Fabrication of Ultra-Fine Ag NPs on TiO2 Thin Films by Alcohol-Assisted Photodeposition Process for Photocatalysis-Related Applications
Abstract
:1. Introduction
2. Materials and Methods
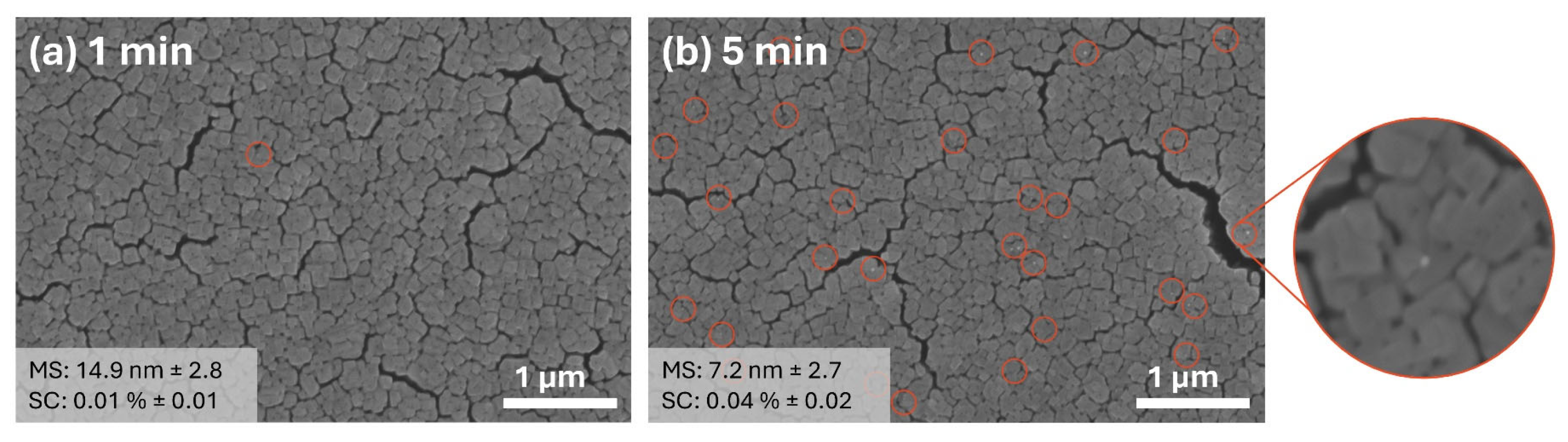
2.1. Fabrication of TiO2 Thin Films
2.2. Photodeposition of Ag NPs on TiO2 Thin Films
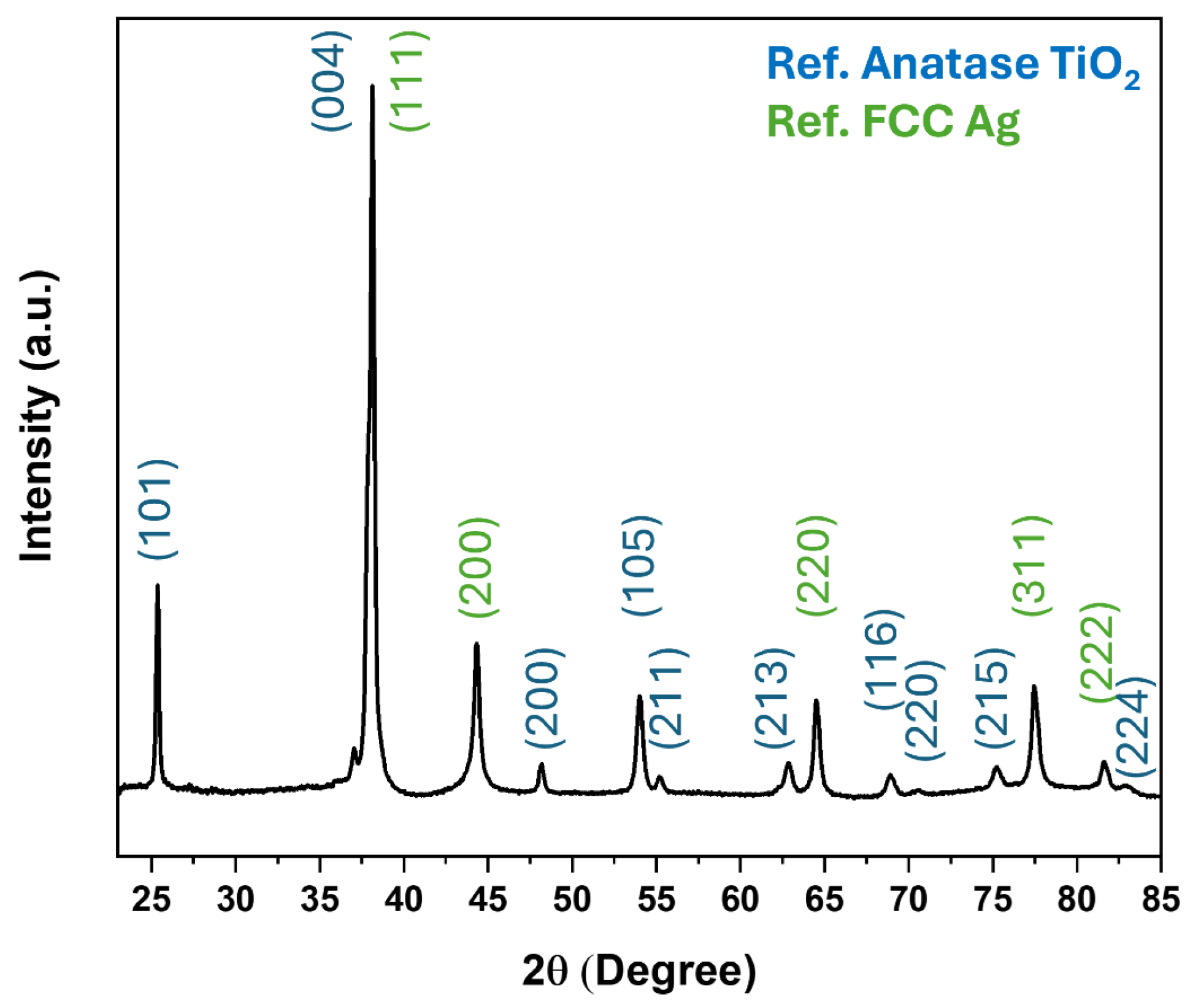
2.3. Materials Characterization
3. Results and Discussion
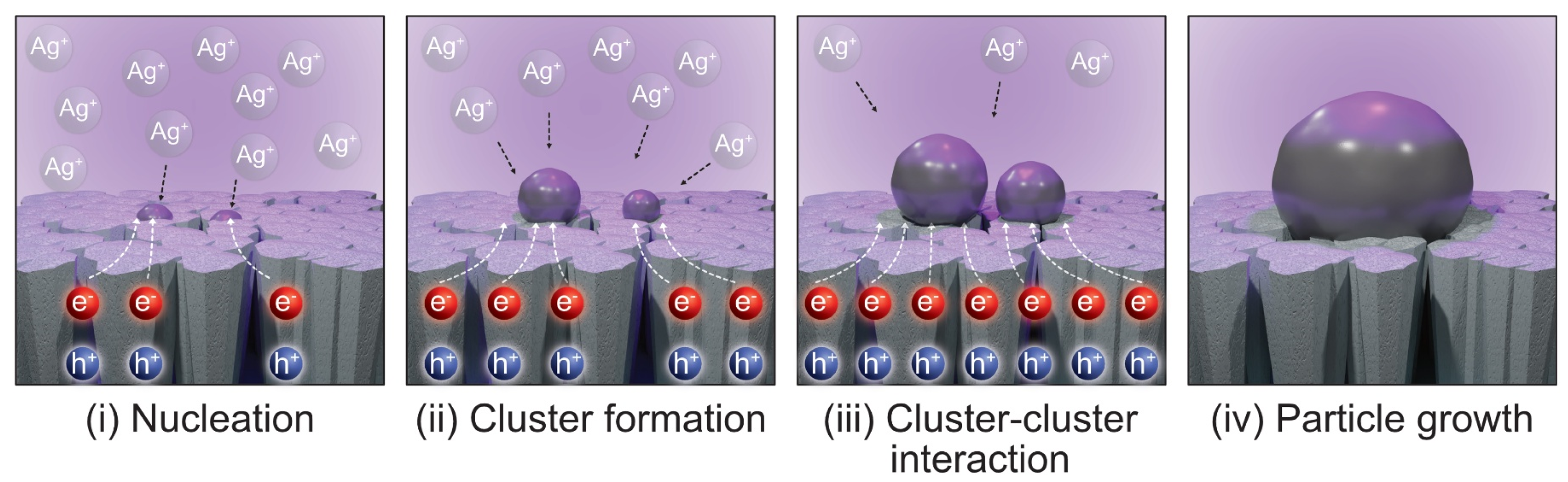
3.1. Ag NPs Formation in Water Media
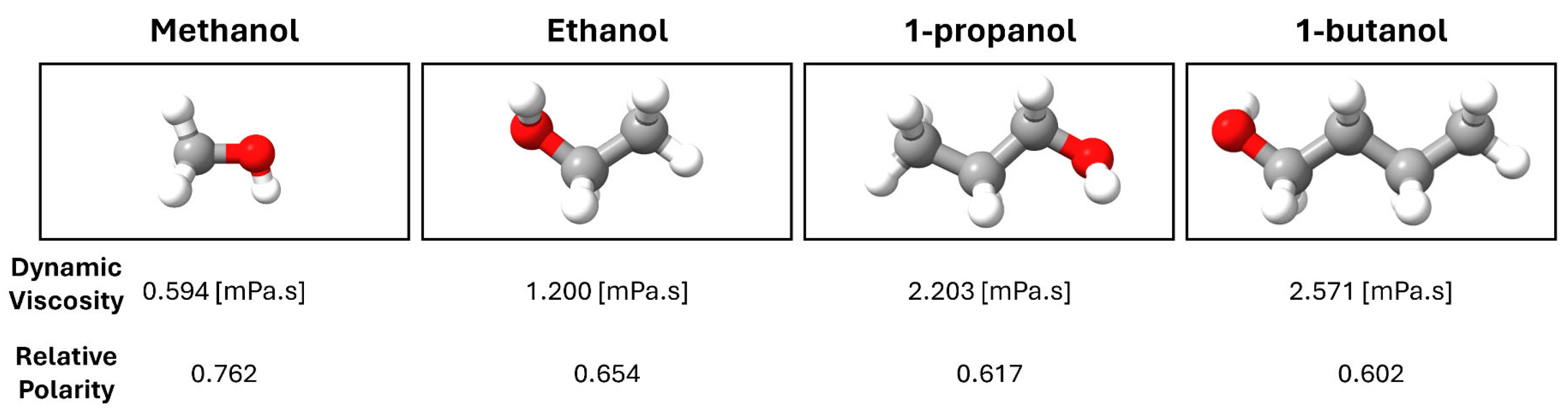
3.2. Ag NPs Formation in 25% Alcohol Media (Methanol, Ethanol, and 1-Propanol)
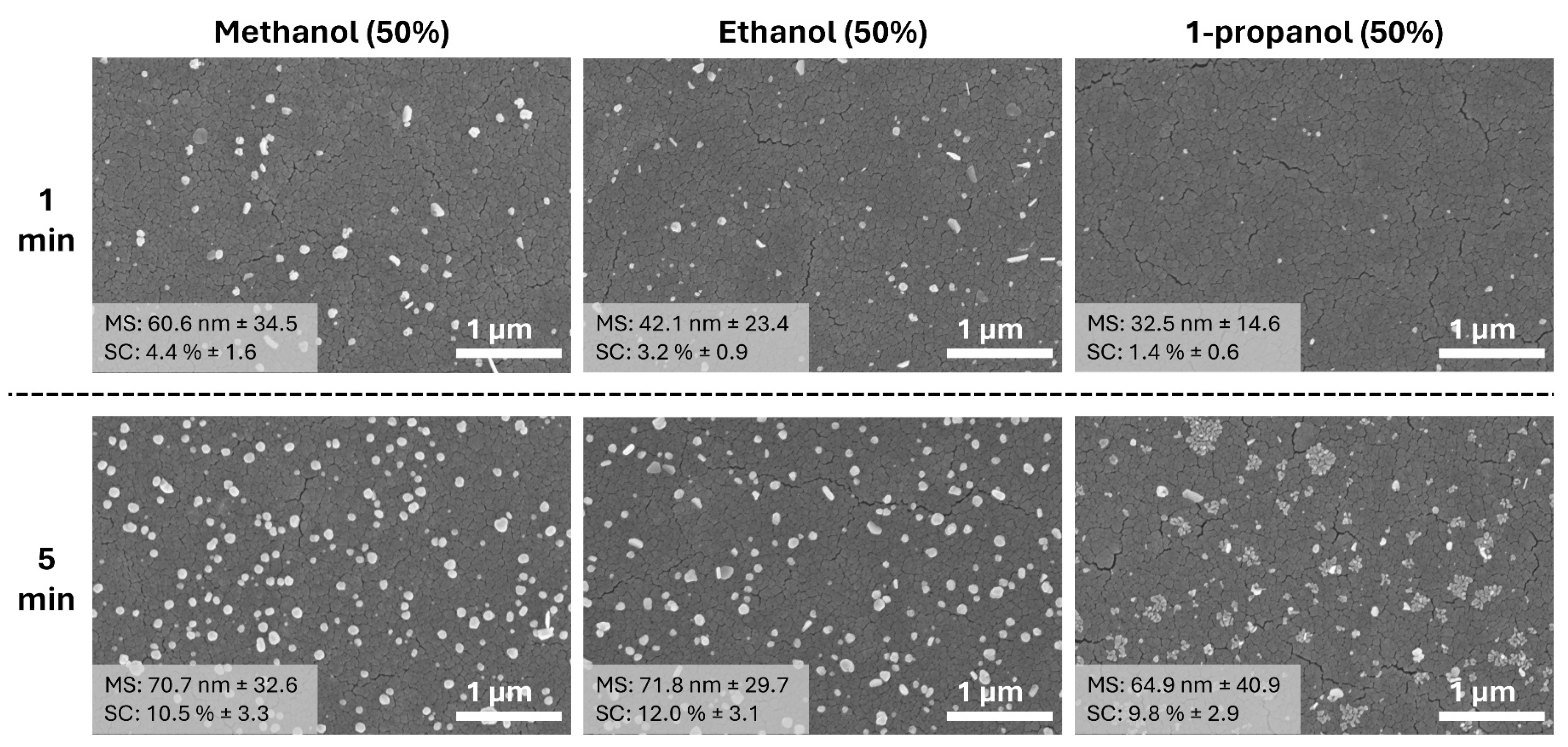
3.3. Ag NPs Formation in 50% Alcohol Media (Methanol, Ethanol, and 1-Propanol)
3.4. Ag NPs Formation in 100% Alcohol Media (Methanol, Ethanol, and 1-Propanol)
3.5. Interfacial Charge Transfer Processes
3.6. Ag NPs Formation in 100% 1-Butanol
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waiskopf, N.; Ben-Shahar, Y.; Banin, U. Photocatalytic Hybrid Semiconductor–Metal Nanoparticles; from Synergistic Properties to Emerging Applications. Adv. Mater. 2018, 30, 1706697. [Google Scholar] [CrossRef]
- Ahmed, S.N.; Haider, W. Heterogeneous Photocatalysis and Its Potential Applications in Water and Wastewater Treatment: A Review. Nanotechnology 2018, 29, 342001. [Google Scholar] [CrossRef]
- Fang, S.; Rahaman, M.; Bharti, J.; Reisner, E.; Robert, M.; Ozin, G.A.; Hu, Y.H. Photocatalytic CO2 Reduction. Nat. Rev. Methods Primers 2023, 3, 61. [Google Scholar] [CrossRef]
- Tao, X.; Zhao, Y.; Wang, S.; Li, C.; Li, R. Recent Advances and Perspectives for Solar-Driven Water Splitting Using Particulate Photocatalysts. Chem. Soc. Rev. 2022, 51, 3561–3608. [Google Scholar] [CrossRef]
- Veziroglu, S.; Röder, K.; Gronenberg, O.; Vahl, A.; Polonskyi, O.; Strunskus, T.; Rubahn, H.-G.; Kienle, L.; Adam, J.; Fiutowski, J.; et al. Cauliflower-like CeO2–TiO2 Hybrid Nanostructures with Extreme Photocatalytic and Self-Cleaning Properties. Nanoscale 2019, 11, 9840–9844. [Google Scholar] [CrossRef]
- Bhuskute, B.D.; Ali-Löytty, H.; Honkanen, M.; Salminen, T.; Valden, M. Influence of the Photodeposition Sequence on the Photocatalytic Activity of Plasmonic Ag–Au/TiO2 Nanocomposites. Nanoscale Adv. 2022, 4, 4335–4343. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, T.; Kim, B.; Choi, S.; Kim, K. Synthesis of TiO2/MoSx/Ag Nanocomposites via Photodeposition for Enhanced Photocatalysis and Membrane Fouling Mitigation. J. Environ. Chem. Eng. 2023, 11, 109266. [Google Scholar] [CrossRef]
- Wenderich, K.; Mul, G. Methods, Mechanism, and Applications of Photodeposition in Photocatalysis: A Review. Chem. Rev. 2016, 116, 14587–14619. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Porto, C.L.; Palumbo, F.; Modic, M.; Cvelbar, U.; Ghobeira, R.; De Geyter, N.; De Vrieze, M.; Kos, Š.; Serša, G.; et al. Synthesis of Antibacterial Composite Coating Containing Nanocapsules in an Atmospheric Pressure Plasma. Mater. Sci. Eng. C 2021, 119, 111496. [Google Scholar] [CrossRef] [PubMed]
- Bai, Q.; Shupyk, I.; Vauriot, L.; Majimel, J.; Labrugere, C.; Delville, M.-H.; Delville, J.-P. Design of Metal@Titanium Oxide Nano-Heterodimers by Laser-Driven Photodeposition: Growth Mechanism and Modeling. ACS Nano 2021, 15, 2947–2961. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Battiato, S.; Pellegrino, A.L.; Paoli, P.; Rossi, P.; Jiménez, C.; Malandrino, G.; Muñoz-Rojas, D. Deposition of Metallic Silver Coatings by Aerosol Assisted MOCVD Using Two New Silver β-Diketonate Adduct Metalorganic Precursors. Dalton Trans. 2017, 46, 10986–10995. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Sharma, D.; Kumari, P.; Pal, B. Influence of Photodeposition Time and Loading Amount of Ag Co-Catalyst on Growth, Distribution and Photocatalytic Properties of Ag@TiO2 Nanocatalysts. Opt. Mater. 2020, 106, 109975. [Google Scholar] [CrossRef]
- Zhao, H.; Mao, Q.; Jian, L.; Dong, Y.; Zhu, Y. Photodeposition of Earth-Abundant Cocatalysts in Photocatalytic Water Splitting: Methods, Functions, and Mechanisms. Chin. J. Catal. 2022, 43, 1774–1804. [Google Scholar] [CrossRef]
- Fresno, F.; Portela, R.; Suárez, S.; Coronado, J.M. Photocatalytic Materials: Recent Achievements and near Future Trends. J. Mater. Chem. A 2014, 2, 2863–2884. [Google Scholar] [CrossRef]
- O’Rourke, C.; Wells, N.; Mills, A. Photodeposition of Metals from Inks and Their Application in Photocatalysis. Catal. Today 2019, 335, 91–100. [Google Scholar] [CrossRef]
- Zhao, F.; Bai, Q.; Xia, C.; Hao, J.; Gayot, M.; Delville, J.-P.; Delville, M.-H. Core–Shell Nanoheterodimers: Laser-Assisted Deposition of Single Bimetallic Au@M (M = Au, Ag, Pd, Pt) Nanodots on TiO2 Nanoparticles. Mater. Adv. 2023, 4, 694–708. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, E.; Park, Y.; Kim, J.; Ryu, W.; Rho, J.; Kim, K. Photodeposited Metal-Semiconductor Nanocomposites and Their Applications. J. Mater. 2018, 4, 83–94. [Google Scholar] [CrossRef]
- Chan, S.C.; Barteau, M.A. Preparation of Highly Uniform Ag/TiO2 and Au/TiO2 Supported Nanoparticle Catalysts by Photodeposition. Langmuir 2005, 21, 5588–5595. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, L.; He, H.; Sun, L.; Wang, H.; Fang, X.; Zhao, Y.; Zheng, D.; Qi, Y.; Li, Z.; et al. In Situ Photodeposition of Platinum Clusters on a Covalent Organic Framework for Photocatalytic Hydrogen Production. Nat. Commun. 2022, 13, 1355. [Google Scholar] [CrossRef] [PubMed]
- Veziroglu, S.; Ghori, M.Z.; Kamp, M.; Kienle, L.; Rubahn, H.-G.; Strunskus, T.; Fiutowski, J.; Adam, J.; Faupel, F.; Aktas, O.C. Photocatalytic Growth of Hierarchical Au Needle Clusters on Highly Active TiO2 Thin Film. Adv. Mater. Interfaces 2018, 5, 1800465. [Google Scholar] [CrossRef]
- Kong, J.; Qin, Y.-H.; Wang, T.-L.; Wang, C.-W. Photodeposition of Pt Nanoparticles onto TiO2@CNT as High-Performance Electrocatalyst for Oxygen Reduction Reaction. Int. J. Hydrogen Energy 2020, 45, 1991–1997. [Google Scholar] [CrossRef]
- Shondo, J.; Veziroglu, S.; Tjardts, T.; Fiutowski, J.; Schröder, S.; Mishra, Y.K.; Strunskus, T.; Rubahn, H.; Faupel, F.; Aktas, O.C. Selective Adsorption and Photocatalytic Clean-Up of Oil by TiO2 Thin Film Decorated with p-V3 D3 Modified Flowerlike Ag Nanoplates. Adv. Mater. Interfaces 2022, 9, 2102126. [Google Scholar] [CrossRef]
- Veziroglu, S.; Obermann, A.-L.; Ullrich, M.; Hussain, M.; Kamp, M.; Kienle, L.; Leißner, T.; Rubahn, H.-G.; Polonskyi, O.; Strunskus, T.; et al. Photodeposition of Au Nanoclusters for Enhanced Photocatalytic Dye Degradation over TiO2 Thin Film. ACS Appl. Mater. Interfaces 2020, 12, 14983–14992. [Google Scholar] [CrossRef]
- Majeed, I.; Ali, H.; Idrees, A.; Arif, A.; Ashraf, W.; Rasul, S.; Khan, M.A.; Nadeem, M.A.; Nadeem, M.A. Understanding the Role of Metal Supported on TiO2 in Photoreforming of Oxygenates. Energy Adv. 2022, 1, 842–867. [Google Scholar] [CrossRef]
- Chen, G.; Li, R.; Huang, L. Advances in Photochemical Deposition for Controllable Synthesis of Heterogeneous Catalysts. Nanoscale 2023, 15, 13909–13931. [Google Scholar] [CrossRef]
- Yan, F.; Wang, Y.; Zhang, J.; Lin, Z.; Zheng, J.; Huang, F. Schottky or Ohmic Metal–Semiconductor Contact: Influence on Photocatalytic Efficiency of Ag/ZnO and Pt/ZnO Model Systems. ChemSusChem 2014, 7, 101–104. [Google Scholar] [CrossRef]
- Ferrah, D.; Tieu, P. Controllable Growth of Copper on TiO2 Nanoparticles by Photodeposition Based on Coupled Effects of Solution Viscosity and Photoreduction Rate for Catalysis-Related Applications. ACS Appl. Nano Mater. 2020, 3, 5855–5861. [Google Scholar] [CrossRef]
- Lisiecki, I. Size, Shape, and Structural Control of Metallic Nanocrystals. J. Phys. Chem. B 2005, 109, 12231–12244. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Fang, J. Particle-Mediated Nucleation and Growth of Solution-Synthesized Metal Nanocrystals: A New Story beyond the LaMer Curve. Nano Today 2016, 11, 145–167. [Google Scholar] [CrossRef]
- Shondo, J.; Veziroglu, S.; Tjardts, T.; Sarwar, T.B.; Mishra, Y.K.; Faupel, F.; Aktas, O.C. Nanoscale Synergetic Effects on Ag–TiO2 Hybrid Substrate for Photoinduced Enhanced Raman Spectroscopy (PIERS) with Ultra-Sensitivity and Reusability. Small 2022, 2203861. [Google Scholar] [CrossRef] [PubMed]
- Vahl, A.; Veziroglu, S.; Henkel, B.; Strunskus, T.; Polonskyi, O.; Aktas, O.C.; Faupel, F. Pathways to Tailor Photocatalytic Performance of TiO2 Thin Films Deposited by Reactive Magnetron Sputtering. Materials 2019, 12, 2840. [Google Scholar] [CrossRef] [PubMed]
- Daniel, L.; Nagai, H.; Yoshida, N.; Sato, M. Photocatalytic Activity of Vis-Responsive Ag-Nanoparticles/TiO2 Composite Thin Films Fabricated by Molecular Precursor Method (MPM). Catalysts 2013, 3, 625–645. [Google Scholar] [CrossRef]
- Ramasamy, P.; Seo, D.-M.; Kim, S.-H.; Kim, J. Effects of TiO2 Shells on Optical and Thermal Properties of Silver Nanowires. J. Mater. Chem. 2012, 22, 11651–11657. [Google Scholar] [CrossRef]
- Sahyun, M.R.V.; Serpone, N. Primary Events in the Photocatalytic Deposition of Silver on Nanoparticulate TiO2. Langmuir 1997, 13, 5082–5088. [Google Scholar] [CrossRef]
- Zhao, S.; Cheng, Z.; Kang, L.; Li, M.; Gao, Z. The Facile Preparation of Ag Decorated TiO2/ZnO Nanotubes and Their Potent Photocatalytic Degradation Efficiency. RSC Adv. 2017, 7, 50064–50071. [Google Scholar] [CrossRef]
- Li, S.; Hu, J.; Yang, Y.; Zhao, L.; Qiao, Y.; Liu, W.; Liu, P.; Chen, M. Ag/Nano-TiO2 Composite Compact Film for Enhanced Performance of Perovskite Solar Cells Based on Carbon Counter Electrodes. Appl. Phys. A 2017, 123, 628. [Google Scholar] [CrossRef]
- Ali, M.H.; Azad, M.A.K.; Khan, K.A.; Rahman, M.O.; Chakma, U.; Kumer, A. Analysis of Crystallographic Structures and Properties of Silver Nanoparticles Synthesized Using PKL Extract and Nanoscale Characterization Techniques. ACS Omega 2023, 8, 28133–28142. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veziroglu, S. Fabrication of Ultra-Fine Ag NPs on TiO2 Thin Films by Alcohol-Assisted Photodeposition Process for Photocatalysis-Related Applications. Materials 2024, 17, 1354. https://doi.org/10.3390/ma17061354
Veziroglu S. Fabrication of Ultra-Fine Ag NPs on TiO2 Thin Films by Alcohol-Assisted Photodeposition Process for Photocatalysis-Related Applications. Materials. 2024; 17(6):1354. https://doi.org/10.3390/ma17061354
Chicago/Turabian StyleVeziroglu, Salih. 2024. "Fabrication of Ultra-Fine Ag NPs on TiO2 Thin Films by Alcohol-Assisted Photodeposition Process for Photocatalysis-Related Applications" Materials 17, no. 6: 1354. https://doi.org/10.3390/ma17061354




