossi, M.; De Martino, V.; Marampon, F.; Borro, L.; Secinaro, A.; Del Fattore, A. Strategies for bone regeneration: From graft to tissue engineering. Int. J. Mol. Sci. 2021, 22, 1128. [Google Scholar] [CrossRef]
- Simon, M.H.; Grünwald, L.; Schenke, M.; Dickschas, J.; Strecker, W. Corrective osteotomies of femur and tibia: Which factors influence bone healing? Arch. Orthop. Trauma. Surg. 2020, 140, 303–311. [Google Scholar] [CrossRef]
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 2041731418776819. [Google Scholar] [CrossRef]
- Schröter, L.; Kaiser, F.; Stein, S.; Gbureck, U.; Ignatius, A. Biological and mechanical performance and degradation characteristics of calcium phosphate cements in large animals and humans. Acta Biomater. 2020, 117, 1–20. [Google Scholar] [CrossRef]
- Ostrowski, N.; Roy, A.; Kumta, P.N. Magnesium phosphate cement systems for hard tissue applications: A review. ACS Biomater. Sci. Eng. 2016, 2, 1067–1083. [Google Scholar] [CrossRef] [PubMed]
- Pina, S.; Ferreira, J.M. Brushite-forming Mg-, Zn-and Sr-substituted bone cements for clinical applications. Materials 2010, 3, 519–535. [Google Scholar] [CrossRef]
- Nabiyouni, M.; Brückner, T.; Zhou, H.; Gbureck, U.; Bhaduri, S.B. Magnesium-based bioceramics in orthopedic applications. Acta Biomater. 2018, 66, 23–43. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Ulrich, H.; Palm, C.; Willbold, E. Biodegradable magnesium scaffolds: Part II: Peri-implant bone remodeling. J. Biomed. Mater. Res. A 2007, 81, 757–765. [Google Scholar] [CrossRef]
- Fuchs, A.; Kreczy, D.; Brückner, T.; Gbureck, U.; Stahlhut, P.; Bengel, M.; Hoess, A.; Nies, B.; Bator, J.; Klammert, U.; et al. Bone regeneration capacity of newly developed spherical magnesium phosphate cement granules. Clin. Oral. Investig. 2021, 26, 2619–2633. [Google Scholar] [CrossRef]
- Ostrowski, N.; Lee, B.; Hong, D.; Enick, P.N.; Roy, A.; Kumta, P.N. Synthesis, Osteoblast, and Osteoclast Viability of Amorphous and Crystalline Tri-Magnesium Phosphate. ACS Biomater. Sci. Eng. 2015, 1, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Bose, S. Osteoclastogenesis and osteoclastic resorption of tricalcium phosphate: Effect of strontium and magnesium doping. J. Biomed. Mater. Res. A 2012, 100, 2450–2461. [Google Scholar] [CrossRef]
- Kanter, B.; Vikman, A.; Bruckner, T.; Schamel, M.; Gbureck, U.; Ignatius, A. Bone regeneration capacity of magnesium phosphate cements in a large animal model. Acta Biomater. 2018, 69, 352–361. [Google Scholar] [CrossRef]
- Kaiser, F.; Schröter, L.; Stein, S.; Krüger, B.; Weichhold, J.; Stahlhut, P.; Ignatius, A.; Gbureck, U. Accelerated bone regeneration through rational design of magnesium phosphate cements. Acta Biomater. 2022, 145, 358–371. [Google Scholar] [CrossRef]
- Wu, F.; Wei, J.; Guo, H.; Chen, F.; Hong, H.; Liu, C. Self-setting bioactive calcium-magnesium phosphate cement with high strength and degradability for bone regeneration. Acta Biomater. 2008, 4, 1873–1884. [Google Scholar] [CrossRef]
- Jia, J.; Zhou, H.; Wei, J.; Jiang, X.; Hua, H.; Chen, F.; Wei, S.; Shin, J.W.; Liu, C. Development of magnesium calcium phosphate biocement for bone regeneration. J. R. Soc. Interface 2010, 7, 1171–1180. [Google Scholar] [CrossRef]
- Kowalewicz, K.; Vorndran, E.; Feichtner, F.; Waselau, A.C.; Brueckner, M.; Meyer-Lindenberg, A. In-Vivo Degradation Behavior and Osseointegration of 3D Powder-Printed Calcium Magnesium Phosphate Cement Scaffolds. Materials 2021, 14, 946. [Google Scholar] [CrossRef]
- Kowalewicz, K.; Waselau, A.C.; Feichtner, F.; Schmitt, A.M.; Brückner, M.; Vorndran, E.; Meyer-Lindenberg, A. Comparison of degradation behavior and osseointegration of 3D powder-printed calcium magnesium phosphate cement scaffolds with alkaline or acid post-treatment. Front. Bioeng. Biotechnol. 2022, 10, 998254. [Google Scholar] [CrossRef]
- Ewald, A.; Kreczy, D.; Bruckner, T.; Gbureck, U.; Bengel, M.; Hoess, A.; Nies, B.; Bator, J.; Klammert, U.; Fuchs, A. Development and Bone Regeneration Capacity of Premixed Magnesium Phosphate Cement Pastes. Materials 2019, 12, 2119. [Google Scholar] [CrossRef]
- Vorndran, E.; Klarner, M.; Klammert, U.; Grover, L.M.; Patel, S.; Barralet, J.E.; Gbureck, U. 3D powder printing of β-tricalcium phosphate ceramics using different strategies. Adv. Eng. Mater. 2008, 10, B67–B71. [Google Scholar] [CrossRef]
- Castilho, M.; Moseke, C.; Ewald, A.; Gbureck, U.; Groll, J.; Pires, I.; Teßmar, J.; Vorndran, E. Direct 3D powder printing of biphasic calcium phosphate scaffolds for substitution of complex bone defects. Biofabrication 2014, 6, 015006. [Google Scholar] [CrossRef] [PubMed]
- Vorndran, E.; Wunder, K.; Moseke, C.; Biermann, I.; Müller, F.A.; Zorn, K.; Gbureck, U. Hydraulic setting Mg3(PO4)2 powders for 3D printing technology. Adv. Appl. Ceram. 2011, 110, 476–481. [Google Scholar] [CrossRef]
- Meininger, S.; Moseke, C.; Spatz, K.; Marz, E.; Blum, C.; Ewald, A.; Vorndran, E. Effect of strontium substitution on the material properties and osteogenic potential of 3D powder printed magnesium phosphate scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 1145–1158. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Klammert, U.; Vorndran, E.; Reuther, T.; Müller, F.A.; Zorn, K.; Gbureck, U. Low temperature fabrication of magnesium phosphate cement scaffolds by 3D powder printing. J. Mater. Sci. Mater. Med. 2010, 21, 2947–2953. [Google Scholar] [CrossRef] [PubMed]
- Schaufler, C.; Schmitt, A.M.; Moseke, C.; Stahlhut, P.; Geroneit, I.; Brückner, M.; Meyer-Lindenberg, A.; Vorndran, E. Physicochemical degradation of calcium magnesium phosphate (stanfieldite) based bone replacement materials and the effect on their cytocompatibility. Biomed. Mater. 2022, 18, 015022. [Google Scholar] [CrossRef] [PubMed]
- ISO 13175-3:2012; Implants for Surgery—Calcium Phosphates—Part 3: Hydroxyapatite and Beta-Tricalcium Phosphate Bone Substitutes. ISO: Geneva, Switzerland, 2012.
- Donath, K.; Breuner, G. A method for the study of undecalcified bones and teeth with attached soft tissues. The Säge-Schliff (sawing and grinding) technique. J. Oral. Pathol. 1982, 11, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Waselau, A.C.; Feichtner, F.; Julmi, S.; Klose, C.; Maier, H.J.; Wriggers, P.; Meyer-Lindenberg, A. In vivo investigation of open-pored magnesium scaffolds LAE442 with different coatings in an open wedge defect. J. Appl. Biomater. Funct. Mater. 2022, 20, 22808000221142679. [Google Scholar] [CrossRef] [PubMed]
- Brosset, T.; Pasquier, G.; Migaud, H.; Gougeon, F. Opening wedge high tibial osteotomy performed without filling the defect but with locking plate fixation (TomoFix™) and early weight-bearing: Prospective evaluation of bone union, precision and maintenance of correction in 51 cases. Orthop. Traumatol. Surg. Res. 2011, 97, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Whelan, D.B.; Bhandari, M.; Stephen, D.; Kreder, H.; McKee, M.D.; Zdero, R.; Schemitsch, E.H. Development of the radiographic union score for tibial fractures for the assessment of tibial fracture healing after intramedullary fixation. J. Trauma 2010, 68, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Litrenta, J.; Tornetta, P., 3rd; Mehta, S.; Jones, C.; O’Toole, R.V.; Bhandari, M.; Kottmeier, S.; Ostrum, R.; Egol, K.; Ricci, W.; et al. Determination of Radiographic Healing: An Assessment of Consistency Using RUST and Modified RUST in Metadiaphyseal Fractures. J. Orthop. Trauma 2015, 29, 516–520. [Google Scholar] [CrossRef]
- Leow, J.M.; Clement, N.D.; Tawonsawatruk, T.; Simpson, C.J.; Simpson, A.H. The radiographic union scale in tibial (RUST) fractures: Reliability of the outcome measure at an independent centre. Bone Joint Res. 2016, 5, 116–121. [Google Scholar] [CrossRef]
- Stengele, A. Systematische Analyse der Abbindereaktion von Magnesiumphosphat mit Polyacrylsäure im Vergleich zu Klassischen Wässrigen Zementsystemen. Ph.D. Thesis, Universität Würzburg, Würzburg, Germany, 2017. [Google Scholar]
- Gefel, E. Zelluläre Resorption 3D-Gedruckter Knochenimplantate auf Basis von Calciummagnesiumphosphaten. Ph.D. Thesis, Universität Würzburg, Würzburg, Germany, 2023. [Google Scholar]
- Zeng, D.; Xia, L.; Zhang, W.; Huang, H.; Wei, B.; Huang, Q.; Wei, J.; Liu, C.; Jiang, X. Maxillary sinus floor elevation using a tissue-engineered bone with calcium-magnesium phosphate cement and bone marrow stromal cells in rabbits. Tissue Eng. Part A 2012, 18, 870–881. [Google Scholar] [CrossRef]
- Klammert, U.; Reuther, T.; Blank, M.; Reske, I.; Barralet, J.E.; Grover, L.M.; Kübler, A.C.; Gbureck, U. Phase composition, mechanical performance and in vitro biocompatibility of hydraulic setting calcium magnesium phosphate cement. Acta Biomater. 2010, 6, 1529–1535. [Google Scholar] [CrossRef]
- Bavya Devi, K.; Lalzawmliana, V.; Saidivya, M.; Kumar, V.; Roy, M.; Kumar Nandi, S. Magnesium Phosphate Bioceramics for Bone Tissue Engineering. Chem. Rec. 2022, 22, e202200136. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Xun, S.; Haoye, M.; Baichuan, S.; Peng, C.; Xuejian, L.; Kaihong, Z.; Xuan, Y.; Jiang, P.; Shibi, L. 3D printed porous ceramic scaffolds for bone tissue engineering: A review. Biomater. Sci. 2017, 5, 1690–1698. [Google Scholar] [CrossRef] [PubMed]
- Castilho, M.; Rodrigues, J.; Vorndran, E.; Gbureck, U.; Quental, C.; Folgado, J.; Fernandes, P.R. Computational design and fabrication of a novel bioresorbable cage for tibial tuberosity advancement application. J. Mech. Behav. Biomed. Mater. 2017, 65, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Gefel, E.; Moseke, C.; Schmitt, A.M.; Dümmler, N.; Stahlhut, P.; Ewald, A.; Meyer-Lindenberg, A.; Vorndran, E. Degradation of 3D-printed magnesium phosphate ceramics in vitro and a prognosis on their bone regeneration potential. Bioact. Mater. 2023, 19, 376–391. [Google Scholar] [CrossRef] [PubMed]
- Castilho, M.; Dias, M.; Vorndran, E.; Gbureck, U.; Fernandes, P.; Pires, I.; Gouveia, B.; Armés, H.; Pires, E.; Rodrigues, J. Application of a 3D printed customized implant for canine cruciate ligament treatment by tibial tuberosity advancement. Biofabrication 2014, 6, 025005. [Google Scholar] [CrossRef] [PubMed]
- Vorndran, E.; Ewald, A.; Müller, F.A.; Zorn, K.; Kufner, A.; Gbureck, U. Formation and properties of magnesium-ammonium-phosphate hexahydrate biocements in the Ca-Mg-PO4 system. J. Mater. Sci. Mater. Med. 2011, 22, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, C.; Yu, S.; Wu, X.; Jie, Z.; Dai, H. Citric acid enhances the physical properties, cytocompatibility and osteogenesis of magnesium calcium phosphate cement. J. Mech. Behav. Biomed. Mater. 2019, 94, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Yamamoto, M.; Ioku, K.; Goto, S. Relationship between compressive strength and pore structure of hardened cement pastes. Adv. Cem. Res. 1997, 9, 25–30. [Google Scholar] [CrossRef]
- Klammert, U.; Ignatius, A.; Wolfram, U.; Reuther, T.; Gbureck, U. In vivo degradation of low temperature calcium and magnesium phosphate ceramics in a heterotopic model. Acta Biomater. 2011, 7, 3469–3475. [Google Scholar] [CrossRef]
- Fiset, S.; Godbout, C.; Crookshank, M.C.; Zdero, R.; Nauth, A.; Schemitsch, E.H. Experimental Validation of the Radiographic Union Score for Tibial Fractures (RUST) Using Micro-Computed Tomography Scanning and Biomechanical Testing in an in-Vivo Rat Model. J. Bone Joint Surg. Am. 2018, 100, 1871–1878. [Google Scholar] [CrossRef]
- Mısır, A.; Yıldız, K.İ.; Kızkapan, T.B.; Uzun, E.; Özçamdallı, M.; Oğuzkaya, S. Reliability of rust and modified rust scores for evaluation of union in pediatric and adult femoral shaft fractures. Acta Orthop. Traumatol. Turc. 2021, 55, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Claes, L.; Recknagel, S.; Ignatius, A. Fracture healing under healthy and inflammatory conditions. Nat. Rev. Rheumatol. 2012, 8, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Féron, J.M.; Mauprivez, R. Fracture repair: General aspects and influence of osteoporosis and anti-osteoporosis treatment. Injury 2016, 47 (Suppl. S1), S10–S14. [Google Scholar] [CrossRef] [PubMed]
- Kanter, B. Osseointegration Kalthärtender Knochenzemente im Schafmodell. Ph.D. Thesis, Ludwig-Maximilians-Universität München, München, Germany, 2014. [Google Scholar]
- Planzer, K. Variable Fixation Locking Screw (VFLS): Investigation of Bone healing with a Device Intended to Optimize Strain and Micromotion for Each Phase of Fracture Healing. Ph.D. Thesis, University of Zurich, Zurich, Switzerland, 2019. [Google Scholar]
- Naghavi, S.A.; Tamaddon, M.; Garcia-Souto, P.; Moazen, M.; Taylor, S.; Hua, J.; Liu, C. A novel hybrid design and modelling of a customised graded Ti-6Al-4V porous hip implant to reduce stress-shielding: An experimental and numerical analysis. Front. Bioeng. Biotechnol. 2023, 11, 1092361. [Google Scholar] [CrossRef] [PubMed]
- Claes, L.; Eckert-Hübner, K.; Augat, P. The effect of mechanical stability on local vascularization and tissue differentiation in callus healing. J. Orthop. Res. 2002, 20, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Perren, S.; Fernandez, A.; Regazzoni, P. Understanding fracture healing biomechanics based on the “strain” concept and its clinical applications. Acta Chir. Orthop. Traumatol. Cech. 2015, 82, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Gelli, R.; Mati, L.; Ridi, F.; Baglioni, P. Tuning the properties of magnesium phosphate-based bone cements: Effect of powder to liquid ratio and aqueous solution concentration. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 95, 248–255. [Google Scholar] [CrossRef]
- Nuss, K.M.; von Rechenberg, B. Biocompatibility issues with modern implants in bone—A review for clinical orthopedics. Open Orthop. J. 2008, 2, 66–78. [Google Scholar] [CrossRef]



| CaxMg3−x(PO4)2 | CaHPO4 | CaCO3 | MgHPO4 3H2O | Mg(OH)2 |
|---|---|---|---|---|
| x = 0.00 | - | - | 2.00 | 1.00 |
| x = 0.75 | 0.50 | 0.25 | 1.50 | 0.75 |
| mRUST Score | Osteotomy Line | Callus |
|---|---|---|
| 1 | Visible | Missing |
| 2 | Visible | Available |
| 3 | Visible | Bridged |
| 4 | Not visible | Completely bridged or remodeled |
| Parameters | Score 0 | Score 1 | Score 2 |
|---|---|---|---|
| Scaffold visibility | Not visible | Partially visible | Fully visible |
| Loss of shape | Wedge shape no longer recognizable | Wedge shape partially recognizable | Wedge shape clearly recognizable |
| Closure of osteotomy gap by callus tissue | Scaffold completely covered by callus | Partial callus formation | No callus formation |
| Scaffold integration | Continuous visible contact surface between scaffold and bone | Contact surface between scaffold and bone partially interrupted | No visible scaffold–bone contact |
| Resorption zone | No resorption zone | Scaffold partially surrounded by resorption zone | Scaffold completely surrounded by resorption zone |
| Delimitable zone within scaffold | No zone delimitable | Zone indistinctly delimitable | Zone clearly delimitable |
| Scaffold fit (on day of surgery) | Scaffold is attached to the cortices | Scaffold does not lie medially or laterally against cortical bone | Scaffold is neither medial nor lateral to cortical bone |
| Bridging of medial osteotomy gap (cis-cortex) | Complete bridging | Partial bridging | No bridging |
| Endosteal callus formation proximal to scaffold | None | Minor | Medium to high |
| Endosteal callus formation distal to scaffold | None | Minor | Medium to high |
| Periosteal callus formation (trans-cortex) | None | Minor | Medium to high |
| Remodeling trans-cortex | Even and narrow = physiological | Loosened and cancellous | Heavily curved |
| Parameters | Score 0 | Score 1 | Score 2 | Score 3 | Score 4 |
|---|---|---|---|---|---|
| Cis-cortex bridging | 76–100% | 51–75% | 26–50% | 1–25% | Not bridged |
| Trans-cortex bridging | 76–100% | 51–75% | 26–50% | 1–25% | Not bridged |
| Cis-cortex remodeling (bone maturation) | Mainly lamellar bone | Woven bone with no to little lamellar bone | Woven bone with cartilage | Cartilage tissue | No remodeling |
| Trans-cortex remodeling (bone maturation) | Mainly lamellar bone | Woven bone with no to little lamellar bone | Woven bone with cartilage | Cartilage tissue | No remodeling |
| Proximal endosteal callus | 76–100% | 51–75% | 26–50% | 1–25% | No callus |
| Distal endosteal callus | 76–100% | 51–75% | 26–50% | 1–25% | No callus |
| Scaffold degradation | 76–100% | 51–75% | 26–50% | 1–25% | No degradation |
| Scaffold integration | 76–100% | 51–75% | 26–50% | 1–25% | No integration |
| Resorption zone | Measured at level of scaffold center in mm | ||||
| Proportion of dark-colored material | Estimated percentage of scaffold | ||||
| Thickness of the trans-cortex in mm | Measured including periosteal callus | ||||
| Brushite | Stanfieldite | Farringtonite | Struvite | Newberyite | Periclase | |
|---|---|---|---|---|---|---|
| MPC | - | - | 81.0 ± 3.2 | 19.0 ± 3.2 | - | - |
| CMPC | 10.9 ± 2.4 | 20.1 ± 4.9 | 10.3 ± 1.4 | - | 58.1 ± 3.9 | 0.6 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hemmerlein, E.; Vorndran, E.; Schmitt, A.-M.; Feichtner, F.; Waselau, A.-C.; Meyer-Lindenberg, A. In Vivo Investigation of 3D-Printed Calcium Magnesium Phosphate Wedges in Partial Load Defects. Materials 2024, 17, 2136. https://doi.org/10.3390/ma17092136
Hemmerlein E, Vorndran E, Schmitt A-M, Feichtner F, Waselau A-C, Meyer-Lindenberg A. In Vivo Investigation of 3D-Printed Calcium Magnesium Phosphate Wedges in Partial Load Defects. Materials. 2024; 17(9):2136. https://doi.org/10.3390/ma17092136
Chicago/Turabian StyleHemmerlein, Elke, Elke Vorndran, Anna-Maria Schmitt, Franziska Feichtner, Anja-Christina Waselau, and Andrea Meyer-Lindenberg. 2024. "In Vivo Investigation of 3D-Printed Calcium Magnesium Phosphate Wedges in Partial Load Defects" Materials 17, no. 9: 2136. https://doi.org/10.3390/ma17092136
APA StyleHemmerlein, E., Vorndran, E., Schmitt, A.-M., Feichtner, F., Waselau, A.-C., & Meyer-Lindenberg, A. (2024). In Vivo Investigation of 3D-Printed Calcium Magnesium Phosphate Wedges in Partial Load Defects. Materials, 17(9), 2136. https://doi.org/10.3390/ma17092136






