Effect of Al/Ni Ratio on the Microstructure and Properties of Nickel–Aluminum Bronze Alloys
Abstract
1. Introduction
2. Experimentation
2.1. Material Preparation
2.2. Experimental Methods
3. Results and Discussion
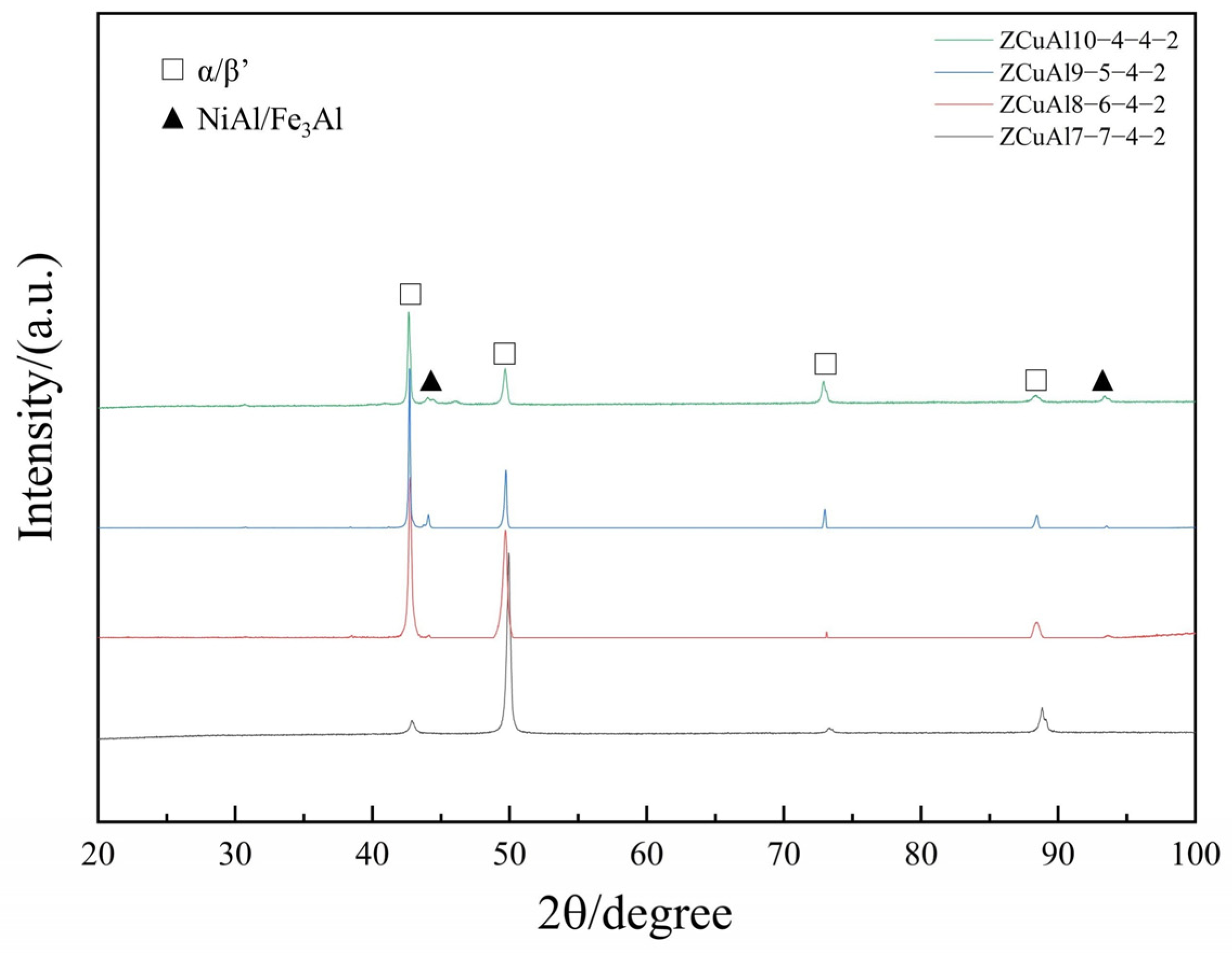
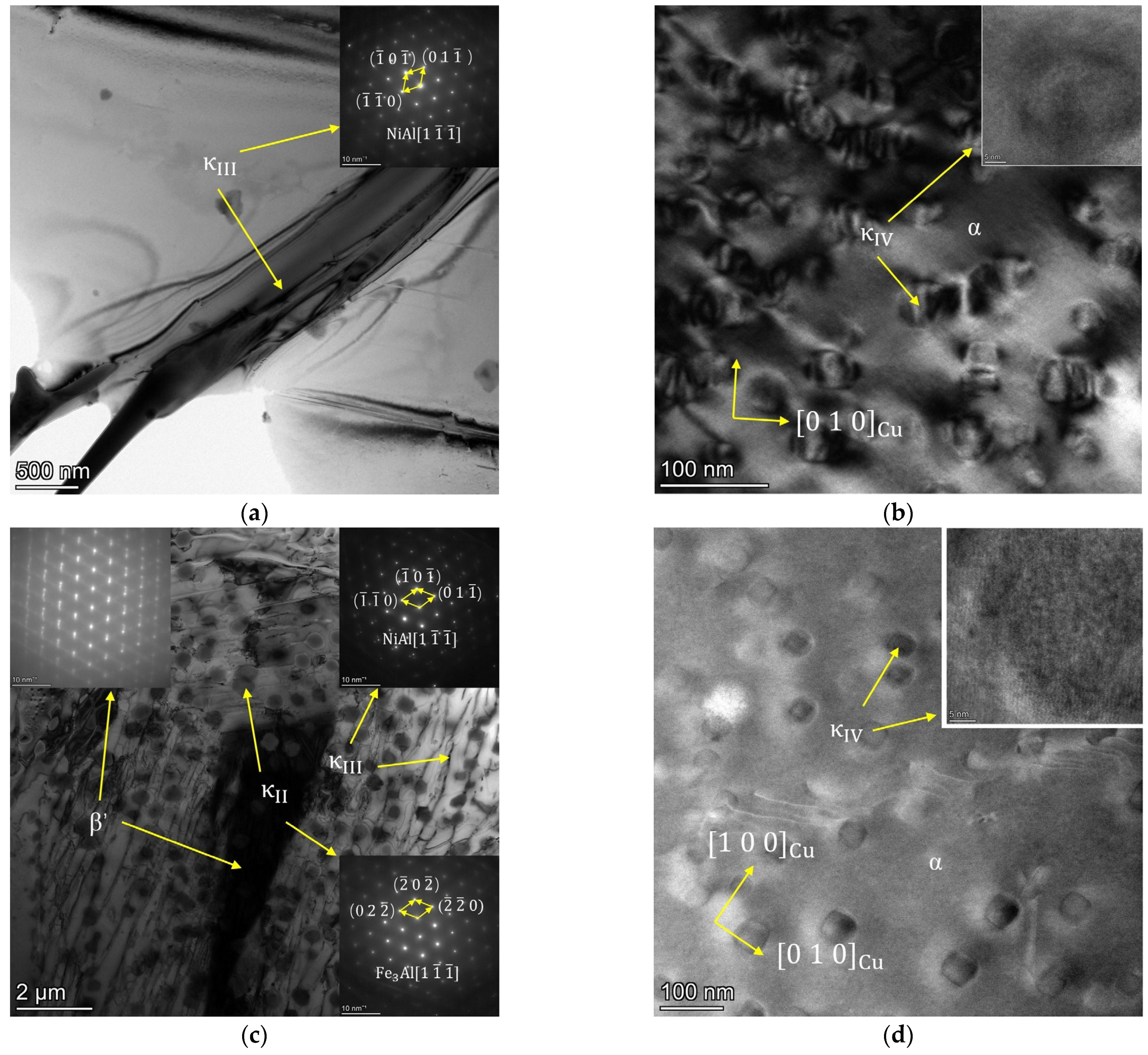
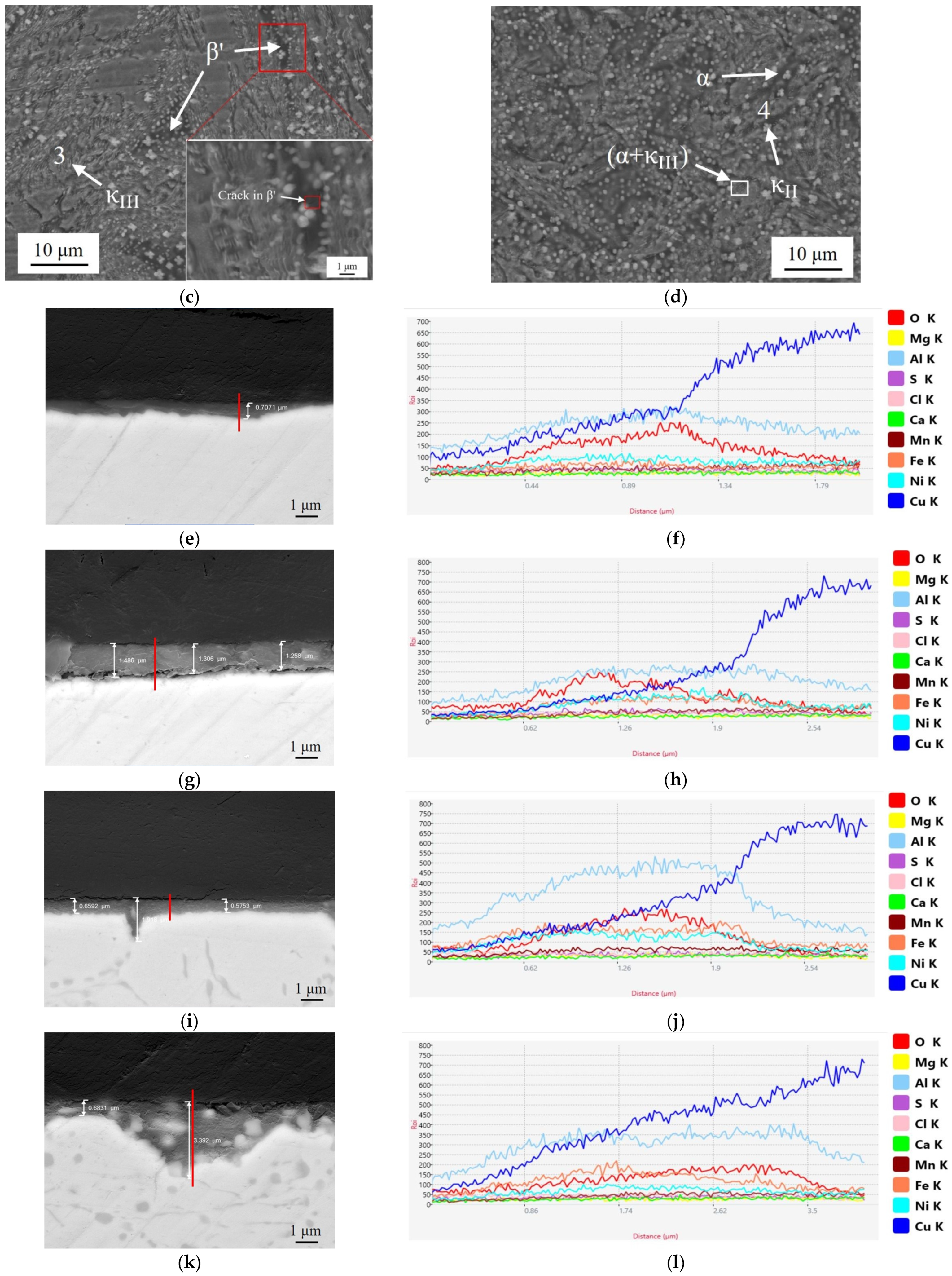
3.1. Microstructure Observation
3.2. Mechanical Testing
3.3. Static Immersion Corrosion
3.4. Electrochemical Testing
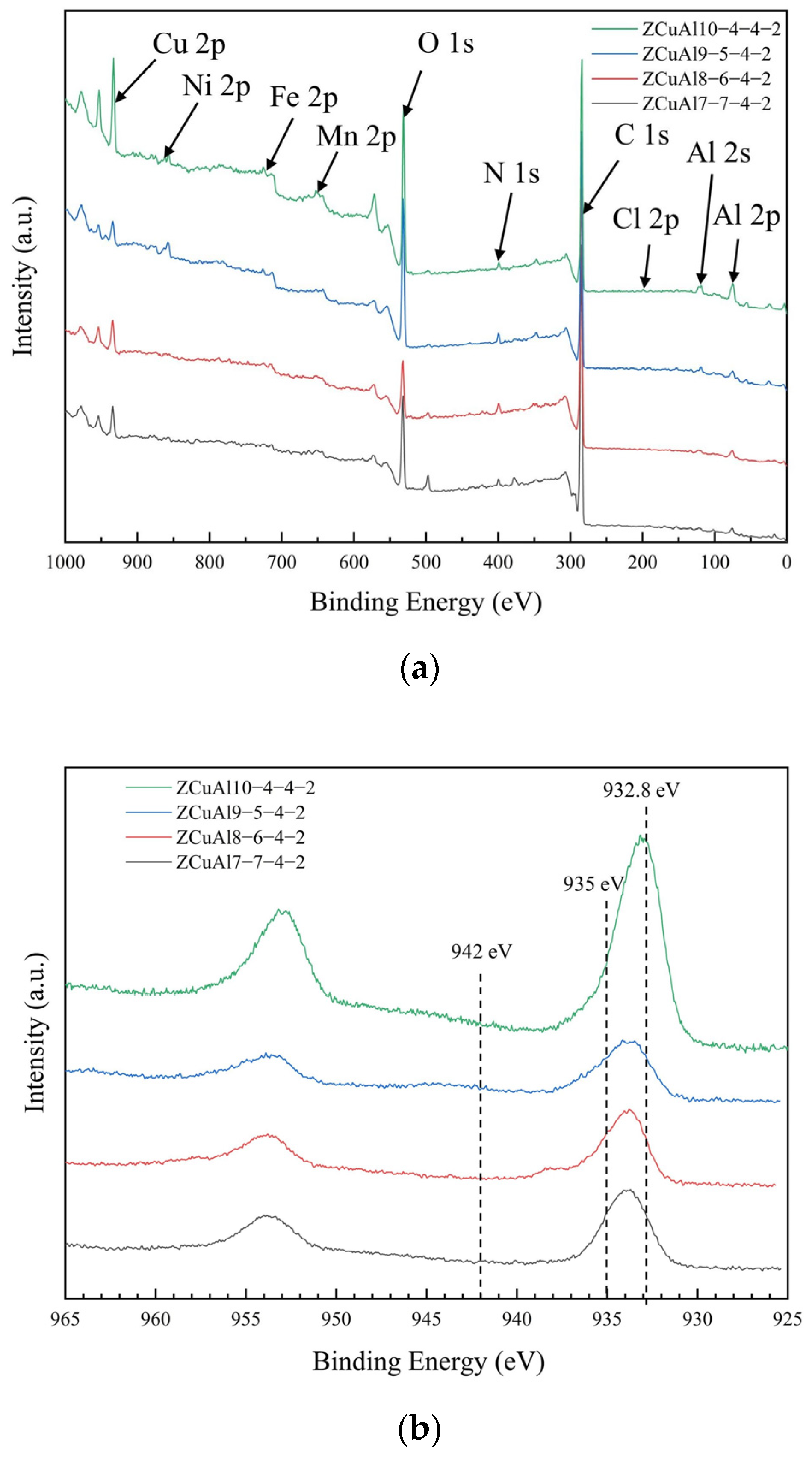
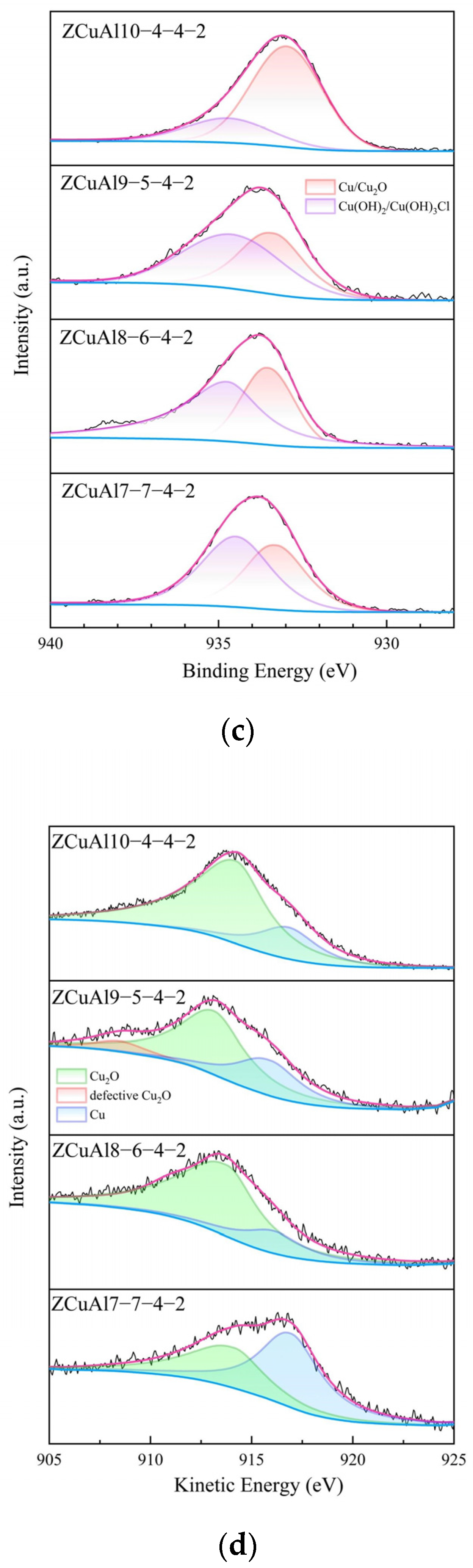
3.5. Corrosion Products and Oxide Films
4. Conclusions
- The morphology and amount of precipitated phase of cast NAB are greatly affected by the Al/Ni ratio in the composition. As the Al/Ni ratio rises from 1 to 2.5, the β′ begins to appear and increases, and the lamellar κIII and globular, petal-like κII precipitate sequentially, with an increase in the content of precipitated phases.
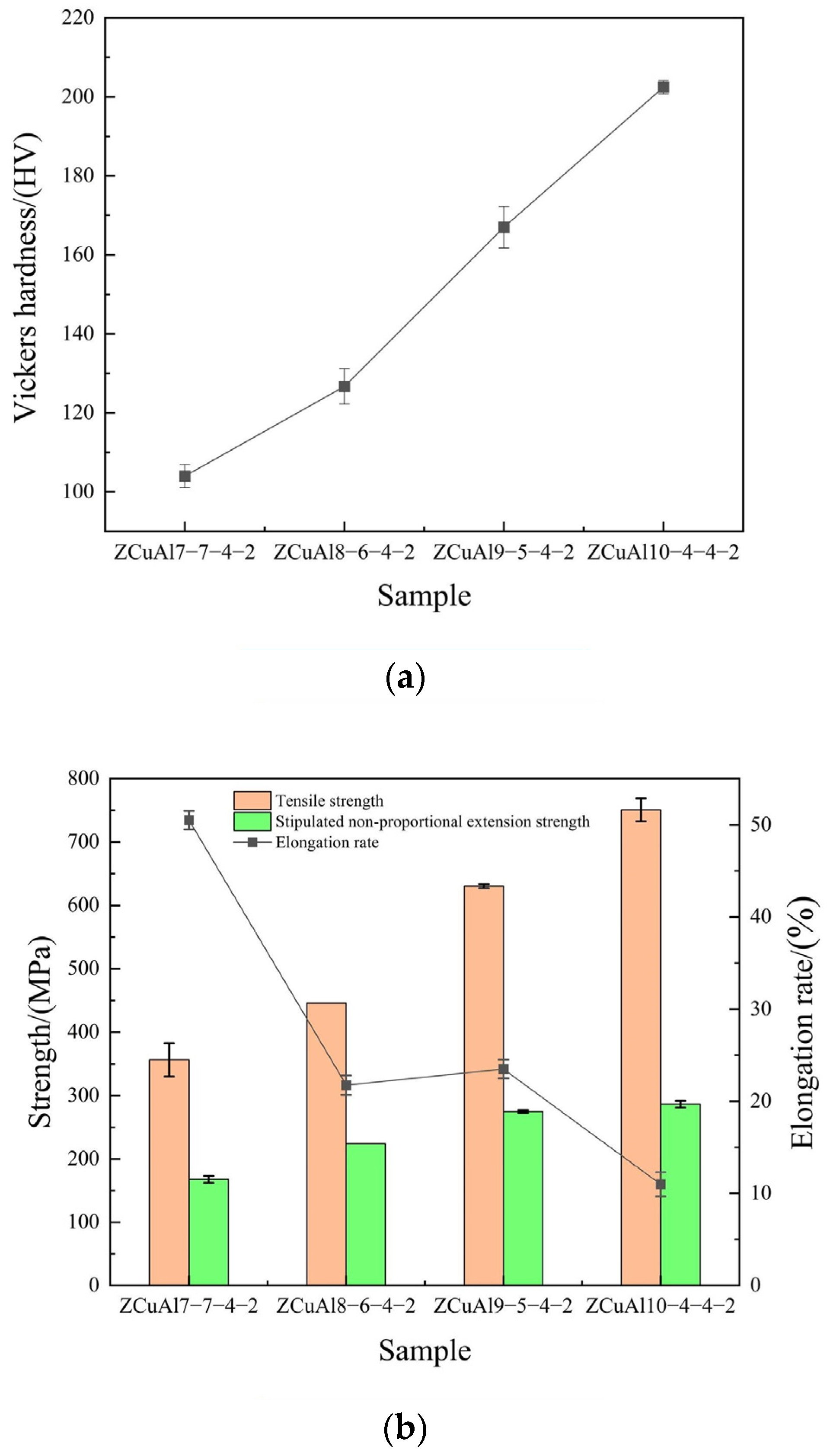
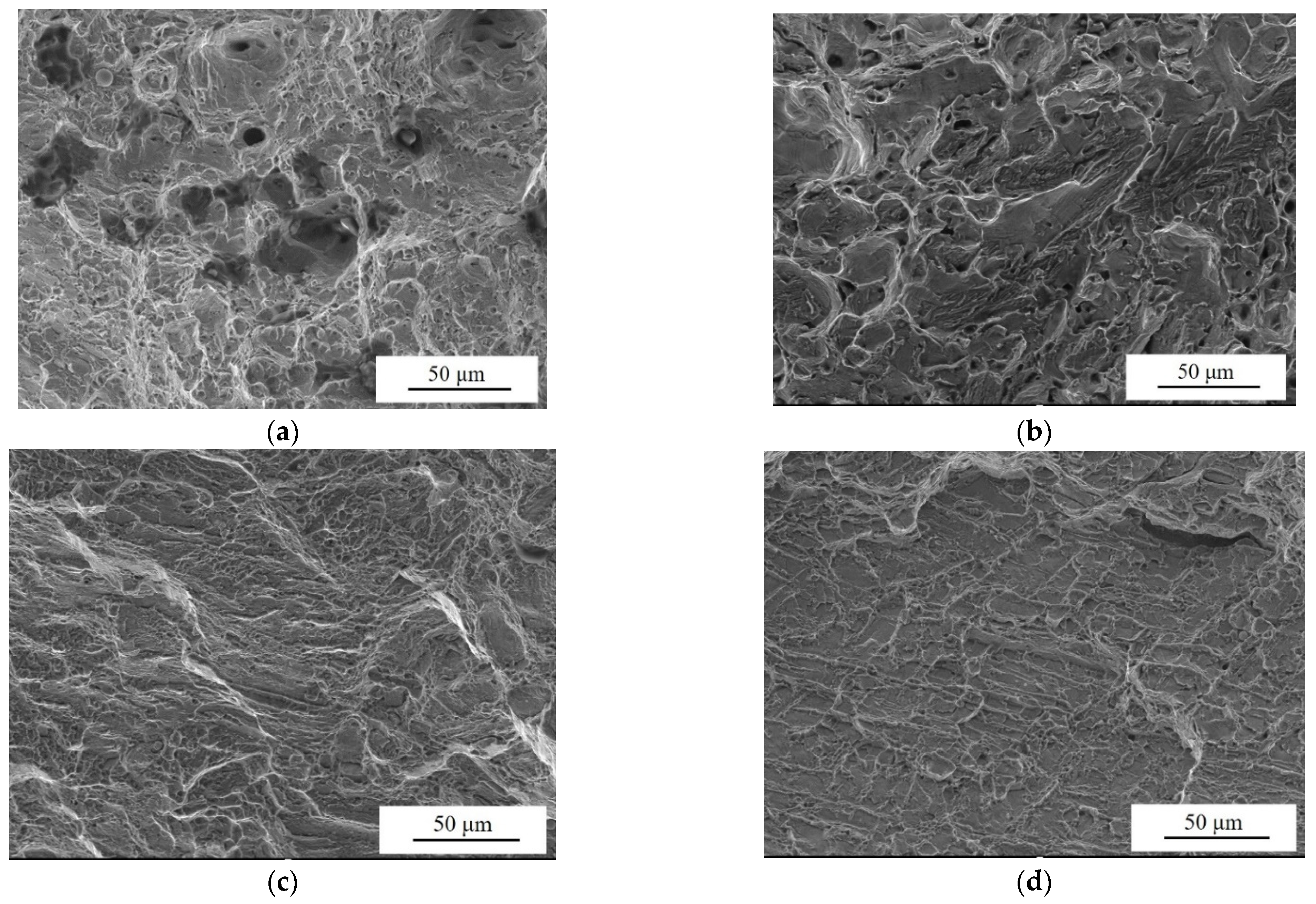
- The Al/Ni ratio can be increased to improve the hardness and strength of the NAB, respectively, from 104.02 HV to 202.46 HV and 356.33 MPa to 750.67 MPa, with an increase of 94.64% and 110.67%. However, the elongation decreases from 50.50% to 11.00%, and the fracture mode of NAB shifts from ductile fracture to brittle fracture, with plasticity deteriorating.
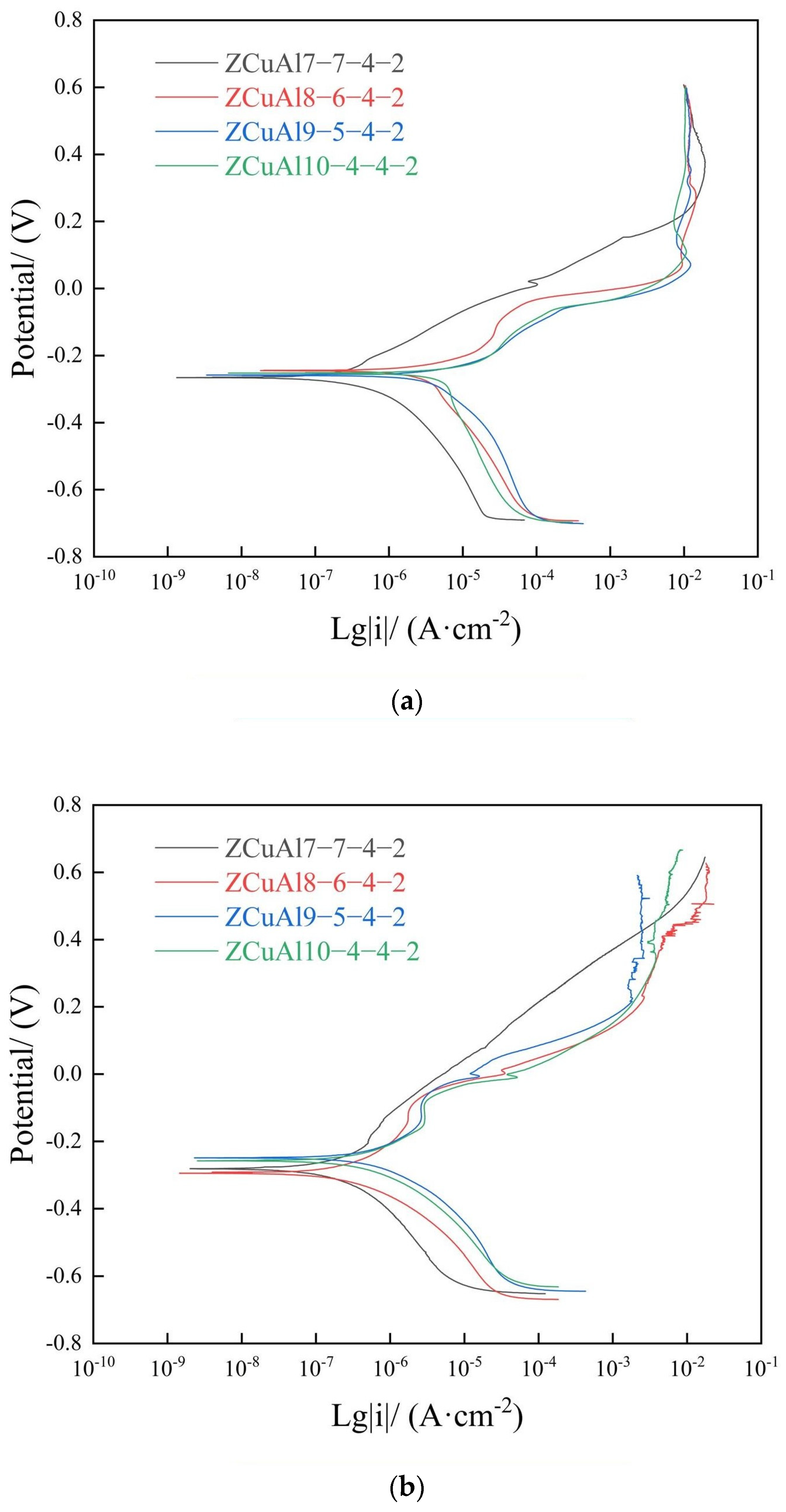
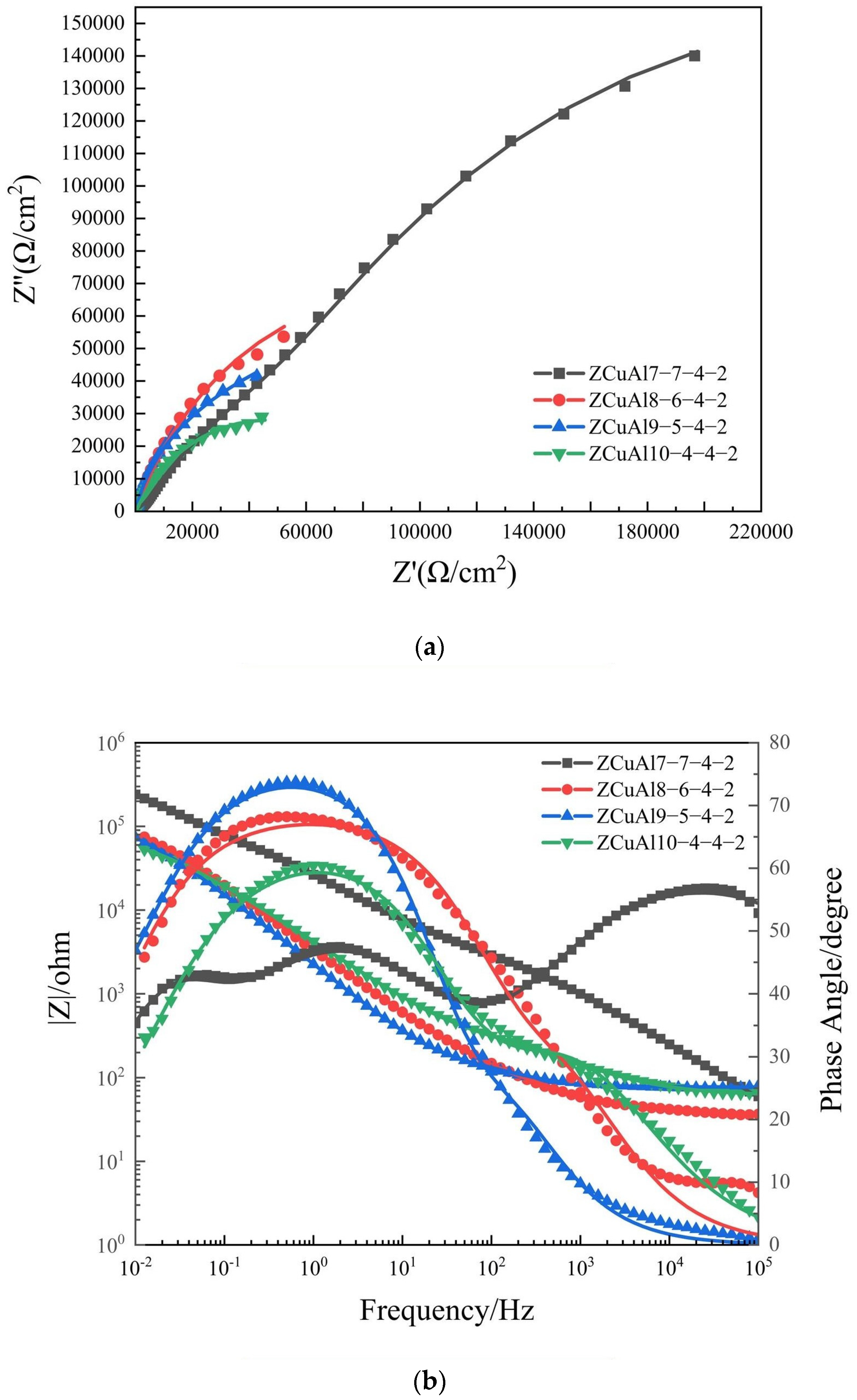
- Under static immersion corrosion in 3.5 wt.% NaCl solution for the same days, the corrosion rates first increase gradually and then approach unanimity, with an increase in the Al/Ni ratio. With a longer corrosion time, the corrosion rate of NAB decreases steadily. Through the dynamic potential polarization curves testing and EIS, it can be seen that with the increase of the Al/Ni ratio, the self-corrosion current density increases while the oxide film resistance Rp decreases. The |Z| value also decreases, which indicates that the corrosion resistance deteriorates.
- In the corrosion process of neutral NaCl solution, NAB has an obvious phenomenon of selective corrosion. Cl− in the neutral solution first reacts with the Al in the κ, which has a lower potential. It eventually hydrolyzes to form dense Al2O3 attached to the surface of the κ, which protects the κ from further corrosion, leading to an increase in the potential of the κ at this time. Subsequently, Cl− reacts with β′, which has the lowest potential now, and then reacts with matrix α. It eventually hydrolyzes to form the protective Cu2O. The final oxide film thus appears as Al2O3 on the inner layer and Cu2O on the outer layer. Through XPS, it can be found that with the increase of the Al/Ni ratio, the phenomenon of selective corrosion becomes more and more serious, and the content of Cu+ in the oxide film gradually increases.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arcos, C.; Ramos-Grez, J.; Sancy, M.; La Fé-Perdomo, I.; Setchi, R.; Guerra, C. Suitability of nickel aluminium bronze alloy fabricated by laser powder bed fusion to be used in the marine environment. Corros. Sci. 2024, 226, 111656. [Google Scholar] [CrossRef]
- GB/T 1176-2013; Casting Copper and Copper Alloys. Standardization Administration of China: Beijing, China, 2013.
- Wu, Z.; Cheng, Y.F.; Liu, L.; Lv, W.; Hu, W. Effect of heat treatment on microstructure evolution and erosion–corrosion behavior of a nickel–aluminum bronze alloy in chloride solution. Corros. Sci. 2015, 98, 260–270. [Google Scholar] [CrossRef]
- Hasan, F.; Lorimer, G.W.; Ridley, N. Crystallography of martensite in a Cu-10Al-5Ni-5Fe alloy. Le J. Phys. Colloq. 1982, 43, C4-653–C4-658. [Google Scholar] [CrossRef]
- Wharton, J.; Barik, R.; Kear, G.; Wood, R.; Stokes, K.; Walsh, F. The corrosion of nickel-aluminium bronze in seawater. Corros. Sci. 2005, 47, 3336–3367. [Google Scholar] [CrossRef]
- Hasan, F.; Jahanafrooz, A.; Lorimer, G.W.; Ridley, N. The morphology, crystallography, and chemistry of phases in as-cast nickel-aluminum bronze. Metall. Trans. A 1982, 13, 1337–1345. [Google Scholar] [CrossRef]
- Thossatheppitak, B.; Suranuntchai, S.; Uthaisangsuk, V.; Manonukul, A.; Mungsuntisuk, P. Mechanical Properties at High Temperatures and Microstructures of a Nickel Aluminum Bronze Alloy. Adv. Mater. Res. 2013, 683, 82–89. [Google Scholar] [CrossRef]
- Nelson, E.A. Microstructural Effects of Multiple Passes during Friction Stir Processing of Nickel-Aluminium Bronze. Ph.D. Thesis, Naval Postgraduate School, Monterey, CA, USA, 2009. [Google Scholar]
- Yuan, J.; Xiaofei, H.; Rong, Y.; Mingfang, L.; Gang, X. Effect of aluminium content on salt water corrosion resistance of nickel-aluminium bronze alloys. Corros. Prot. 2020, 41, 7. [Google Scholar] [CrossRef]
- Yang, F.; Kang, H.; Chen, Z.; Guo, E.; Zeng, Y.; Wang, W.; Wang, T. Electrochemical corrosion mechanisms of nickel-aluminium bronze with different nickel contents using the rotating disc electrode. Corros. Sci. 2019, 157, 438–449. [Google Scholar] [CrossRef]
- Meigh, H.J. Cast and Wrought Aluminium Bronzes-Properties, Processes and Structure; Institute of Materials: London, UK, 2000. [Google Scholar]
- Saud, S.N.; Hamzah, E.; Abubakar, T.; Bakhsheshi-Rad, H.R.; Zamri, M.; Tanemura, M. Effects of Mn Additions on the Structure, Mechanical Properties, and Corrosion Behavior of Cu-Al-Ni Shape Memory Alloys. J. Mater. Eng. Perform. 2014, 23, 3620–3629. [Google Scholar] [CrossRef]
- Zhang, X.-M.; Chen, Z.-Y.; Luo, H.-F.; Zhou, T.; Zhao, Y.-L.; Ling, Z.-C. Corrosion resistances of metallic materials in environments containing chloride ions: A review. Trans. Nonferrous Met. Soc. China 2022, 32, 377–410. [Google Scholar] [CrossRef]
- Yang, F.; Kang, H.; Guo, E.; Li, R.; Chen, Z.; Zeng, Y.; Wang, T. The role of nickel in mechanical performance and corrosion behaviour of nickel-aluminium bronze in 3.5 wt.% NaCl solution. Corros. Sci. 2018, 139, 333–345. [Google Scholar] [CrossRef]
- Basumatary, J.; Wood, R. Synergistic effects of cavitation erosion and corrosion for nickel aluminium bronze with oxide film in 3.5% NaCl solution. Wear 2017, 377, 1286–1297. [Google Scholar] [CrossRef]
- Tavares, S.; Mota, N.; da Igreja, H.; Barbosa, C.; Pardal, J. Microstructure, mechanical properties, and brittle fracture of a cast nickel-aluminum-bronze (NAB) UNS C95800. Eng. Fail. Anal. 2021, 128, 105606. [Google Scholar] [CrossRef]
- Thapliyal, S.; Dwivedi, D.K. On cavitation erosion behavior of friction stir processed surface of cast nickel aluminium bronze. Wear 2017, 376, 1030–1042. [Google Scholar] [CrossRef]
- GB/T 228-2002; Metallic Materials—Tensile Testing at Ambient Temperature. Standardization Administration of China: Beijing, China, 2002.
- Luo, Q.; Qin, Z.; Wu, Z.; Shen, B.; Liu, L.; Hu, W. The corrosion behavior of Ni-Cu gradient layer on the nickel aluminum-bronze (NAB) alloy. Corros. Sci. 2018, 138, 8–19. [Google Scholar] [CrossRef]
- Anantapong, J.; Uthaisangsuk, V.; Suranuntchai, S.; Manonukul, A. Effect of hot working on microstructure evolution of as-cast Nickel Aluminum Bronze alloy. Mater. Des. 2014, 60, 233–243. [Google Scholar] [CrossRef]
- Dharmendra, C.; Hadadzadeh, A.; Amirkhiz, B.; Ram, G.J.; Mohammadi, M. Microstructural evolution and mechanical behavior of nickel aluminum bronze Cu-9Al-4Fe-4Ni-1Mn fabricated through wire-arc additive manufacturing. Addit. Manuf. 2019, 30, 100872. [Google Scholar] [CrossRef]
- JB/T 7901-2001; Metals Materials—Uniform Corrosion—Methods of Laboratory Immersion Testing. Standardization Administration of China: Beijing, China, 2001.
- Wang, W.; Zhang, W.; Liu, Y.; Zou, J.; Mi, X.; Li, D.; Li, K.; Huang, G. Tailoring microstructure of a nickel aluminium bronze by hot extrusion and its impact on mechanical and corrosion behaviour. Corros. Sci. 2023, 215, 111049. [Google Scholar] [CrossRef]
- Huttunen-Saarivirta, E.; Isotahdon, E.; Metsäjoki, J.; Salminen, T.; Carpén, L.; Ronkainen, H. Tribocorrosion behaviour of aluminium bronze in 3.5 wt.% NaCl solution. Corros. Sci. 2018, 144, 207–223. [Google Scholar] [CrossRef]
- Nair, S.; Sellamuthu, R.; Saravanan, R. Effect of Nickel content on hardness and wear rate of surface modified cast aluminum bronze alloy. Mater. Today Proc. 2018, 5, 6617–6625. [Google Scholar] [CrossRef]
- Barik, R.; Wharton, J.; Wood, R.; Stokes, K. Further studies into the flow corrosion cathodic mass transfer kinetics of copper and nickel-aluminium bronze wall-jet electrodes. Corros. Sci. 2020, 170, 108660. [Google Scholar] [CrossRef]
- Qin, Z.; Zhang, Q.; Luo, Q.; Wu, Z.; Shen, B.; Liu, L.; Hu, W. Microstructure design to improve the corrosion and cavitation corrosion resistance of a nickel-aluminum bronze. Corros. Sci. 2018, 139, 255–266. [Google Scholar] [CrossRef]
- Song, Q.; Zheng, Y.; Ni, D.; Ma, Z. Studies of the nobility of phases using scanning Kelvin probe microscopy and its relationship to corrosion behaviour of Ni–Al bronze in chloride media. Corros. Sci. 2015, 92, 95–103. [Google Scholar] [CrossRef]
- Drach, A.; Tsukrov, I.; DeCew, J.; Aufrecht, J.; Grohbauer, A.; Hofmann, U. Field studies of corrosion behaviour of copper alloys in natural seawater. Corros. Sci. 2013, 10, 453–464. [Google Scholar] [CrossRef]
- Pidaparti, R.M.; Aghazadeh, B.S.; Whitfield, A.; Rao, A.; Mercier, G.P. Classification of corrosion defects in NiAl bronze through image analysis. Corros. Sci. 2010, 52, 3661–3666. [Google Scholar] [CrossRef]
- Schüssler, A.; Exner, H. The corrosion of nickel-aluminium bronzes in seawater—I. Protective layer formation and the passivation mechanism. Corros. Sci. 1993, 34, 1793–1802. [Google Scholar] [CrossRef]
- Seah, M.P.; Gilmore, I.S.; Beamson, G. XPS: Binding energy calibration of electron spectrometers 5—Re-evaluation of the reference energies. Surf. Interface Anal. 1998, 26, 642–649. [Google Scholar] [CrossRef]
- Losev, A.; Rostov, K.; Tyuliev, G. Electron beam induced reduction of CuO in the presence of a surface carbonaceous layer: An XPS/HREELS study. Surf. Sci. 1989, 213, 564–579. [Google Scholar] [CrossRef]
- Moulder, J.F.; Chastain, J.; King, R.C. Handbook of x-ray photoelectron spectroscopy: A reference book of standard spectra for identification and interpretation of XPS data. Chem. Phys. Lett. 1992, 220, 7–10. [Google Scholar]
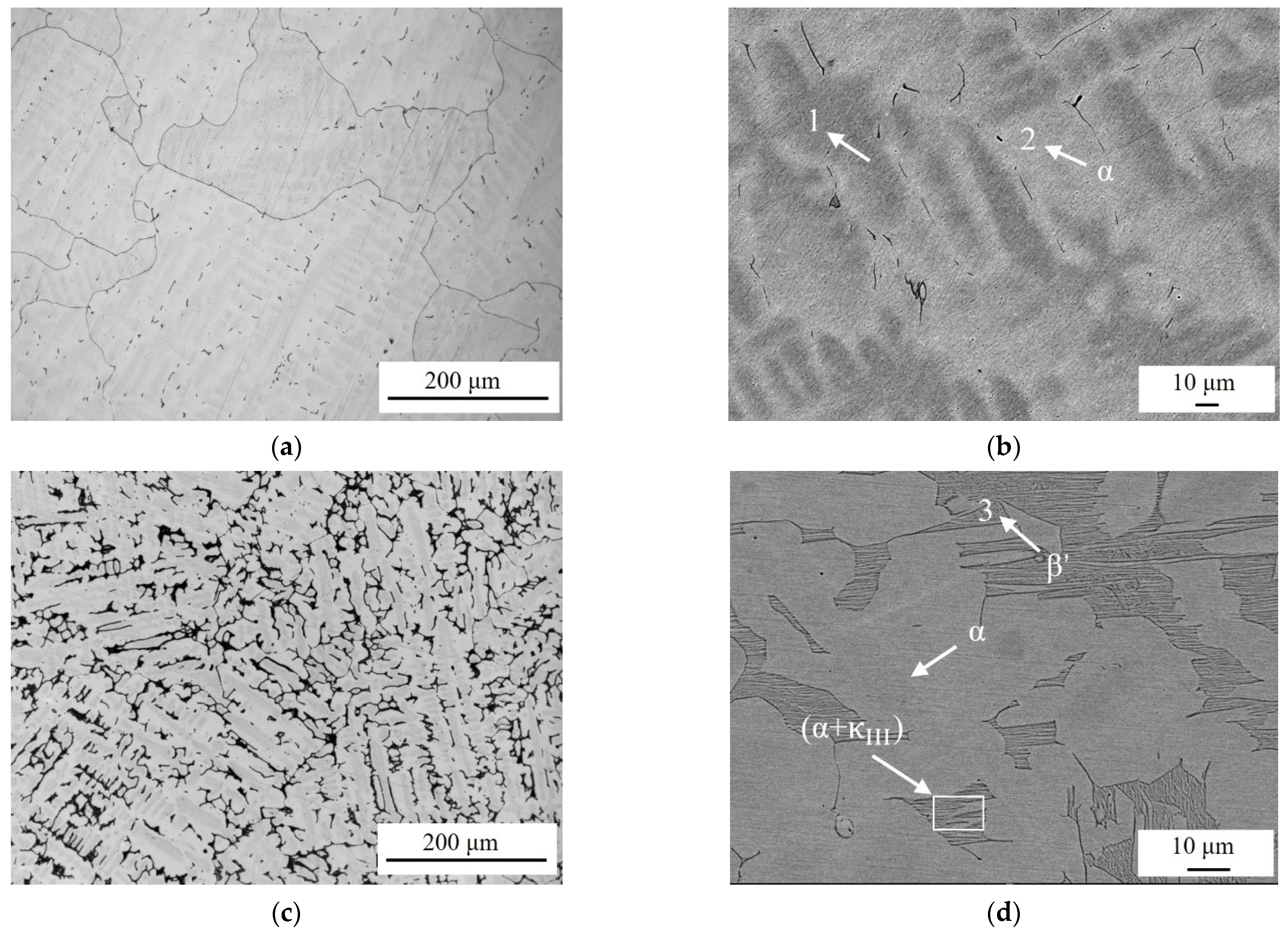
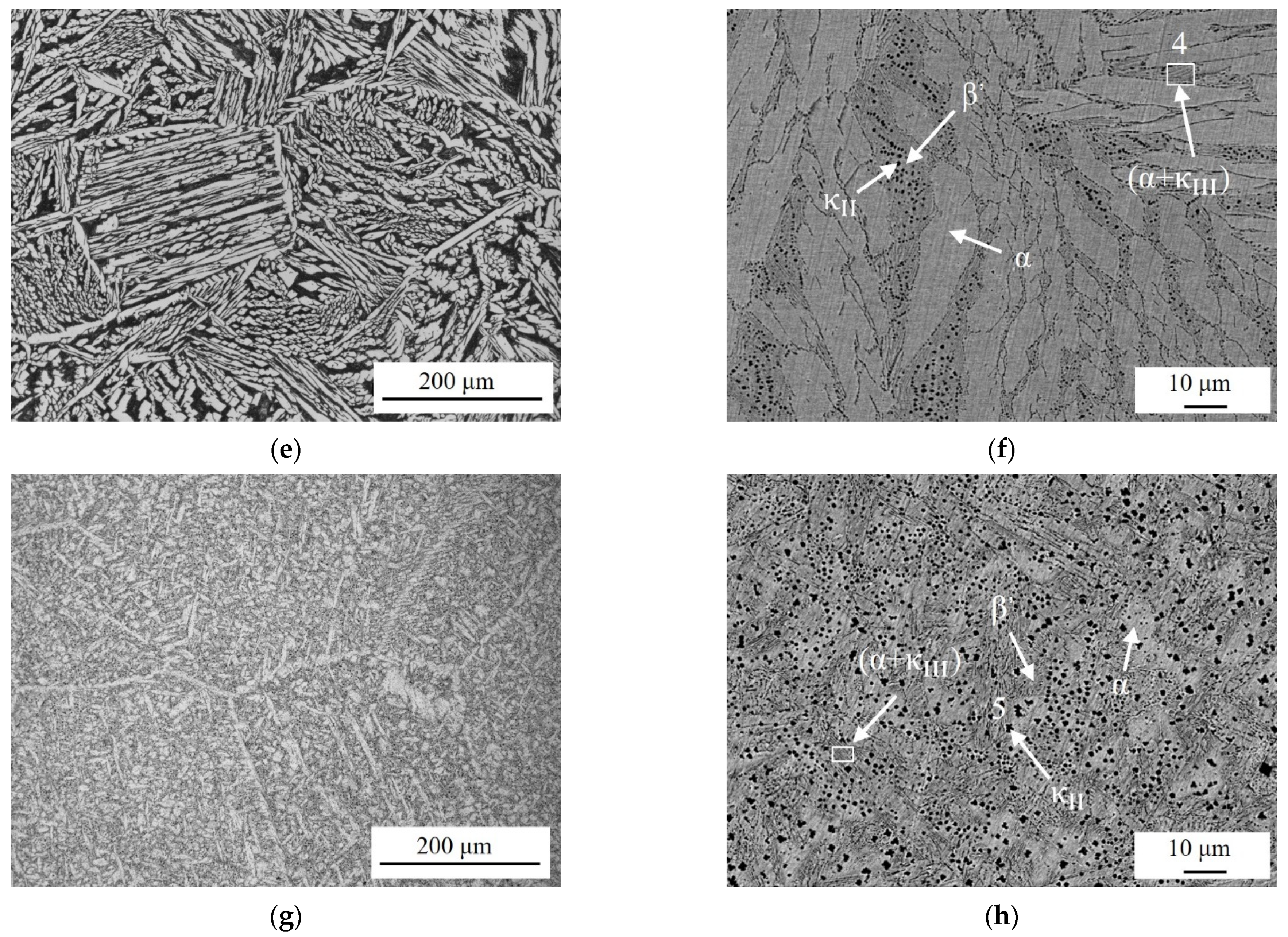




| Alloy | Al(wt.%) | Ni(wt.%) | Fe(wt.%) | Mn(wt.%) | Cu(wt.%) | Al/Ni Ratio |
|---|---|---|---|---|---|---|
| ZCuAl7-7-4-2 | 6.94 | 6.82 | 4.10 | 1.72 | Bal. | 1.0 |
| ZCuAl8-6-4-2 | 7.90 | 6.13 | 3.92 | 2.12 | Bal. | 1.3 |
| ZCuAl9-5-4-2 | 8.94 | 5.18 | 4.12 | 2.18 | Bal. | 1.8 |
| ZCuAl10-4-4-2 | 9.94 | 3.99 | 3.94 | 1.82 | Bal. | 2.5 |
| Phase | Al(wt.%) | Ni(wt.%) | Fe(wt.%) | Mn(wt.%) | Cu(wt.%) |
|---|---|---|---|---|---|
| 1 | 8.88 | 7.81 | 5.46 | 1.79 | 76.06 |
| 2(α) | 6.09 | 7.66 | 4.09 | 2.06 | 80.10 |
| 3(β′) | 9.51 | 6.40 | 2.97 | 2.26 | 78.86 |
| 4() | 13.15 | 11.62 | 9.96 | 2.44 | 62.83 |
| 5() | 16.11 | 9.51 | 44.02 | 3.65 | 26.71 |
| Sample | Exposure Time (D) | ba (mV/Dec) | bc (mV/Dec) | Ecorr (mV) | icorr (μA/cm2) | Rp (×103Ω∙cm2) |
|---|---|---|---|---|---|---|
| ZCuAl7-7-4-2 | 10D | 199.19 | −165.66 | −265.72 | 0.50 | 78.12 |
| ZCuAl8-6-4-2 | 10D | 77.53 | −182.92 | −245.06 | 1.34 | 17.68 |
| ZCuAl9-5-4-2 | 10D | 72.74 | −157.85 | −258.23 | 1.77 | 12.20 |
| ZCuAl10-4-4-2 | 10D | 73.25 | −220.34 | −252.73 | 2.16 | 11.07 |
| ZCuAl7-7-4-2 | 30D | 327.22 | −244.19 | −281.48 | 0.35 | 173.48 |
| ZCuAl8-6-4-2 | 30D | 201.92 | −140.96 | −295.55 | 0.36 | 100.12 |
| ZCuAl9-5-4-2 | 30D | 205.54 | −147.79 | −249.35 | 0.69 | 54.41 |
| ZCuAl10-4-4-2 | 30D | 211.85 | −179.19 | −257.75 | 0.78 | 54.04 |
| ZCuAl7-7-4-2 | ZCuAl8-6-4-2 | ZCuAl9-5-4-2 | ZCuAl10-4-4-2 | |
|---|---|---|---|---|
| Rs/(Ω cm2) | 5.4 | 37.3 | 77.3 | 60.8 |
| Q1/(μF cm−2 sn−1) | 2.6 | 38.5 | 47.6 | 33.0 |
| n1 | 0.662 | 0.750 | 0.820 | 0.639 |
| Rf (Rf1)/(Ω cm2) | 3967 | 134 | 144 | 407 |
| Q2/(μF cm−2 sn−1) | 8.9 | 32.9 | 45.9 | 31.5 |
| n2 | 0.625 | 0.784 | 0.877 | 0.770 |
| Rf2/(Ω cm2) | 153,900 | - | - | - |
| Q3/(μF cm−2 sn−1) | 19.2 | - | - | - |
| n3 | 0.847 | - | - | - |
| Rct/(Ω cm2) | 363,500 | 196,300 | 111,400 | 90,300 |
| Rp/(Ω cm2) | 521,367 | 196,434 | 111,544 | 90,707 |
| Wd/(×10−3 S cm−2 s1/2) | - | - | 0.12 | 1.76 |
| χ2/(×10−3) | 0.13 | 3.11 | 0.58 | 0.68 |
| Area | Al(wt.%) | Ni(wt.%) | Fe(wt.%) | Mn(wt.%) | O(wt.%) | Cl(wt.%) | Cu(wt.%) |
|---|---|---|---|---|---|---|---|
| 1 | 6.08 | 10.42 | 8.44 | 1.66 | 6.92 | 0.15 | 66.33 |
| 2(α) | 7.95 | 6.07 | 6.07 | 2.54 | 13.87 | 0.35 | 63.14 |
| 3() | 19.31 | 18.87 | 17.23 | 3.17 | 13.09 | 0.42 | 27.92 |
| 4() | 16.88 | 10.83 | 24.95 | 3.42 | 18.67 | 1.02 | 24.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Y.; Xie, H.; Feng, X.; Huang, S.; Zhang, W.; Peng, L.; Wang, W.; Zhao, Y. Effect of Al/Ni Ratio on the Microstructure and Properties of Nickel–Aluminum Bronze Alloys. Materials 2024, 17, 1330. https://doi.org/10.3390/ma17061330
Tan Y, Xie H, Feng X, Huang S, Zhang W, Peng L, Wang W, Zhao Y. Effect of Al/Ni Ratio on the Microstructure and Properties of Nickel–Aluminum Bronze Alloys. Materials. 2024; 17(6):1330. https://doi.org/10.3390/ma17061330
Chicago/Turabian StyleTan, Yinxun, Haofeng Xie, Xue Feng, Shuhui Huang, Wenjing Zhang, Lijun Peng, Wanyu Wang, and Yizhi Zhao. 2024. "Effect of Al/Ni Ratio on the Microstructure and Properties of Nickel–Aluminum Bronze Alloys" Materials 17, no. 6: 1330. https://doi.org/10.3390/ma17061330
APA StyleTan, Y., Xie, H., Feng, X., Huang, S., Zhang, W., Peng, L., Wang, W., & Zhao, Y. (2024). Effect of Al/Ni Ratio on the Microstructure and Properties of Nickel–Aluminum Bronze Alloys. Materials, 17(6), 1330. https://doi.org/10.3390/ma17061330






