Advances in Nanoarchitectonics: A Review of “Static” and “Dynamic” Particle Assembly Methods
Abstract
1. Introduction
2. Self-Assembly
3. Static Assembly Methods
3.1. Layer-by-Layer Assembly
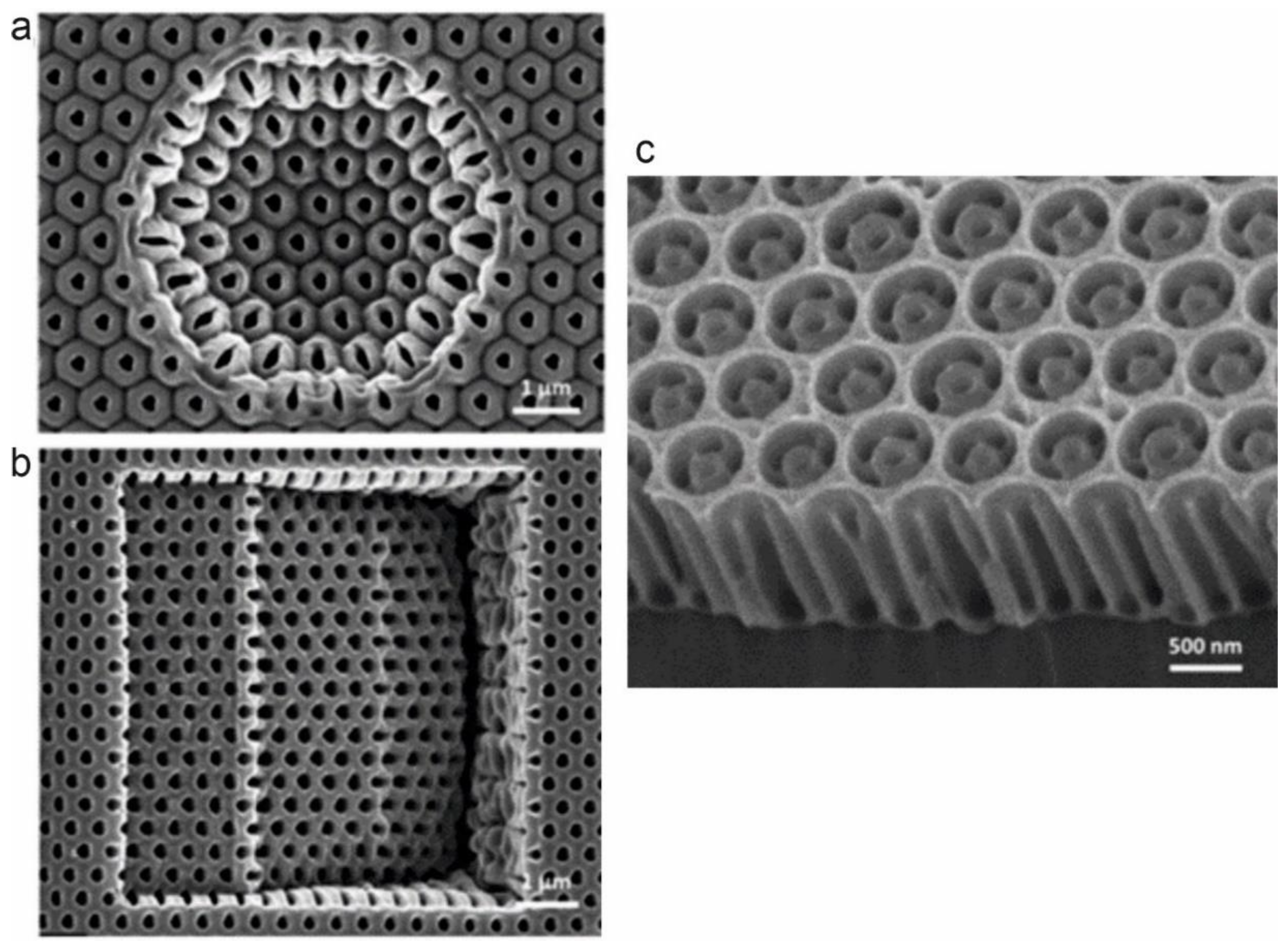
3.2. Template-Assisted Assembly
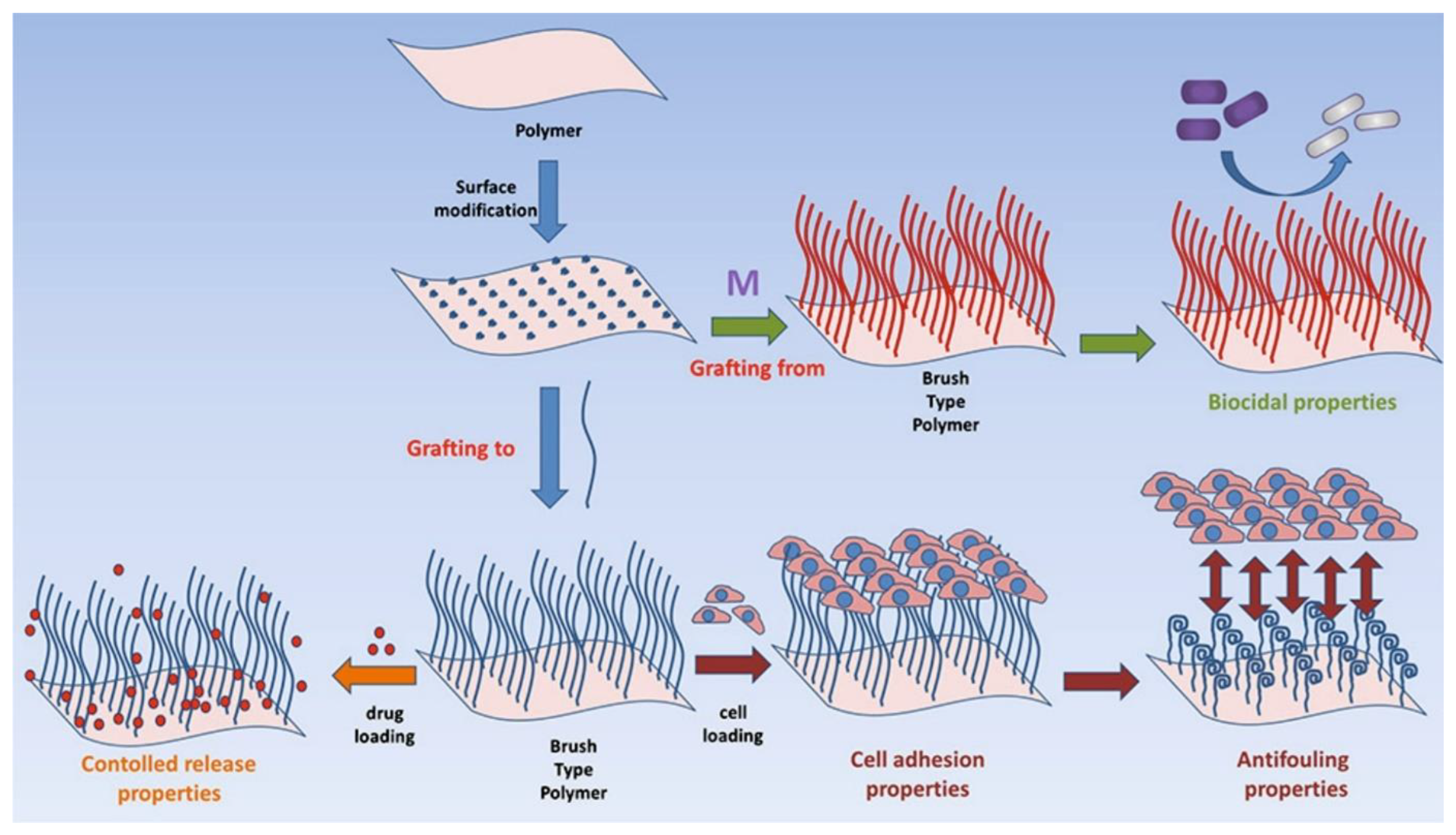
3.3. Polymer Brushes for Surface Assembly
4. Dynamic Assembly Methods
4.1. Shear-Driven Assembly
4.1.1. Fluidic Assembly
Drop Coating
Microfluidic Devices
Capillary Assembly
4.1.2. Dip Coating
4.1.3. Spin Coating
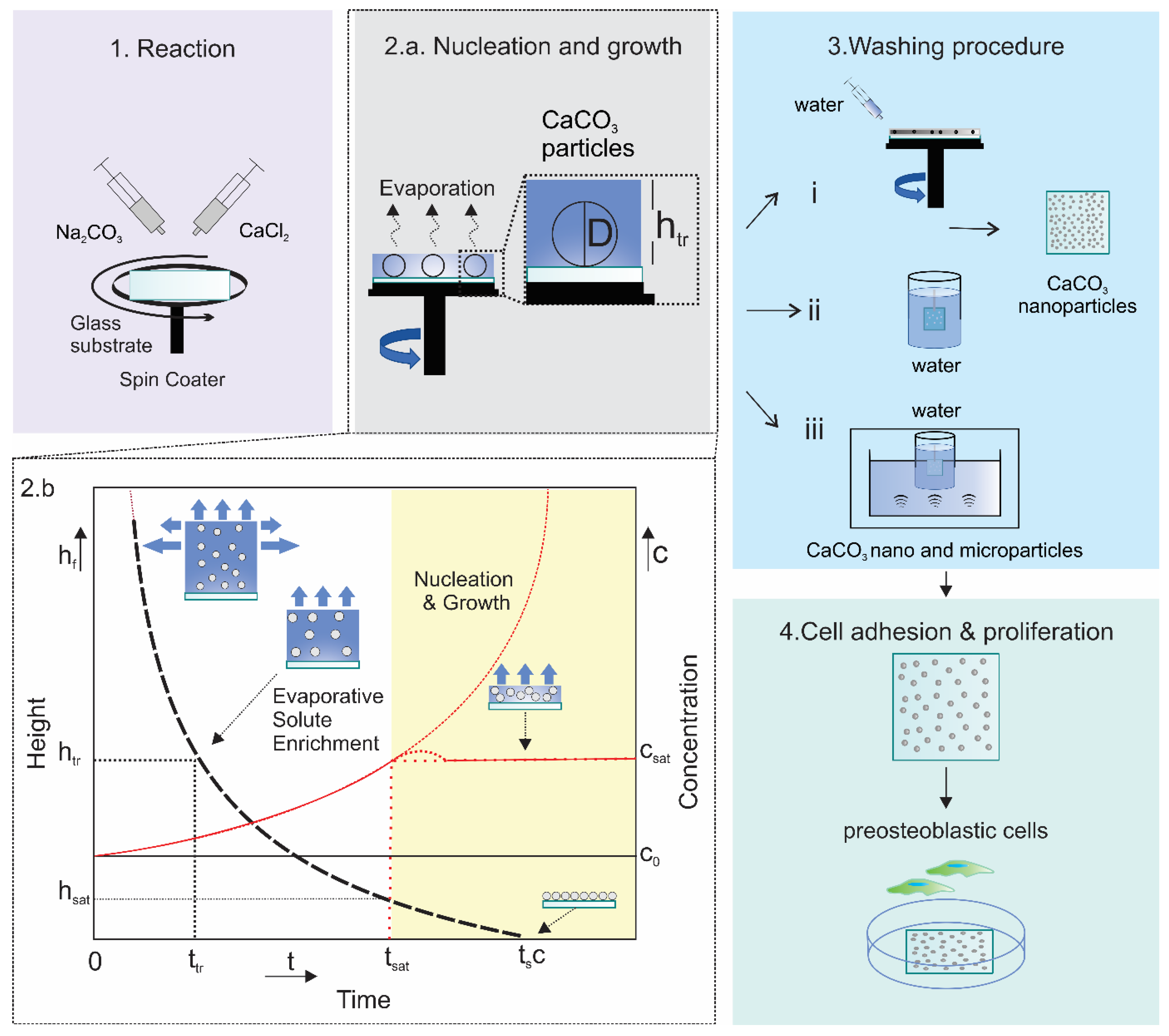
4.1.4. Other Dynamic Methods
4.2. Field-Driven Assembly
4.2.1. Electromagnetic Field
4.2.2. Electric Field
4.2.3. Magnetic Field
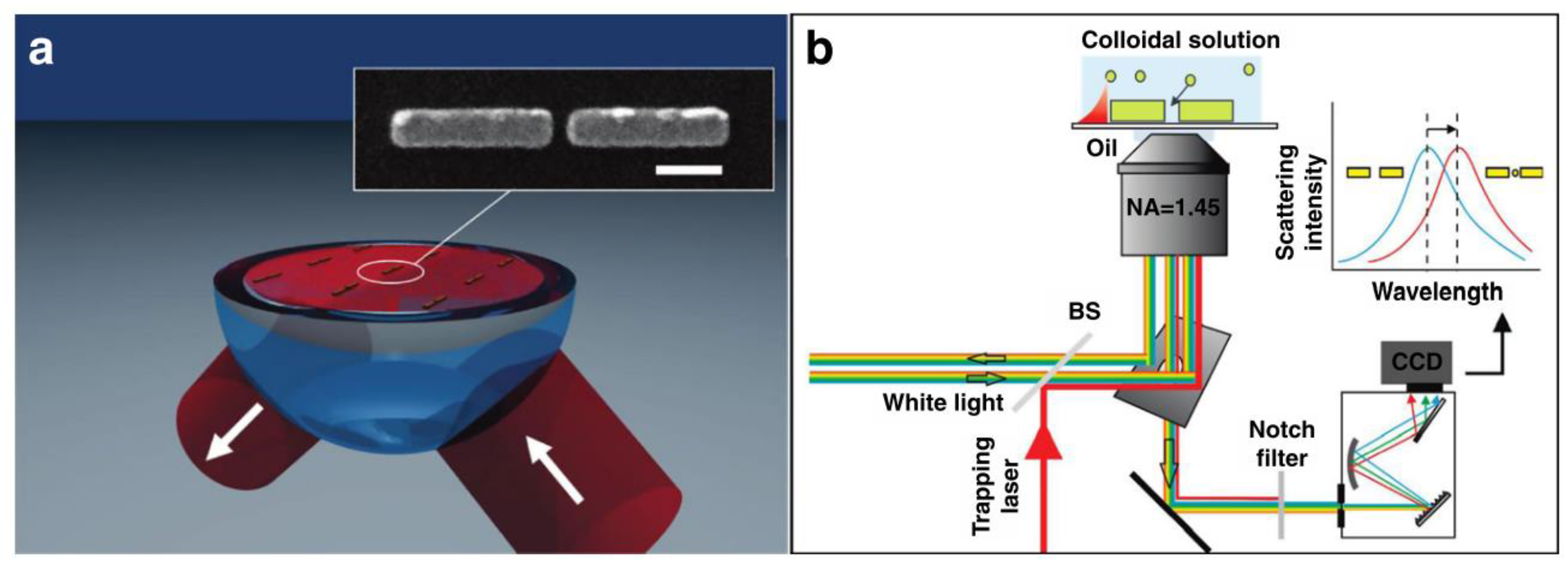
4.2.4. Acoustic Field
5. Advantages and Limitations
6. Nanoarchitectonics in Biomaterial Sciences
6.1. Nanoarchitectonics for Drug Delivery
6.2. Nanoarchitectonics for Tissue Engineering
6.3. Nanoarchitectonics for Biosensors
6.4. Nanoarchitectonics for Imaging and Diagnostics
7. Comparative Analysis of Static and Dynamic Methods: Challenges and Future Perspectives
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, J.; Lin, Z.; Ju, Y.; Rahim, M.A.; Richardson, J.J.; Caruso, F. Polyphenol-Mediated Assembly for Particle Engineering. Acc. Chem. Res. 2020, 53, 1269–1278. [Google Scholar] [CrossRef]
- Cao, L.; Huang, Y.; Parakhonskiy, B.; Skirtach, A.G. Nanoarchitectonics beyond Perfect Order—Not Quite Perfect but Quite Useful. Nanoscale 2022, 14, 15964–16002. [Google Scholar] [CrossRef]
- Siddiqui, A.; Thawarkar, S.; Singh, S.P. A Novel Perylenediimide Molecule: Synthesis, Structural Property Relationship and Nanoarchitectonics. J. Solid State Chem. 2022, 306, 122687. [Google Scholar] [CrossRef]
- Ariga, K.; Aono, M. Nanoarchitectonics. Jpn. J. Appl. Phys. 2016, 55, 1102A6. [Google Scholar] [CrossRef]
- Avinash, M.B.; Govindaraju, T. Architectonics: Design of Molecular Architecture for Functional Applications. Acc. Chem. Res. 2018, 51, 414–426. [Google Scholar] [CrossRef]
- Saveleva, M.S.; Eftekhari, K.; Abalymov, A.A.; Douglas, T.E.L.; Volodkin, D.V.; Parakhonskiy, B.V.; Skirtach, A.G. Hierarchy of Hybrid Materials—The Place of Inorganics-in-Organics in It, Their Composition and Applications. Front. Chem. 2019, 7, 179. [Google Scholar] [CrossRef]
- Song, J.; Vikulina, A.S.; Parakhonskiy, B.V.; Skirtach, A.G. Hierarchy of Hybrid Materials. Part-II: The Place of Organics-on-Inorganics in It, Their Composition and Applications. Front. Chem. 2023, 11, 1078840. [Google Scholar] [CrossRef]
- Ariga, K.; Jia, X.; Song, J.; Hill, J.P.; Leong, D.T.; Jia, Y.; Li, J. Nanoarchitectonics beyond Self-Assembly: Challenges to Create Bio-Like Hierarchic Organization. Angew. Chemie—Int. Ed. 2020, 59, 15424–15446. [Google Scholar] [CrossRef]
- Ariga, K.; Lvov, Y.; Decher, G. There Is Still Plenty of Room for Layer-by-Layer Assembly for Constructing Nanoarchitectonics-Based Materials and Devices. Phys. Chem. Chem. Phys. 2022, 24, 4097–4115. [Google Scholar] [CrossRef]
- Ariga, K.; Ji, Q.; Hill, J.P.; Bando, Y.; Aono, M. Forming Nanomaterials as Layered Functional Structures toward Materials Nanoarchitectonics. NPG Asia Mater. 2012, 4, e17. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Li, J.; Wang, Y. Directional and Reconfigurable Assembly of Metallodielectric Patchy Particles. ACS Nano 2021, 15, 5439–5448. [Google Scholar] [CrossRef]
- Shrestha, L.K.; Mori, T.; Ariga, K. Dynamic Nanoarchitectonics: Supramolecular Polymorphism and Differentiation, Shape-Shifter and Hand-Operating Nanotechnology. Curr. Opin. Colloid Interface Sci. 2018, 35, 68–80. [Google Scholar] [CrossRef]
- Müller, M. Process-Directed Self-Assembly of Copolymers: Results of and Challenges for Simulation Studies. Prog. Polym. Sci. 2020, 101, 101198. [Google Scholar] [CrossRef]
- Quist, A.P.; Pavlovic, E.; Oscarsson, S. Recent Advances in Microcontact Printing. Anal. Bioanal. Chem. 2005, 381, 591–600. [Google Scholar] [CrossRef]
- Ariga, K. Biomimetic and Biological Nanoarchitectonics. Int. J. Mol. Sci. 2022, 23, 3577. [Google Scholar] [CrossRef]
- Ruiz-Clavijo, A.; Caballero-Calero, O.; Martín-González, M. Revisiting Anodic Alumina Templates: From Fabrication to Applications. Nanoscale 2021, 13, 2227–2265. [Google Scholar] [CrossRef]
- Varney, M.C.M.; Zhang, Q.; Senyuk, B.; Smalyukh, I.I. Self-Assembly of Colloidal Particles in Deformation Landscapes of Electrically Driven Layer Undulations in Cholesteric Liquid Crystals. Phys. Rev. E 2016, 94, 042709. [Google Scholar] [CrossRef]
- Ariga, K.; Watanabe, S.; Mori, T.; Takeya, J. Soft 2D Nanoarchitectonics. NPG Asia Mater. 2018, 10, 90–106. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Grzybowski, B. Self-Assembly at All Scales. Science 2002, 295, 1155–2421. [Google Scholar] [CrossRef]
- Lehn, J.M. Toward Self-Organization and Complex Matter. Science 2002, 295, 2400–2403. [Google Scholar] [CrossRef]
- Cui, X.; Shih, E.M.; Jauregui, L.A.; Chae, S.H.; Kim, Y.D.; Li, B.; Seo, D.; Pistunova, K.; Yin, J.; Park, J.H.; et al. Lowerature Ohmic Contact to Monolayer MoS2 by van Der Waals Bonded Co/h-BN Electrodes. Nano Lett. 2017, 17, 4781–4786. [Google Scholar] [CrossRef]
- Lehn, J.-M. Supramolecular Chemistry—Scope and Perspectives Molecules, Supermolecules, and Molecular Devices(Nobel Lecture). Angew. Chem. Int. Ed. Engl. 1988, 27, 89–112. [Google Scholar] [CrossRef]
- Wang, C.; Murugadoss, V.; Kong, J.; He, Z.; Mai, X.; Shao, Q.; Chen, Y.; Guo, L.; Liu, C.; Angaiah, S.; et al. Overview of Carbon Nanostructures and Nanocomposites for Electromagnetic Wave Shielding. Carbon 2018, 140, 696–733. [Google Scholar] [CrossRef]
- Caulder, D.L.; Raymond, K.N. Supermolecules by Design. Acc. Chem. Res. 1999, 32, 975–982. [Google Scholar] [CrossRef]
- Kim, S.M.; Jung, J.I.; Chai, C.; Imm, J.Y. Characteristics and Glucose Uptake Promoting Effect of Chrysin-Loaded Phytosomes Prepared with Different Phospholipid Matrices. Nutrients 2019, 11, 2549. [Google Scholar] [CrossRef]
- Ariga, K.; Nishikawa, M.; Mori, T.; Takeya, J.; Shrestha, L.K.; Hill, J.P. Self-Assembly as a Key Player for Materials Nanoarchitectonics. Sci. Technol. Adv. Mater. 2019, 20, 51–95. [Google Scholar] [CrossRef]
- Lehn, J.M. Programmed Chemical Systems: Multiple Subprograms and Multiple Processing/Expression of Molecular Information. Chem.—A Eur. J. 2000, 6, 2097–2102. [Google Scholar] [CrossRef]
- Cortez, M.L.; Lorenzo, A.; Marmisollé, W.A.; von Bilderling, C.; Maza, E.; Pietrasanta, L.; Battaglini, F.; Ceolín, M.; Azzaroni, O. Highly-Organized Stacked Multilayers via Layer-by-Layer Assembly of Lipid-like Surfactants and Polyelectrolytes. Stratified Supramolecular Structures for (Bio)Electrochemical Nanoarchitectonics. Soft Matter 2018, 14, 1939–1952. [Google Scholar] [CrossRef]
- Ariga, K.; Jia, X.; Song, J.; Hsieh, C.T.; Hsu, S. hui Materials Nanoarchitectonics as Cell Regulators. ChemNanoMat 2019, 5, 692–702. [Google Scholar] [CrossRef]
- Mokrab, Y.; Sansom, M.S.P. Interaction of Diverse Voltage Sensor Homologs with Lipid Bilayers Revealed by Self-Assembly Simulations. Biophys. J. 2011, 100, 875–884. [Google Scholar] [CrossRef]
- Qiang, X.; Wang, X.; Ji, Y.; Li, S.; He, L. Liquid-Crystal Self-Assembly of Lipid Membranes on Solutions: A Dissipative Particle Dynamic Simulation Study. Polymer 2017, 115, 1–11. [Google Scholar] [CrossRef]
- Baccile, N.; Poirier, A.; Seyrig, C.; Le Griel, P.; Perez, J.; Hermida-Merino, D.; Pernot, P.; Roelants, S.L.K.W.; Soetaert, W. Chameleonic Amphiphile: The Unique Multiple Self-Assembly Properties of a Natural Glycolipid in Excess of Water. J. Colloid Interface Sci. 2023, 630, 404–415. [Google Scholar] [CrossRef]
- Min, Y.; Akbulut, M.; Kristiansen, K.; Golan, Y.; Israelachvili, J. The Role of Interparticle and External Forces in Nanoparticle Assembly. Nat. Mater. 2008, 7, 527–538. [Google Scholar] [CrossRef]
- Boles, M.A.; Ling, D.; Hyeon, T.; Talapin, D.V. The Surface Science of Nanocrystals. Nat. Mater. 2016, 15, 141–153. [Google Scholar] [CrossRef]
- Kovalenko, M.V.; Scheele, M.; Talapin, D.V. Colloidal Nanocrystals with Molecular Metal Chalcogenide Surface Ligands. Science 2009, 324, 1417–1420. [Google Scholar] [CrossRef]
- Fafarman, A.T.; Koh, W.; Diroll, B.T.; Kim, D.K.; Ko, D.-K.; Oh, S.J.; Ye, X.; Doan-Nguyen, V.; Crump, M.R.; Reifsnyder, D.C.; et al. Thiocyanate-Capped Nanocrystal Colloids: Vibrational Reporter of Surface Chemistry and Solution-Based Route to Enhanced Coupling in Nanocrystal Solids. J. Am. Chem. Soc. 2011, 133, 15753–15761. [Google Scholar] [CrossRef]
- Lee, J.; Sundar, V.C.; Heine, J.R.; Bawendi, M.G.; Jensen, K.F. Full Color Emission from II-VI Semiconductor Quantum Dot-Polymer Composites. Adv. Mater. 2000, 12, 1102–1105. [Google Scholar] [CrossRef]
- Boles, M.A.; Engel, M.; Talapin, D.V. Self-Assembly of Colloidal Nanocrystals: From Intricate Structures to Functional Materials. Chem. Rev. 2016, 116, 11220–11289. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.M.; Jaeger, H.M.; Sorensen, C.M.; Klabunde, K.J. Formation of Long-Range-Ordered Nanocrystal Superlattices on Silicon Nitride Substrates. J. Phys. Chem. B 2001, 105, 3353–3357. [Google Scholar] [CrossRef]
- Murray, C.B.; Kagan, C.R.; Bawendi, M.G. Self-Organization of CdSe Nanocrystallites into Three-Dimensional Quantum Dot Superlattices. Science 1995, 270, 1335–1338. [Google Scholar] [CrossRef]
- Diroll, B.T.; Doan-Nguyen, V.V.T.; Cargnello, M.; Gaulding, E.A.; Kagan, C.R.; Murray, C.B. X-Ray Mapping of Nanoparticle Superlattice Thin Films. ACS Nano 2014, 8, 12843–12850. [Google Scholar] [CrossRef]
- Talapin, D.V.; Murray, C.B. PbSe Nanocrystal Solids for N- and p-Channel Thin Film Field-Effect Transistors. Science 2005, 310, 86–89. [Google Scholar] [CrossRef]
- Bigioni, T.P.; Lin, X.-M.; Nguyen, T.T.; Corwin, E.I.; Witten, T.A.; Jaeger, H.M. Kinetically Driven Self Assembly of Highly Ordered Nanoparticle Monolayers. Nat. Mater. 2006, 5, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Bodnarchuk, M.I.; Kovalenko, M.V.; Heiss, W.; Talapin, D.V. Energetic and Entropic Contributions to Self-Assembly of Binary Nanocrystal Superlattices: Temperature as the Structure-Directing Factor. J. Am. Chem. Soc. 2010, 132, 11967–11977. [Google Scholar] [CrossRef]
- Bodnarchuk, M.I.; Kovalenko, M.V.; Pichler, S.; Fritz-Popovski, G.; Hesser, G.; Heiss, W. Large-Area Ordered Superlattices from Magnetic Wüstite/Cobalt Ferrite Core/Shell Nanocrystals by Doctor Blade Casting. ACS Nano 2010, 4, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Dong, A.; Chen, J.; Vora, P.M.; Kikkawa, J.M.; Murray, C.B. Binary Nanocrystal Superlattice Membranes Self-Assembled at the Liquid–Air Interface. Nature 2010, 466, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Rupich, S.M.; Shevchenko, E.V.; Bodnarchuk, M.I.; Lee, B.; Talapin, D.V. Size-Dependent Multiple Twinning in Nanocrystal Superlattices. J. Am. Chem. Soc. 2010, 132, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, H.M.; Nagel, S.R.; Behringer, R.P. Granular Solids, Liquids, and Gases. Rev. Mod. Phys. 1996, 68, 1259–1273. [Google Scholar] [CrossRef]
- Klajn, R.; Bishop, K.J.M.; Grzybowski, B.A. Light-Controlled Self-Assembly of Reversible and Irreversible Nanoparticle Suprastructures. Proc. Natl. Acad. Sci. USA 2007, 104, 10305–10309. [Google Scholar] [CrossRef]
- Zhuang, J.; Wu, H.; Yang, Y.; Cao, Y.C. Supercrystalline Colloidal Particles from Artificial Atoms. J. Am. Chem. Soc. 2007, 129, 14166–14167. [Google Scholar] [CrossRef]
- Lengert, E.V.; Koltsov, S.I.; Li, J.; Ermakov, A.V.; Parakhonskiy, B.V.; Skorb, E.V.; Skirtach, A.G. Nanoparticles in Polyelectrolyte Multilayer Layer-by-Layer (LbL) Films and Capsules—Key Enabling Components of Hybrid Coatings. Coatings 2020, 10, 1131. [Google Scholar] [CrossRef]
- Rydzek, G.; Ji, Q.; Li, M.; Schaaf, P.; Hill, J.P.; Boulmedais, F.; Ariga, K. Electrochemical Nanoarchitectonics and Layer-by-Layer Assembly: From Basics to Future. Nano Today 2015, 10, 138–167. [Google Scholar] [CrossRef]
- Ariga, K.; Shrestha, L.K. Intelligent Nanoarchitectonics for Self-Assembling Systems. Adv. Intell. Syst. 2020, 2, 1900157. [Google Scholar] [CrossRef]
- Iler, R.K. Multilayers of colloidal particles. J. Colloid Interface Sci. 1966, 21, 569–594. [Google Scholar] [CrossRef]
- Decher, G. Fuzzy Nanoassemblies: Toward Layered Polymeric Multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Donath, E.; Sukhorukov, G.B.; Caruso, F.; Davis, S.A.; Möhwald, H. Novel Hollow Polymer Shells by Colloid-Templated Assembly of Polyelectrolytes. Angew. Chem. Int. Ed. 1998, 37, 2201–2205. [Google Scholar] [CrossRef]
- Caruso, F.; Caruso, R.; Möhwald, H. Nanoengineering of Inorganic and Hybrid Hollow Spheres by Colloidal Templating. Science 1998, 282, 1111–1114. [Google Scholar] [CrossRef]
- Kim, Y.H.; Park, J.; Yoo, P.J.; Hammond, P.T. Selective Assembly of Colloidal Particles on a Nanostructured Template Coated with Polyelectrolyte Multilayers. Adv. Mater. 2007, 19, 4426–4430. [Google Scholar] [CrossRef]
- Pappa, A.M.; Inal, S.; Roy, K.; Zhang, Y.; Pitsalidis, C.; Hama, A.; Pas, J.; Malliaras, G.G.; Owens, R.M. Polyelectrolyte Layer-by-Layer Assembly on Organic Electrochemical Transistors. ACS Appl. Mater. Interfaces 2017, 9, 10427–10434. [Google Scholar] [CrossRef]
- Yuan, W.; Weng, G.M.; Lipton, J.; Li, C.M.; Van Tassel, P.R.; Taylor, A.D. Weak Polyelectrolyte-Based Multilayers via Layer-by-Layer Assembly: Approaches, Properties, and Applications. Adv. Colloid Interface Sci. 2020, 282, 102200. [Google Scholar] [CrossRef]
- Ariga, K.; Yamauchi, Y. Nanoarchitectonics from Atom to Life. Chem. –Asian J. 2020, 15, 718–728. [Google Scholar] [CrossRef]
- Ariga, K.; Leong, D.T.; Mori, T. Nanoarchitectonics for Hybrid and Related Materials for Bio-Oriented Applications. Adv. Funct. Mater. 2018, 28, 1702905. [Google Scholar] [CrossRef]
- Zhang, R.; Köhler, K.; Kreft, O.; Skirtach, A.; Möhwald, H.; Sukhorukov, G.B. Salt-Induced Fusion of Microcapsules of Polyelectrolytes. Soft Matter 2010, 6, 4742. [Google Scholar] [CrossRef]
- Elbert, D.L.; Herbert, C.B.; Hubbell, J.A. Thin Polymer Layers Formed by Polyelectrolyte Multilayer Techniques on Biological Surfaces. Langmuir 1999, 15, 5355–5362. [Google Scholar] [CrossRef]
- Picart, C.; Mutterer, J.; Richert, L.; Luo, Y.; Prestwich, G.D.; Schaaf, P.; Voegel, J.C.; Lavalle, P. Molecular Basis for the Explanation of the Exponential Growth of Polyelectrolyte Multilayers. Proc. Natl. Acad. Sci. USA 2002, 99, 12531–12535. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, E.; Ritacco, H.; Rubio, J.E.F.; Rubio, R.G.; Ortega, F. Salt-Induced Changes in the Growth of Polyelectrolyte Layers of Poly(Diallyl-Dimethylammonium Chloride) and Poly(4-Styrene Sulfonate of Sodium). Soft Matter 2009, 5, 2130. [Google Scholar] [CrossRef]
- Zhao, S.; Caruso, F.; Dähne, L.; Decher, G.; De Geest, B.G.; Fan, J.; Feliu, N.; Gogotsi, Y.; Hammond, P.T.; Hersam, M.C.; et al. The Future of Layer-by-Layer Assembly: A Tribute to ACS Nano Associate Editor Helmuth Möhwald. ACS Nano 2019, 13, 6151–6169. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Parakhonskiy, B.V.; Skirtach, A.G. A Decade of Developing Applications Exploiting the Properties of Polyelectrolyte Multilayer Capsules. Chem. Commun. 2023, 59, 807–835. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Van der Meeren, L.; Verduijn, J.; Parakhonskiy, B.V.; Skirtach, A.G. New All-Nanoparticle Microcapsules for Ultrasound Release and Laser Remote Killing of Cancer Cells. Mater. Today Commun. 2022, 33, 104287. [Google Scholar] [CrossRef]
- Li, J.; Khalenkow, D.; Volodkin, D.; Lapanje, A.; Skirtach, A.G.; Parakhonskiy, B.V. Surface Enhanced Raman Scattering (SERS)-Active Bacterial Detection by Layer-by-Layer (LbL) Assembly All-Nanoparticle Microcapsules. Colloids Surf. A Physicochem. Eng. Asp. 2022, 650, 129547. [Google Scholar] [CrossRef]
- Skirtach, A.G.; Muñoz Javier, A.; Kreft, O.; Köhler, K.; Piera Alberola, A.; Möhwald, H.; Parak, W.J.; Sukhorukov, G.B. Laser-Induced Release of Encapsulated Materials inside Living Cells. Angew. Chem. Int. Ed. 2006, 45, 4612–4617. [Google Scholar] [CrossRef]
- Sadasivan, V.; Richter, C.P.; Menon, L.; Williams, P.F. Electrochemical Self-Assembly of Porous Alumina Templates. AIChE J. 2005, 51, 649–655. [Google Scholar] [CrossRef]
- Ariga, K.; Matsumoto, M.; Mori, T.; Shrestha, L.K. Materials Nanoarchitectonics at Two-Dimensional Liquid Interfaces. Beilstein J. Nanotechnol. 2019, 10, 1559–1587. [Google Scholar] [CrossRef] [PubMed]
- Vikulina, A.S.; Stetsyura, I.Y.; Onses, M.S.; Yilmaz, E.; Skirtach, A.G.; Volodkin, D. Mesoporous One-Component Gold Microshells as 3D SERS Substrates. Biosensors 2021, 11, 380. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, L.; Zhang, Z.; Wang, D. Recent Advances of Raman Spectroscopy for the Analysis of Bacteria. Anal. Sci. Adv. 2023, 4, 81–95. [Google Scholar] [CrossRef]
- Lu, K. Newfound Capability of Focused Ion Beam Patterning Guided Anodization. Electrochim. Acta 2012, 63, 256–262. [Google Scholar] [CrossRef]
- Wang, X.; Xu, S.; Cong, M.; Li, H.; Gu, Y.; Xu, W. Hierarchical Structural Nanopore Arrays Fabricated by Pre-patterning Aluminum Using Nanosphere Lithography. Small 2012, 8, 972–976. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.E.; Wood, G.C. Anodic Films on Aluminium; Elsevier: Amsterdam, The Netherlands, 1983; pp. 205–329. [Google Scholar]
- Benfedda, B.; Hamadou, L.; Benbrahim, N.; Kadri, A.; Chainet, E.; Charlot, F. Electrochemical Impedance Investigation of Anodic Alumina Barrier Layer. J. Electrochem. Soc. 2012, 159, C372–C381. [Google Scholar] [CrossRef]
- Zakeri, R.; Watts, C.; Wang, H.; Kohli, P. Synthesis and Characterization of Nonlinear Nanopores in Alumina Films. Chem. Mater. 2007, 19, 1954–1963. [Google Scholar] [CrossRef]
- Lv, M.; Wang, Q.; Meng, Q.; Zhao, T.; Liu, H.; Jiang, L. Self-Assembly of Alumina Nanowires into Controllable Micro-Patterns by Laser-Assisted Solvent Spreading: Towards Superwetting Surfaces. CrystEngComm 2015, 17, 540–545. [Google Scholar] [CrossRef]
- Binks, B.P. Particles as Surfactants—Similarities and Differences. Curr. Opin. Colloid Interface Sci. 2002, 7, 21–41. [Google Scholar] [CrossRef]
- Kim, J.U.; O’Shaughnessy, B. Morphology Selection of Nanoparticle Dispersions by Polymer Media. Phys. Rev. Lett. 2002, 89, 238301. [Google Scholar] [CrossRef]
- Tokareva, M.; Ohar, H.; Tokarev, S.; Stetsyshyn, Y. Synthesis, Structure and Properties of the Grafted Peptidomimetic Polymer Brushes Based on Poly(N-Methacryloyl-L-Proline). Chem. Chem. Technol. 2021, 15, 26–32. [Google Scholar] [CrossRef]
- Pinchasik, B.-E.; Tauer, K.; Möhwald, H.; Skirtach, A.G. Polymer Brush Gradients by Adjusting the Functional Density Through Temperature Gradient. Adv. Mater. Interfaces 2014, 1, 1300056. [Google Scholar] [CrossRef]
- Ionov, L.; Minko, S. Mixed Polymer Brushes with Locking Switching. ACS Appl. Mater. Interfaces 2012, 4, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Açarı, İ.K.; Sel, E.; Özcan, İ.; Ateş, B.; Köytepe, S.; Thakur, V.K. Chemistry and Engineering of Brush Type Polymers: Perspective towards Tissue Engineering. Adv. Colloid Interface Sci. 2022, 305, 102694. [Google Scholar] [CrossRef]
- Leong, K.; Boardman, A.K.; Ma, H.; Jen, A.K.-Y. Single-Cell Patterning and Adhesion on Chemically Engineered Poly(Dimethylsiloxane) Surface. Langmuir 2009, 25, 4615–4620. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.J.; Hohman, J.N.; Morin, E.I.; Weiss, P.S. Molecular Flux Dependence of Chemical Patterning by Microcontact Printing. ACS Appl. Mater. Interfaces 2013, 5, 10310–10316. [Google Scholar] [CrossRef]
- Qiu, S.; Ji, J.; Sun, W.; Pei, J.; He, J.; Li, Y.; Li, J.J.; Wang, G. Recent Advances in Surface Manipulation Using Micro-Contact Printing for Biomedical Applications. Smart Mater. Med. 2021, 2, 65–73. [Google Scholar] [CrossRef]
- Yang, W.; Qin, Y.; Wang, Z.; Yu, T.; Chen, Y.; Ge, Z. Recent Advance in Cell Patterning Techniques: Approaches, Applications and Future Prospects. Sens. Actuators A Phys. 2022, 333, 113229. [Google Scholar] [CrossRef]
- Cherniavskaya, O.; Adzic, A.; Knutson, C.; Gross, B.J.; Zang, L.; Liu, R.; Adams, D.M. Edge Transfer Lithography of Molecular and Nanoparticle Materials. Langmuir 2002, 18, 7029–7034. [Google Scholar] [CrossRef]
- Stein, A.; Wright, G.; Yager, K.G.; Doerk, G.S.; Black, C.T. Selective Directed Self-Assembly of Coexisting Morphologies Using Block Copolymer Blends. Nat. Commun. 2016, 7, 12366. [Google Scholar] [CrossRef] [PubMed]
- Suh, H.S.; Kim, D.H.; Moni, P.; Xiong, S.; Ocola, L.E.; Zaluzec, N.J.; Gleason, K.K.; Nealey, P.F. Sub-10-Nm Patterning via Directed Self-Assembly of Block Copolymer Films with a Vapour-Phase Deposited Topcoat. Nat. Nanotechnol. 2017, 12, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.W.; Hur, Y.H.; Kim, H.J.; Kim, J.M.; Park, W.I.; Kim, M.J.; Kim, B.J.; Jung, Y.S. Proximity Injection of Plasticizing Molecules to Self-Assembling Polymers for Large-Area, Ultrafast Nanopatterning in the Sub-10-Nm Regime. ACS Nano 2013, 7, 6747–6757. [Google Scholar] [CrossRef] [PubMed]
- Dolejsi, M.; Moni, P.; Bezik, C.T.; Zhou, C.; de Pablo, J.J.; Gleason, K.K.; Nealey, P.F. Ultrathin Initiated Chemical Vapor Deposition Polymer Interfacial Energy Control for Directed Self-Assembly Hole-Shrink Applications. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. Mater. Process. Meas. Phenom. 2019, 37, 061804. [Google Scholar] [CrossRef]
- Kim, Y.C.; Kim, S.Y. A Single Crystal 2D Hexagonal Array in a Centimeter Scale with a Self-Directed Assembly of Diblock Copolymer Spheres. ACS Nano 2022, 16, 3870–3880. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jin, M.; He, T.; Zhou, G.; Shui, L. Microfluidic Induced Controllable Microdroplets Assembly in Confined Channels. Micromachines 2015, 6, 1331–1345. [Google Scholar] [CrossRef]
- Chen, A.; Pan, T. Three-Dimensional Fit-to-Flow Microfluidic Assembly. Biomicrofluidics 2011, 5, 046505. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.E.; Snezhko, A. Driving Self-Assembly and Emergent Dynamics in Colloidal Suspensions by Time-Dependent Magnetic Fields. Rep. Prog. Phys. 2013, 76, 126601. [Google Scholar] [CrossRef]
- Liljeström, V.; Chen, C.; Dommersnes, P.; Fossum, J.O.; Gröschel, A.H. Active Structuring of Colloids through Field-Driven Self-Assembly. Curr. Opin. Colloid Interface Sci. 2019, 40, 25–41. [Google Scholar] [CrossRef]
- Al-Ali, A.; Waheed, W.; Abu-Nada, E.; Alazzam, A. A Review of Active and Passive Hybrid Systems Based on Dielectrophoresis for the Manipulation of Microparticles. J. Chromatogr. A 2022, 1676, 463268. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, J.; Yuan, D.; Li, W. Hybrid Microfluidics Combined with Active and Passive Approaches for Continuous Cell Separation. Electrophoresis 2017, 38, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Xing, X.; Evans, J.; He, S. Optofluidic Vortex Arrays Generated by Graphene Oxide for Tweezers, Motors and Self-Assembly. NPG Asia Mater. 2016, 8, e257. [Google Scholar] [CrossRef]
- Huang, Z.; Shao, G.; Zhou, D.; Deng, X.; Qiao, J.; Li, L. 3D Printing of High-Precision and Ferromagnetic Functional Devices. Int. J. Extrem. Manuf. 2023, 5, 035501. [Google Scholar] [CrossRef]
- Bal, S.; Das, K.; Ahmed, S.; Das, D. Chemically Fueled Dissipative Self-Assembly That Exploits Cooperative Catalysis. Angew. Chem. 2019, 131, 250–253. [Google Scholar] [CrossRef]
- Xie, X.; Zhang, Y.; Liang, Y.; Wang, M.; Cui, Y.; Li, J.; Liu, C. Programmable Transient Supramolecular Chiral G-quadruplex Hydrogels by a Chemically Fueled Non-equilibrium Self-Assembly Strategy. Angew. Chem. 2022, 134, e202114471. [Google Scholar] [CrossRef]
- Mondal, D.; Bandyopadhyay, S.N.; Goswami, D. Elucidating Optical Field Directed Hierarchical Self-Assembly of Homogenous versus Heterogeneous Nanoclusters with Femtosecond Optical Tweezers. PLoS ONE 2019, 14, e0223688. [Google Scholar] [CrossRef]
- Xie, Q.; Davies, G.B.; Harting, J. Direct Assembly of Magnetic Janus Particles at a Droplet Interface. ACS Nano 2017, 11, 11232–11239. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, K.S.; Di Carlo, D.; Chung, A.J. Optofluidic Fabrication for 3D-Shaped Particles. Nat. Commun. 2015, 6, 6976. [Google Scholar] [CrossRef]
- Eftekhari, K.; Van der Meeren, L.; Depla, D.; Parakhonskiy, B.; Skirtach, A.G. PM2.5 and PM10 Adsorption onto Filters and Surfaces Functionalized with Calcium Carbonate Particle Assembly. Colloids Surf. A Physicochem. Eng. Asp. 2024, 680, 132617. [Google Scholar] [CrossRef]
- Maestro, A. Tailoring the Interfacial Assembly of Colloidal Particles by Engineering the Mechanical Properties of the Interface. Curr. Opin. Colloid Interface Sci. 2019, 39, 232–250. [Google Scholar] [CrossRef]
- Sempels, W.; De Dier, R.; Mizuno, H.; Hofkens, J.; Vermant, J. Auto-Production of Biosurfactants Reverses the Coffee Ring Effect in a Bacterial System. Nat. Commun. 2013, 4, 1757. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.Y.; Kim, Y.W.; Yoo, J.Y. Inward Flow of Micro-Particles in an Evaporating Di-Dispersed Colloid Droplet on Hydrophilic Surface. In Proceedings of the ASME 2009 7th International Conference on Nanochannels, Microchannels, and Minichannels, Pohang, Republic of Korea, 22–24 June 2009; Volume 81, pp. 1051–1054. [Google Scholar] [CrossRef]
- Janjua, M.; Nudurupati, S.; Singh, P.; Aubry, N. Electric Field-induced Self-assembly of Micro- and Nanoparticles of Various Shapes at Two-fluid Interfaces. Electrophoresis 2011, 32, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, S.J.; Warzecha, C.C.; Yadavali, S.; El-Mayta, R.; Alameh, M.G.; Wang, L.; Weissman, D.; Wilson, J.M.; Issadore, D.; Mitchell, M.J. Scalable MRNA and SiRNA Lipid Nanoparticle Production Using a Parallelized Microfluidic Device. Nano Lett. 2021, 21, 5671–5680. [Google Scholar] [CrossRef] [PubMed]
- Maeki, M.; Saito, T.; Sato, Y.; Yasui, T.; Kaji, N.; Ishida, A.; Tani, H.; Baba, Y.; Harashima, H.; Tokeshi, M. A Strategy for Synthesis of Lipid Nanoparticles Using Microfluidic Devices with a Mixer Structure. RSC Adv. 2015, 5, 46181–46185. [Google Scholar] [CrossRef]
- Ni, S.; Leemann, J.; Wolf, H.; Isa, L. Insights into Mechanisms of Capillary Assembly. Faraday Discuss. 2015, 181, 225–242. [Google Scholar] [CrossRef]
- Ni, S.; Isa, L.; Wolf, H. Capillary Assembly as a Tool for the Heterogeneous Integration of Micro- and Nanoscale Objects. Soft Matter 2018, 14, 2978–2995. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Manoharan, B.; Sivakumar, N. Introduction. In Perovskite Photovoltaics; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–24. [Google Scholar]
- Zhang, Z.; Peng, F.; Kornev, K. The Thickness and Structure of Dip-Coated Polymer Films in the Liquid and Solid States. Micromachines 2022, 13, 982. [Google Scholar] [CrossRef]
- Brinker, C.J.; Frye, G.C.; Hurd, A.J.; Ashley, C.S. Fundamentals of Sol-Gel Dip Coating. Thin Solid Films 1991, 201, 97–108. [Google Scholar] [CrossRef]
- Tyona, M.D. A Theoritical Study on Spin Coating Technique. Adv. Mater. Res. 2013, 2, 195–208. [Google Scholar] [CrossRef]
- Eftekhari, K.; Danglad-Flores, J.A.; Li, J.; Riegler, H.; Parakhonskiy, B.V.; Skirtach, A.G. Calcium Carbonate Particle Synthesis in a Confined and Dynamically Thinning Layer on a Spin-Coater—In Situ Deposition for Cell Adhesion. Mater. Chem. Phys. 2023, 310, 128462. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Triani, G.; Sorrell, C.C. Morphology and Photocatalytic Activity of Highly Oriented Mixed Phase Titanium Dioxide Thin Films. Surf. Coat. Technol. 2011, 205, 3658–3664. [Google Scholar] [CrossRef]
- Mustafa, H.A.M.; Jameel, D.A. Modeling and the Main Stages of Spin Coating Process: A Review. J. Appl. Sci. Technol. Trends 2021, 2, 91–95. [Google Scholar] [CrossRef]
- Danglad-Flores, J.; Eftekhari, K.; Skirtach, A.G.; Riegler, H. Controlled Deposition of Nanosize and Microsize Particles by Spin-Casting. Langmuir 2019, 35, 3404–3412. [Google Scholar] [CrossRef]
- Eickelmann, S.; Riegler, H. Rupture of Ultrathin Solution Films on Planar Solid Substrates Induced by Solute Crystallization. J. Colloid Interface Sci. 2018, 528, 63–69. [Google Scholar] [CrossRef]
- Karpitschka, S.; Weber, C.M.; Riegler, H. Spin Casting of Dilute Solutions: Vertical Composition Profile during Hydrodynamic-Evaporative Film Thinning. Chem. Eng. Sci. 2015, 129, 243–248. [Google Scholar] [CrossRef]
- Meyerhofer, D. Characteristics of Resist Films Produced by Spinning. J. Appl. Phys. 1978, 49, 3993–3997. [Google Scholar] [CrossRef]
- Emslie, A.G.; Bonner, F.T.; Peck, L.G. Flow of a Viscous Liquid on a Rotating Disk. J. Appl. Phys. 1958, 29, 858–862. [Google Scholar] [CrossRef]
- Ariga, K.; Yamauchi, Y.; Mori, T.; Hill, J.P. 25th Anniversary Article: What Can Be Done with the Langmuir-Blodgett Method? Recent Developments and Its Critical Role in Materials Science. Adv. Mater. 2013, 25, 6477–6512. [Google Scholar] [CrossRef]
- Oliveira, O.N.; Caseli, L.; Ariga, K. The Past and the Future of Langmuir and Langmuir–Blodgett Films. Chem. Rev. 2022, 122, 6459–6513. [Google Scholar] [CrossRef]
- Deák, A.; Bancsi, B.; Tóth, A.L.; Kovács, A.L.; Hórvölgyi, Z. Complex Langmuir-Blodgett Films from Silica Nanoparticles: An Optical Spectroscopy Study. Colloids Surf. A Physicochem. Eng. Asp. 2006, 278, 10–16. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, J.; Li, Y.; Lu, K. Preparation and Freezing Behavior of TiO2 Nanoparticle Suspensions. Ceram. Int. 2016, 42, 15597–15602. [Google Scholar] [CrossRef]
- Lu, K.; Zhu, X. Freeze Casting as a Nanoparticle Material-Forming Method. In Progress in Nanotechnology; Wiley: Hoboken, NJ, USA, 2009; pp. 3–11. [Google Scholar]
- Rogers, C.; Pun, D.; Fu, Q.; Zhang, H. Fabricating MOF/Polymer Composites via Freeze Casting for Water Remediation. Ceramics 2018, 1, 353–363. [Google Scholar] [CrossRef]
- dos Santos Aguilera, L.; Marçal, R.L.S.B.; de Campos, J.B.; da Silva, M.H.P.; da Silva Figueiredo, A.B.H. Magnetic Filter Produced by ZnFe2O4 Nanoparticles Using Freeze Casting. J. Mater. Res. Technol. 2018, 7, 350–355. [Google Scholar] [CrossRef]
- Boymelgreen, A.; Schiffbauer, J.; Khusid, B.; Yossifon, G. Synthetic Electrically Driven Colloids: A Platform for Understanding Collective Behavior in Soft Matter. Curr. Opin. Colloid Interface Sci. 2022, 60, 101603. [Google Scholar] [CrossRef]
- Wang, W.; Lv, X.; Moran, J.L.; Duan, S.; Zhou, C. A Practical Guide to Active Colloids: Choosing Synthetic Model Systems for Soft Matter Physics Research. Soft Matter 2020, 16, 3846–3868. [Google Scholar] [CrossRef]
- Han, K. Electric and Magnetic Field-Driven Dynamic Structuring for Smart Functional Devices. Micromachines 2023, 14, 661. [Google Scholar] [CrossRef]
- Jonas, U.; Krüger, C. The Effect of Polar, Nonpolar, and Electrostatic Interactions and Wetting Behavior on the Particle Assembly at Patterned Surfaces. J. Supramol. Chem. 2002, 2, 255–270. [Google Scholar] [CrossRef]
- Lucarini, S.; Hossain, M.; Garcia-Gonzalez, D. Recent Advances in Hard-Magnetic Soft Composites: Synthesis, Characterisation, Computational Modelling, and Applications. Compos. Struct. 2022, 279, 114800. [Google Scholar] [CrossRef]
- Jones, M.W.; Hunt, T. Electromagnetic-Field Theories of Qualia: Can They Improve upon Standard Neuroscience? Front. Psychol. 2023, 14, 1015967. [Google Scholar] [CrossRef]
- Kamal, M.A.; Petukhov, A.V.; Pal, A. Path-Dependent Self-Assembly of Magnetic Anisotropic Colloidal Peanuts. J. Phys. Chem. B 2020, 124, 5754–5760. [Google Scholar] [CrossRef]
- Llera, M.; Codnia, J.; Jorge, G.A. Formation and Kinetics of Self-Assembled Structures of Magnetic Microparticles in Rotating Fields. IEEE Trans. Magn. 2013, 49, 4725–4728. [Google Scholar] [CrossRef]
- Spatafora-Salazar, A.; Lobmeyer, D.M.; Cunha, L.H.P.; Joshi, K.; Biswal, S.L. Hierarchical Assemblies of Superparamagnetic Colloids in Time-Varying Magnetic Fields. Soft Matter 2021, 17, 1120–1155. [Google Scholar] [CrossRef]
- Jeon, S.; Nam, J.; Lee, W.; Jang, G. Selective Navigating and Unclogging Motions of an Intravascular Helical Magnetic Millirobot Actuated by External Biaxial Rotating Magnetic Fields. IEEE/ASME Trans. Mechatron. 2017, 22, 1456–1464. [Google Scholar] [CrossRef]
- He, L.; Wang, M.; Ge, J.; Yin, Y. Magnetic Assembly Route to Colloidal Responsive Photonic Nanostructures. Acc. Chem. Res. 2012, 45, 1431–1440. [Google Scholar] [CrossRef]
- Wang, Z.; Mu, Y.; Lyu, D.; Wu, M.; Li, J.; Wang, Z.; Wang, Y. Engineering Shapes of Active Colloids for Tunable Dynamics. Curr. Opin. Colloid Interface Sci. 2022, 61, 101608. [Google Scholar] [CrossRef]
- Martínez-Pedrero, F. Static and Dynamic Behavior of Magnetic Particles at Fluid Interfaces. Adv. Colloid Interface Sci. 2020, 284, 102233. [Google Scholar] [CrossRef]
- Karthik, V.; Poornima, S.; Vigneshwaran, A.; Raj, D.P.R.D.D.; Subbaiya, R.; Manikandan, S.; Saravanan, M. Nanoarchitectonics Is an Emerging Drug/Gene Delivery and Targeting Strategy—A Critical Review. J. Mol. Struct. 2021, 1243, 130844. [Google Scholar] [CrossRef]
- Amadi, E.V.; Venkataraman, A.; Papadopoulos, C. Nanoscale Self-Assembly: Concepts, Applications and Challenges. Nanotechnology 2022, 33, 132001. [Google Scholar] [CrossRef]
- Grier, D.G. A Revolution in Optical Manipulation. Nature 2003, 424, 810–816. [Google Scholar] [CrossRef]
- Grover, S.C.; Gauthier, R.C.; Skirtach, A.G. Analysis of the Behaviour of Erythrocytes in an Optical Trapping System. Opt. Express 2000, 7, 533. [Google Scholar] [CrossRef]
- Roy, B.; Arya, M.; Thomas, P.; Jürgschat, J.K.; Venkata Rao, K.; Banerjee, A.; Malla Reddy, C.; Roy, S. Self-Assembly of Mesoscopic Materials To Form Controlled and Continuous Patterns by Thermo-Optically Manipulated Laser Induced Microbubbles. Langmuir 2013, 29, 14733–14742. [Google Scholar] [CrossRef]
- Sharma, V.; Paul, D.; Chaubey, S.K.; Tiwari, S.; Kumar, G.V.P. Large-Scale Optothermal Assembly of Colloids Mediated by a Gold Microplate. J. Phys. Condens. Matter 2020, 32, 324002. [Google Scholar] [CrossRef]
- Trivedi, M.; Saxena, D.; Ng, W.K.; Sapienza, R.; Volpe, G. Self-Organized Lasers from Reconfigurable Colloidal Assemblies. Nat. Phys. 2022, 18, 939–944. [Google Scholar] [CrossRef]
- Paul, D.; Chand, R.; Kumar, G.V.P. Optothermal Evolution of Active Colloidal Matter in a Defocused Laser Trap. ACS Photonics 2022, 9, 3440–3449. [Google Scholar] [CrossRef]
- Yu, S.; Lu, J.; Ginis, V.; Kheifets, S.; Lim, S.W.D.; Qiu, M.; Gu, T.; Hu, J.; Capasso, F. On-Chip Optical Tweezers Based on Freeform Optics. Optica 2021, 8, 409. [Google Scholar] [CrossRef]
- Neale, S.L. Assembly of Mesoscopic to Macroscopic Particles with Optoelectronic Tweezers (OET). In Proceedings of the Optical Trapping and Optical Micromanipulation XV, San Diego, CA, USA, 19–23 August 2018; Dholakia, K., Spalding, G.C., Eds.; p. 71. [Google Scholar]
- Kirkham, G.R.; Britchford, E.; Upton, T.; Ware, J.; Gibson, G.M.; Devaud, Y.; Ehrbar, M.; Padgett, M.; Allen, S.; Buttery, L.D.; et al. Precision Assembly of Complex Cellular Microenvironments Using Holographic Optical Tweezers. Sci. Rep. 2015, 5, 8577. [Google Scholar] [CrossRef] [PubMed]
- Melzer, J.E.; McLeod, E. Assembly of Multicomponent Structures from Hundreds of Micron-Scale Building Blocks Using Optical Tweezers. Microsyst. Nanoeng. 2021, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Righini, M.; Ghenuche, P.; Cherukulappurath, S.; Myroshnychenko, V.; García de Abajo, F.J.; Quidant, R. Nano-Optical Trapping of Rayleigh Particles and Escherichia Coli Bacteria with Resonant Optical Antennas. Nano Lett. 2009, 9, 3387–3391. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huang, L.; Santschi, C.; Martin, O.J.F. Trapping and Sensing 10 Nm Metal Nanoparticles Using Plasmonic Dipole Antennas. Nano Lett. 2010, 10, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Peng, H.; Xu, L.; Wang, B.; Lan, K.; Zhao, T.; Che, R.; Li, W.; Zhao, D. Anisotropic Self-Assembly of Asymmetric Mesoporous Hemispheres with Tunable Pore Structures at Liquid–Liquid Interfaces. J. Am. Chem. Soc. 2022, 144, 15754–15763. [Google Scholar] [CrossRef]
- Du, J.; Feng, A.; Poelman, D. Temperature Dependency of Trap-Controlled Persistent Luminescence. Laser Photon. Rev. 2020, 14, 2000060. [Google Scholar] [CrossRef]
- Lin, L.; Peng, X.; Wang, M.; Scarabelli, L.; Mao, Z.; Liz-Marzán, L.M.; Becker, M.F.; Zheng, Y. Light-Directed Reversible Assembly of Plasmonic Nanoparticles Using Plasmon-Enhanced Thermophoresis. ACS Nano 2016, 10, 9659–9668. [Google Scholar] [CrossRef]
- Liu, W.; Shao, J.; Jia, Y.; Tao, Y.; Ding, Y.; Jiang, H.; Ren, Y. Trapping and Chaining Self-Assembly of Colloidal Polystyrene Particles over a Floating Electrode by Using Combined Induced-Charge Electroosmosis and Attractive Dipole–Dipole Interactions. Soft Matter 2015, 11, 8105–8112. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Lee, G.-B.; Fu, L.-M.; Lee, K.-H.; Yang, R.-J. Electrokinetically Driven Active Micro-Mixers Utilizing Zeta Potential Variation Induced by Field Effect. J. Micromechanics Microengineering 2004, 14, 1390–1398. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, L.; Fang, F.; Fu, Y.; Yin, Z. A Review on Colloidal Self-Assembly and Their Applications. Curr. Nanosci. 2016, 12, 725–746. [Google Scholar] [CrossRef]
- Rivero, P.; Goicoechea, J.; Arregui, F. Layer-by-Layer Nano-Assembly: A Powerful Tool for Optical Fiber Sensing Applications. Sensors 2019, 19, 683. [Google Scholar] [CrossRef] [PubMed]
- Dies, H.; Bottomley, A.; Nicholls, D.L.; Stamplecoskie, K.; Escobedo, C.; Docoslis, A. Electrokinetically-Driven Assembly of Gold Colloids into Nanostructures for Surface-Enhanced Raman Scattering. Nanomaterials 2020, 10, 661. [Google Scholar] [CrossRef]
- Zhao, Y.; Hubarevich, A.; Iarossi, M.; Borzda, T.; Tantussi, F.; Huang, J.; De Angelis, F. Hyperbolic Nanoparticles on Substrate with Separate Optical Scattering and Absorption Resonances: A Dual Function Platform for SERS and Thermoplasmonics. Adv. Opt. Mater. 2021, 9, 2100888. [Google Scholar] [CrossRef]
- Michałowska, A.; Krajczewski, J.; Kudelski, A. Magnetic Iron Oxide Cores with Attached Gold Nanostructures Coated with a Layer of Silica: An Easily, Homogeneously Deposited New Nanomaterial for Surface-Enhanced Raman Scattering Measurements. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 277, 121266. [Google Scholar] [CrossRef] [PubMed]
- Sin, M.L.Y.; Gau, V.; Liao, J.C.; Haake, D.A.; Wong, P.K. Active Manipulation of Quantum Dots Using AC Electrokinetics. J. Phys. Chem. C 2009, 113, 6561–6565. [Google Scholar] [CrossRef]
- Lochab, V.; Prakash, S. Combined Electrokinetic and Shear Flows Control Colloidal Particle Distribution across Microchannel Cross-Sections. Soft Matter 2021, 17, 611–620. [Google Scholar] [CrossRef]
- Xue, X.; Wang, J.; Furlani, E.P. Self-Assembly of Crystalline Structures of Magnetic Core–Shell Nanoparticles for Fabrication of Nanostructured Materials. ACS Appl. Mater. Interfaces 2015, 7, 22515–22524. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, G.; Sreenivasulu, G.; Benoit, C.; Petrov, V.M.; Chavez, F. Magnetic Field Directed Assembly of Superstructures of Ferrite-Ferroelectric Core-Shell Nanoparticles and Studies on Magneto-Electric Interactions. J. Appl. Phys. 2015, 117, 17B904. [Google Scholar] [CrossRef]
- Abe, Y.; Zhang, B.; Gordillo, L.; Karim, A.M.; Francis, L.F.; Cheng, X. Dynamic Self-Assembly of Charged Colloidal Strings and Walls in Simple Fluid Flows. Soft Matter 2017, 13, 1681–1692. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Bloom, M.; Bae, S.C.; Luijten, E.; Granick, S. Linking Synchronization to Self-Assembly Using Magnetic Janus Colloids. Nature 2012, 491, 578–581. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhu, Z.; Li, Q.; Zhang, J.; Wang, C.-F.; Wu, G.; Li, S.S.; Xiao, J.J.; Chen, S. Rapid Visualized Hydrophobic-Force-Driving Self-Assembly towards Brilliant Photonic Crystals. Chem. Eng. J. 2021, 420, 127582. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, K.; Menq, C.H. Design, Implementation, and Force Modeling of Quadrupole Magnetic Tweezers. IEEE/ASME Trans. Mechatron. 2010, 15, 704–713. [Google Scholar] [CrossRef]
- Guduri, B.B.R.; Khoathane, C.; Anandjiwala, R.D.; De Veries, A.; Sadiku, E.R.; Van Wyk, L.V. Effect of Water Absorption on Mechanical Properties of Flax Fibre Reinforced Composites. In Proceedings of the Sixth International Conference on Composite Science and Technology, Durban, South Africa, 22–24 January 2007. [Google Scholar]
- Amblard, M.; Fehrentz, J.A.; Martinez, J.; Subra, G. Methods and Protocols of Modern Solid Phase Peptide Synthesis. Mol. Biotechnol. 2006, 33, 239–254. [Google Scholar] [CrossRef]
- Harraq, A.A.; Choudhury, B.D.; Bharti, B. Field-Induced Assembly and Propulsion of Colloids. Langmuir 2022, 38, 3001–3016. [Google Scholar] [CrossRef]
- De Vlaminck, I.; Dekker, C. Recent Advances in Magnetic Tweezers. Annu. Rev. Biophys. 2012, 41, 453–472. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Kim, H.G.; Shon, M.J.; Yoon, T. High-Resolution Magnetic Tweezers. Annu. Rev. Biochem. 2022, 91, 33–59. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Liu, Y.; Yang, Y.; Yang, X.; Tan, R.; Shen, Y. Self-Assembly Magnetic Chain Unit for Bulk Biomaterial Actuation. IEEE Robot. Autom. Lett. 2019, 4, 262–268. [Google Scholar] [CrossRef]
- Haber, C.; Wirtz, D. Magnetic Tweezers for DNA Micromanipulation. Rev. Sci. Instrum. 2000, 71, 4561–4570. [Google Scholar] [CrossRef]
- Smith, S.M. Twisting DNA Molecules. Biophys. J. 1998, 74, 1609–1610. [Google Scholar] [CrossRef]
- Allewell, N.M. Thematic Minireview Series: Single-Molecule Measurements in Biochemistry and Molecular Biology. J. Biol. Chem. 2010, 285, 18959–18960. [Google Scholar] [CrossRef]
- Lipfert, J.; Hao, X.; Dekker, N.H. Quantitative Modeling and Optimization of Magnetic Tweezers. Biophys. J. 2009, 96, 5040–5049. [Google Scholar] [CrossRef] [PubMed]
- Vach, P.J.; Faivre, D. The Triathlon of Magnetic Actuation: Rolling, Propelling, Swimming with a Single Magnetic Material. Sci. Rep. 2015, 5, 9364. [Google Scholar] [CrossRef]
- Pigliaru, L.; Paleari, L.; Bragaglia, M.; Nanni, F.; Ghidini, T.; Rinaldi, M. Poly-Ether-Ether-Ketone—Neodymium-Iron-Boron Bonded Permanent Magnets via Fused Filament Fabrication. Synth. Met. 2021, 279, 116857. [Google Scholar] [CrossRef]
- Du, X.; Graedel, T.E. Global Rare Earth In-Use Stocks in NdFeB Permanent Magnets. J. Ind. Ecol. 2011, 15, 836–843. [Google Scholar] [CrossRef]
- Xuan, X. Recent Advances in Direct Current Electrokinetic Manipulation of Particles for Microfluidic Applications. Electrophoresis 2019, 40, 2484–2513. [Google Scholar] [CrossRef] [PubMed]
- Ragan, R.; Darvishzadeh-Varcheie, M.; Capolino, F.; Thrift, W. Templated Electrokinetic Directed Chemical Assembly for the Fabrication of Close-Packed Plasmonic Metamolecules. In Proceedings of the Plasmonics: Design, Materials, Fabrication, Characterization, and Applications XV, San Diego, CA, USA, 6–10 August 2017; Tanaka, T., Tsai, D.P., Eds.; p. 62. [Google Scholar]
- Work, A.H.; Williams, S.J. Characterization of 2D Colloids Assembled by Optically-Induced Electrohydrodynamics. Soft Matter 2015, 11, 4266–4272. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, W.K.; Belytschko, T.; Patankar, N.; To, A.C.; Kopacz, A.; Chung, J.-H. Immersed Electrokinetic Finite Element Method. Int. J. Numer. Methods Eng. 2007, 71, 379–405. [Google Scholar] [CrossRef]
- Zhou, T.; Chen, J.; Kropp, E.; Kulinsky, L. Guided Electrokinetic Assembly of Polystyrene Microbeads onto Photopatterned Carbon Electrode Arrays. ACS Appl. Mater. Interfaces 2020, 12, 35647–35656. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.; García-Sánchez, P.; Morgan, H. AC Electrokinetics of Conducting Microparticles: A Review. Curr. Opin. Colloid Interface Sci. 2016, 24, 79–90. [Google Scholar] [CrossRef]
- Moncada-Hernandez, H.; Nagler, E.; Minerick, A.R. Theoretical and Experimental Examination of Particle–Particle Interaction Effects on Induced Dipole Moments and Dielectrophoretic Responses of Multiple Particle Chains. Electrophoresis 2014, 35, 1803–1813. [Google Scholar] [CrossRef]
- Ramos, A.; Morgan, H.; Green, N.G.; Castellanos, A. Ac Electrokinetics: A Review of Forces in Microelectrode Structures. J. Phys. D. Appl. Phys. 1998, 31, 2338–2353. [Google Scholar] [CrossRef]
- Liu, Z.; Cui, J.; Zhan, W. Dipolar Janus Liposomes: Formation, Electrokinetic Motion and Self-Assembly. Soft Matter 2020, 16, 2177–2184. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Y.; Onck, P.; den Toonder, J. A Concise Review of Microfluidic Particle Manipulation Methods. Microfluid. Nanofluidics 2020, 24, 24. [Google Scholar] [CrossRef]
- Vegerhof, A.; Rudinzky, A.; Beiderman, Y.; Duadi, H.; Popovtzer, R.; Zalevsky, Z. Manipulated Magnetic Nano Particles for Photonic Biomedical Mapping. Nanosci. Nanotechnol. Lett. 2015, 7, 861–869. [Google Scholar] [CrossRef]
- Grzelczak, M.; Vermant, J.; Furst, E.M.; Liz-Marzán, L.M. Directed Self-Assembly of Nanoparticles. ACS Nano 2010, 4, 3591–3605. [Google Scholar] [CrossRef]
- Lash, M.H.; Fedorchak, M.V.; McCarthy, J.J.; Little, S.R. Scaling up Self-Assembly: Bottom-up Approaches to Macroscopic Particle Organization. Soft Matter 2015, 11, 5597–5609. [Google Scholar] [CrossRef]
- Cai, Z.; Li, Z.; Ravaine, S.; He, M.; Song, Y.; Yin, Y.; Zheng, H.; Teng, J.; Zhang, A. From Colloidal Particles to Photonic Crystals: Advances in Self-Assembly and Their Emerging Applications. Chem. Soc. Rev. 2021, 50, 5898–5951. [Google Scholar] [CrossRef]
- Jitkang, L.; Pin, Y.S.; Xin, C.H.; Chun, L.S. Characterization of Magnetic Nanoparticle by Dynamic Light Scattering. Nanoscale Res. Lett. 2013, 8, 308–381. [Google Scholar]
- Guex, A.G.; Di Marzio, N.; Eglin, D.; Alini, M.; Serra, T. The Waves That Make the Pattern: A Review on Acoustic Manipulation in Biomedical Research. Mater. Today Bio 2021, 10, 100110. [Google Scholar] [CrossRef] [PubMed]
- Çetin, B.; Özer, M.B.; Solmaz, M.E. Microfluidic Bio-Particle Manipulation for Biotechnology. Biochem. Eng. J. 2014, 92, 63–82. [Google Scholar] [CrossRef]
- Sánchez-Iglesias, A.; Grzelczak, M.; Altantzis, T.; Goris, B.; Pérez-Juste, J.; Bals, S.; Van Tendeloo, G.; Donaldson, S.H.; Chmelka, B.F.; Israelachvili, J.N.; et al. Hydrophobic Interactions Modulate Self-Assembly of Nanoparticles. ACS Nano 2012, 6, 11059–11065. [Google Scholar] [CrossRef] [PubMed]
- Moradi Kashkooli, F.; Soltani, M.; Souri, M. Controlled Anti-Cancer Drug Release through Advanced Nano-Drug Delivery Systems: Static and Dynamic Targeting Strategies. J. Control Release 2020, 327, 316–349. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Balla, V.K. A Review on the Use of Magnetic Fields and Ultrasound for Non-Invasive Cancer Treatment. J. Adv. Res. 2018, 14, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kohane, D.S. External Triggering and Triggered Targeting Strategies for Drug Delivery. Nat. Rev. Mater. 2017, 2, 17020. [Google Scholar] [CrossRef]
- Mi, P. Stimuli-Responsive Nanocarriers for Drug Delivery, Tumor Imaging, Therapy and Theranostics. Theranostics 2020, 10, 4557–4588. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K. Nanoarchitectonics: A Navigator from Materials to Life. Mater. Chem. Front. 2017, 1, 208–211. [Google Scholar] [CrossRef]
- Zafar, B.; Campbell, J.; Cooke, J.; Skirtach, A.G.; Volodkin, D. Modification of Surfaces with Vaterite CaCO3 Particles. Micromachines 2022, 13, 473. [Google Scholar] [CrossRef] [PubMed]
- Kotoulas, K.T.; Campbell, J.; Skirtach, A.G.; Volodkin, D.; Vikulina, A. Surface Modification with Particles Coated or Made of Polymer Multilayers. Pharmaceutics 2022, 14, 2483. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Li, J.; Fei, J.; Ji, Q.; Hill, J.P. Nanoarchitectonics for Dynamic Functional Materials from Atomic-/Molecular-Level Manipulation to Macroscopic Action. Adv. Mater. 2016, 28, 1251–1286. [Google Scholar] [CrossRef] [PubMed]
- Kryuchkova, M.; Batasheva, S.; Naumenko, E.; Rozhina, E.; Akhatova, F.; Panchal, A.; Lvov, Y.; Fakhrullin, R. Self-Assembly of Concentric Microrings of Tubule and Platy Nanoclays for Cell Patterning and Capturing. Appl. Clay Sci. 2020, 195, 105707. [Google Scholar] [CrossRef]
- Lishchynskyi, O.; Stetsyshyn, Y.; Raczkowska, J.; Awsiuk, K.; Orzechowska, B.; Abalymov, A.; Skirtach, A.G.; Bernasik, A.; Nastyshyn, S.; Budkowski, A. Fabrication and Impact of Fouling-Reducing Temperature-Responsive POEGMA Coatings with Embedded CaCO3 Nanoparticles on Different Cell Lines. Materials 2021, 14, 1417. [Google Scholar] [CrossRef]
- Dwivedi, C.; Verma, S. Preparation and Characterization of Liposomes with Application. J. Sci. Innov. Res. 2013, 2, 486–510. [Google Scholar]
- Ahmed, K.S.; Hussein, S.A.; Ali, A.H.; Korma, S.A.; Lipeng, Q.; Jinghua, C. Liposome: Composition, Characterisation, Preparation, and Recent Innovation in Clinical Applications. J. Drug Target. 2019, 27, 742–761. [Google Scholar] [CrossRef]
- Voit, B.I.; Lederer, A. Hyperbranched and Highly Branched Polymer Architectures—Synthetic Strategies and Major Characterization Aspects. Chem. Rev. 2009, 109, 5924–5973. [Google Scholar] [CrossRef]
- Hiremath, A.; Murthy, A.A.; Thipperudrappa, S.; KN, B. Nanoparticles Filled Polymer Nanocomposites: A Technological Review. Cogent Eng. 2021, 8, 1991229. [Google Scholar] [CrossRef]
- Fu, S.; Sun, Z.; Huang, P.; Li, Y.; Hu, N. Some Basic Aspects of Polymer Nanocomposites: A Critical Review. Nano Mater. Sci. 2019, 1, 2–30. [Google Scholar] [CrossRef]
- Skirtach, A.G.; Antipov, A.A.; Shchukin, D.G.; Sukhorukov, G.B. Remote Activation of Capsules Containing Ag Nanoparticles and IR Dye by Laser Light Remote Activation of Capsules Containing Ag Nanoparticles and IR Dye by Laser Light. Langmuir 2004, 20, 6988–6992. [Google Scholar] [CrossRef]
- Parakhonskiy, B.V.; Bedard, M.F.; Bukreeva, T.V.; Sukhorukov, G.B.; Möhwald, H.; Skirtach, A.G. Nanoparticles on Polyelectrolytes at Low Concentration: Controlling Concentration and Size. J. Phys. Chem. C 2010, 114, 1996–2002. [Google Scholar] [CrossRef]
- Parakhonskiy, B.V.; Gorin, D.A.; Bäumler, H.; Skirtach, A.G. Temperature Rise around Nanoparticles. J. Therm. Anal. Calorim. 2017, 127, 895–904. [Google Scholar] [CrossRef]
- Bukreeva, T.V.; Parakhonsky, B.V.; Skirtach, A.G.; Susha, A.S.; Sukhorukov, G.B. Preparation of Polyelectrolyte Microcapsules with Silver and Gold Nanoparticles in a Shell and the Remote Destruction of Microcapsules under Laser Irradiation. Crystallogr. Rep. 2006, 51, 863–869. [Google Scholar] [CrossRef]
- Richbourg, N.R.; Peppas, N.A.; Sikavitsas, V.I. Tuning the Biomimetic Behavior of Scaffolds for Regenerative Medicine through Surface Modifications. J. Tissue Eng. Regen. Med. 2019, 13, 1275–1293. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Schwartz, Z.; Kahn, A.; Li, X.; Shao, Z.; Sun, M.; Ao, Y.; Boyan, B.D.; Chen, H. Advances in Porous Scaffold Design for Bone and Cartilage Tissue Engineering and Regeneration. Tissue Eng. Part B Rev. 2019, 25, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Dave, K.; Gomes, V.G. Interactions at Scaffold Interfaces: Effect of Surface Chemistry, Structural Attributes and Bioaffinity. Mater. Sci. Eng. C 2019, 105, 110078. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Tan, A.; Pastorin, G.; Ho, H.K. Nanomaterial Scaffolds for Stem Cell Proliferation and Differentiation in Tissue Engineering. Biotechnol. Adv. 2013, 31, 654–668. [Google Scholar] [CrossRef]
- Zaidi, S.A.; Shin, J.H. Recent Developments in Nanostructure Based Electrochemical Glucose Sensors. Talanta 2016, 149, 30–42. [Google Scholar] [CrossRef]
- Hannah, S.; Blair, E.; Corrigan, D.K. Developments in Microscale and Nanoscale Sensors for Biomedical Sensing. Curr. Opin. Electrochem. 2020, 23, 7–15. [Google Scholar] [CrossRef]
- Tian, K.; Prestgard, M.; Tiwari, A. A Review of Recent Advances in Nonenzymatic Glucose Sensors. Mater. Sci. Eng. C 2014, 41, 100–118. [Google Scholar] [CrossRef]
- Wang, J.; Qu, X. Recent Progress in Nanosensors for Sensitive Detection of Biomolecules. Nanoscale 2013, 5, 3589. [Google Scholar] [CrossRef]
- Geraldes, C.F.G.C.; Laurent, S. Classification and Basic Properties of Contrast Agents for Magnetic Resonance Imaging. Contrast Media Mol. Imaging 2009, 4, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Esteban, B.; Riba, J.-R.; Baquero, G.; Rius, A.; Puig, R. Temperature Dependence of Density and Viscosity of Vegetable Oils. Biomass Bioenergy 2012, 42, 164–171. [Google Scholar] [CrossRef]
- Gao, Q.; Zhang, J.; Gao, J.; Zhang, Z.; Zhu, H.; Wang, D. Gold Nanoparticles in Cancer Theranostics. Front. Bioeng. Biotechnol. 2021, 9, 647905. [Google Scholar] [CrossRef]
- Tian, S.; He, J.; Lyu, D.; Li, S.; Xu, Q.-H. Aggregation Enhanced Photoactivity of Photosensitizer Conjugated Metal Nanoparticles for Multimodal Imaging and Synergistic Phototherapy below Skin Tolerance Threshold. Nano Today 2022, 45, 101534. [Google Scholar] [CrossRef]
- Wang, W.; Duan, W.; Ahmed, S.; Sen, A.; Mallouk, T.E. From One to Many: Dynamic Assembly and Collective Behavior of Self-Propelled Colloidal Motors. Acc. Chem. Res. 2015, 48, 1938–1946. [Google Scholar] [CrossRef] [PubMed]
- Krissanaprasit, A.; Key, C.M.; Pontula, S.; LaBean, T.H. Self-Assembling Nucleic Acid Nanostructures Functionalized with Aptamers. Chem. Rev. 2021, 121, 13797–13868. [Google Scholar] [CrossRef]

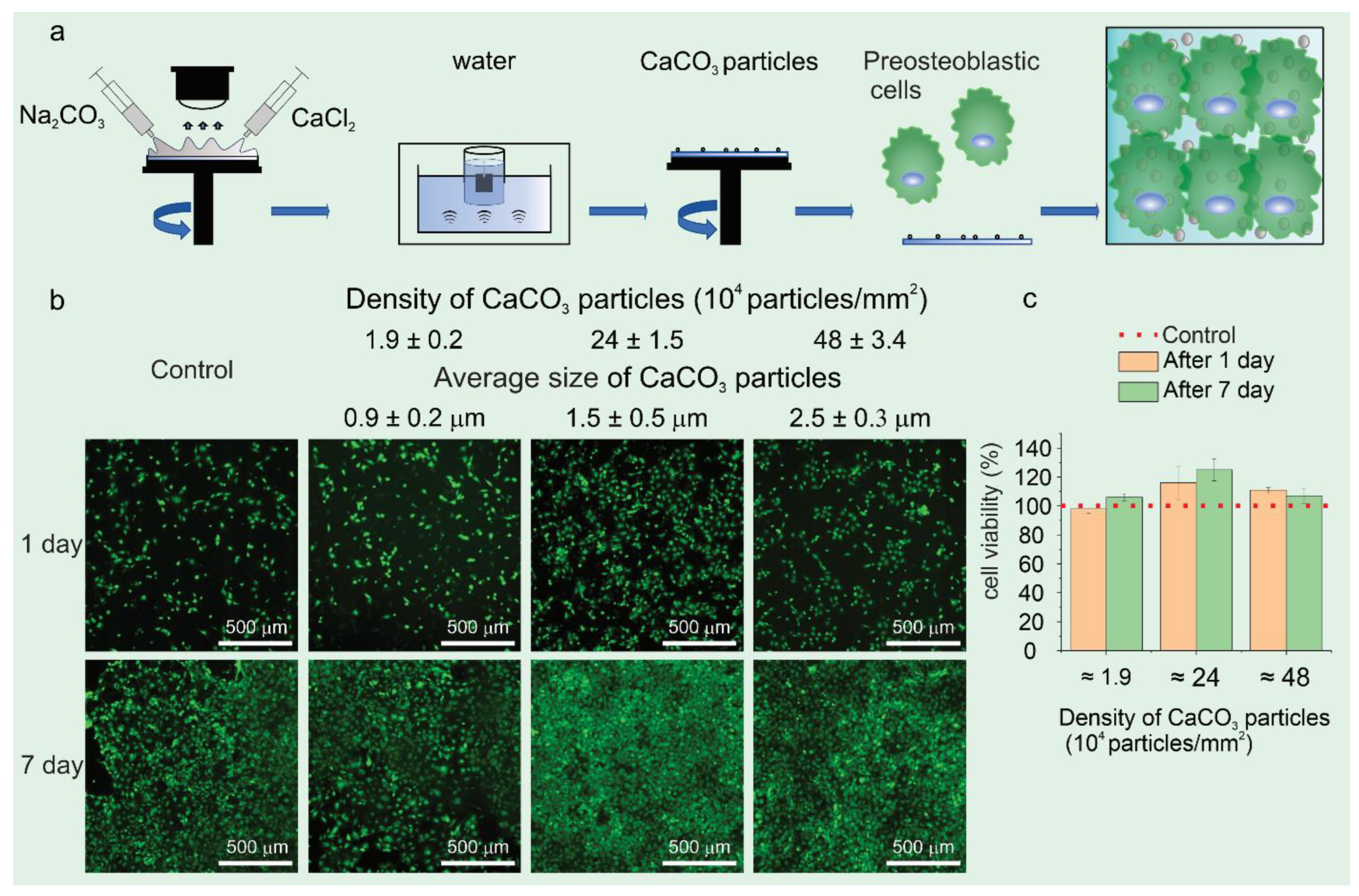
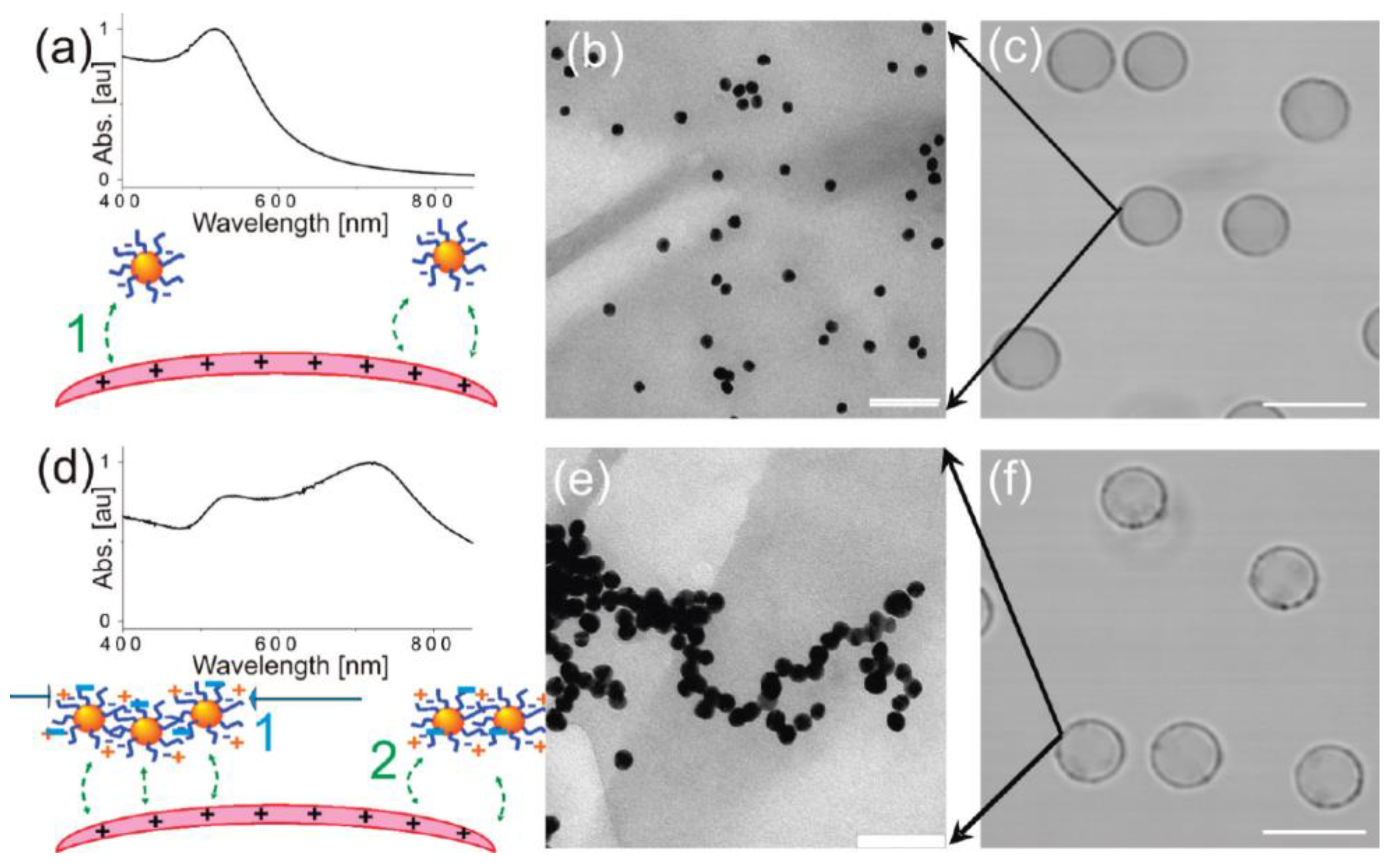
| Category | Assembly Method | Advantages | Disadvantages | |
|---|---|---|---|---|
| Static and dynamic | Self-Assembly | Uses weak interactions (e.g., van der Waals forces, hydrogen bonding) for the organization of nanoparticles | Limited in performing specific advanced functions due to conventional molecules’ limitations | [10,18,19,20,21] |
| Static | Layer-by-Layer Assembly | Exceptional control and versatility, applicable in various fields, allows the formation of multilayered structures | The complex physical mechanisms governing adsorbed quantities require a specifically charged substrate | [51,52,53,58,59,60,61,62] |
| Template-Assisted Assembly | Precise assembly of nanoparticles, high degree of structural regularity, versatile applications | Requires specialized substrates or templates for organization | [2,10,16,29,70,72,73,74,75] | |
| Polymer Brushes | Can engage nanoparticles without aggregation, offers adjustability in properties | Weak interaction between polymer brushes and nanoparticles may lead to aggregation | [83,84,85,86] | |
| Dynamic | Drop Coating | Offer precisely controlled, thin layers on a wide range of substrates. Versatile applications like thin films, protective coatings, and the deposition of materials onto surfaces. Homogeneous deposition of particles and control over particle distribution | The evaporating liquid can leave behind a ring-like deposit of particles. Dependency on variables, such as temperature and ethanol concentration, and substrate roughness | [110,111,112] |
| Microfluidic Devices | Suitable for handling minute quantities of fluids, applicable in drug delivery systems, sensors, and pumps | Handling and control of fluids in microchannels can be challenging | [110,115] | |
| Capillary Assembly | Employs capillary forces for precise positioning of components, applicable in microelectronics and optics | Specialized technique requiring precise control of capillary forces | [118,119] | |
| Dip Coating | Simple, cost effective, and suitable for creating thin-film coatings | Control of coating thickness can be challenging; a potential slowdown in production due to drying and curing times | [120,121,122] | |
| Spin Coating | Widely used in semiconductor manufacturing, can produce films with thicknesses of less than 10 nanometers | Limitations due to specific assumptions in the dynamic method; factors like solvent diffusion and substrate surface roughness | [123,124,126,127,128,129,130,131] | |
| Langmuir–Blodgett | Precise method for assembling monolayers into nanostructured films, instrumental in surface science and nanotechnology | Not included in the primary focus of the study; relevance is limited to specific applications | [132,133,134] | |
| Freeze Casting | Forms materials with high porosity and complex geometries, useful in fabricating nanocomposites | Not included in the primary focus of the study; precision required in packing and pore directionality | [135,136,137,138] | |
| Magnetic Field | High precision in the manipulation of biomolecules and polymers, plays a pivotal role in biophysics and nanotechnology | Requires understanding of electromagnetism; limited approach with single-pole tweezers | [26,101,178,179,180,181,182,183,184,185,186,187,188,189,190] | |
| Acoustic Field | Rapid parallel fabrication of objects from a solution, mechanical stability, and self-sustaining properties of assembled structures | Early-stage technology with potential long-term effects on cells; complex dynamics surrounding cell proliferation and differentiation | [177,197,198,199,200,201,202,203,205] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eftekhari, K.; Parakhonskiy, B.V.; Grigoriev, D.; Skirtach, A.G. Advances in Nanoarchitectonics: A Review of “Static” and “Dynamic” Particle Assembly Methods. Materials 2024, 17, 1051. https://doi.org/10.3390/ma17051051
Eftekhari K, Parakhonskiy BV, Grigoriev D, Skirtach AG. Advances in Nanoarchitectonics: A Review of “Static” and “Dynamic” Particle Assembly Methods. Materials. 2024; 17(5):1051. https://doi.org/10.3390/ma17051051
Chicago/Turabian StyleEftekhari, Karaneh, Bogdan V. Parakhonskiy, Dmitry Grigoriev, and Andre G. Skirtach. 2024. "Advances in Nanoarchitectonics: A Review of “Static” and “Dynamic” Particle Assembly Methods" Materials 17, no. 5: 1051. https://doi.org/10.3390/ma17051051
APA StyleEftekhari, K., Parakhonskiy, B. V., Grigoriev, D., & Skirtach, A. G. (2024). Advances in Nanoarchitectonics: A Review of “Static” and “Dynamic” Particle Assembly Methods. Materials, 17(5), 1051. https://doi.org/10.3390/ma17051051









