A High Copper Concentration Copper-Quadrol Complex Electroless Solution for Chip Bonding Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Optimization of Copper–Quadrol Complex Solution
2.1.1. Taguchi Method
2.1.2. Sample Preparation and Coating Characterization
2.2. Concept Testing
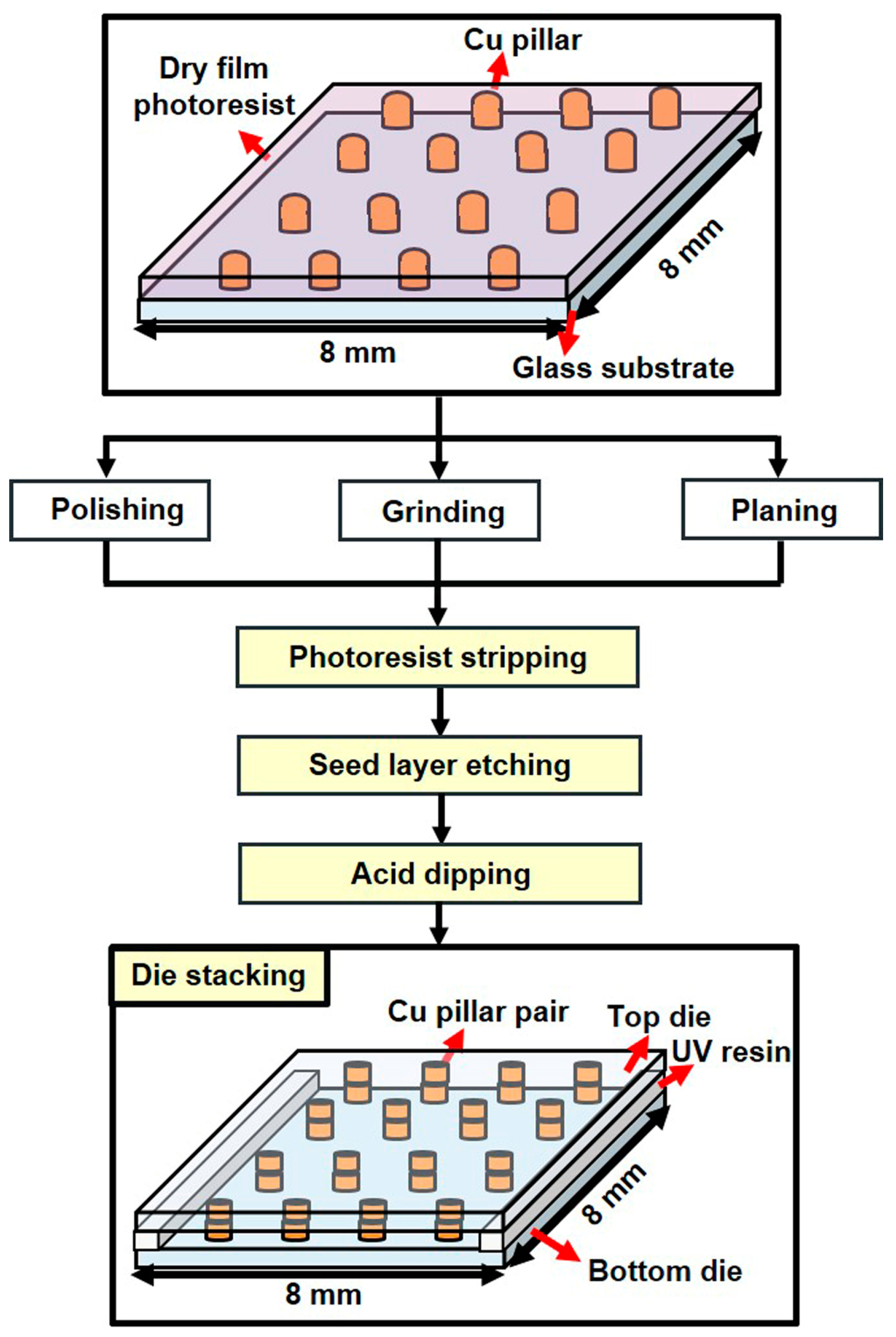
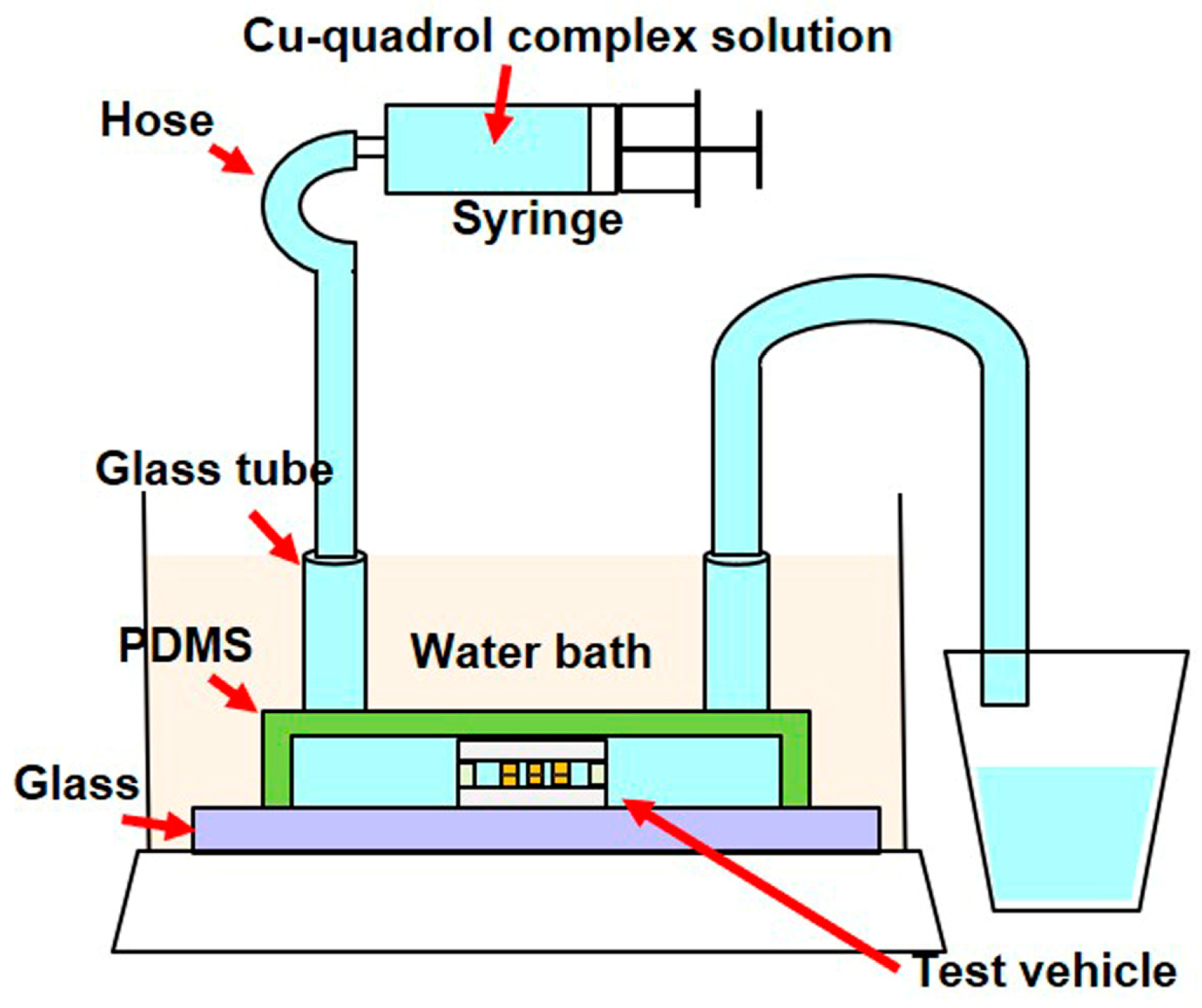
2.2.1. Fabrication of the Test Vehicle
2.2.2. Electroless Cu Plating Process
3. Results and Discussion
3.1. Effect of Plating Parameters on Plating Rate and Decomposition Time
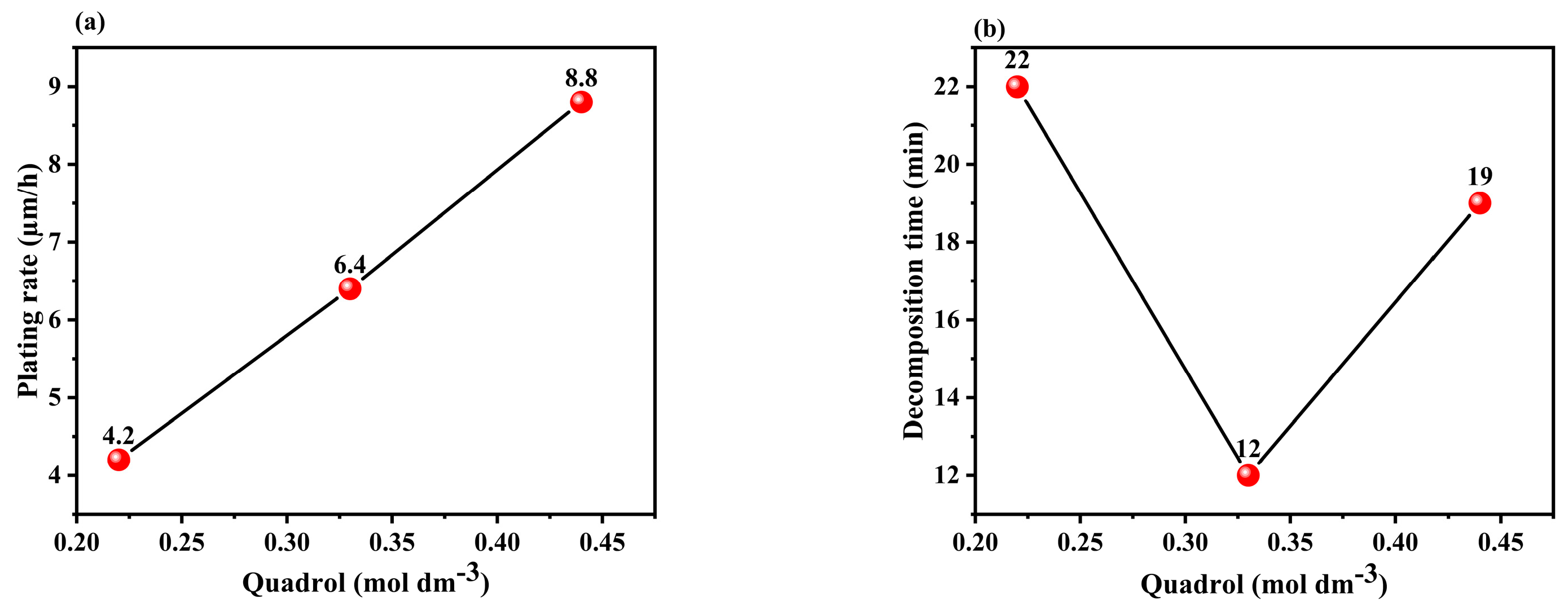
3.1.1. Effect of the Concentration of Quadrol in the Solution
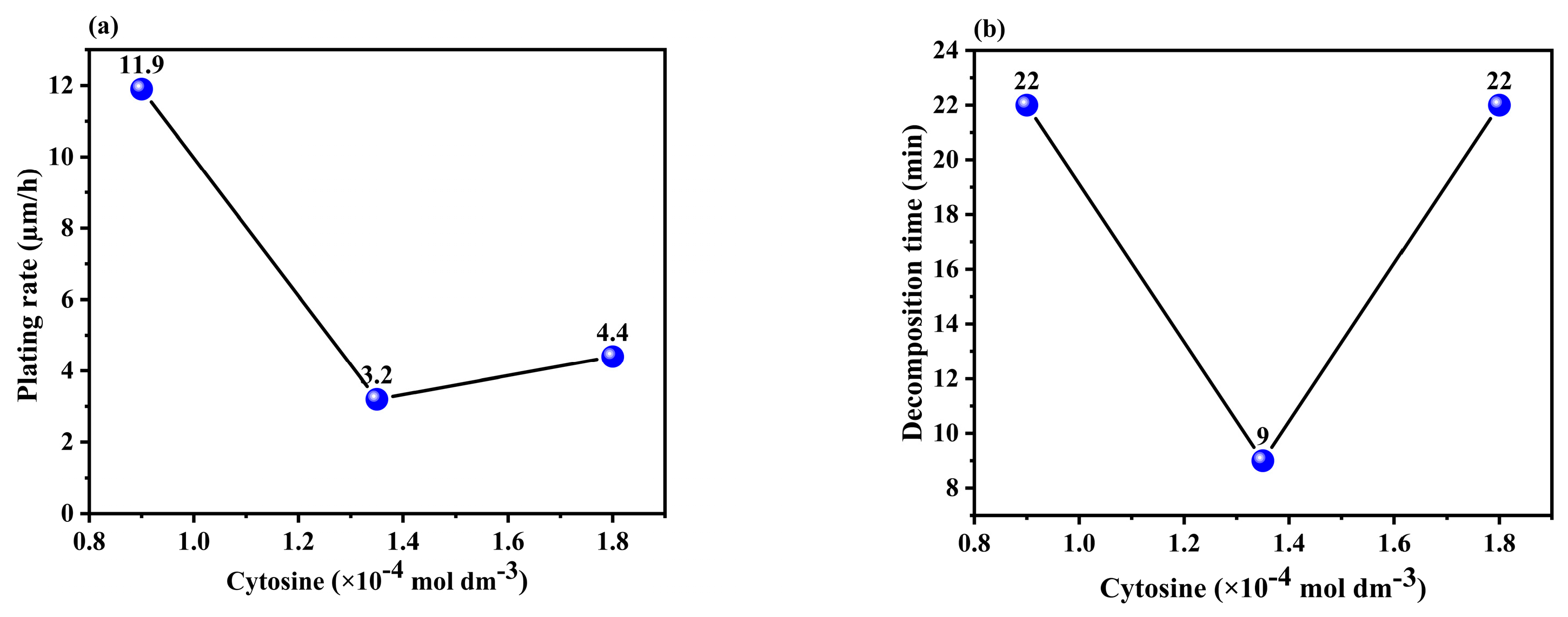
3.1.2. Effect of the Concentration of Cytosine in the Solution
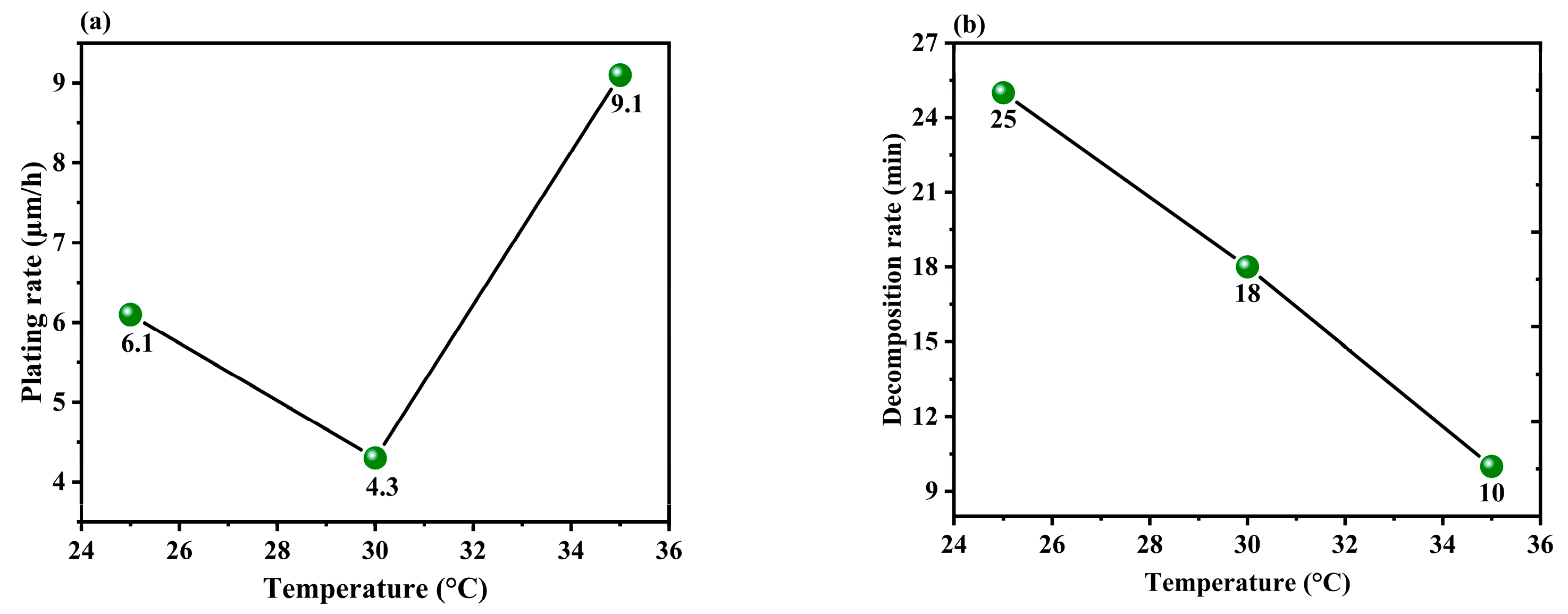
3.1.3. Effect of the Temperature of the Solution
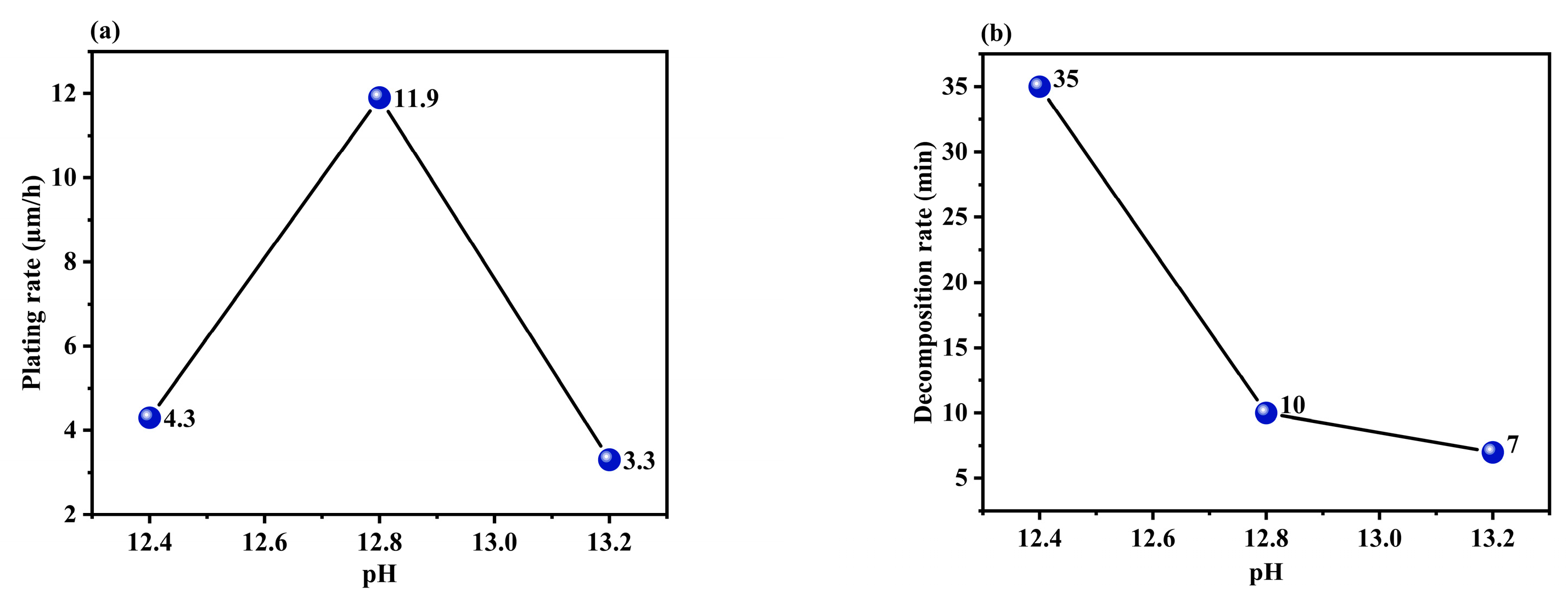
3.1.4. Effect of the pH of the Solution
3.2. Plating Condition of Electroless Cu Plating Process
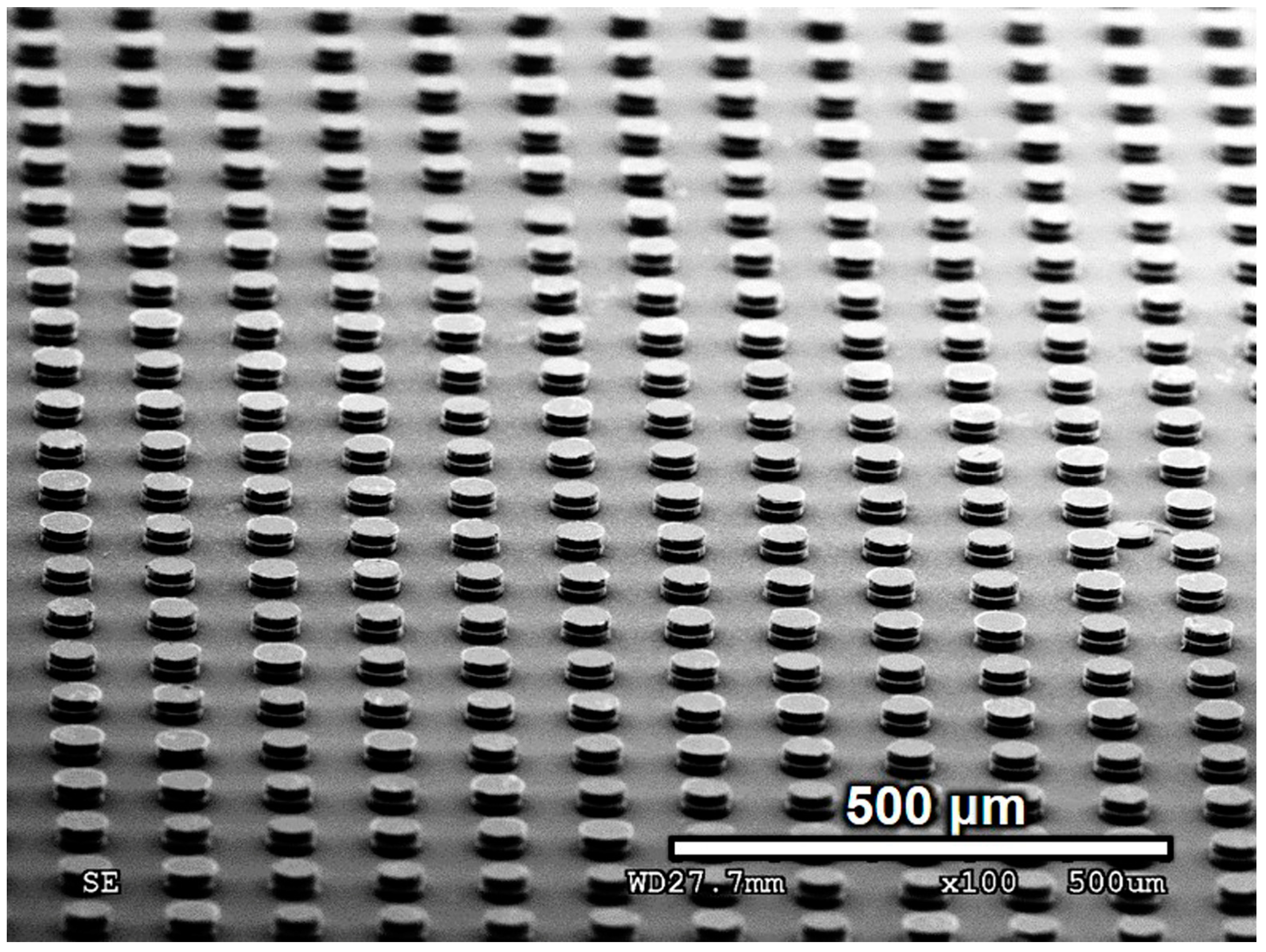
3.2.1. Morphology of Bonded Cu Pillars
3.2.2. Cross Section of Electroless-Plated Cu
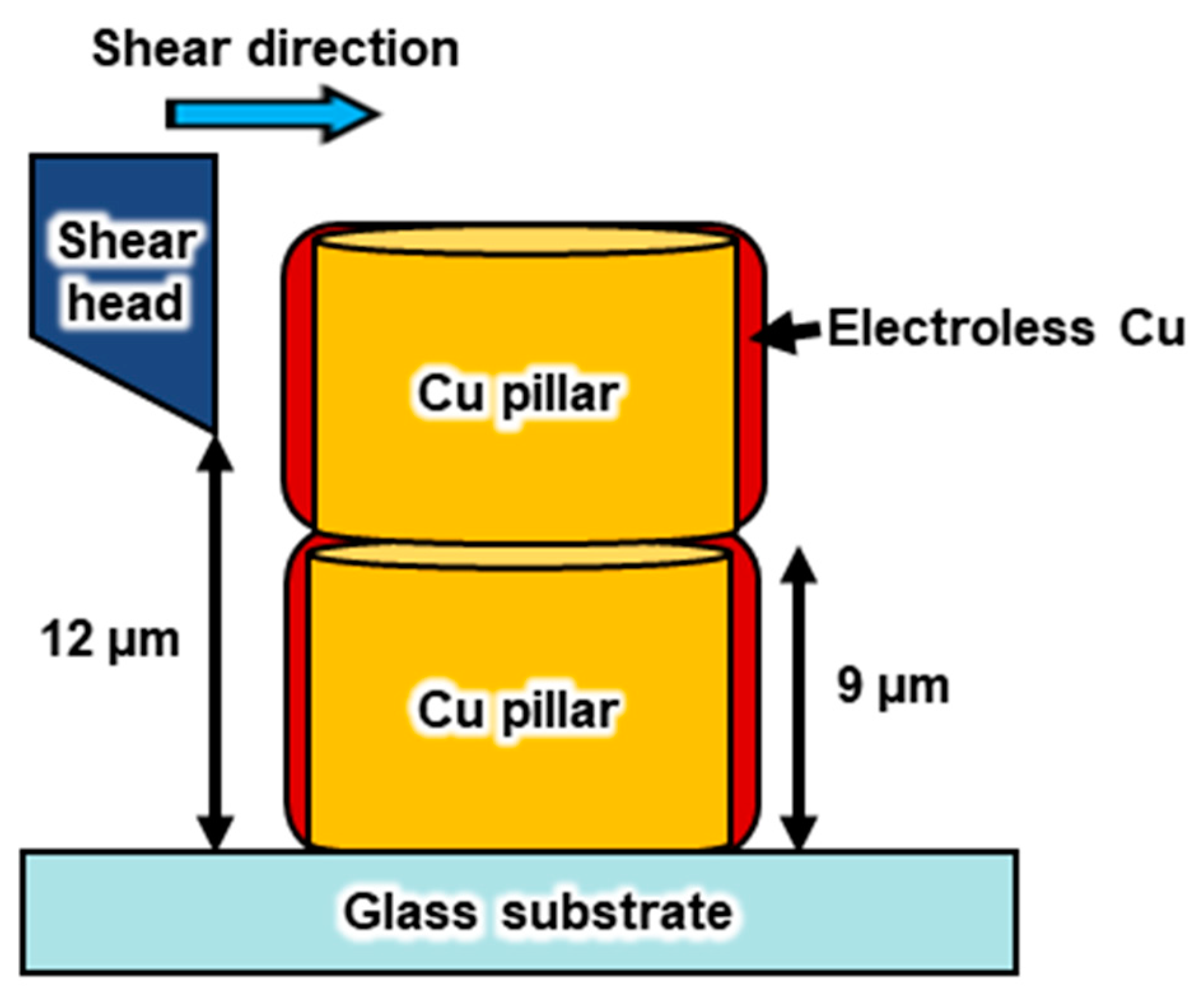
3.2.3. Mechanical Property Measurements
3.2.4. Comparison of this Method with Existing Technologies
4. Conclusions
5. Future Works
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tu, K.-N. Reliability challenges in 3D IC packaging technology. Microelectron. Reliab. 2011, 51, 517–523. [Google Scholar] [CrossRef]
- Moreau, S.; Jourdon, J.; Lhostis, S.; Bouchu, D.; Ayoub, B.; Arnaud, L.; Fremont, H. Hybrid bonding-based interconnects: A status on the last robustness and reliability achievements. ECS J. Solid State Sci. Technol. 2022, 11, 024001. [Google Scholar] [CrossRef]
- Chen, G.; Wang, Y.; Xiao, X.; Yan, Y.; Zhu, W. Failure Analysis of Anisotropic Conductive Adhesive Packages in Narrow-Pitch Flip Chip Packaging. In Proceedings of the 2021 22nd International Conference on Electronic Packaging Technology (ICEPT), Xiamen, China, 11–14 August 2021; pp. 1–5. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Zhang, H.; Chen, Z.; Zhou, C.; Liu, X.; Zhu, W. The thermal cycling reliability of copper pillar solder bump in flip chip via thermal compression bonding. Microelectron. Reliab. 2020, 104, 113543. [Google Scholar] [CrossRef]
- Osborn, T.; Galiba, N.; Kohl, P.A. Electroless copper deposition with PEG suppression for all-copper flip-chip connections. J. Electrochem. Soc. 2009, 156, D226. [Google Scholar] [CrossRef]
- Shacham-Diamand, Y.; Osaka, T.; Okinaka, Y.; Sugiyama, A.; Dubin, V. 30 years of electroless plating for semiconductor and polymer micro-systems. Microelectron. Eng. 2015, 132, 35–45. [Google Scholar] [CrossRef]
- Hajdu, J. Electroless plating: The past is prologue. Plat. Surf. Finish. 1996, 83, 29–33. [Google Scholar]
- He, A.; Osborn, T.; Allen, S.A.B.; Kohl, P.A. Low-temperature bonding of copper pillars for all-copper chip-to-substrate interconnections. Electrochem. Solid-State Lett. 2006, 9, C192. [Google Scholar] [CrossRef]
- Hung, H.; Ma, Z.; Shih, P.; Huang, J.; Kao, L.; Yang, C.; Renganathan, V.; Kao, C.; Hung, Y.; Kao, C. Highly uniform microfluidic electroless interconnections for chip stacking applications. Electrochim. Acta 2021, 376, 138032. [Google Scholar] [CrossRef]
- Shih, P.; Shen, C.; Chen, Y.; Huang, C.; Gräfner, S.; Huang, J.; Kao, C. A novel method of low temperature, pressureless interconnection for wafer level scale 3D packaging. In Proceedings of the 2022 IEEE 72nd Electronic Components and Technology Conference (ECTC), San Diego, CA, USA, 31–33 June 2022; pp. 1294–1299. [Google Scholar] [CrossRef]
- Gräfner, S.; Huang, J.; Shih, P.; Renganathan, V.; Kung, P.; Chen, Y.; Huang, C.; Chen, C.; Kao, C. Numerical fluidic-chemical multi-physics simulation of a mass production model for electroless plating of fine-pitch interconnections in a microchannel for chip packaging applications. In Proceedings of the 2022 IEEE 24th Electronics Packaging Technology Conference (EPTC), Singapore, 7–9 December 2022; pp. 644–649. [Google Scholar] [CrossRef]
- Gräfner, S.J.; Huang, J.-H.; Renganathan, V.; Kung, P.-Y.; Wu, P.-Y.; Kao, C. Fluidic-chemical characteristics of electroless copper deposition of ordered mass-fabricated pillars in a microchannel for chip packaging applications. Chem. Eng. Sci. 2023, 269, 118474. [Google Scholar] [CrossRef]
- Huang, J.; Shih, P.; Renganathan, V.; Gr, S.; Chen, Y.; Huang, C.; Kao, C.; Lin, Y.; Hung, Y.; Kao, C. Development of high copper concentration, low operating temperature, and environmentally friendly electroless copper plating using a copper-glycerin complex solution. Electrochim. Acta 2022, 425, 140710. [Google Scholar] [CrossRef]
- Huang, J.-H.; Shih, P.-S.; Shen, C.-H.; Renganathan, V.; Gräfner, S.J.; Lin, Y.-C.; Kao, C.-L.; Lin, Y.-S.; Hung, Y.-C.; Chiang, C.-W. A novel and simple method of low temperature, low process time, pressureless interconnection for 3D packaging. In Proceedings of the 2023 IEEE 73rd Electronic Components and Technology Conference (ECTC), Orlando, FL, USA, 30 May–2 June 2023; pp. 1660–1663. [Google Scholar] [CrossRef]
- Chatterjee, B. Electroless copper plating of resin-coated optical fibres. Surf. Technol. 1984, 23, 333–340. [Google Scholar] [CrossRef]
- Hanna, F.; Hamid, Z.A.; Aal, A.A. Controlling factors affecting the stability and rate of electroless copper plating. Mater. Lett. 2004, 58, 104–109. [Google Scholar] [CrossRef]
- Pauliukaitė, R.; Stalnionis, G.; Jusys, Z.; Vaškelis, A. Effect of Cu (II) ligands on electroless copper deposition rate in formaldehyde solutions: An EQCM study. J. Appl. Electrochem. 2006, 36, 1261–1269. [Google Scholar] [CrossRef]
- McCormack, J.F.; Nuzzi, F.J. Electroless Copper Deposition Process Having Faster Plating Rates. U.S. Patent No 4,301,196, 17 November 1981. [Google Scholar]
- Karna, S.K.; Sahai, R. An overview on Taguchi method. Int. J. Eng. Math. Sci. 2012, 1, 11–18. [Google Scholar]
- Karanam, S.A.K.; Rathinam, R.; Mouria, P.K.; Alguno, A.C.; Capangpangan, R.Y.; Achamyeleh, T.; Gonzales, J.L.A.; Sharma, V.K.; Livingston, T.S. Optimizing the Parameters of Spark Plasma Sintering to Enhance the Hardness of MgO/TiC Composites. Adv. Mater. Sci. Eng. 2023, 2023, 1335481. [Google Scholar] [CrossRef]
- Shravani, D.; Lakshmi, P.; Balasubramaniam, J. Preparation and optimization of various parameters of enteric coated pellets using the Taguchi L9 orthogonal array design and their characterization. Acta Pharm. Sin. B 2011, 1, 56–63. [Google Scholar] [CrossRef]
- Lu, J.; Wang, M.; Deng, X.; Yan, J.; Yun, J.; Jiao, S. Evaluation of K3Fe (CN) 6 on deposition behavior and structure of electroless copper plating. Electrochemistry 2019, 87, 214–219. [Google Scholar] [CrossRef]
- Schoenberg, L.N. The use of organic additives to stabilize and enhance the deposition rate of electroless copper plating. J. Electrochem. Soc. 1972, 119, 1491. [Google Scholar] [CrossRef]
- Hocalar, M.E. Defining the Effectiveness of Factors in Process of Drying Industrial Bakers Yeast by Using Taguchi Method and Regression Analysis, and Comparing the Results. Int. J. Qual. Res. 2007, 1, 215–220. [Google Scholar]
- Salicio-Paz, A.; Ugarte, I.; Sort, J.; Pellicer, E.; García-Lecina, E. Full optimization of an electroless nickel solution: Boosting the performance of low-phosphorous coatings. Materials 2021, 14, 1501. [Google Scholar] [CrossRef]
- Arun, K.; Kumar, S. Parametric Optimization of Electroless Ni-P Coating for Impact Resistance-A Taguchi Approach. World Appl. Sci. J. 2012, 17, 524–531. [Google Scholar]
- Shu, J.; Grandjean, B.; Kaliaguine, S. Effect of Cu (OH)2 on electroless copper plating. Ind. Eng. Chem. Res. 1997, 36, 1632–1636. [Google Scholar] [CrossRef]
- Jeyaraj, S.; Arulshri, K.; Sivasankaran, S. Investigations on effect of process parameters of electrodeposited Ni-Al2O3 composite coating using orthogonal array approach and mathematical modeling. Arch. Civ. Mech. Eng. 2016, 16, 168–177. [Google Scholar] [CrossRef]
- Joo, Y.-K.; Zhang, S.-H.; Yoon, J.-H.; Cho, T.-Y. Optimization of the adhesion strength of arc ion plating TiAlN films by the Taguchi method. Materials 2009, 2, 699–709. [Google Scholar] [CrossRef]
- Jeyapaul, R.; Shahabudeen, P.; Krishnaiah, K. Quality management research by considering multi-response problems in the Taguchi method–a review. Int. J. Adv. Manuf. Technol. 2005, 26, 1331–1337. [Google Scholar] [CrossRef]
- Orhan, G.; Gürmen, S.; Timur, S. The behavior of organic components in copper recovery from electroless plating bath effluents using 3D electrode systems. J. Hazard. Mater. 2004, 112, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Luo, G.; Li, M.; Shen, Q.; Wang, C.; Zhang, L. Effect of 2, 2′-dipyridyl on the plating rate, microstructure and performance of copper-coated tungsten composite powders prepared using electroless plating. Appl. Surf. Sci. 2014, 301, 85–90. [Google Scholar] [CrossRef]
- Duda, L. Effect of isomeric dipyridyls on electroless copper deposition from EDTA solutions. Plat. Surf. Finish. 1998, 85, 60–62. [Google Scholar]
- Deckert, C. Electroless copper plating, a review: Part I. Plat. Surf. Finish. 1995, 82, 48–55. [Google Scholar]
- Georgieva, M.; Avdeev, G.; Stoychev, D.; Petrova, M. Microstructure and texture investigation of chemically deposited copper coatings. Trans. IMF 2015, 93, 97–103. [Google Scholar] [CrossRef]
- Georgieva, M.; Avdeev, G.; Milusheva, V.; Lazarova, D.; Petrova, M. Investigation of the structure of copper coatings obtained by chemical deposition from formaldehyde-free solution on dielectrics. Bulg. Chem. Commun. 2020, 52, 28–34. [Google Scholar]
- Schumacher, R.; Pesek, J.; Melroy, O. Kinetic analysis of electroless deposition of copper. J. Phys. Chem. 1985, 89, 4338–4342. [Google Scholar] [CrossRef]
- Li, J.; Kohl, P.A. The acceleration of nonformaldehyde electroless copper plating. J. Electrochem. Soc. 2002, 149, C631. [Google Scholar] [CrossRef]
- Kao, L.; Hung, H.; Chen, Y.; Kao, C. Bonding of Copper Pillars Using Electroless Cu Plating. In Proceedings of the 2019 International Conference on Electronics Packaging (ICEP), Niigata, Japan, 17–20 April 2019; pp. 220–222. [Google Scholar] [CrossRef]
- Koo, H.-C.; Saha, R.; Kohl, P.A. Copper electroless bonding of dome-shaped pillars for chip-to-package interconnect. J. Electrochem. Soc. 2011, 158, D698. [Google Scholar] [CrossRef]
- Pan, J.; Toleno, B.J.; Chou, T.C.; Dee, W.J. The effect of reflow profile on SnPb and SnAgCu solder joint shear strength. Solder. Surf. Mt. Technol. 2006, 18, 48–56. [Google Scholar] [CrossRef]



| Bath Parameters | Value |
|---|---|
| Copper sulfate | 0.2 (mol dm−3) |
| Formaldehyde | 0.27 (mol dm−3) |
| PLURONIC F-127 | 0.001 g/L |
| Quadrol | 0.22–0.44 (mol dm−3) |
| Cytosine | 0.9–1.8 × 10−4 (mol dm−3) |
| Temperature | 25–35 °C |
| pH | 12.4–13.2 |
| Design Factors | Levels | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Quadrol (mol dm−3) | 0.22 | 0.33 | 0.44 |
| Cytosine (×10−4 mol dm−3) | 0.9 | 1.35 | 1.8 |
| Temperature (°C) | 25 | 30 | 35 |
| pH | 12.4 | 12.8 | 13.2 |
| Results | ||||||
|---|---|---|---|---|---|---|
| Test. No. | A: Quadrol (mol dm−3) | B: Cytosine (×10−4 mol dm−3) | C: Temperature (°C) | D: pH | Plating Rate (μm/h) | Decomposition Time (min) |
| 1 | 0.22 | 0.9 | 25 | 12.4 | 7 | 52 |
| 2 | 0.22 | 1.35 | 30 | 12.8 | 4.2 | 7 |
| 3 | 0.22 | 1.8 | 35 | 13.2 | 1.5 | 8 |
| 4 | 0.33 | 0.9 | 30 | 13.2 | 6.5 | 6 |
| 5 | 0.33 | 1.35 | 35 | 12.4 | 3.5 | 12.5 |
| 6 | 0.33 | 1.8 | 25 | 12.8 | 9.3 | 16 |
| 7 | 0.44 | 0.9 | 35 | 12.8 | 22.2 | 8 |
| 8 | 0.44 | 1.35 | 25 | 13.2 | 1.9 | 8 |
| 9 | 0.44 | 1.8 | 30 | 12.4 | 2.3 | 41 |
| Level | Factor | |||
|---|---|---|---|---|
| Quadrol | Cytosine | Temperature | pH | |
| 1 | 4.2 | 11.9 | 6.1 | 4.3 |
| 2 | 6.4 | 3.2 | 4.3 | 11.9 |
| 3 | 8.8 | 4.4 | 9.1 | 3.3 |
| Delta | 4.6 | 8.7 | 4.8 | 8.6 |
| Rank | 4 | 1 | 3 | 2 |
| Level | Factor | |||
|---|---|---|---|---|
| Quadrol | Cytosine | Temperature | pH | |
| 1 | 22 | 22 | 25 | 35 |
| 2 | 12 | 9 | 18 | 10 |
| 3 | 19 | 22 | 10 | 7 |
| Delta | 10 | 13 | 15 | 28 |
| Rank | 4 | 3 | 2 | 1 |
| Average Pillar Height | Average Pillar Height Difference | Bonding Percentage | |
|---|---|---|---|
| Original sample | 15.6–19.2 μm | 3.6 μm | 0% |
| Sample after polishing | 14.3–17.3 μm | 3 μm | 3% |
| Sample after grinding | 8–10.2 μm | 2.2 μm | 70% |
| Sample after surface planing | 8.5–8.9 μm | 0.4 μm | >99% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.-H.; Shih, P.-S.; Renganathan, V.; Gräfner, S.J.; Lin, Y.-C.; Kao, C.-L.; Lin, Y.-S.; Hung, Y.-C.; Kao, C.R. A High Copper Concentration Copper-Quadrol Complex Electroless Solution for Chip Bonding Applications. Materials 2024, 17, 1638. https://doi.org/10.3390/ma17071638
Huang J-H, Shih P-S, Renganathan V, Gräfner SJ, Lin Y-C, Kao C-L, Lin Y-S, Hung Y-C, Kao CR. A High Copper Concentration Copper-Quadrol Complex Electroless Solution for Chip Bonding Applications. Materials. 2024; 17(7):1638. https://doi.org/10.3390/ma17071638
Chicago/Turabian StyleHuang, Jeng-Hau, Po-Shao Shih, Vengudusamy Renganathan, Simon Johannes Gräfner, Yu-Chun Lin, Chin-Li Kao, Yung-Sheng Lin, Yun-Ching Hung, and Chengheng Robert Kao. 2024. "A High Copper Concentration Copper-Quadrol Complex Electroless Solution for Chip Bonding Applications" Materials 17, no. 7: 1638. https://doi.org/10.3390/ma17071638
APA StyleHuang, J.-H., Shih, P.-S., Renganathan, V., Gräfner, S. J., Lin, Y.-C., Kao, C.-L., Lin, Y.-S., Hung, Y.-C., & Kao, C. R. (2024). A High Copper Concentration Copper-Quadrol Complex Electroless Solution for Chip Bonding Applications. Materials, 17(7), 1638. https://doi.org/10.3390/ma17071638








