1. Introduction
Electrolysis Manganese Residue (EMR) is the waste residue generated during solid–liquid separation in the filtering process of electrolytic production of manganese metal [
1]. At present, there are about 160 million tons of untreated electrolytic manganese slag in China, and the rate of increase is 10 million tons per year [
2], which not only occupies a large amount of land resources, but also causes serious pollution to the surrounding soil and water due to the presence of a large number of heavy metals in manganese slag, which can easily leach out and enter into the soil, surface water, or groundwater under the action of rainfall [
3]. With the introduction and implementation of a series of policies, such as the Action Program for In-Depth Protection and Restoration of the Yangtze River and the Opinions of the CPC Central Committee–State Council on In-Depth Prevention and Control of Pollution, the pollution problem of EMR is a problem that restricts the sustainable development of the manganese electrolysis industry, and it is also the key to realizing ecological governance within the country. Therefore, there is a need to render EMR non-hazardous and resourceful. The harmless treatment of electrolytic manganese slag mainly includes water washing [
4], acid leaching [
5], electrokinetic restoration [
6], solidification/stabilization (solidification/stabilization, S/S) technology [
7], and other routes. The first three methods have high processing costs and complex technical routes, with scholars now favoring efficient, energy-saving, easy-to-operate S/S technology; compared with other S/S technologies, geopolymers have the advantages of fast setting and early strengthening, good durability, inexpensive costs, and low carbon emissions, which have attracted the attention of researchers [
8,
9,
10].
Geopolymer is a kind of inorganic, highly polymerized, cementitious material prepared using an alkali activator with aluminosilicate raw materials (ASMs) as the raw material, and the physical form is a three-dimensional network gel with amorphous and quasi-crystalline characteristics [
11]. Geopolymer has developed significantly, and the choice of raw materials and activators has broadened considerably, with a wide range of sources of ASMs for the preparation of geopolymers that extends from kaolinite [
12] to fly ash (FA) [
13], steel slag [
14], sludge [
15], coal gangue (CG) [
16], and other solid wastes [
17]. EMR has high silica–aluminum content and is a potential ASM for the preparation of geopolymer. The preparation of geopolymer from EMR can solve the environmental problems caused by EMR and realize the harmless treatment of EMR, but only using EMR as a raw material cannot provide enough ASM for the geopolymerization reaction. Numerous studies have shown that CG and FA are good silica–aluminum feedstocks for the preparation of geopolymer materials and can replace a portion of EMR to prepare geopolymer with excellent properties [
18]. Wang et al. [
19] produced a geopolymer with a 28d strength of 22 MPa through alkaline activation of uncalcined gangue and fly ash at a ratio of 3:7. Beata Figiela et al. [
20] used mining wastes such as gangue to prepare geopolymers, exploring the possibility that these solid wastes could be used as materials in the wider construction industry. Liet al. [
21] calcined electrolytic manganese slag mixed with sodium hydroxide at a mass ratio of 1:0.2 at 200 °C, then calcined with fly ash at a mass ratio of 3:2. After mixing with fly ash at the ratio of 3:2, a geopolymer of 16 MPa was produced at 15 MPa, which demonstrated good environmental stability and leaching toxicity in accordance with the national standard. Meanwhile, the related experiments showed that the geopolymer had better performance in heavy metal curing [
22,
23,
24]. In recent years, even though there have been many attempts to use EMR, the consumption of EMR is still relatively low [
25]. There are fewer studies on the use of higher dosages of EMR or the preparation of cementitious materials with high dosages of EMR without the aid of pressure molding. The aim of this study was to increase the percentage of EMR in the raw material without using pressure molding. To achieve more efficient treatment of EMR and alleviate the environmental problems caused by EMR stockpiling, the use of pour molding can lower energy consumption and reduce carbon emissions. It provides a new idea for the harmless and resourceful utilization of EMR.
In this study, a large amount of manganese waste residue produced in the manganese electrolysis industry, waste gangue produced in coal mining, and fly ash were utilized as raw materials. Geopolymers were prepared by cast molding, i.e., without using applied pressure molding. The mixing ratio and optimization were designed using Design-Expert software (V8.0.6.1), which can avoid the disadvantage of orthogonal experiments that cannot provide visual images. The best mixing ratio was then derived under the condition of disposing of as much EMR as possible. The effects of the modulus of alkali activation solution, the dosage of alkali activation solution, and the curing conditions on the geopolymers were then investigated. In addition, the performance of electrolytic manganese slag–solid waste-based geopolymer (SW-GP) was evaluated by analyzing the mineral phase, morphology, and leaching concentration of the geopolymer.
2. Experimental Section
2.1. Materials
EMR with a moisture content of 14% was taken from a manganese electrolysis enterprise in Guizhou, China, the CG was taken from Yangquan, Shanxi, and the FA used in this study belonged to low-calcium fly ash and was purchased from Wuhan, China, from a materials company. The physical and chemical properties of EMR, CG, and FA are listed in
Table 1. The silica–alumina content of CG and FA, containing more than 80% of the sum, is very suitable as a raw material for the preparation of geopolymers, and the silica–alumina content of EMR is about 40%, which has the potential to be used as a raw material for the preparation of geopolymers. Sodium hydroxide (NaOH) particles were purchased from Sinopharm Reagent (Shanghai, China), and sodium silicate (Na
2SiO
3) powder with a modulus of 3.14 was purchased from Aladdin Co. (Shanghai, China) All reagents were analytically pure reagents.
2.2. Preparation of EMR–Solid Waste-Based Mass Polymers
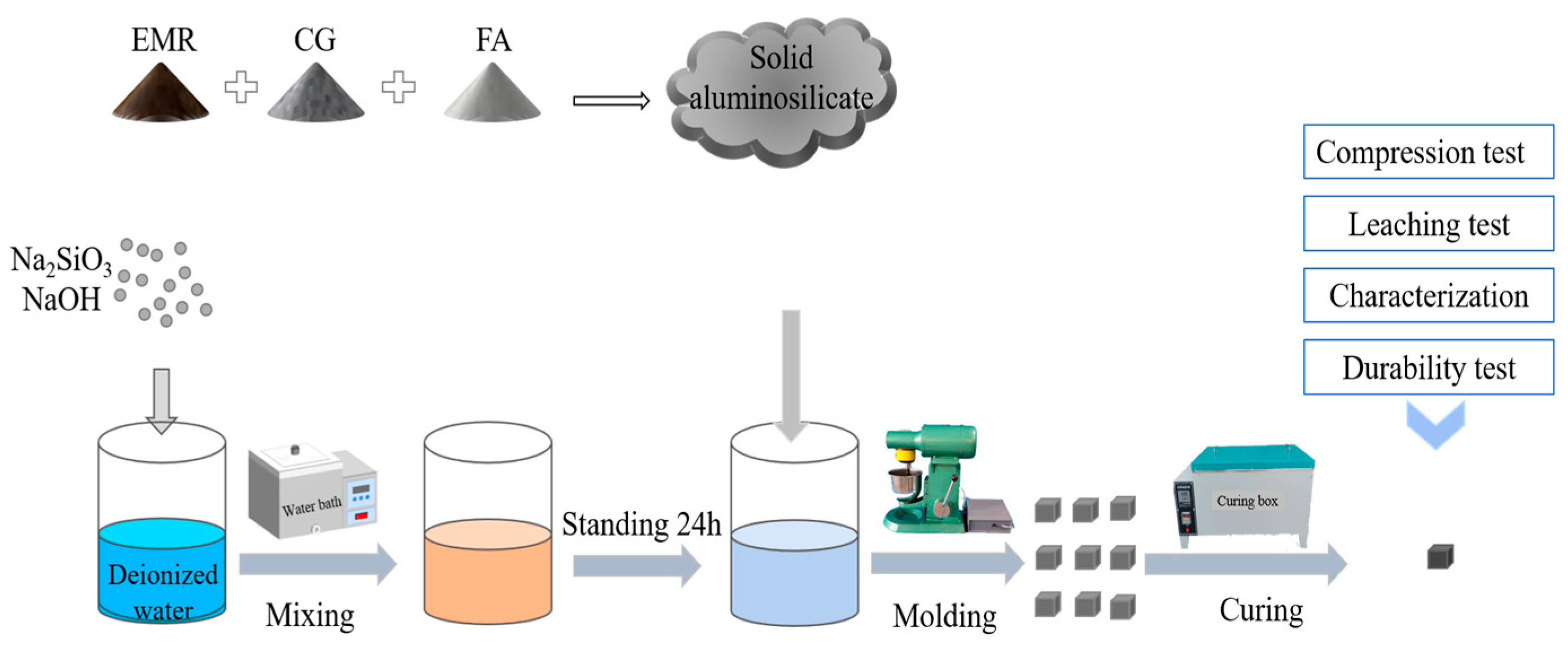
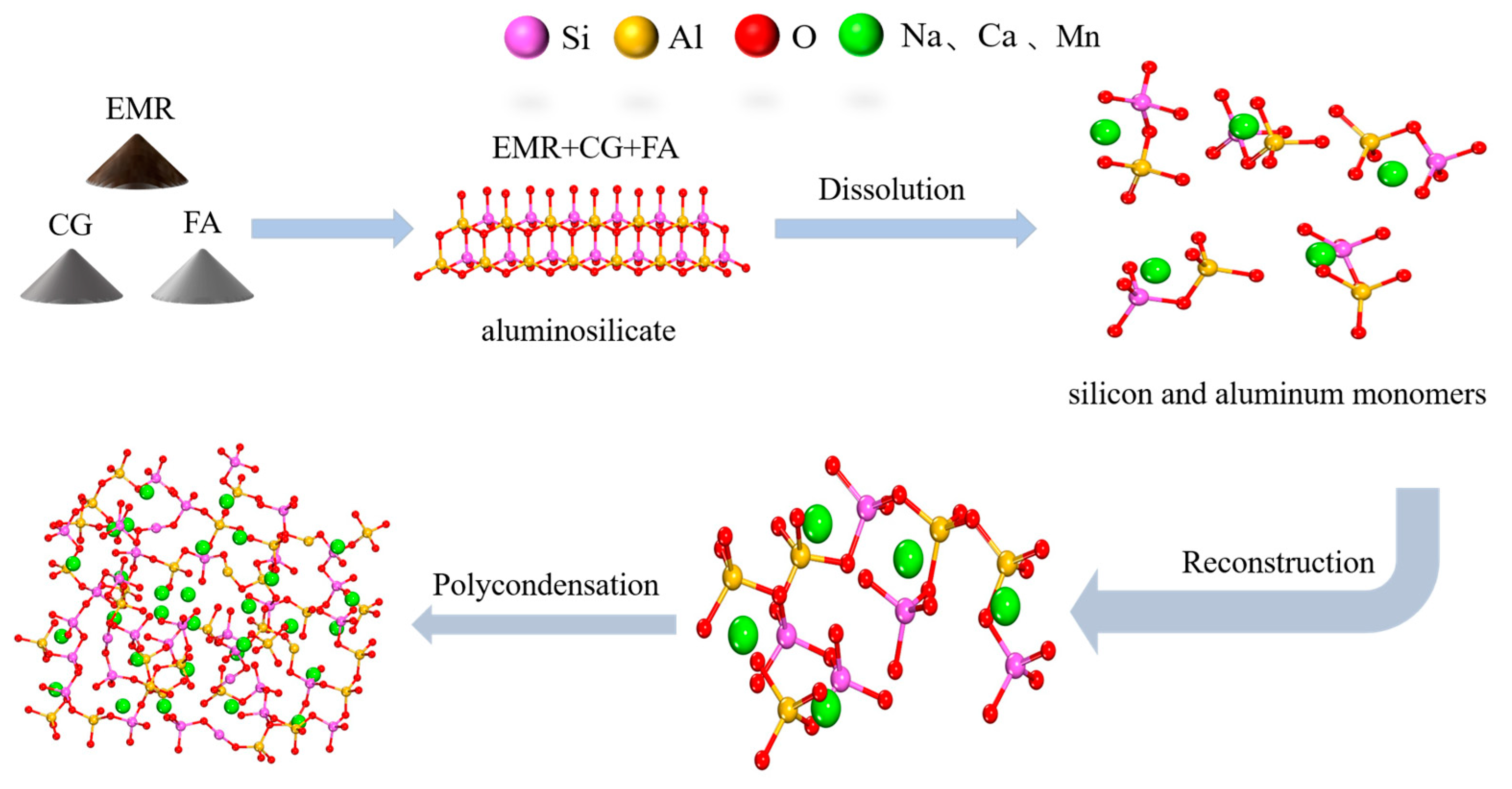
A schematic diagram of the preparation of geopolymer is shown in
Figure 1. (1) EMR, CG, and FA were all dried in an oven at 105 °C for 4 h and ground in a ball mill for 60 min before being cooled to room temperature; the materials were sieved using a 100 mesh sieve before being set aside. (2) The solid Na
2SiO
3 powder with a modulus of 3.14 was dissolved in distilled water, and sodium hydroxide was then added to obtain a sodium silicate solution with a modulus of 1.5 (60% H
2O, 24% SiO
2, 16% Na
2O) which was then allowed to cool to room temperature. (3) The results show that the geopolymer slurry has better fluidity when the liquid–solid (L/S) ratio is 0.9 and is suitable for casting. (4) The mixing experimental design was designed according to
Table 2, and the EMR, CG, and FA were then weighed according to the proportion in
Table 3 before being poured into a planetary stirring pot and stirred for 2 min, followed by the addition of alkali exciters according to the L/S mass ratio of 0.9, stirring at a low speed for 2 min, and stirring at a high speed for 3 min to obtain a black slurry which was then poured into a 20 mm × 20 mm × 20 mm mold. (5) After the gas in the slurry was expelled by the shaker, it was placed in an incubator with a constant temperature of 60 °C and humidity of 96% to cure for 24 h and delaminate to obtain the final geopolymers. The resulting specimens were then cured in the incubator for 1d to obtain the final geopolymer. Finally, the samples were placed in sealed bags and stored at room temperature, ready for compressive strength testing and other characterization at the appropriate age.
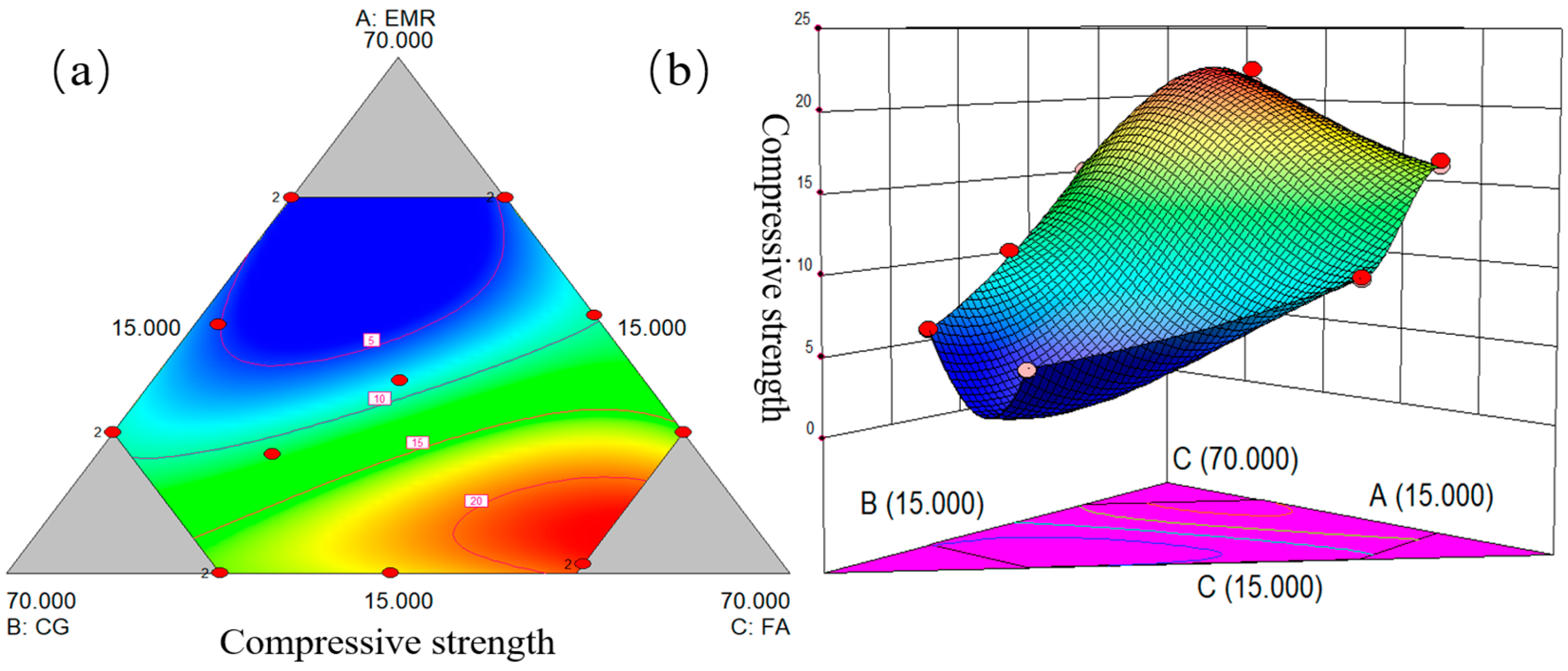
The mix ratios of the mixes were designed using Design-Expert software. The contents of EMR, CG, and FA were set as independent variables and compressive strength was selected as a dependent variable. D-optimal mixes were then chosen in the design of the response surface test, and the interactions of EMR, CG, and FA were then analyzed to establish a response surface of the proportion of raw materials and the mechanical strength of the geopolymer. The optimal response surface model was derived by analyzing the fit of the surface’s model, and the optimization conditions were finally set to derive the optimal fit ratio. The experimental factorial design values are shown in
Table 2. It is found that when the amount of EMR is greater than 55%, the geopolymer has low strength and cannot be molded smoothly, and when the amount of EMR is less than 15%, the mechanical strength of the geopolymer is mainly provided by CG and FA, which is of less value for the present study.
2.3. Test Methods
2.3.1. Compressive Strength
The compressive strength test was carried out using a TYE-3000 compression testing machine (Wuxi Jianyi Instrument & Machinery Co., Ltd., Wuxi, China). The samples were 20 × 20 × 20 cm cubes, and the loading rate was 0.3 MPa/s. A total of 3 specimens were tested in each group and the average value was taken [
26]. Strength was compared with that of standard no-burn bricks [
27]. Compressive strength was calculated according to Equation (1).
where
Rc (MPa) is the compressive strength of the geopolymer and
Fc (N) is the maximum pressure on the geopolymer.
A (mm
2) is the pressurized area of the geopolymer, which is 4 × 10
2 mm
2 in this study.
2.3.2. Leaching Toxicity
The toxicity leaching experiments of geopolymers were conducted according to the solid waste–leaching toxicity–horizontal oscillation method (HJ/T 557-2010) [
28]. A Labs Prodigy7 plasma emission spectrometer (Prodigy7, Leeman Labs, Hudson, NH, USA) from Leeman Labs was used to determine the concentration of heavy metals in the leachate; a Lambda 750S ultraviolet-visible near-infrared spectrophotometer (Lambda 750S, PerkinElmer, Waltham, MA, USA) from PerkinElmer was used to test the leachate’s ammonia nitrogen content.
The leaching concentrations of the samples were measured and compared with the limits of heavy metals in leachate (total Mn < 2 mg/L) and ammonia nitrogen in leachate (ammonia nitrogen < 15 mg/L) according to hazardous waste leaching toxicity identification criteria (GB5085.3-2007) [
29].
2.4. Characterization
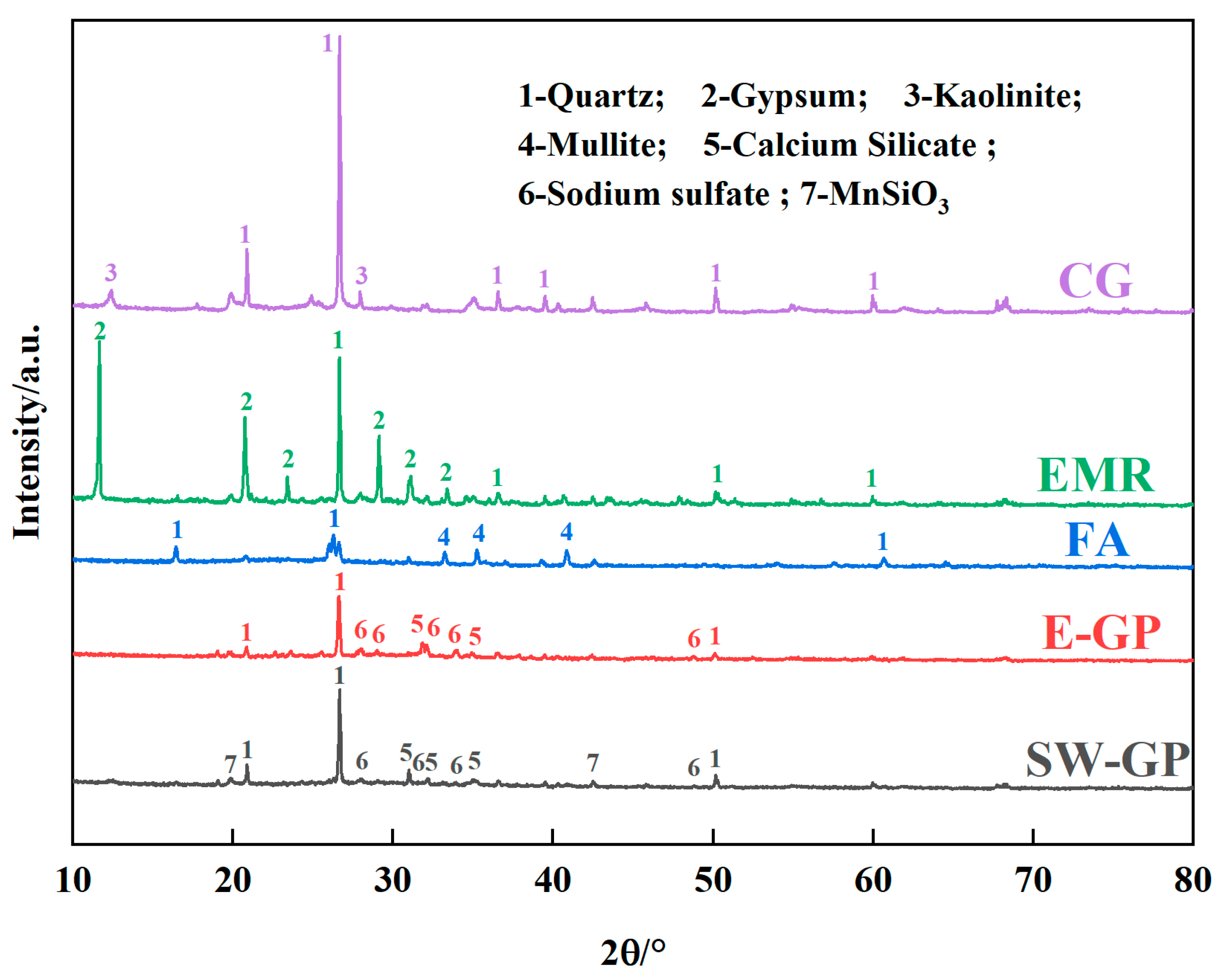
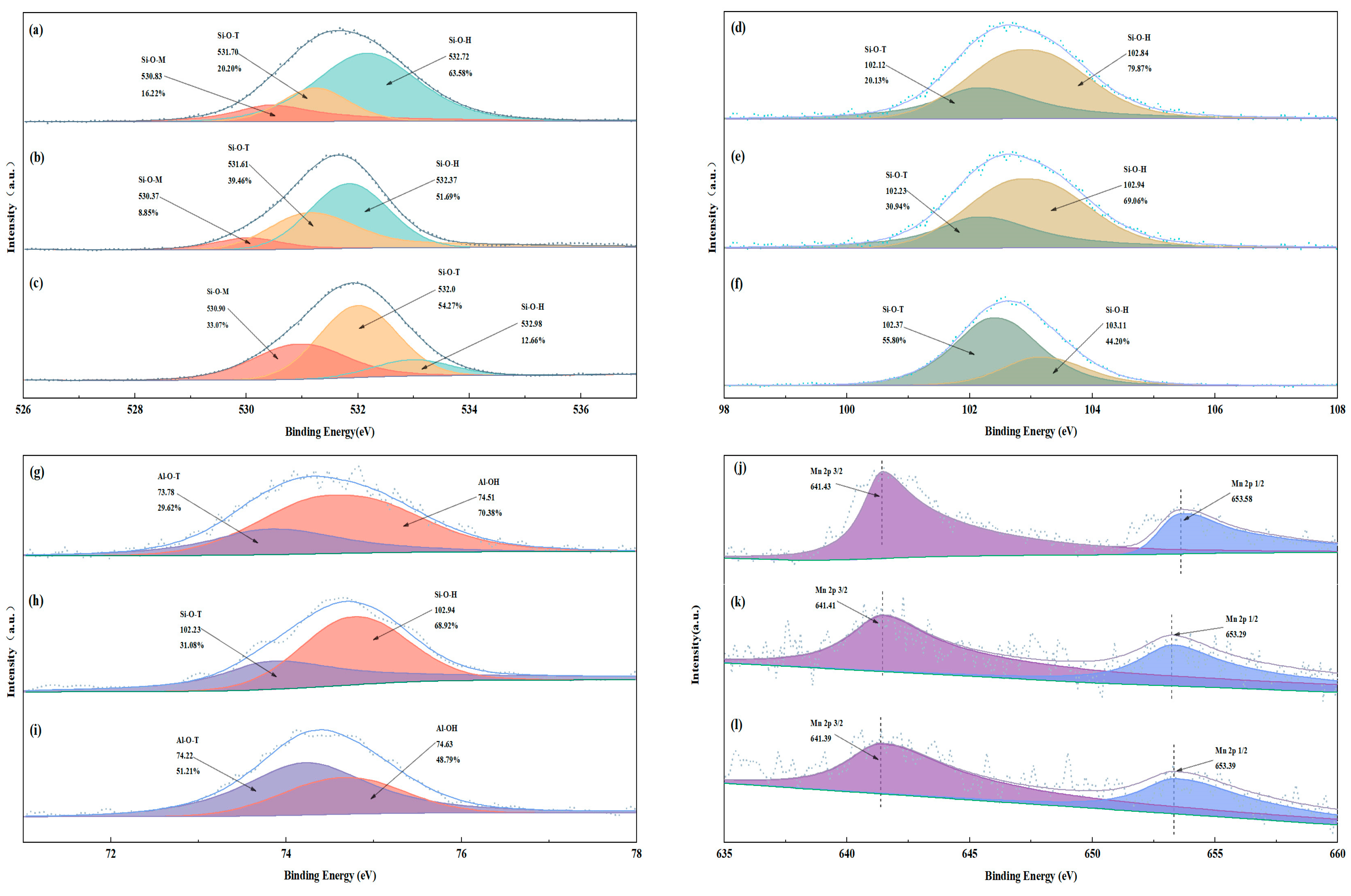
The composition of the raw materials was analyzed using an X-ray fluorescence (XRF) spectrometer (Zetium, PANalytical B.V., Almelo, The Netherlands). The physical phases of the raw materials and geopolymers were analyzed using an X-ray diffractometer (XRD) (D/Max-RB, Rigaku, Tokyo, Japan). Surface morphology was analyzed by scanning electron microscopy (SEM) (JSM5610, JEOL, Akishima, Japan) and energy dispersive spectroscopy (EDS) at an accelerating voltage of 5 kV. A Fourier transform infrared (FTIR) spectrometer (Nexus, Thermo Nicolet, Waltham, MA, USA) was used. The EMR and geopolymer surfaces, the chemical states of O, Si, Al, and Mn, and molecular structural information were analyzed using an X-ray photoelectron (XPS) spectrometer (ESCALAB 250Xi, Thermo Fisher, Waltham, MA, USA), and all peaks were corrected using C-C (284.8 eV).
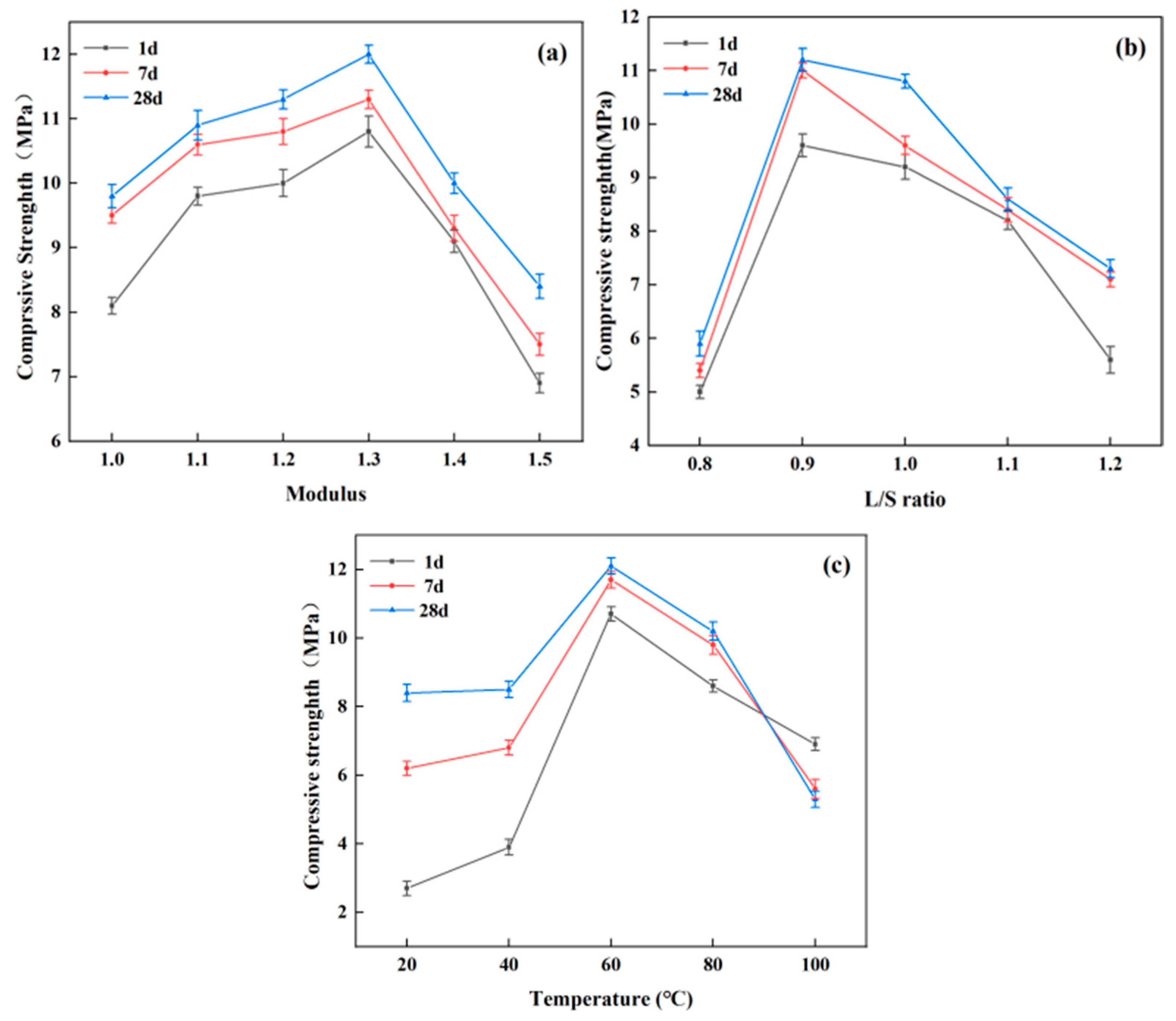
4. Conclusions
SW-GP was prepared through excitation with a Na2SiO3 solution using EMR, CG, and FA as raw materials. Under the conditions of 43% EMR, 34% CG, 23% FA, an exciter modulus of 1.3, a 0.9 L/S ratio, a 60 °C curing temperature, and a 24 h curing time, the strength of the prepared geopolymer reached 12.0 MPa, exceeding the strength of MU7.5 no-burn bricks. The results of toxicity leaching experiments showed that the leaching concentrations of the main heavy metal (Mn) and ammonia nitrogen in geopolymer were 0.123 mg/L and 0.538 mg/L, respectively, which were lower than the national standards. It was shown that EMR, CG, and FA were able to be used as raw materials in the preparation of geopolymer blocks in which a large amount of gelling material was formed (such as geopolymer gels and hydrated calcium silicate gels), and free Mn2+ was wrapped by the geopolymer network in addition to the generation of stabilized MnSiO3 such that the geopolymer had good mechanical properties and chemical stability. This method can treat and utilize a large amount of EMR, which has high economic and environmental significance.