Cemented vs. Cementless Fixation in Primary Knee Replacement: A Narrative Review
Abstract
1. Introduction
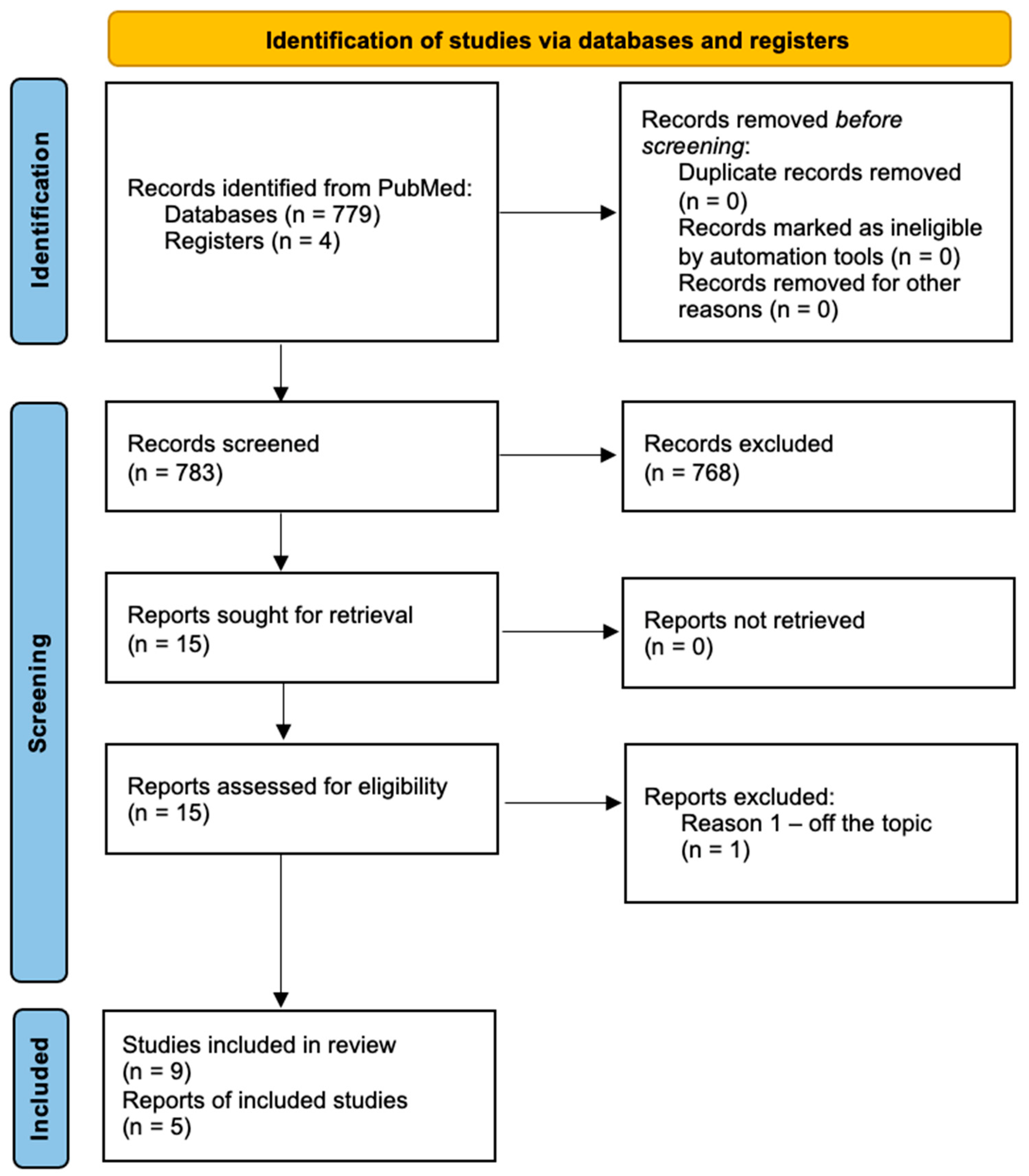
2. Materials and Methods
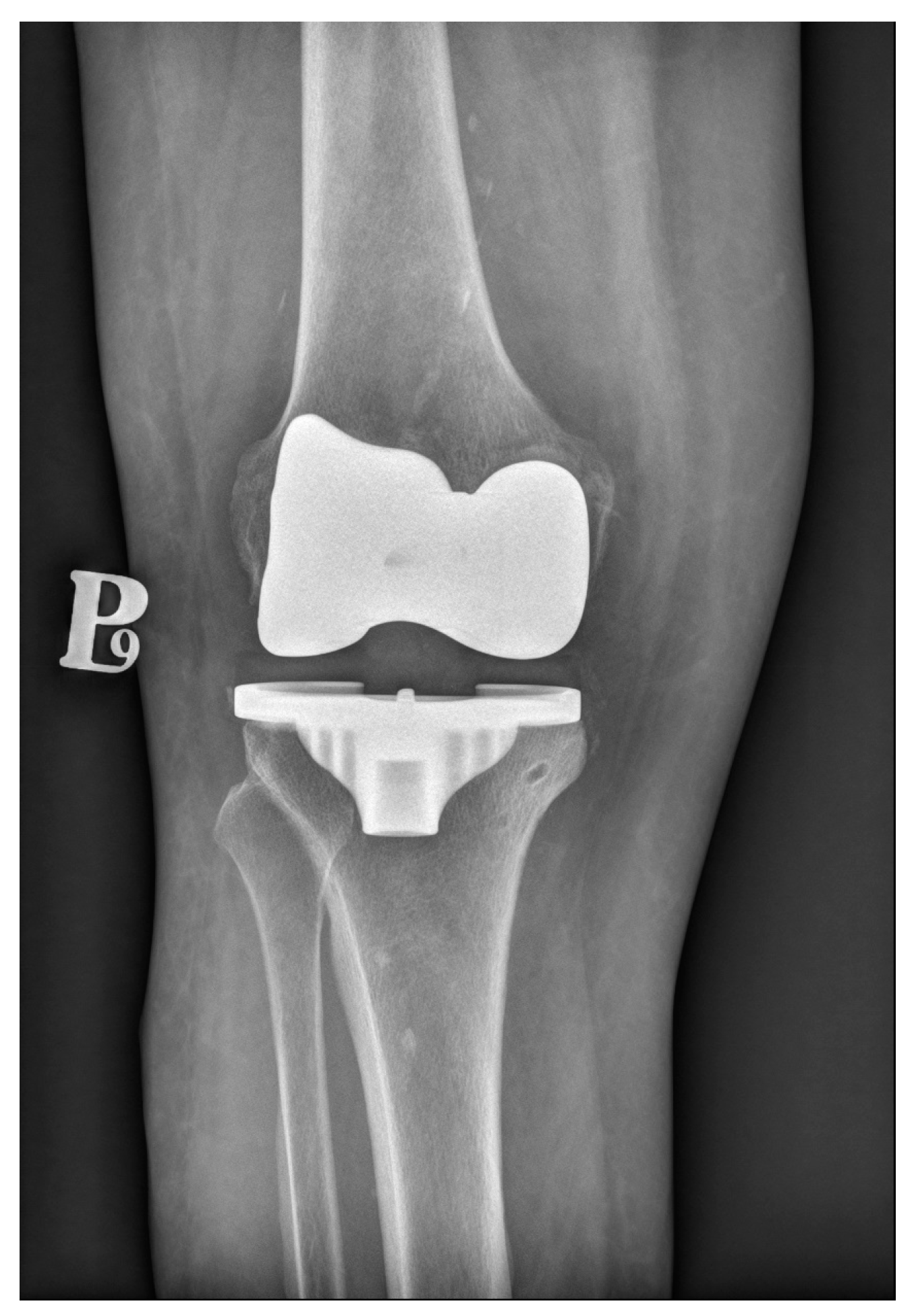
3. Cemented Knee Replacement
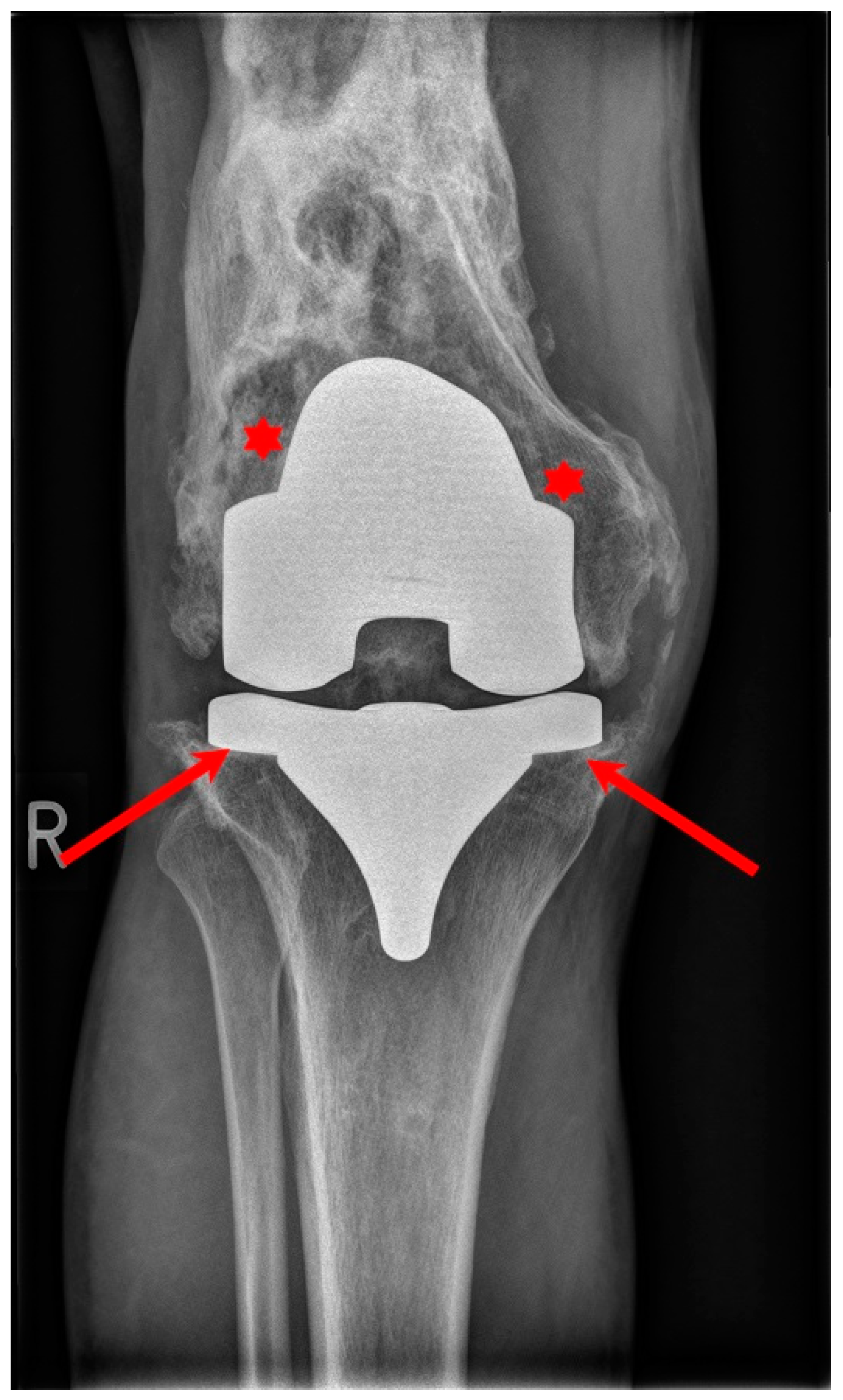
4. Cementless Knee Replacement
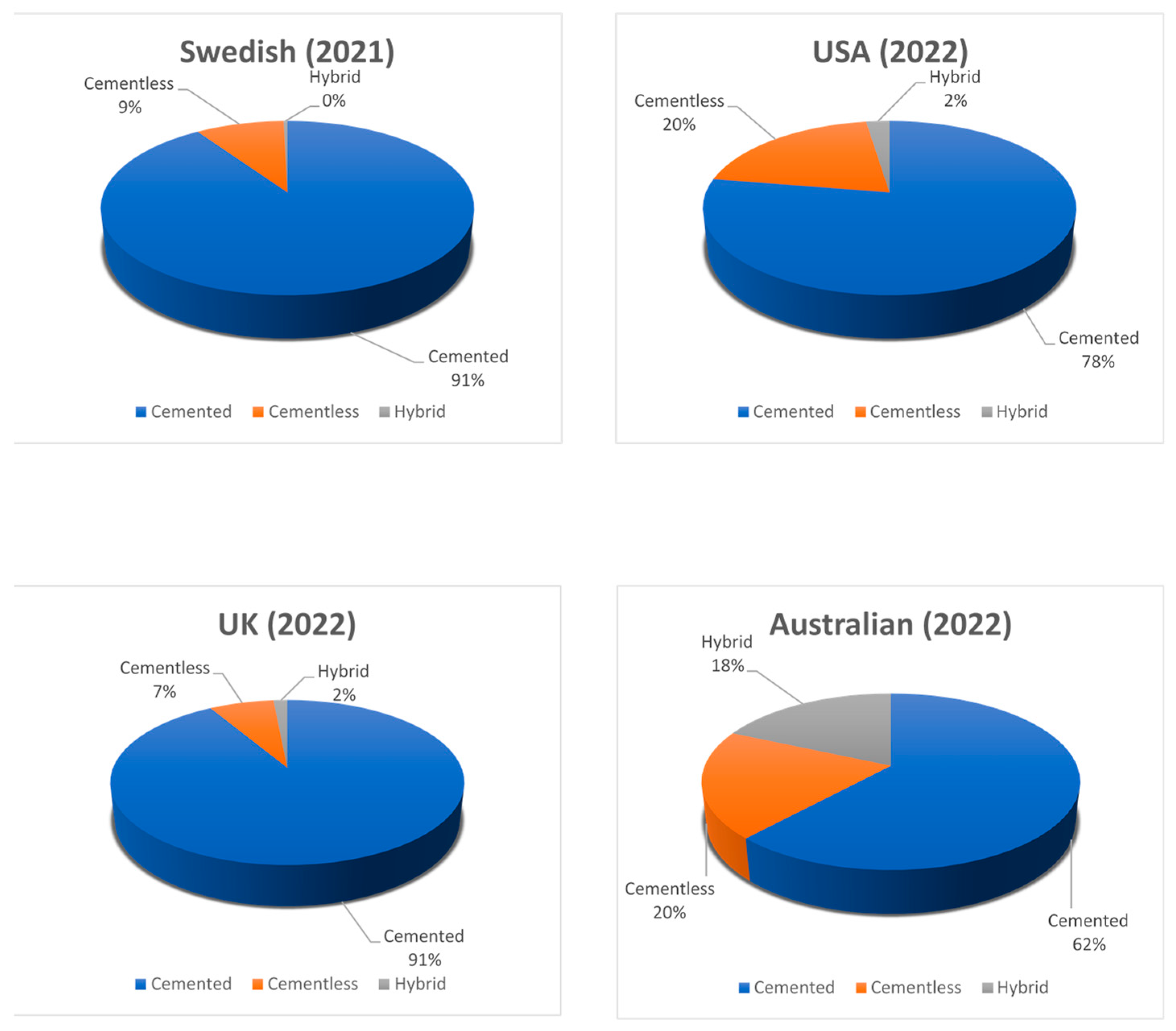
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cameron, K.; Hsiao, M.; Owens, B.; Burks, R.; Svoboda, S.J. Incidence of Physician-Diagnosed Osteoarthritis AmongActive Duty United States Military Service Members. Arthritis Rheumatol. 2011, 63, 2974–2982. [Google Scholar] [CrossRef]
- Martin-Rodriguez, E.; Guillen-Grima, F.; Martí, A.; Brugos-Larumbe, A. Comorbidity Associated with Obesity in a Large Population: The APNA Study. Obes. Res. Clin. Pract. 2015, 9, 435–447. [Google Scholar] [CrossRef]
- Flegal, K.M.; Panagiotou, O.A.; Graubard, B.I. Estimating Population Attributable Fractions to Quantify the Health Burden of Obesity. Ann. Epidemiol. 2015, 25, 201–207. [Google Scholar] [CrossRef]
- Dulay, G.S.; Cooper, C.; Dennison, E.M. Knee Pain, Knee Injury, Knee Osteoarthritis & Work. Best. Pract. Res. Clin. Rheumatol. 2015, 29, 454–461. [Google Scholar] [CrossRef]
- Papadopoulos, E.C.; Parvizi, J.; Lai, C.H.; Lewallen, D.G. Total Knee Arthroplasty Following Prior Distal Femoral Fracture. Knee 2002, 9, 267–274. [Google Scholar] [CrossRef]
- Giorgino, R.; Albano, D.; Fusco, S.; Peretti, G.M.; Mangiavini, L.; Messina, C. Knee Osteoarthritis: Epidemiology, Pathogenesis, and Mesenchymal Stem Cells: What Else Is New? An Update. Int. J. Mol. Sci. 2023, 24, 6405. [Google Scholar] [CrossRef]
- van den Bosch, M.H.J.; van Lent, P.L.E.M.; van der Kraan, P.M. Identifying Effector Molecules, Cells, and Cytokines of Innate Immunity in OA. Osteoarthr. Cartil. 2020, 28, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, R. Knee Joint Osteoarthritis Diagnosis Based on Selected Acoustic Signal Discriminants Using Machine Learning. Appl. Comput. Sci. 2022, 18, 71–85. [Google Scholar] [CrossRef]
- Mehana, E.-S.E.; Khafaga, A.F.; El-Blehi, S.S. The Role of Matrix Metalloproteinases in Osteoarthritis Pathogenesis: An Updated Review. Life Sci. 2019, 234, 116786. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhao, S.; Yang, H.; Zhang, C.; Kang, Q.; Deng, J.; Xu, Y.; Ding, Y.; Li, S. Potential Novel Prediction of TMJ-OA: MiR-140-5p Regulates Inflammation Through Smad/TGF-β Signaling. Front. Pharmacol. 2019, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Emmert, D.; Rasche, T.; Stieber, C.; Conrad, R.; Mücke, M. Knee pain-symptoms, diagnosis and therapy of osteoarthritis. MMW Fortschr. Med. 2018, 160, 58–64. [Google Scholar] [CrossRef]
- Krakowski, P.; Karpiński, R.; Maciejewski, R.; Jonak, J.; Jurkiewicz, A. Short-Term Effects of Arthroscopic Microfracturation of Knee Chondral Defects in Osteoarthritis. Appl. Sci. 2020, 10, 8312. [Google Scholar] [CrossRef]
- Leach, R.E.; Gregg, T.; Siber, F.J. Weight-Bearing Radiography in Osteoarthritis of the Knee. Radiology 1970, 97, 265–268. [Google Scholar] [CrossRef]
- Kohn, M.D.; Sassoon, A.A.; Fernando, N.D. Classifications in Brief: Kellgren-Lawrence Classification of Osteoarthritis. Clin. Orthop. Relat. Res. 2016, 474, 1886. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, R.; Krakowski, P.; Jonak, J.; Machrowska, A.; Maciejewski, M.; Nogalski, A. Diagnostics of Articular Cartilage Damage Based on Generated Acoustic Signals Using ANN—Part I: Femoral-Tibial Joint. Sensors 2022, 22, 2176. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, R.; Krakowski, P.; Jonak, J.; Machrowska, A.; Maciejewski, M.; Nogalski, A. Diagnostics of Articular Cartilage Damage Based on Generated Acoustic Signals Using ANN—Part II: Patellofemoral Joint. Sensors 2022, 22, 3765. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, R.; Krakowski, P.; Jonak, J.; Machrowska, A.; Maciejewski, M. Comparison of Selected Classification Methods Based on Machine Learning as a Diagnostic Tool for Knee Joint Cartilage Damage Based on Generated Vibroacoustic Processes. Appl. Comput. Sci. 2023, 19, 136–150. [Google Scholar] [CrossRef]
- Talesa, G.; Manfreda, F.; Pace, V.; Ceccarini, P.; Antinolfi, P.; Rinonapoli, G.; Caraffa, A. The Treatment of Knee Cartilage Lesions: State of the Art. Acta Biomed. 2022, 93, e2022099. [Google Scholar] [CrossRef]
- Bedi, A.; Feeley, B.T.; Williams, R.J. Management of Articular Cartilage Defects of the Knee. J. Bone Jt. Surg. Am. 2010, 92, 994–1009. [Google Scholar] [CrossRef] [PubMed]
- Falah, M.; Nierenberg, G.; Soudry, M.; Hayden, M.; Volpin, G. Treatment of Articular Cartilage Lesions of the Knee. Int. Orthop. 2010, 34, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Øiestad, B.E.; Juhl, C.B.; Culvenor, A.G.; Berg, B.; Thorlund, J.B. Knee Extensor Muscle Weakness Is a Risk Factor for the Development of Knee Osteoarthritis: An Updated Systematic Review and Meta-Analysis Including 46 819 Men and Women. Br. J. Sports Med. 2022, 56, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Driban, J.B.; Hootman, J.M.; Sitler, M.R.; Harris, K.P.; Cattano, N.M. Is Participation in Certain Sports Associated with Knee Osteoarthritis? A Systematic Review. J. Athl. Train. 2017, 52, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Zengini, E.; Finan, C.; Wilkinson, J.M. The Genetic Epidemiological Landscape of Hip and Knee Osteoarthritis: Where Are We Now and Where Are We Going? J. Rheumatol. 2016, 43, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Ryder, J.J.; Garrison, K.; Song, F.; Hooper, L.; Skinner, J.; Loke, Y.; Loughlin, J.; Higgins, J.P.T.; MacGregor, A.J. Genetic Associations in Peripheral Joint Osteoarthritis and Spinal Degenerative Disease: A Systematic Review. Ann. Rheum. Dis. 2008, 67, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jordan, J.M. Epidemiology of Osteoarthritis. Clin. Geriatr. Med. 2010, 26, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Cieza, A.; Causey, K.; Kamenov, K.; Hanson, S.W.; Chatterji, S.; Vos, T. Global Estimates of the Need for Rehabilitation Based on the Global Burden of Disease Study 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2021, 396, 2006–2017. [Google Scholar] [CrossRef]
- GBD 2019 Diseases and Injuries Collaborators. Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Liu, Q.; Yin, H.; Wang, K.; Diao, N.; Zhang, Y.; Lin, J.; Guo, A. Prevalence Trends of Site-Specific Osteoarthritis From 1990 to 2019: Findings From the Global Burden of Disease Study 2019. Arthritis Rheumatol. 2022, 74, 1172–1183. [Google Scholar] [CrossRef]
- Lim, W.B.; Al-Dadah, O. Conservative Treatment of Knee Osteoarthritis: A Review of the Literature. World J. Orthop. 2022, 13, 212–229. [Google Scholar] [CrossRef]
- Moseng, T.; Vliet Vlieland, T.P.M.; Battista, S.; Beckwée, D.; Boyadzhieva, V.; Conaghan, P.G.; Costa, D.; Doherty, M.; Finney, A.G.; Georgiev, T.; et al. EULAR Recommendations for the Non-Pharmacological Core Management of Hip and Knee Osteoarthritis: 2023 Update. Ann. Rheum. Dis. 2024. [Google Scholar] [CrossRef]
- Arden, N.K.; Perry, T.A.; Bannuru, R.R.; Bruyère, O.; Cooper, C.; Haugen, I.K.; Hochberg, M.C.; McAlindon, T.E.; Mobasheri, A.; Reginster, J.-Y. Non-Surgical Management of Knee Osteoarthritis: Comparison of ESCEO and OARSI 2019 Guidelines. Nat. Rev. Rheumatol. 2021, 17, 59–66. [Google Scholar] [CrossRef]
- Michael, J.W.-P.; Schlüter-Brust, K.U.; Eysel, P. The Epidemiology, Etiology, Diagnosis, and Treatment of Osteoarthritis of the Knee. Dtsch. Arztebl. Int. 2010, 107, 152–162. [Google Scholar] [CrossRef]
- Kan, H.S.; Chan, P.K.; Chiu, K.Y.; Yan, C.H.; Yeung, S.S.; Ng, Y.L.; Shiu, K.W.; Ho, T. Non-Surgical Treatment of Knee Osteoarthritis. Hong Kong Med. J. 2019, 25, 127–133. [Google Scholar] [CrossRef]
- Gilat, R.; Haunschild, E.D.; Knapik, D.M.; Evuarherhe, A.; Parvaresh, K.C.; Cole, B.J. Hyaluronic Acid and Platelet-Rich Plasma for the Management of Knee Osteoarthritis. Int. Orthop. 2021, 45, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, R.W.; Huizinga, M.R.; Duivenvoorden, T.; van Raaij, T.M.; Verhagen, A.P.; Bierma-Zeinstra, S.M.; Verhaar, J.A. Osteotomy for Treating Knee Osteoarthritis. Cochrane Database Syst. Rev. 2014, 2014, CD004019. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, X.; Zhang, K.; He, X. Unicompartmental Knee Replacement and High Tibial Osteotomy for Medial Unicompartmental Knee Osteoarthritis. Medicine 2020, 99, e23454. [Google Scholar] [CrossRef]
- Hussain, S.M.; Neilly, D.W.; Baliga, S.; Patil, S.; Meek, R. Knee Osteoarthritis: A Review of Management Options. Scott. Med. J. 2016, 61, 7–16. [Google Scholar] [CrossRef]
- Peng, H.; Ou, A.; Huang, X.; Wang, C.; Wang, L.; Yu, T.; Zhang, Y.; Zhang, Y. Osteotomy Around the Knee: The Surgical Treatment of Osteoarthritis. Orthop. Surg. 2021, 13, 1465–1473. [Google Scholar] [CrossRef]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Higashi, H.; Barendregt, J.J. Cost-Effectiveness of Total Hip and Knee Replacements for the Australian Population with Osteoarthritis: Discrete-Event Simulation Model. PLoS ONE 2011, 6, e25403. [Google Scholar] [CrossRef]
- Ruiz, D.; Koenig, L.; Dall, T.M.; Gallo, P.; Narzikul, A.; Parvizi, J.; Tongue, J. The Direct and Indirect Costs to Society of Treatment for End-Stage Knee Osteoarthritis. J. Bone Jt. Surg. Am. 2013, 95, 1473–1480. [Google Scholar] [CrossRef]
- Postler, A.E.; Lützner, C.; Goronzy, J.; Lange, T.; Deckert, S.; Günther, K.P.; Lützner, J. When Are Patients with Osteoarthritis Referred for Surgery? Best. Pract. Res. Clin. Rheumatol. 2023, 37, 101835. [Google Scholar] [CrossRef]
- Halawi, M.J.; Jongbloed, W.; Baron, S.; Savoy, L.; Williams, V.J.; Cote, M.P. Patient Dissatisfaction After Primary Total Joint Arthroplasty: The Patient Perspective. J. Arthroplast. 2019, 34, 1093–1096. [Google Scholar] [CrossRef]
- Palazzuolo, M.; Antoniadis, A.; Mahlouly, J.; Wegrzyn, J. Total Knee Arthroplasty Improves the Quality-Adjusted Life Years in Patients Who Exceeded Their Estimated Life Expectancy. Int. Orthop. 2021, 45, 635–641. [Google Scholar] [CrossRef]
- Gelber, A.C.; Hochberg, M.C.; Mead, L.A.; Wang, N.Y.; Wigley, F.M.; Klag, M.J. Joint Injury in Young Adults and Risk for Subsequent Knee and Hip Osteoarthritis. Ann. Intern. Med. 2000, 133, 321–328. [Google Scholar] [CrossRef]
- Huétink, K.; Stoel, B.C.; Watt, I.; Kloppenburg, M.; Bloem, J.L.; Malm, S.H.; Van’t Klooster, R.; Nelissen, R.G.H.H. Identification of Factors Associated with the Development of Knee Osteoarthritis in a Young to Middle-Aged Cohort of Patients with Knee Complaints. Clin. Rheumatol. 2015, 34, 1769–1779. [Google Scholar] [CrossRef]
- Singer, S.P.; Dammerer, D.; Krismer, M.; Liebensteiner, M.C. Maximum Lifetime Body Mass Index Is the Appropriate Predictor of Knee and Hip Osteoarthritis. Arch. Orthop. Trauma. Surg. 2018, 138, 99–103. [Google Scholar] [CrossRef]
- Papas, P.V.; Congiusta, D.; Cushner, F.D. Cementless versus Cemented Fixation in Total Knee Arthroplasty. J. Knee Surg. 2019, 32, 596–599. [Google Scholar] [CrossRef]
- Duffy, G.P.; Berry, D.J.; Rand, J.A. Cement versus Cementless Fixation in Total Knee Arthroplasty. Clin. Orthop. Relat. Res. 1998, 356, 66–72. [Google Scholar] [CrossRef]
- Brown, T.E.; Harper, B.L.; Bjorgul, K. Comparison of Cemented and Uncemented Fixation in Total Knee Arthroplasty. Orthopedics 2013, 36, 380–387. [Google Scholar] [CrossRef]
- Irmola, T.; Ponkilainen, V.; Mäkelä, K.T.; Robertsson, O.; W-Dahl, A.; Furnes, O.; Fenstad, A.M.; Pedersen, A.B.; Schrøder, H.M.; Eskelinen, A.; et al. Association between Fixation Type and Revision Risk in Total Knee Arthroplasty Patients Aged 65 Years and Older: A Cohort Study of 265,877 Patients from the Nordic Arthroplasty Register Association 2000–2016. Acta Orthop 2021, 92, 91–96. [Google Scholar] [CrossRef]
- Prudhon, J.-L.; Verdier, R. Cemented or Cementless Total Knee Arthroplasty?: Comparative Results of 200 Cases at a Minimum Follow-up of 11 Years. SICOT-J. 2017, 3, 70. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Chockalingam, S.; Scott, G. The Outcome of Cemented vs. Cementless Fixation of a Femoral Component in Total Knee Replacement (TKR) with the Identification of Radiological Signs for the Prediction of Failure. Knee 2000, 7, 233–238. [Google Scholar] [CrossRef]
- The Tenth Annual Report of the AJRR on Hip and Knee Arthroplasty Annual Report 2023. Available online: https://www.aaos.org/registries/publications/ajrr-annual-report/ (accessed on 17 December 2023).
- Annual Report. 2022 The Swedish Arthroplasty Register. Available online: https://sar.registercentrum.se/news/download-the-sar-annual-report-2022 (accessed on 27 November 2023).
- Australian Orthopaedic Association National Joint Replacement Registry 2023 Annual Report. Available online: https://aoanjrr.sahmri.com/annual-reports-2023 (accessed on 5 January 2024).
- The National Joint Registry 20th Annual Report. 2023. Available online: https://www.njrcentre.org.uk/njr-annual-report-2022/ (accessed on 27 November 2023).
- Karpiński, R.; Szabelski, J.; Maksymiuk, J. Seasoning Polymethyl Methacrylate (PMMA) Bone Cements with Incorrect Mix Ratio. Materials 2019, 12, 3073. [Google Scholar] [CrossRef]
- Karpiński, R.; Szabelski, J.; Maksymiuk, J. Effect of Physiological Fluids Contamination on Selected Mechanical Properties of Acrylate Bone Cement. Materials 2019, 12, 3963. [Google Scholar] [CrossRef]
- Szabelski, J.; Karpiński, R.; Krakowski, P.; Jojczuk, M.; Jonak, J.; Nogalski, A. Analysis of the Effect of Component Ratio Imbalances on Selected Mechanical Properties of Seasoned, Medium Viscosity Bone Cements. Materials 2022, 15, 5577. [Google Scholar] [CrossRef]
- Karpiński, R.; Szabelski, J.; Krakowski, P.; Jojczuk, M.; Jonak, J.; Nogalski, A. Evaluation of the Effect of Selected Physiological Fluid Contaminants on the Mechanical Properties of Selected Medium-Viscosity PMMA Bone Cements. Materials 2022, 15, 2197. [Google Scholar] [CrossRef]
- Szabelski, J.; Karpiński, R.; Krakowski, P.; Jonak, J. The Impact of Contaminating Poly (Methyl Methacrylate) (PMMA) Bone Cements on Their Compressive Strength. Materials 2021, 14, 2555. [Google Scholar] [CrossRef]
- Machrowska, A.; Karpiński, R.; Jonak, J.; Szabelski, J.; Krakowski, P. Numerical Prediction of the Component-Ratio-Dependent Compressive Strength of Bone Cement. Appl. Comput. Sci. 2020, 16, 88–101. [Google Scholar] [CrossRef]
- Kühn, K.-D. Chapter 3.1: Properties of Bone Cement-What Is Bone Cement? The Well-Cemented Total Hip Arthroplasty: Theory and Practice. In The Well-Cemented Total Hip Arthroplasty; Springer: Berlin/Heidelberg, Germany, 2005; pp. 52–59. ISBN 978-3-540-24197-3. [Google Scholar]
- Charnley, J. Anchorage of the Femoral Head Prosthesis to the Shaft of the Femur. J. Bone Jt. Surg. Br. 1960, 42-B, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Arora, M.; Chan, E.K.; Gupta, S.; Diwan, A.D. Polymethylmethacrylate Bone Cements and Additives: A Review of the Literature. World J. Orthop. 2013, 4, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, R.; Szabelski, J.; Krakowski, P.; Jonak, J. Effect of Physiological Saline Solution Contamination on Selected Mechanical Properties of Seasoned Acrylic Bone Cements of Medium and High Viscosity. Materials 2021, 14, 110. [Google Scholar] [CrossRef] [PubMed]
- Machrowska, A.; Szabelski, J.; Karpiński, R.; Krakowski, P.; Jonak, J.; Jonak, K. Use of Deep Learning Networks and Statistical Modeling to Predict Changes in Mechanical Parameters of Contaminated Bone Cements. Materials 2020, 13, 5419. [Google Scholar] [CrossRef]
- Conner, E.M.; Grisham, M.B. Inflammation, Free Radicals, and Antioxidants. Nutrition 1996, 12, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Winrow, V.R.; Winyard, P.G.; Morris, C.J.; Blake, D.R. Free Radicals in Inflammation: Second Messengers and Mediators of Tissue Destruction. Br. Med. Bull. 1993, 49, 506–522. [Google Scholar] [CrossRef] [PubMed]
- Vale, F.M.; Castro, M.; Monteiro, J.; Couto, F.S.; Pinto, R.; Rico, J.M.G.T. Acrylic Bone Cement Induces the Production of Free Radicals by Cultured Human Fibroblasts. Biomaterials 1997, 18, 1133–1135. [Google Scholar] [CrossRef]
- Gandhi, R.; Tsvetkov, D.; Davey, J.R.; Mahomed, N.N. Survival and Clinical Function of Cemented and Uncemented Prostheses in Total Knee Replacement: A Meta-Analysis. J. Bone Jt. Surg. Br. 2009, 91, 889–895. [Google Scholar] [CrossRef]
- Lawrie, C.M.; Schwabe, M.; Pierce, A.; Nunley, R.M.; Barrack, R.L. The Cost of Implanting a Cemented versus Cementless Total Knee Arthroplasty. Bone Jt. J. 2019, 101-B, 61–63. [Google Scholar] [CrossRef]
- Chiou, D.; Li, A.K.; Upfill-Brown, A.; Arshi, A.; Hsiue, P.; Chen, K.; Stavrakis, A.; Photopoulos, C.D. Cementless Compared to Cemented Total Knee Arthroplasty Is Associated with More Revisions Within 1 Year of Index Surgery. Arthroplast. Today 2023, 21, 101122. [Google Scholar] [CrossRef]
- Mohammad, H.R.; Judge, A.; Murray, D.W. A Matched Comparison of the Long-Term Outcomes of Cemented and Cementless Total Knee Replacements: An Analysis from the National Joint Registry of England, Wales, Northern Ireland and the Isle of Man. J. Bone Jt. Surg. Am. 2021, 103, 2270–2280. [Google Scholar] [CrossRef]
- Edgar, M.; Harvey, J.; Jiang, S.; Walters, J.; Kozina, E.; Kaplan, N.; Redondo, M.; Zabawa, L.; Chmell, S. Cemented Total Knee Arthroplasty Provides Greater Knee Range of Motion at 2 Years than Cementless Technique. Eur. J. Orthop. Surg. Traumatol. 2023, 33, 3561–3568. [Google Scholar] [CrossRef]
- Mercurio, M.; Gasparini, G.; Sanzo, V.; Familiari, F.; Castioni, D.; Galasso, O. Cemented Total Knee Arthroplasty Shows Less Blood Loss but a Higher Rate of Aseptic Loosening Compared with Cementless Fixation: An Updated Meta-Analysis of Comparative Studies. J. Arthroplast. 2022, 37, 1879–1887.e4. [Google Scholar] [CrossRef]
- Owens, J.; Otero, J.E.; Noiseux, N.O.; Springer, B.D.; Martin, J.R. Risk Factors for Post-Operative Blood Transfusion Following Total Knee Arthroplasty. Iowa Orthop. J. 2020, 40, 69–73. [Google Scholar]
- Hungerford, D.S.; Krackow, K.A.; Kenna, R.V. Cementless Total Knee Replacement in Patients 50 Years Old and Under. Orthop. Clin. North. Am. 1989, 20, 131–145. [Google Scholar]
- Yamamoto, S.; Nakata, S.; Kondoh, Y. A Follow-up Study of an Uncemented Knee Replacement. The Results of 312 Knees Using the Kodama-Yamamoto Prosthesis. J. Bone Jt. Surg. Br. 1989, 71, 505–508. [Google Scholar] [CrossRef]
- Schwabe, M.T.; Hannon, C.P. The Evolution, Current Indications and Outcomes of Cementless Total Knee Arthroplasty. J. Clin. Med. 2022, 11, 6608. [Google Scholar] [CrossRef] [PubMed]
- Søballe, K.; Toksvig-Larsen, S.; Gelineck, J.; Fruensgaard, S.; Hansen, E.S.; Ryd, L.; Lucht, U.; Bünger, C. Migration of Hydroxyapatite Coated Femoral Prostheses. A Roentgen Stereophotogrammetric Study. J. Bone Jt. Surg. Br. 1993, 75, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.K.; Tan, J.H.S.; Bedair, H.S.; Dawson-Bowling, S.; Hanna, S.A. Cemented vs. Cementless Fixation in Primary Total Knee Arthroplasty: A Systematic Review and Meta-Analysis. EFORT Open Rev. 2020, 5, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Ritter, M.A.; Meneghini, R.M. Twenty-Year Survivorship of Cementless Anatomic Graduated Component Total Knee Arthroplasty. J. Arthroplast. 2010, 25, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Muth, J.; Poggie, M.; Kulesha, G.; Michael Meneghini, R. Novel Highly Porous Metal Technology in Artificial Hip and Knee Replacement: Processing Methodologies and Clinical Applications. JOM 2013, 65, 318–325. [Google Scholar] [CrossRef]
- Netravali, N.A.; Shen, F.; Park, Y.; Bargar, W.L. A Perspective on Robotic Assistance for Knee Arthroplasty. Adv. Orthop. 2013, 2013, 970703. [Google Scholar] [CrossRef]
- Nugent, M.; Wyatt, M.C.; Frampton, C.M.; Hooper, G.J. Despite Improved Survivorship of Uncemented Fixation in Total Knee Arthroplasty for Osteoarthritis, Cemented Fixation Remains the Gold Standard: An Analysis of a National Joint Registry. J. Arthroplast. 2019, 34, 1626–1633. [Google Scholar] [CrossRef]
- Olivecrona, C.; Lapidus, L.J.; Benson, L.; Blomfeldt, R. Tourniquet Time Affects Postoperative Complications after Knee Arthroplasty. Int. Orthop. 2013, 37, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Dalury, D.F. Cementless Total Knee Arthroplasty: Current Concepts Review. Bone Jt. J. 2016, 98-B, 867–873. [Google Scholar] [CrossRef]
- Whitehouse, M.R.; Atwal, N.S.; Pabbruwe, M.; Blom, A.W.; Bannister, G.C. Osteonecrosis with the Use of Polymethylmethacrylate Cement for Hip Replacement: Thermal-Induced Damage Evidenced in Vivo by Decreased Osteocyte Viability. Eur. Cell Mater. 2014, 27, 50–62, discussion 62–63. [Google Scholar] [CrossRef] [PubMed]
- Karpinski, R.; Szabelski, J.; Maksymiuk, J. Analysis of the Properties of Bone Cement with Respect to Its Manufacturing and Typical Service Lifetime Conditions. MATEC Web Conf. 2018, 244, 01004. [Google Scholar] [CrossRef]
- Wang, K.; Sun, H.; Zhang, K.; Li, S.; Wu, G.; Zhou, J.; Sun, X. Better Outcomes Are Associated with Cementless Fixation in Primary Total Knee Arthroplasty in Young Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicine 2020, 99, e18750. [Google Scholar] [CrossRef]
- Stotter, C.; von Roth, P. Diagnosis of loosening after knee arthroplasty. Orthopade 2021, 50, 972–978. [Google Scholar] [CrossRef]
- Rodriguez, S.; Ranawat, A.S. The Future Is Non-Cemented Total Knee Arthroplasty: Volume Trends at the Hospital for Special Surgery. Indian. J. Orthop. 2021, 55, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Park, J.-W.; Jang, Y.-S. The 22 to 25-Year Survival of Cemented and Cementless Total Knee Arthroplasty in Young Patients. J. Arthroplast. 2021, 36, 566–572. [Google Scholar] [CrossRef]
- Mohammad, H.R.; Judge, A.; Murray, D.W. A Comparison of the Periprosthetic Fracture Rate of Cemented and Cementless Total Knee Arthroplasties: An Analysis of Data from the National Joint Registry. J. Arthroplast. 2023, S0883-5403(23)01192-0. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, A.; Björkman, A.; Besjakov, J.; Onsten, I. Cemented Tibial Component Fixation Performs Better than Cementless Fixation: A Randomized Radiostereometric Study Comparing Porous-Coated, Hydroxyapatite-Coated and Cemented Tibial Components over 5 Years. Acta Orthop. 2005, 76, 362–369. [Google Scholar] [CrossRef]
- Rahman, A.; Martin, B.; Jenkins, C.; Mohammad, H.; Barker, K.; Dodd, C.; Jackson, W.; Price, A.; Mellon, S.; Murray, D.W. Less Pain Reported 5 Years after Cementless Compared to Cemented Unicompartmental Knee Replacement: An Analysis of Pain, Neuropathy, and Co-Morbidity Scores. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 5180–5189. [Google Scholar] [CrossRef]
- Law, T.Y.; Kurowicki, J.; Rosas, S.; Sabeh, K.; Summers, S.; Hubbard, Z.; Roche, M. Cannabis Use Increases Risk for Revision after Total Knee Arthroplasty. J. Long. Term. Eff. Med. Implant. 2018, 28, 125–130. [Google Scholar] [CrossRef]
- Denduluri, S.K.; Woolson, S.T.; Indelli, P.F.; Mariano, E.R.; Harris, A.H.S.; Giori, N.J. Cannabinoid and Opioid Use Among Total Joint Arthroplasty Patients: A 6-Year, Single-Institution Study. Orthopedics 2021, 44, e101–e106. [Google Scholar] [CrossRef] [PubMed]
- Zywiel, M.G.; Stroh, D.A.; Lee, S.Y.; Bonutti, P.M.; Mont, M.A. Chronic Opioid Use Prior to Total Knee Arthroplasty. J. Bone Jt. Surg. Am. 2011, 93, 1988–1993. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, N.M.; Parry, J.A.; Taunton, M.J. Patients at Risk: Large Opioid Prescriptions After Total Knee Arthroplasty. J. Arthroplast. 2017, 32, 2395–2398. [Google Scholar] [CrossRef]
- Watts, C.D.; Houdek, M.T.; Wagner, E.R.; Abdel, M.P.; Taunton, M.J. Insulin Dependence Increases the Risk of Failure After Total Knee Arthroplasty in Morbidly Obese Patients. J. Arthroplast. 2016, 31, 256–259. [Google Scholar] [CrossRef]
- Sloan, M.; Sheth, N.P.; Nelson, C.L. Obesity and Hypoalbuminaemia Are Independent Risk Factors for Readmission and Reoperation Following Primary Total Knee Arthroplasty. Bone Jt. J. 2020, 102-B, 31–35. [Google Scholar] [CrossRef]
- Jiang, S.L.; Schairer, W.W.; Bozic, K.J. Increased Rates of Periprosthetic Joint Infection in Patients with Cirrhosis Undergoing Total Joint Arthroplasty. Clin. Orthop. Relat. Res. 2014, 472, 2483–2491. [Google Scholar] [CrossRef] [PubMed]
- Issa, K.; Boylan, M.R.; Naziri, Q.; Perfetti, D.C.; Maheshwari, A.V.; Mont, M.A. The Impact of Hepatitis C on Short-Term Outcomes of Total Joint Arthroplasty. J. Bone Jt. Surg. Am. 2015, 97, 1952–1957. [Google Scholar] [CrossRef] [PubMed]
- Pour, A.E.; Matar, W.Y.; Jafari, S.M.; Purtill, J.J.; Austin, M.S.; Parvizi, J. Total Joint Arthroplasty in Patients with Hepatitis C. J. Bone Jt. Surg. Am. 2011, 93, 1448–1454. [Google Scholar] [CrossRef] [PubMed]
- Jergesen, H.E.; Thielen, Z.P.; Roever, J.A.; Vashon, T.T.; Wu, H.-H.; Yi, P.H. Primary Hip and Knee Arthroplasty in a Safety Net Hospital: Substance Abuse and Other Factors Affecting Short-Term Complications. J. Arthroplast. 2018, 33, 3003–3008. [Google Scholar] [CrossRef]
- Tay, K.S.; Cher, E.W.L.; Zhang, K.; Tan, S.B.; Howe, T.S.; Koh, J.S.B. Comorbidities Have a Greater Impact Than Age Alone in the Outcomes of Octogenarian Total Knee Arthroplasty. J. Arthroplast. 2017, 32, 3373–3378. [Google Scholar] [CrossRef]
- Ottesen, T.D.; Zogg, C.K.; Haynes, M.S.; Malpani, R.; Bellamkonda, K.S.; Grauer, J.N. Dialysis Patients Undergoing Total Knee Arthroplasty Have Significantly Increased Odds of Perioperative Adverse Events Independent of Demographic and Comorbidity Factors. J. Arthroplast. 2018, 33, 2827–2834. [Google Scholar] [CrossRef]
- Sahota, S.; Lovecchio, F.; Harold, R.E.; Beal, M.D.; Manning, D.W. The Effect of Smoking on Thirty-Day Postoperative Complications After Total Joint Arthroplasty: A Propensity Score-Matched Analysis. J. Arthroplast. 2018, 33, 30–35. [Google Scholar] [CrossRef]
- Ward, D.T.; Metz, L.N.; Horst, P.K.; Kim, H.T.; Kuo, A.C. Complications of Morbid Obesity in Total Joint Arthroplasty: Risk Stratification Based on BMI. J. Arthroplast. 2015, 30, 42–46. [Google Scholar] [CrossRef]
- Wagner, E.R.; Kamath, A.F.; Fruth, K.; Harmsen, W.S.; Berry, D.J. Effect of Body Mass Index on Reoperation and Complications After Total Knee Arthroplasty. J. Bone Jt. Surg. Am. 2016, 98, 2052–2060. [Google Scholar] [CrossRef]
- Christensen, T.C.; Wagner, E.R.; Harmsen, W.S.; Schleck, C.D.; Berry, D.J. Effect of Physical Parameters on Outcomes of Total Knee Arthroplasty. J. Bone Jt. Surg. Am. 2018, 100, 1829–1837. [Google Scholar] [CrossRef]
- George, J.; Piuzzi, N.S.; Ng, M.; Sodhi, N.; Khlopas, A.A.; Mont, M.A. Association Between Body Mass Index and Thirty-Day Complications After Total Knee Arthroplasty. J. Arthroplast. 2018, 33, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.R.; Watts, C.D.; Taunton, M.J. Bariatric Surgery Does Not Improve Outcomes in Patients Undergoing Primary Total Knee Arthroplasty. Bone Jt. J. 2015, 97-B, 1501–1505. [Google Scholar] [CrossRef] [PubMed]
- Katakam, A.; Melnic, C.M.; Bragdon, C.R.; Sauder, N.; Collins, A.K.; Bedair, H.S. Low Body Mass Index Is a Predictor for Mortality and Increased Length of Stay Following Total Joint Arthroplasty. J. Arthroplast. 2021, 36, 72–77. [Google Scholar] [CrossRef] [PubMed]
| Study Type | Data Source | Population | Follow-Up | Conclusions | Period | Authors |
|---|---|---|---|---|---|---|
| Systematic review + meta-analysis of randomized controlled trials | Medline + Embase | 6 studies—755 TKA (356 cemented, 399 cementless); mean age—62.5 years (range: 43–80); male-to-female ratio—1:3 | 8.4 years (2.0–16.6) | An overall significant difference in post-operative outcomes, including all-cause revision rate and knee function was not found. Increased postoperative pain in cementless group. | From the earliest to November 2018 | Prasad et al. (2020) [68] |
| Systematic review + meta-analysis of randomized controlled trials | PubMed, Embase, Medline, Web of Science, and full Cochrane Library | 6 studies—510 TKA (255 cemented, 255 cementless); age range 33–65 | 12 years (2–16.6) | Complication and survival rates were similar between groups. Cementless is superior to cemented TKA in young patients. Better clinical outcomes in cementless group. | From inception to July 2018 | Wang et al. (2020) [76] |
| Prospectively followed and retrospectively analyzed trial | Surgeries performed by a single surgeon at single academic institution | 261 patients (522 knees) who underwent bilateral simultaneous TKAs (one cemented, second cementless—order was randomized); mean age—62.5 years ± 5.5; 180 women: 81 men; BMI 27.2 ± 5.3 | 23.8 years (22–25) | Comparable outcomes and survivorship. Rate of survival at 25-year follow-up is 98% for cemented and 97% for cementless. | Between January 1995 and February 1998 | Kim et al. (2021) [80] |
| An analysis from the National Joint Registry | National Joint Registry of England, Wales, Northern Ireland and Isle of Man | Propensity matched scoring techniques matched 44,954 cemented and cementless TKRs (22,477 cemented, 22,477 cementless) | 7.2 years (SD 3.8) | Ten-year implant survival 95% in both groups (96% cemented vs. 95.5% cementless). The rates of revision for infection were lower in the cementless group, although rates of revision for pain were higher. | Between 1 January 2004 and 31 December 2018 | Mohammad et al. (2021) [60] |
| Updated meta-analysis of comparative studies | PubMed, MEDLINE, Scopus and the Cochrane Central | 18 studies—5222 patients (2734 cemented, 2343 cementless); mean age—64.4 years ± 9.4 (cemented) and 62.3 years ± 8.6 (cementless); female 48% (cemented) and 51.4% (cementless); BMI—33.2 ± 6 (cemented), 33.8 ± 6.6 (cementless) | 107.9 months ± 30 (cemented) and 104.3 months ± 10 (cementless) | Comparable functional outcomes and reoperation rates. Cemented TKA showed less blood loss but a higher rate of manipulation under anesthesia and aseptic loosening. | Searched in January 2022 published over the past 15 years | Mercurio et al. (2022) [62] |
| Original research—retrospective review | PearlDiver patient record database. International Classification of Diseases and Current Procedural Terminology codes were used to identify patients who had undergone cemented and cementless TKAs and subsequent surgical revisions | 324,508 patients, 312,988 (cemented, 11,520 cementless); females (57.67% cementless, 63.48% cemented); mean age—(64.02 years ± 9.2 cementless vs. 66.4 years ± 8.9 cemented) | 90 days and 1 year after TKA | Decreased rate of I&D, one-component revision and arthroscopy with LOA in cemented group | From 2015 to 2019 | Chiou et al. (2023) [59] |
| Retrospective review | Institutional review board. Current Procedural Terminology codes were used to identify patients who had undergone cemented or cementless TKA. | 168 patients (80 cemented, 88 cementless); average age—57.94 (SD 14.13) in cemented group, 55.94 (SD 14.65) in cementless group; average BMI—33.98 (SD 5.43) in cemented group, 34.86 (6.90) in cementless group | More or equal to 2-year | Cemented group required fewer MUAs and had greater final ROM. Cementless group had lower tourniquet time. | Between January 2015 and June 2017 | Edgar et al. (2023) [61] |
| Analysis of data from the National Joint Registry | National Joint Registry | Propensity matched scoring techniques matched 44,954 TKRs (22,477 cemented, 22,477 cementless) | 3-month and 10-year fracture rates | Comparable rates of periprosthetic fracture at 3 months (0.02 cemented vs. 0.04 cementless) and 10 years (1.2% cemented vs. 1.4% cementless) | Between 1st January 2004 and 31st December 2018 | Mohammad et al. (2023) [78] |
| Retrospective study | Procedures performed by 4 knee surgeons at 2 hospitals in the UK. During the period of this study, clinicians transitioned from use of the cemented UKR to the cementless UKR. | 524 patients, 262 cemented and 262 cementless UKR | Patients were reviewed 5 years after UKR | UKR group had remarkably lower pain levels compared to TKR scores reported in the literature. Significantly less pain in cementless UKR group compared to cemented UKR group. | From 2006 to 2012 | Rahman et al. (2023) [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilczyński, M.; Bieniek, M.; Krakowski, P.; Karpiński, R. Cemented vs. Cementless Fixation in Primary Knee Replacement: A Narrative Review. Materials 2024, 17, 1136. https://doi.org/10.3390/ma17051136
Wilczyński M, Bieniek M, Krakowski P, Karpiński R. Cemented vs. Cementless Fixation in Primary Knee Replacement: A Narrative Review. Materials. 2024; 17(5):1136. https://doi.org/10.3390/ma17051136
Chicago/Turabian StyleWilczyński, Mikołaj, Michał Bieniek, Przemysław Krakowski, and Robert Karpiński. 2024. "Cemented vs. Cementless Fixation in Primary Knee Replacement: A Narrative Review" Materials 17, no. 5: 1136. https://doi.org/10.3390/ma17051136
APA StyleWilczyński, M., Bieniek, M., Krakowski, P., & Karpiński, R. (2024). Cemented vs. Cementless Fixation in Primary Knee Replacement: A Narrative Review. Materials, 17(5), 1136. https://doi.org/10.3390/ma17051136





