Recycled or Bio-Based Solvents for the Synthesis of ZnO Nanoparticles: Characterization and Validation in Organic Solar Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
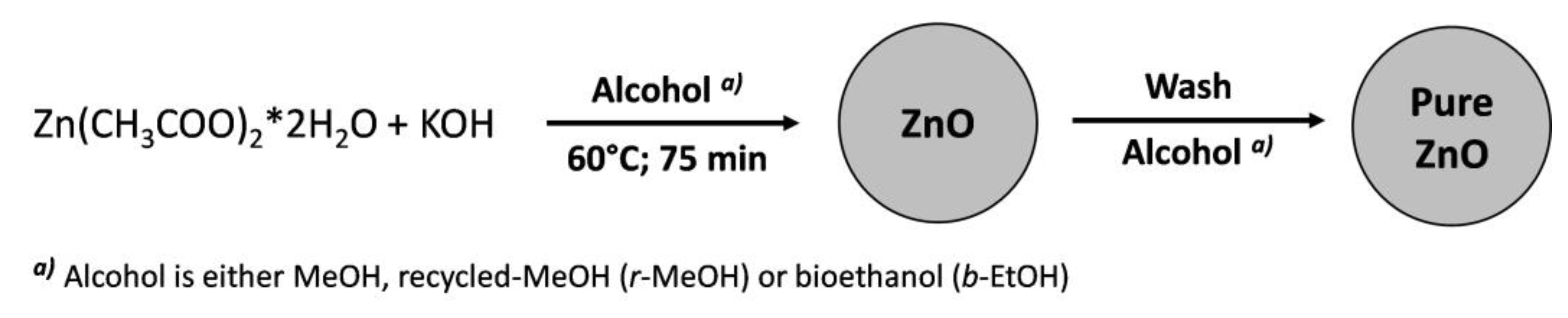
2.2. Preparation of the ZnO NP Inks
2.2.1. Synthesis of the Ref-ZnO NPs
2.2.2. Synthesis of r-ZnO NPs
2.2.3. Synthesis of b-ZnO NPs
2.3. Characterization Methods
2.4. Device Fabrication
3. Results
3.1. ZnO Nanoparticle Inks
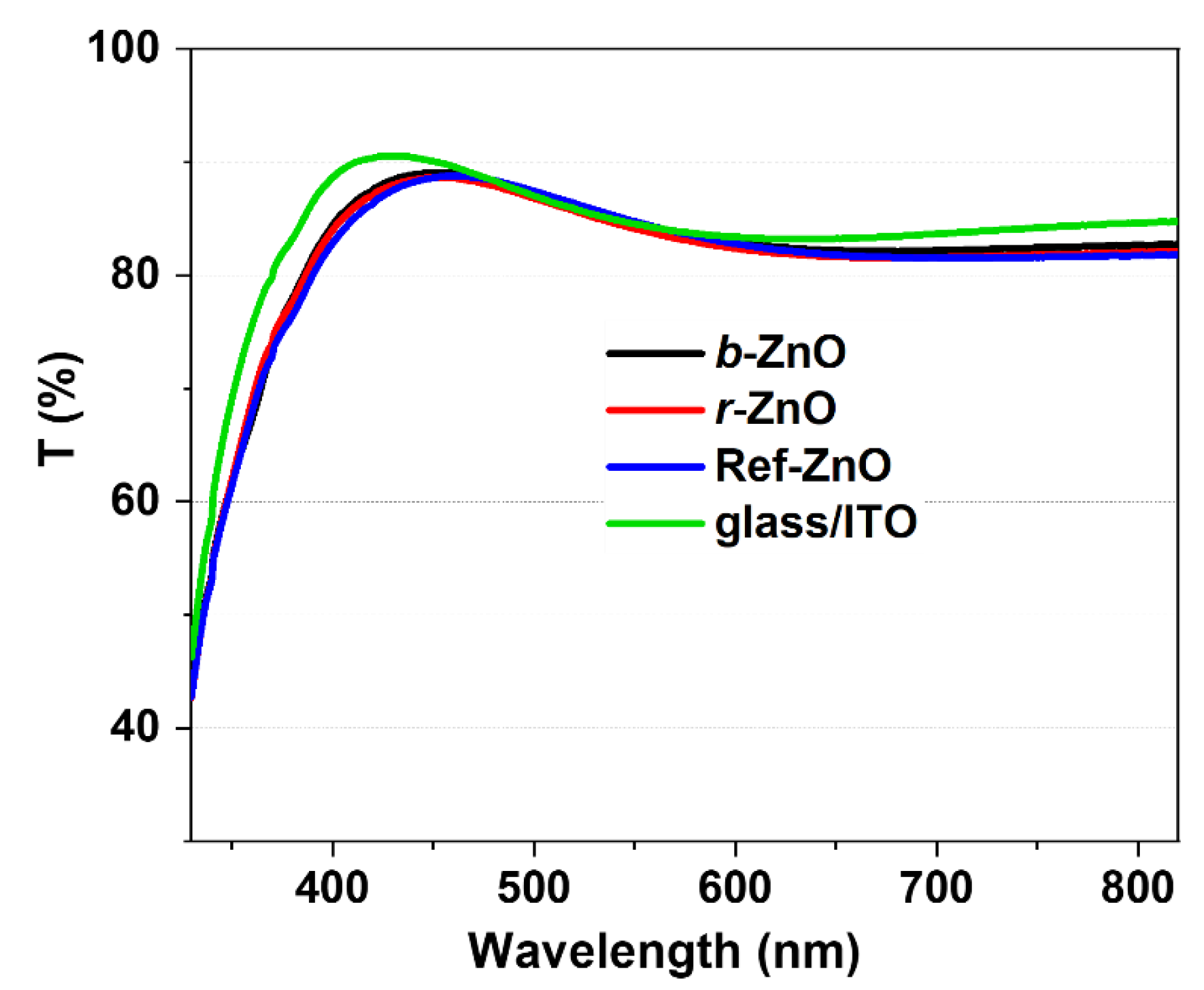
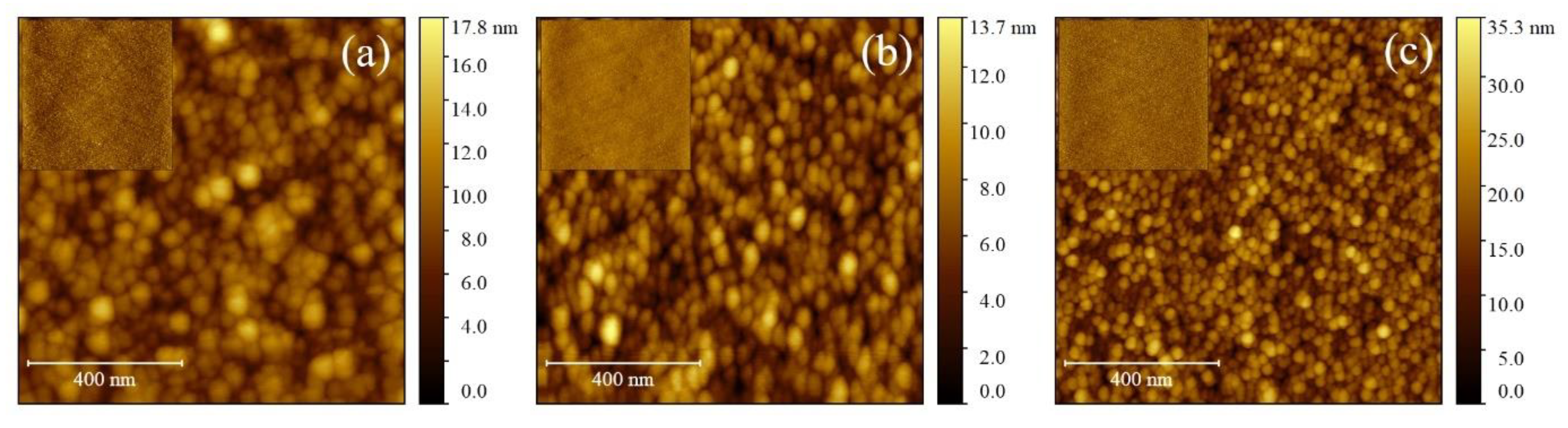
3.2. ZnO Thin-Films
(Φbulk-T − Φbulk-ITO) + (VD-T − VD-ITO) = (Φbulk-T − Φbulk-ITO) + A
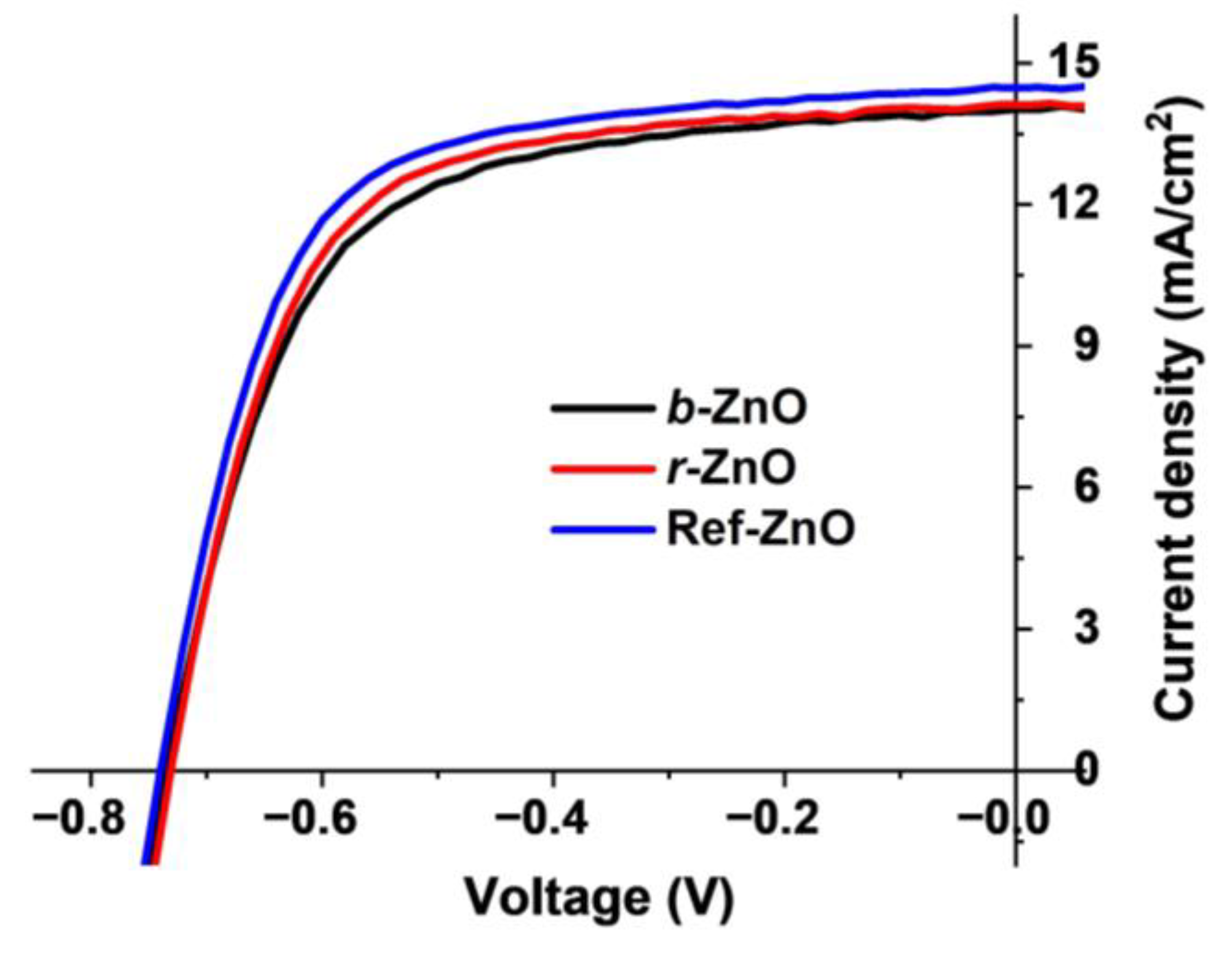
3.3. Photovoltaic Devices
| NPs as the ETL | VOC (V) | JSC (mA/cm2) | FF (%) | PCE (%) |
|---|---|---|---|---|
| b-ZnO | 0.733 ± 0.003 | 14.0 ± 0.3 | 63 ± 1 | 6.5 ± 0.2 |
| r-ZnO | 0.731 ± 0.010 | 14.1 ± 0.4 | 66 ± 2 | 6.8 ± 0.2 |
| Ref-ZnO | 0.738 ± 0.004 | 14.5 ± 0.4 | 65 ± 1 | 6.9 ± 0.2 |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mandal, A.K.; Katuwal, S.; Tettey, F.; Gupta, A.; Bhattarai, S.; Jaisi, S.; Bhandari, D.P.; Shah, A.K.; Bhattarai, N.; Parajuli, N. Current Research on Zinc Oxide Nanoparticles: Synthesis, Characterization, and Biomedical Applications. Nanomaterials 2022, 12, 3066. [Google Scholar] [CrossRef]
- Meyer, J.; Hamwi, S.; Kröger, M.; Kowalsky, W.; Riedl, T.; Kahn, A. Transition Metal Oxides for Organic Electronics: Energetics, Device Physics and Applications. Adv. Mater. 2012, 24, 5408–5427. [Google Scholar] [CrossRef]
- Yu, X.; Marks, T.J.; Facchetti, A. Metal Oxides for Optoelectronic Applications. Nat. Mater. 2016, 15, 383–396. [Google Scholar] [CrossRef]
- Yin, Z.; Wei, J.; Zheng, Q. Interfacial Materials for Organic Solar Cells: Recent Advances and Perspectives. Adv. Sci. 2016, 3, 1500362. [Google Scholar] [CrossRef]
- Albonetti, C.; El Qacemi, V.; Limage, S.; Versini, C.; Kauffmann, L.D.; Muccini, M.; Seri, M. Enhanced Thermal Stability of Inverted Polymer Solar Cells Based on Solution-Processed WOx as an Anode Interlayer. Phys. Status Solidi (A) Appl. Mater. Sci. 2021, 218, 2000748. [Google Scholar] [CrossRef]
- Liu, C.; Xiao, C.; Li, W. Zinc Oxide Nanoparticles as Electron Transporting Interlayer in Organic Solar Cells. J. Mater. Chem. C 2021, 9, 14093–14114. [Google Scholar] [CrossRef]
- Yip, H.-L.; Jen, A.K.-Y. Recent Advances in Solution-Processed Interfacial Materials for Efficient and Stable Polymer Solar Cells. Energy Environ. Sci. 2012, 5, 5994–6011. [Google Scholar] [CrossRef]
- White, M.S.; Olson, D.C.; Shaheen, S.E.; Kopidakis, N.; Ginley, D.S. Inverted Bulk-Heterojunction Organic Photovoltaic Device Using a Solution-Derived ZnO Underlayer. Appl. Phys. Lett. 2006, 89, 143517. [Google Scholar] [CrossRef]
- Arrabito, G.; Aleeva, Y.; Pezzilli, R.; Ferrara, V.; Medaglia, P.G.; Pignataro, B.; Prestopino, G. Printing ZnO Inks: From Principles to Devices. Crystals 2020, 10, 449. [Google Scholar] [CrossRef]
- Sutharappa Kaliyamoorthy, T.; Subramaniyan, V.; Renganathan, S.; Elavarasan, V.; Ravi, J.; Prabhakaran Kala, P.; Subramaniyan, P.; Vijayakumar, S. Sustainable Environmental-Based ZnO Nanoparticles Derived from Pisonia Grandis for Future Biological and Environmental Applications. Sustainability 2022, 14, 17009. [Google Scholar] [CrossRef]
- Xu, J.; Huang, Y.; Zhu, S.; Abbes, N.; Jing, X.; Zhang, L. A Review of the Green Synthesis of ZnO Nanoparticles Using Plant Extracts and Their Prospects for Application in Antibacterial Textiles. J. Eng. Fibers Fabr. 2021, 16, 155892502110462. [Google Scholar] [CrossRef]
- Faisal, S.; Jan, H.; Shah, S.A.; Shah, S.; Khan, A.; Akbar, M.T.; Rizwan, M.; Jan, F.; Wajidullah; Akhtar, N.; et al. Green Synthesis of Zinc Oxide (ZnO) Nanoparticles Using Aqueous Fruit Extracts of Myristica Fragrans: Their Characterizations and Biological and Environmental Applications. ACS Omega 2021, 6, 9709–9722. [Google Scholar] [CrossRef] [PubMed]
- Rathnasamy, R.; Thangasamy, P.; Thangamuthu, R.; Sampath, S.; Alagan, V. Green Synthesis of ZnO Nanoparticles Using Carica Papaya Leaf Extracts for Photocatalytic and Photovoltaic Applications. J. Mater. Sci. Mater. Electron. 2017, 28, 10374–10381. [Google Scholar] [CrossRef]
- Shashanka, R.; Esgin, H.; Yilmaz, V.M.; Caglar, Y. Fabrication and Characterization of Green Synthesized ZnO Nanoparticle Based Dye-Sensitized Solar Cells. J. Sci. Adv. Mater. Devices 2020, 5, 185–191. [Google Scholar] [CrossRef]
- Yang, S.; Yu, H. The Modification of ZnO Surface with Natural Antioxidants to Fabricate Highly Efficient and Stable Inverted Organic Solar Cells. Chem. Eng. J. 2023, 452, 139658. [Google Scholar] [CrossRef]
- Marchi, L.; Dinelli, F.; Maccagnani, P.; Costa, V.; Chenet, T.; Belletti, G.; Natali, M.; Cocchi, M.; Bertoldo, M.; Seri, M. Sodium Alginate as a Natural Substrate for Efficient and Sustainable Organic Solar Cells. ACS Sustain. Chem. Eng. 2022, 10, 15608–15617. [Google Scholar] [CrossRef]
- Bolognesi, M.; Prosa, M.; Seri, M. Biocompatible and Biodegradable Organic Electronic Materials. In Sustainable Strategies in Organic Electronics; Marrocchi, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 297–338. ISBN 978-0-12-823147-0. [Google Scholar]
- Li, W.; Liu, Q.; Zhang, Y.; Li, C.; He, Z.; Choy, W.C.H.; Low, P.J.; Sonar, P.; Kyaw, A.K.K. Biodegradable Materials and Green Processing for Green Electronics. Adv. Mater. 2020, 32, 2001591. [Google Scholar] [CrossRef]
- Gowda, A.; Kumar, S. Recent Advances in Discotic Liquid Crystal-Assisted Nanoparticles. Materials 2018, 11, 382. [Google Scholar] [CrossRef]
- Sun, D.; Wong, M.; Sun, L.; Li, Y.; Miyatake, N.; Sue, H.-J. Purification and Stabilization of Colloidal ZnO Nanoparticles in Methanol. J. Sol-Gel Sci. Technol. 2007, 43, 237–243. [Google Scholar] [CrossRef]
- Hammad, T.M.; Salem, J.K.; Harrison, R.G. The Influence of Annealing Temperature on the Structure, Morphologies and Optical Properties of ZnO Nanoparticles. Superlattices Microstruct. 2010, 47, 335–340. [Google Scholar] [CrossRef]
- Kim, J.Y.; Biswas, S.; Lee, Y.; Lee, H.W.; Jeon, J.M.; Kim, H. Highly Efficient Inverted Polymer Solar Cells Using an Indium Gallium Zinc Oxide Interfacial Layer. Sol. RRL 2021, 5, 2000673. [Google Scholar] [CrossRef]
- Wang, G.; Jiu, T.; Tang, G.; Li, J.; Li, P.; Song, X.; Lu, F.; Fang, J. Interface Modification of ZnO-Based Inverted PTB7:PC 71 BM Organic Solar Cells by Cesium Stearate and Simultaneous Enhancement of Device Parameters. ACS Sustain. Chem. Eng. 2014, 2, 1331–1337. [Google Scholar] [CrossRef]
- Available online: https://uk.vwr.com/store/product/733114/null (accessed on 7 March 2024).
- Pietri, E.; El Qacemi, V.; Kauffman, L.D.; Versini, C.; Didane, Y. Nanoparticle-Based Ink Formulations. WO2015000796, 8 January 2015. [Google Scholar]
- Goh, E.G.; Xu, X.; McCormick, P.G. Effect of Particle Size on the UV Absorbance of Zinc Oxide Nanoparticles. Scr. Mater. 2014, 78–79, 49–52. [Google Scholar] [CrossRef]
- Viswanatha, R.; Sapra, S.; Satpati, B.; Satyam, P.V.; Dev, B.N.; Sarma, D.D. Understanding the Quantum Size Effects in ZnO Nanocrystals. J. Mater. Chem. 2004, 14, 661–668. [Google Scholar] [CrossRef]
- Kumar Jangir, L.; Kumari, Y.; Kumar, A.; Kumar, M.; Awasthi, K. Investigation of Luminescence and Structural Properties of ZnO Nanoparticles, Synthesized with Different Precursors. Mater. Chem. Front. 2017, 1, 1413–1421. [Google Scholar] [CrossRef]
- Souza, T.G.F.; Ciminelli, V.S.T.; Mohallem, N.D.S. A Comparison of TEM and DLS Methods to Characterize Size Distribution of Ceramic Nanoparticles. J. Phys. Conf. Ser. 2016, 733, 012039. [Google Scholar] [CrossRef]
- Heuer-Jungemann, A.; Feliu, N.; Bakaimi, I.; Hamaly, M.; Alkilany, A.; Chakraborty, I.; Masood, A.; Casula, M.F.; Kostopoulou, A.; Oh, E.; et al. The Role of Ligands in the Chemical Synthesis and Applications of Inorganic Nanoparticles. Chem. Rev. 2019, 119, 4819–4880. [Google Scholar] [CrossRef]
- Maguire, C.M.; Rösslein, M.; Wick, P.; Prina-Mello, A. Characterisation of Particles in Solution—A Perspective on Light Scattering and Comparative Technologies. Sci. Technol. Adv. Mater. 2018, 19, 732–745. [Google Scholar] [CrossRef]
- Saadatkhah, N.; Carillo Garcia, A.; Ackermann, S.; Leclerc, P.; Latifi, M.; Samih, S.; Patience, G.S.; Chaouki, J. Experimental Methods in Chemical Engineering: Thermogravimetric Analysis—TGA. Can. J. Chem. Eng. 2020, 98, 34–43. [Google Scholar] [CrossRef]
- Alonso Ipiña, A.; Lázaro Urrutia, M.; Lázaro Urrutia, D.; Alvear Portilla, D. Thermal Oxidative Decomposition Estimation Combining TGA and DSC as Optimization Targets for PMMA. J. Phys. Conf. Ser. 2018, 1107, 032011. [Google Scholar] [CrossRef]
- Sakohara, S.; Ishida, M.; Anderson, M.A. Visible Luminescence and Surface Properties of Nanosized ZnO Colloids Prepared by Hydrolyzing Zinc Acetate. J. Phys. Chem. B 1998, 102, 10169–10175. [Google Scholar] [CrossRef]
- Wang, G.; Adil, M.A.; Zhang, J.; Wei, Z. Large-Area Organic Solar Cells: Material Requirements, Modular Designs, and Printing Methods. Adv. Mater. 2019, 31, 1805089. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Li, S.; Xiao, L.; Zhang, S.; Han, B.; Kang, J.; Zhou, H. Development and Application of Blade-Coating Technique in Organic Solar Cells. Nano Res. 2023, 16, 11571–11588. [Google Scholar] [CrossRef]
- Alamdari, S.; Sasani Ghamsari, M.; Lee, C.; Han, W.; Park, H.-H.; Tafreshi, M.J.; Afarideh, H.; Ara, M.H.M. Preparation and Characterization of Zinc Oxide Nanoparticles Using Leaf Extract of Sambucus Ebulus. Appl. Sci. 2020, 10, 3620. [Google Scholar] [CrossRef]
- Ghaffarian, H.R.; Saiedi, M.; Sayyadnejad, M.A.; Rashidi, A.M. Synthesis of ZnO Nanoparticles by Spray Pyrolysis Method. Iran. J. Chem. Chem. Eng. 2011, 30, 1–6. [Google Scholar] [CrossRef]
- Portale, G.; Sciortino, L.; Albonetti, C.; Giannici, F.; Martorana, A.; Bras, W.; Biscarini, F.; Longo, A. Influence of Metal–Support Interaction on the Surface Structure of Gold Nanoclusters Deposited on Native SiOx/Si Substrates. Phys. Chem. Chem. Phys. 2014, 16, 6649–6656. [Google Scholar] [CrossRef] [PubMed]
- Canet-Ferrer, J.; Coronado, E.; Forment-Aliaga, A.; Pinilla-Cienfuegos, E. Correction of the Tip Convolution Effects in the Imaging of Nanostructures Studied through Scanning Force Microscopy. Nanotechnology 2014, 25, 395703. [Google Scholar] [CrossRef] [PubMed]
- Klapetek, P.; Valtr, M.; Nečas, D.; Salyk, O.; Dzik, P. Atomic Force Microscopy Analysis of Nanoparticles in Non-Ideal Conditions. Nanoscale Res. Lett. 2011, 6, 514. [Google Scholar] [CrossRef] [PubMed]
- Douhéret, O.; Swinnen, A.; Bertho, S.; Haeldermans, I.; D’Haen, J.; D’Olieslaeger, M.; Vanderzande, D.; Manca, J. V High-resolution Morphological and Electrical Characterisation of Organic Bulk Heterojunction Solar Cells by Scanning Probe Microscopy. Prog. Photovolt. Res. Appl. 2007, 15, 713–726. [Google Scholar] [CrossRef]
- Albonetti, C.; Olivieri, G.; Shehu, A.; Quiroga, S.D.; Murgia, M.; Biscarini, F. Unravelling Molecular Disorder at SAM-Functionalized Charge Injection Interfaces in Organic Field-Effect Transistors. Org. Electron. 2022, 100, 106360. [Google Scholar] [CrossRef]
- Kawano, H. Effective Work Functions of the Elements. Prog. Surf. Sci. 2022, 97, 100583. [Google Scholar] [CrossRef]
- Liscio, A.; Palermo, V.; Samorì, P. Nanoscale Quantitative Measurement of the Potential of Charged Nanostructures by Electrostatic and Kelvin Probe Force Microscopy: Unraveling Electronic Processes in Complex Materials. Acc. Chem. Res. 2010, 43, 541–550. [Google Scholar] [CrossRef]
- MSE Supplies-ITO-Coated Glass. Available online: https://www.msesupplies.com/products/mse-pro-1-1-mm-7-10-ohm-sq-ito-coated-glass-substrate?variant=19984542596 (accessed on 4 January 2024).
- Kim, J.S.; Cacialli, F.; Cola, A.; Gigli, G.; Cingolani, R. Increase of Charge Carriers Density and Reduction of Hall Mobilities in Oxygen-Plasma Treated Indium–Tin–Oxide Anodes. Appl. Phys. Lett. 1999, 75, 19–21. [Google Scholar] [CrossRef]
- Novikov, S.N.; Timoshenkov, S.P. Measurements of Contact Potential Difference (Work Functions) of Metals and Semiconductors Surface by the Static Ionized Capacitor Method. Adv. Colloid Interface Sci. 2003, 105, 329–339. [Google Scholar] [CrossRef]
- Deng, X.; Nie, R.; Li, A.; Wei, H.; Zheng, S.; Huang, W.; Mo, Y.; Su, Y.; Wang, Q.; Li, Y.; et al. Ultra-Low Work Function Transparent Electrodes Achieved by Naturally Occurring Biomaterials for Organic Optoelectronic Devices. Adv. Mater. Interfaces 2014, 1, 1400215. [Google Scholar] [CrossRef]
- He, K.; Yang, X.; Yan, H.; Wu, Z.; Li, Z.; Zhong, S.; Ou, Q.; Liang, R. Work Function Changes of Plasma Treated Indium Tin Oxide. Org. Electron. 2014, 15, 1731–1737. [Google Scholar] [CrossRef]
- Zheng, Z.; Zu, X.; Zhang, Y.; Zhou, W. Rational Design of Type-II Nano-Heterojunctions for Nanoscale Optoelectronics. Mater. Today Phys. 2020, 15, 100262. [Google Scholar] [CrossRef]
- Zhang, L.; Jaroniec, M. Toward Designing Semiconductor-Semiconductor Heterojunctions for Photocatalytic Applications. Appl. Surf. Sci. 2018, 430, 2–17. [Google Scholar] [CrossRef]
- Lattante, S. Electron and Hole Transport Layers: Their Use in Inverted Bulk Heterojunction Polymer Solar Cells. Electronics 2014, 3, 132–164. [Google Scholar] [CrossRef]
- Yu, Z.; Perera, I.R.; Daeneke, T.; Makuta, S.; Tachibana, Y.; Jasieniak, J.J.; Mishra, A.; Bäuerle, P.; Spiccia, L.; Bach, U. Indium Tin Oxide as a Semiconductor Material in Efficient P-Type Dye-Sensitized Solar Cells. NPG Asia Mater. 2016, 8, e305. [Google Scholar] [CrossRef]
- Sundaram, K.B.; Khan, A. Work Function Determination of Zinc Oxide Films. J. Vac. Sci. Technol. A Vac. Surf. Film. 1997, 15, 428–430. [Google Scholar] [CrossRef]
- Mishra, S.K.; Srivastava, R.K.; Prakash, S.G. ZnO Nanoparticles: Structural, Optical and Photoconductivity Characteristics. J. Alloys Compd. 2012, 539, 1–6. [Google Scholar] [CrossRef]
- Hussain, B.; Aslam, A.; Khan, T.; Creighton, M.; Zohuri, B. Electron Affinity and Bandgap Optimization of Zinc Oxide for Improved Performance of ZnO/Si Heterojunction Solar Cell Using PC1D Simulations. Electronics 2019, 8, 238. [Google Scholar] [CrossRef]
- Ellmer, K. Electrical Properties. In Transparent Conductive Zinc Oxide. Basics and Applications in Thin Film Solar Cells; Ellmer, K., Klein, A., Rech, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 35–78. ISBN 978-3-540-73612-7. [Google Scholar]
- Maragliano, C.; Lilliu, S.; Dahlem, M.S.; Chiesa, M.; Souier, T.; Stefancich, M. Quantifying Charge Carrier Concentration in ZnO Thin Films by Scanning Kelvin Probe Microscopy. Sci. Rep. 2014, 4, 4203. [Google Scholar] [CrossRef]
- Huang, J.; Yin, Z.; Zheng, Q. Applications of ZnO in Organic and Hybrid Solar Cells. Energy Environ. Sci. 2011, 4, 3861–3877. [Google Scholar] [CrossRef]



| NPs | λsh (nm) | Hydrodynamic Diameter (d(H)) (nm) | Viscosity at 20 °C (cP) | Weight Loss (a,b) (%) | |
|---|---|---|---|---|---|
| After Synthesis (a) | Final Ink | ||||
| b-ZnO | 350 | 15.8 ± 1.2 | 14.5 ± 1.2 | 3 ± 1 | 5.2 ± 0.2 |
| r-ZnO | 338 | 11.0 ± 1.3 | 7.2 ± 1.3 | 3 ± 1 | 8.9 ± 0.3 |
| Ref-ZnO | 333 | 19.5 ± 1.3 | 6.4 ± 1.3 | 3 ± 1 | 9.2 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albonetti, C.; Alkarsifi, R.; El Qacemi, V.; Dhuiege, B.; Ruani, G.; Seri, M. Recycled or Bio-Based Solvents for the Synthesis of ZnO Nanoparticles: Characterization and Validation in Organic Solar Cells. Materials 2024, 17, 1332. https://doi.org/10.3390/ma17061332
Albonetti C, Alkarsifi R, El Qacemi V, Dhuiege B, Ruani G, Seri M. Recycled or Bio-Based Solvents for the Synthesis of ZnO Nanoparticles: Characterization and Validation in Organic Solar Cells. Materials. 2024; 17(6):1332. https://doi.org/10.3390/ma17061332
Chicago/Turabian StyleAlbonetti, Cristiano, Riva Alkarsifi, Virginie El Qacemi, Benjamin Dhuiege, Giampiero Ruani, and Mirko Seri. 2024. "Recycled or Bio-Based Solvents for the Synthesis of ZnO Nanoparticles: Characterization and Validation in Organic Solar Cells" Materials 17, no. 6: 1332. https://doi.org/10.3390/ma17061332
APA StyleAlbonetti, C., Alkarsifi, R., El Qacemi, V., Dhuiege, B., Ruani, G., & Seri, M. (2024). Recycled or Bio-Based Solvents for the Synthesis of ZnO Nanoparticles: Characterization and Validation in Organic Solar Cells. Materials, 17(6), 1332. https://doi.org/10.3390/ma17061332







