How Does Ethylenediaminetetraacetic Acid Irrigation Affect Biodentine? A Multimethod Ex Vivo Study
Abstract
:1. Introduction
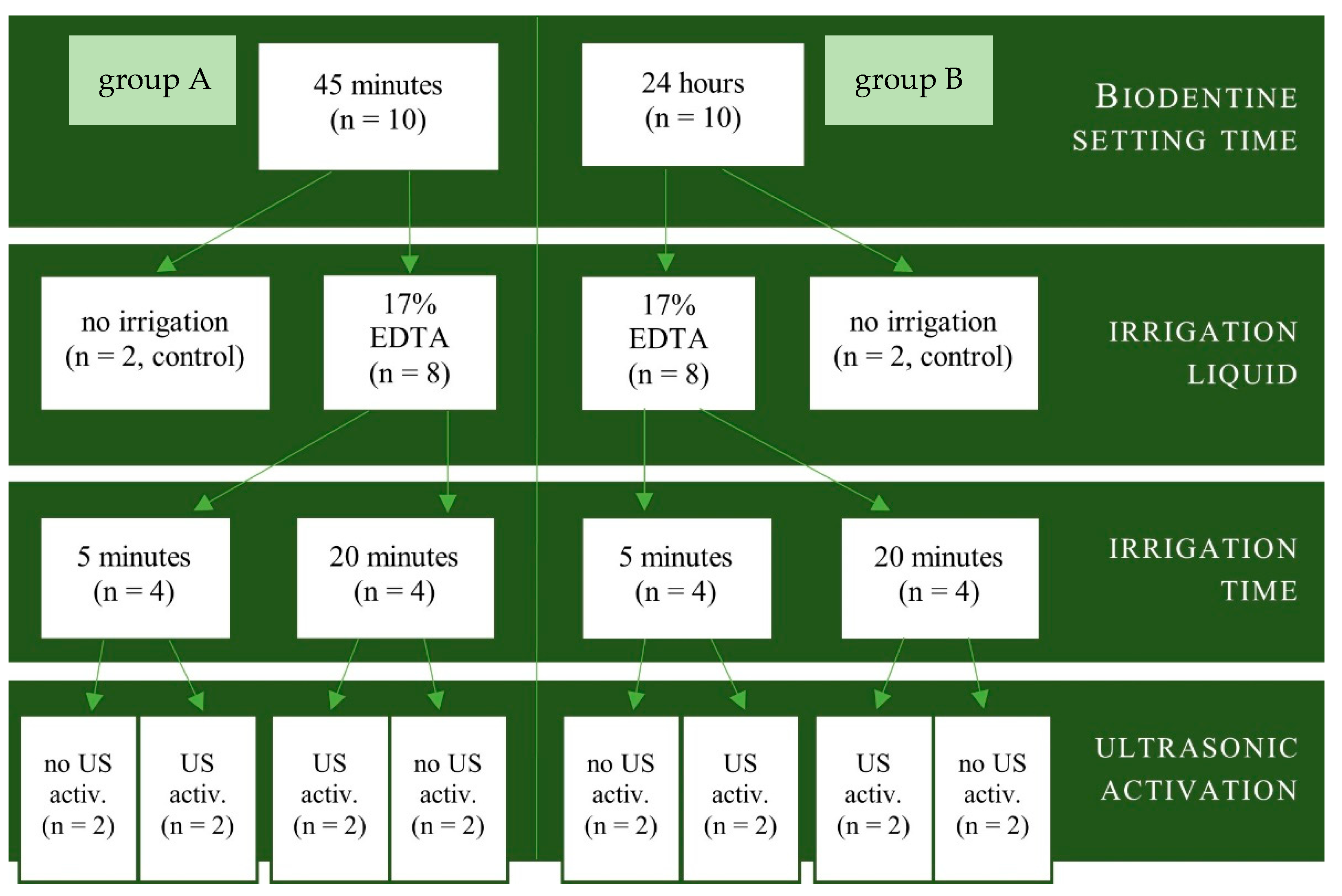
2. Materials and Methods
3. Results
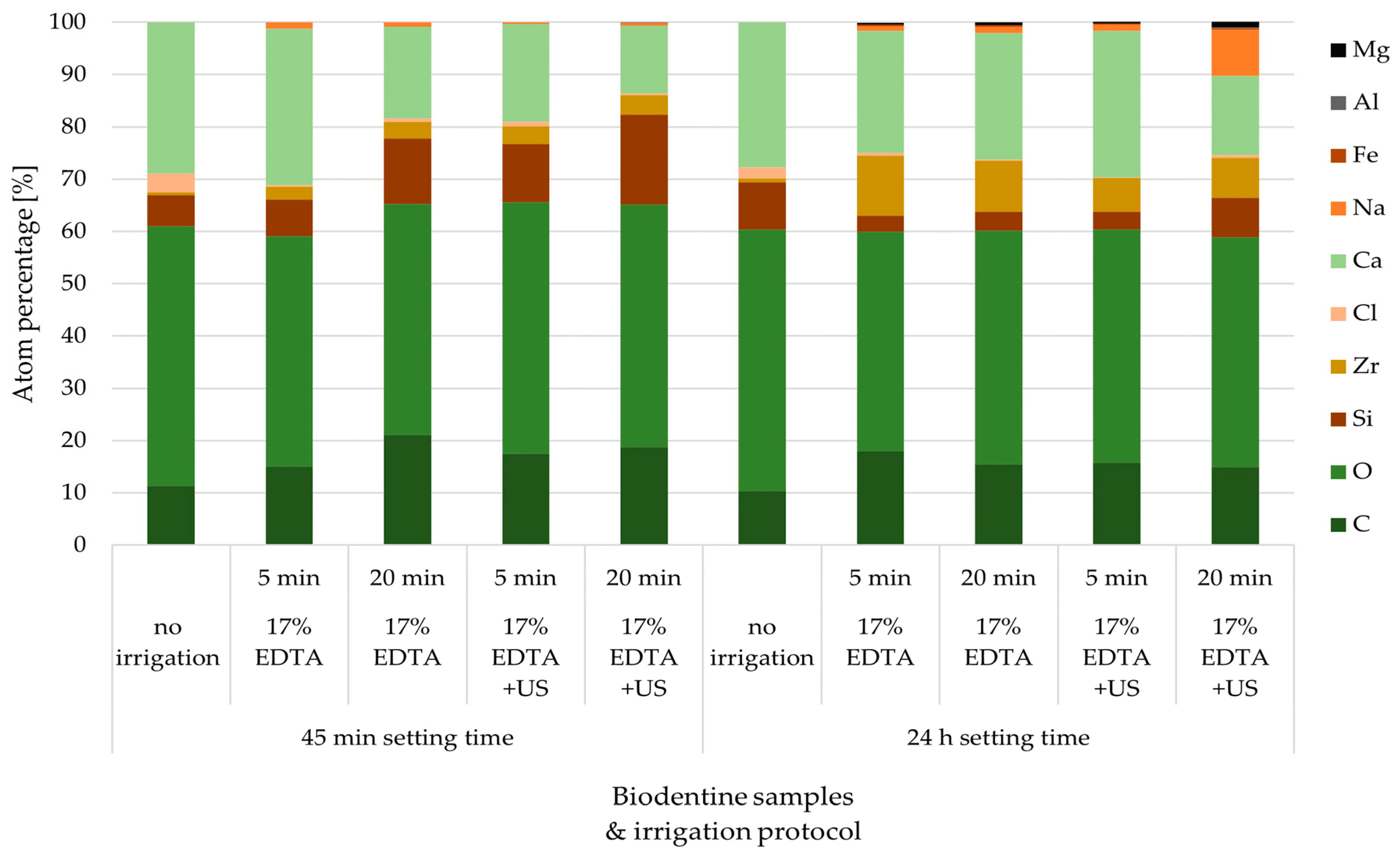
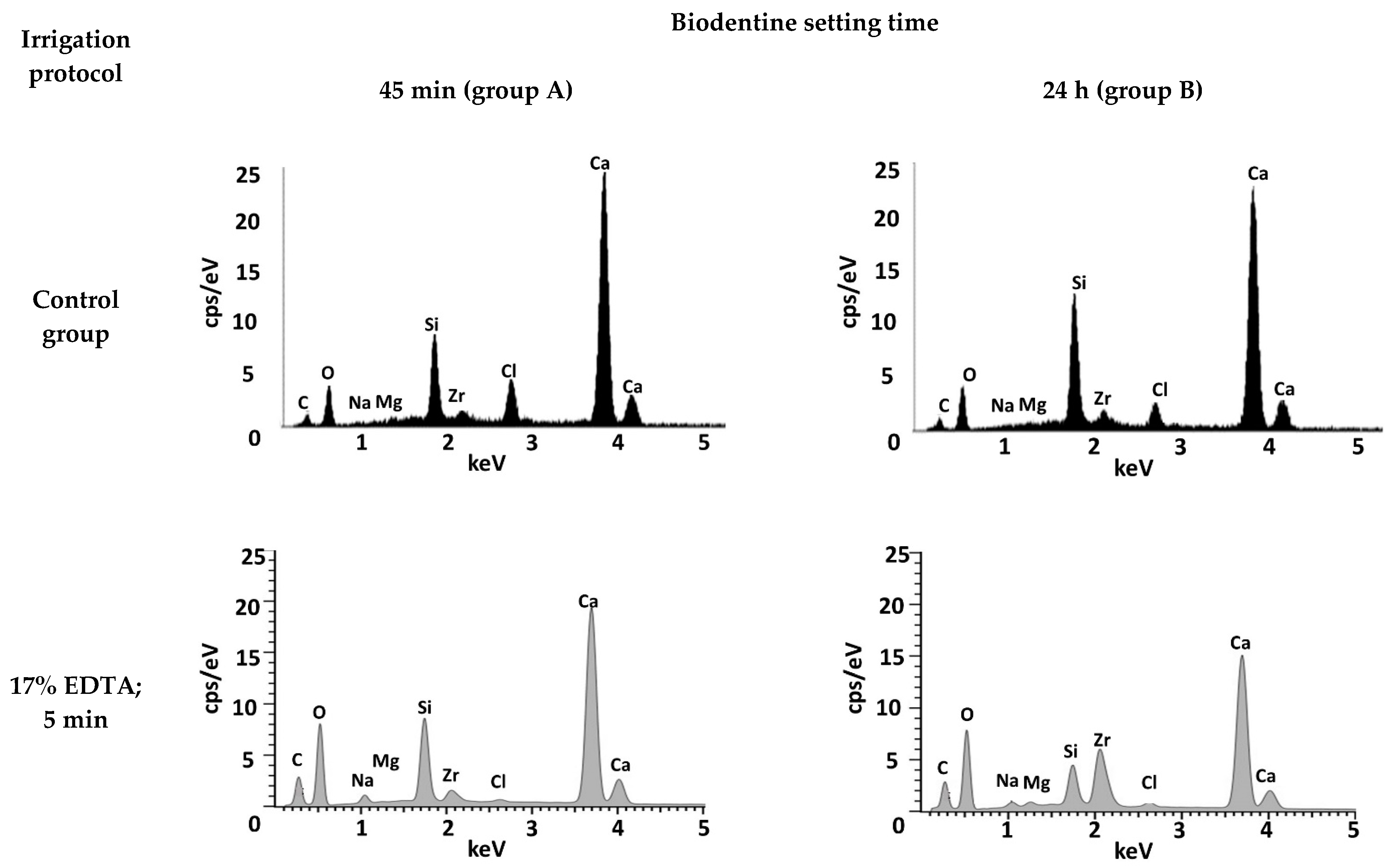
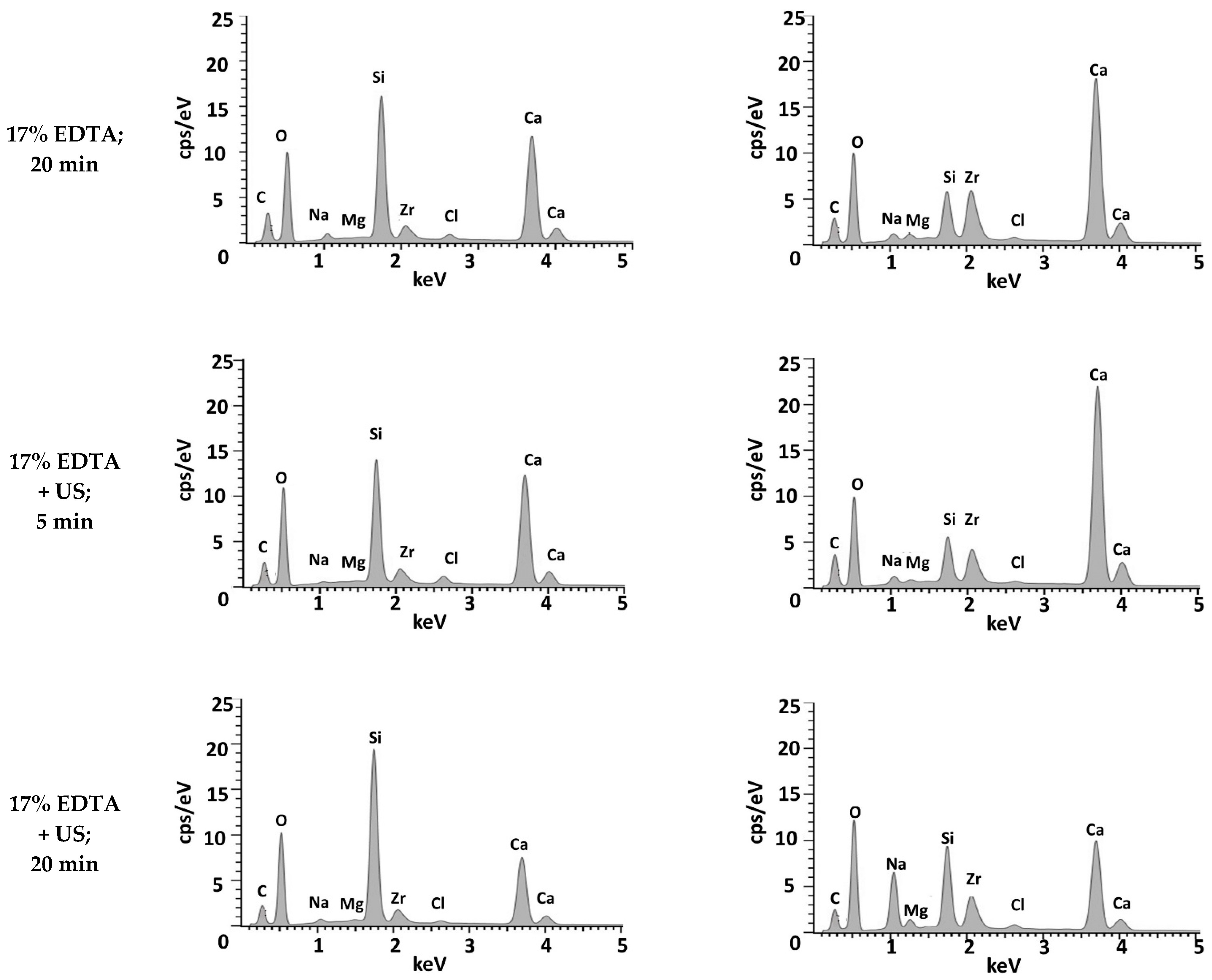
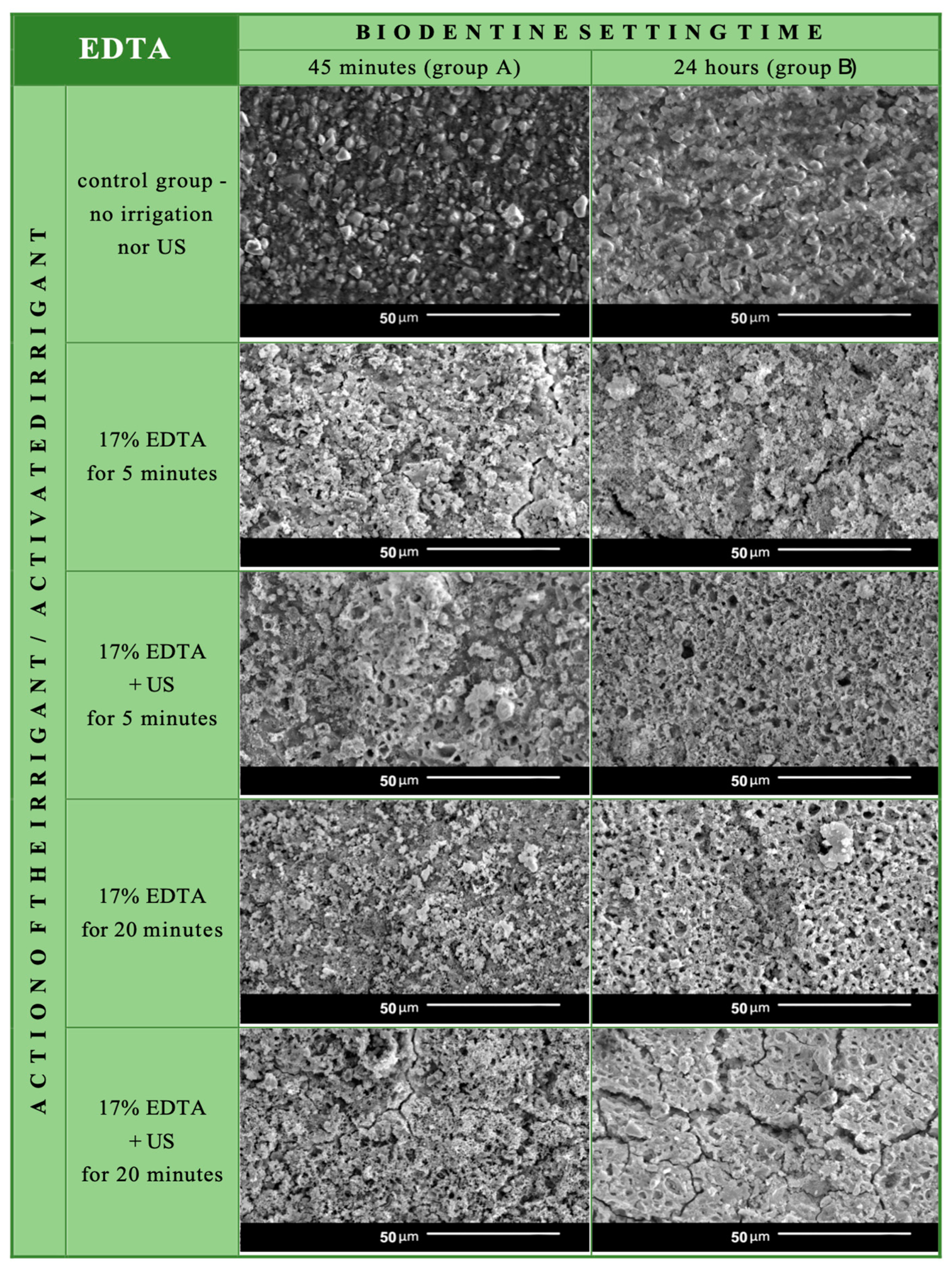
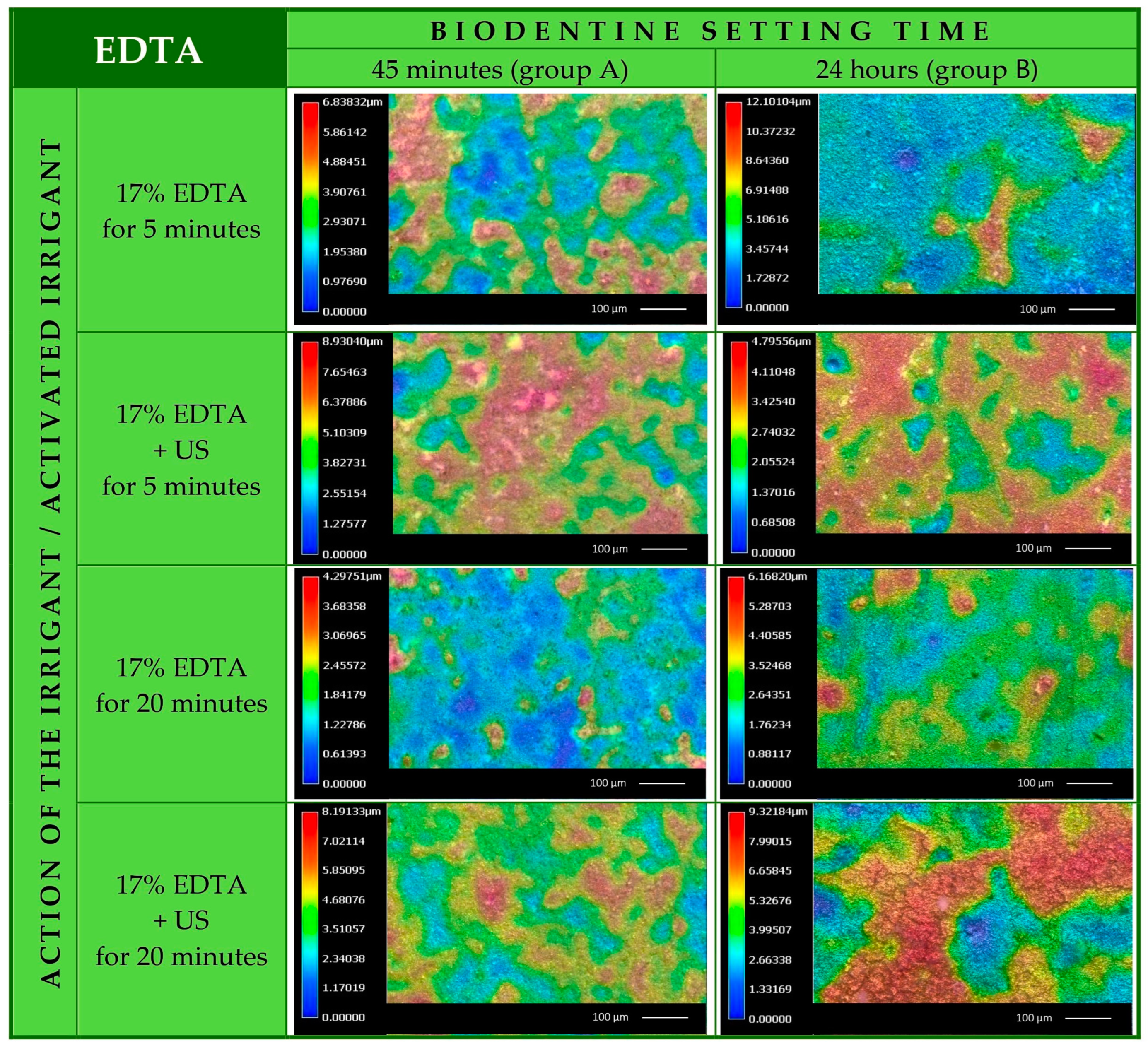
3.1. Biodentine Surface Chemical Composition
3.2. Biodentine Surface Appearance
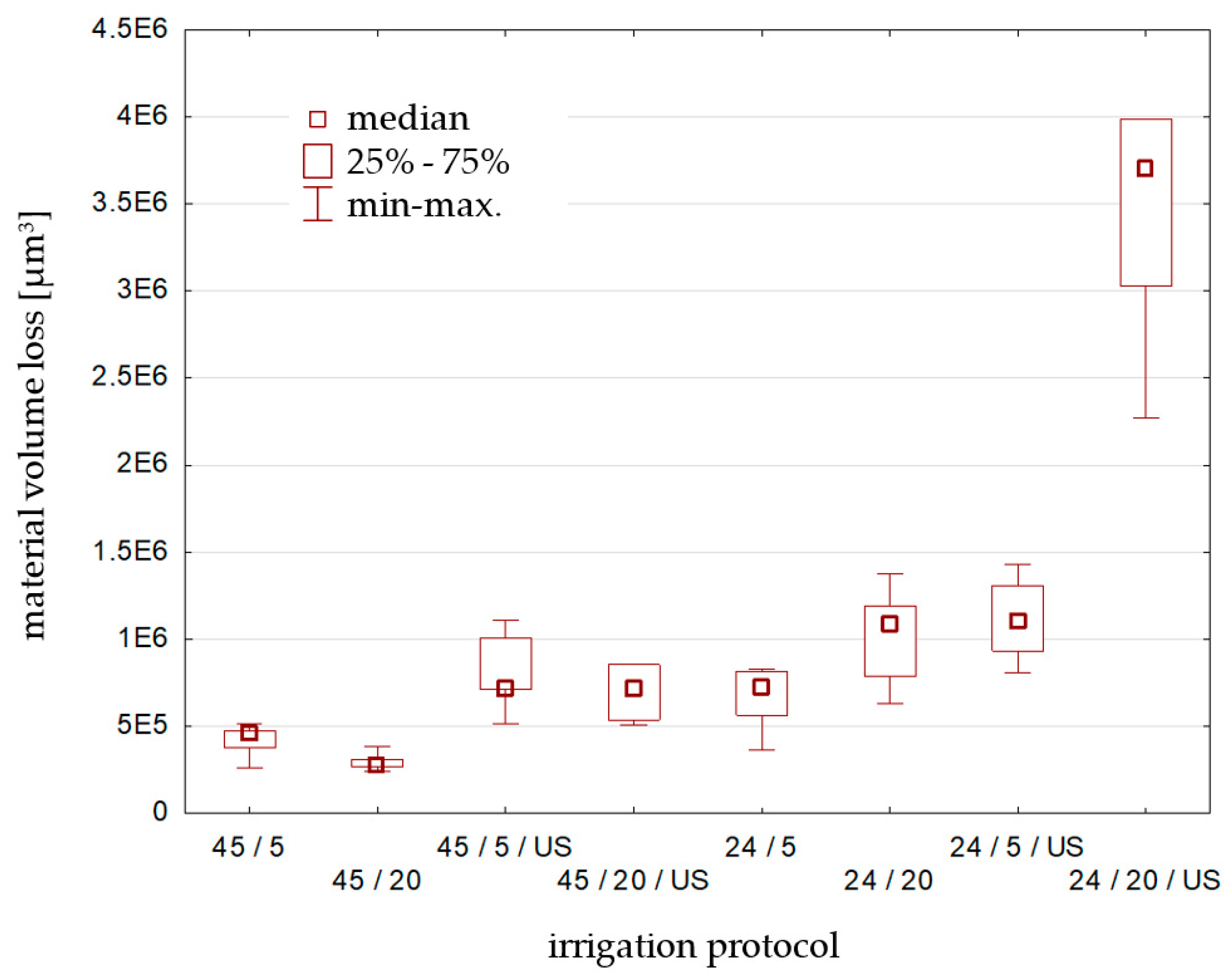
3.3. Biodentine Surface Volume Loss
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dawood, A.E.; Parashos, P.; Wong, R.H.K.; Reynolds, E.C.; Manton, D.J. Calcium Silicate-Based Cements: Composition, Properties, and Clinical Applications. J. Investig. Clin. Dent. 2017, 8, e12195. [Google Scholar] [CrossRef]
- Torabinejad, M.; Parirokh, M.; Dummer, P.M.H. Mineral Trioxide Aggregate and Other Bioactive Endodontic Cements: An Updated Overview—Part II: Other Clinical Applications and Complications. Int. Endod. J. 2018, 51, 284–317. [Google Scholar] [CrossRef]
- Falkowska, J.; Chady, T.; Dura, W.; Droździk, A.; Tomasik, M.; Marek, E.; Safranow, K.; Lipski, M. The Washout Resistance of Bioactive Root-End Filling Materials. Materials 2023, 16, 5757. [Google Scholar] [CrossRef]
- Jitaru, S.; Hodisan, I.; Timis, L.; Lucian, A.; Bud, M. The Use of Bioceramics in Endodontics—Literature Review. Clujul Med. 2016, 89, 470–473. [Google Scholar] [CrossRef]
- Khan, A.S.; Syed, M.R. A Review of Bioceramics-Based Dental Restorative Materials. Dent. Mater. J. 2019, 38, 163–176. [Google Scholar] [CrossRef]
- Camilleri, J. Hydration Characteristics of Biodentine and Theracal Used as Pulp Capping Materials. Dent. Mater. 2014, 30, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Awawdeh, L.; Al-Qudah, A.; Hamouri, H.; Chakra, R.J. Outcomes of Vital Pulp Therapy Using Mineral Trioxide Aggregate or Biodentine: A Prospective Randomized Clinical Trial. J. Endod. 2018, 44, 1603–1609. [Google Scholar] [CrossRef] [PubMed]
- Kunert, M.; Lukomska-Szymanska, M. Bio-Inductive Materials in Direct and Indirect Pulp Capping—A Review Article. Materials 2020, 13, 1204. [Google Scholar] [CrossRef] [PubMed]
- Cuadros-Fernández, C.; Lorente Rodríguez, A.I.; Sáez-Martínez, S.; García-Binimelis, J.; About, I.; Mercadé, M. Short-Term Treatment Outcome of Pulpotomies in Primary Molars Using Mineral Trioxide Aggregate and Biodentine: A Randomized Clinical Trial. Clin. Oral Investig. 2016, 20, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Taha, N.A.; Abdulkhader, S.Z. Full Pulpotomy with Biodentine in Symptomatic Young Permanent Teeth with Carious Exposure. J. Endod. 2018, 44, 932–937. [Google Scholar] [CrossRef] [PubMed]
- About, I. Biodentine: From Biochemical and Bioactive Properties to Clinical Applications. G. Ital. Endod. 2016, 30, 81–88. [Google Scholar] [CrossRef]
- Caron, G.; Azérad, J.; Faure, M.O.; Machtou, P.; Boucher, Y. Use of a New Retrograde Filling Material (Biodentine) for Endodontic Surgery: Two Case Reports. Int. J. Oral Sci. 2014, 6, 250–253. [Google Scholar] [CrossRef]
- Camilleri, J. Investigation of Biodentine as Dentine Replacement Material. J. Dent. 2013, 41, 600–610. [Google Scholar] [CrossRef]
- Parirokh, M.; Torabinejad, M.; Dummer, P.M.H. Mineral Trioxide Aggregate and Other Bioactive Endodontic Cements: An Updated Overview—Part I: Vital Pulp Therapy. Int. Endod. J. 2018, 51, 177–205. [Google Scholar] [CrossRef]
- Solanki, N.; Venkappa, K.; Shah, N. Biocompatibility and Sealing Ability of Mineral Trioxide Aggregate and Biodentine as Root-End Filling Material: A Systematic Review. J. Conserv. Dent. 2018, 21, 10–15. [Google Scholar] [PubMed]
- Camilleri, J.; Grech, L.; Galea, K.; Keir, D.; Fenech, M.; Formosa, L.; Damidot, D.; Mallia, B. Porosity and Root Dentine to Material Interface Assessment of Calcium Silicate-Based Root-End Filling Materials. Clin. Oral Investig. 2014, 18, 1437–1446. [Google Scholar] [CrossRef]
- Buła, K.; Palatyńska-Ulatowska, A.; Klimek, L. Biodentine Management and Setting Time with Vicat and Vickers Evaluation; a Survey-Based Study on Clinicians’ Experience. Arch. Mater. Sci. Eng. 2020, 103, 75–85. [Google Scholar] [CrossRef]
- American Association of Endodontists. AAE Glossary of Endodontic Terms. Available online: https://www.aae.org/specialty/clinical-resources/glossary-endodontic-terms/%0Ahttp://www.nxtbook.com/nxtbooks/aae/endodonticglossary2016/#/0 (accessed on 20 May 2023).
- Estrela, C.; Decurcio, D. de A.; Rossi-Fedele, G.; Silva, J.A.; Guedes, O.A.; Borges, Á.H. Root Perforations: A Review of Diagnosis, Prognosis and Materials. Braz. Oral Res. 2018, 32, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Palatyńska-Ulatowska, A.; Buła, K.; Klimek, L. Influence of Sodium Hypochlorite and Ultrasounds on Surface Features and Chemical Composition of Biodentine Tricalcium Silicate-Based Material. Dent. Mater. J. 2020, 39, 587–592. [Google Scholar] [CrossRef]
- Dąbrowska, K.; Palatyńska-Ulatowska, A.; Klimek, L. The Effect of Irrigation with Citric Acid on Biodentine Tricalcium Silicate-Based Cement: SEM-EDS In Vitro Study. Materials 2022, 15, 3467. [Google Scholar] [CrossRef] [PubMed]
- Zehnder, M. Root Canal Irrigants. J. Endod. 2006, 32, 389–398. [Google Scholar] [CrossRef]
- de Arruda, J.A.A.; Schuch, L.F.; Pereira, A.; Monteiro, J.L.G.C.; Melo-Júnior, P.M.R.; Mesquita, R.A.; Moreno, A.; Callou, G. Investigation of Different Sodium Hypochlorite Volumes, Concentrations and Times of Irrigation in Endodontic Therapy: A Systematic Review. Arch. Health Investig. 2019, 8, 185–191. [Google Scholar] [CrossRef]
- Moreira, R.N.; Pinto, E.B.; Galo, R.; Falci, S.G.M.; Mesquita, A.T. Passive Ultrasonic Irrigation in Root Canal: Systematic Review and Meta-Analysis. Acta Odontol. Scand. 2019, 77, 55–60. [Google Scholar] [CrossRef]
- Dioguardi, M.; Di Gioia, G.; Illuzzi, G.; Laneve, E.; Cocco, A.; Troiano, G. Endodontic Irrigants: Different Methods to Improve Efficacy and Related Problems. Eur. J. Dent. 2018, 12, 459–466. [Google Scholar] [CrossRef]
- Ballal, V.; Marques, J.N.; Campos, C.N.; Lima, C.O.; Simão, R.A.; Prado, M. Effects of Chelating Agent and Acids on Biodentine. Aust. Dent. J. 2018, 63, 170–176. [Google Scholar] [CrossRef]
- Sethi, S.; Bhushan, J.; Joshi, R.; Singla, R.; Sidhu, K. Effect of Different Irrigants on the Push-out Bond Strength of Biodentine and TheraCal LC when Used for Perforation Repair in Simulated Condition. J. Conserv. Dent. 2023, 26, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Rebolloso de Barrio, E.; Gancedo-Caravia, L.; García-Barbero, E.; Pérez-Higueras, J.J. Effect of Exposure to Root Canal Irrigants on the Push-out Bond Strength of Calcium Silicate–Based Cements. Clin. Oral Investig. 2021, 25, 3267–3274. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, M.A.; Islam, M.S.; Elbeltagy, K.; Nassar, M. Effect of Different Chelating Agents on the Shear Bond Strength of Calcium Silicate-Based Cements to Coronal Dentin. Aust. Endod. J. 2023, 49, 426–462. [Google Scholar] [CrossRef] [PubMed]
- Eren, S.K.; Örs, S.A.; Aksel, H.; Canay, Ş.; Karasan, D. Effect of Irrigants on the Color Stability, Solubility, and Surface Characteristics of Calcium-Silicate Based Cements. Restor. Dent. Endod. 2022, 47, e10. [Google Scholar] [CrossRef] [PubMed]
- Septodont Biodentine Active Biosilicate Technology Scientific File. In Vitro. 2010. Available online: http://www.oraverse.com/bio/img/Biodentine-ScientificFile.pdf (accessed on 15 May 2020).
- Borkar, S.A.; Ataide, I. Biodentine Pulpotomy Several Days after Pulp Exposure: Four Case Reports. J. Conserv. Dent. 2015, 18, 73–78. [Google Scholar] [CrossRef]
- Villat, C.; Grosgogeat, B.; Seux, D.; Farge, P. Conservative Approach of a Symptomatic Carious Immature Permanent Tooth Using a Tricalcium Silicate Cement (Biodentine): A Case Report. Restor. Dent. Endod. 2013, 38, 258–264. [Google Scholar] [CrossRef]
- Mai, S.; Kim, Y.K.; Arola, D.D.; Gu, L.S.; Kim, J.R.; Pashley, D.H.; Tay, F.R. Differential Aggressiveness of Ethylenediamine Tetraacetic Acid in Causing Canal Wall Erosion in the Presence of Sodium Hypochlorite. J. Dent. 2010, 38, 201–206. [Google Scholar] [CrossRef]
- Qian, W.; Shen, Y.; Haapasalo, M. Quantitative Analysis of the Effect of Irrigant Solution Sequences on Dentin Erosion. J. Endod. 2011, 37, 1437–1441. [Google Scholar] [CrossRef]
- Chandler, N.; Chellappa, D. Lubrication during Root Canal Treatment. Aust. Endod. J. 2019, 45, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Grech, L.; Mallia, B.; Camilleri, J. Investigation of the Physical Properties of Tricalcium Silicate Cement-Based Root-End Filling Materials. Dent. Mater. 2013, 29, e20–e28. [Google Scholar] [CrossRef] [PubMed]
- ASTM C125-20; Standard Terminology Relating to Concrete and Concrete Aggregates. ASTM International: West Conshohocken, PA, USA, 2020; pp. 1–9. [CrossRef]
- Sears, M.E. Chelation: Harnessing and Enhancing Heavy Metal Detoxification—A Review. Sci. World J. 2013, 2013, 219840. [Google Scholar] [CrossRef] [PubMed]

| Element | 45 min Setting Time | 24 h Setting Time | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No Irrigation | 17% EDTA 5 min | 17% EDTA 20 min | 17% EDTA + US 5 min | 17% EDTA + US 20 min | No Irrigation | 17% EDTA 5 min | 17% EDTA 20 min | 17% EDTA + US 5 min | 17% EDTA + US 20 min | |
| Carbon | 11.35 | 15.0 | 21.1 | 17.5 | 18.8 | 10.41 | 18.0 | 15.5 | 15.8 | 14.9 |
| Oxygen | 49.61 | 44.1 | 44.2 | 48.1 | 46.3 | 49.93 | 41.9 | 44.7 | 44.6 | 44.0 |
| Silicon | 5.96 | 7.0 | 12.5 | 11.1 | 17.2 | 9.09 | 3.1 | 3.6 | 3.4 | 7.5 |
| Zirconium | 0.56 | 2.5 | 3.2 | 3.4 | 3.8 | 0.74 | 1.,5 | 9.7 | 6.4 | 7.7 |
| Chlorine | 3.64 | 0.3 | 0.6 | 0.9 | 0.3 | 2.09 | 0.5 | 0.3 | 0.2 | 0.5 |
| Calcium | 28.87 | 29.9 | 17.5 | 18.7 | 12.9 | 27.74 | 23.4 | 24.2 | 28.0 | 15.2 |
| Natrium | 0.0 | 1.2 | 0.9 | 0.3 | 0.6 | 0.0 | 0.8 | 1.1 | 1.2 | 8.8 |
| Iron | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 0.3 | 0.2 | 0.3 |
| Aluminum | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 |
| Magnesium | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.4 | 0.6 | 0.3 | 1.1 |
| Parameter | Irrigation Protocol | ||||
|---|---|---|---|---|---|
| No Irrigation (Control) | 17% EDTA 5 min | 17% EDTA 20 min | 17% EDTA + US 5 min | 17% EDTA + US 20 min | |
| Group A—45 min Setting | |||||
| total volume loss | 602,927.89 µm3 | 417,313.69 µm3 | 295,658.41 µm3 | 811,488.96 µm3 | 692,396.96 µm3 |
| volume loss per 1 µm2 | 5.03 µm3 | 3.98 µm3 | 2.95 µm3 | 7.74 µm3 | 6.57 µm3 |
| standard deviation | 298,351.33 µm3 | 99,037.94 µm3 | 54,593.00 µm3 | 242,789.08 µm3 | 166,958.76 µm3 |
| Group B—24 h Setting | |||||
| total volume loss | 602,927.89 µm3 | 660,091.36 µm3 | 1,012,729.76 µm3 | 1,115,836.11 µm3 | 3,394,783.66 µm3 |
| volume loss per 1 µm2 | 5.03 µm3 | 6.30 µm3 | 10.64 µm3 | 11.70 µm3 | 32.71 µm3 |
| standard deviation | 298,351.33 µm3 | 196,107.81 µm3 | 302,416.00 µm3 | 256,712.78 µm3 | 738,214.44 µm3 |
| Group A | Group B | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 17% EDTA, 5 min | 17% EDTA, 20 min | 17% EDTA + US, 5 min | 17% EDTA + US, 20 min | 17% EDTA, 5 min | 17% EDTA, 20 min | 17% EDTA + US, 5 min | 17% EDTA + US, 20 min | ||
| group A | 17% EDTA, 5 min | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 0.3591 | 0.1064 | 0.0014 | |
| 17% EDTA, 20 min | 1.0000 | 0.5023 | 1.0000 | 1.0000 | 0.0750 | 0.0183 | 0.0001 | ||
| 17% EDTA + US, 5 min | 1.0000 | 0.5023 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 0.7695 | ||
| 17% EDTA + US, 20 min | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 0.2533 | ||
| group B | 17% EDTA, 5 min | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 0.1623 | |
| 17% EDTA, 20 min | 0.3591 | 0.0750 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | ||
| 17% EDTA + US, 5 min | 0.1064 | 0.0183 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | ||
| 17% EDTA + US, 20 min | 0.0014 | 0.0001 | 0.7695 | 0.2533 | 0.1623 | 1.0000 | 1.0000 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dąbrowska, K.; Palatyńska-Ulatowska, A.; Klimek, L. How Does Ethylenediaminetetraacetic Acid Irrigation Affect Biodentine? A Multimethod Ex Vivo Study. Materials 2024, 17, 1230. https://doi.org/10.3390/ma17061230
Dąbrowska K, Palatyńska-Ulatowska A, Klimek L. How Does Ethylenediaminetetraacetic Acid Irrigation Affect Biodentine? A Multimethod Ex Vivo Study. Materials. 2024; 17(6):1230. https://doi.org/10.3390/ma17061230
Chicago/Turabian StyleDąbrowska, Katarzyna, Aleksandra Palatyńska-Ulatowska, and Leszek Klimek. 2024. "How Does Ethylenediaminetetraacetic Acid Irrigation Affect Biodentine? A Multimethod Ex Vivo Study" Materials 17, no. 6: 1230. https://doi.org/10.3390/ma17061230






