Effect of Heteroatom Doping on Electrochemical Properties of Olivine LiFePO4 Cathodes for High-Performance Lithium-Ion Batteries
Abstract
:1. Introduction
2. Experimental Section
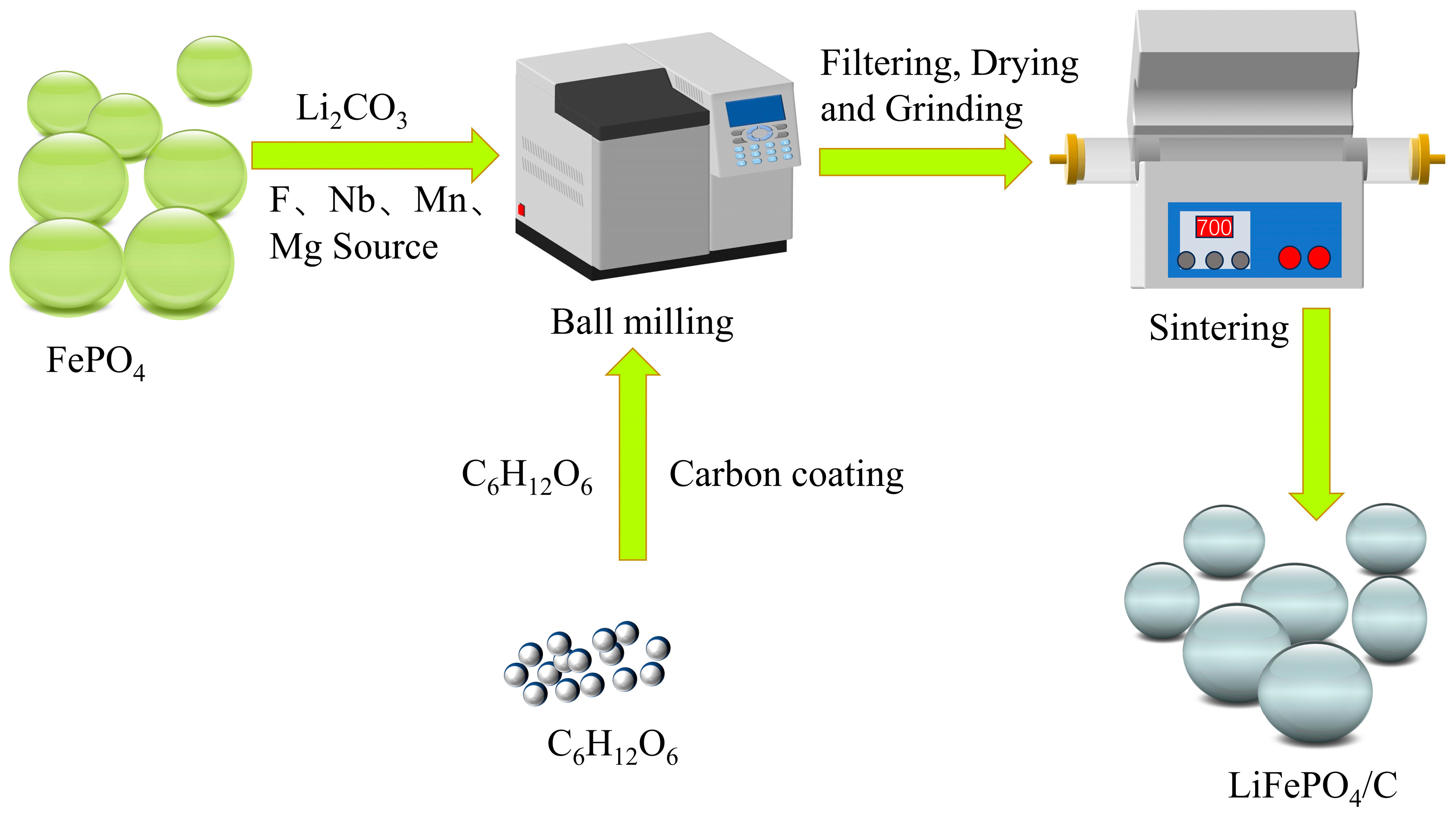
2.1. Synthesis and Doping of LFP/C
2.2. Material Characterization
2.3. Electrochemical Characterization
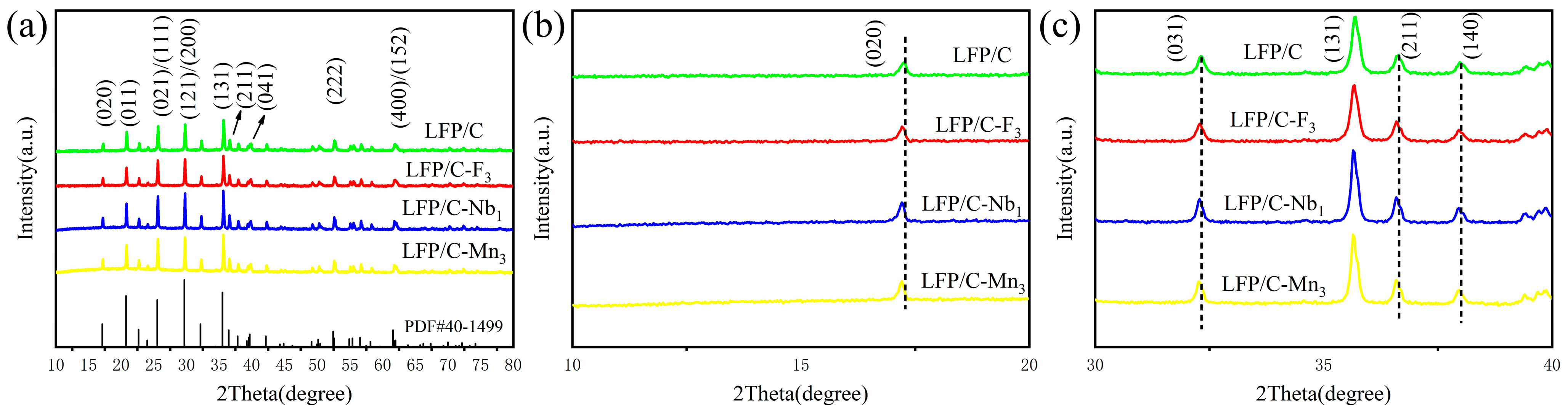
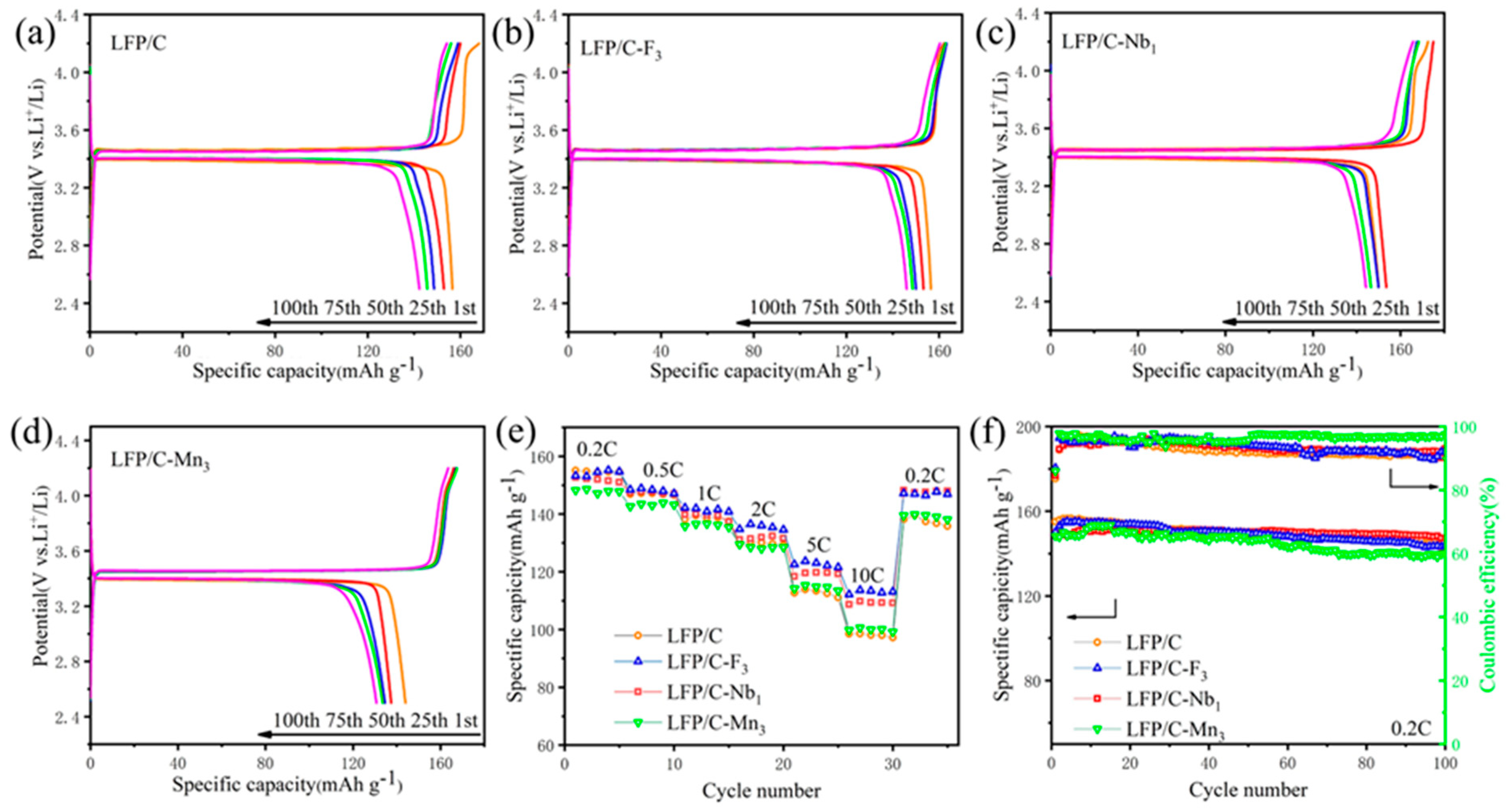
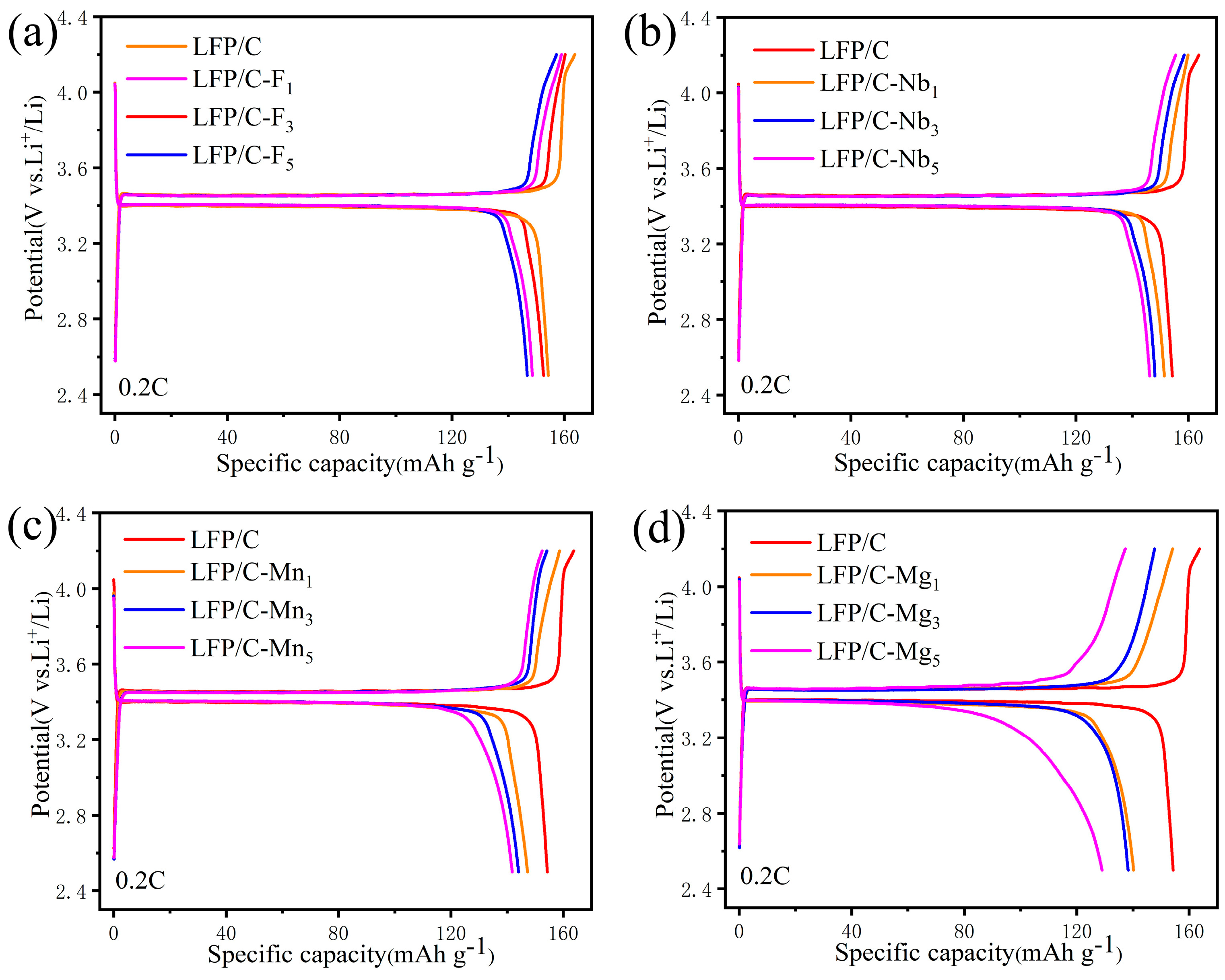
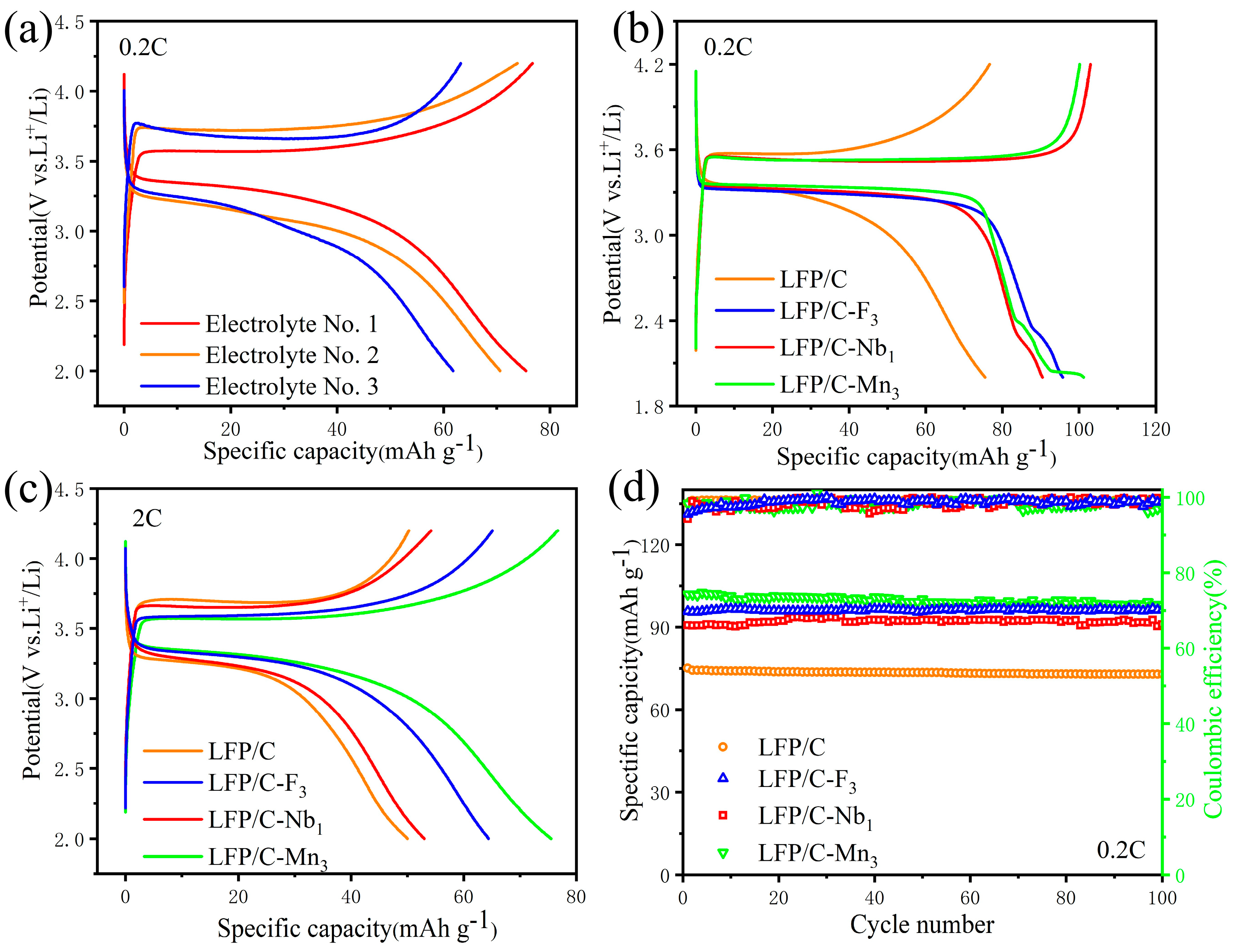
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LFP | LiFePO4 |
| FP | FePO4 |
| LFP/C | Carbon–coated LiFePO4 |
| LFP/C–Fn | Carbon–coated LiFePO4 doped with n at.% F |
| LFP/C–Nbn | Carbon–coated LiFePO4 doped with n at.% Nb |
| LFP/C–Mnn | Carbon–coated LiFePO4 doped with n at.% Mn |
| LFMP | LiFeMnPO4 |
| LFP/C–Xn | Carbon–coated LiFePO4 doped with n at.% X dopant element |
References
- Karami, M.; Masoudpanah, S.M.; Rezaie, H.R. Solution combustion synthesis of hierarchical porous LiFePO4 powders as cathode materials for lithium-ion batteries. Adv. Powder Technol. 2021, 32, 1935–1942. [Google Scholar] [CrossRef]
- Huang, J.; Zhu, Y.; Feng, Y.; Han, Y.; Gu, Z.; Liu, R.; Yang, D.; Chen, K.; Zhang, X.; Sun, W.; et al. Research Progress on Key Materials and Technologies for Secondary Batteries. Acta Phys.-Chim. Sin. 2022, 38, 2208008. [Google Scholar] [CrossRef]
- Choi, J.; Zabihi, O.; Ahmadi, M.; Naebe, M. Advancing structural batteries: Cost-efficient high-performance carbon fiber-coated LiFePO4 cathodes. RSC Adv. 2023, 13, 30633–30642. [Google Scholar] [CrossRef]
- Catenaro, E.; Onori, S. Experimental data of lithium-ion batteries under galvanostatic discharge tests at different rates and temperatures of operation. Data Brief 2021, 35, 106894. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.W.; Wang, C.H.; Huang, A.F.; Su, W.N.; Hwang, B.J. Green chemical delithiation of lithium iron phosphate for energy storage application. Chem. Eng. J. 2021, 418, 129191. [Google Scholar] [CrossRef]
- Apachitei, G.; Heymer, R.; Lain, M.; Dogaru, D.; Hidalgo, M.; Marco, J.; Copley, M. Scale-Up of Lithium Iron Phosphate Cathodes with High Active Materials Contents for Lithium Ion Cells. Batteries 2023, 9, 518. [Google Scholar] [CrossRef]
- Gadomski, K.; Buchberger, D.A.; Pietrzak, T.K. Synthesis and electrical properties of glassy and nanocrystalline LiFePO4. J. Non Cryst. Solids 2024, 625, 122771. [Google Scholar] [CrossRef]
- Bal, B.; Ozdogru, B.; Nguyen, D.T.; Li, Z.; Murugesan, V.; Capraz, O.O. Probing the Formation of Cathode-Electrolyte Interphase on Lithium Iron Phosphate Cathodes via Operando Mechanical Measurements. ACS Appl. Interfaces 2023, 15, 42449–42459. [Google Scholar] [CrossRef]
- Dos Santos Junior, G.A.; Fortunato, V.D.S.; Gandra, F.G.; Nascentes, C.C.; Silva, G.G.; Ortega, P.F.R.; Lavall, R.L. Electrochemical performance of hybrid supercapacitor device based on Mn-doped LiFePO4/C and imidazolium ionic liquid electrolyte. J. Energy Storage 2023, 74, 109112. [Google Scholar] [CrossRef]
- Kanagaraj, A.B.; Chaturvedi, P.; Alkindi, T.S.; Susantyoko, R.A.; An, B.H.; Patole, S.P.; Shanmugam, K.; AlMheiri, S.; AlDahmani, S.; AlFadaq, H.; et al. Mechanical, thermal and electrical properties of LiFePO4/MWCNTs composite electrodes. Mater. Lett. 2018, 230, 57–60. [Google Scholar] [CrossRef]
- Mayer, S.F.; de la Calle, C.; Fernandez-Diaz, M.T.; Amarilla, J.M.; Alonso, J.A. Nitridation effect on lithium iron phosphate cathode for rechargeable batteries. RSC Adv. 2022, 12, 3696–3707. [Google Scholar] [CrossRef] [PubMed]
- Nitou, M.V.M.; Pang, Y.; Wan, Z.; Li, W.; Zhong, Z.; Muhammad, W.; Muhammad, S.; Muhammad, S.; Niu, Y.; Lv, W. LiFePO4 as a dual-functional coating for separators in lithium-ion batteries: A new strategy for improving capacity and safety. J. Energy Chem. 2023, 86, 490–498. [Google Scholar] [CrossRef]
- Reizabal, A.; Fidalgo-Marijuan, A.; Goncalves, R.; Gutierrez-Pardo, A.; Aguesse, F.; Perez-Alvarez, L.; Vilas-Vilela, J.L.; Costa, C.M.; Lanceros-Mendez, S. Silk fibroin and sericin polymer blends for sustainable battery separators. J. Colloid Interface Sci. 2022, 611, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Fagundes, W.S.; Xavier, F.F.S.; Santana, L.K.; Azevedo, M.E.; Canobre, S.C.; Amaral, F.A. PAni-coated LiFePO4 Synthesized by a Low Temperature Solvothermal Method. Mater. Res. 2018, 22, e20180566. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, D. Effect of organic carbon coating prepared by hydrothermal method on performance of lithium iron phosphate battery. Alex. Eng. J. 2023, 80, 1–7. [Google Scholar] [CrossRef]
- Apachitei, G.; Hidalgo, M.; Dogaru, D.; Lain, M.; Heymer, R.; Marco, J.; Copley, M. Optimisation of Industrially Relevant Electrode Formulations for LFP Cathodes in Lithium Ion Cells. Batteries 2023, 9, 192. [Google Scholar] [CrossRef]
- Bezerra, C.A.G.; Davoglio, R.A.; Biaggio, S.R.; Bocchi, N.; Rocha-Filho, R.C. High-purity LiFePO4 prepared by a rapid one-step microwave-assisted hydrothermal synthesis. J. Mater. Sci. 2021, 56, 10018–10029. [Google Scholar]
- Kroff, M.; Hevia, S.A.; O’Shea, J.N.; Muro, I.G.; Palomares, V.; Rojo, T.; Del Rio, R. Lithium Iron Phosphate/Carbon (LFP/C) Composite Using Nanocellulose as a Reducing Agent and Carbon Source. Polymers 2023, 15, 2628. [Google Scholar] [CrossRef] [PubMed]
- Kanagaraj, A.B.; Al Shibli, H.; Alkindi, T.S.; Susantyoko, R.A.; An, B.H.; AlMheiri, S.; AlDahmani, S.; Fadaq, H.; Choi, D.S. Hydrothermal synthesis of LiFePO4 micro-particles for fabrication of cathode materials based on LiFePO4/carbon nanotubes nanocomposites for Li-ion batteries. Ionics 2018, 24, 3685–3690. [Google Scholar] [CrossRef]
- Alsamet, M.A.M.M.; Burgaz, E. Synthesis and characterization of nano-sized LiFePO4 by using consecutive combination of sol-gel and hydrothermal methods. Electrochim. Acta 2021, 367, 137530. [Google Scholar] [CrossRef]
- Rikka, V.R.; Sahu, S.R.; Chatterjee, A.; Prakash, R.; Sundararajan, G.; Gopalan, R. Enhancing cycle life and usable energy density of fast charging LiFePO4-graphite cell by regulating electrodes’ lithium level. iScience 2022, 25, 104831. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Hu, Z.; Long, Z.; Tang, M.; Qiu, X. Improvement of electrochemical properties of lithium iron phosphate cathode by rare earth oxides modification. J. Alloys Compd. 2023, 947, 169581. [Google Scholar] [CrossRef]
- Sellami, M.; Barre, M.; Dammak, M.; Toumi, M. Local Structure, Thermal, Optical and Electrical Properties of LiFePO4 Polycrystalline Synthesized by Co-Precipitation Method. Braz. J. Phys. 2021, 51, 1521–1528. [Google Scholar] [CrossRef]
- Yi, W.; Sun, C.; Jiang, W.; Zhai, Y.; Gao, Y. Effect of different carbon and nitrogen co-doping on electrochemical performance of LiFePO4/CN. Mater. Lett. 2022, 324, 132713. [Google Scholar] [CrossRef]
- Jiao, L.X.; Li, Z.Q.; Zhu, Y.Z.; Wei, Z.; Liang, Y.; Wang, X.L.; Cui, Y.; Zhang, Z.H.; He, M.; Song, B. Enhanced electrical conductivity and lithium ion diffusion rate of LiFePO4 by Fe site and P site doping. AIP Adv. 2023, 13, 075306. [Google Scholar] [CrossRef]
- Hu, H.; Li, H.; Lei, Y.; Liu, J.; Liu, X.; Wang, R.; Peng, J.; Wang, X. Mg-doped LiMn0.8Fe0.2PO4/C nano-plate as a high-performance cathode material for lithium-ion batteries. J. Energy Storage 2023, 73, 109006. [Google Scholar] [CrossRef]
- Jiang, F.; Qu, K.; Wang, M.; Chen, J.; Liu, Y.; Xu, H.; Huang, Y.; Li, J.; Gao, P.; Zheng, J.; et al. Atomic scale insight into the fundamental mechanism of Mn doped LiFePO4. Sustain. Energy Fuels 2020, 4, 2741–2751. [Google Scholar] [CrossRef]
- Suarso, E.; Setyawan, F.A.; Subhan, A.; Ramli, M.M.; Ismail, N.S.; Zainuri, M.; Arifin, Z.; Darminto. Enhancement of LiFePO4 (LFP) electrochemical performance through the insertion of coconut shell-derived rGO-like carbon as cathode of Li-ion battery. J. Mater. Sci. Mater. Electron. 2021, 32, 28297–28306. [Google Scholar] [CrossRef]
- Hua, W.; Yang, X.; Casati, N.P.M.; Liu, L.; Wang, S.; Baran, V.; Knapp, M.; Ehrenberg, H.; Indris, S. Probing thermally-induced structural evolution during the synthesis of layered Li−, Na−, or K− containing 3d transition-metal oxides. eScience 2022, 2, 183–191. [Google Scholar] [CrossRef]
- Chuang, H.C.; Teng, J.W.; Kuan, W.F. Supercritical CO2-enhanced surface modification on LiFePO4 cathodes through ex-situ carbon coating for lithium-ion batteries. Colloids Surf. A Physicochem. Eng. Asp. 2024, 684, 133110. [Google Scholar] [CrossRef]
- Torabi, M.; Sadrnezhaad, S.K. Nanostructured-microfibrillar polypyrrole coated NiTi current collectors for high power and shape memory LiFePO4 cathodes for Li-ion batteries. J. Alloys Compd. 2023, 969, 172467. [Google Scholar] [CrossRef]
- Yang, G.; Liang, X.; Zheng, S.; Chen, H.; Zhang, W.; Li, S.; Pan, F. Li-rich channels as the material gene for facile lithium diffusion in halide solid electrolytes. eScience 2022, 2, 79–86. [Google Scholar] [CrossRef]
- Sun, C.; Rajasekhara, S.; Goodenough, J.B.; Zhou, F. Monodisperse porous LiFePO4 microspheres for a high power Li-ion battery cathode. J. Am. Chem. Soc. 2011, 133, 2132–2135. [Google Scholar] [CrossRef] [PubMed]
- Chai, S.; Zhang, Y.; Wang, Y.; He, Q.; Zhou, S.; Pan, A. Biodegradable composite polymer as advanced gel electrolyte for quasi-solid-state lithium-metal battery. eScience 2022, 2, 494–508. [Google Scholar] [CrossRef]
- Dose, W.M.; Peebles, C.; Blauwkamp, J.; Jansen, A.N.; Liao, C.; Johnson, C.S. Synthesis of high-density olivine LiFePO4 from paleozoic siderite FeCO3 and its electrochemical performance in lithium batteries. APL Mater. 2022, 10, 041113. [Google Scholar] [CrossRef]
- Li, B.; Xiao, J.; Zhu, X.; Wu, Z.; Zhang, X.; Han, Y.; Niu, J.; Wang, F. Enabling high-performance lithium iron phosphate cathodes through an interconnected carbon network for practical and high-energy lithium-ion batteries. J. Colloid Interface Sci. 2024, 653, 942–948. [Google Scholar] [CrossRef]
- Li, Z.; Ren, X.; Tian, W.; Zheng, Y.; Sun, J.; An, L.; Wen, L.; Wang, L.; Liang, G. High Volumetric Energy Density of LiFePO4/KB Cathode Materials Based on Ketjen Black Additive. ChemElectroChem 2020, 7, 2174–2183. [Google Scholar] [CrossRef]
- Krishnan, S.; Yadav, V.; Devotta, I.; Srivastava, U.; Ssv, R. Effect of multi-walled carbon nanotubes diameter in electrochemical activity of lithium iron phosphate. Diam. Relat. Mater. 2024, 141, 110651. [Google Scholar] [CrossRef]
- Li, T.; Chang, X.; Xin, Y.; Liu, Y.; Tian, H. Synergistic Strategy Using Doping and Polymeric Coating Enables High-Performance High-Nickel Layered Cathodes for Lithium-Ion Batteries. J. Phys. Chem. C 2023, 127, 8448–8461. [Google Scholar] [CrossRef]
- Galaguz, V.; Korduban, O.; Panov, E.; Malovanyi, S. The use of Raman and XPS spectroscopy to study the cathode material of LiFePO4/C. J. Serbian Chem. Soc. 2020, 85, 1047–1054. [Google Scholar] [CrossRef]
- Connor, W.D.; Arisetty, S.; Yao, K.P.; Fu, K.; Advani, S.G.; Prasad, A.K. Analysis of solvent-free lithium-ion electrodes formed under high pressure and heat. J. Power Sources 2022, 546, 231972. [Google Scholar] [CrossRef]
- Levin, O.V.; Eliseeva, S.N.; Alekseeva, E.V.; Tolstopjatova, E.G.; Kondratiev, V.V. Composite LiFePO4/poly-3,4-ethylenedioxythiophene Cathode for Lithium-Ion Batteries with Low Content of Non-Electroactive Components. Int. J. Electrochem. Sci. 2015, 10, 8175–8189. [Google Scholar] [CrossRef]
- Valvo, M.; Chien, Y.C.; Liivat, A.; Tai, C.W. Detecting voltage shifts and charge storage anomalies by iron nanoparticles in three-electrode cells based on converted iron oxide and lithium iron phosphate. Electrochim. Acta 2023, 440, 141747. [Google Scholar] [CrossRef]
- Naik, A.; Zhou, J.; Gao, C.; Wang, L. Microwave Assisted Solid State Synthesis of LiFePO4/C Using Two Different Carbon Sources. Int. J. Electrochem. Sci. 2014, 9, 6124–6133. [Google Scholar] [CrossRef]
- Sundarayya, Y.; Vijeth, H.; Nagaraju, D.; Kumara Swamy, K.C.; Sunandana, C.S. Isovalent substitution of vanadium in LiFePO4: Evolution of monoclinic α-Li3Fe2(PO4)3 phase. Inorg. Chem. Commun. 2023, 150, 110530. [Google Scholar] [CrossRef]
- Rastgoo-Deylami, M.; Javanbakht, M.; Ghaemi, M.; Naji, L.; Omidvar, H.; Ganjali, M.R. Synthesis and Electrochemical Properties of Rhombohedral LiFePO4/C Microcrystals Via a Hydrothermal Route for Lithium Ion Batteries. Int. J. Electrochem. Sci. 2014, 9, 3199–3208. [Google Scholar] [CrossRef]
- Ma, C.; Wang, X.; Song, Y.; Hu, H.; Li, W.; Qiu, Z.; Cui, Y.; Xing, W. Low-temperature performance optimization of LiFePO4-based batteries. Asia-Pac. J. Chem. Eng. 2022, 18, e2841. [Google Scholar] [CrossRef]
- Na, Y.; Sun, X.; Fan, A.; Cai, S.; Zheng, C. Methods for enhancing the capacity of electrode materials in low-temperature lithium-ion batteries. Chin. Chem. Lett. 2021, 32, 973–982. [Google Scholar] [CrossRef]
- Myalo, Z.; Ikpo, C.O.; Nwanya, A.C.; Ndipingwi, M.M.; Duoman, S.F.; Mokwebo, K.V.; Iwuoha, E.I. Graphenised Lithium Iron Phosphate and Lithium Manganese Silicate Hybrid Cathodes: Potentials for Application in Lithium-ion Batteries. Electroanalysis 2020, 32, 2982–2999. [Google Scholar] [CrossRef]
- Zaki, N.H.M.; Ahmad, S.I.; Sazman, F.N.; Badrudin, F.W.; Abdullah, A.L.A.; Taib, M.F.M.; Hassan, O.H.; Yahya, M.Z.A. The influence of Cl doping on the structural, electronic properties and Li-ion migration of LiFePO4: A DFT study. Comput. Theor. Chem. 2023, 1221, 114029. [Google Scholar] [CrossRef]
- Seo, S.B.; Song, Y.; Choi, Y.R.; Kang, M.; Choi, G.B.; Kim, J.H.; Han, J.H.; Hong, S.; Muramatsu, H.; Kim, M.Y.; et al. Double-walled carbon nanotubes as effective conducting agents for lithium iron phosphate cathodes. Carbon 2023, 218, 118731. [Google Scholar] [CrossRef]
- Sharmila, V.; Parthibavarman, M. Lithium manganese phosphate associated with MWCNT: Enhanced positive electrode for lithium hybrid batteries. J. Alloys Compd. 2021, 858, 157715. [Google Scholar] [CrossRef]
- Jheng, S.C.; Chen, J.S. The Synthesis of LiFePO4/C Composite by the Precipitation Between Two Water/Oil Emulsions. Int. J. Electrochem. Sci. 2013, 8, 4901–4913. [Google Scholar] [CrossRef]
- Shaji, N.; Jiang, F.; Sung, J.Y.; Nanthagopal, M.; Kim, T.; Jeong, B.J.; Jung, S.P.; Lee, C.W. Heteroatoms-doped carbon effect on LiFePO4 cathode for Li-ion batteries. J. Energy Storage 2023, 72, 108710. [Google Scholar] [CrossRef]
- Vásquez, F.A.; Rosero-Navarro, N.C.; Miura, A.; Goto, Y.; Tadanaga, K.; Calderón, J.A. Beneficial Effect of LiFePO4/C coating on Li0.9Mn1.6Ni0.4O4 obtained by microwave heating. Electrochim. Acta 2023, 437, 141544. [Google Scholar] [CrossRef]
- Zhu, H.; Li, Z.; Li, C.; Jia, H.; Fang, H.; Qiao, L.; Lv, P.; Li, X. Near-in-situ electrochemical impedance spectroscopy analysis based on lithium iron phosphate electrode. Electrochim. Acta 2023, 464, 142919. [Google Scholar] [CrossRef]
- Yücel, Y.D.; Zenkert, D.; Lindström, R.W.; Lindbergh, G. LiFePO4-coated carbon fibers as positive electrodes in structural batteries: Insights from spray coating technique. Electrochem. Commun. 2024, 160, 107670. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Meng, Y.; Xiao, M.; Kang, T.; Gao, H.; Huang, L.; Zhu, F. Preparation and electrochemical properties of Co doped core-shell cathode material on a lithium iron phosphate surface. J. Alloys Compd. 2022, 923, 166326. [Google Scholar] [CrossRef]
- Kim, J.; Song, S.; Lee, C.S.; Lee, M.; Bae, J. Prominent enhancement of stability under high current density of LiFePO4-based multidimensional nanocarbon composite as cathode for lithium-ion batteries. J. Colloid Interface Sci. 2023, 650, 1958–1965. [Google Scholar] [CrossRef]
- Karimzadeh, S.; Safaei, B.; Huang, W.; Jen, T.C. Theoretical investigation on niobium doped LiFePO4 cathode material for high performance lithium-ion batteries. J. Energy Storage 2023, 67, 107572. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Xin, Y.; He, B.; Zhang, F.; Tian, H. Effect of Heteroatom Doping on Electrochemical Properties of Olivine LiFePO4 Cathodes for High-Performance Lithium-Ion Batteries. Materials 2024, 17, 1299. https://doi.org/10.3390/ma17061299
Jiang X, Xin Y, He B, Zhang F, Tian H. Effect of Heteroatom Doping on Electrochemical Properties of Olivine LiFePO4 Cathodes for High-Performance Lithium-Ion Batteries. Materials. 2024; 17(6):1299. https://doi.org/10.3390/ma17061299
Chicago/Turabian StyleJiang, Xiukun, Yan Xin, Bijiao He, Fang Zhang, and Huajun Tian. 2024. "Effect of Heteroatom Doping on Electrochemical Properties of Olivine LiFePO4 Cathodes for High-Performance Lithium-Ion Batteries" Materials 17, no. 6: 1299. https://doi.org/10.3390/ma17061299



