Theoretical Study of Electric, Dielectric, and Optical Properties in Ion Doped Multiferroic SrFe12O19 Nanoparticles
Abstract
1. Introduction
2. The Model
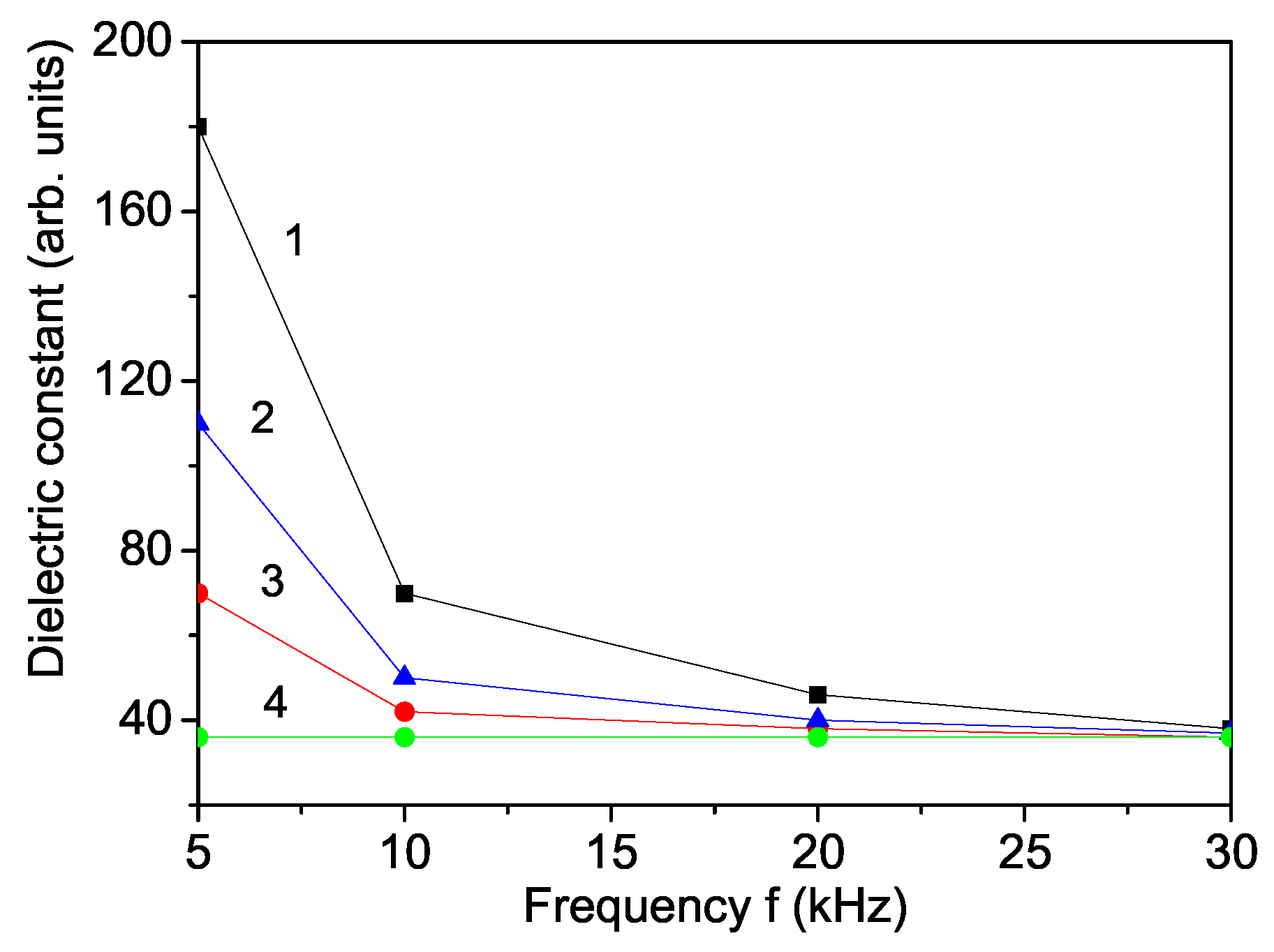
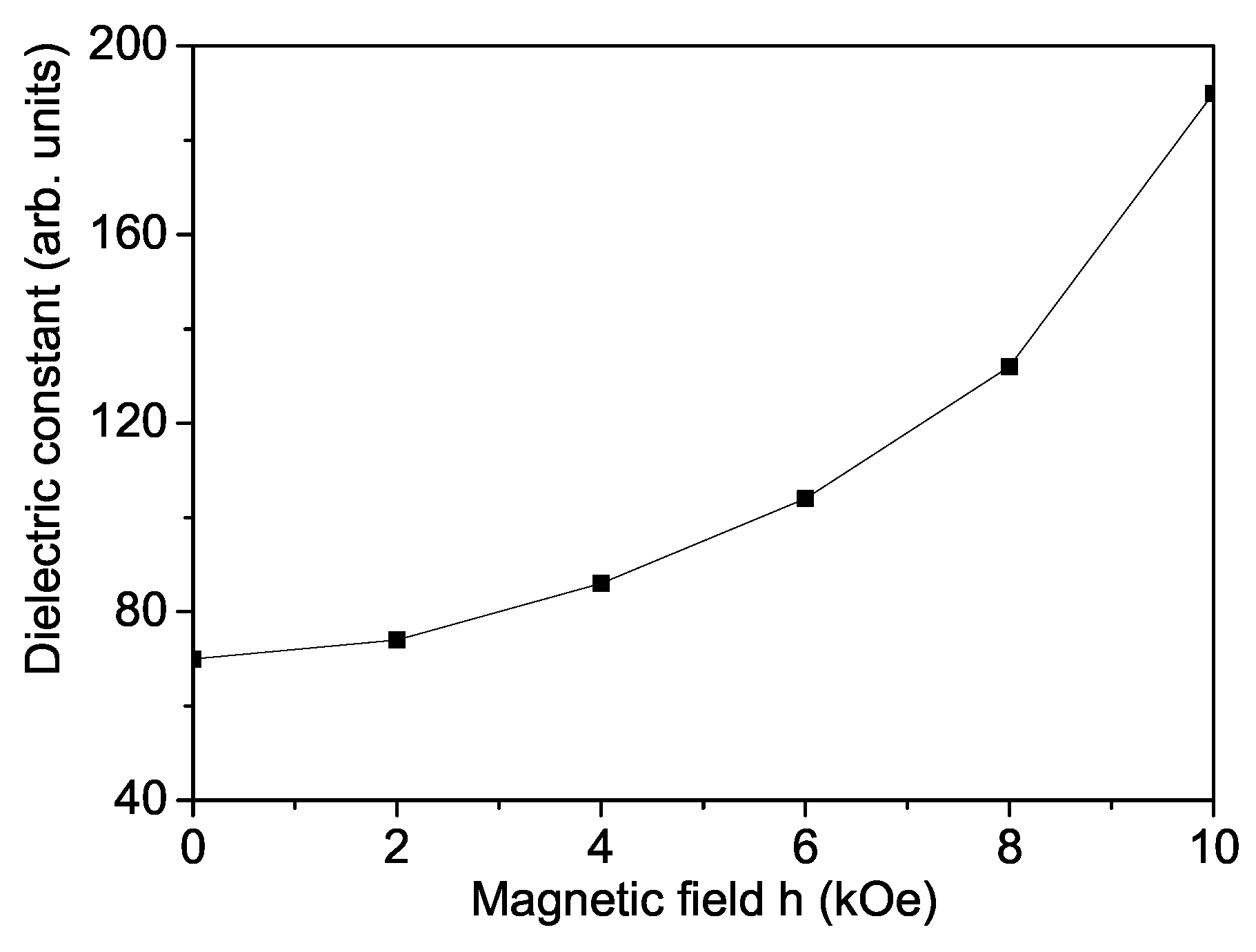
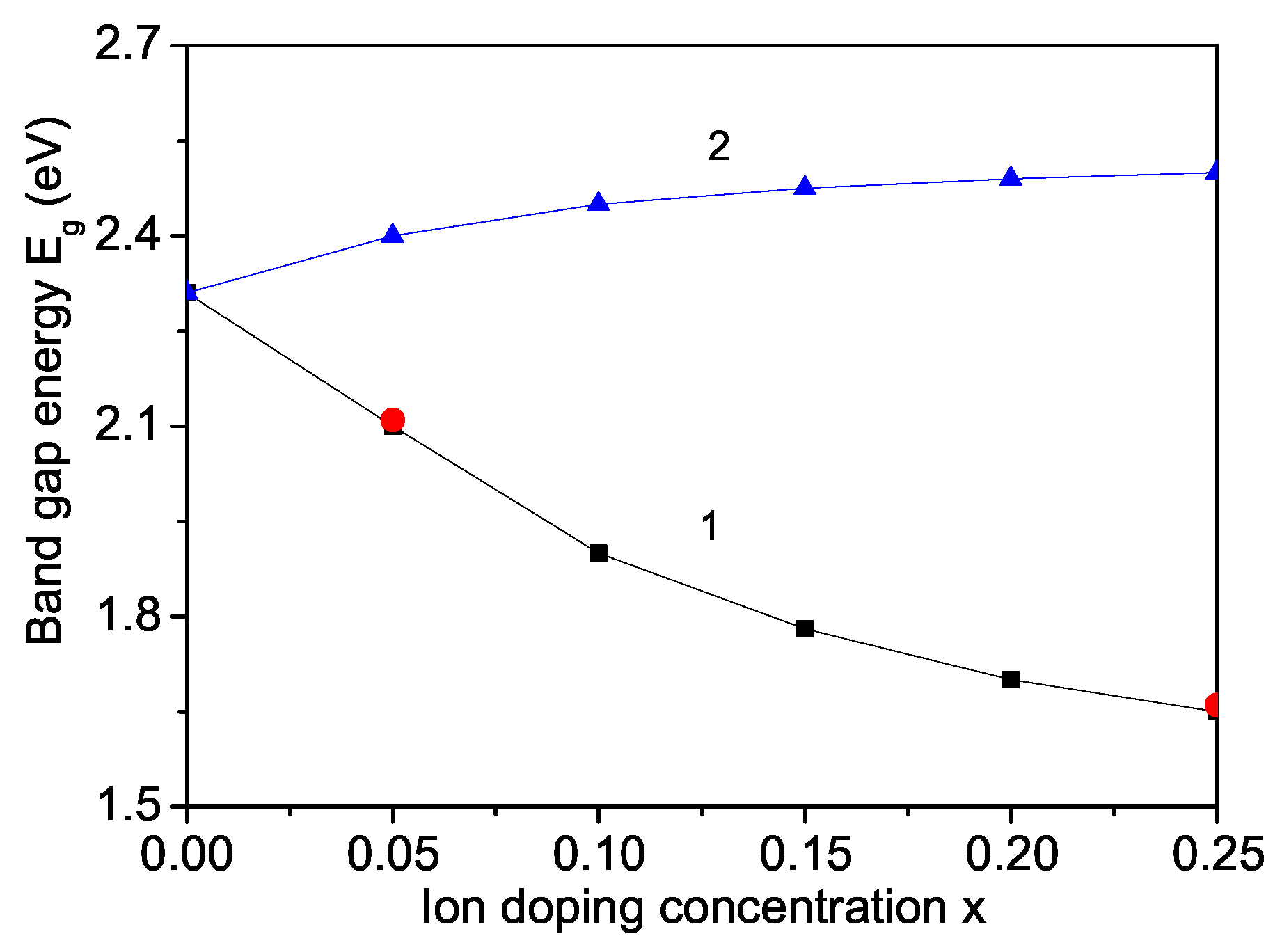
3. Numerical Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pullar, R.C. Hexagonal ferrites: A review of the synthesis, properties and applications of hexaferrite ceramics. Prog. Mater. Sci. 2012, 57, 1191. [Google Scholar] [CrossRef]
- Oezguer, U.; Alivov, Y.; Morkoc, H. Microwave ferrites, part 1: Fundamental properties. J. Mater. Sci. Mater. Electron. 2009, 20, 789–834. [Google Scholar] [CrossRef]
- Singh, V.P.; Jasrotia, R.; Kumar, R.; Raizada, P.; Thakur, S.; Batoo, K.M.; Singh, M. A Current Review on the Synthesis and Magnetic Properties of M-Type Hexaferrites Material. World J. Cond. Matter Phys. 2018, 8, 36–61. [Google Scholar] [CrossRef]
- Adam, A.; Elshafaie, A.; Ibrahim, E.M.M. Structural and magnetic properties of Dy doped SrFe12O19 ferrites. Mater. Today Commun. 2023, 35, 105884. [Google Scholar] [CrossRef]
- Sun, R.; Li, X.; Xia, A.; Su, S.; Jin, C. Hexagonal SrFe12O19 ferrite with high saturation magnetization. Ceram. Intern. 2018, 44, 13551. [Google Scholar] [CrossRef]
- Zi, Z.F.; Sun, Y.P.; Zhu, X.B.; Yang, Z.R.; Dai, J.M.; Song, W.H. Structural and magnetic properties of SrFe12O19 hexaferrite synthesized by a modified chemical co-precipitation method. J. Magn. Magn. Mater. 2008, 320, 2746. [Google Scholar] [CrossRef]
- Kikuchi, T.; Nakamura, T.; Yamasaki, T.; Nakanishi, M.; Fujii, T.; Takada, J.; Ikeda, Y. Magnetic properties of La-Co substituted M-type strontium hexaferrites prepared by polymerizable complex method. J. Magn. Magn. Mater. 2010, 322, 2381. [Google Scholar] [CrossRef]
- Hou, Y.H.; Chen, X.; Guo, X.L.; Li, W.; Huang, Y.L.; Tao, X.M. Effects of intrinsic defects and doping on SrFe12O19: A first-principles exploration of the structural, electronic and magnetic properties. J. Magn. Magn. Mater. 2021, 538, 168257. [Google Scholar] [CrossRef]
- Bercoff, P.G.; Herme, C.; Jacobo, S.E. The influence of Nd-Co substitution on the magnetic properties of non-stoichiometric strontium hexaferrite nanoparticles. J. Magn. Magn. Mater. 2009, 321, 2245. [Google Scholar] [CrossRef]
- Liu, R.; Wu, D.; Yu, X.; Zhou, N.; Liu, D.; Wang, L.; Xu, Z.; Gong, H.; Zhao, T.; Sun, J.; et al. Improved magnetic properties of self-composite SrFe12O19 powder prepared by Fe3O4 nanoparticles. Arabian J. Chem. 2022, 15, 104071. [Google Scholar] [CrossRef]
- Gajbhiye, N.S.; Vijayajakshmi, A. Magnetic Properties of Nanosized SrFe12O19 Particles. J. Magn. Soc. Jpn. 1998, 22 (Suppl. S1), 252. [Google Scholar] [CrossRef] [PubMed]
- Ruikar, D.V.; Kashid, P.B.; Supugade, S.; Pisal, N.; Puri, V. Structural, Electrical and Magnetic Properties of SrCoxFe12−xO19 Prepared by Co-precipitation Method. Adv. Ceram. Sci. Eng. (ACSE) 2013, 2, 72. Available online: https://api.semanticscholar.org/CorpusID:139004495 (accessed on 25 March 2024).
- Omari, L.H.; Lekdadri, A.; Lassri, H.; Mentre, O.; Minaud, C.; Dhahri, E.; Jama, C. Effect of low amount Mn doping on structural and magnetic properties of SrFe12O19: Effective magnetic anisotropy study by Stoner-Wohlfarth model. Mater. Today Commun. 2021, 27, 102257. [Google Scholar] [CrossRef]
- Ghimire, M.L.; Kunwar, D.L.; Dahal, J.N.; Neupane, D.; Yoon, S.; Mishra, S.R. Co-Doped Rare-Earth (La, Pr) and Co-Al Substituted M-Type Strontium Hexaferrite: Structural, Magnetic, and Mossbauer Spectroscopy Study. Mater. Sci. Appl. 2020, 11, 474–493. [Google Scholar] [CrossRef]
- Suo, X.; Li, J.; Zhang, W.; Li, P. Effect of La3+ Substitution on the Structure and Magnetic Properties of M-type Sr Hexaferrites. J. Supercond. Novel Magn. 2022, 36, 197. [Google Scholar] [CrossRef]
- Liu, X.; Zhong, W.; Yang, S.; Yu, Z.; Gu, B.; Du, Y. Influences of La3+ substitution on the structure and magnetic properties of M-type strontium ferrites. J. Magn. Magn. Mater. 2002, 238, 207. [Google Scholar] [CrossRef]
- Irshad, Z.; Bibi, I.; Ghafoor, A.; Majid, F.; Kamal, S.; Ezzine, S.; Elqahtani, Z.M.; Alwadai, N.; El Messaoudi, N. Ni doped SrFe12O19 nanoparticles synthesized via micro-emulsion route and photocatalytic activity evaluation for the degradation of crystal violet under visible light irradiation. Results Phys. 2022, 42, 106006. [Google Scholar] [CrossRef]
- Nazeer, Z.; Ismat; Majid, F.; Kamal, S.; Ibrahim, S.M.; Iqbal, M. Energy band gap tuning of BaFe12O19 doped with Cr and Zn ions to enhance the optical, dielectric, ferroelectric, and photocatalytic properties. Optical Mater. 2022, 125, 112090. [Google Scholar] [CrossRef]
- Paul, S.; Anand, V.; Noorjahan; Duggal, V.; Manokamna. Preparation and performance of a Sr2+ doped LaMnO3 cathode for low temperature solid oxide fuel cells. Mater. Today Proc. 2023; in press. [Google Scholar] [CrossRef]
- Gaona-Esquivel, A.; Hernandez-M, D.S.; Hernandez-Rodriguez, Y.M.; Cigarroa-Mayorga, O.E. The role of Nd as a dopant in Mn3O4 NPs on the heat induction of artificial breast tissue due to the irradiation of microwaves. Mater. Chem. Phys. 2022, 292, 126822. [Google Scholar] [CrossRef]
- Qiang, G.; Jin, Y.; Lu, X.; Cui, X.; Deng, D.; Kang, B.; Yang, W.; Cao, S.; Zhang, J. Temperature effect on the magnetic property and ferroelectricity in hexaferrite SrFe12O19. Appl. Phys. A 2016, 122, 681. [Google Scholar] [CrossRef]
- Tan, G.; Huang, Y.; Sheng, H. Magnetoelectric Response in Multiferroic SrFe12O19 Ceramics. PLoS ONE 2016, 11, e0167084. [Google Scholar] [CrossRef]
- Huang, Y.; Tan, G. Antiferroelectric and Magnetodielectric Coupling Response of La0.2Sr0.7Fe12O19 Ceramics. arXiv 2018, arXiv:1803.00731. [Google Scholar] [CrossRef]
- Yin, J.-H.; Tan, G.-L.; Duan, C.-C. Antiferroelectrics and Magnetoresistance in La0.5Sr0.5Fe12O19 Multiferroic System. Materials 2023, 16, 492. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.-C.; Tan, G.-L. Tuning ferroelectrics to antiferroelectrics in multiferroic LaxSr1-xFe12O19 ceramics. J. Mater. Res. 2022, 37, 1651. [Google Scholar] [CrossRef]
- Tan, G.; Nan, N.; Sharma, P.; Kumar, A. Antiferroelectric and magnetic performance in La0.2Sr0.7Fe12O19 system. J. Mater. Sci. Mater. Electron. 2021, 32, 21697–21708. [Google Scholar] [CrossRef]
- Xue, F.; Liang, L.; Gu, Y.; Takeuchi, I.; Kalinin, S.V.; Chen, L.-Q. Composition- and pressure-induced ferroelectric to antiferroelectric phase transitions in Sm-doped BiFeO3 system. Appl. Phys. Lett. 2015, 106, 012903. [Google Scholar] [CrossRef]
- Azim, M.; Atiq, S.; Riaz, S.; Naseem, S. Phase Stablization and Frequency Dependent Dielectric Response of La-doped Sr-hexaferrites. Mater. Today Proc. 2015, 2 Pt B, 5578–5581. [Google Scholar] [CrossRef]
- Nyathani, M.; Sriramulu, G.; Tadiboyina, A.B.; Prasad, N.V.K.; Ravinder, D.; Katlakunta, S. Crystal Chemistry, Magnetic and Dielectric Properties of Nickel Doped Strontium Ferrites. Biointerf. Res. Appl. Chem. 2022, 12, 929–939. [Google Scholar] [CrossRef]
- Altaf, F.; Atiq, S.; Riazand, S.; Naseem, S. Optimizing the Preparation Conditions of Co-Doped SrFe12O19 and Investigating Its Structural and Dialectical Properties. Sci. Int. 2013, 25, 63–69. [Google Scholar]
- Rasheed, A.; Bibi, I.; Majid, F.; Kamal, S.; Taj, B.; Raza, M.A.S.; Khaliq, N.; Katubi, K.M.; Ezzine, S.; Alwadai, N.; et al. Mn doped SrFe12O19 fabricated via facile microemulsion route and solar-light-driven photocatalytic removal of crystal violet dye. Physica B 2022, 646, 414303. [Google Scholar] [CrossRef]
- Iqbal, M.J.; Ashiq, M.N.; Gul, I.H. Physical, electrical and dielectric properties of Ca-substituted strontium hexaferrite (SrFe12O19) nanoparticles synthesized by co-precipitation method. J. Magn. Magn. Mater. 2010, 322, 1720–1726. [Google Scholar] [CrossRef]
- Manikandan, M.; Kumar, K.S.; Aparnadevi, N.; Venkateswaran, C. Hopkinson effect and temperaturedependent dielectric properties of single domain SrFe12O19 particles. Phys. Status Solidi A 2015, 212, 2179–2185. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Liu, S.; Pan, D.; Chen, L.; Chang, H. Site preference, magnetic properties and energy band gap of SrFe12−xInxO19. J. Magn. Magn. Mater. 2020, 493, 165731. [Google Scholar] [CrossRef]
- Apostolova, I.N.; Apostolov, A.T.; Trimper, S.; Wesselinowa, J.M. Multiferroic Properties of Pure, Transition-Metal and Rare-Earth–Doped BaFe12O19 Nanoparticles. Phys. Status Solidi B 2021, 258, 2100069. [Google Scholar] [CrossRef]
- Thakre, A.; Thakre, N.; Choi, G.; Oh, S.; Ryu, J.; Lee, H.S. First principle understanding of antiferroelectric ordering in La-doped silver niobate. Physica B 2022, 640, 414040. [Google Scholar] [CrossRef]
- Tan, G.-L.; Li, W. Ferroelectricity and Ferromagnetism of M-Type Lead Hexaferrite. J. Am. Ceram. Soc. 2015, 98, 1812. [Google Scholar] [CrossRef]
- Vaks, V.G. Introduction to the Microscopic Theory of Ferroelectrics; Nauka: Moscow, Russia, 1973; p. 158. (In Russian) [Google Scholar]
- Fuchikami, N. Magnetic Anisotropy of Magnetoplumbite BaFe12O19. J. Phys. Soc. Jpn. 1965, 20, 760. [Google Scholar] [CrossRef]
- Zinenko, S.N.; Murakhovski, A.A.; Olkhovik, L.P.; Sizova, Z.I.; Shurinova, E.V.; Kamzin, A.S. Theoretical prediction of a surface-induced spin-reorientation phase transition in BaFe12O19 nanocrystals. J. Exp. Theor. Phys. 2003, 96, 945. [Google Scholar] [CrossRef]
- Golubenko, Z.V.; Olkhovik, L.P.; Popkov, Y.A.; Sizova, Z.I.; Kamzin, A.S. Magnetic anisotropy of a system of nanocrystalline BaO-6Fe2O3 particles. Phys. Solid State 1998, 40, 1718. [Google Scholar] [CrossRef]
- Liu, J.-L.; Zhang, P.; Zhang, X.-K.; Xie, Q.-Q.; Pan, D.-J.; Zhang, J.; Zhang, M. Synthesis and microwave absorbing properties of La-doped Sr-hexaferrite nanopowders via sol-gel auto-combustion method. Rare Met. 2017, 36, 704–710. [Google Scholar] [CrossRef]
- Blinc, R.; Zeks, B. Soft Modes in Ferroelectrics and Antferroelectrics; North-Holland: Amsterdam, The Netherlands, 1974. [Google Scholar]
- Wang, P.S.; Xiang, H.J. Room-Temperature Ferrimagnet with Frustrated Antiferroelectricity: Promising Candidate toward Multiple-State Memory. Phys. Rev. X 2014, 4, 011035. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, A.; Dube, A.; Li, Q.; Varshney, D. Structural and dielectric properties of La and Ni-doped M-type BaFe12O19 ceramics. AIP Conf. Proc. 2016, 1731, 140010. [Google Scholar] [CrossRef]
- Wesselinowa, J.M.; Apostolova, I. Impact of defects on the properties of ferromagnetic nanoparticles. J. Appl. Phys. 2008, 103, 073910. [Google Scholar] [CrossRef]
- Zhang, T.; Peng, X.; Li, J.; Yang, Y.; Xu, J.; Wang, P.; Jin, D.; Jin, H.; Hong, B.; Wang, X.; et al. Structural, magnetic and electromagnetic properties of SrFe12O19 ferrite with particles aligned in a magnetic field. J. Alloys Comp. 2017, 690, 936. [Google Scholar] [CrossRef]
- Liu, Y.; Lai, J.; Liu, Y. Preparation, Characterization, and Microwave Absorption Properties of Cobalt-Doped SrFe12O19 nanoparticles. J. Nanoelectr. Optoelectr. 2021, 16, 998–1004. [Google Scholar] [CrossRef]
- Thanh, T.D.; Tran, N.; Chinh, N.T.V.; Anh, N.T.N.; Manh, D.H.; Tuan, N.Q. Excellent microwave absorption performances of cobalt-doped SrFe12O19 hexaferrite with varying incident angles. J. All. Comp. 2023, 952, 170060. [Google Scholar] [CrossRef]
- Tenorio-Gonzalez, F.N.; Bolarin-Miro, A.M.; Jesus, F.S.; Vera-Serna, P.; Menendez-Gonzalez, N.; Sanchez-Marcos, J. Crystal structure and magnetic properties of high Mn-doped strontium hexaferrite. J. All. Comp. 2017, 695, 2083–2090. [Google Scholar] [CrossRef]
- Bibi, F.; Iqbal, S.; Kalsoom, A.; Jamshaid, M.; Ahmed, A.; Mirza, M.; Qureshi, W.A. Magnetically separable Nd and Mn co-doped SrFe12O19 hexa-ferrites nanostructures for the evaluation of structural, magnetic and photo-catalytic studies under solar irradiation for the crystal violet dye removal from the industrial wastes. Ceram. Int. 2023, 49, 15990–16001. [Google Scholar] [CrossRef]
- Jimenez-Miramontes, J.A.; Melendez-Zaragoza, M.J.; Salinas-Gutierrez, J.M.; Lopez-Ortiz, A.; Collins-Martinez, V. Synthesis and Evaluation of the Phases SrFe2O4 and SrFe12O19 for Hydrogen Production from Photocathalytic Water Spliting; XVII Int; Congress of the Mexican Hydrogen Soc.: Mexico City, Mexico, 2017. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apostolov, A.T.; Apostolova, I.N.; Wesselinowa, J.M. Theoretical Study of Electric, Dielectric, and Optical Properties in Ion Doped Multiferroic SrFe12O19 Nanoparticles. Materials 2024, 17, 1544. https://doi.org/10.3390/ma17071544
Apostolov AT, Apostolova IN, Wesselinowa JM. Theoretical Study of Electric, Dielectric, and Optical Properties in Ion Doped Multiferroic SrFe12O19 Nanoparticles. Materials. 2024; 17(7):1544. https://doi.org/10.3390/ma17071544
Chicago/Turabian StyleApostolov, Angel T., Iliana N. Apostolova, and Julia Mihailowa Wesselinowa. 2024. "Theoretical Study of Electric, Dielectric, and Optical Properties in Ion Doped Multiferroic SrFe12O19 Nanoparticles" Materials 17, no. 7: 1544. https://doi.org/10.3390/ma17071544
APA StyleApostolov, A. T., Apostolova, I. N., & Wesselinowa, J. M. (2024). Theoretical Study of Electric, Dielectric, and Optical Properties in Ion Doped Multiferroic SrFe12O19 Nanoparticles. Materials, 17(7), 1544. https://doi.org/10.3390/ma17071544





