The Microstructure and Mechanical Properties of a 15-6 PH Stainless Steel with Improved Thermal Aging Embrittlement Resistance
Abstract
1. Introduction
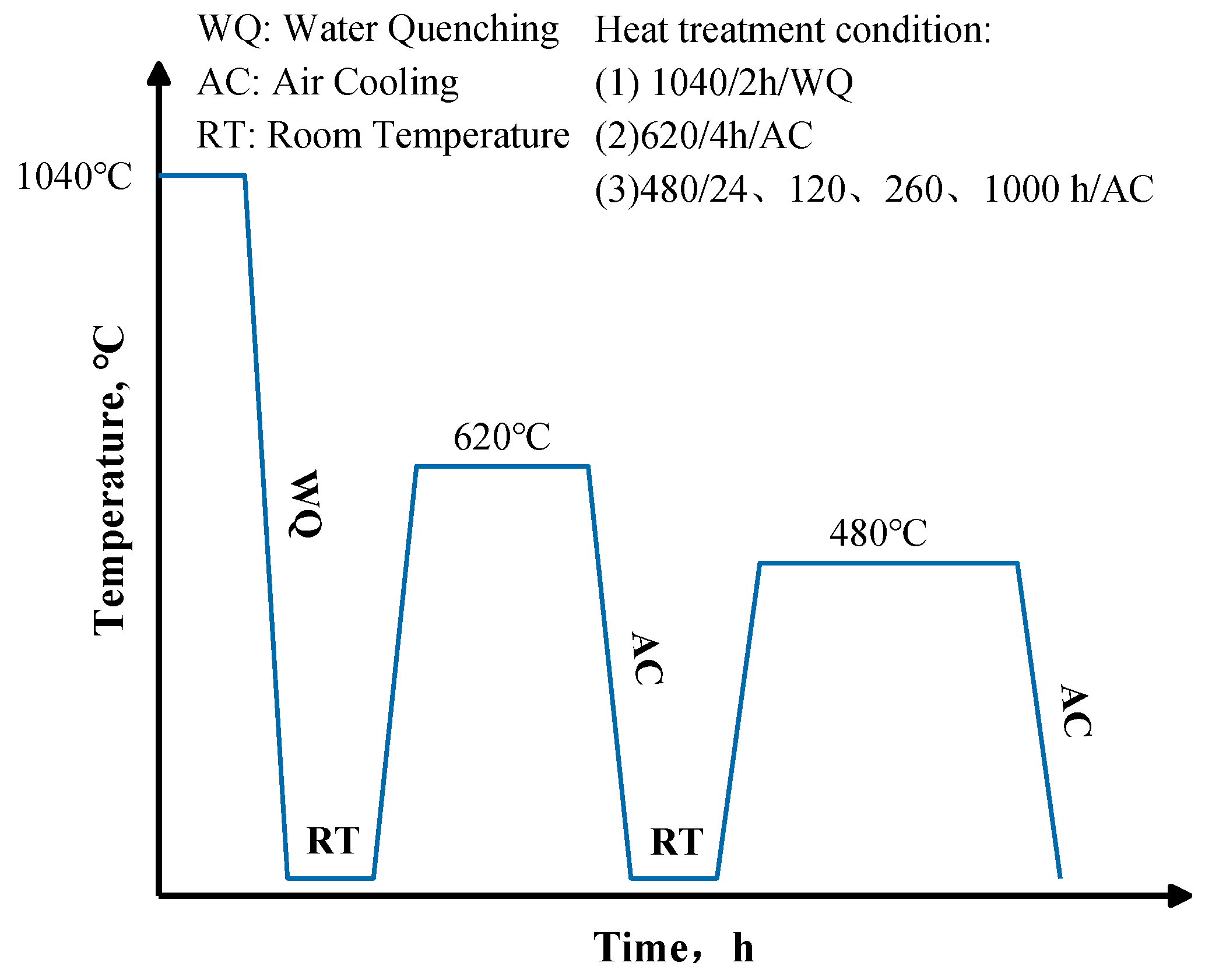
2. Experimental Materials and Methods
3. Results and Analysis
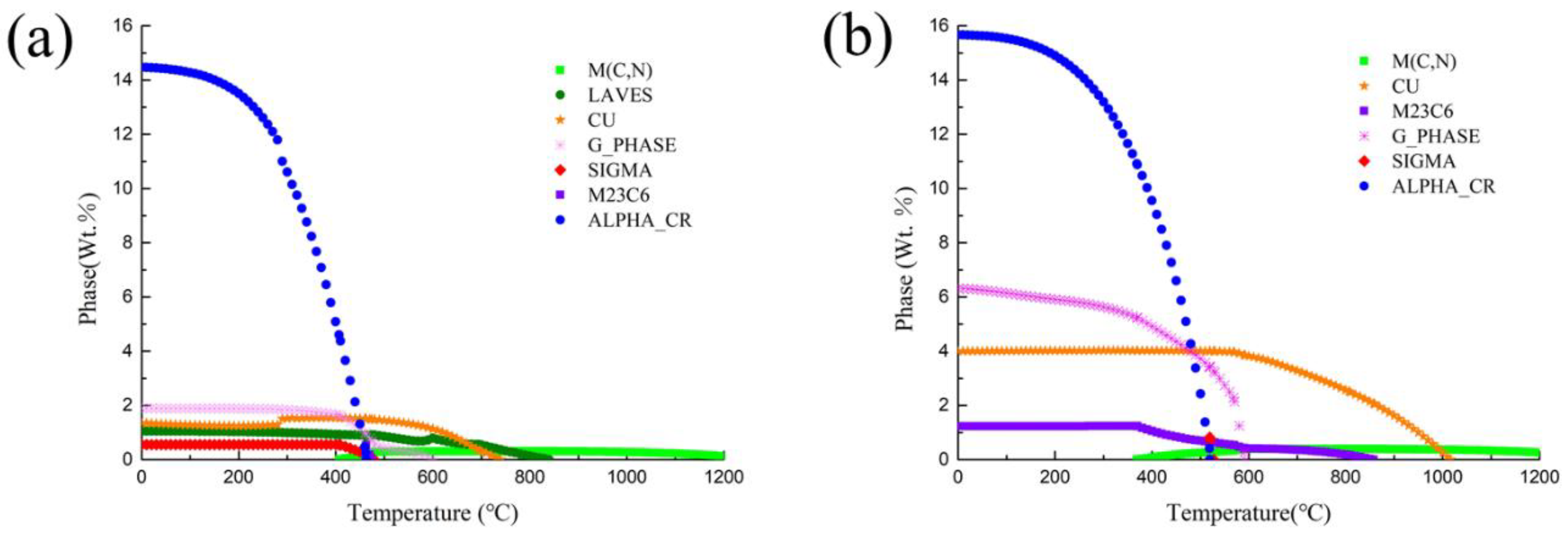
3.1. Thermodynamic Calculation and Phase Analysis
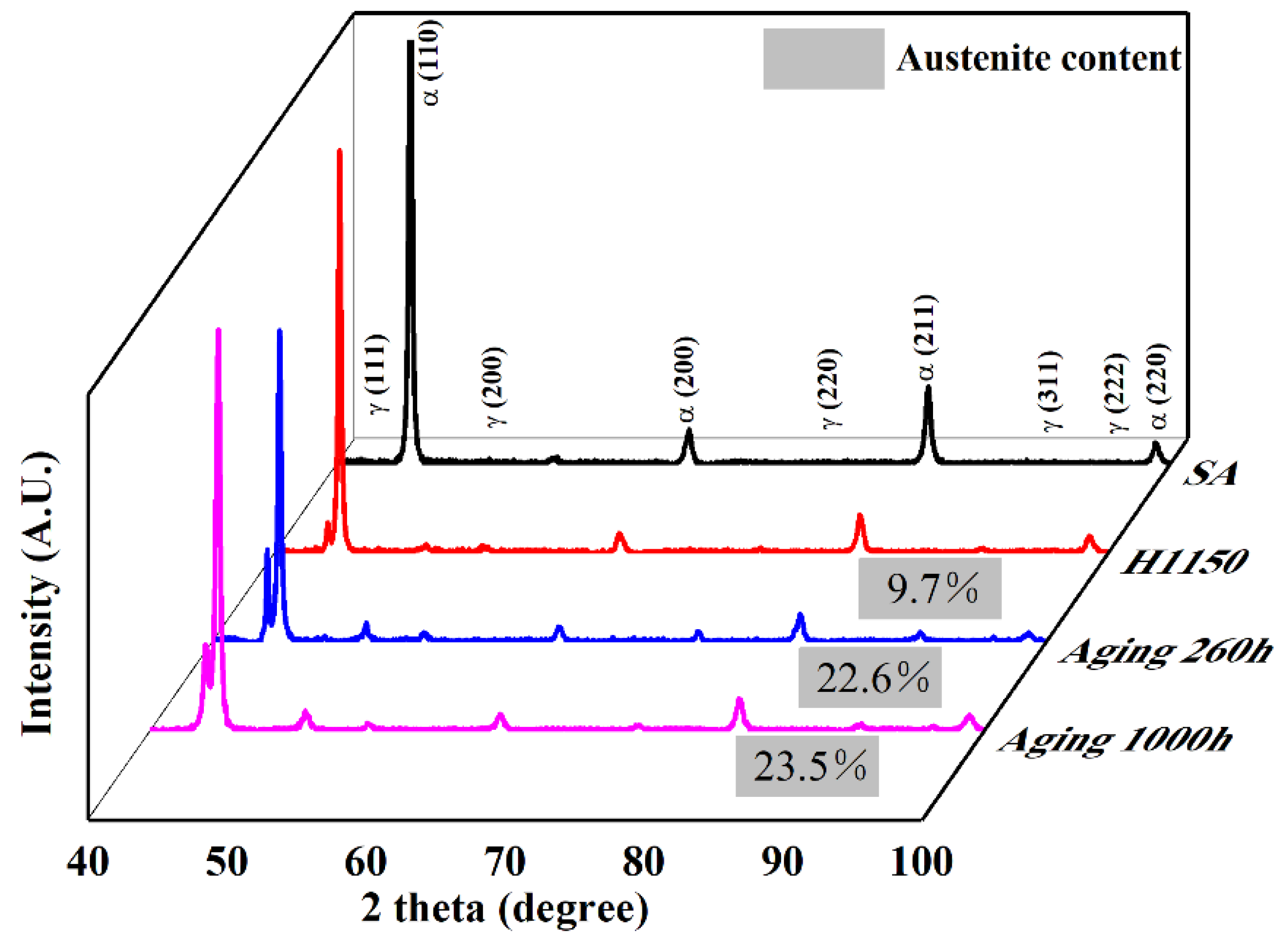
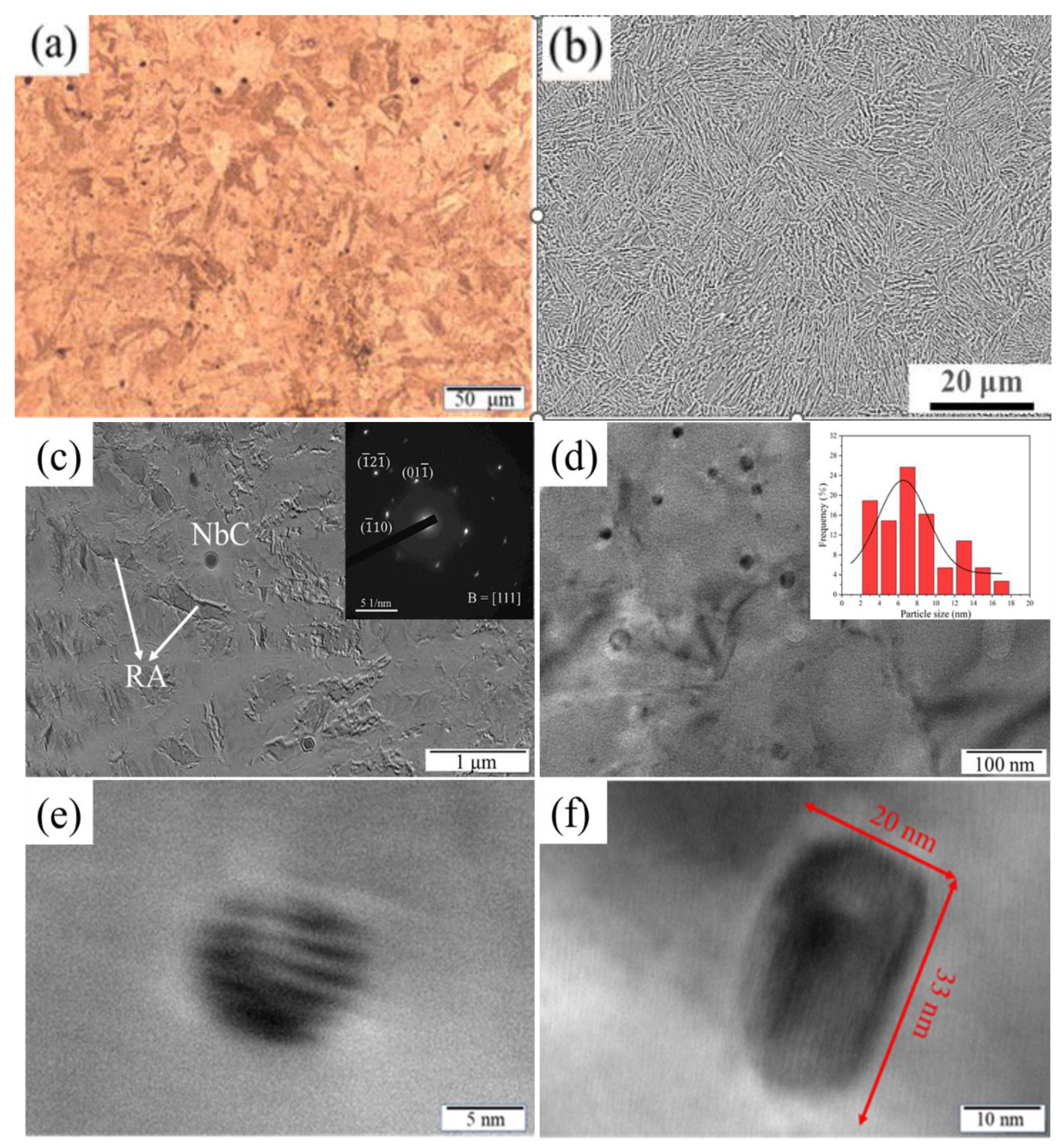
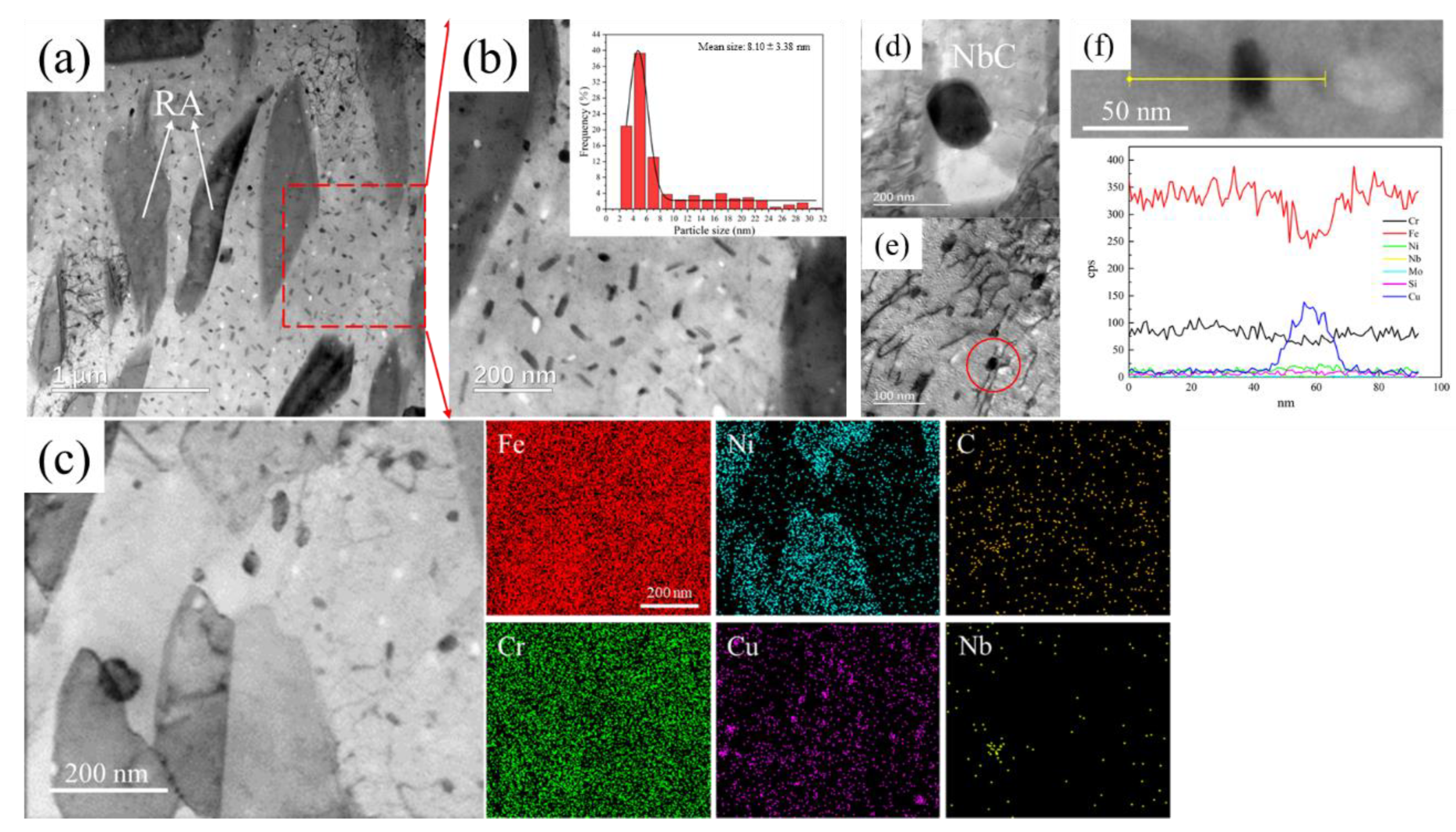
3.2. Microstructure of 15-6 PH after H1150 Heat Treatment
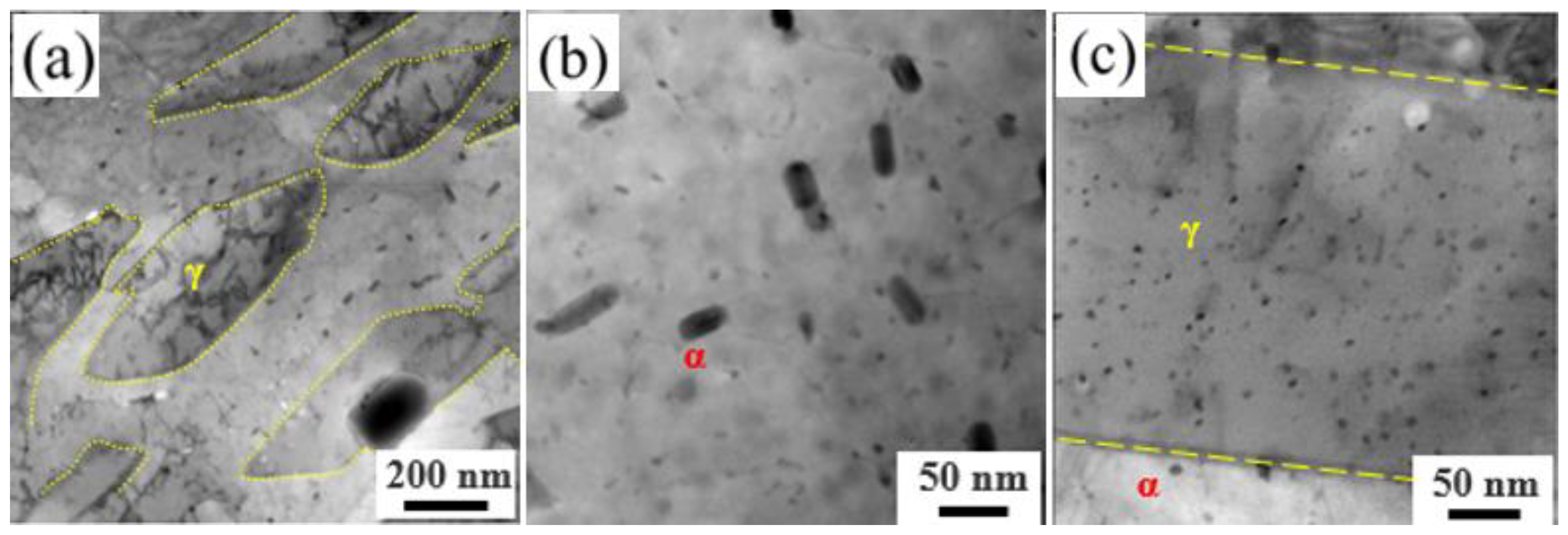
3.3. Microstructure Evolution during Thermal Aging at 480 °C
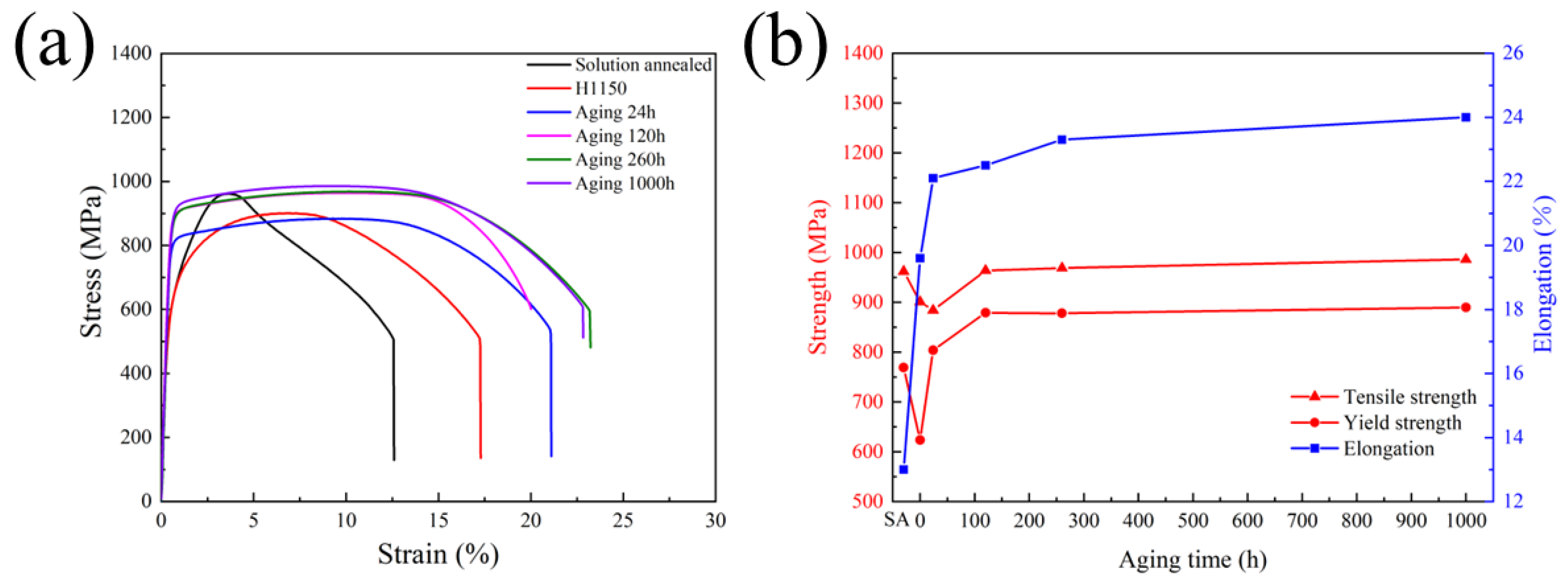
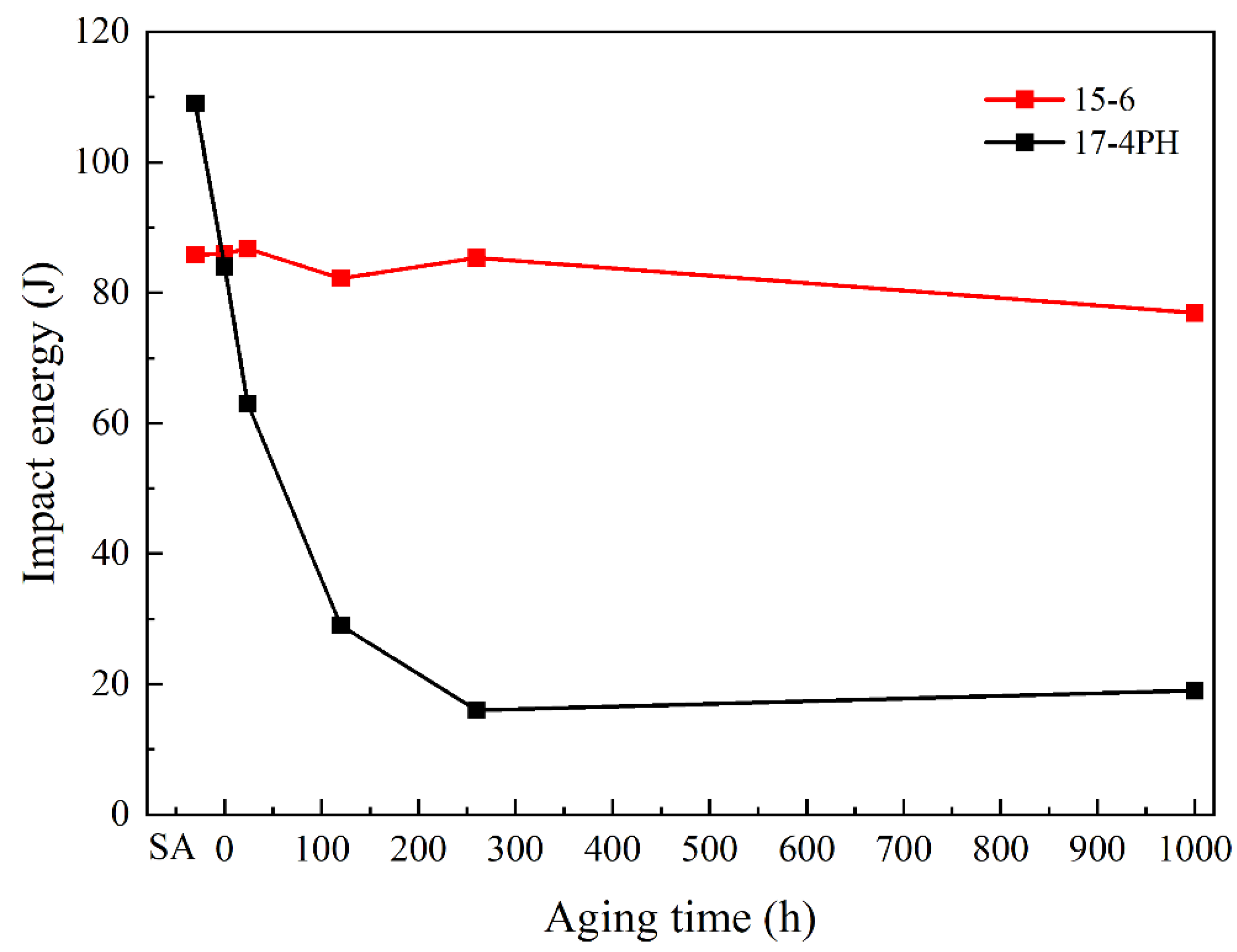
3.4. Changes of Mechanical Properties during Thermal Aging
4. Conclusions
- (1)
- Reversed austenite formed after H1150 heat treatment in the 15-6 PH steel and its content increased following thermal aging at 480 °C.
- (2)
- The Cu-rich precipitate coarsened and the crystal structure changed from the 9R to the FCC structure during thermal aging at 480 °C, while very fine Cu-rich particles precipitated in the reversed austenite during thermal aging at 480 °C.
- (3)
- The tensile strength was quite stable after thermal aging at 480 °C for different lengths of time, while the elongation obviously increased compared with a solution-treated material.
- (4)
- The 0 °C impact energy of 15-6 PH remained about 80 J after thermal aging at 480 °C up to 1000 h, which is nearly four times that of 17-4 PH. This demonstrated that the 15-6 PH material shows promising aging embrittlement resistance, while the procession and material cost are comparable with 17-4 PH. A longer aging time is necessary to evaluate the long-term performance of the steel in the near future.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tavares, S.S.M.; Machado, C.L.C.; Oliveira, I.G.; Martins, T.R.B.; Masoumi, M. Damage associated with the interaction between hydrogen and microstructure in a high sulfur 17-4PH steel for studs. Eng. Fail. Anal. 2017, 82, 642–647. [Google Scholar] [CrossRef]
- Ping, L.; Cai, Q.Z.; Wei, B.K.; Zhang, X.Z. Effect of Aging Temperature on Erosion-Corrosion Behavior of 17-4PH Stainless Steels in Dilute Sulphuric Acid Slurry. J. Iron Steel Res. Int. 2006, 13, 73–78. [Google Scholar]
- Raj, S.V.; Ghosn, L.J.; Lerch, B.A.; Hebsur, M.; Cosgriff, L.M.; Fedor, J. Mechanical properties of 17-4PH stainless steel foam panels. Mater. Sci. Eng. A 2007, 456, 305–316. [Google Scholar] [CrossRef]
- Hara, T.; Semba, H.; Amaya, H. Pipe and Tube Steels for Oil and Gas Industry and Thermal Power Plant. Encycl. Mater. Met. Alloys 2022, 2, 140–152. [Google Scholar]
- Macha, J.H.; Kirby, M.L.; Hickey, W.F.; Riha, D.S.; Alston, J.K.; Holden, B.B. Investigation of Thermal Embrittlement of 17-4PH Stainless Steel Main Steam Isolation Valves. J. Fail. Anal. Prev. 2021, 21, 1445–1465. [Google Scholar] [CrossRef]
- Jin, C.; Zhou, H.; Lai, Y.; Li, B.; Zhang, K.; Chen, H.; Zhao, J. Microstructure and mechanical properties of 15-5 PH stainless steel under different aging temperature. Met. Res. Technol. 2021, 118, 601. [Google Scholar] [CrossRef]
- Brandl, D.; Lukas, M.; Stockinger, M.; Ploberger, S.; Ressel, G. Evidence of austenite memory in PH 15-5 and assessment of its formation mechanism. Mater. Des. 2019, 176, 107841. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, J.; Xie, D.; Lv, F.; Zhang, Z.; Wang, H. Improved mechanical properties of laser-repaired 15-5PH stainless steel by in-situ heat treatment and grain refinement. Opt. Laser Technol. 2022, 150, 107999. [Google Scholar] [CrossRef]
- Yeli, G.; Auger, M.A.; Wilford, K.; Smith, G.D.; Bagot, P.A.; Moody, M.P. Sequential nucleation of phases in a 17-4PH steel: Microstructural characterisation and mechanical properties. Acta Mater. 2017, 125, 38–49. [Google Scholar] [CrossRef]
- Viswanathan, U.K.; Banerjee, S.; Krishnan, R. Effects of aging on the microstructure of 17-4PH stainless steel. Mater. Sci. Eng. A 1988, 104, 181–189. [Google Scholar] [CrossRef]
- Hsiao, C.N.; Chiou, C.S.; Yang, J.R. Aging reactions in a 17-4PH stainless steel. Mater. Chem. Phys. 2002, 74, 134–142. [Google Scholar] [CrossRef]
- Habibi Bajguirani, H.R. The effect of ageing upon the microstructure and mechanical properties of type 15-5PH stainless steel. Mater. Sci. Eng. A 2002, 338, 142–159. [Google Scholar] [CrossRef]
- Couturier, L.; De Geuser, F.; Descoins, M.; Deschamps, A. Evolution of the microstructure of a 15-5PH martensitic stainless steel during precipitation hardening heat treatment. Mater. Des. 2016, 107, 416–425. [Google Scholar] [CrossRef]
- Wang, J.; Zou, H.; Li, C.; Qiu, S.Y.; Shen, B.L. The effect of microstructural evolution on hardening behavior of type 17-4PH stainless steel in long-term aging at 350 °C. Mater. Charact. 2006, 57, 274–280. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Shen, Q.; Liu, W.; Wang, Z. Nano-precipitates Evolution and Their Effects on Mechanical Properties of 17-4 Precipitation-hardening Stainless Steel. Acta Mater. 2018, 156, 158–171. [Google Scholar] [CrossRef]
- Murayama, M.; Hono, K.; Katayama, Y. Microstructural evolution in a 17-4 PH stainless steel after aging at 400 °C. Metall. Mater. Trans. A 1999, 30, 345–353. [Google Scholar] [CrossRef]
- Bai, B.; Hu, R.; Zhang, C.; Xue, J.; Yang, W. Effect of precipitates on hardening of 17-4PH martensitic stainless steel serviced at 300 °C in nuclear power plant. Ann. Nucl. Energy 2021, 154, 108123. [Google Scholar] [CrossRef]
- Zhou, T.; Babu, R.P.; Odqvist, J.; Yu, H.; Hedström, P. Quantitative electron microscopy and physically based modelling of Cu precipitation in precipitation-hardening martensitic stainless steel 15-5 PH. Mater. Des. 2018, 143, 141–149. [Google Scholar] [CrossRef]
- Lee, T.H.; Kim, Y.O.; Kim, S.J. Crystallographic model for bcc-to-9R martensitic transformation of Cu precipitates in ferritic steel. Philos. Mag. A 2007, 87, 209–224. [Google Scholar] [CrossRef]
- Sun, H.; Li, D.; Diao, Y.; He, Y.; Yan, L.; Pang, X.; Gao, K. Nanoscale Cu particle evolution and its impact on the mechanical properties and strengthening mechanism in precipitation-hardening stainless steel. Mater. Charact. 2022, 188, 111885. [Google Scholar] [CrossRef]
- Othen, P.J.; Jenkins, M.L.; Smith, G.D.W.; Phythian, W.J. Transmission electron microscope investigations of the structure of copper precipitates in thermally-aged Fe-Cu and Fe-Cu-Ni. Philos. Mag. Lett. 1991, 64, 383–391. [Google Scholar] [CrossRef]
- Othen, P.J.; Jenkins, M.L.; Smith, G.D.W. High-resolution electron microscopy studies of the structure of Cu precipitates in α-Fe. Philos. Mag. A 1994, 70, 1–24. [Google Scholar] [CrossRef]
- Blackburn, M.J.; Nutting, J. Metallography of an iron-21% chromium alloy subjected to 475 °C embrittlement. J. Iron Steel Inst. 1964, 202, 610–613. [Google Scholar]
- Shiao, J.J.; Tsai, C.H.; Kai, J.J.; Huang, J.H. Aging embrittlement and lattice image analysis in a Fe–Cr–Ni duplex stainless steel aged at 400 °C. J. Nucl. Mater. 1994, 217, 269–278. [Google Scholar] [CrossRef]
- Vitek, J.M.; David, S.A.; Alexander, D.J.; Keiser, J.R.; Nanstad, R.K. Low temperature aging behavior of type 308 stainless steel weld metal. Acta Metall. Et. Mater. 1991, 39, 503–516. [Google Scholar] [CrossRef]
- Hättestrand, M.; Larsson, P.; Chai, G.; Nilsson, J.O.; Odqvist, J. Study of decomposition of ferrite in a duplex stainless steel cold worked and aged at 450–500 °C. Mater. Sci. Eng. A 2009, 499, 489–492. [Google Scholar] [CrossRef]
- Wang, J.; Zou, H.; Li, C.; Peng, Y.; Qiu, S.; Shen, B. The spinodal decomposition in 17-4PH stainless steel subjected to long-term aging at 350 °C. Mater. Charact. 2008, 59, 587–591. [Google Scholar] [CrossRef]
- Malouines, P.; Grandemange, J.M. RCC-M: Content, working approach and future evolutions. Press. Vessel. Pip. Conf. 2011, 44519, 769–783. [Google Scholar]
- Pareige, C.; Emo, J.; Saillet, S.; Domain, C.; Pareige, P. Kinetics of G-phase precipitation and spinodal decomposition in very long aged ferrite of a Mo-free duplex stainless steel. J. Nucl. Mater. 2015, 465, 383–389. [Google Scholar] [CrossRef]
- Lach, T.G.; Collins, D.A.; Sang Byun, T. Evolution of the role of molybdenum in duplex stainless steels during thermal aging: From enhancing spinodal decomposition to forming heterogeneous precipitates. J. Nucl. Mater. 2021, 557, 153268. [Google Scholar] [CrossRef]
- ASTM A693-22; Standard Specification for Precipitation-Hardening Stainless and Heat-Resisting Steel Plate, Sheet, and Strip. American Society of Testing Materials: Philadelphia, PA, USA, 2022.
- Tanaka, M.; Choi, C.S. The effects of carbon contents and Ms temperature on the hardness of martensitic Fe-Ni-C alloys. Trans. Iron Steel Inst. Jpn. 1972, 12, 16–25. [Google Scholar] [CrossRef]
- Yamabe, J.; Sezgin, J.G.; Wada, K. Interpretation of complex, tensile-fracture phenomena in precipitation-hardened, martensitic stainless steels, 17–4PH, in presence of hydrogen. Mater. Sci. Eng. A 2021, 823, 141717. [Google Scholar] [CrossRef]
- Lashgari, H.R.; Kong, C.; Adabifiroozjaei, E.; Li, S. Microstructure, post thermal treatment response, and tribological properties of 3D printed 17-4 PH stainless steel. Wear 2020, 456–457, 203367. [Google Scholar] [CrossRef]
- Song, Y.Y.; Li, X.Y.; Rong, L.J.; Li, Y.Y.; Nagai, T. Reversed austenite in 0Cr13Ni4Mo martensitic stainless steels. Mater. Chem. Phys. 2014, 143, 728–734. [Google Scholar] [CrossRef]
- Bhambroo, R.; Roychowdhury, S.; Kain, V.; Raja, V.S. Effect of reverted austenite on mechanical properties of precipitation hardenable 17-4 stainlesssteel. Mater. Sci. Eng. A 2013, 568, 127–133. [Google Scholar] [CrossRef]
- LeBrun, T.; Nakamoto, T.; Horikawa, K.; Kobayashi, H. Effect of retained austenite on subsequent thermal processing and resultant mechanical properties of selective laser melted 17-4 PH stainless steel. Mater. Des. 2015, 81, 44–53. [Google Scholar] [CrossRef]
- Russell, K.C.; Brown, L.M. A dispersion strengthening model based on differing elastic moduli applied to the iron-copper system. Acta Metall. 1972, 20, 969–974. [Google Scholar] [CrossRef]
- Fujii, K.; Fukuya, K.; Kasada, R.; Kimura, A.; Ohkubo, T. Effects of tensile stress on Cu clustering in irradiated Fe–Cu alloy. J. Nucl. Mater. 2015, 458, 281–287. [Google Scholar] [CrossRef]
- Murr, L.E.; Martinez, E.; Hernandez, J.; Collins, S.; Amato, K.N.; Gaytan, S.M.; Shindo, P.W. Microstructures and Properties of 17-4 PH Stainless Steel Fabricated by Selective Laser Melting. J. Mater. Res. Technol. 2012, 1, 167–177. [Google Scholar] [CrossRef]
- Chung, C.Y.; Tzeng, Y.C. Effects of aging treatment on the precipitation behavior of ε-Cu phase and mechanical properties of metal injection molding 17-4PH stainless steel. Mater. Lett. 2019, 237, 228–231. [Google Scholar] [CrossRef]
| Steel | Cr | Ni | Cu | W | Mn | Si | C | Nb | Fe |
|---|---|---|---|---|---|---|---|---|---|
| 15-6 PH | 15.04 | 5.99 | 1.54 | 0.66 | 0.06 | 0.30 | 0.03 | 0.38 | Bal. |
| 17-4 PH | 17.20 | 3.87 | 3.52 | - | 0.85 | 0.43 | 0.05 | 0.21 | Bal. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, R.; Yin, C.; Bai, B.; Yang, W.; Zhou, Z. The Microstructure and Mechanical Properties of a 15-6 PH Stainless Steel with Improved Thermal Aging Embrittlement Resistance. Materials 2024, 17, 1179. https://doi.org/10.3390/ma17051179
Lv R, Yin C, Bai B, Yang W, Zhou Z. The Microstructure and Mechanical Properties of a 15-6 PH Stainless Steel with Improved Thermal Aging Embrittlement Resistance. Materials. 2024; 17(5):1179. https://doi.org/10.3390/ma17051179
Chicago/Turabian StyleLv, Runtao, Chenxin Yin, Bing Bai, Wen Yang, and Zhangjian Zhou. 2024. "The Microstructure and Mechanical Properties of a 15-6 PH Stainless Steel with Improved Thermal Aging Embrittlement Resistance" Materials 17, no. 5: 1179. https://doi.org/10.3390/ma17051179
APA StyleLv, R., Yin, C., Bai, B., Yang, W., & Zhou, Z. (2024). The Microstructure and Mechanical Properties of a 15-6 PH Stainless Steel with Improved Thermal Aging Embrittlement Resistance. Materials, 17(5), 1179. https://doi.org/10.3390/ma17051179






