Fabrication and Characterization of Al2O3-Siloxane Composite Thermal Pads for Thermal Interface Materials
Abstract
1. Introduction
2. Materials and Experimental Methods
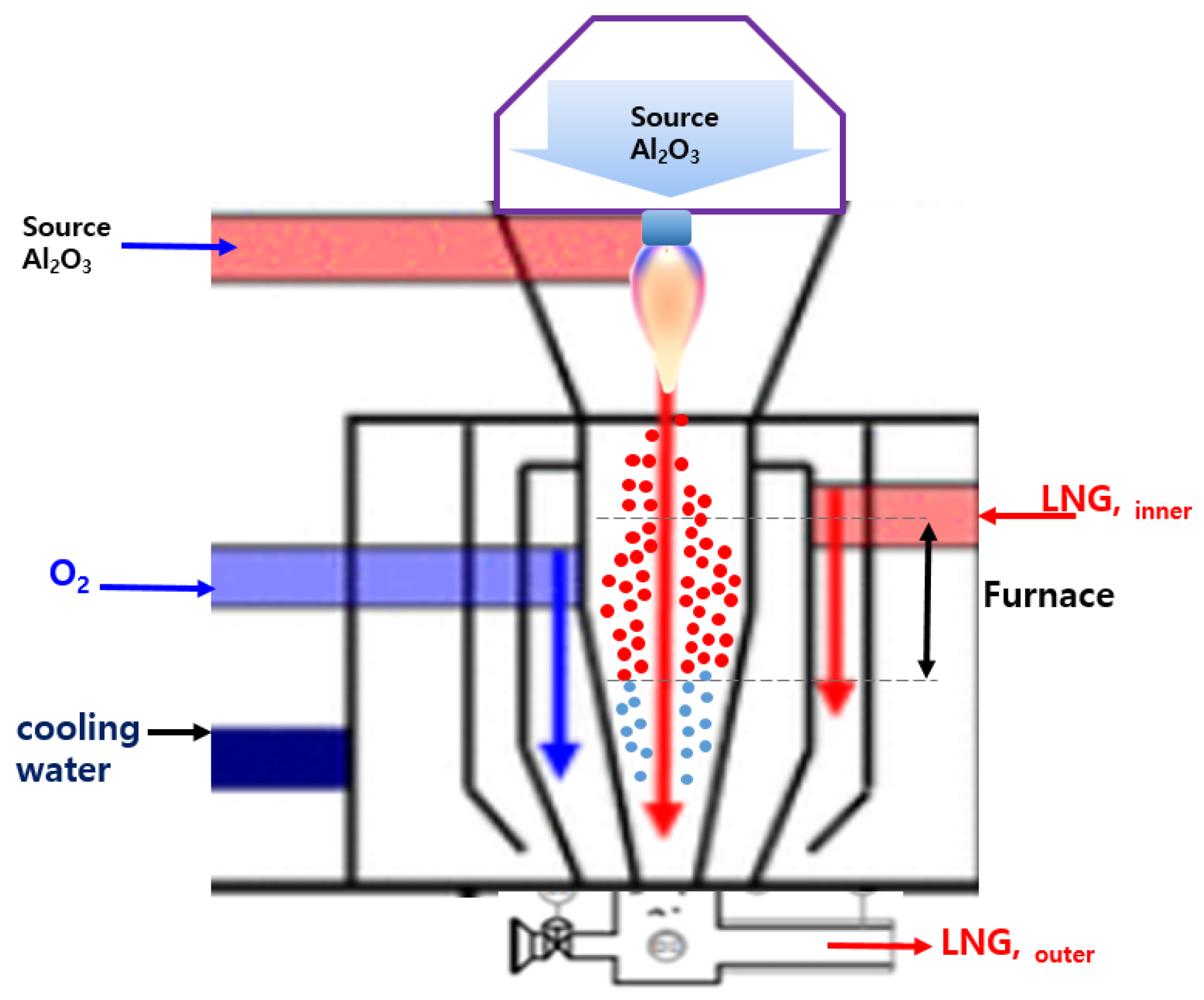
2.1. Preparation of Al2O3 Nanoparticle Powder
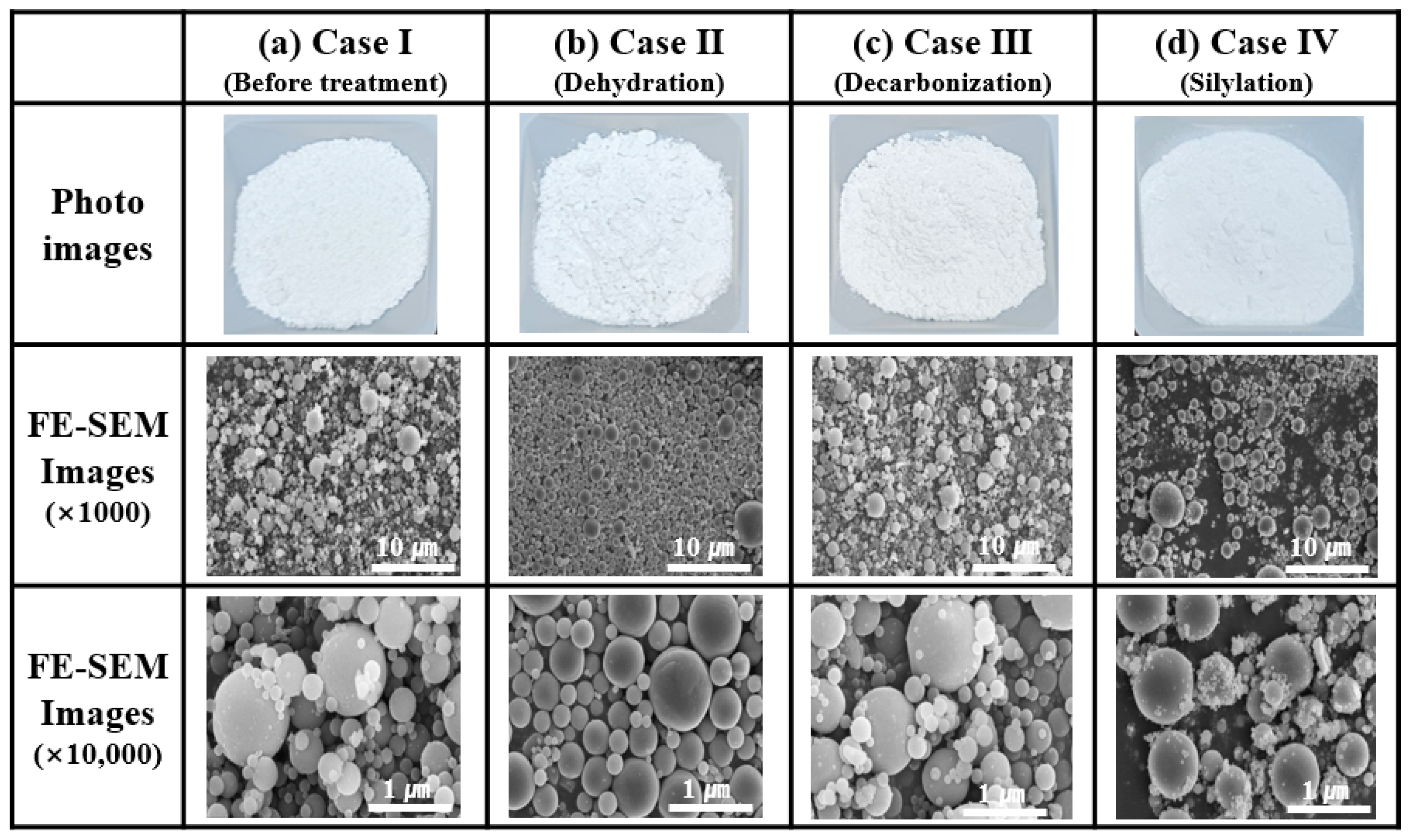
2.2. Surface Treatment of the Synthesized Al2O3 Nanoparticle Powders
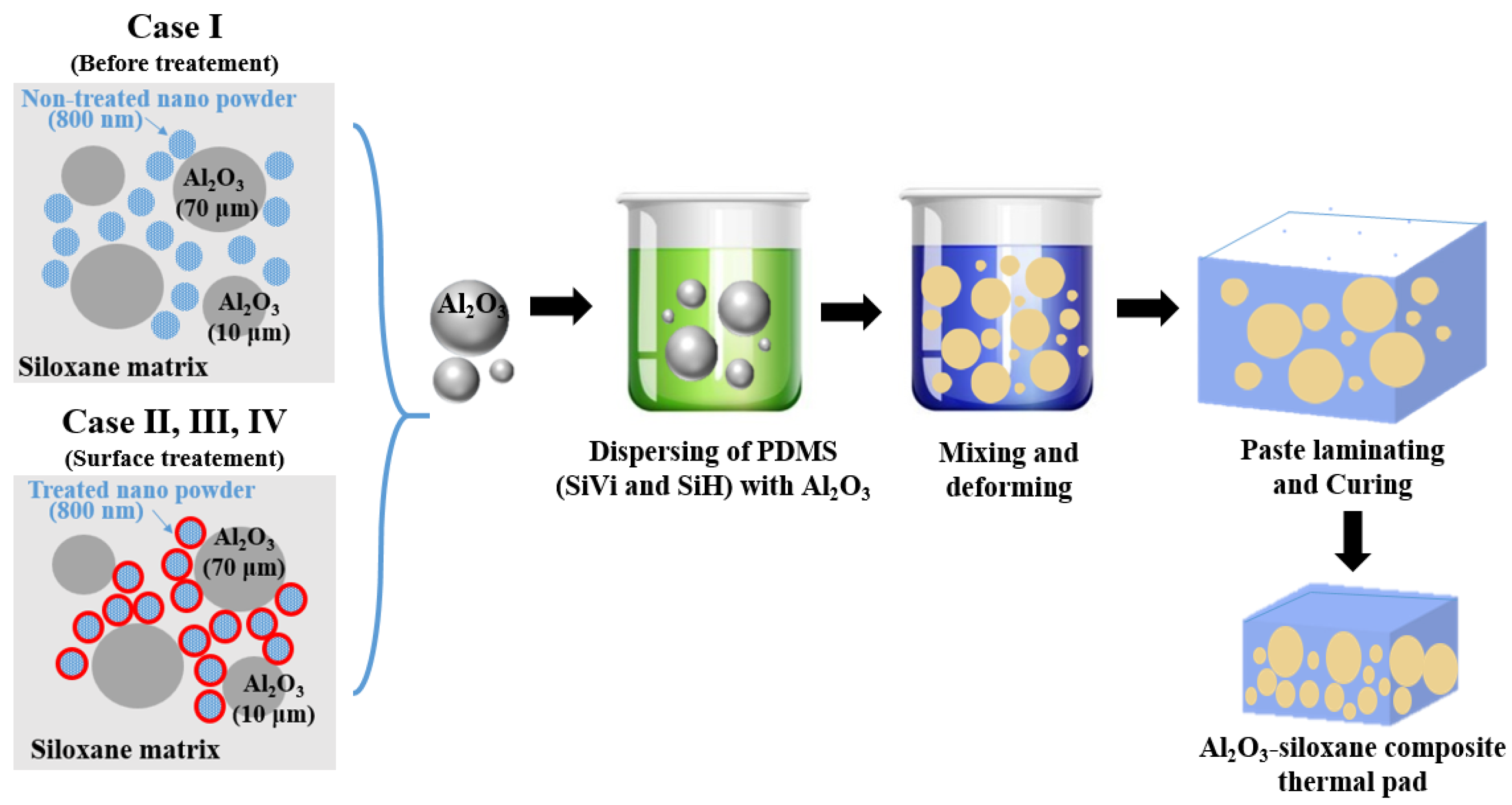
2.3. Fabrication of Al2O3-Siloxane Composite Thermal Pads Using the Synthesized Al2O3 Nanoparticle Powder
2.4. Analysis and Characterization
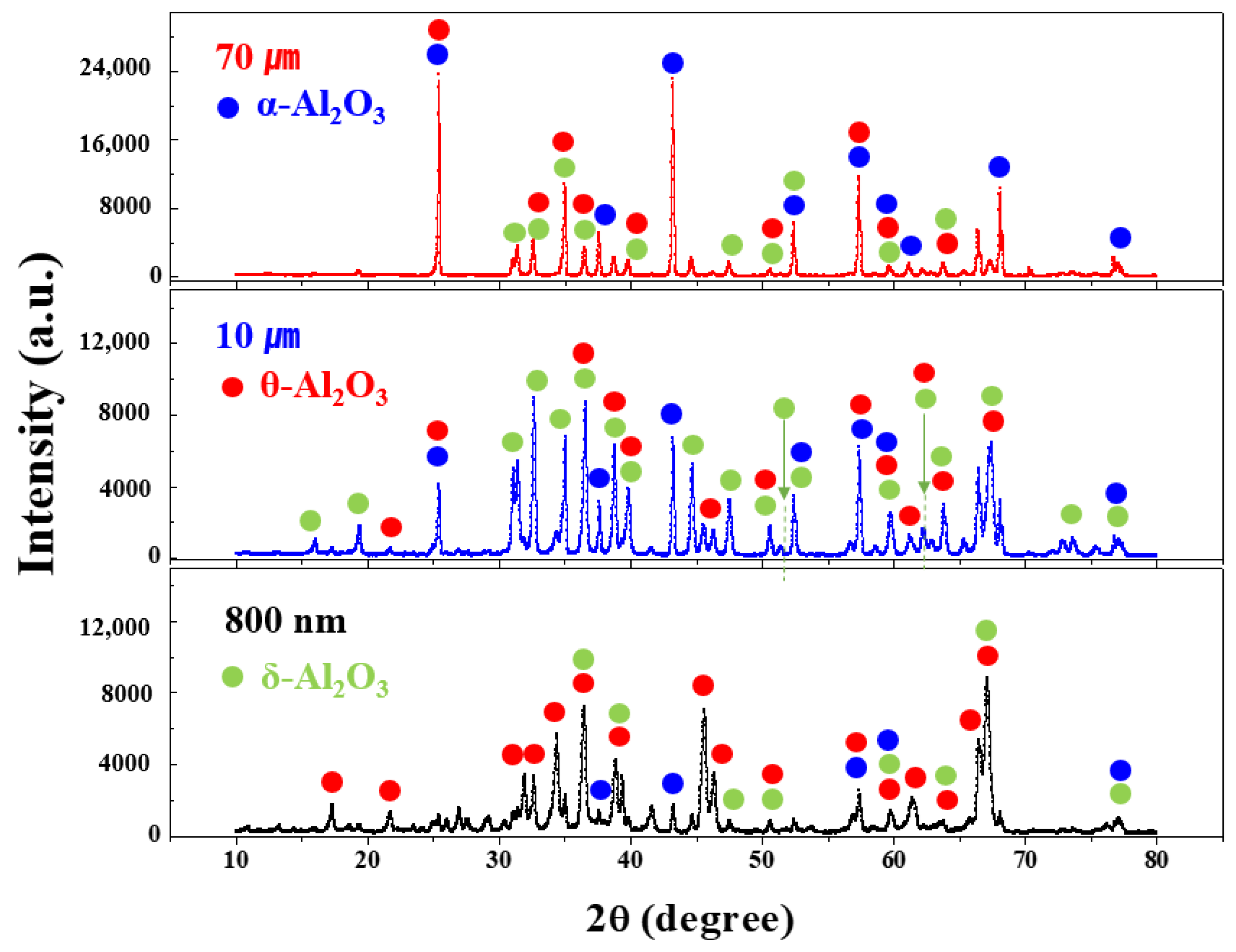
2.4.1. X-ray Diffraction
2.4.2. Field Emission Scanning Electron Microscopy
2.4.3. Fourier Transform Infrared Spectroscopy
2.4.4. Inductively Coupled Plasma–Optical Emission Spectrometry
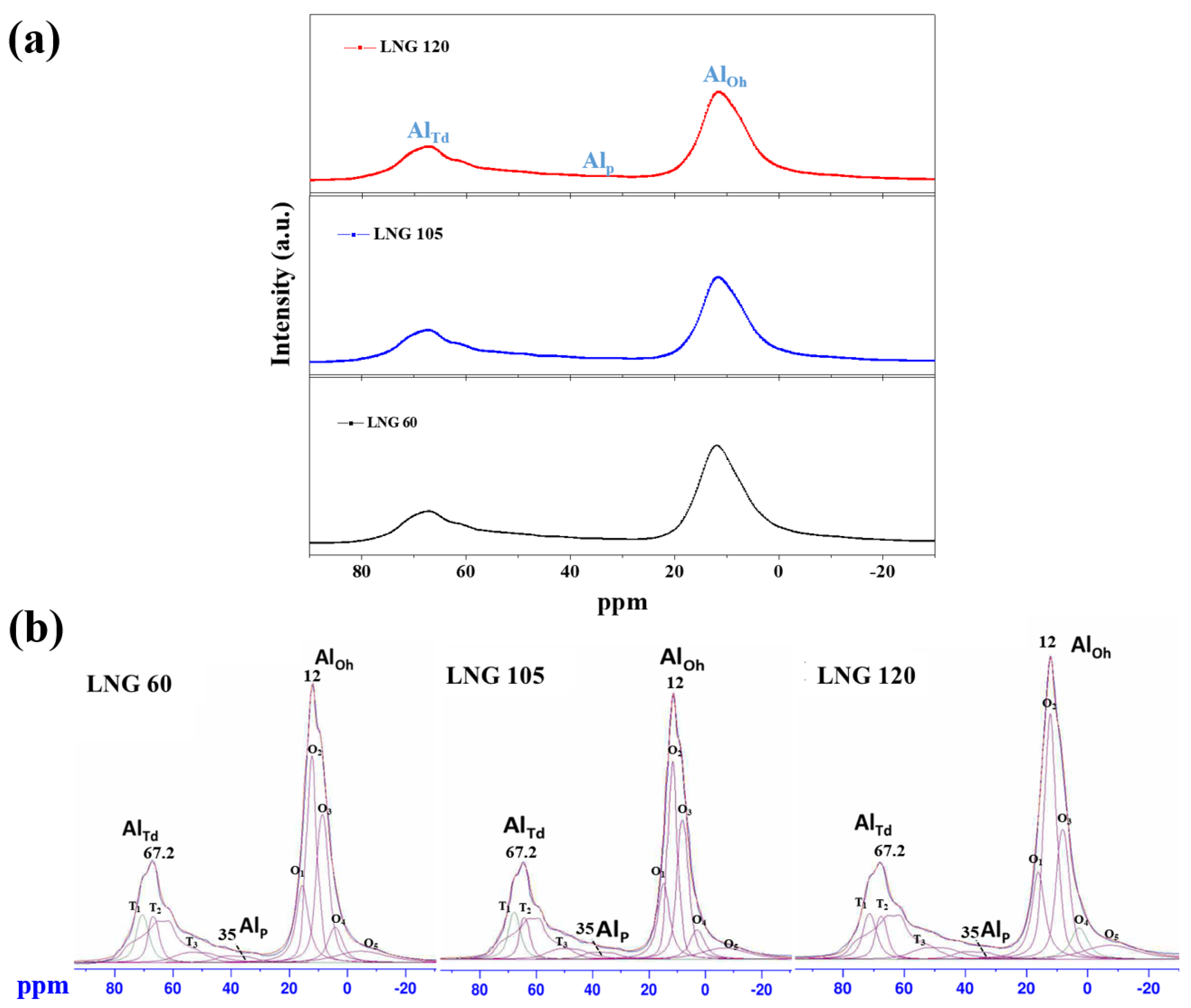
2.4.5. Nuclear Magnetic Resonance
2.4.6. Thermomechanical and Electrical Properties
3. Results and Discussion
3.1. Synthesis of the Al2O3 Nanoparticle Powder
3.2. Surface Treatment of the Al2O3 Powder
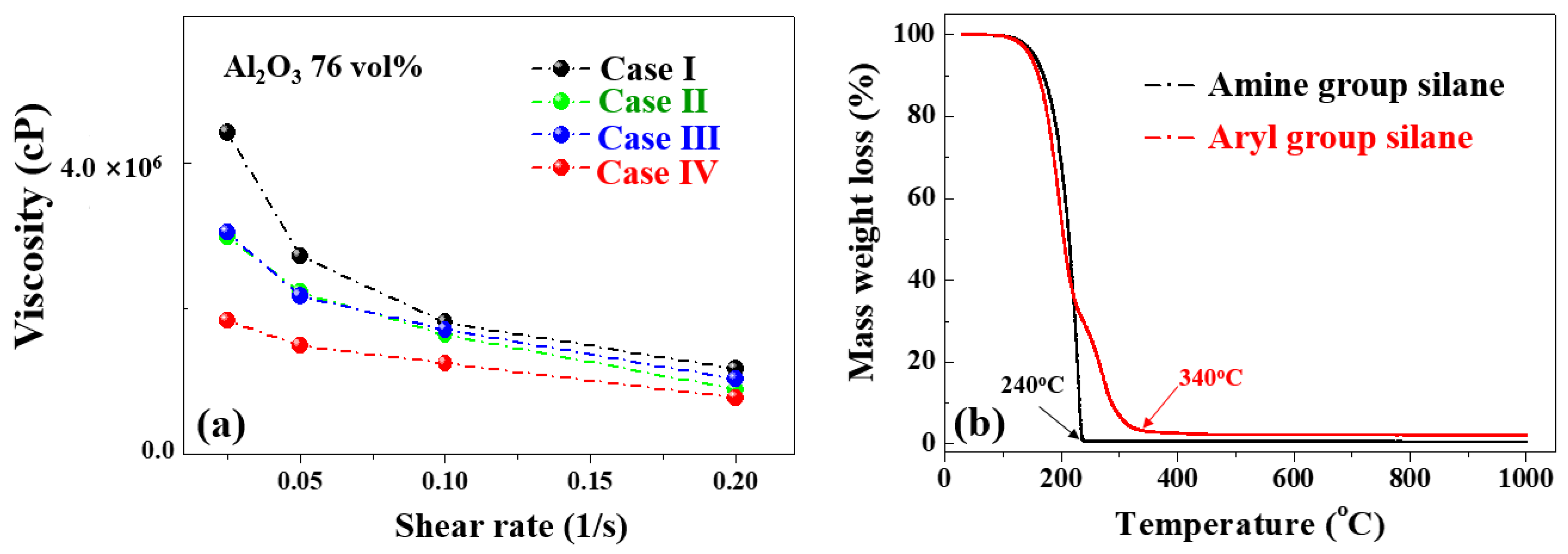
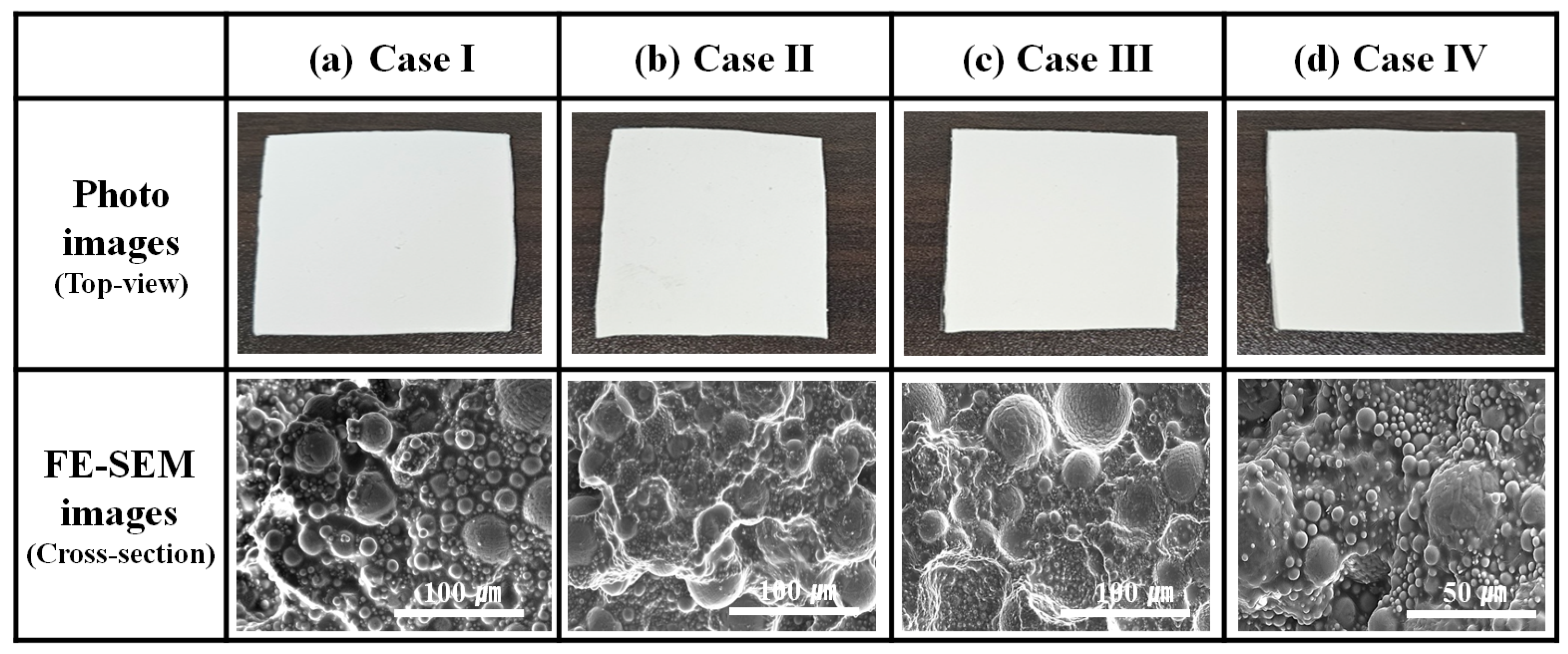
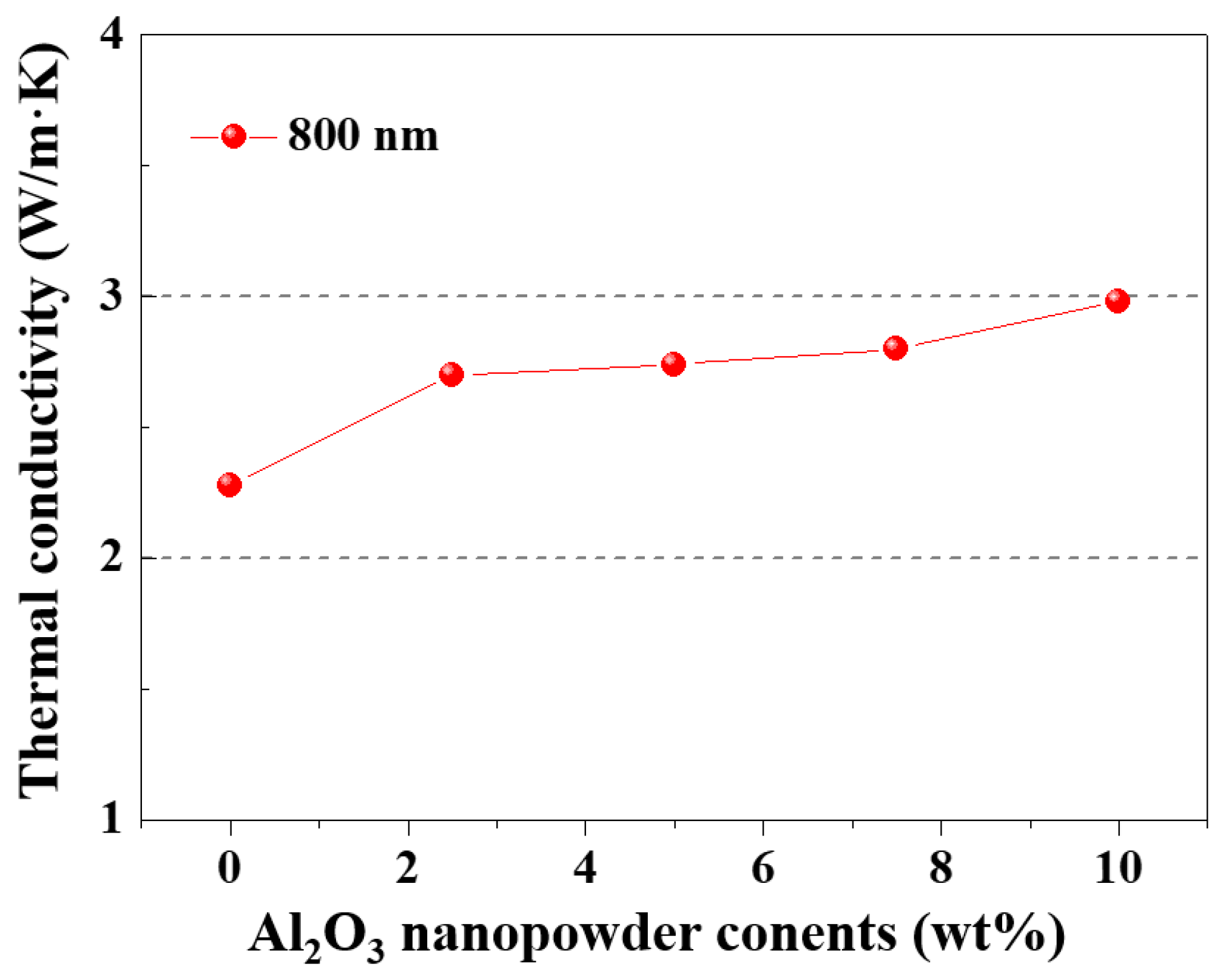
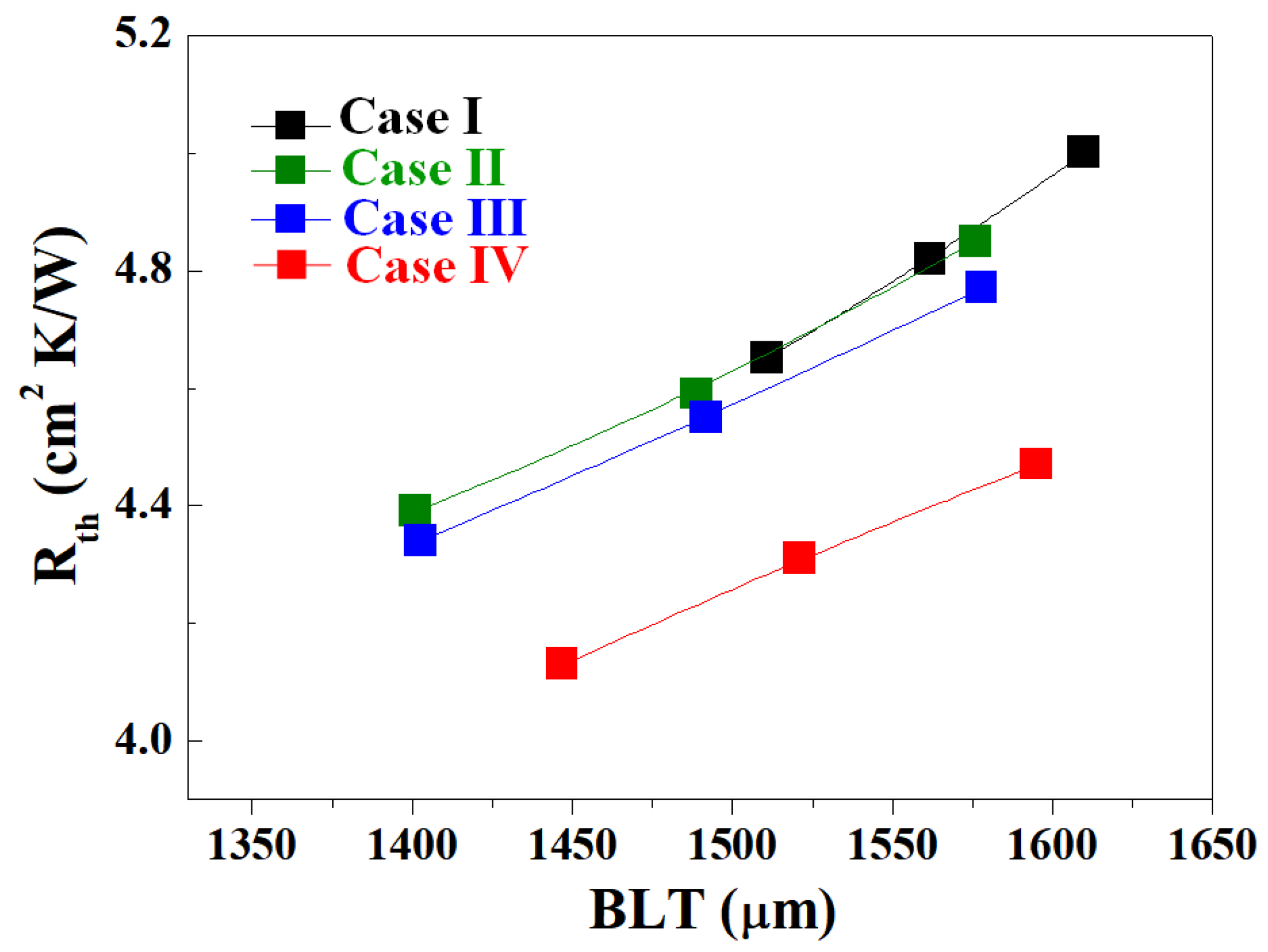
3.3. Fabrication of Al2O3–Siloxane Composite Thermal Pads
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, Z.; Zhang, C.; Li, Y.; Zou, J.; Li, Y.; Yang, B.; Hu, R.; Qian, Q. Effect of the alumina micro-particle sizes on the thermal conductivity and dynamic mechanical property of epoxy resin. PLoS ONE 2023, 18, e0292878. [Google Scholar] [CrossRef] [PubMed]
- Klimov, A.; Bakeev, I.; Zenin, A. Electron-beam processing of aluminum-containing ceramics in the forevacuum pressure range. Ceramics 2023, 6, 2098–2116. [Google Scholar] [CrossRef]
- Dong, X.; An, Q.; Zhang, S.; Yu, H.; Wang, M. Porous ceramics based on high-thermal-stability Al2O3–ZrO2 nanofibers for thermal insulation and sound absorption applications. Ceram. Int. 2023, 49, 31035–31045. [Google Scholar] [CrossRef]
- He, S.; Li, Y.; Zong, X.; Wu, S. The effect of AlN content on the properties of Al2O3-AlN composite ceramics fabricated by digital light processing. Crystals 2023, 13, 107. [Google Scholar] [CrossRef]
- Xu, X.; Chen, J.; Zhou, J.; Li, B. Thermal conductivity of polymers and their nanocomposites. Adv. Mater. 2018, 30, 1705544. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J. Rheological behaviors of plasticized polyvinyl chloride thermally conductive composites with oriented flaky fillers: A case study on graphite and mica. J. Appl. Polym. Sci. 2022, 139, 52186. [Google Scholar] [CrossRef]
- Varenik, M.; Nadiv, R.; Levy, I.; Vasilyev, G.; Regev, O. Breaking through the solid/liquid processability barrier: Thermal conductivity and rheology in hybrid graphene–graphite polymer composites. ACS Appl. Mater. Interfaces 2017, 9, 7556–7564. [Google Scholar] [CrossRef]
- Wen, H.L.; Chen, Y.Y.; Yen, F.S.; Huang, C.Y. Size characterization of θ-and α-Al2O3 crystallites during phase transformation. Nanostruct. Mater. 1999, 11, 89–101. [Google Scholar] [CrossRef]
- Eom, S.H.; Pee, J.H.; Lee, J.K.; Hwang, K.T.; Cho, W.S.; Kim, K.J. Effects of Chemical composition and particle size of starting aluminum source on the spheroidization in the flame fusion process. J. Powder Mater. 2009, 16, 431–437. [Google Scholar] [CrossRef][Green Version]
- Barnes, H.A.; Hutton, J.F.; Walters, K. An Introduction to Rheology, 3rd ed.; Elsevier: Amsterdam, the Netherlands, 1993; pp. 1–199. [Google Scholar]
- Culham, J.R.; Teertstra, P.; Savija, I.; Yovanovich, M.M. Design, Assembly and commissioning of a test apparatus for characterizing thermal interface materials. In Proceedings of the ITherm 2002—Eighth Intersociety Conference on Thermal and Thermomechanical Phenomena in Electronic Systems, San Diego, CA, USA, 30 May–1 June 2002; pp. 128–135. [Google Scholar]
- Kempers, R.; Kolodner, P.; Lyons, A.; Robinson, A.J. A high-precision apparatus for the characterization of thermal interface materials. Rev. Sci. Instr. 2009, 80, 095111. [Google Scholar] [CrossRef]
- Kovarik, L.; Bowden, M.; Andersen, A.; Jaegers, N.R.; Washton, N.; Szanyi, J. Quantification of high-temperature transition Al2O3 and their phase transformations. Angew. Chem. Int. Ed. 2020, 59, 21719–21727. [Google Scholar] [CrossRef] [PubMed]
- O’Dell, L.A.; Savin, S.L.; Chadwick, A.V.; Smith, M.E. A 27Al MAS NMR study of a sol–gel produced alumina: Identification of the NMR parameters of the θ-Al2O3 transition alumina phase. Solid State Nucl. Mag. Resonance 2007, 31, 169–173. [Google Scholar] [CrossRef]
- Xu, S.; Jaegers, N.R.; Hu, W.; Kwak, J.H.; Bao, X.; Sun, J.; Wang, Y.; Hu, J.Z. High-field one-dimensional and two-dimensional 27Al magic-angle spinning nuclear magnetic resonance study of θ-, δ-, and γ-Al2O3 dominated aluminum oxides: Toward understanding the Al sites in γ-Al2O3. ACS Omega 2021, 6, 4090–4099. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, K.; Han, P.; Yang, B.; Feng, L. Calculation and analysis of the structure and viscosity of B2O3-regulated CaO-Al2O3-based mold fluxes. J. Chem. 2020, 2020, 8844392. [Google Scholar] [CrossRef]
- Köck, E.M.; Kogler, M.; Bielz, T.; Klötzer, B.; Penner, S. In situ FT-IR spectroscopic study of CO2 and CO adsorption on Y2O3, ZrO2, and yttria-stabilized ZrO2. J. Phys. Chem. C 2013, 117, 17666–17673. [Google Scholar] [CrossRef]
- Miller, D.C.; Kempe, M.D.; Muller, M.T.; Gray, M.H.; Araki, K.; Kurtz, S.R. Durability of polymeric encapsulation materials in a PMMA/glass concentrator photovoltaic system. Prog. Photovolt. Res. Appl. 2016, 24, 1385–1409. [Google Scholar] [CrossRef]
- Goldberg, M.A.; Protsenko, P.V.; Smirnov, V.V.; Antonova, O.S.; Smirnov, S.V.; Konovalov, A.A.; Vorckachev, K.G.; Kudryavtsev, E.A.; Barinov, S.M.; Komlev, V.S. The enhancement of hydroxyapatite thermal stability by Al doping. J. Mater. Res. Technol. 2020, 9, 76–88. [Google Scholar] [CrossRef]
- Sergeeva, A.V.; Zhitova, E.S.; Nuzhdaev, A.A.; Zolotarev, A.A.; Bocharov, V.N.; Ismagilova, R.M. Infrared and Raman spectroscopy of ammoniovoltaite,(NH4)2Fe2+ 5Fe3+ 3Al (SO4)12(H2O)18. Minerals 2020, 10, 781. [Google Scholar] [CrossRef]
- Ahmad, S.; Farrukh, M.A. Anti-microbial activities of sulfonamides using disc diffusion method. Park. J. Pharm. Sci. 2012, 25, 839–844. [Google Scholar]
- Atayde, C.; Doi, I. Highly stable hydrophilic surfaces of PDMS thin layer obtained by UV radiation and oxygen plasma treatments. Physica Status Solidi C 2010, 7, 189–192. [Google Scholar] [CrossRef]
- Drake, K.; Mukherjee, I.; Mirza, K.; Ji, H.F.; Wei, Y. Phenylethynyl and phenol end-capping studies of polybiphenyloxydiphenylsilanes for cross-linking and enhanced thermal stability. Macromolecules 2011, 44, 4107–4115. [Google Scholar] [CrossRef]
- Osswald, S.; Yushin, G.; Mochalin, V.; Kucheyev, S.O.; Gogotsi, Y. Control of sp2/sp3 carbon ratio and surface chemistry of nanodiamond powders by selective oxidation in air. J. Am. Chem. Soc. 2006, 128, 11635–11642. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.M.; Murphy, T.E.; Reipa, V. Ultraviolet photoluminescence from 6H silicon carbide nanoparticles. Appl. Phys. Lett. 2008, 92, 253112. [Google Scholar] [CrossRef]
- Mohanachandran Nair Sindhu, S.; Sankaranarayana Iyer, S. Generalized theory of thermal conductivity for different media: Solids to nanofluids. J. Phys. Chem. C 2019, 123, 23264–23271. [Google Scholar] [CrossRef]
- Ko, E.; Choi, S.S. Characterization and formation of chemical bonds of silica-coupling agent-rubber. Elastomers Compos. 2014, 49, 239–244. [Google Scholar] [CrossRef][Green Version]
- Dou, Z.; Zhang, B.; Xu, P.; Fu, Q.; Wu, K. Dry-contact thermal interface material with the desired bond line thickness and ultralow applied thermal resistance. ACS Appl. Mater. Interfaces 2023, 15, 57602–57612. [Google Scholar] [CrossRef]
- Li, Y.-T.; Liu, W.-J.; Shen, F.-X.; Zhang, G.-D.; Gong, L.-X.; Zhao, L.; Song, P.; Gao, J.-F.; Tang, L.-C. Processing, thermal conductivity and flame retardant properties of silicone rubber filled with different geometries of thermally conductive fillers: A comparative study. Compos. Part B 2022, 238, 109907. [Google Scholar] [CrossRef]






| The Synthesized Spherical Al2O3 Powder | Particle Size of the Al2O3 Powder | ||
|---|---|---|---|
| 70 μm | 10 μm | 800 nm | |
| Mixing ratio (wt. %) | 55 | 36 | 9 |
| Crystal Phase (%) | LNG Flow Rates (Nm3/h) | ||
|---|---|---|---|
| 60 Nm3/h | 105 Nm3/h | 120 Nm3/h | |
| δ | 85 | 87 | 88 |
| θ | 8 | 10 | 10 |
| α | 7 | 3 | 2 |
| LNG Flow Rates (Nm3/h) | AlTd (%) | AlP (%) | AlOh (%) | AlOh/AlTd |
|---|---|---|---|---|
| 60 Nm3/h | 33 | 2.6 | 100 | 3.0 |
| 105 Nm3/h | 34 | 2.9 | 100 | 2.9 |
| 120 Nm3/h | 31 | 2.7 | 100 | 3.2 |
| Case Study | Element Compositions (mg/L) | ||||
|---|---|---|---|---|---|
| Si | Fe | Ca | Mg | Na | |
| Case I (before treatment) | 0.16 | <0.01 | 0.51 | 0.08 | 5.02 |
| Case II | 0.12 | <0.01 | 0.65 | 0.04 | 0.74 |
| Case III | 0.11 | <0.01 | <0.01 | <0.01 | 4.46 |
| Crystal Phase (%) | Particle Size of Al2O3 Powder | ||
|---|---|---|---|
| 800 nm | 10 µm | 70 µm | |
| δ | 91 | 19 | 1 |
| θ | 2 | 62 | 35 |
| α | 7 | 19 | 64 |
| Case Studies | Volume Fraction (%) | Thickness (mm) | Hardness (HS) | Thermal Conductivity (W/m·K) |
|---|---|---|---|---|
| Case I | 60 | 1.92 | 46 | 2.53 |
| 69 | 2.40 | 55 | 2.62 | |
| 73 | 2.40 | 67 | 2.79 | |
| 76 | 2.43 | 69 | 3.05 | |
| Case II | 60 | 2.20 | 59 | 2.41 |
| 69 | 2.28 | 57 | 2.86 | |
| 73 | 2.21 | 72 | 3.44 | |
| 76 | 2.35 | 77 | 3.57 | |
| 79 | 2.38 | 80 | 3.93 | |
| Case III | 60 | 2.52 | 53 | 2.47 |
| 69 | 3.09 | 51 | 2.75 | |
| 73 | 3.02 | 71 | 3.11 | |
| 76 | 3.01 | 79 | 3.66 | |
| 79 | 3.13 | 80 | 3.96 | |
| Case IV | 60 | 2.52 | 57 | 2.77 |
| 69 | 2.92 | 72 | 3.15 | |
| 73 | 3.05 | 77 | 3.56 | |
| 76 | 2.90 | 79 | 3.71 | |
| 79 | 3.02 | 85 | 4.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-K.; Koo, Y.-J.; Kim, H.S.; Lee, J.-K.; Jeong, K.; Lee, Y.; Jung, E.Y. Fabrication and Characterization of Al2O3-Siloxane Composite Thermal Pads for Thermal Interface Materials. Materials 2024, 17, 914. https://doi.org/10.3390/ma17040914
Kim S-K, Koo Y-J, Kim HS, Lee J-K, Jeong K, Lee Y, Jung EY. Fabrication and Characterization of Al2O3-Siloxane Composite Thermal Pads for Thermal Interface Materials. Materials. 2024; 17(4):914. https://doi.org/10.3390/ma17040914
Chicago/Turabian StyleKim, Seul-Ki, Yeong-Jin Koo, Hyun Sik Kim, Jong-Keun Lee, Kyounghoon Jeong, Younki Lee, and Eun Young Jung. 2024. "Fabrication and Characterization of Al2O3-Siloxane Composite Thermal Pads for Thermal Interface Materials" Materials 17, no. 4: 914. https://doi.org/10.3390/ma17040914
APA StyleKim, S.-K., Koo, Y.-J., Kim, H. S., Lee, J.-K., Jeong, K., Lee, Y., & Jung, E. Y. (2024). Fabrication and Characterization of Al2O3-Siloxane Composite Thermal Pads for Thermal Interface Materials. Materials, 17(4), 914. https://doi.org/10.3390/ma17040914






