Influence of V on the Microstructure and Precipitation Behavior of High-Carbon Hardline Steel during Continuous Cooling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Prepared
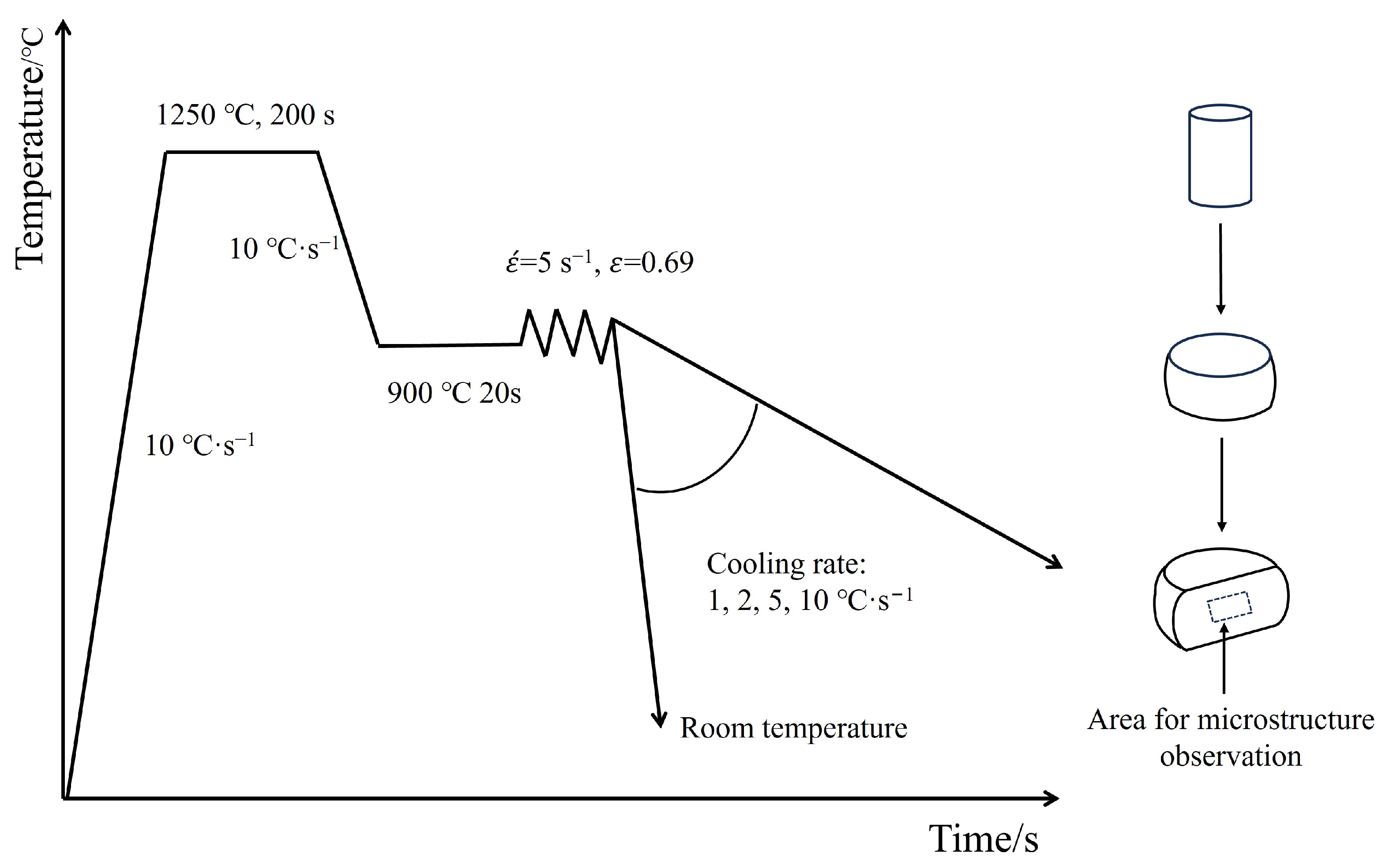
2.2. Thermo-Mechanical Processing
2.3. Characterization Methods
3. Results and Discussion
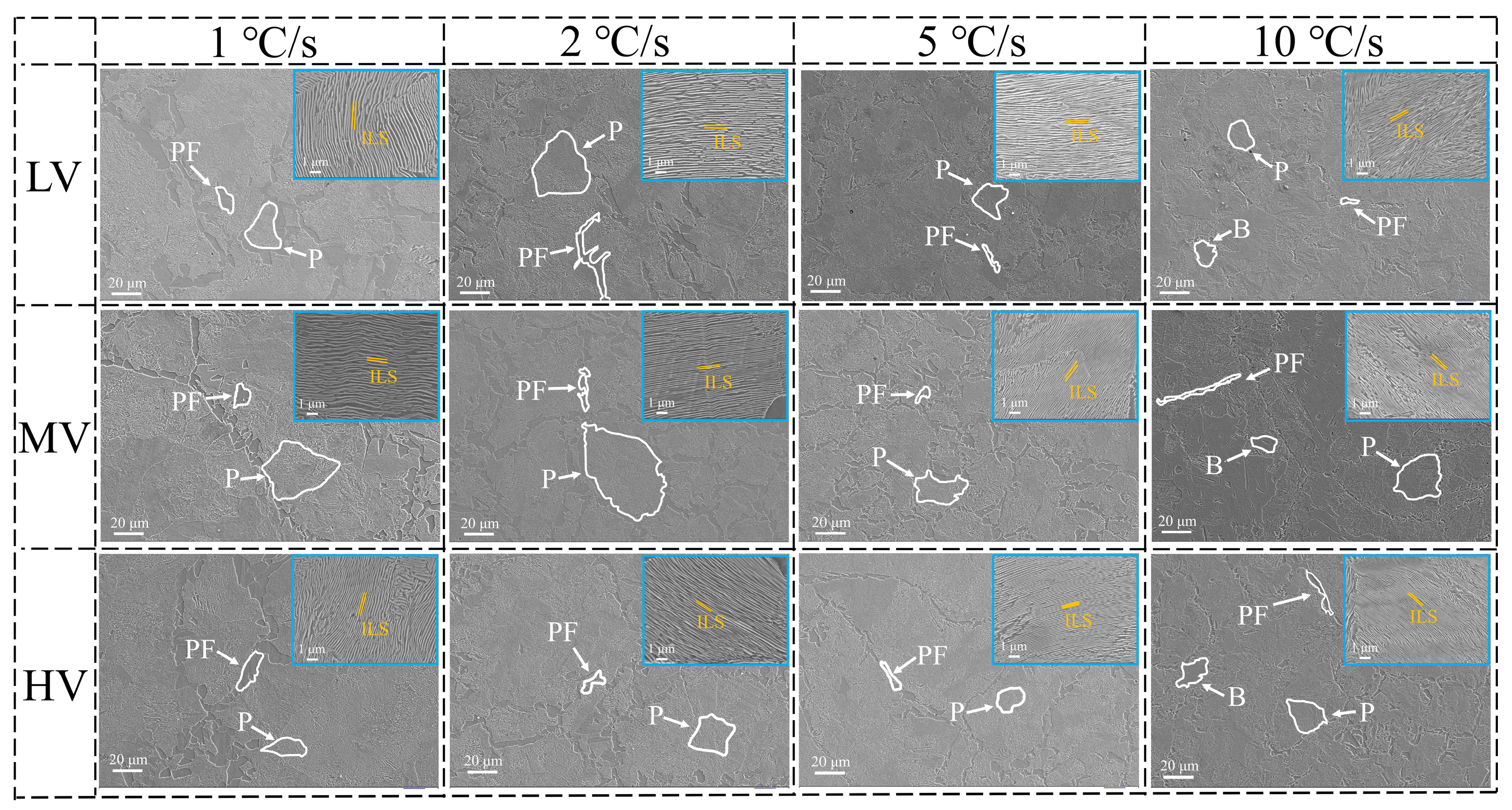
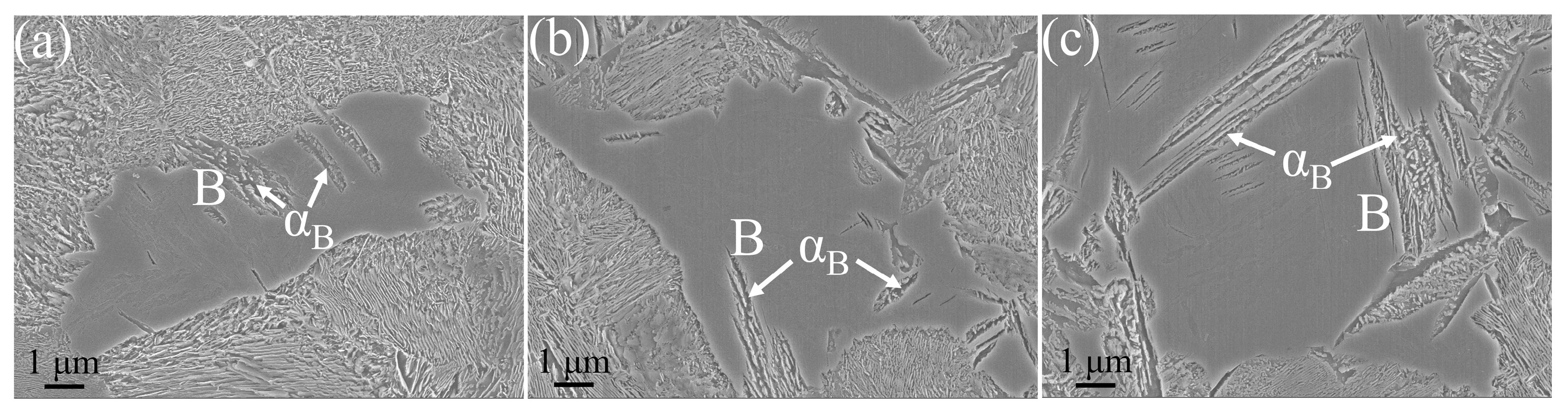
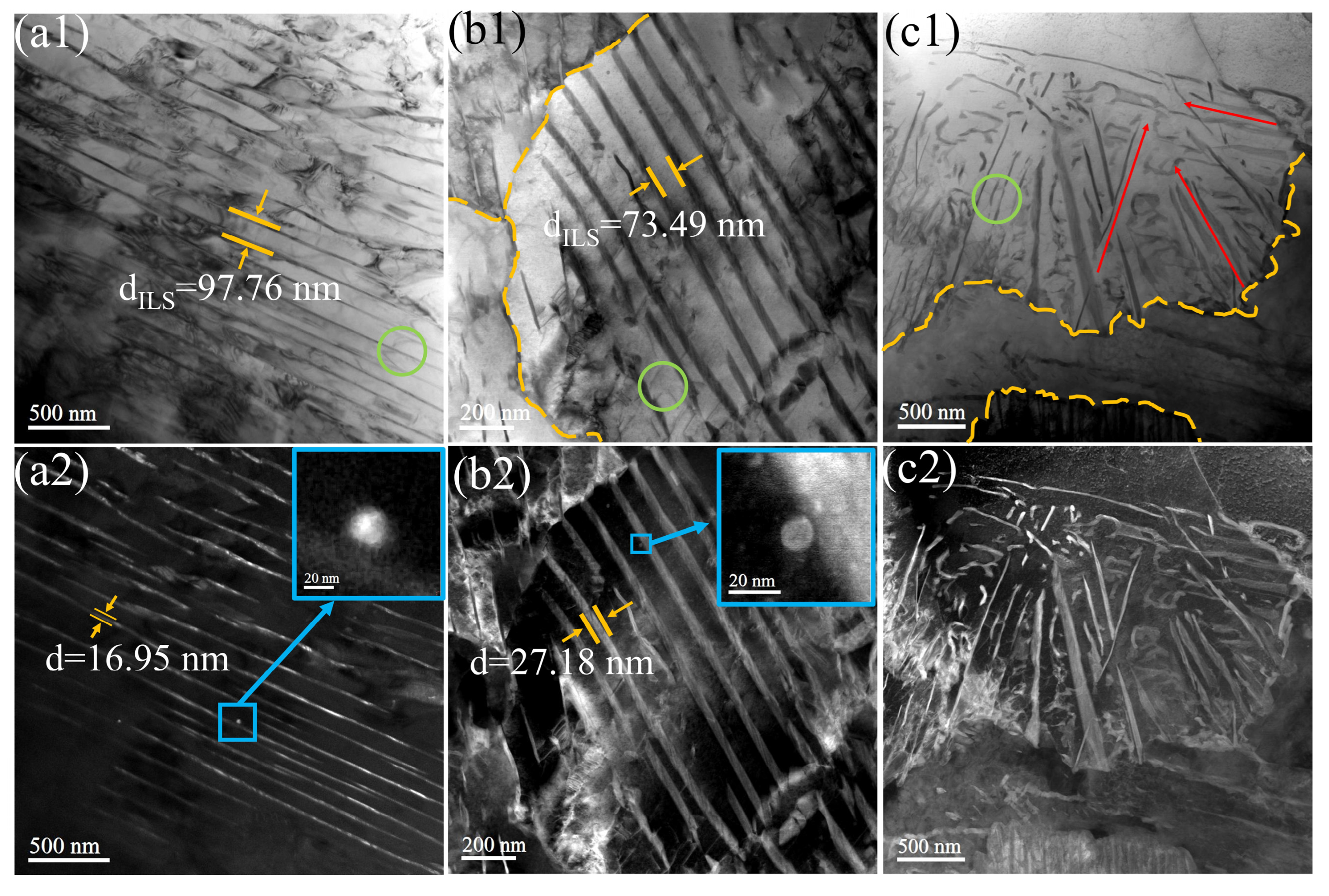
3.1. Microstructure
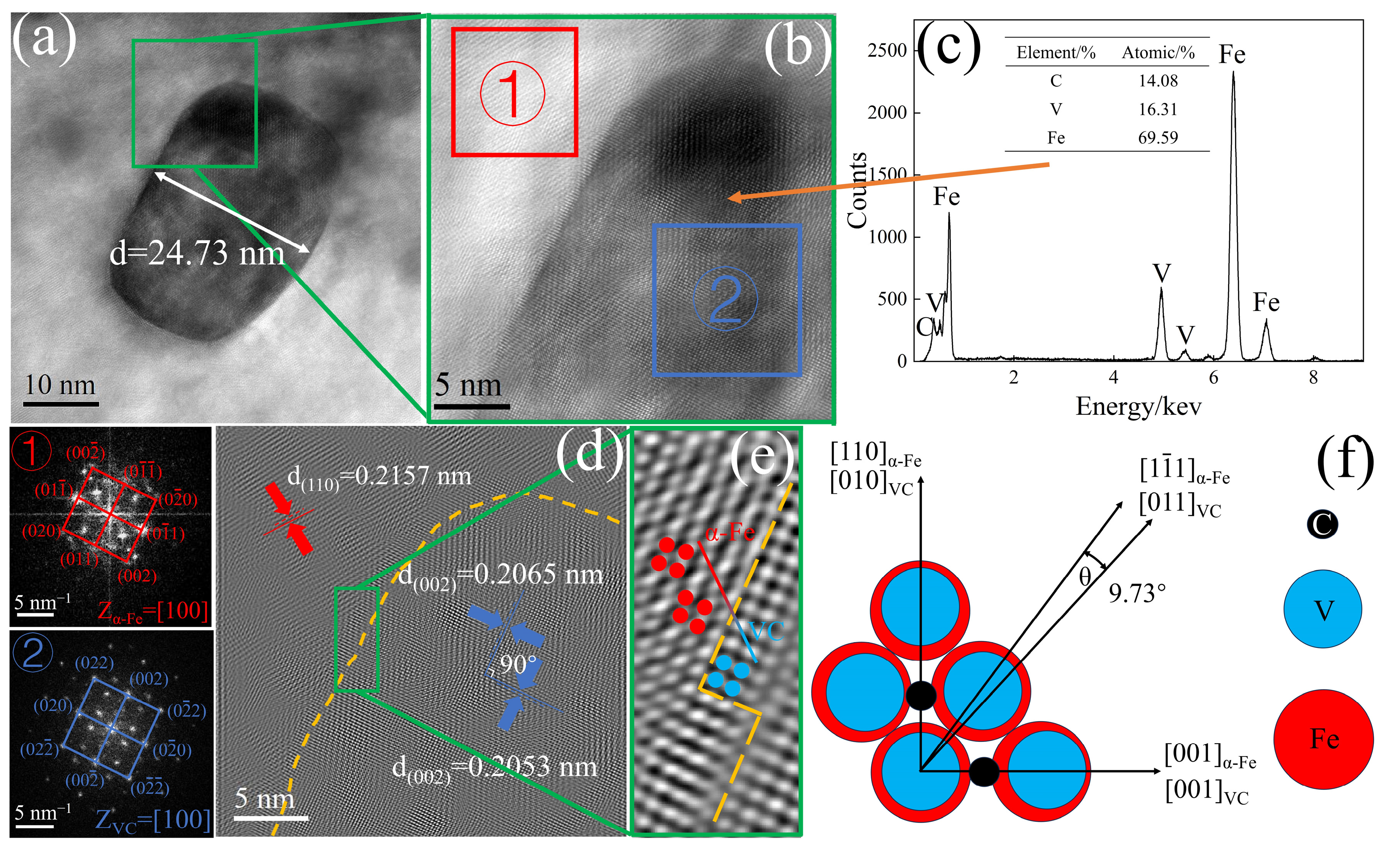
3.2. Precipitate
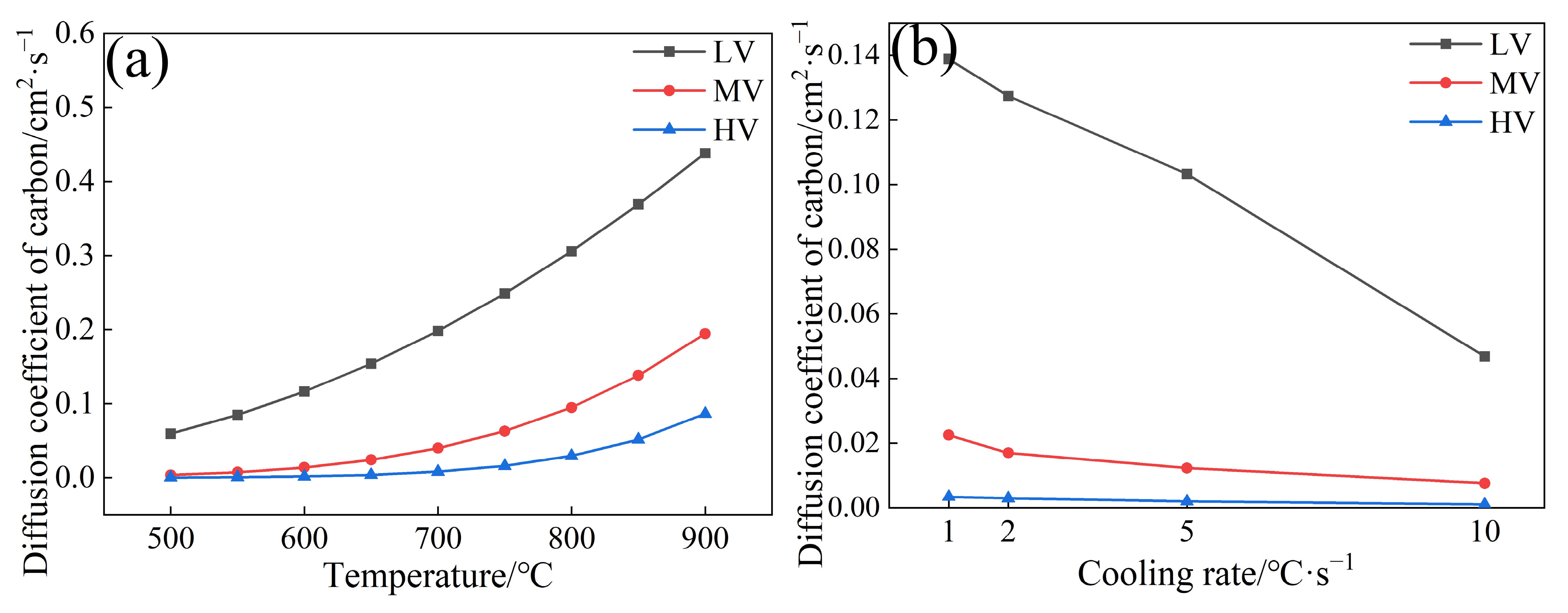
3.3. Effect of V Content on the Carbon Diffusion Coefficient
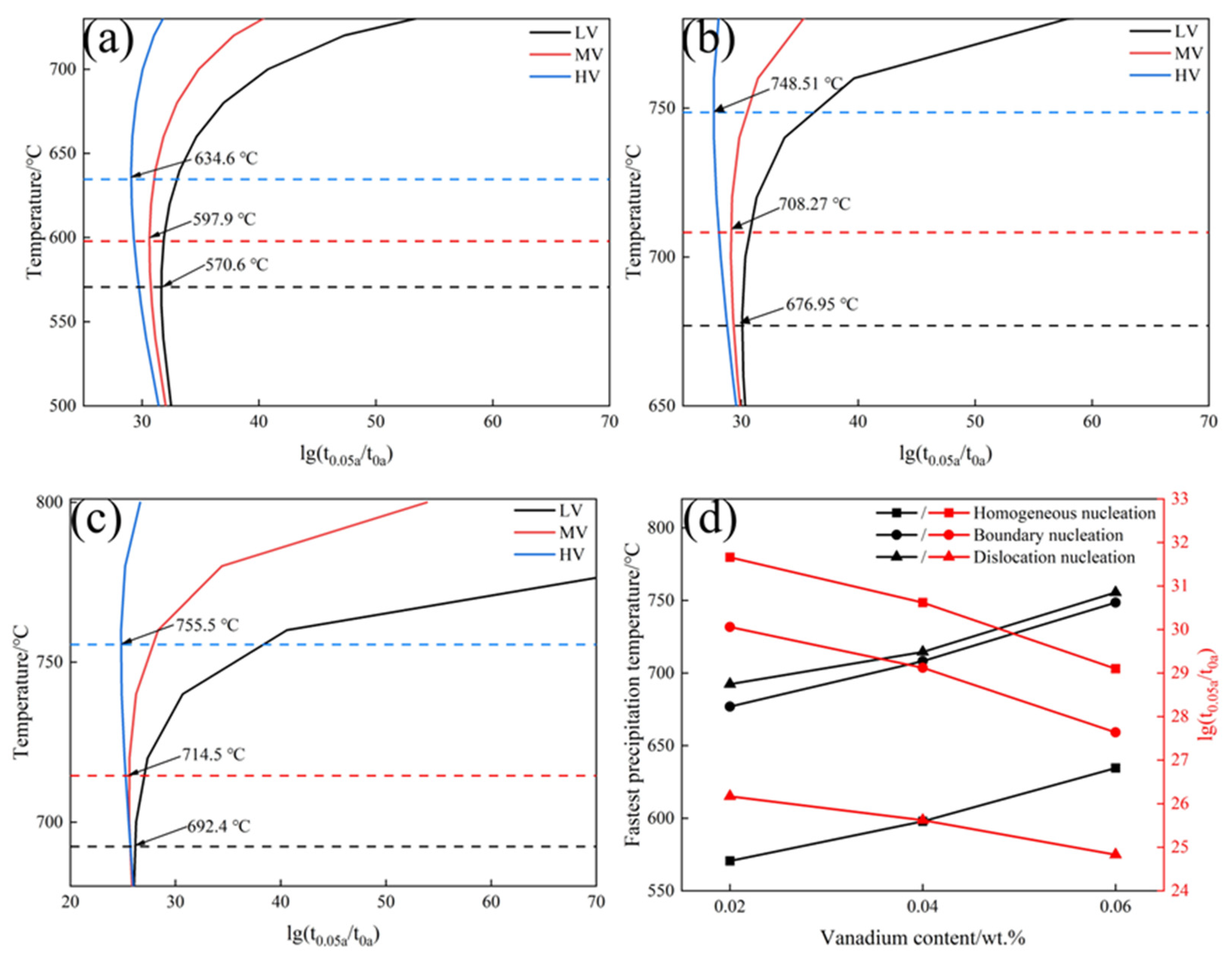
3.4. Effect of V Content on the Precipitation Behavior of VC
4. Conclusions
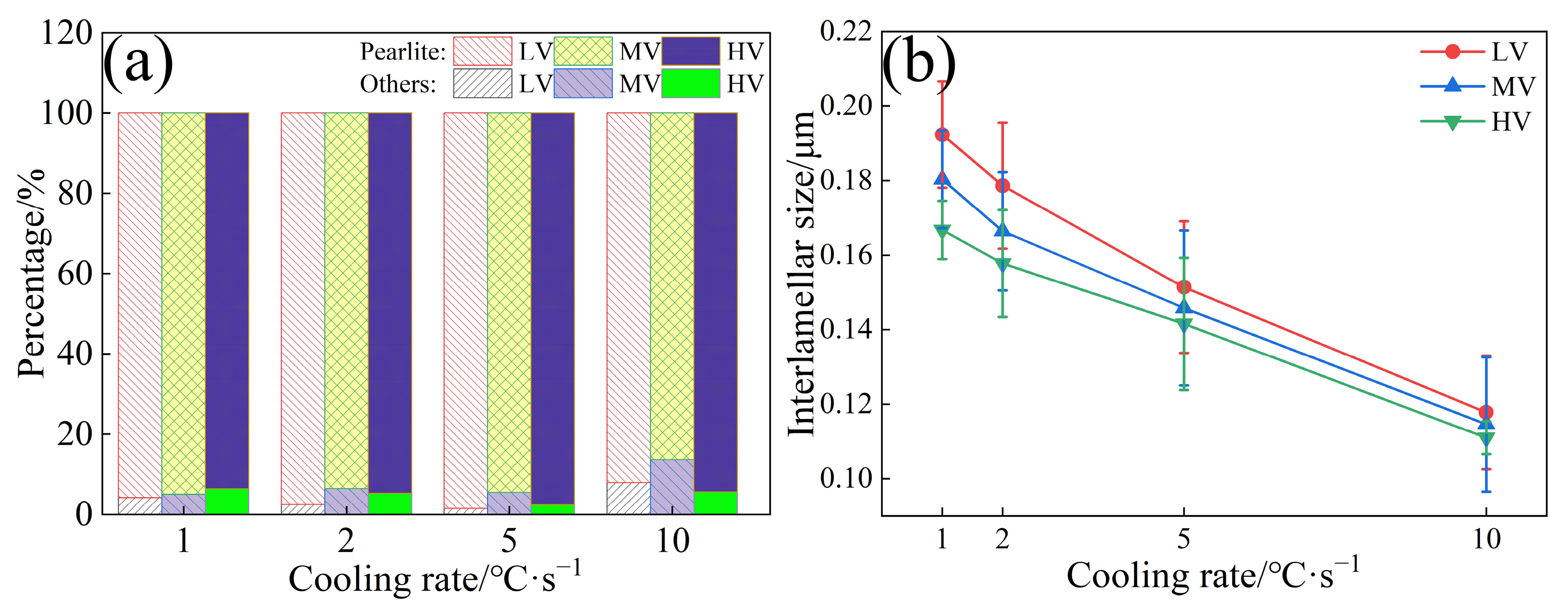
- The ILS for three experimental steels were in the order of LV > MV > HV. As the V content in the steels increased from 0.02 wt.% to 0.06 wt.%, the ILS of the experimental steels at four cooling rates (1, 2, 5, and 10 °C·s−1) decreased from 0.192, 0.178, 0.151, and 0.117 μm to 0.166, 0.157, 0.141, and 0.110 μm, respectively. At a cooling rate of 10 °C·s−1, a small amount of bainite is observed in the experimental steel, accompanied by a decrease in the content of pearlite.
- The carbon diffusion coefficients for three experimental steels were in the order of LV > MV > HV. As the V content increased from 0.02 wt.% to 0.06 wt.%, the carbon diffusion coefficients of the experimental steel at four cooling rates (1, 2, 5, and 10 °C·s−1) decreased from 0.138, 0.127, 0.103, and 0.0467 cm2·s−1 to 3.41 × 10−3, 2.93 × 10−3, 1.95 × 10−3, and 0.98 × 10−3 cm2·s−1, respectively.
- The precipitated phase was VC, with a diameter of approximately 24.73 nm. The misfit between α-Fe and VC was 5.02%, forming a semi-coherent interface between them. VC atoms gradually adjusted their positions to grow along the α-Fe direction. The ΔG of VC values for the three experimental steels were in the order HV > MV > LV. Within the same nucleation mechanism, the critical nucleation temperatures for the experimental steels are in the order HV > MV > LV. With an increase in V content, the PTT curves shift to the left, and the critical nucleation temperatures for homogeneous nucleation, grain boundary nucleation, and dislocation line nucleation increase from 570.6, 676.9, and 692.4 °C to 634.6, 748.5, and 755.5 °C, respectively.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Masoumi, M.; Loureiro, R.C.P.; Mohtadi-Bonab, M.A.; Béreš, M.; de Abreu, H.F.G. Analysis of Crystallographic Orientation and Crack Behavior in Armor Wire with Spheroidized Pearlite: Simulation and Modeling with Linear Elastic Fracture Mechanics. Eng. Fail. Anal. 2024, 159, 108126. [Google Scholar] [CrossRef]
- Matteis, P. Automatic Measurement of Pearlite Spacing by Spectral Analysis. Metallogr. Microstruc. 2020, 9, 152–158. [Google Scholar] [CrossRef]
- Rodriguez-Galeano, K.F.; Nutter, J.; Azakli, Y.; Slater, C.; Rainforth, W.M. Influence of Cr and Cr+Nb on the Interphase Precipitation and Mechanical Properties of V–Mo Microalloyed Steels. Mater. Sci. Eng. A. 2024, 893, 146140. [Google Scholar] [CrossRef]
- Montaña, Y.; Idoyaga, Z.; Gutiérrez, I.; Iza-Mendia, A. Pearlite Spheroidisation and Microstructure Refinement through Heavy Warm Deformation of Hot Rolled 55Vnb Microalloyed Steel. Metall. Mater. Trans. A 2022, 53, 2586–2599. [Google Scholar] [CrossRef]
- Singh, P.P.; Ghosh, S.; Mula, S. Strengthening Behaviour and Failure Analysis of Hot-Rolled Nb+V Microalloyed Steel Processed at Various Coiling Temperatures. Mater. Sci. Eng. A 2022, 859, 144210. [Google Scholar] [CrossRef]
- Wang, Y.; Kang, J.; Liu, D.; Li, Y.; Wang, C.; Wu, Z.; Niu, N.; Yuan, G. Effect of V and Nb on the Microstructure and Properties of High Strength and Toughness Steel for Petroleum Casing. J. Mater. Res. Technol. 2024, 29, 1403–1413. [Google Scholar] [CrossRef]
- Tian, J.; Wang, H.; Zhu, M.; Zhou, M.; Zhang, Q.; Su, X.; Guo, A.; Xu, G. Improving Mechanical Properties in High-Carbon Pearlitic Steels by Replacing Partial V with Nb. Mater. Sci. Eng. A 2022, 834, 142622. [Google Scholar] [CrossRef]
- Solano-Alvarez, W.; Fernandez Gonzalez, L.; Bhadeshia, H.K.D.H. The Effect of Vanadium Alloying on the Wear Resistance of Pearlitic Rails. Wear 2019, 436–437, 203004. [Google Scholar] [CrossRef]
- Wang, B.; Xie, Y.; Zhao, S.; Li, J.; Hu, L. Density Functional Theory Study of the Influence of Ti and V Partitioning to Cementite in Ferritic Steels. Phys. Status Solidi. B 2014, 251, 950–957. [Google Scholar] [CrossRef]
- Wang, Z.; Hui, W.; Chen, Z.; Zhang, Y.; Zhao, X. Effect of Vanadium on Microstructure and Mechanical Properties of Bainitic Forging Steel. Mater. Sci. Eng. A 2020, 771, 138653. [Google Scholar] [CrossRef]
- Pereloma, E.; Cortie, D.; Singh, N.; Casillas, G.; Niessen, F. Uncovering the Mechanism of Dislocation Interaction with Nanoscale (<4 Nm) Interphase Precipitates in Microalloyed Ferritic Steels. Mater. Res. Lett. 2020, 8, 341–347. [Google Scholar]
- Singh, N.; Casillas, G.; Wexler, D.; Killmore, C.; Pereloma, E. Application of Advanced Experimental Techniques to Elucidate the Strengthening Mechanisms Operating in Microalloyed Ferritic Steels with Interphase Precipitation. Acta Mater. 2020, 201, 386–402. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Detemple, E.; Eggeler, G. Revealing the Two-Step Nucleation and Growth Mechanism of Vanadium Carbonitrides in Microalloyed Steels. Scr. Mater. 2020, 187, 350–354. [Google Scholar] [CrossRef]
- Ioannidou, C.; Arechabaleta, Z.; Rijkenberg, A.; Dalgliesh, R.M.; van Well, A.A.; Offerman, S.E. Vc-Precipitation Kinetics Studied by Small-Angle Neutron Scattering in Nano-Steels. Mater. Sci. Forum 2018, 941, 236–244. [Google Scholar] [CrossRef]
- Gong, P.; Liu, X.G.; Rijkenberg, A.; Rainforth, W.M. The Effect of Molybdenum on Interphase Precipitation and Microstructures in Microalloyed Steels Containing Titanium and Vanadium. Acta Mater. 2018, 161, 374–387. [Google Scholar] [CrossRef]
- Moon, J.; Lee, T.; Kim, S.; Lee, C.; Jang, J.H.; Shin, J.; Lee, J.; Lee, B.H.; Suh, D. Isothermal Transformation of Austenite to Ferrite and Precipitation Behavior in 9Cr-1.5Mo-1.25Co-0.1C-Vnb Heat-Resistant Steel. Mater. Charact. 2020, 170, 110677. [Google Scholar] [CrossRef]
- Bikmukhametov, I.; Beladi, H.; Wang, J.; Tari, V.; Rollett, A.D.; Hodgson, P.D.; Timokhina, I. Interface Characteristics and Precipitation During the Austenite-to-Ferrite Transformation of a Ti-Mo Microalloyed Steel. J. Alloys Compd. 2022, 893, 162224. [Google Scholar] [CrossRef]
- Ioannidou, C.; Arechabaleta, Z.; Navarro-López, A.; Rijkenberg, A.; Dalgliesh, R.M.; Kölling, S.; Bliznuk, V.; Pappas, C.; Sietsma, J.; van Well, A.A.; et al. Interaction of Precipitation with Austenite-to-Ferrite Phase Transformation in Vanadium Micro-Alloyed Steels. Acta Mater. 2019, 181, 10–24. [Google Scholar] [CrossRef]
- Kant Thakur, S.; Harish, L.; Kumar Das, A.; Rath, S.; Pathak, P.; Kumar Jha, B. Hot Deformation Behavior and Processing Map of Nb-V-Ti Micro-Alloyed Steel. Mater. Today Proc. 2020, 28, 1973–1979. [Google Scholar] [CrossRef]
- Liang, B.; Ding, Z.; Xu, Z.; Yan, F.; Tian, R.; Chen, C. 3D Growth of Intragranular Pearlite in an Aged 100Mn13 Steel. Steel Res. Int. 2021, 92, 2100226. [Google Scholar] [CrossRef]
- Miyamoto, G.; Hori, R.; Poorganji, B.; Furuhara, T. Interphase Precipitation of Vc and Resultant Hardening in V-Added Medium Carbon Steels. ISIJ Int. 2011, 51, 1733–1739. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Clark, S.J.; Janik, V.; Heenan, R.K.; Venero, D.A.; Yan, K.; McCartney, D.G.; Sridhar, S.; Lee, P.D. Investigating Nano-Precipitation in a V-Containing Hsla Steel Using Small Angle Neutron Scattering. Acta Mater. 2018, 145, 84–96. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Miyamoto, G.; Shinbo, K.; Furuhara, T.; Ohmura, T.; Suzuki, T.; Tsuzaki, K. Effects of Transformation Temperature on Vc Interphase Precipitation and Resultant Hardness in Low-Carbon Steels. Acta Mater. 2015, 84, 375–384. [Google Scholar] [CrossRef]
- Gu, C.; Scott, C.; Fazeli, F.; Wang, X.; Bassim, N.; Zurob, H. Site-Specific Analysis of Precipitates During the Coiling of an Hsla Steel Containing V and Nb. J. Mater. Res. Technol. 2023, 27, 6308–6318. [Google Scholar] [CrossRef]
- Singh, P.P.; Mula, S.; Ghosh, S. Grain Refinement, Strain Hardening and Fracture in Thermomechanically Processed Ultra-Strong Microalloyed Steel. Mater. Today Commun. 2024, 38, 107582. [Google Scholar] [CrossRef]
- Maejima, T.; Yonemura, M.; Kawano, K.; Miyamoto, G. Lattice Strain and Strength Evaluation on V Microalloyed Pearlite Steel. ISIJ Int. 2020, 60, 1810–1818. [Google Scholar] [CrossRef]
- Ballard, T.J.; Speer, J.G.; Findley, K.O.; De Moor, E. Evolution of Austenite Microstructure and Microalloy Precipitation State During Double-Twist Torsion Testing on Nb-Ti-Bearing Steels. Materialia 2023, 31, 101879. [Google Scholar] [CrossRef]
- Yong, Q.L. Secondary Phases in Steels; Metallurgical Industry Press: Beijing, China, 2006. [Google Scholar]
- Rao, G.R.; Rakesha, K.K.; Babu, T.S.; Adarsh, K.S.; Vidhyasagar, M.; Kumar, D.S.; Balachandran, G. Development of Ultra High Strength 27Mnsivs6 Micro-Alloyed Steel by Austempering and Forced Cooling Conditions. Sādhanā Acad. Proc. Eng. Sci. 2022, 47, 206. [Google Scholar] [CrossRef]
- Donthula, H.; Neogy, S.; Sarkar, N.K.; Vishwanadh, B.; Ghosh, S.K.; Chakrabarti, D.; Singh, S.B.; Tewari, R. Quantification of Microstructure Obtained During Isothermal Bainite Transformation: A Novel Dilatometry-Based Model. Scr. Mater. 2024, 242, 115939. [Google Scholar] [CrossRef]
- Franceschi, M.; Bertolini, R.; Fabrizi, A.; Dabalà, M.; Pezzato, L. Effect of Ausforming Temperature on Bainite Morphology in a 3.2% Si Carbide-Free Bainitic Steel. Mater. Sci. Eng. A 2023, 864, 144553. [Google Scholar] [CrossRef]
- Shin, S.; Lee, S.; Hwang, B. In-Situ Sem/Ebsd Investigation of Tensile Deformation-Driven Microstructural Changes in Low-Carbon Hsla Steels with Ferrite-Bainite Microstructures. Mater. Charact. 2023, 206, 113418. [Google Scholar] [CrossRef]
- GB/T 7232-2012; Terminology of Metal Heat Treatment. Standards Press of China: Beijing, China, 2012.
- Dey, I.; Saha, R.; Ghosh, S.K. Influence of Microalloying and Isothermal Treatment on Microstructure and Mechanical Properties of High Carbon Steel. Met. Mater. Int. 2022, 28, 1662–1677. [Google Scholar] [CrossRef]
- Morales, E.V.; Betancourt, G.; Fernandes, J.R.; Batista, G.Z.; Bott, I.S. Hardening Mechanisms in a High Wall Thickness Sour Service Pipe Steel Api 5L X65 Before and After Post-Welding Heat Treatments. Mater. Sci. Eng. A 2022, 851, 143612. [Google Scholar] [CrossRef]
- Mishra, K.; Khiratkar, V.N.; Singh, A. Improvement of Sub-Critical Fatigue Crack Growth Life by Nano-Structuring of Pearlite. Int. J. Fatigue 2019, 122, 84–92. [Google Scholar] [CrossRef]
- Isavand, S.; Assempour, A. Strain Localization and Deformation Behavior in Ferrite-Pearlite Steel Unraveled by High-Resolution in-Situ Testing Integrated with Crystal Plasticity Simulations. Int. J. Mech. Sci. 2021, 200, 106441. [Google Scholar] [CrossRef]
- Li, J.K.; Yang, Z.N.; Hua, M.; Chen, C.; Zhang, F.C. A Medium-C Martensite Steel with 2.6 Gpa Tensile Strength and Large Ductility. Scr. Mater. 2023, 228, 115327. [Google Scholar]
- Bramfitt, B.L. The Effect of Carbide and Nitride Additions on the Heterogeneous Nucleation Behavior of Liquid Iron. Metall. Trans. B 1970, 1, 1987–1995. [Google Scholar] [CrossRef]
- StJohn, D.H.; Easton, M.A.; Qian, M.; Taylor, J.A. Grain Refinement of Magnesium Alloys: A Review of Recent Research, Theoretical Developments, and Their Application. Metall. Mater. Trans. A 2013, 44, 2935–2949. [Google Scholar] [CrossRef]
- Chen, L.H.; Li, C.R.; Li, Z.Y.; Li, Z.S.; Liu, Z.L. Heterogeneous Nucleation Analysis and Grain Refinement of Second Phase VC in HRB500E Steel. Mater. Repr. 2020, 34, 436–439. [Google Scholar]
- Hu, G.X.; Cai, X.; Rong, Y.H. Fundamental of Materials Science; Shanghai Jiaotong University Press: Shanghai, China, 2011. [Google Scholar]
- Tian, J.; Xu, G.; Wang, L.; Zhou, M.; Hu, H. In Situ Observation of the Lengthening Rate of Bainite Sheaves During Continuous Cooling Process in a Fe–C–Mn–Si Superbainitic Steel. T. Indian I. Metals 2018, 71, 185–194. [Google Scholar] [CrossRef]
- Lee, K.J.; Lee, J.K. Modelling of Γ/A Transformation in Niobium-Containing Microalloyed Steels. Scr. Mater. 1999, 40, 831–836. [Google Scholar] [CrossRef]
- Hillert, M.; Staffansson, L. The Regular Solution Model for Stoichiometric Phases and Ionic Melts. Acta Chem Scand. 1970, 24, 3618–3626. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of Phase Change. II Transformation-Time Relations for Random Distribution of Nuclei. J. Chem. Phy. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Karmakar, A.; Biswas, S.; Mukherjee, S.; Chakrabarti, D.; Kumar, V. Effect of Composition and Thermo-Mechanical Processing Schedule on the Microstructure, Precipitation and Strengthening of Nb-Microalloyed Steel. Mater. Sci. Eng. A 2017, 690, 158–169. [Google Scholar] [CrossRef]
- Bansal, G.K.; Srivastava, V.C.; Ghosh Chowdhury, S. Role of Solute Nb in Altering Phase Transformations During Continuous Cooling of a Low-Carbon Steel. Mater. Sci. Eng. A 2019, 767, 138416. [Google Scholar] [CrossRef]
- Dey, I.; Ghosh, S.K.; Saha, R. Effects of Cooling Rate and Strain Rate on Phase Transformation, Microstructure and Mechanical Behaviour of Thermomechanically Processed Pearlitic Steel. J. Mater. Res. Technol. 2019, 8, 2685–2698. [Google Scholar] [CrossRef]
- Hutten, E.; Liang, S.; Bellhouse, E.; Sarkar, S.; Lu, Y.; Langelier, B.; Zurob, H.S. Mechanical Properties and Precipitation Behavior of High Strength Hot-Rolled Ferritic Steel Containing Nb and V. J. Mater. Res. Technol. 2021, 14, 2061–2070. [Google Scholar] [CrossRef]


| Element | C | Si | Mn | P | S | Cr | Ni | Cu | V | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| LV | 0.69 | 0.34 | 0.63 | 0.033 | 0.034 | 0.12 | 0.23 | 0.11 | 0.02 | Bal. |
| MV | 0.69 | 0.35 | 0.62 | 0.034 | 0.032 | 0.11 | 0.24 | 0.12 | 0.04 | Bal. |
| HV | 0.68 | 0.35 | 0.63 | 0.032 | 0.031 | 0.13 | 0.26 | 0.11 | 0.06 | Bal. |
| [hkl]VC | [hkl]α-Fe | d[hkl]VC | d[hkl]α-Fe | θ,deg | d[hkl]VCcosθ |
|---|---|---|---|---|---|
| [010] | [110] | 4.162 | 4.145 | 0 | 4.162 |
| [011] | 2.943 | 2.539 | 9.73 | 2.901 | |
| [001] | [001] | 2.943 | 2.931 | 0 | 2.943 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Gu, S.; Wang, J.; Wei, F.; Li, Z.; Zeng, Z.; Shen, B.; Li, C. Influence of V on the Microstructure and Precipitation Behavior of High-Carbon Hardline Steel during Continuous Cooling. Materials 2024, 17, 1392. https://doi.org/10.3390/ma17061392
Zhang J, Gu S, Wang J, Wei F, Li Z, Zeng Z, Shen B, Li C. Influence of V on the Microstructure and Precipitation Behavior of High-Carbon Hardline Steel during Continuous Cooling. Materials. 2024; 17(6):1392. https://doi.org/10.3390/ma17061392
Chicago/Turabian StyleZhang, Junxiang, Shangjun Gu, Jie Wang, Fulong Wei, Zhiying Li, Zeyun Zeng, Bin Shen, and Changrong Li. 2024. "Influence of V on the Microstructure and Precipitation Behavior of High-Carbon Hardline Steel during Continuous Cooling" Materials 17, no. 6: 1392. https://doi.org/10.3390/ma17061392





