Crystal Structure, Luminescence and Electrical Conductivity of Pure and Mg2+-Doped β-Ga2O3-In2O3 Solid Solutions Synthesized in Oxygen or Argon Atmospheres
Abstract
:1. Introduction
2. Experimental Details
3. Results
3.1. Phase Composition and Crystal Structure Parameters
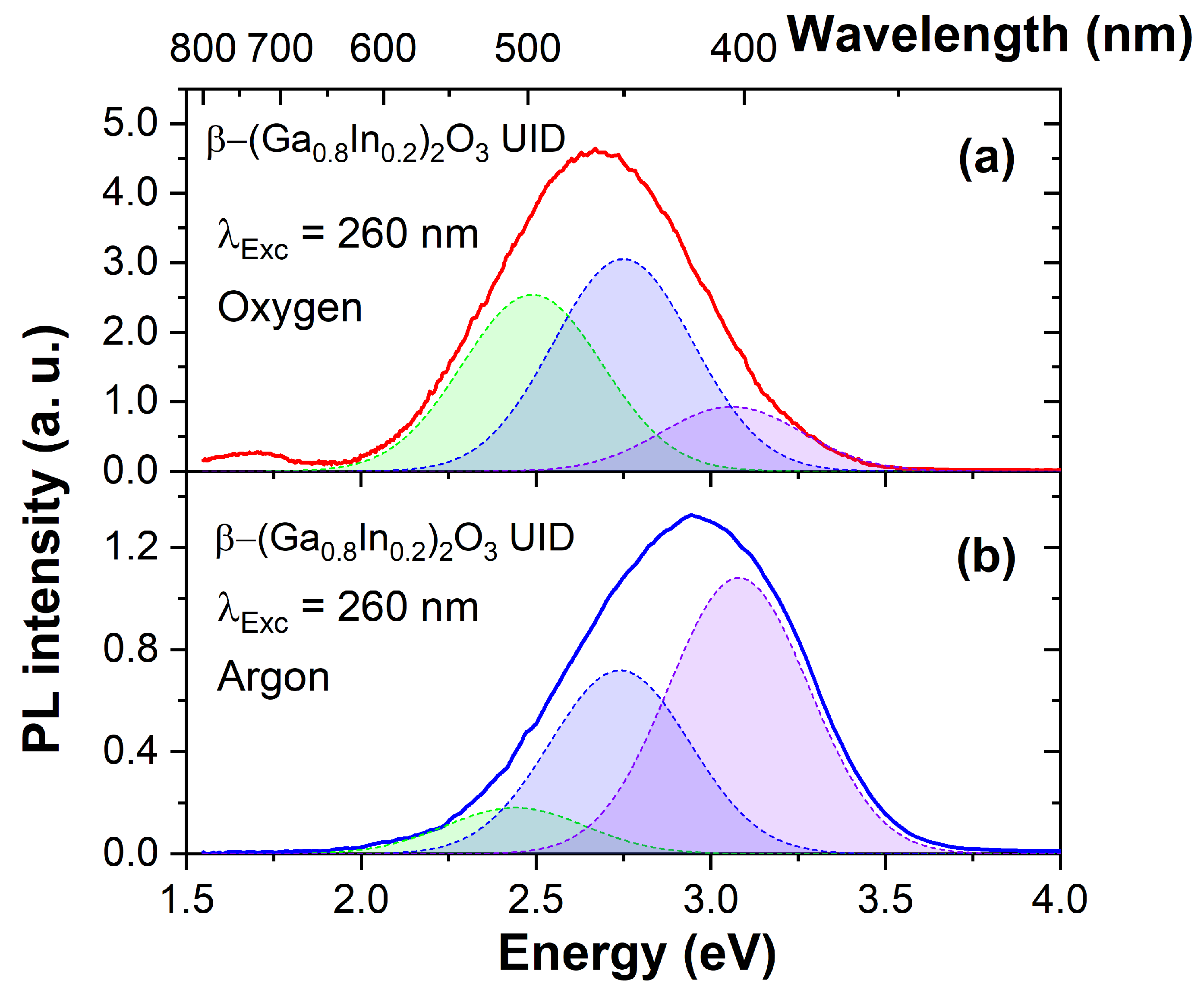
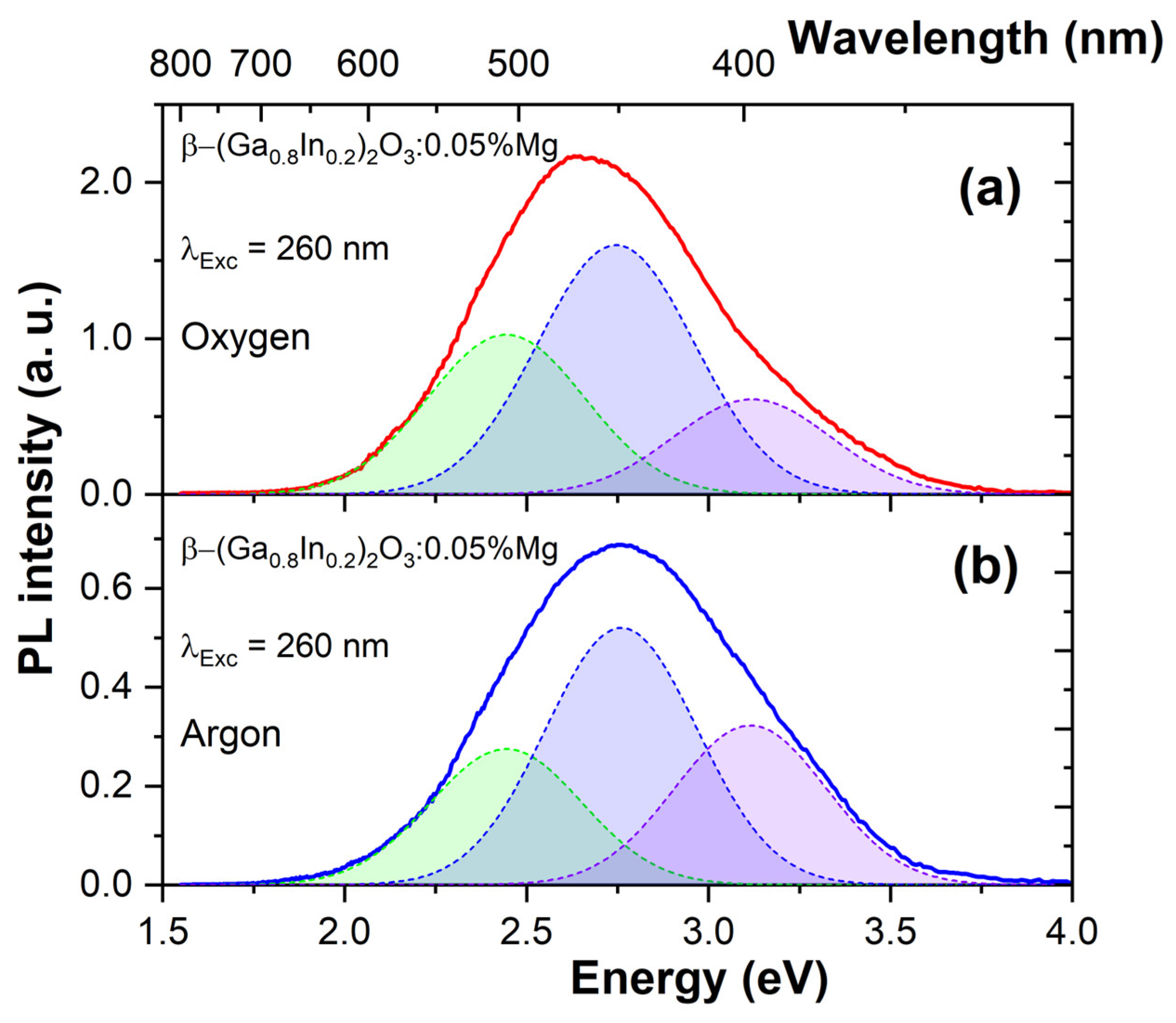
3.2. Photoluminescent Properties
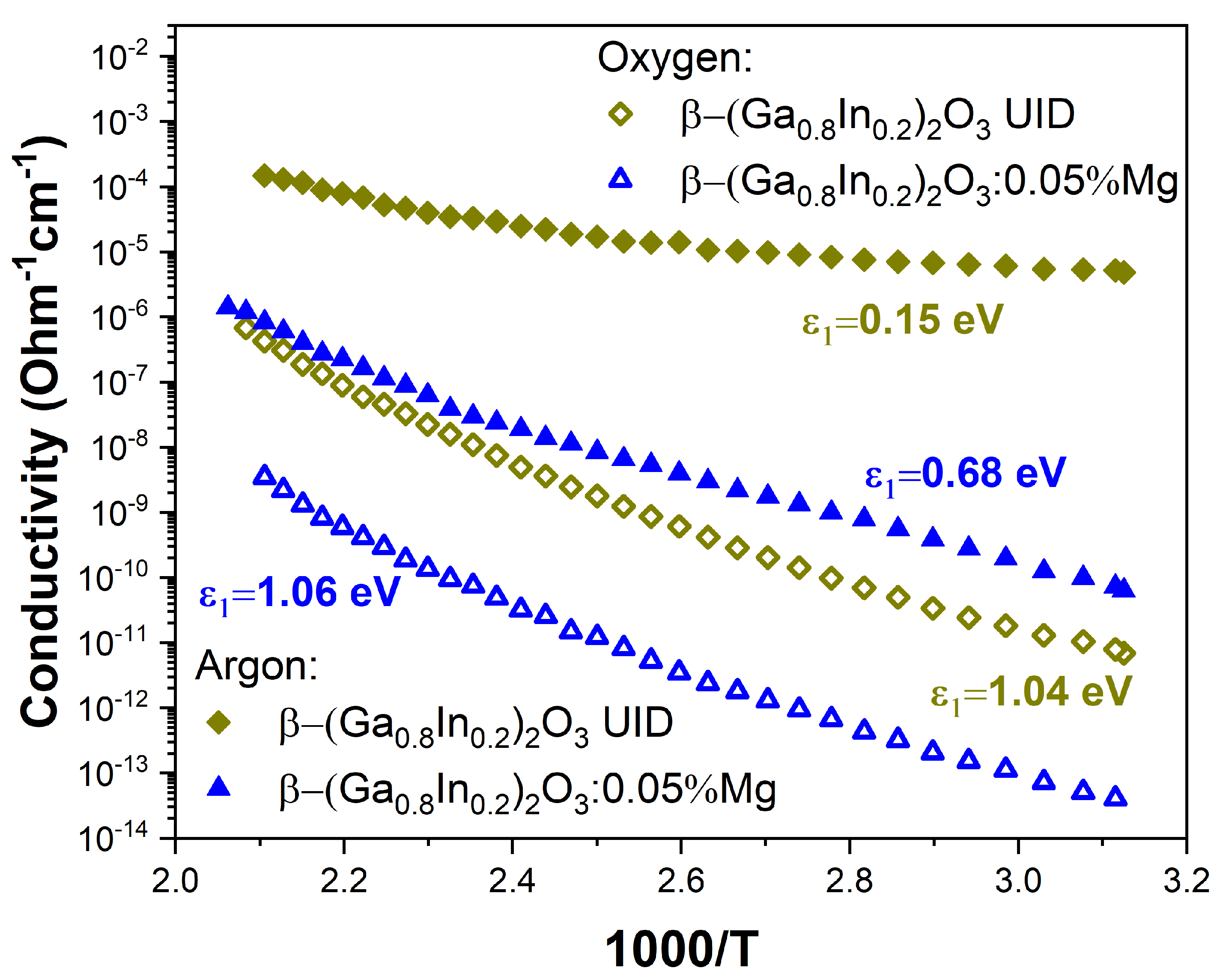
3.3. Electrical Conductivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Pearton, S.J.; Yang, J.; Cary, P.H.; Ren, F.; Kim, J.; Tadjer, M.J.; Mastro, M.A. A review of Ga2O3 materials, processing, and devices. Appl. Phys. Rev. 2018, 5, 011301. [Google Scholar] [CrossRef]
- Mastro, M.A.; Kuramata, A.; Calkins, J.; Kim, J.; Ren, F.; Pearton, S.J. Perspective—Opportunities and future directions for Ga2O3. ECS J. Solid State Sci. Technol. 2017, 6, 356–359. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, Z.; Ha, S.; Li, D.; Zhang, K.; Zhang, H.; Feng, J. Gallium oxide for gas sensor applications: A comprehensive review. Materials 2022, 15, 7339. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Guo, Q.; Chen, Z.; Wu, Z.; Li, P.; Tang, W.J. Review of Ga2O3-based optoelectronic devices. Mater. Today Phys. 2019, 11, 100157. [Google Scholar] [CrossRef]
- Chi, Z.; Asher, J.J.; Jennings, M.R.; Chikoidze, E.; Pérez-Tomás, A. Ga2O3 and related ultra-wide bandgap power semiconductor oxides: New energy electronics solutions for CO2 emission mitigation. Materials 2022, 15, 1164. [Google Scholar] [CrossRef]
- Miyata, T.; Nakatani, T.; Minami, T. Gallium oxide as host material for multicolor emitting phosphors. J. Lumin. 2000, 87, 1183–1185. [Google Scholar] [CrossRef]
- Roy, R.; Hill, V.G.; Osborn, E.F. Polymorphism of Ga2O3 and the System Ga2O3−H2O. J. Am. Chem. Soc. 1952, 74, 719–722. [Google Scholar] [CrossRef]
- Geller, S. Crystal Structure of β-Ga2O3. J. Chem. Phys. 1960, 33, 676–684. [Google Scholar] [CrossRef]
- Villora, E.G.; Shimamura, K.; Yoshikawa, Y.; Aoki, K.; Ichinose, N. Large-size β-Ga2O3 single crystals and wafers. J. Cryst. Growth 2004, 270, 420–426. [Google Scholar] [CrossRef]
- Yusa, H.; Tsuchiya, T.; Sata, N.; Ohishi, Y. Rh2O3 (II)-type structures in Ga2O3 and In2O3 under high pressure: Experiment and theory. Phys. Rev. B 2008, 77, 064107. [Google Scholar] [CrossRef]
- von Wenckstern, H. Properties of (In,Ga)2O3 Alloys. In Gallium Oxide: Technol. Devices Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 119–148. [Google Scholar] [CrossRef]
- Galazka, Z.; Ganschow, S.; Fiedler, A.; Bertram, R.; Klimm, D.; Irmscher, K.; Schewski, R.; Pietsch, M.; Albrecht, M.; Bickermann, M. Doping of Czochralski-grown bulk β-Ga2O3 single crystals with Cr, Ce and Al. J. Cryst. Growth 2018, 486, 82–90. [Google Scholar] [CrossRef]
- Swallow, J.E.; Palgrave, R.G.; Murgatroyd, P.A.; Regoutz, A.; Lorenz, M.; Hassa, A.; Grundmann, M.; von Wenckstern, H.; Varley, J.B.; Veal, T.D. Indium gallium oxide alloys: Electronic structure, optical gap, surface space charge, and chemical trends within common-cation semiconductors. ACS Appl. Mater. Interfaces 2021, 13, 2807–2819. [Google Scholar] [CrossRef] [PubMed]
- Zhydachevskyy, Y.; Mykhaylyk, V.; Stasiv, V.; Bulyk, L.I.; Hreb, V.; Lutsyuk, I.; Luchechko, A.; Hayama, S.; Vasylechko, L.; Suchocki, A. Chemical Tuning, Pressure, and Temperature Behavior of Mn4+ Photoluminescence in Ga2O3–Al2O3 Alloys. Inorg. Chem. 2022, 61, 18135–18146. [Google Scholar] [CrossRef] [PubMed]
- Vasil’tsiv, V.I.; Zakharko, Y.M.; Rym, Y.I. UV excitation bands and thermoluminescence of (Ga1−xInx)2O3 solid solutions. J. Appl. Spectrosc. 1993, 58, 289–292. [Google Scholar] [CrossRef]
- Galazka, Z.; Fiedler, A.; Popp, A.; Ganschow, S.; Kwasniewski, A.; Seyidov, P.; Pietsch, M.; Dittmar, A.; Anooz, S.B.; Irmscher, K.; et al. Bulk single crystals and physical properties of β-(AlxGa1−x)2O3 (x = 0–0.35) grown by the Czochralski method. J. Appl. Phys. 2023, 133, 035702. [Google Scholar] [CrossRef]
- Stasiv, V.; Zhydachevskyy, Y.; Stadnik, V.; Hreb, V.; Mykhaylyk, V.; Vasylechko, L.; Luchechko, A.; Wojciechowski, T.; Sybilski, P.; Suchocki, A. Chemical tuning of photo- and persistent luminescence of Cr3+-activated β-Ga2O3 by alloying with Al2O3 and In2O3. J. Alloys Compd. 2024, 982, 173827. [Google Scholar] [CrossRef]
- Kim, S.; Ryou, H.; Moon, J.; Lee, I.G.; Hwang, W.S. Codoping of Al and In atoms in β-Ga2O3 semiconductors. J. Alloys Compd. 2023, 931, 167502. [Google Scholar] [CrossRef]
- Li, H.; Wu, Z.; Wu, S.; Tian, P.; Fang, Z. (AlxGa1-x)2O3-based materials: Growth, properties, and device applications. J. Alloys Compd. 2023, 960, 170671. [Google Scholar] [CrossRef]
- Qi, K.; Fu, S.; Wang, Y.; Han, Y.; Fu, R.; Gao, C.; Ma, J.; Xu, H.; Li, B.; Shen, A.; et al. High-detectivity solar-blind deep UV photodetectors based on cubic/monoclinic mixed-phase (InxGa1−x)2O3 thin films. J. Alloys Compd. 2023, 965, 171473. [Google Scholar] [CrossRef]
- Welch, E.; Talukder, M.A.A.; Martins, N.R.; Borges, P.D.; Droopad, R.; Scolfaro, L. Indium defect complexes in (InxGa1−x)2O3: A combined experimental and hybrid density functional theory study. J. Phys. D Appl. Phys. 2024, 57, 145302. [Google Scholar] [CrossRef]
- Galazka, Z. β-Ga2O3 for wide-bandgap electronics and optoelectronics. Semicond. Sci. Technol. 2018, 33, 113001. [Google Scholar] [CrossRef]
- Akselrud, L.; Grin, Y. WinCSD: Software package for crystallographic calculations (Version 4). J. Appl. Cryst. 2014, 47, 803–805. [Google Scholar] [CrossRef]
- Åhman, J.; Svensson, G.; Albertsson, J. A Reinvestigation of β-Gallium Oxide. Acta Crystallogr. C 1996, 52, 1336–1338. [Google Scholar] [CrossRef]
- Shannon, R.D.; Prewitt, C.T. Synthesis and structure of phases in the In2O3 Ga2O3 system. J. Inorg. Nucl. Chem. 1968, 30, 1389–1398. [Google Scholar] [CrossRef]
- Pasquevich, A.F.; Uhrmacher, M.; Ziegeler, L.; Lieb, K.P. Hyperfine interactions of 111Cd in Ga2O3. Phys. Rev. B 1993, 48, 10052. [Google Scholar] [CrossRef]
- Vasyltsiv, V.I.; Rym, Y.I.; Zakharko, Y.M. Optical Absorption and Photoconductivity at the Band Edge of β-Ga2−xInxO3. Phys. Status Solidi (B) 1996, 195, 653–658. [Google Scholar] [CrossRef]
- Edwards, D.D.; Folkins, P.E.; Mason, T.O. Phase Equilibria in the Ga2O3In2O3 System. J. Am. Ceram. Soc. 1997, 80, 253. [Google Scholar] [CrossRef]
- Zhong, J.; Zhuo, Y.; Du, F.; Zhang, H.; Zhao, W.; Brgoch, J. Efficient and Tunable Luminescence in Ga2-xInxO3:Cr3+ for Near-Infrared Imaging. ACS Appl. Mater. Interfaces 2021, 13, 31835. [Google Scholar] [CrossRef]
- Kranert, C.; Lenzner, J.; Jenderka, M.; Lorenz, M.; von Wenckstern, H.; Schmidt-Grund, R.; Grundmann, M. Lattice Parameters and Raman-Active Phonon Modes of (InxGa1–x)2O3 for x < 0.4. J. Appl. Phys. 2014, 116, 013505. [Google Scholar] [CrossRef]
- Edwards, D.D.; Mason, T.O. Subsolidus Phase Relations in the Ga2O3-In2O3-SnO2 System. J. Am. Ceram. Soc. 1998, 81, 3285–3292. [Google Scholar] [CrossRef]
- Tomm, Y.; Ko, J.M.; Yoshikawa, A.; Fukuda, T. Floating Zone Growth of β-Ga2O3: A New Window Material for Optoelectronic Device Applications. Sol. Energy Mater. Sol. Cells 2001, 66, 369–374. [Google Scholar] [CrossRef]
- Luchechko, A.; Vasyltsiv, V.; Ploch, D.; Kostyk, L.; Kushlyk, M.; Slobodzyan, D.; Hreb, V.; Vasylechko, L.; Yang, G.; Shpotyuk, Y. Effect of sintering atmosphere on structural, luminescence and electrical properties of β-Ga2O3 ceramics. Appl. Nanosci. 2023, 13, 7327–7334. [Google Scholar] [CrossRef]
- Binet, L.; Gourier, D. Origin of the blue luminescence of β-Ga2O3. J. Phys. Chem. Solids 1998, 59, 1241–1249. [Google Scholar] [CrossRef]
- Onuma, T.; Nakata, Y.; Sasaki, K.; Masui, T.; Yamaguchi, T.; Honda, T.; Kuramata, A.; Yamakoshi, S.; Higashiwaki, M. Modeling and interpretation of UV and blue luminescence intensity in β-Ga2O3 by silicon and nitrogen doping. J. Appl. Phys. 2018, 124, 075103. [Google Scholar] [CrossRef]
- Luchechko, A.; Vasyltsiv, V.; Kushlyk, M.; Slobodzyan, D.; Baláž, M.; Cebulski, J.; Szmuc, K.; Szlęzak, J.; Shpotyuk, Y. Structural and luminescence characterization of β-Ga2O3 nanopowders obtained via high-energy ball milling. Appl. Nanosci. 2023, 13, 5149–5155. [Google Scholar] [CrossRef]
- Vasyltsiv, V.; Kostyk, L.; Tsvetkova, O.; Lys, R.; Kushlyk, M.; Pavlyk, B.; Luchechko, A. Luminescence and Conductivity of β-Ga2O3 and β-Ga2O3: Mg Single Crystals. Acta Phys. Pol. A 2022, 141, 312–318. [Google Scholar] [CrossRef]
- Vasyltsiv, V.; Luchechko, A.; Zhydachevskyy, Y.; Kostyk, L.; Lys, R.; Slobodzyan, D.; Jakieła, R.; Pavlyk, B.; Suchocki, A. Correlation between electrical conductivity and luminescence properties in β-Ga2O3: Cr3+ and β-Ga2O3: Cr, Mg single crystals. J. Vac. Sci. Technol. A 2021, 39, 033201. [Google Scholar] [CrossRef]
- Ho, Q.D.; Frauenheim, T.; Deák, P. Origin of photoluminescence in β-Ga2O3. Phys. Rev. B 2018, 97, 115163. [Google Scholar] [CrossRef]
- Luchechko, A.; Vasyltsiv, V.; Kostyk, L.; Tsvetkova, O.; Pavlyk, B. Thermally stimulated luminescence and conductivity of β-Ga2O3 crystals. J. Nano-Electron. Phys. 2019, 11, 03035. [Google Scholar] [CrossRef]
- Wu, S.; Liu, Z.; Yang, H.; Wang, Y. Effects of Annealing on Surface Residual Impurities and Intrinsic Defects of β-Ga2O3. Crystals 2023, 13, 1045. [Google Scholar] [CrossRef]
- Frodason, Y.K.; Johansen, K.M.; Vines, L.; Varley, J.B. Self-trapped hole and impurity-related broad luminescence in β-Ga2O3. J. Appl. Phys. 2020, 127, 075701. [Google Scholar] [CrossRef]
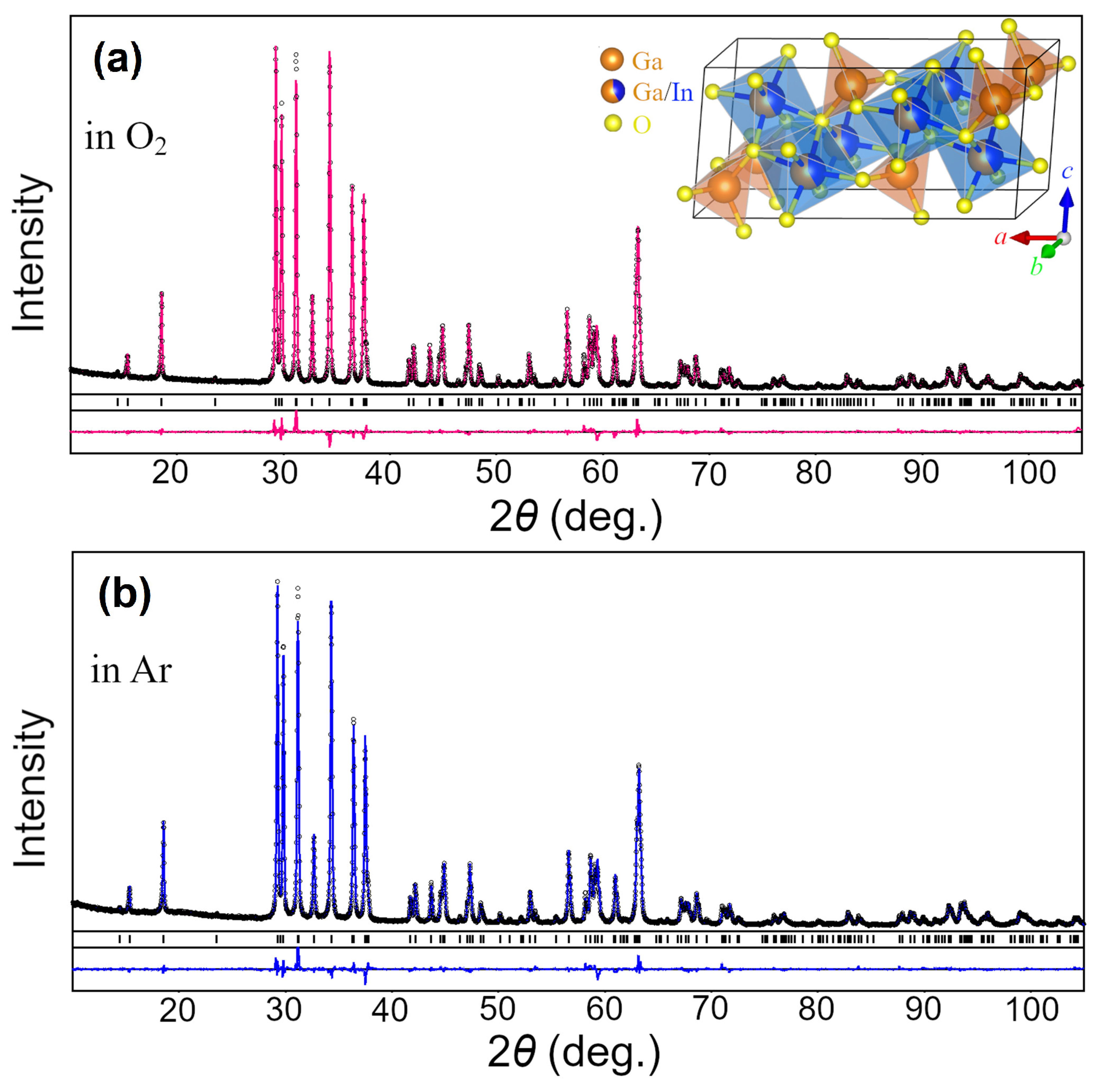
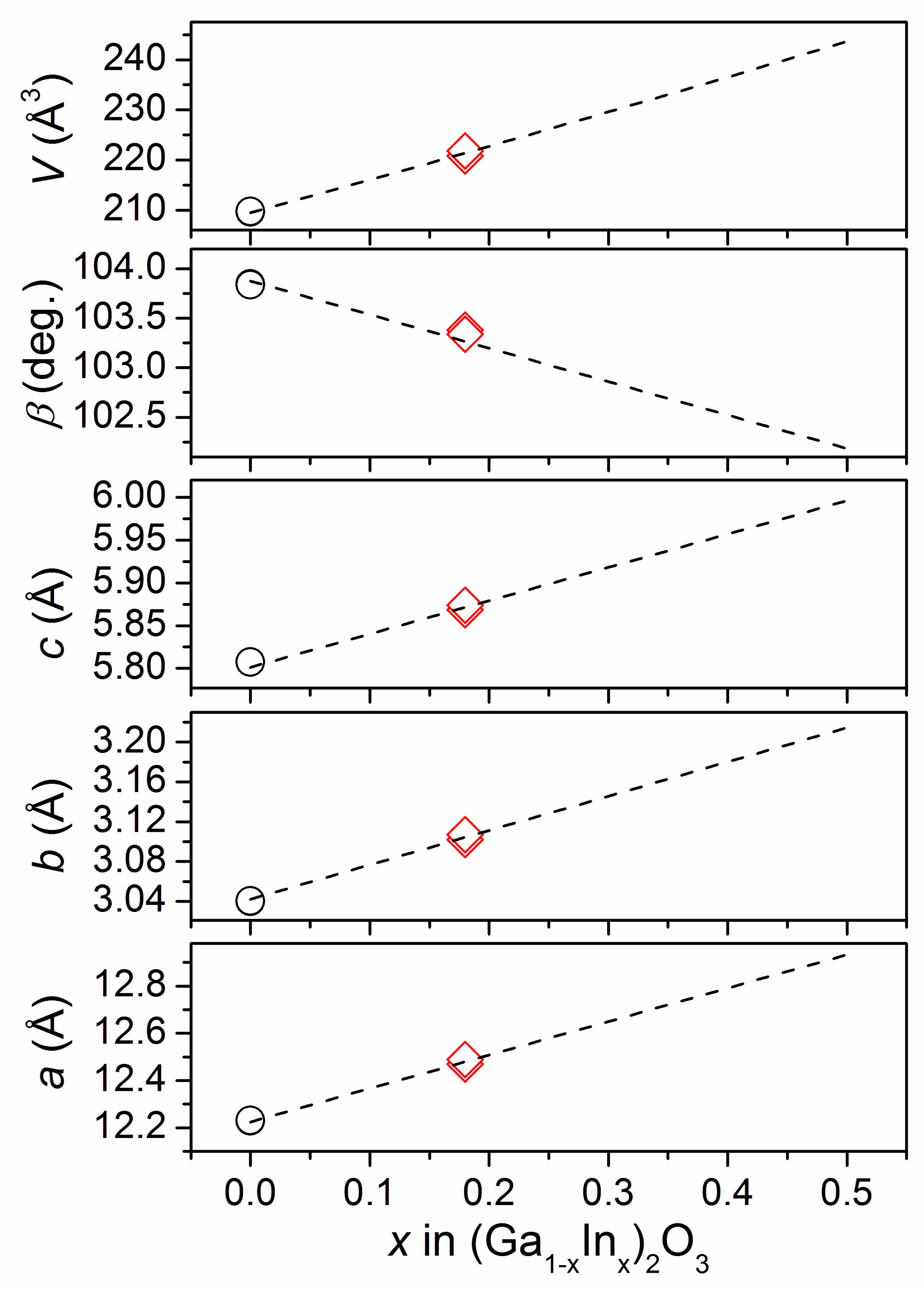


| Lattice Parameters | Atoms, Sites | x/a | y/b | z/c | Biso/eq, Å2 | Occupancy |
|---|---|---|---|---|---|---|
| β-(Ga1−xInx)2O3 in O2; RI = 0.0381, RP = 0.0713 | ||||||
| a = 12.4697(2) Å | Ga1, 4i | 0.09048(10) | 0 | 0.2929(3) | 0.82(3) | 1.01(1) Ga3+ |
| b = 3.10206(5) Å | Ga2, 4i | 0.34367(7) | 0 | 0.1885(2) | 0.93(3) | 0.63(2) Ga3+ + 0.38(2) In3+ |
| c = 5.86855(9) Å | O1, 4i | 0.1628(4) | 0 | 0.6187(11) | 1.1(2) | O2− |
| β = 103.379(1) o | O2, 4i | 0.1714(4) | 0 | 0.0653(14) | 1.6(2) | O2− |
| V = 220.85(1) Å3 | O3, 4i | 0.5123(4) | 0 | 0.2460(8) | 0.7(2) | O2− |
| Texture axis and parameter: [1 0 0] 0.563(5) | ||||||
| β-(Ga1−xInx)2O3 in Ar; RI = 0.0454, RP = 0.0733 | ||||||
| a = 12.4886(2) Å | Ga1, 4i | 0.09041(10) | 0 | 0.2926(3) | 0.76(3) | 1.00(1) Ga3+ |
| b = 3.10717(6) Å | Ga2, 4i | 0.34347(7) | 0 | 0.1884(2) | 0.78(3) | 0.65(2) Ga3+ + 0.35(2) In3+ |
| c = 5.87368(10) Å | O1, 4i | 0.1629(4) | 0 | 0.6191(12) | 1.9(2) | O2− |
| β = 103.337(1) o | O2, 4i | 0.1707(4) | 0 | 0.0658(13) | 1.0(2) | O2− |
| V = 221.78(1) Å3 | O3, 4i | 0.5137(4) | 0 | 0.2487(9) | 1.6(2) | O2− |
| Texture axis and parameter: [1 0 0] 0.584(5) | ||||||
| Atoms | Ga2O3 Ref. [24] | (Ga1−xInx)2O3 | Atoms | Ga2O3 Ref. [24] | (Ga1−xInx)2O3 | ||
|---|---|---|---|---|---|---|---|
| in O2 | in Ar | in O2 | in Ar | ||||
| Ga-O3 (×2) | 1.832 | 1.819(3) | 1.812(3) | M-O1 (×2) | 1.937 | 1.933(4) | 1.934(4) |
| Ga-O2 | 1.863 | 1.850(7) | 1.842(7) | M-O3 | 1.936 | 2.051(5) | 2.073(5) |
| Ga-O1 | 1.835 | 1.916(7) | 1.923(7) | M-O2 | 2.005 | 2.102(6) | 2.112(6) |
| M-O2 (×2) | 2.074 | 2.128(5) | 2.134(5) | ||||
| Ga-O4(ave), increment | 1.841 | 1.851 +0.54% | 1.847 +0.33% | MO6(ave), increment | 1.994 | 2.046 +2.61% | 2.054 +3.00% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luchechko, A.; Vasyltsiv, V.; Kushlyk, M.; Hreb, V.; Slobodzyan, D.; Vasylechko, L.; Zhydachevskyy, Y. Crystal Structure, Luminescence and Electrical Conductivity of Pure and Mg2+-Doped β-Ga2O3-In2O3 Solid Solutions Synthesized in Oxygen or Argon Atmospheres. Materials 2024, 17, 1391. https://doi.org/10.3390/ma17061391
Luchechko A, Vasyltsiv V, Kushlyk M, Hreb V, Slobodzyan D, Vasylechko L, Zhydachevskyy Y. Crystal Structure, Luminescence and Electrical Conductivity of Pure and Mg2+-Doped β-Ga2O3-In2O3 Solid Solutions Synthesized in Oxygen or Argon Atmospheres. Materials. 2024; 17(6):1391. https://doi.org/10.3390/ma17061391
Chicago/Turabian StyleLuchechko, Andriy, Vyacheslav Vasyltsiv, Markiyan Kushlyk, Vasyl Hreb, Dmytro Slobodzyan, Leonid Vasylechko, and Yaroslav Zhydachevskyy. 2024. "Crystal Structure, Luminescence and Electrical Conductivity of Pure and Mg2+-Doped β-Ga2O3-In2O3 Solid Solutions Synthesized in Oxygen or Argon Atmospheres" Materials 17, no. 6: 1391. https://doi.org/10.3390/ma17061391





