Study of the Durability of Membrane Electrode Assemblies in Various Accelerated Stress Tests for Proton-Exchange Membrane Water Electrolysis
Abstract
1. Introduction
2. Experimental
2.1. Membrane Electrode Assemblies and Test Bench Setup
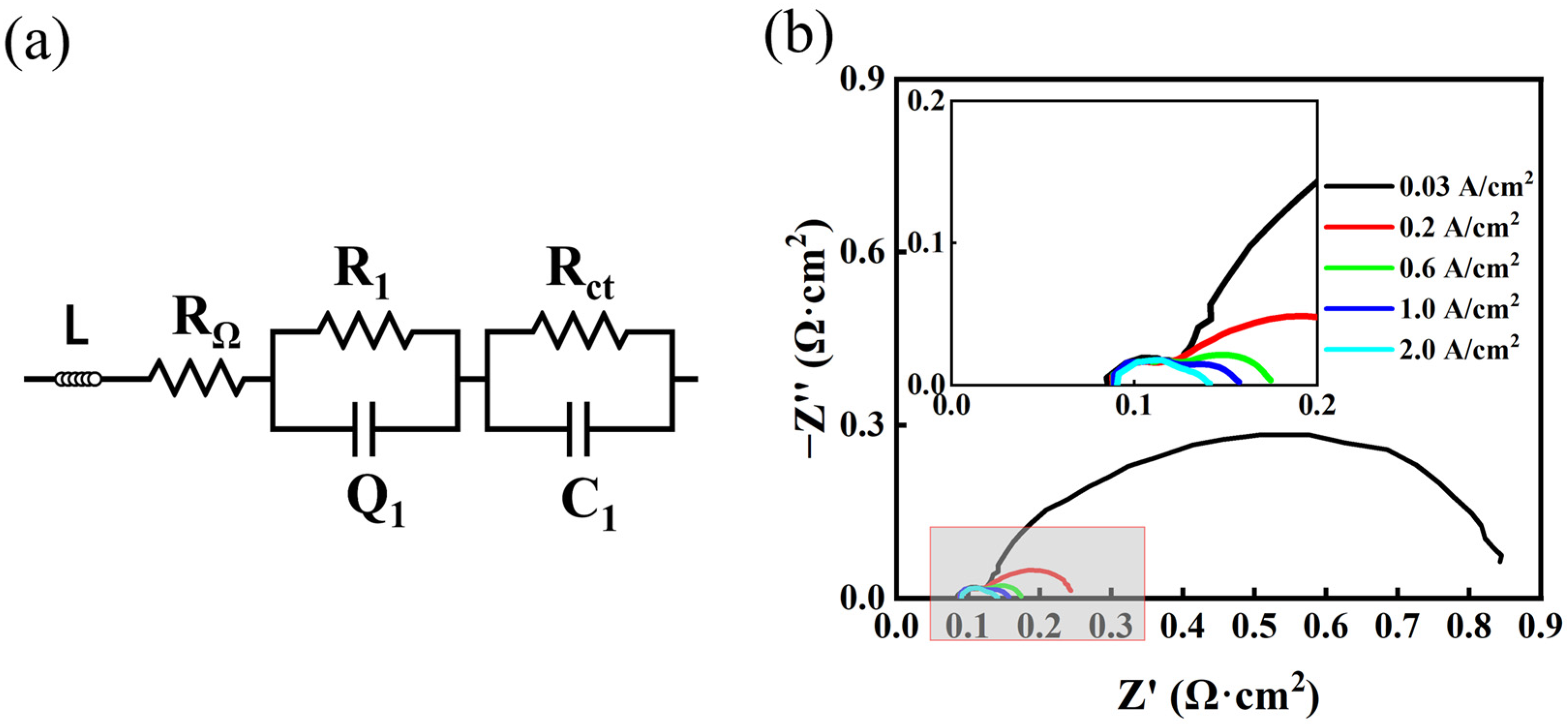
2.2. Electrochemical Measurements
- (1)
- Activation process: Before the performance test, an activation procedure was applied to the electrolyzer using ITECH’s DC power supply IT-M3110 (ITECH, Nanjing, China) to provide current, and it was carried out in the following order: 0.2 A/cm2 for 1 h, 1 A/cm2 for 1 h, 2 V for 0.5 h, 1.7 V for 2 h, and 2 V for 0.5 h.
- (2)
- Accelerated stress tests (ASTs): Three types of ASTs were performed. The first one was the constant-current mode test at 1 A/cm2, 2 A/cm2, and 3 A/cm2; The second was square-wave mode tests fluctuating in the range of 1–2 A/cm2 and 1–3 A/cm2; a cycle of the square-wave mode consisted of two parts, a current-holding part and a current-change part. In this investigation, the current-holding part was used for 28 s dwell time, while the current-varying part was used for different step times (2 s, 12 s, and 22 s). The last AST simulated a solar fluctuation, using the power generated in Qinghai, China, during 13 h of sunshine on a day in June 2022. Detailed parameters of the ASTs are displayed in Table 1, and the corresponding test waveforms are shown in Figure 1a–d.
3. Results and Discussion
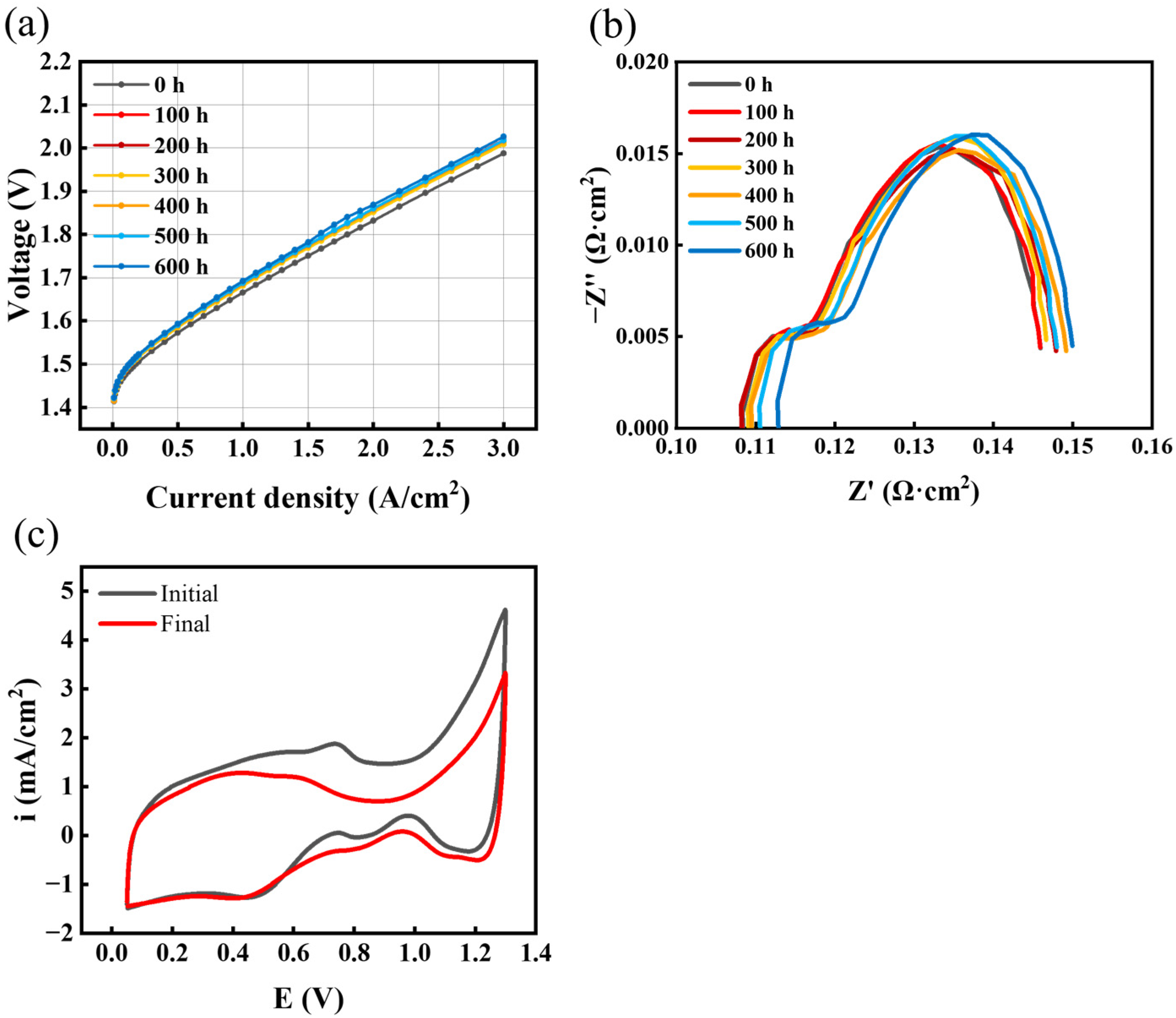
3.1. PEMWE Performance Change
3.2. Analyzing the Evolution of Resistance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ziegler, M.S.; Mueller, J.M.; Pereira, G.D.; Song, J.; Ferrara, M.; Chiang, Y.-M.; Trancik, J.E. Storage Requirements and Costs of Shaping Renewable Energy Toward Grid Decarbonization. Joule 2019, 3, 2134–2153. [Google Scholar] [CrossRef]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Kojima, H.; Nagasawa, K.; Todoroki, N.; Ito, Y.; Matsui, T.; Nakajima, R. Influence of renewable energy power fluctuations on water electrolysis for green hydrogen production. Int. J. Hydrogen Energy 2023, 48, 4572–4593. [Google Scholar] [CrossRef]
- Moradi Nafchi, F.; Baniasadi, E.; Afshari, E.; Javani, N. Performance assessment of a solar hydrogen and electricity production plant using high temperature PEM electrolyzer and energy storage. Int. J. Hydrogen Energy 2018, 43, 5820–5831. [Google Scholar] [CrossRef]
- Makhsoos, A.; Kandidayeni, M.; Pollet, B.G.; Boulon, L. A perspective on increasing the efficiency of proton exchange membrane water electrolyzers–a review. Int. J. Hydrog. Energy 2023, 48, 15341–15370. [Google Scholar] [CrossRef]
- Schnuelle, C.; Wassermann, T.; Fuhrlaender, D.; Zondervan, E. Dynamic hydrogen production from PV & wind direct electricity supply–Modeling and techno-economic assessment. Int. J. Hydrogen Energy 2020, 45, 29938–29952. [Google Scholar]
- Frensch, S.H.; Serre, G.; Fouda-Onana, F.; Jensen, H.C.; Christensen, M.L.; Araya, S.S.; Kær, S.K. Impact of iron and hydrogen peroxide on membrane degradation for polymer electrolyte membrane water electrolysis: Computational and experimental investigation on fluoride emission. J. Power Sources 2019, 420, 54–62. [Google Scholar] [CrossRef]
- Alia, S.M.; Rasimick, B.; Ngo, C.; Neyerlin, K.; Kocha, S.S.; Pylypenko, S.; Xu, H.; Pivovar, B.S. Activity and durability of iridium nanoparticles in the oxygen evolution reaction. J. Electrochem. Soc. 2016, 163, F3105–F3112. [Google Scholar] [CrossRef]
- Gago, A.S.; Ansar, A.S.; Gazdzicki, P.; Wagner, N.; Arnold, J.; Friedrich, K.A. Low cost bipolar plates for large scale PEM electrolyzers. ECS Trans. 2014, 64, 1039. [Google Scholar] [CrossRef]
- Wong, K.H.; Kjeang, E.J.C. Mitigation of chemical membrane degradation in fuel cells: Understanding the effect of cell voltage and iron ion redox cycle. ChemSusChem 2015, 8, 1072–1082. [Google Scholar] [CrossRef]
- Fonseca, C.; Barbosa, M.J.C.S. Corrosion behaviour of titanium in biofluids containing H2O2 studied by electrochemical impedance spectroscopy. Corros. Sci. 2001, 43, 547–559. [Google Scholar] [CrossRef]
- Cherevko, S.; Geiger, S.; Kasian, O.; Mingers, A.; Mayrhofer, K.J. Oxygen evolution activity and stability of iridium in acidic media. Part 2.–Electrochemically grown hydrous iridium oxide. J. Electroanal. Chem. 2016, 774, 102–110. [Google Scholar] [CrossRef]
- Lettenmeier, P.; Wang, R.; Abouatallah, R.; Helmly, S.; Morawietz, T.; Hiesgen, R.; Kolb, S.; Burggraf, F.; Kallo, J.; Gago, A. Durable membrane electrode assemblies for proton exchange membrane electrolyzer systems operating at high current densities. Electrochim. Acta 2016, 210, 502–511. [Google Scholar] [CrossRef]
- Scohy, M.; Abbou, S.; Martin, V.; Gilles, B.; Sibert, E.; Dubau, L.; Maillard, F. Probing surface oxide formation and dissolution on/of Ir single crystals via X-ray photoelectron spectroscopy and inductively coupled plasma mass spectrometry. ACS Catal. 2019, 9, 9859–9869. [Google Scholar] [CrossRef]
- Martin, A.; Trinke, P.; Stähler, M.; Stähler, A.; Scheepers, F.; Bensmann, B.; Carmo, M.; Lehnert, W.; Hanke-Rauschenbach, R. The Effect of Cell Compression and Cathode Pressure on Hydrogen Crossover in PEM Water Electrolysis. J. Electrochem. Soc. 2022, 169, 014502. [Google Scholar] [CrossRef]
- Feng, Q.; Yuan, X.Z.; Liu, G.; Wei, B.; Zhang, Z.; Li, H.; Wang, H. A review of proton exchange membrane water electrolysis on degradation mechanisms and mitigation strategies. J. Power Sources 2017, 366, 33–55. [Google Scholar] [CrossRef]
- Pham, C.V.; Escalera-López, D.; Mayrhofer, K.; Cherevko, S.; Thiele, S. Essentials of High Performance Water Electrolyzers–From Catalyst Layer Materials to Electrode Engineering. Adv. Energy Mater. 2021, 11, 2101998. [Google Scholar] [CrossRef]
- Siracusano, S.; Hodnik, N.; Jovanovic, P.; Ruiz-Zepeda, F.; Šala, M.; Baglio, V.; Aricò, A.S. New insights into the stability of a high performance nanostructured catalyst for sustainable water electrolysis. Nano Energy 2017, 40, 618–632. [Google Scholar] [CrossRef]
- Chandesris, M.; Medeau, V.; Guillet, N.; Chelghoum, S.; Thoby, D.; Fouda-Onana, F. Membrane degradation in PEM water electrolyzer: Numerical modeling and experimental evidence of the influence of temperature and current density. Int. J. Hydrogen Energy 2015, 40, 1353–1366. [Google Scholar] [CrossRef]
- Xiuying, G.; Xianming, L.; Zhuang, X.; Guangli, H.; Ping, M. Cost analysis of hydrogen production by electrolysis of renewable energy. Energy Storage Sci. Technol. 2020, 9, 688. [Google Scholar]
- Li, N.; Araya, S.S.; Kær, S.K. Investigating low and high load cycling tests as accelerated stress tests for proton exchange membrane water electrolysis. Electrochim. Acta 2021, 370, 137748. [Google Scholar] [CrossRef]
- Rakousky, C.; Reimer, U.; Wippermann, K.; Carmo, M.; Lueke, W.; Stolten, D. An analysis of degradation phenomena in polymer electrolyte membrane water electrolysis. J. Power Sources 2016, 326, 120–128. [Google Scholar] [CrossRef]
- Siracusano, S.; Trocino, S.; Briguglio, N.; Pantò, F.; Aricò, A.S. Analysis of performance degradation during steady-state and load-thermal cycles of proton exchange membrane water electrolysis cells. J. Power Sources 2020, 468, 228390. [Google Scholar] [CrossRef]
- Fouda-Onana, F.; Chandesris, M.; Médeau, V.; Chelghoum, S.; Thoby, D.; Guillet, N. Investigation on the degradation of MEAs for PEM water electrolysers part I: Effects of testing conditions on MEA performances and membrane properties. Int. J. Hydrogen Energy 2016, 41, 16627–16636. [Google Scholar] [CrossRef]
- Rakousky, C.; Reimer, U.; Wippermann, K.; Kuhri, S.; Carmo, M.; Lueke, W.; Stolten, D. Polymer electrolyte membrane water electrolysis: Restraining degradation in the presence of fluctuating power. J. Power Sources 2017, 342, 38–47. [Google Scholar] [CrossRef]
- Frensch, S.H.; Fouda-Onana, F.; Serre, G.; Thoby, D.; Araya, S.S.; Kær, S.K. Influence of the operation mode on PEM water electrolysis degradation. Int. J. Hydrogen Energy 2019, 44, 29889–29898. [Google Scholar] [CrossRef]
- Babic, U.; Tarik, M.; Schmidt, T.J.; Gubler, L. Understanding the effects of material properties and operating conditions on component aging in polymer electrolyte water electrolyzers. J. Power Sources 2020, 451, 227778. [Google Scholar] [CrossRef]
- Alia, S.M.; Stariha, S.; Borup, R.L. Electrolyzer Durability at Low Catalyst Loading and with Dynamic Operation. J. Electrochem. Soc. 2019, 166, F1164. [Google Scholar] [CrossRef]
- Böhm, D.; Beetz, M.; Gebauer, C.; Bernt, M.; Schröter, J.; Kornherr, M.; Zoller, F.; Bein, T.; Fattakhova-Rohlfing, D. Highly conductive titania supported iridium oxide nanoparticles with low overall iridium density as OER catalyst for large-scale PEM electrolysis. Appl. Mater. Today 2021, 24, 101134. [Google Scholar] [CrossRef]
- Aßmann, P.; Gago, A.S.; Gazdzicki, P.; Friedrich, K.A.; Wark, M. Toward developing accelerated stress tests for proton exchange membrane electrolyzers. Curr. Opin. Electrochem. 2020, 21, 225–233. [Google Scholar] [CrossRef]
- Ayers, K.E.; Anderson, E.B.; Capuano, C.; Carter, B.; Dalton, L.; Hanlon, G.; Manco, J.; Niedzwiecki, M. Research Advances towards Low Cost, High Efficiency PEM Electrolysis. ECS Trans. 2010, 33, 3. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Himabindu, V. Hydrogen production by PEM water electrolysis–A review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- Alia, S.M.; Reeves, K.S.; Baxter, J.S.; Cullen, D.A. The Impact of Ink and Spray Variables on Catalyst Layer Properties, Electrolyzer Performance, and Electrolyzer Durability. J. Electrochem. Soc. 2020, 167, 144512. [Google Scholar] [CrossRef]
- Alia, S.M.; Reeves, K.S.; Yu, H.; Park, J.; Kariuki, N.; Kropf, A.J.; Myers, D.J.; Cullen, D.A. Electrolyzer Performance Loss from Accelerated Stress Tests and Corresponding Changes to Catalyst Layers and Interfaces. J. Electrochem. Soc. 2022, 169, 054517. [Google Scholar] [CrossRef]
- Yuan, X.-Z.; Shaigan, N.; Song, C.; Aujla, M.; Neburchilov, V.; Kwan, J.T.H.; Wilkinson, D.P.; Bazylak, A.; Fatih, K. The porous transport layer in proton exchange membrane water electrolysis: Perspectives on a complex component. Sustain. Energy Fuels 2022, 6, 1824–1853. [Google Scholar] [CrossRef]
- Kulkarni, D.; Huynh, A.; Satjaritanun, P.; O’Brien, M.; Shimpalee, S.; Parkinson, D.; Shevchenko, P.; DeCarlo, F.; Danilovic, N.; Ayers, K.E.; et al. Elucidating effects of catalyst loadings and porous transport layer morphologies on operation of proton exchange membrane water electrolyzers. Appl. Catal. B Environ. 2022, 308, 121213. [Google Scholar] [CrossRef]
- Barsoukov, E.; Macdonald, J.R. Impedance Spectroscopy: Theory, Experiment, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Damjanovic, A. Electron transfer through thin anodic films in oxygen evolution at Pt electrodes in alkaline solutions. Electrochim. Acta 1992, 37, 2533–2539. [Google Scholar] [CrossRef]
- Siracusano, S.; Trocino, S.; Briguglio, N.; Baglio, V.; Aricò, A.S. Electrochemical impedance spectroscopy as a diagnostic tool in polymer electrolyte membrane electrolysis. Materials 2018, 11, 1368. [Google Scholar] [CrossRef]
- Moya, A.A. Electrochemical Impedance of Ion-Exchange Membranes with Interfacial Charge Transfer Resistances. J. Phys. Chem. C 2016, 120, 6543–6552. [Google Scholar] [CrossRef]
- Cai, X.; Lin, R.; Xu, J.; Lu, Y. Construction and analysis of photovoltaic directly coupled conditions in PEM electrolyzer. Int. J. Hydrogen Energy 2022, 47, 6494–6507. [Google Scholar] [CrossRef]
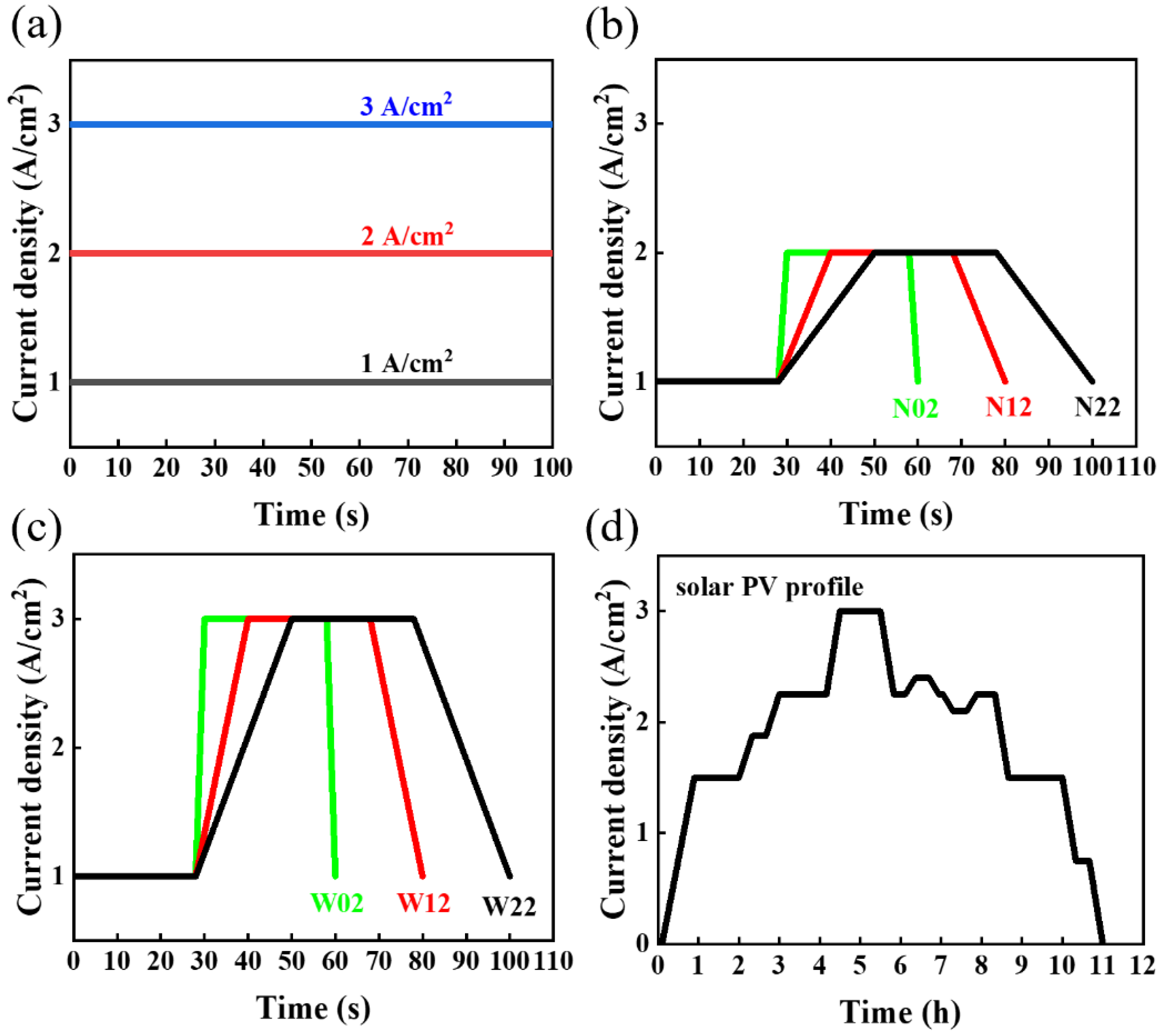


| Operation Mode | Test Condition | Mode of Change (Name) |
|---|---|---|
| Constant current | 600 h, 80 °C | 1 A/cm2 (C1) |
| 2 A/cm2 (C2) | ||
| 3 A/cm2 (C3) | ||
| Narrow square wave | 1~2 A/cm2 28 s dwell time 600 h, 80 °C | 2 s step time (N02) |
| 12 s step time (N12) | ||
| 22 s step time (N22) | ||
| Wide square wave | 1~3 A/cm2 28 s dwell time 600 h, 80 °C | 2 s step time (W02) |
| 12 s step time (W12) | ||
| 22 s step time (W22) | ||
| Solar photovoltaic mode | 0~3 A/cm2 1540 h, 80 °C | Simulating fluctuation (Solar) |
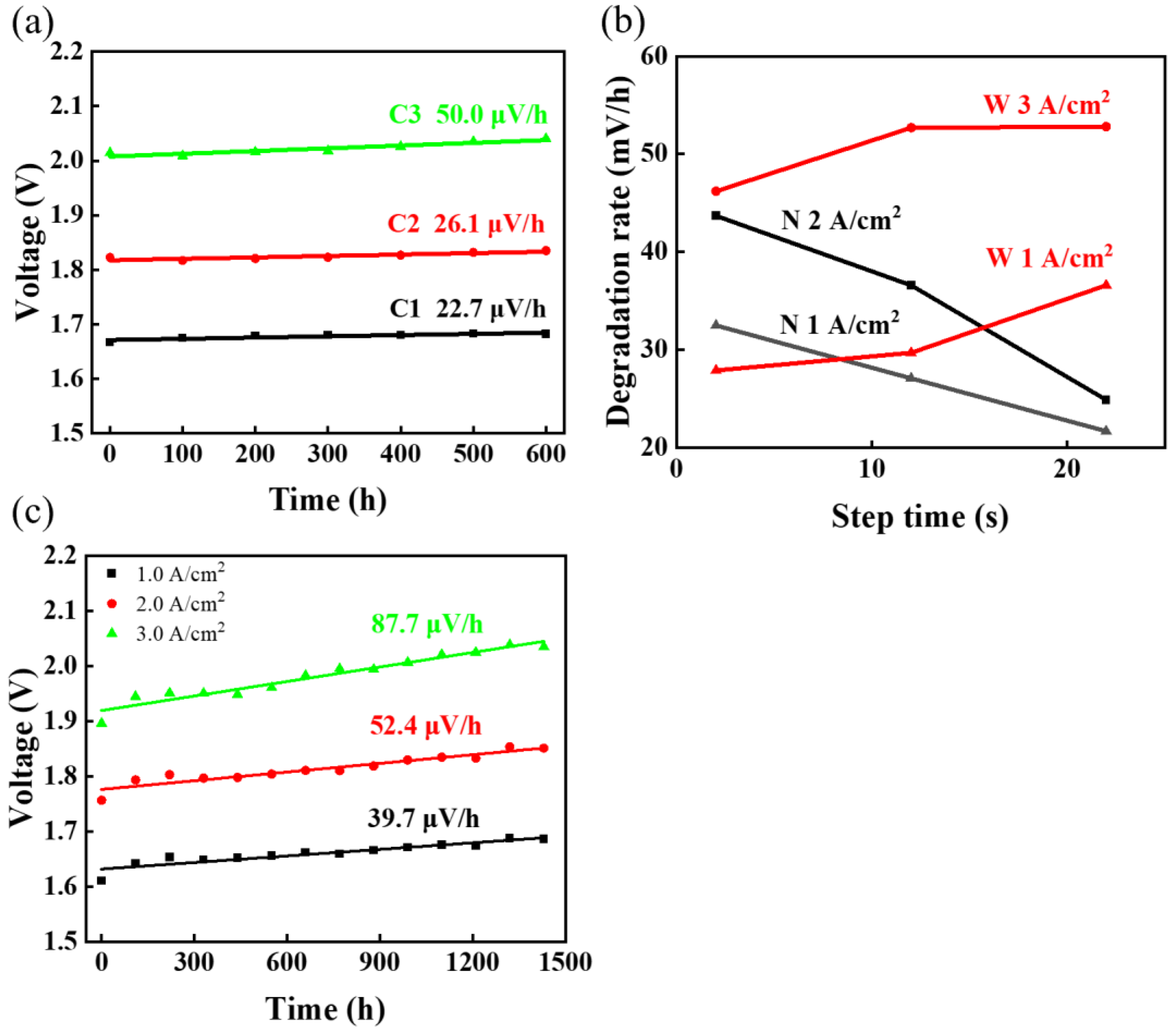
| Test Mode | AST | Degradation Rate | Impact on Degradation |
|---|---|---|---|
| Constant-current mode | C1 | 22. 7 μV/h @ 1 A/cm2 | High current density would accelerate degradation. |
| C2 | 26.1 μV/h @ 2 A/cm2 | ||
| C3 | 50.0 μV/h @ 3 A/cm2 | ||
| Narrow square-wave mode (1–2 A/cm2) | N02 | 32.5 μV/h @ 1 A/cm2 47.3 μV/h @ 2 A/cm2 | Fluctuates in the range of 1–2 A/cm2, decreasing step time of current would accelerate degradation. |
| N12 | 27.1 μV/h @ 1 A/cm2 36.6 μV/h @ 2 A/cm2 | ||
| N22 | 21.7 μV/h @ 1 A/cm2 24.9 μV/h @ 2 A/cm2 | ||
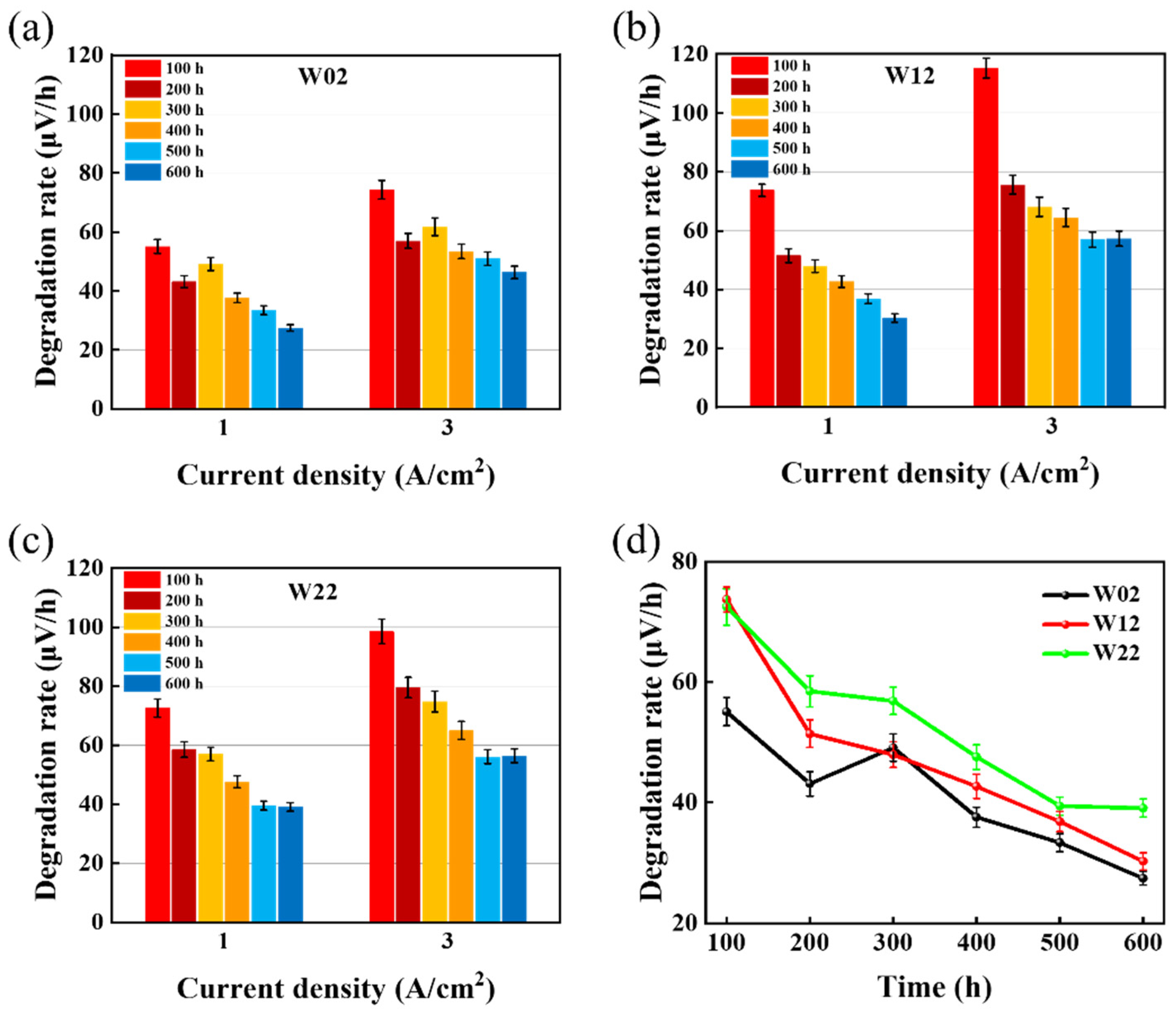
| Wide square-wave mode (1–3 A/cm2) | W02 | 27.9 μV/h @ 1 A/cm2 46.2 μV/h @ 3 A/cm2 | Fluctuates in the range of 1–3 A/cm2, increasing step time of current would accelerate degradation, but there seems to be an upper limit. |
| W12 | 29.8 μV/h @ 1 A/cm2 52.7 μV/h @ 3 A/cm2 | ||
| W22 | 36.7 μV/h @ 1 A/cm2 52.8 μV/h @ 3 A/cm2 | ||
| Simulating solar mode | Solar | 39.7 μV/h @ 1 A/cm2 52.4 μV/h @ 2 A/cm2 87.7 μV/h @ 3 A/cm2 | Near 2–3 A/cm2, increasing step time, the maximum degradation rate occurs. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Z.; Liu, J.; Li, P.; Liang, C. Study of the Durability of Membrane Electrode Assemblies in Various Accelerated Stress Tests for Proton-Exchange Membrane Water Electrolysis. Materials 2024, 17, 1331. https://doi.org/10.3390/ma17061331
Su Z, Liu J, Li P, Liang C. Study of the Durability of Membrane Electrode Assemblies in Various Accelerated Stress Tests for Proton-Exchange Membrane Water Electrolysis. Materials. 2024; 17(6):1331. https://doi.org/10.3390/ma17061331
Chicago/Turabian StyleSu, Zhengquan, Jun Liu, Pengfei Li, and Changhao Liang. 2024. "Study of the Durability of Membrane Electrode Assemblies in Various Accelerated Stress Tests for Proton-Exchange Membrane Water Electrolysis" Materials 17, no. 6: 1331. https://doi.org/10.3390/ma17061331
APA StyleSu, Z., Liu, J., Li, P., & Liang, C. (2024). Study of the Durability of Membrane Electrode Assemblies in Various Accelerated Stress Tests for Proton-Exchange Membrane Water Electrolysis. Materials, 17(6), 1331. https://doi.org/10.3390/ma17061331







