Dynamics of Tribofilm Formation in Boundary Lubrication Investigated Using In Situ Measurements of the Friction Force and Contact Voltage
Abstract
:1. Introduction
2. Experimental Methods
2.1. Lubricant Formulations
2.2. Specimens
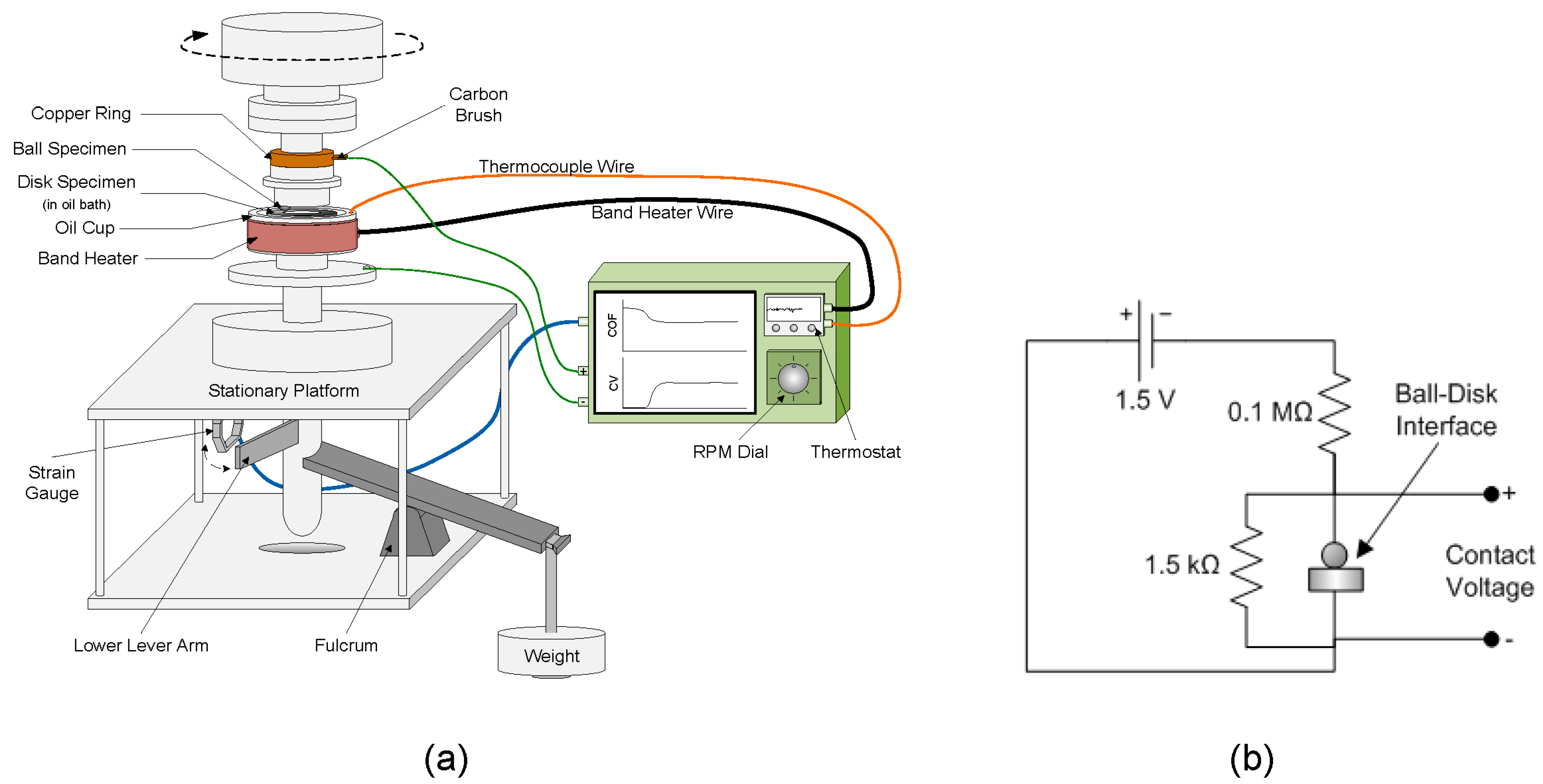
2.3. Friction Experiments
2.4. Coefficient of Friction and Contact Voltage Measurements
3. Results and Discussion
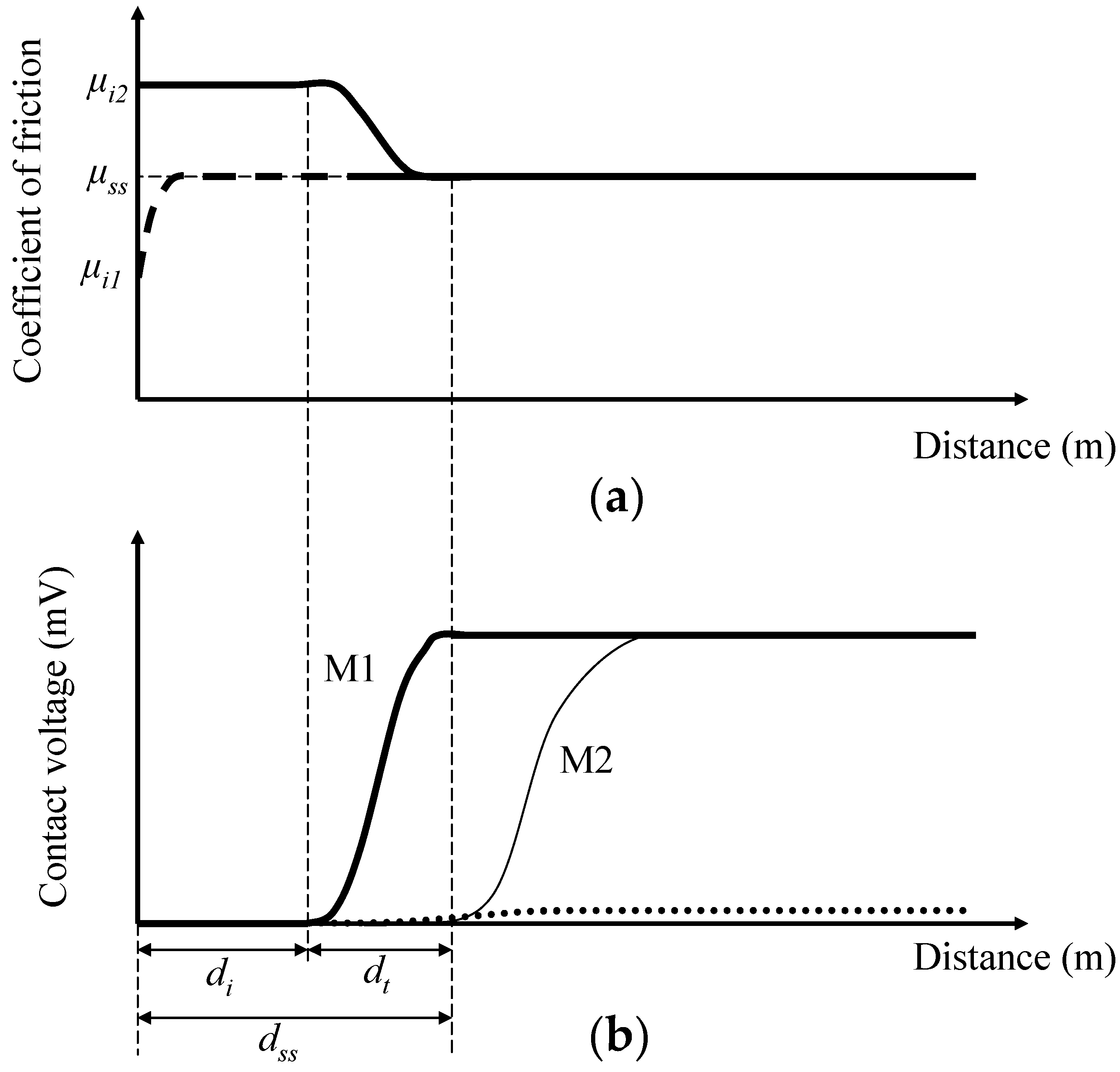
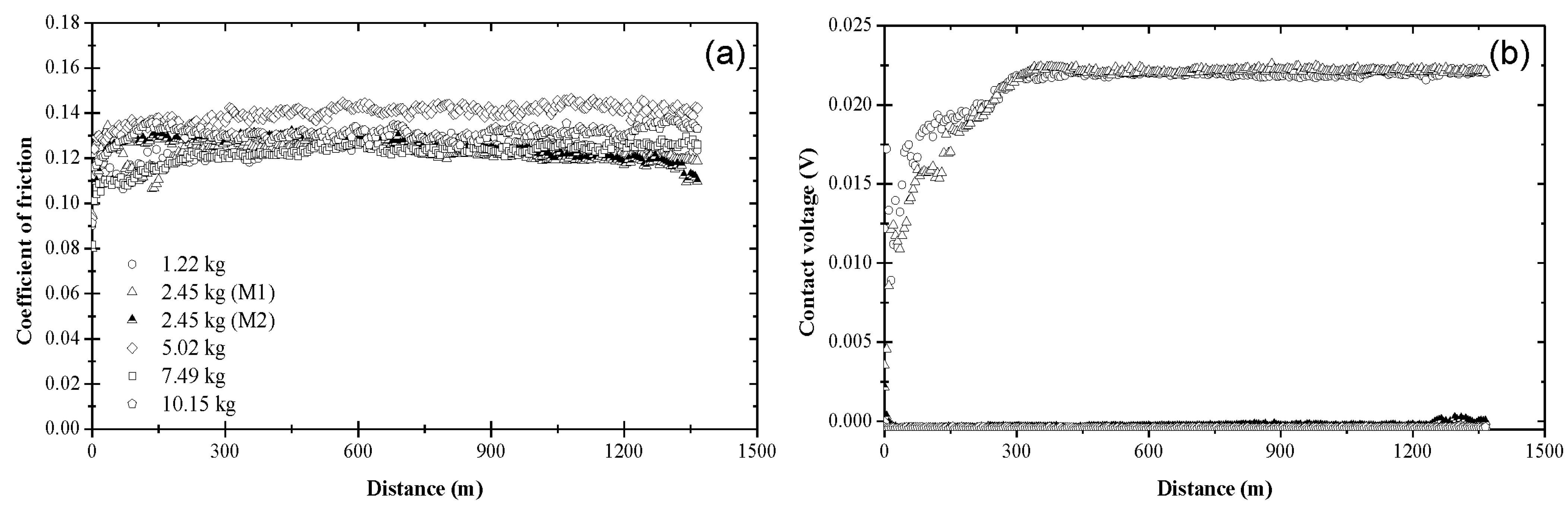
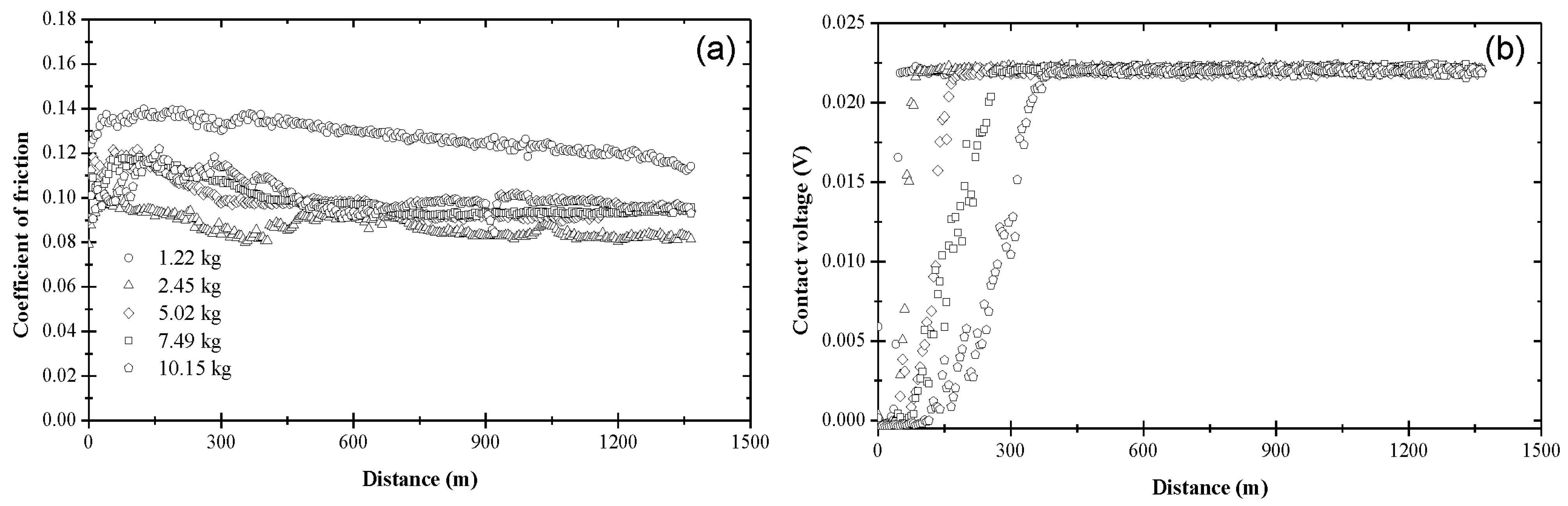
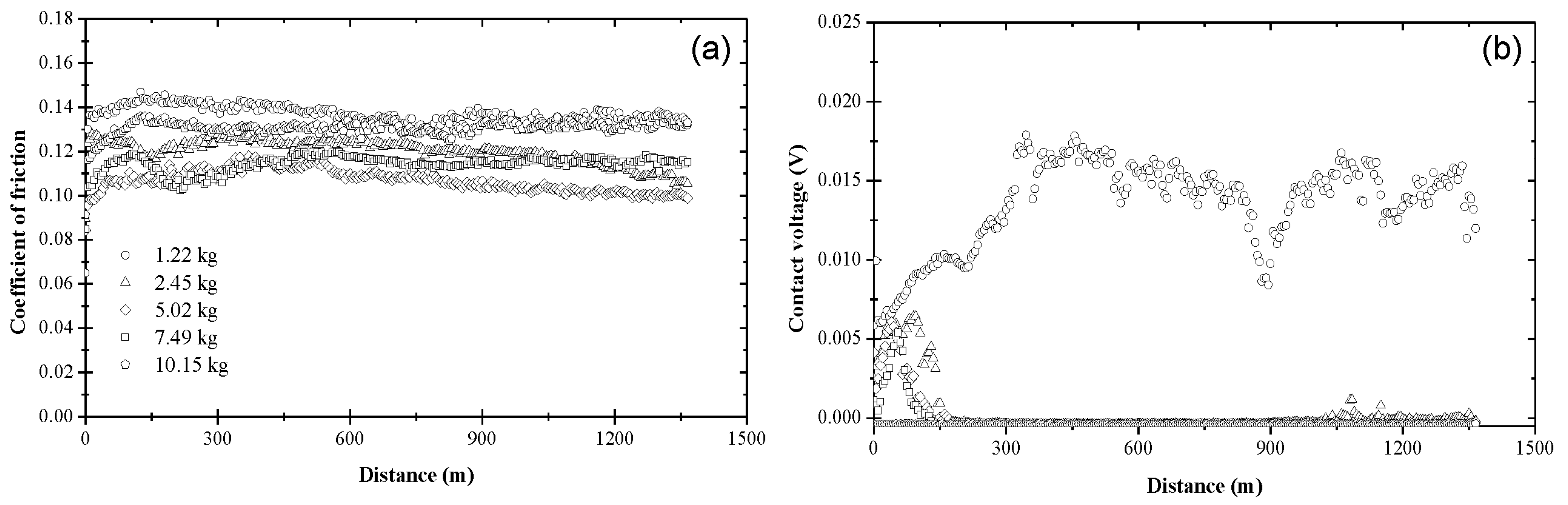
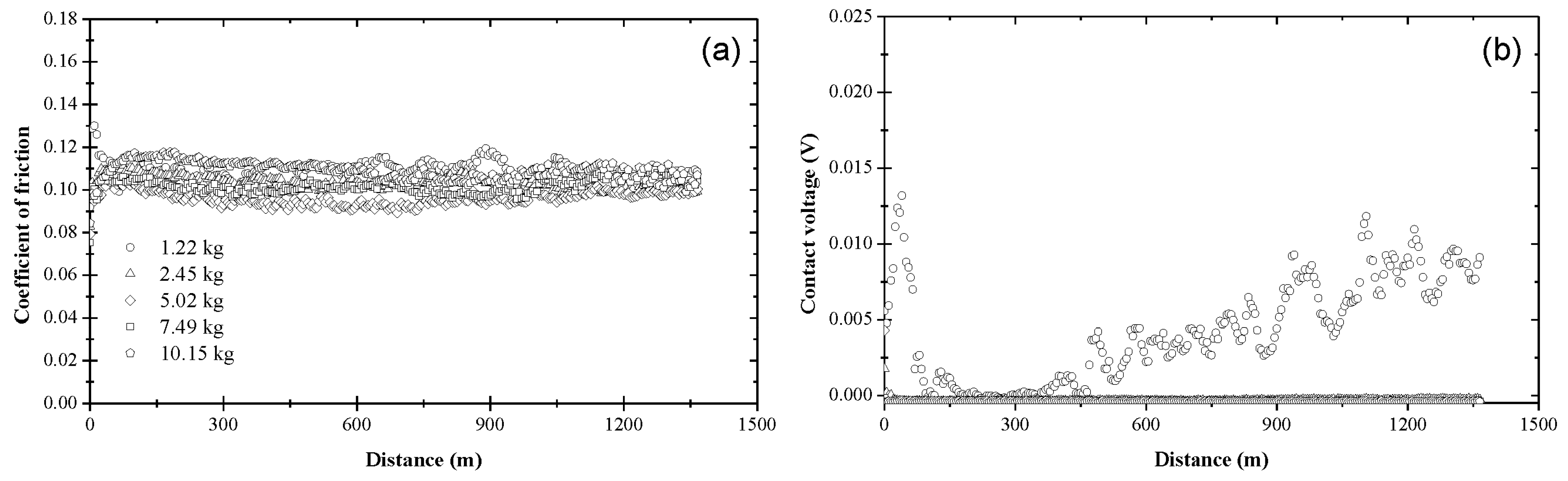
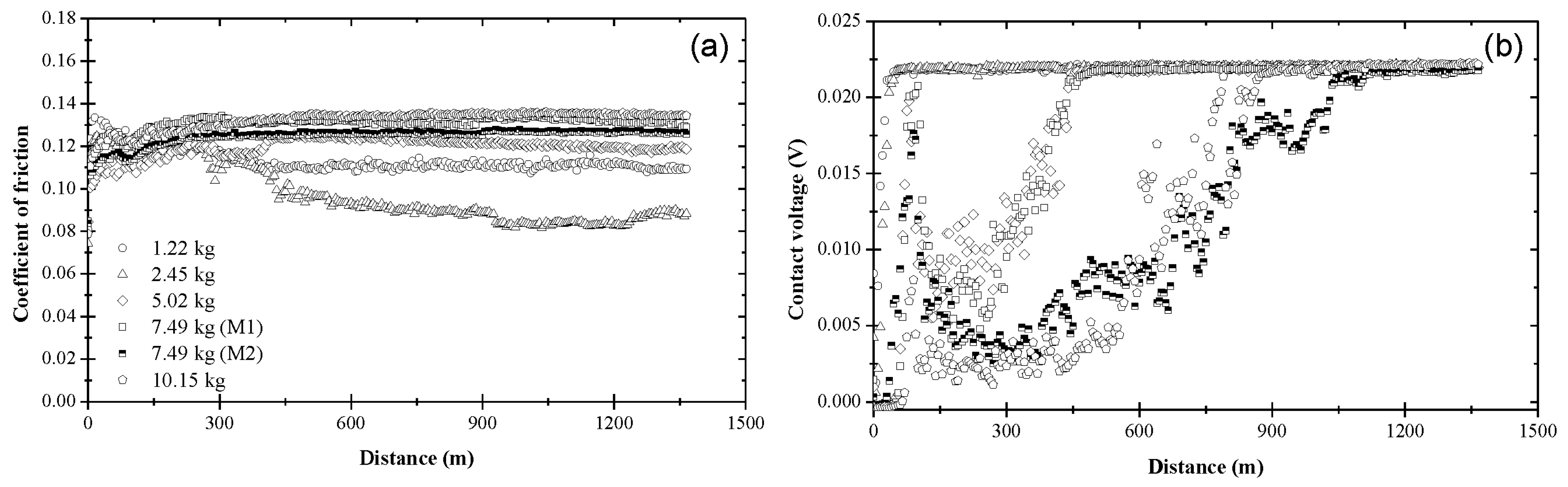
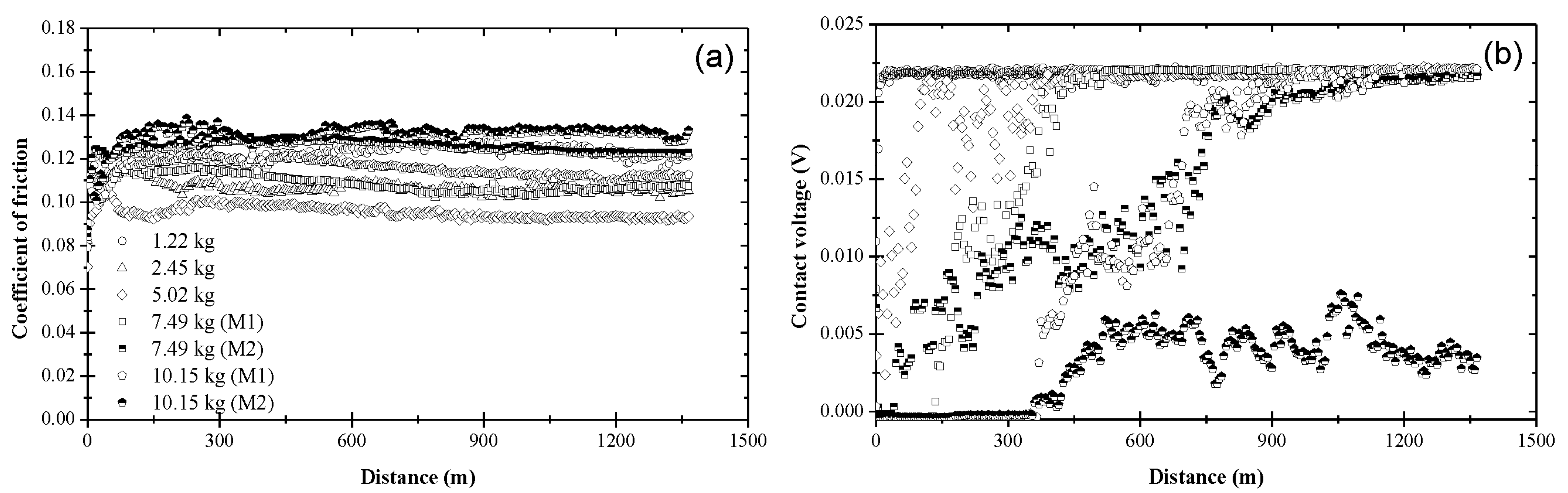
3.1. Coefficient of Friction and Contact Voltage
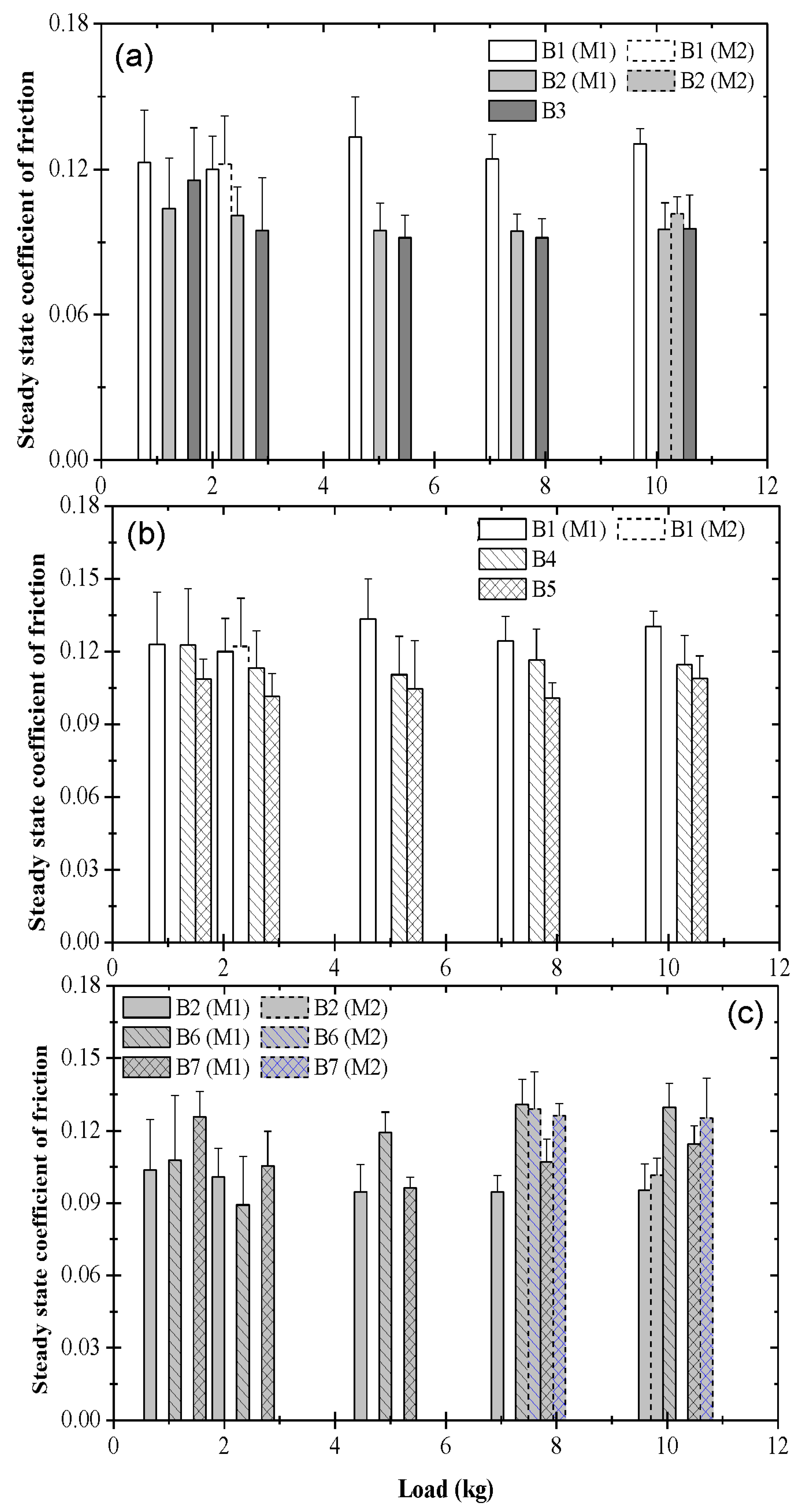
3.2. Steady-State Coefficient of Friction
3.3. Critical Distance for Stable Tribofilm Formation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kar, P.; Asthana, P.; Liang, H. Formation and Characterization of Tribofilms. ASME J. Tribol. 2008, 130, 042301. [Google Scholar] [CrossRef]
- Hsu, S.M.; Gates, R.S. Boundary Lubricating Films: Formation and Lubrication Mechanism. Tribol. Int. 2005, 38, 305–312. [Google Scholar] [CrossRef]
- Pereira, G.; Lachenwitzer, A.; Kasrai, M.; Bancroft, G.M.; Norton, P.R.; Abrecht, M.; Gilbert, P.U.P.A.; Regier, T.; Blyth, R.I.R.; Thompson, J. Chemical and Mechanical Analysis of Tribofilms from Fully Formulated Oils. Part 1—Films on 52100 Steel. Tribology 2007, 1, 48–61. [Google Scholar] [CrossRef]
- Chen, Y.; Renner, P.; Liang, H. A Review of Current Understanding in Tribochemical Reactions Involving Lubricant Additives. Friction 2023, 11, 489–512. [Google Scholar] [CrossRef]
- Biswas, S.K. Some Mechanisms of Tribofilm Formation in Metal/Metal and Ceramic/Metal Sliding Interactions. Wear 2000, 245, 178–189. [Google Scholar] [CrossRef]
- Mortier, R.M.; Orszulik, S.T. Chemistry and Technology of Lubricants, 2nd ed.; Chapman and Hall: London, UK, 1997; pp. 74–250. [Google Scholar]
- Barnes, A.M.; Bartle, K.D.; Thibon, V.R.A. A Review of Zinc Dialkyldithiophosphates: Characterization and Role in the Lubricating Oil. Tribol. Int. 2001, 38, 389–395. [Google Scholar] [CrossRef]
- Spedding, H.; Watkins, R.C. The Antiwear Mechanism of ZDDP’s. Tribol. Int. 1982, 3, 1–12. [Google Scholar]
- Bardasz, E.A.; Lamb, G.D. Additives for Crankcase Lubricant Applications. In Lubricant Additives: Chemistry and Applications; Rudnick, L.R., Ed.; Marcel Dekker: New York, NY, USA, 2003; pp. 387–428. [Google Scholar]
- Spikes, H. The History and Mechanisms of ZDDP. Tribol. Lett. 2004, 17, 469–489. [Google Scholar] [CrossRef]
- Zhang, J.; Spikes, H. On the Mechanism of ZDDP Antiwear Film Formation. Tribol. Lett. 2016, 63, 24. [Google Scholar] [CrossRef]
- Soltanahmadi, S.; Morina, A.; van Eijk, M.C.P.; Nedelcu, I.; Neville, A. Experimental Observation of Zinc Dialkyl Dithiophosphate (ZDDP)-induced Iron Sulphide Formation. Appl. Surf. Sci. 2017, 414, 41–51. [Google Scholar] [CrossRef]
- Morina, A.; Neville, A. Tribofilms: Aspects of Formation, Stability and Removal. J. Phys. D 2007, 40, 5476–5487. [Google Scholar] [CrossRef]
- Zhao, Y.; Geng, Z.; Li, D.; Wang, L.; Lu, Z.; Zhang, G. An Investigation on the Tribological Properties of Graphene and ZDDP as Additives in PAO4 Oil. Diam. Relat. Mater. 2021, 120, 108635. [Google Scholar] [CrossRef]
- Gosvami, N.N.; Bares, J.A.; Mangolini, F.; Konicek, A.R.; Yablon, D.G.; Carpick, R.W. Mechanisms of Antiwear Tribofilm Growth Revealed In Situ by Single-Asperity Sliding Contacts. Science 2015, 348, 102–106. [Google Scholar] [CrossRef]
- Dorgham, A.; Parsaeian, P.; Azam, A.; Wang, C.; Morina, A.; Neville, A. Single-Asperity Study of the Reaction Kinetics of P-Based Triboreactive Films. Tribol. Int. 2019, 133, 288–296. [Google Scholar] [CrossRef]
- Fang, L.; Korres, S.; Lamberti, W.A.; Webster, M.N.; Carpick, R.W. What Stress Components Drive Mechanochemistry? A Study of ZDDP Tribofilm Formation. Faraday Discuss. 2023, 241, 394–412. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Wang, C.; Espejo, C.; Wang, J.; Neville, A.; Morina, A. Understanding the Friction Reduction Mechanism Based on Molybdenum Disulfide Tribofilm Formation and Removal. Langmuir 2018, 34, 13523–13533. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Gabler, C.; Doerr, N.; Aswath, P.B. Mechanism of Tribofilm Formation with P and S Containing Ionic Liquids. Tribol. Int. 2015, 92, 353–364. [Google Scholar] [CrossRef]
- Vyavhare, K.; Sharma, V.; Sharma, V.; Erdemir, A.; Aswath, P.B. XANES Study of Tribofilm Formation with Low Phosphorus Additive Mixtures of Phosphonium Ionic Liquid and Borate Ester. Front. Mech. Eng. 2021, 7, 671457. [Google Scholar] [CrossRef]
- Wijanarko, W.; Khanmohammadi, H.; Espallargas, N. Ionic Liquids as Boundary Additives in Water-Based and PAO Lubricants. Friction 2022, 10, 1405–1423. [Google Scholar] [CrossRef]
- Lahouij, I.; Gould, B.; Demas, N.; Greco, A.; Chen, Z.; Cooper, G.D.; Jackson, A.; Carpick, R.W. Inhibition of Micro-Pitting by Tribofilm-Forming ZrO2 Nanocrystal Lubricant Additives: A Micro-Pitting Rig and Transmission Electron Microscope Study. Tribol. Lett. 2022, 70, 13. [Google Scholar] [CrossRef]
- Ripoll, M.R.; Tomala, A.M.; Pirker, L.; Remškar, M. In-Situ Formation of MoS2 and WS2 Tribofilms by the Synergy Between Transition Metal Oxide Nanoparticles and Sulphur-Containing Oil Additives. Tribol. Lett. 2020, 68, 41. [Google Scholar] [CrossRef]
- Kim, S.M.; Sit, C.Y.; Komvopoulos, K.; Yamaguchi, E.S.; Ryason, P.R. Boundary Lubrication of Steel Surfaces with Borate, Phosphorus, and Sulfur Containing Lubricants at Relatively Low and Elevated Temperatures. Tribol. Trans. 2000, 43, 569–578. [Google Scholar] [CrossRef]
- Komvopoulos, K.; Chiaro, V.; Pakter, B.; Yamaguchi, E.S.; Ryason, P.R. Antiwear Tribofilm Formation on Steel Surfaces Lubricated with Gear Oil Containing Borate, Phosphorus, and Sulfur Additives. Tribol. Trans. 2002, 45, 568–575. [Google Scholar] [CrossRef]
- Komvopoulos, K.; Do, V.; Yamaguchi, E.S.; Ryason, P.R. Effect of Sulfur- and Phosphorus-Containing Additives and Metal Deactivator on the Tribological Properties of Boundary-Lubricated Steel Surfaces. Tribol. Trans. 2003, 46, 315–325. [Google Scholar] [CrossRef]
- Komvopoulos, K.; Pernama, S.A.; Ma, J.; Yamaguchi, E.S.; Ryason, P.R. Synergistic Effects of Boron-, Sulfur-, and Phosphorus-Containing Lubricants in Boundary Lubrication of Steel Surfaces. Tribol. Trans. 2005, 48, 218–229. [Google Scholar] [CrossRef]
- Chhattal, M.; Rosenkranz, A.; Zaki, S.; Ren, K.; Ghaffar, A.; Gong, Z.; Grützmacher, P.G. Unveiling the Tribological Potential of MXenes-Current Uunderstanding and Future Perspectives. Adv. Colloid Interface Sci. 2023, 321, 103021. [Google Scholar] [CrossRef] [PubMed]
- Boidi, G.; de Queiróz, J.C.F.; Profito, F.J.; Rosenkranz, A. Ti3C2Tx MXene Nanosheets as Lubricant Additives to Lower Friction under High Loads, Sliding Ratios, and Elevated Temperatures. ACS Appl. Nano Mater. 2023, 6, 729–737. [Google Scholar] [CrossRef]
- Miao, X.; Li, Z.; Liu, S.; Wang, J.; Yang, S. MXenes in Tribology: Current Status and Perspectives. Adv. Powder Mater. 2023, 2, 100092. [Google Scholar] [CrossRef]
- Somayaji, A.; Aswath, P. Antiwear Behaviour of ZDDP and Flourinated ZDDP in the Presence of Alkylated Diphenyl Amine Antioxidants. Tribol. Trans. 2008, 51, 403–412. [Google Scholar] [CrossRef]
- Wollenberg, R.H.; Plavac, F.; Erdman, T.R. Modified Succinimides. U.S. Patent 4,612,132, 16 September 1986. [Google Scholar]
- Ruhe, W.R., Jr.; Stokes, C.D. Lubricating Oil Additive Composition and Method of Making the Same. U.S. Patent 0247386 A1, 2 November 2006. [Google Scholar]
- Yamaguchi, E.S.; Ryason, P.R.; Yeh, S.W.; Hansen, T.P. Boundary Film Formation by ZnDTPs and Detergents Using ECR. Tribol. Trans. 1998, 41, 262–272. [Google Scholar] [CrossRef]
- Yamaguchi, E.S.; Roby, S.H.; Francisco, M.M.; Ruelas, S.G. Antiwear Film Formation by ZnDTP, Detergent, and Dispersant Components of Passenger Car Motor Oils. Tribol. Trans. 2002, 45, 425–429. [Google Scholar] [CrossRef]


| Blend | Composition | Concentration (ppm) | |||
|---|---|---|---|---|---|
| N | P | Zn | S | ||
| 1 | base oil | – | – | – | 19 |
| 2 | base oil + 0.05 wt% ZDDP | 1.7 | 513 | 545 | 1042 |
| 3 | base oil + 0.08 wt% ZDDP | – | 803 | 868 | 1615 |
| 4 | base oil + 0.1 wt% dispersant A | 894 | – | – | 57 |
| 5 | base oil + 0.1 wt% dispersant B | 175 | – | – | 26.7 |
| 6 | base oil + 0.05 wt% ZDDP + 0.1 wt% dispersant A | 888 | 516 | 552 | 1102 |
| 7 | base oil + 0.05 wt% ZDDP + 0.1 wt% dispersant B | 175 | 519 | 551 | 1081 |
| Property | Specimen | |
|---|---|---|
| Ball | Disk | |
| Yield strength (MPa) | 560 | 560 |
| Rockwell C hardness | 62 ± 3 | 60 ± 3 |
| Ultimate tensile strength (MPa) | 700 | 700 |
| Elastic modulus (GPa) | 193 | 193 |
| Shear modulus (GPa) | 66.5 | 66.5 |
| Poisson’s ratio | 0.30–0.31 | 0.30–0.31 |
| Elongation in 5 cm (%) | 25 | 25 |
| Reduction in area (%) | 57 | 57 |
| Root-mean-square roughness (µm) | <50.8 | (25.4–50.8) * |
| Diameter (cm) | 0.8 | 3.2 |
| Thickness (cm) | n/a | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, A.E.; Komvopoulos, K. Dynamics of Tribofilm Formation in Boundary Lubrication Investigated Using In Situ Measurements of the Friction Force and Contact Voltage. Materials 2024, 17, 1335. https://doi.org/10.3390/ma17061335
Tsai AE, Komvopoulos K. Dynamics of Tribofilm Formation in Boundary Lubrication Investigated Using In Situ Measurements of the Friction Force and Contact Voltage. Materials. 2024; 17(6):1335. https://doi.org/10.3390/ma17061335
Chicago/Turabian StyleTsai, Anna E., and Kyriakos Komvopoulos. 2024. "Dynamics of Tribofilm Formation in Boundary Lubrication Investigated Using In Situ Measurements of the Friction Force and Contact Voltage" Materials 17, no. 6: 1335. https://doi.org/10.3390/ma17061335






