The Influence of Contemporary Denture Base Fabrication Methods on Residual Monomer Content, Flexural Strength and Microhardness
Abstract
1. Introduction
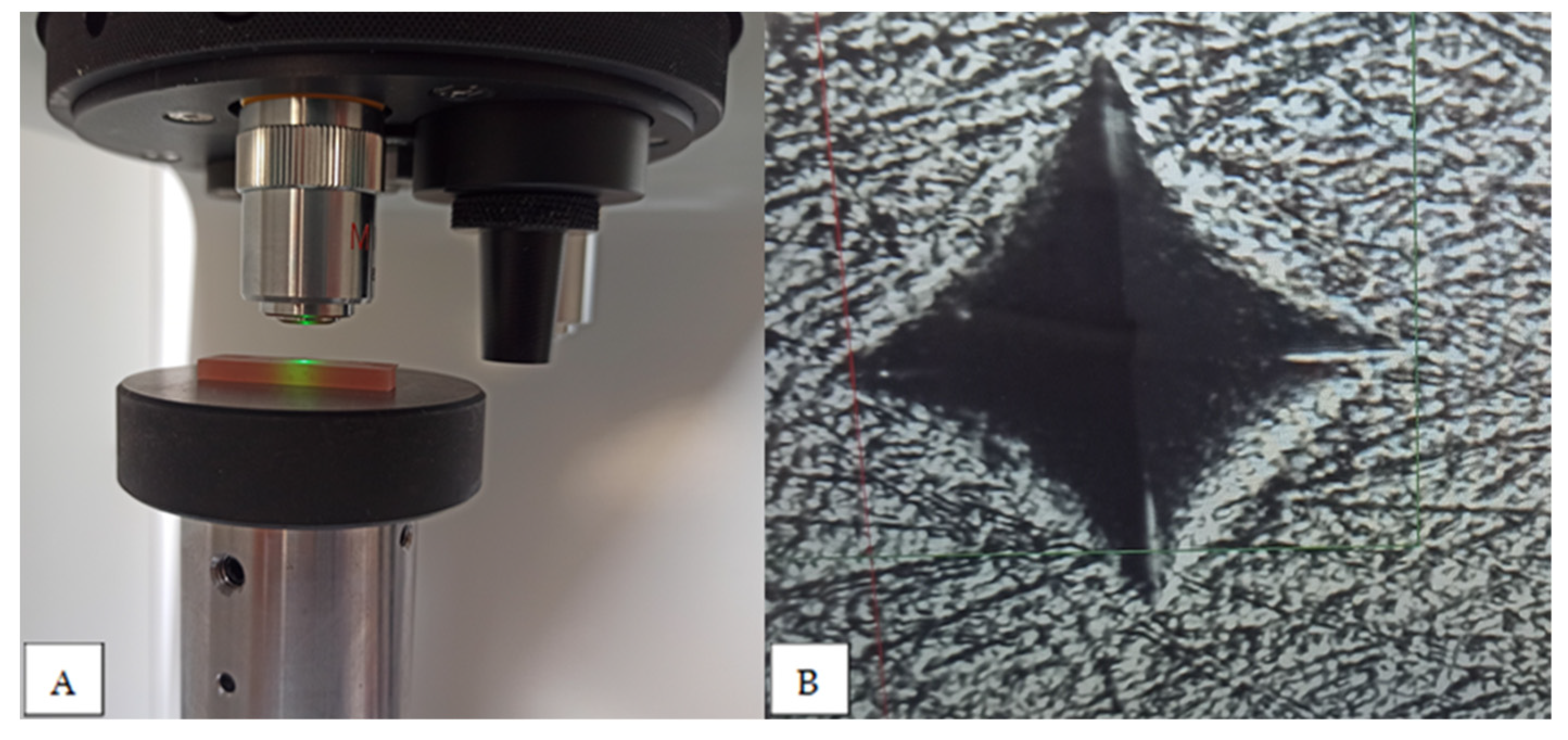
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Srinivasan, M.; Kamnoedboon, P.; McKenna, G.; Angst, L.; Schimmel, M.; Özcan, M.; Müller, F. CAD-CAM Removable Complete Dentures: A Systematic Review and Meta-Analysis of Trueness of Fit, Biocompatibility, Mechanical Properties, Surface Characteristics, Color Stability, Time-Cost Analysis, Clinical and Patient-Reported Outcomes. J. Dent. 2021, 113, 103777. [Google Scholar] [CrossRef] [PubMed]
- Perea-Lowery, L.; Gibreel, M.; Vallittu, P.K.; Lassila, L.V. 3D-Printed vs. Heat-Polymerizing and Autopolymerizing Denture Base Acrylic Resins. Materials 2021, 14, 5781. [Google Scholar] [CrossRef]
- Keul, C.; Seidl, J.; Güth, J.-F.; Liebermann, A. Impact of Fabrication Procedures on Residual Monomer Elution of Conventional Polymethyl Methacrylate (PMMA)—A Measurement Approach by UV/Vis Spectrophotometry. Clin. Oral Investig. 2020, 24, 4519–4530. [Google Scholar] [CrossRef] [PubMed]
- Casucci, A.; Verniani, G.; Barbieri, A.L.; Ricci, N.M.; Ferrari Cagidiaco, E.; Ferrari, M. Flexural Strength Analysis of Different Complete Denture Resin-Based Materials Obtained by Conventional and Digital Manufacturing. Materials 2023, 16, 6559. [Google Scholar] [CrossRef] [PubMed]
- Al-Dulaijan, Y.A.; Alsulaimi, L.; Alotaibi, R.; Alboainain, A.; Alalawi, H.; Alshehri, S.; Khan, S.Q.; Alsaloum, M.; AlRumaih, H.S.; Alhumaidan, A.A.; et al. Comparative Evaluation of Surface Roughness and Hardness of 3D Printed Resins. Materials 2022, 15, 6822. [Google Scholar] [CrossRef]
- Alharethi, N.A. Evaluation of the Influence of Build Orientation on the Surface Roughness and Flexural Strength of 3D-Printed Denture Base Resin and Its Comparison with CAD-CAM Milled Denture Base Resin. Eur. J. Dent. 2023, s-0043-1768972. [Google Scholar] [CrossRef]
- Alqutaibi, A.Y.; Baik, A.; Almuzaini, S.A.; Farghal, A.E.; Alnazzawi, A.A.; Borzangy, S.; Aboalrejal, A.N.; AbdElaziz, M.H.; Mahmoud, I.I.; Zafar, M.S. Polymeric Denture Base Materials: A Review. Polymers 2023, 15, 3258. [Google Scholar] [CrossRef]
- Bidra, A.S.; Taylor, T.D.; Agar, J.R. Computer-Aided Technology for Fabricating Complete Dentures: Systematic Review of Historical Background, Current Status, and Future Perspectives. J. Prosthet. Dent. 2013, 109, 361–366. [Google Scholar] [CrossRef]
- Mubaraki, M.Q.; Moaleem, M.M.A.; Alzahrani, A.H.; Shariff, M.; Alqahtani, S.M.; Porwal, A.; Al-Sanabani, F.A.; Bhandi, S.; Tribst, J.P.M.; Heboyan, A.; et al. Assessment of Conventionally and Digitally Fabricated Complete Dentures: A Comprehensive Review. Materials 2022, 15, 3868. [Google Scholar] [CrossRef]
- Steinmassl, P.-A.; Wiedemair, V.; Huck, C.; Klaunzer, F.; Steinmassl, O.; Grunert, I.; Dumfahrt, H. Do CAD/CAM Dentures Really Release Less Monomer than Conventional Dentures? Clin. Oral Investig. 2017, 21, 1697–1705. [Google Scholar] [CrossRef]
- Altarazi, A.; Haider, J.; Alhotan, A.; Silikas, N.; Devlin, H. 3D Printed Denture Base Material: The Effect of Incorporating TiO2 Nanoparticles and Artificial Ageing on the Physical and Mechanical Properties. Dent. Mater. J. 2023, 39, 1122–1136. [Google Scholar] [CrossRef]
- Becerra, J.; Mainjot, A.; Hüe, O.; Sadoun, M.; Nguyen, J. Influence of High-Pressure Polymerization on Mechanical Properties of Denture Base Resins. J. Prosthodont. 2021, 30, 128–134. [Google Scholar] [CrossRef]
- Pacquet, W.; Benoit, A.; Hatège-Kimana, C.; Wulfman, C. Mechanical Properties of CAD/CAM Denture Base Resins. Int. J. Prosthodont. 2018, 32, 104–106. [Google Scholar] [CrossRef]
- Baba, N.Z.; Goodacre, B.J.; Goodacre, C.J.; Müller, F.; Wagner, S. CAD/CAM Complete Denture Systems and Physical Properties: A Review of the Literature. J. Prosthodont. 2021, 30, 113–124. [Google Scholar] [CrossRef]
- Gad, M.M.; Fouda, S.M.; Abualsaud, R.; Alshahrani, F.A.; Al-Thobity, A.M.; Khan, S.Q.; Akhtar, S.; Ateeq, I.S.; Helal, M.A.; Al-Harbi, F.A. Strength and Surface Properties of a 3D-Printed Denture Base Polymer. J. Prosthodont. 2022, 31, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Kattadiyil, M.T.; AlHelal, A. An Update on Computer-Engineered Complete Dentures: A Systematic Review on Clinical Outcomes. J. Prosthet. Dent. 2017, 117, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.S.; Kim, J.-E.; Jeong, S.H.; Choi, Y.J.; Ryu, J.J. Printing Accuracy, Mechanical Properties, Surface Characteristics, and Microbial Adhesion of 3D-Printed Resins with Various Printing Orientations. J. Prosthet. Dent. 2020, 124, 468–475. [Google Scholar] [CrossRef] [PubMed]
- AlGhamdi, M.A.; Fouda, S.M.; Taymour, N.; Akhtar, S.; Khan, S.Q.; Ali, M.S.; Elakel, A.M.; Nassar, E.A.; Gad, M.M. Comparative Evaluation of TiO2 Nanoparticle Addition and Postcuring Time on the Flexural Properties and Hardness of Additively Fabricated Denture Base Resins. Nanomaterials 2023, 13, 3061. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-E.; Alauddin, M.S.; Mohd Ghazali, M.I.; Said, Z.; Mohamad Zol, S. Effect of Different Vat Polymerization Techniques on Mechanical and Biological Properties of 3D-Printed Denture Base. Polymers 2023, 15, 1463. [Google Scholar] [CrossRef] [PubMed]
- Lung, C.Y.K.; Darvell, B.W. Minimization of the Inevitable Residual Monomer in Denture Base Acrylic. Dent. Mater. 2005, 21, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, E.A.; Durkan, R.; Koroglu, A.; Bagis, B. Comparative Effect of Different Polymerization Techniques on Residual Monomer and Hardness Properties of PMMA-Based Denture Resins. J. Appl. Biomater. Funct. Mater. 2014, 12, 228–233. [Google Scholar] [CrossRef]
- Bartoloni, J.A.; Murchison, D.F.; Wofford, D.T.; Sarkar, N.K. Degree of Conversion in Denture Base Materials for Varied Polymerization Techniques 1. J. Oral Rehabil. 2000, 27, 488–493. [Google Scholar] [CrossRef]
- Farina, A.P.; Cecchin, D.; Soares, R.G.; Botelho, A.L.; Takahashi, J.M.F.K.; Mazzetto, M.O.; Mesquita, M.F. Evaluation of Vickers Hardness of Different Types of Acrylic Denture Base Resins with and without Glass Fibre Reinforcement. Gerodontology 2012, 29, e155–e160. [Google Scholar] [CrossRef]
- Ayman, A.-D. The Residual Monomer Content and Mechanical Properties of CAD\CAM Resins Used in the Fabrication of Complete Dentures as Compared to Heat Cured Resins. Electron. Physician 2017, 9, 4766–4772. [Google Scholar] [CrossRef]
- Engler, M.L.P.D.; Güth, J.-F.; Keul, C.; Erdelt, K.; Edelhoff, D.; Liebermann, A. Residual Monomer Elution from Different Conventional and CAD/CAM Dental Polymers during Artificial Aging. Clin. Oral Investig. 2020, 24, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Gungor, H.; Gundogdu, M.; Alkurt, M.; Yesil Duymus, Z. Effect of Polymerization Cycles on Flexural Strengths and Microhardness of Different Denture Base Materials. Dent. Mater. J. 2017, 36, 168–173. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Seo, R.S.; Vergani, C.E.; Giampaolo, E.T.; Pavarina, A.C.; Machado, A.L. Effect of post-polymerization treatments on the flexural strength and vickers hardness of reline and acrylic denture base resins. J. Appl. Oral Sci. 2007, 15, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Sadamori, S.; Kotani, H.; Hamada, T. The Usage Period of Dentures and Their Residual Monomer Contents. J. Prosthet. Dent. 1992, 68, 374–376. [Google Scholar] [CrossRef] [PubMed]
- De Andrade Lima Chaves, C.; Machado, A.L.; Vergani, C.E.; De Souza, R.F.; Giampaolo, E.T. Cytotoxicity of Denture Base and Hard Chairside Reline Materials: A Systematic Review. J. Prosthet. Dent. 2012, 107, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Rashid, H.; Sheikh, Z.; Vohra, F. Allergic Effects of the Residual Monomer Used in Denture Base Acrylic Resins. Eur. J. Dent. 2015, 9, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, B.C.; Chen, J.-H.; Kontogiorgos, E.D.; Murchison, D.F.; Nagy, W.W. Flexural Strength of Denture Base Acrylic Resins Processed by Conventional and CAD-CAM Methods. J. Prosthet. Dent. 2020, 123, 641–646. [Google Scholar] [CrossRef]
- Al-Dwairi, Z.N.; Tahboub, K.Y.; Baba, N.Z.; Goodacre, C.J. A Comparison of the Flexural and Impact Strengths and Flexural Modulus of CAD/CAM and Conventional Heat-Cured Polymethyl Methacrylate (PMMA). J. Prosthodont. 2020, 29, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Rueggeberg, F.A.; Craig, R.G. Correlation of Parameters Used to Estimate Monomer Conversion in a Light-Cured Composite. J. Dent. Res. 1988, 67, 932–937. [Google Scholar] [CrossRef]
- Lee, H.-H.; Lee, C.-J.; Asaoka, K. Correlation in the Mechanical Properties of Acrylic Denture Base Resins. Dent. Mater. J. 2012, 31, 157–164. [Google Scholar] [CrossRef] [PubMed]
- ISO 20795-1:2013; Dentistry—Base Polymers—Part 1: Denture Base Polymers. International Organisation for Standardization: Geneva, Switzerland, 2013.
- Blagojevic, V.; Murphy, V.M. Microwave Polymerization of Denture Base Materials. A Comparative Study. J. Oral Rehabil. 1999, 26, 804–808. [Google Scholar] [CrossRef]
- Abualsaud, R.; Gad, M. Flexural Strength of CAD/CAM Denture Base Materials: Systematic Review and Meta-Analysis of in-Vitro Studies. J. Int. Soc. Prevent Communit Dent. 2022, 12, 160. [Google Scholar] [CrossRef] [PubMed]
- Raszewski, Z. Acrylic Resins in the CAD/CAM Technology: A Systematic Literature Review. Dent. Med. Probl. 2020, 57, 449–454. [Google Scholar] [CrossRef]
- Bayraktar, G.; Duran, O.; Guvener, B. Effect of Glass Fibre Reinforcement on Residual Methyl Methacrylate Content of Denture Base Polymers. J. Dent. 2003, 31, 297–302. [Google Scholar] [CrossRef]
- Goiato, M.; Freitas, E.; Dos Santos, D.; De Medeiros, R.; Sonego, M. Acrylic Resin Cytotoxicity for Denture Base: Literature Review. Adv. Clin. Exp. Med. 2015, 24, 679–686. [Google Scholar] [CrossRef]
- Bural, C.; AktaŞ, E.; Deniz, G.; Ünlüçerçi, Y.; Bayraktar, G. Effect of Leaching Residual Methyl Methacrylate Concentrations on in Vitro Cytotoxicity of Heat Polymerized Denture Base Acrylic Resin Processed with Different Polymerization Cycles. J. Appl. Oral. Sci. 2011, 19, 306–312. [Google Scholar] [CrossRef]
- Borak, J.; Fields, C.; Andrews, L.S.; Pemberton, M.A. Methyl Methacrylate and Respiratory Sensitization: A Critical Review. Crit. Rev. Toxicol. 2011, 41, 230–268. [Google Scholar] [CrossRef]
- Kedjarune, U.; Charoenworaluk, N.; Koontongkaew, S. Release of Methyl Methacrylate from Heat-curved and Autopolymerized Resins: Cytotoxicity Testing Related to Residual Monomer. Aust. Dent. J. 1999, 44, 25–30. [Google Scholar] [CrossRef]
- Sadamori, S.; Ganefiyanti, T.; Hamada, T.; Arima, T. Influence of Thickness and Location on the Residual Monomer Content of Denture Base Cured by Three Processing Methods. J. Prosthet. Dent. 1994, 72, 19–22. [Google Scholar] [CrossRef]
- Vallittu, P.K.; Miettinen, V.; Alakuijala, P. Residual Monomer Content and Its Release into Water from Denture Base Materials. Dent. Mater. 1995, 11, 338–342. [Google Scholar] [CrossRef]
- Murakami, N.; Wakabayashi, N.; Matsushima, R.; Kishida, A.; Igarashi, Y. Effect of High-Pressure Polymerization on Mechanical Properties of PMMA Denture Base Resin. J. Mech. Behav. Biomed. Mater. 2013, 20, 98–104. [Google Scholar] [CrossRef]
- Lourinho, C.; Salgado, H.; Correia, A.; Fonseca, P. Mechanical Properties of Polymethyl Methacrylate as Denture Base Material: Heat-Polymerized vs. 3D-Printed—Systematic Review and Meta-Analysis of In Vitro Studies. Biomedicines 2022, 10, 2565. [Google Scholar] [CrossRef]
- Iwaki, M.; Kanazawa, M.; Arakida, T.; Minakuchi, S. Mechanical Properties of a Polymethyl Methacrylate Block for CAD/CAM Dentures. J. Oral. Sci. 2020, 62, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Alp, G.; Murat, S.; Yilmaz, B. Comparison of Flexural Strength of Different CAD/CAM PMMA-Based Polymers. J. Prosthodont. 2019, 28, e491–e495. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, M.; Nanditha Kumar, M.; RaghavendraSwamy, K.N.; Thippeswamy, H.M. Flexural Strength and Impact Strength of Heat-Cured Acrylic and 3D Printed Denture Base Resins- A Comparative in Vitro Study. J. Oral Biol. Craniofac Res. 2022, 12, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Prpić, V.; Schauperl, Z.; Ćatić, A.; Dulčić, N.; Čimić, S. Comparison of Mechanical Properties of 3D-Printed, CAD/CAM, and Conventional Denture Base Materials. J. Prosthodont. 2020, 29, 524–528. [Google Scholar] [CrossRef] [PubMed]
- al-Qarni, F.D.; Gad, M.M. Printing Accuracy and Flexural Properties of Different 3D-Printed Denture Base Resins. Materials 2022, 15, 2410. [Google Scholar] [CrossRef]
- Fiore, A.D.; Meneghello, R.; Brun, P.; Rosso, S.; Gattazzo, A.; Stellini, E.; Yilmaz, B. Comparison of the Flexural and Surface Properties of Milled, 3D-Printed, and Heat Polymerized PMMA Resins for Denture Bases: An In Vitro Study. J. Prosthodont. Res. 2022, 66, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Pituru, S.M.; Greabu, M.; Totan, A.; Imre, M.; Pantea, M.; Spinu, T.; Tancu, A.M.C.; Popoviciu, N.O.; Stanescu, I.-I.; Ionescu, E. A Review on the Biocompatibility of PMMA-Based Dental Materials for Interim Prosthetic Restorations with a Glimpse into Their Modern Manufacturing Techniques. Materials 2020, 13, 2894. [Google Scholar] [CrossRef] [PubMed]
- Steinmassl, O.; Offermanns, V.; Stöckl, W.; Dumfahrt, H.; Grunert, I.; Steinmassl, P.-A. In Vitro Analysis of the Fracture Resistance of CAD/CAM Denture Base Resins. Materials 2018, 11, 401. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, S.; Li, R.; Wang, Y.; Fan, X.; Gong, W.; Ma, Y. The Mechanical and Antibacterial Properties of Boron Nitride/Silver Nanocomposite Enhanced Polymethyl Methacrylate Resin for Application in Oral Denture Bases. Biomimetics 2022, 7, 138. [Google Scholar] [CrossRef]
- Alshaikh, A.A.; Khattar, A.; Almindil, I.A.; Alsaif, M.H.; Akhtar, S.; Khan, S.Q.; Gad, M.M. 3D-Printed Nanocomposite Denture-Base Resins: Effect of ZrO2 Nanoparticles on the Mechanical and Surface Properties In Vitro. Nanomaterials 2022, 12, 2451. [Google Scholar] [CrossRef] [PubMed]
- Unkovskiy, A.; Bui, P.H.-B.; Schille, C.; Geis-Gerstorfer, J.; Huettig, F.; Spintzyk, S. Objects Build Orientation, Positioning, and Curing Influence Dimensional Accuracy and Flexural Properties of Stereolithographically Printed Resin. Dent. Mater. 2018, 34, e324–e333. [Google Scholar] [CrossRef] [PubMed]
- Altarazi, A.; Haider, J.; Alhotan, A.; Silikas, N.; Devlin, H. Assessing the Physical and Mechanical Properties of 3D Printed Acrylic Material for Denture Base Application. Dent. Mater. 2022, 38, 1841–1854. [Google Scholar] [CrossRef]
- Zeidan, A.E.; Abd Elrahim, R.; Abd El Hakim, A.; Harby, N.; Helal, M. Evaluation of Surface Properties and Elastic Modulus of CAD-CAM Milled, 3D Printed, and Compression Moulded Denture Base Resins: An in Vitro Study. J. Int. Soc. Prevent Communit Dent. 2022, 12, 630. [Google Scholar] [CrossRef]



| Name of the Material | Manufacturer | Description and Purpose of the Material |
|---|---|---|
| Meliodent heat cure | Kulzer, Hanau, Germany | Denture base material, PMMA, heat cured |
| Vertex Thermosens | Vetex Dental, Soesterberg, The Netherlands | Denture base material, polyamide, injection technique |
| Ivobase CAD pink V | Ivoclar Vivadent, Schaan, Liechtenstein | CAD-CAM denture base material, subtractive manufacturing |
| Polident pink CAD-CAM disc basic | Polident d.o.o., Volčja draga, Slovenia | CAD-CAM denture base material, subtractive manufacturing |
| Anaxdent pink blank U medium pink | Anaxdent GmbH, Stuttgart, Germany | CAD-CAM denture base material, subtractive manufacturing |
| Freeprint denture | Detax, Ettlingen, Germany | CAD-CAM denture base material, additive manufacturing |
| Imprimo LC denture | Scheu, Iserlohn, Germany | CAD-CAM denture base material, additive manufacturing |
| Chemical Name | Manufacturer | Purity |
|---|---|---|
| Acetone | Acros Organics, Geel, Belgium | 99.8% |
| Diclofenac sodium | Sigma Aldrich, St. Louis, MO, USA | ≥98% |
| Hydroquinone | Fluka, Gillingham, UK | ≥99% |
| Methanol | J.T. Baker, Phillipsburg, NJ, USA | ≥99.9% |
| Methil methacrylate, stabilized | Acros Organics, Geel, Belgium | ≥99% |
| Formic acid | Fischer Scientific, Waltham, MA, USA | ≥99% |
| Residual Monomer [% Mass Fraction] | Flexural Strength [MPa] | Microhardness [VHN] | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| 1. Meliodent Heat Cure | 0.53 3,5 | 0.07 | 97.06 2,3,7 | 6.25 | 20.58 2,3,4,5,7 | 0.52 |
| 2. Vertex Thermosens | 62.57 1,4,5,6 | 5.69 | 10.61 1,3,4,5,6,7 | 0.24 | ||
| 3. Ivobase CAD Pink | 3.05 1,4,6,7 | 0.58 | 79.06 1,6 | 4.65 | 17.23 1,2,4,5,6 | 0.99 |
| 4. Polident Pink CAD-CAM | 0.05 3,5 | 0.03 | 96.27 2,7 | 5.81 | 22.86 1,2,3,4,6,7 | 0.72 |
| 5. Anaxdent Pink Blank | 3.20 1,4,6,7 | 1.14 | 83.31 2,6 | 3.21 | 18.83 1,2,3,4,6,7 | 0.48 |
| 6. Freeprint Denture | 0.36 3,5 | 0.16 | 103.33 2,3,5,7 | 16.71 | 21.30 2,3,4,5,7 | 0.45 |
| 7. Imprimo LC Denture | 0.34 3,5 | 0.13 | 69.75 1,4,6 | 7.63 | 16.55 1,2,4,5,6 | 0.81 |
| Microhardness (VHN) | Residual Monomer (% Mass Fraction) | Flexural Strength (Mpa) | ||
|---|---|---|---|---|
| MELIODENT | ||||
| MICROHARDNESS (VHN) | Pearson Correlation | 1.000 | 0.655 | −0.822 |
| P | 0.078 | 0.088 | ||
| RESIDUAL MONOMER (% mass fraction) | Pearson Correlation | 0.655 | 1.000 | −0.976 |
| P | 0.078 | 0.004 * | ||
| FLEXURAL STRENGTH (MPa) | Pearson Correlation | −0.822 | −0.976 | 1.000 |
| P | 0.088 | 0.004 * | ||
| VERTEX THERMOSENS | ||||
| MICROHARDNESS (VHN) | Pearson Correlation | 1.000 | / | −0.096 |
| P | / | 0.878 | ||
| RESIDUAL MONOMER (% mass fraction) | Pearson Correlation | / | / | / |
| P | / | / | / | |
| FLEXURAL STRENGTH (MPa) | Pearson Correlation | −0.096 | / | 1.000 |
| P | 0.878 | / | ||
| SUBTRACTIVE MANUFACTURED MATERIALS | ||||
| MICROHARDNESS (VHN) | Pearson Correlation | 1 | −0.815 | 0.826 |
| P | 0.000 * | 0.000 * | ||
| RESIDUAL MONOMER (% mass fraction) | Pearson Correlation | −0.815 | 1 | −0.756 |
| P | 0.000 * | 0.001 * | ||
| FLEXURAL STRENGTH (MPa) | Pearson Correlation | 0.826 | −0.756 | 1 |
| P | 0.000 * | 0.001 * | ||
| ADDITIVE MANUFACTURED MATERIALS | ||||
| MICROHARDNESS (VHN) | Pearson Correlation | 1 | 0.074 | 0.765 |
| P | 0.786 | 0.010 * | ||
| RESIDUAL MONOMER (% mass fraction) | Pearson Correlation | 0.074 | 1 | 0.215 |
| P | 0.786 | 0.551 | ||
| FLEXURAL STRENGTH (MPa) | Pearson Correlation | 0.765 | 0.215 | 1 |
| P | 0.010 * | 0.551 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vuksic, J.; Pilipovic, A.; Poklepovic Pericic, T.; Kranjcic, J. The Influence of Contemporary Denture Base Fabrication Methods on Residual Monomer Content, Flexural Strength and Microhardness. Materials 2024, 17, 1052. https://doi.org/10.3390/ma17051052
Vuksic J, Pilipovic A, Poklepovic Pericic T, Kranjcic J. The Influence of Contemporary Denture Base Fabrication Methods on Residual Monomer Content, Flexural Strength and Microhardness. Materials. 2024; 17(5):1052. https://doi.org/10.3390/ma17051052
Chicago/Turabian StyleVuksic, Josip, Ana Pilipovic, Tina Poklepovic Pericic, and Josip Kranjcic. 2024. "The Influence of Contemporary Denture Base Fabrication Methods on Residual Monomer Content, Flexural Strength and Microhardness" Materials 17, no. 5: 1052. https://doi.org/10.3390/ma17051052
APA StyleVuksic, J., Pilipovic, A., Poklepovic Pericic, T., & Kranjcic, J. (2024). The Influence of Contemporary Denture Base Fabrication Methods on Residual Monomer Content, Flexural Strength and Microhardness. Materials, 17(5), 1052. https://doi.org/10.3390/ma17051052









