Development of a Recycling Process and Characterization of EVA, PVDF, and PET Polymers from End-of-Life PV Modules
Abstract
1. Introduction
2. Materials and Methods
2.1. Photovoltaic Modules
2.2. Swelling of EVA and Separation Process
2.2.1. Density
2.2.2. Dynamic Viscosity
2.3. Analysis Methods: FT-IR Spectrometry and Elemental Analysis
2.4. Differential Scanning Calorimetry
3. Results and Discussion
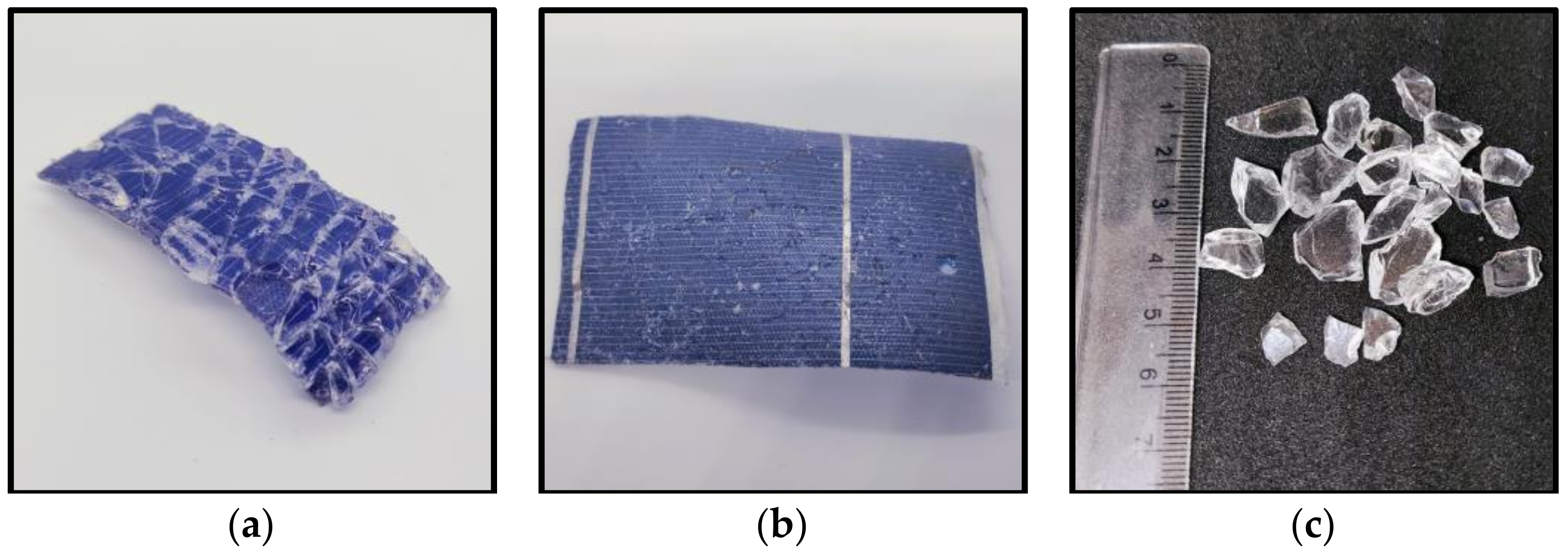
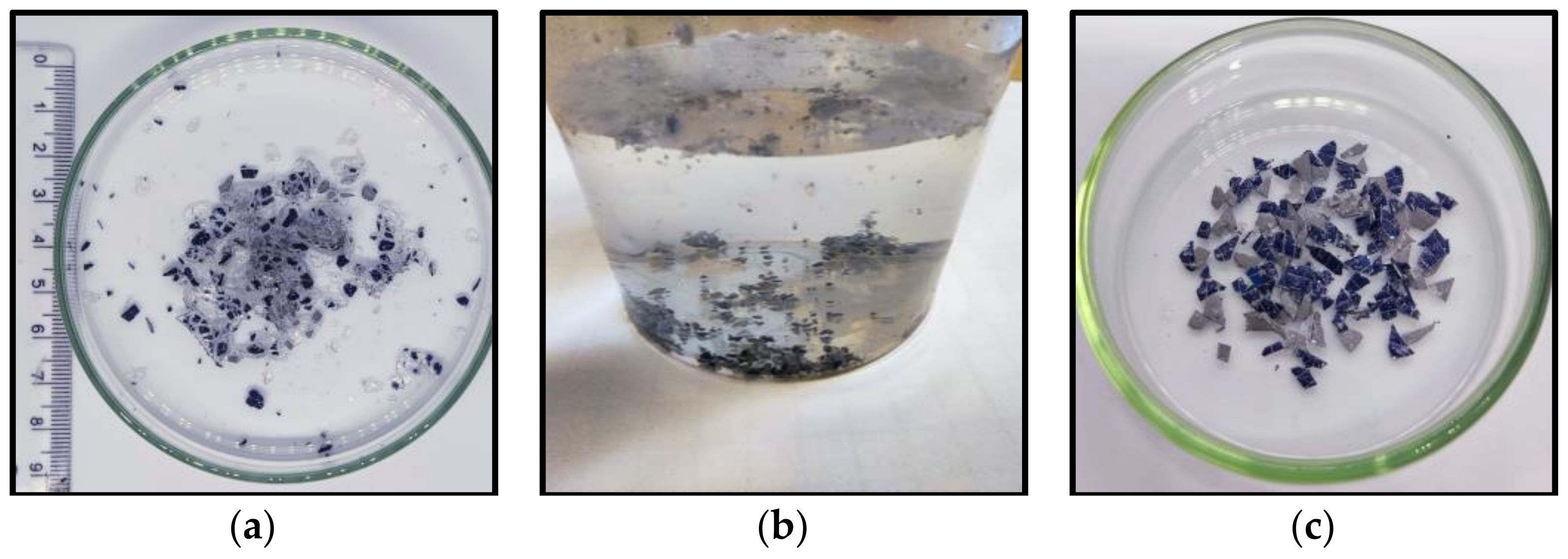
3.1. Thermal Separation of the Glass Layer
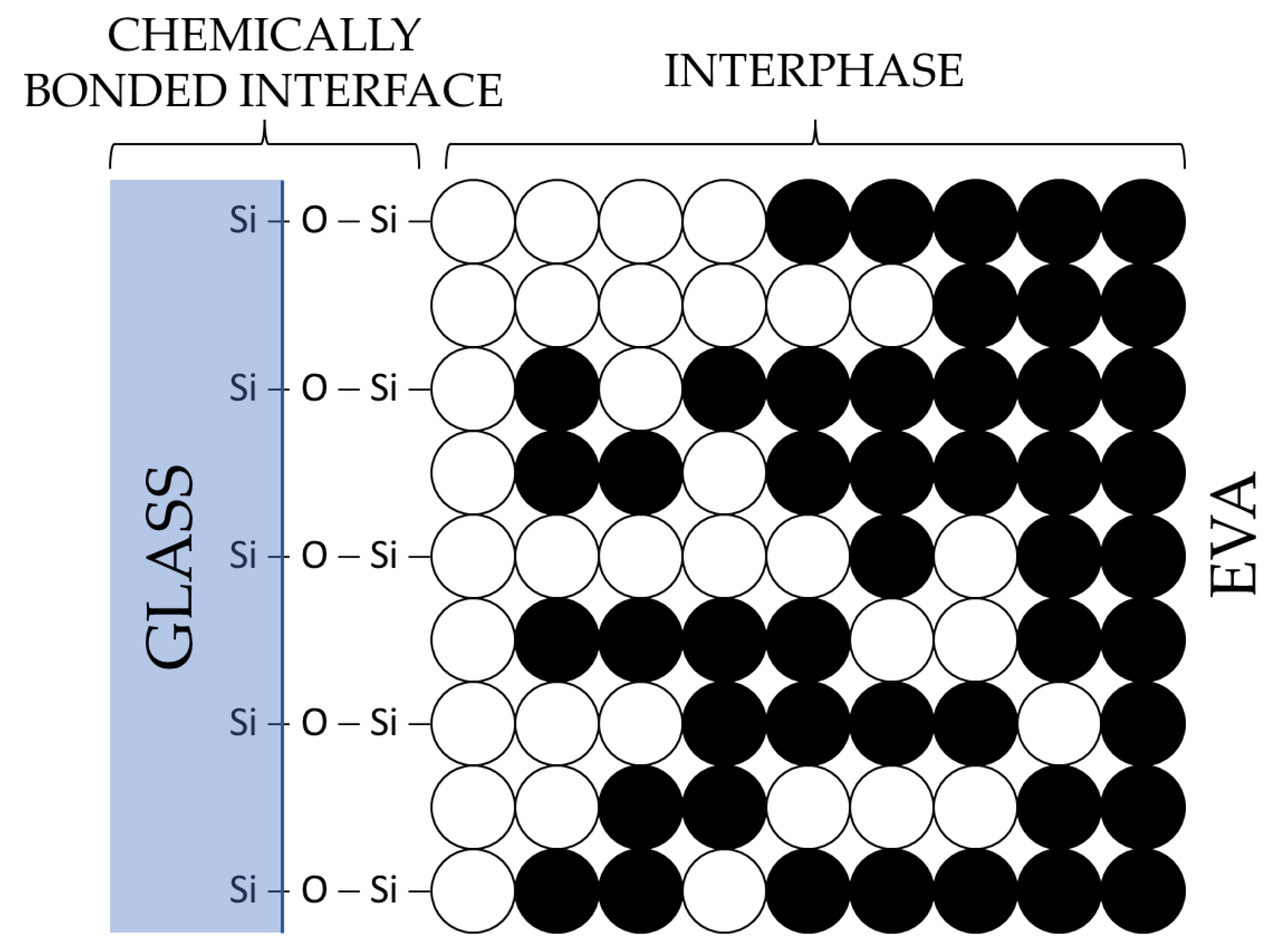
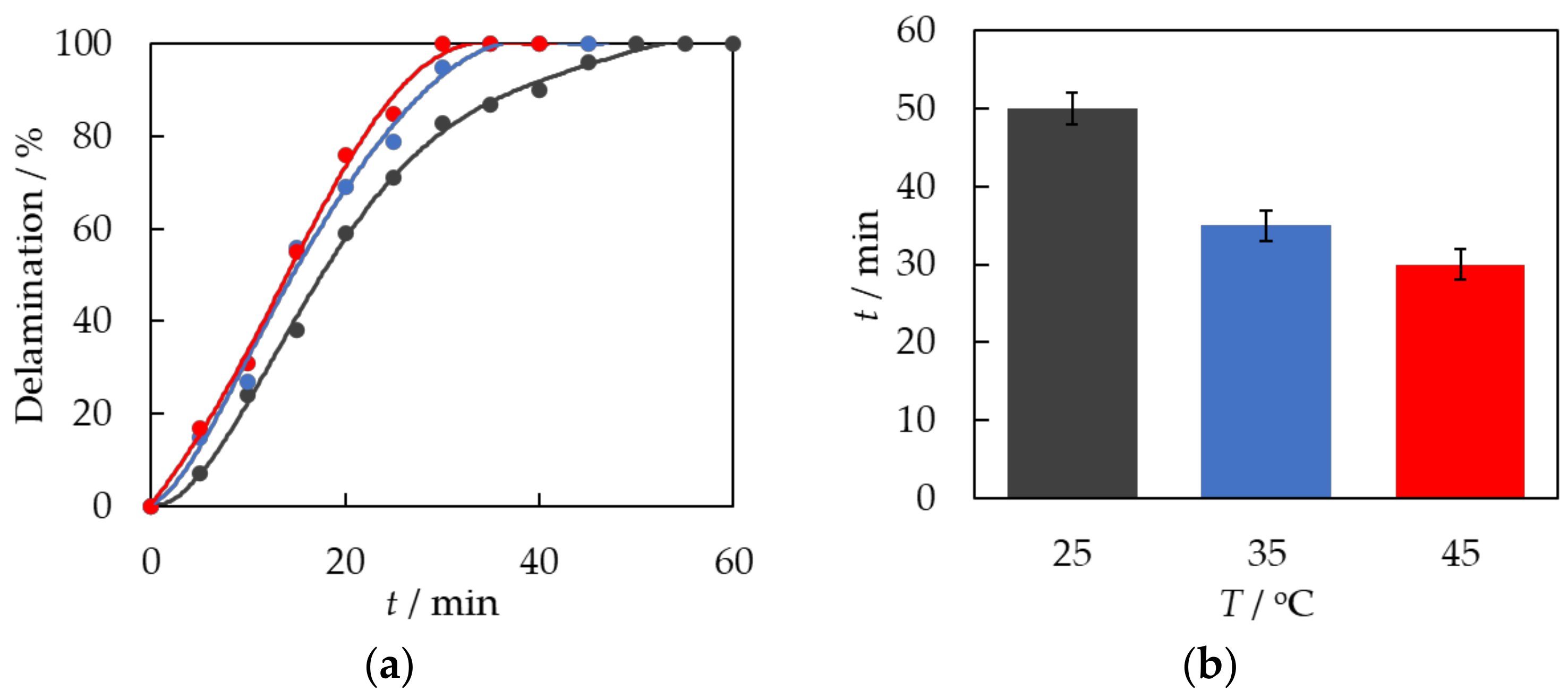
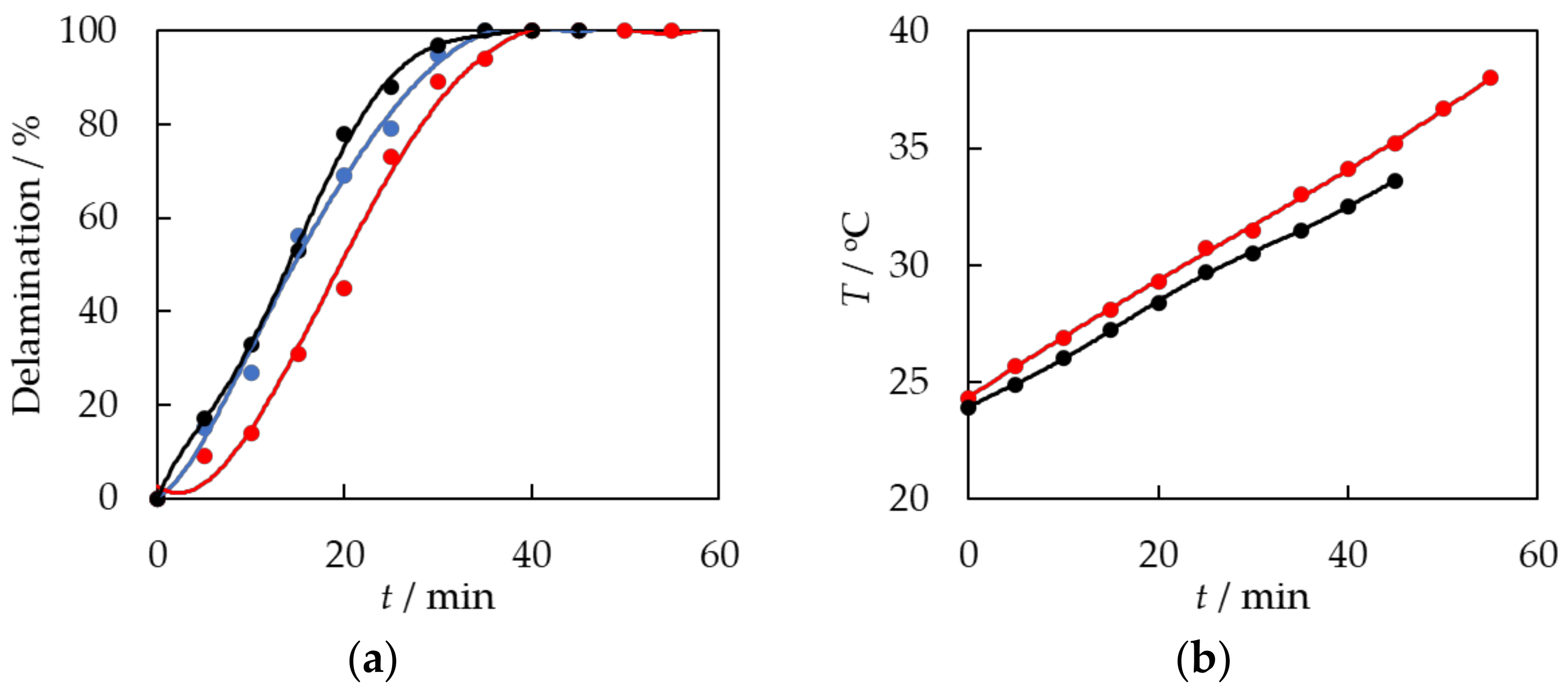
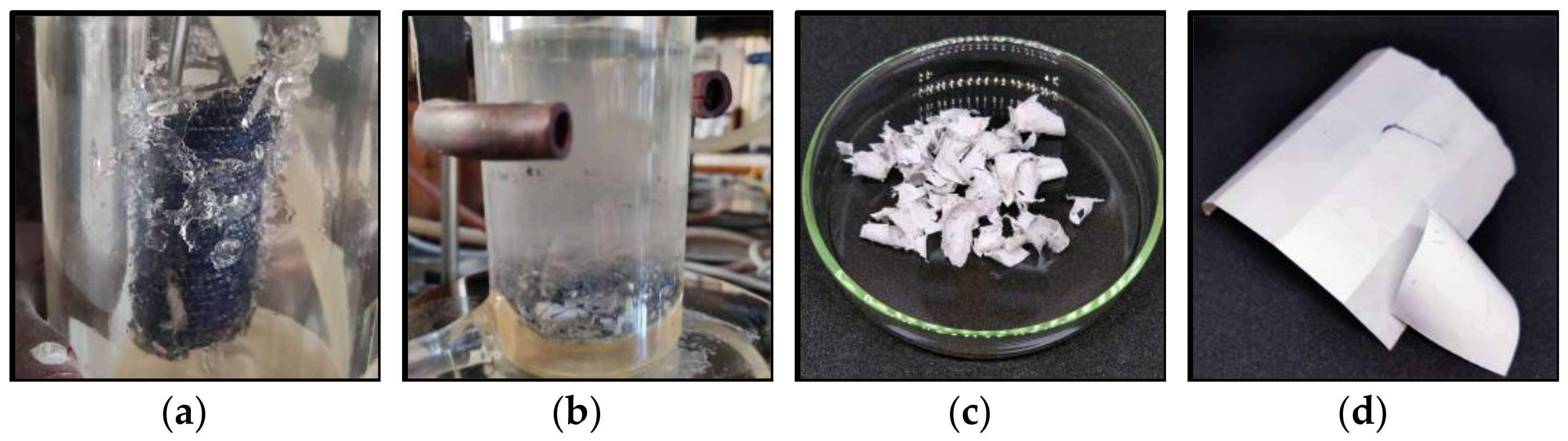
3.2. Chemical Treatment of EVA
3.3. Characteristics of Separated Materials
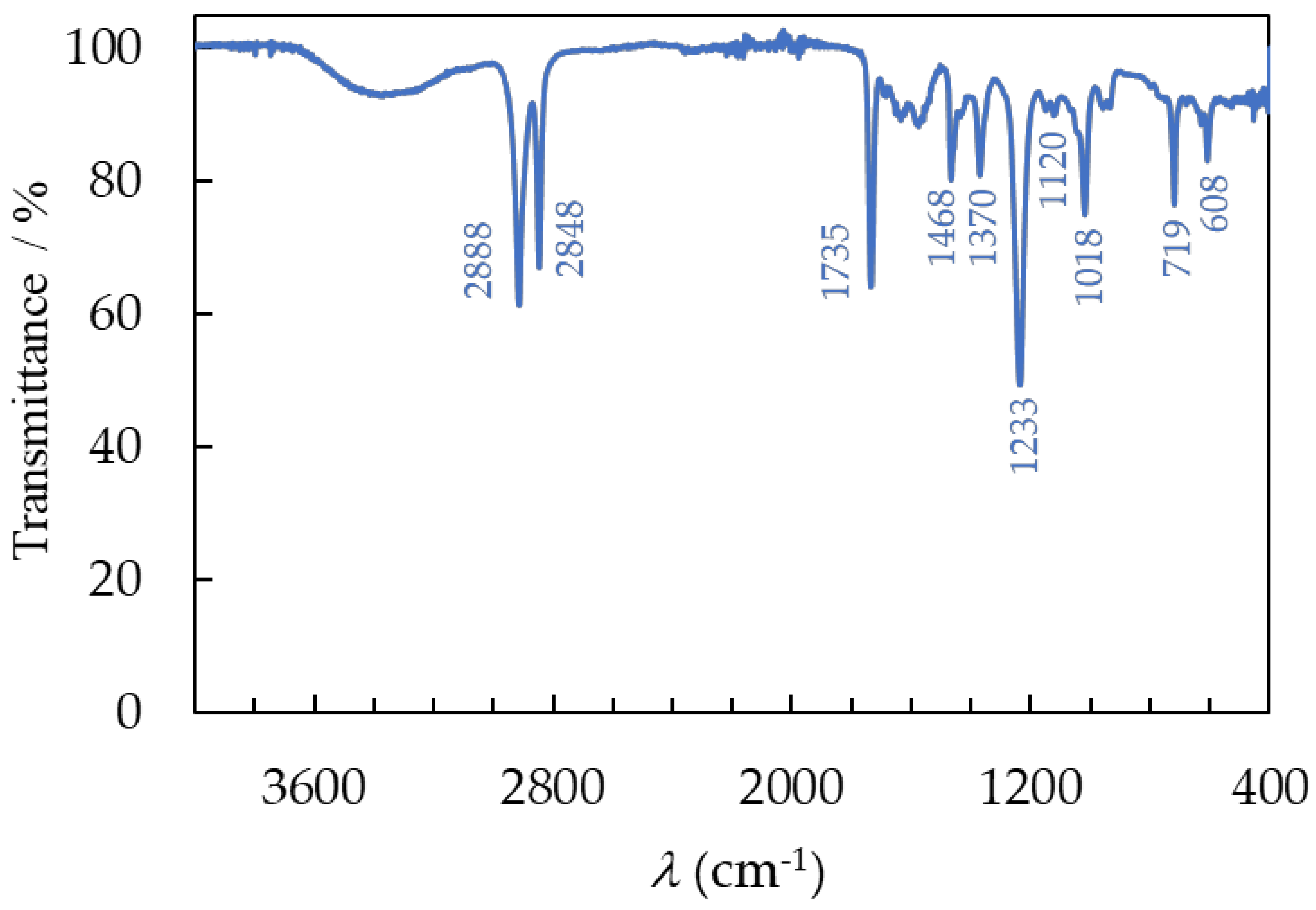
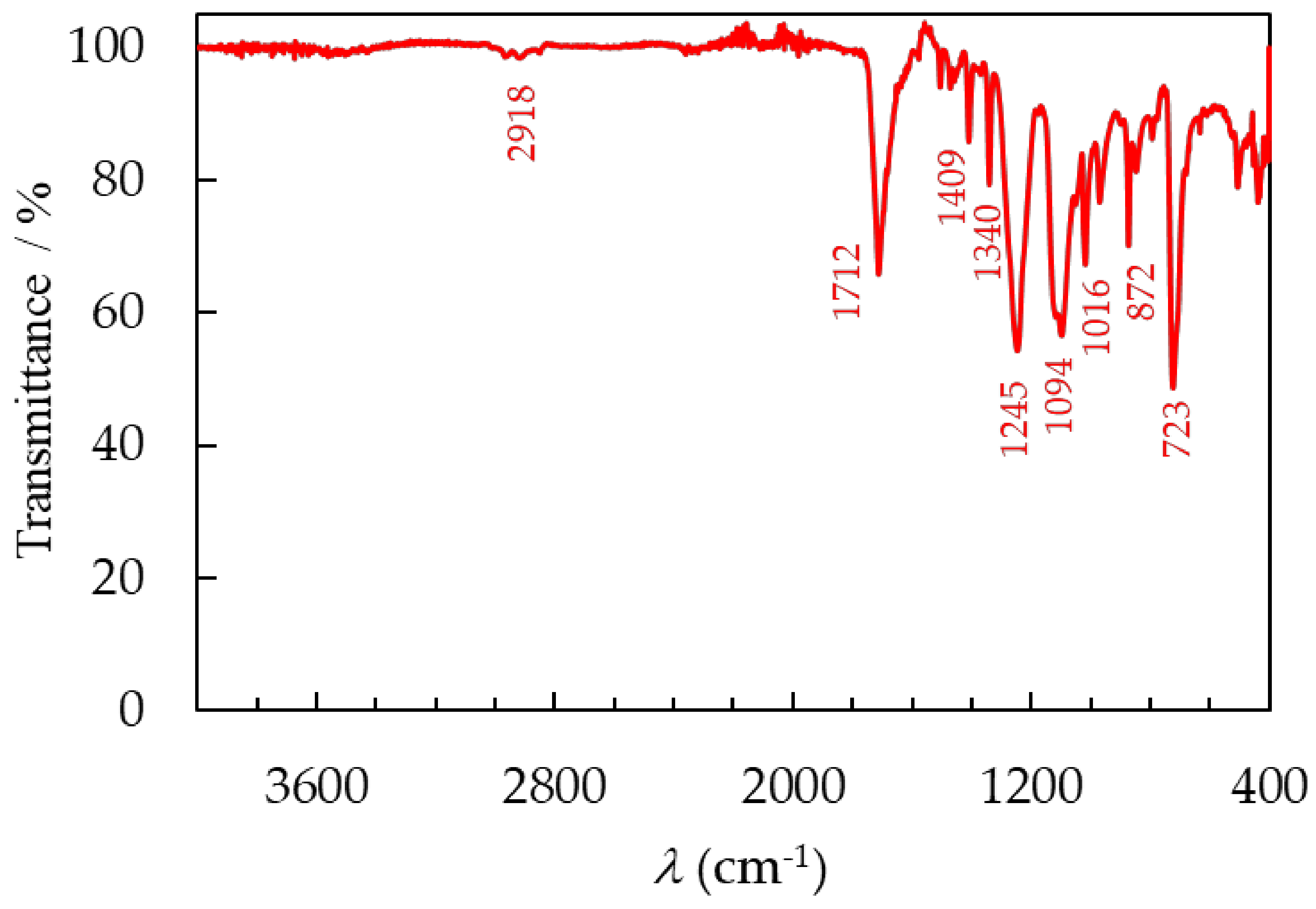
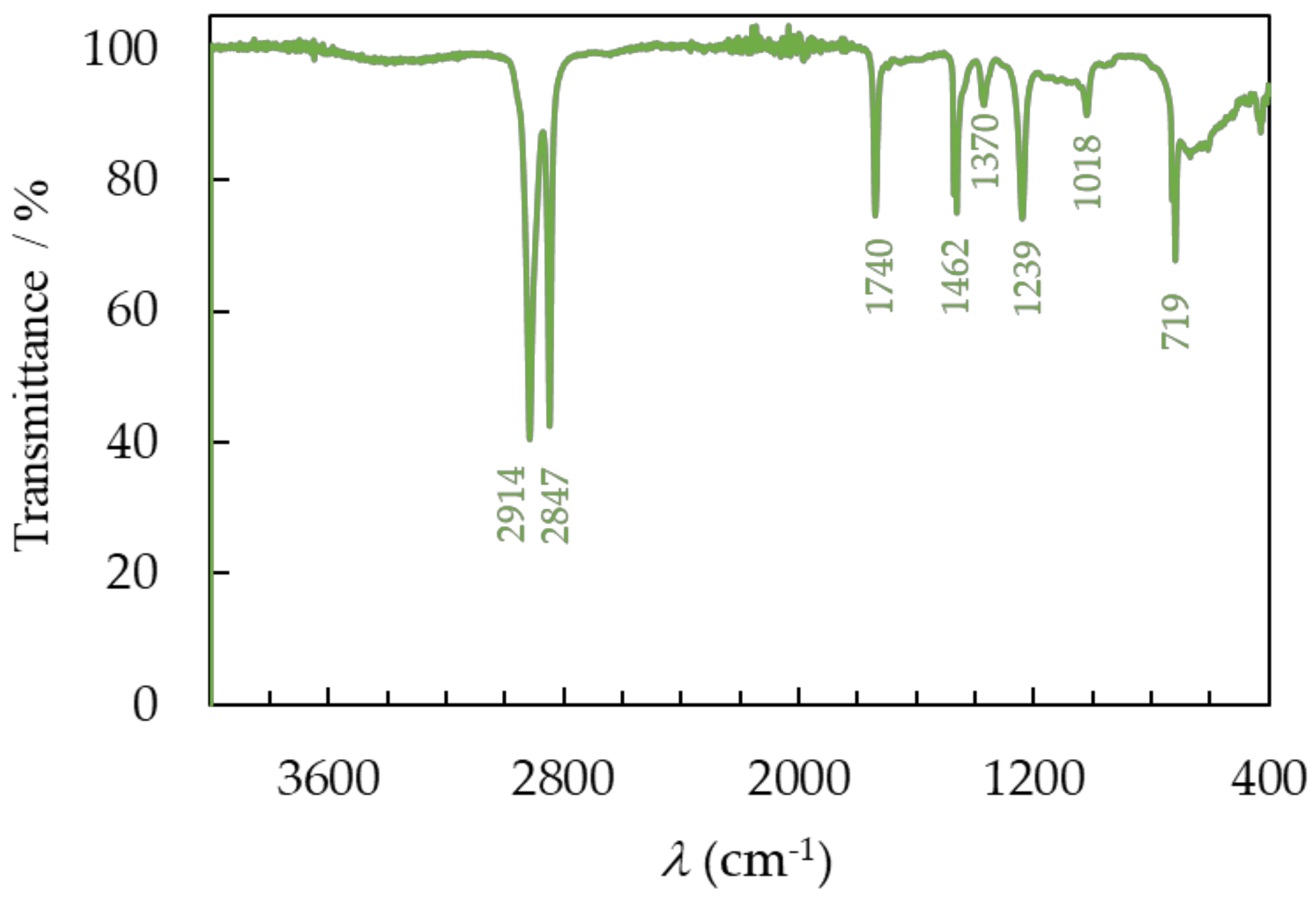
3.3.1. FTIR Spectroscopy
3.3.2. Elemental Analysis
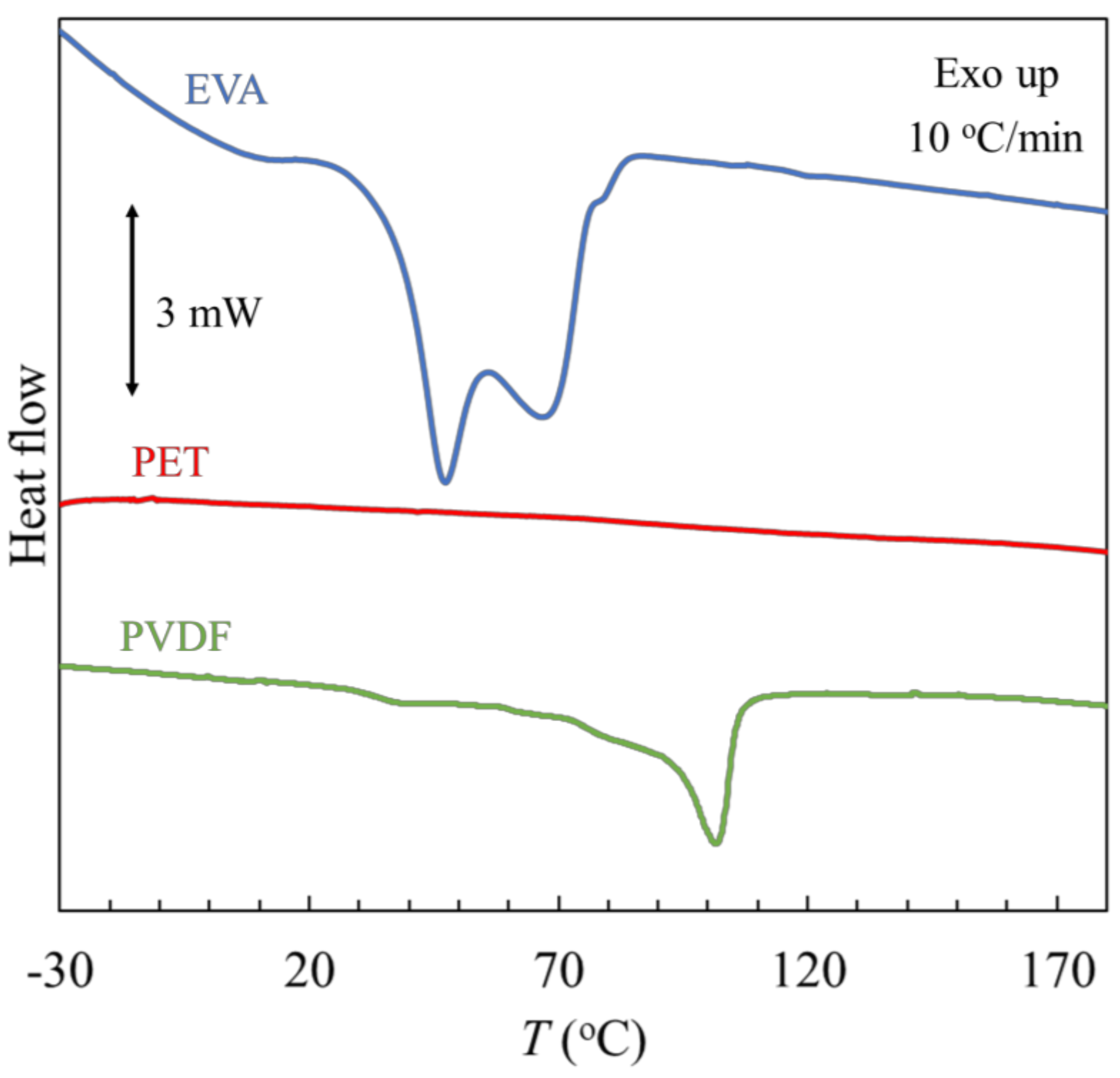
3.3.3. Differential Scanning Calorimetry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Snapshot 2023—IEA-PVPS. Available online: https://iea-pvps.org/snapshot-reports/snapshot-2023/ (accessed on 5 December 2023).
- Xu, Y.; Li, J.; Tan, Q.; Peters, A.L.; Yang, C. Global Status of Recycling Waste Solar Panels: A Review. Waste Manag. 2018, 75, 450–458. [Google Scholar] [CrossRef]
- Heath, G.A.; Silverman, T.J.; Kempe, M.; Deceglie, M.; Ravikumar, D.; Remo, T.; Cui, H.; Sinha, P.; Libby, C.; Shaw, S.; et al. Research and Development Priorities for Silicon Photovoltaic Module Recycling to Support a Circular Economy. Nat. Energy 2020, 5, 502–510. [Google Scholar] [CrossRef]
- Chowdhury, M.S.; Rahman, K.S.; Chowdhury, T.; Nuthammachot, N.; Techato, K.; Akhtaruzzaman, M.; Tiong, S.K.; Sopian, K.; Amin, N. An Overview of Solar Photovoltaic Panels’ End-of-Life Material Recycling. Energy Strategy Rev. 2020, 27, 100431. [Google Scholar] [CrossRef]
- Singh, S.; Powar, S.; Dhar, A. End of Life Management of Crystalline Silicon and Cadmium Telluride Photovoltaic Modules Utilising Life Cycle Assessment. Resour. Conserv. Recycl. 2023, 197, 107097. [Google Scholar] [CrossRef]
- Cerchier, P.; Brunelli, K.; Pezzato, L.; Audoin, C.; Rakotoniaina, J.P.; Sessa, T.; Tammaro, M.; Sabia, G.; Attanasio, A.; Forte, C.; et al. Innovative Recycling of End of Life Silicon PV Panels: ReSiELP. Detritus 2021, 16, 41. [Google Scholar] [CrossRef]
- Gahlot, R.; Mir, S.; Dhawan, N. Recycling of Discarded Photovoltaic Solar Modules for Metal Recovery: A Review and Outlook for the Future. Energy Fuels 2022, 36, 14554–14572. [Google Scholar] [CrossRef]
- Song, B.P.; Zhang, M.Y.; Fan, Y.; Jiang, L.; Kang, J.; Gou, T.T.; Zhang, C.L.; Yang, N.; Zhang, G.J.; Zhou, X. Recycling Experimental Investigation on End of Life Photovoltaic Panels by Application of High Voltage Fragmentation. Waste Manag. 2020, 101, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Song, E.; Zhang, C.; Zhuang, X.; Ma, E.; Bai, J.; Yuan, W.; Wang, J. Pyrolysis-Based Separation Mechanism for Waste Crystalline Silicon Photovoltaic Modules by a Two-Stage Heating Treatment. RSC Adv. 2019, 9, 18115–18123. [Google Scholar] [CrossRef] [PubMed]
- Sasai, M.; Yamashita, T.; Inoue, D. Development of Low-Temperature Thermal Decomposition Recycling Technology from Photovoltaic Modules to Flat Glass Applications. Jpn. J. Appl. Phys. 2023, 62, SK1043. [Google Scholar] [CrossRef]
- Fiandra, V.; Sannino, L.; Andreozzi, C.; Graditi, G. End-of-Life of Silicon PV Panels: A Sustainable Materials Recovery Process. Waste Manag. 2019, 84, 91–101. [Google Scholar] [CrossRef]
- Fiandra, V.; Sannino, L.; Andreozzi, C.; Corcelli, F.; Graditi, G. Silicon Photovoltaic Modules at End-of-Life: Removal of Polymeric Layers and Separation of Materials. Waste Manag. 2019, 87, 97–107. [Google Scholar] [CrossRef]
- Azeumo, M.F.; Conte, G.; Ippolito, N.M.; Medici, F.; Piga, L.; Santilli, S. Photovoltaic Module Recycling, a Physical and a Chemical Recovery Process. Sol. Energy Mater. Sol. Cells 2019, 193, 314–319. [Google Scholar] [CrossRef]
- Subramanian, V.; Tembo, P.; Heninger, M. An Investigation of the Recovery of Silicon Photovoltaic Cells by Application of an Organic Solvent Method. ECS J. Solid State Sci. Technol. 2021, 10, 025001. [Google Scholar] [CrossRef]
- Xu, X.; Lai, D.; Wang, G.; Wang, Y. Nondestructive Silicon Wafer Recovery by a Novel Method of Solvothermal Swelling Coupled with Thermal Decomposition. Chem. Eng. J. 2021, 418, 129457. [Google Scholar] [CrossRef]
- Kang, S.; Yoo, S.; Lee, J.; Boo, B.; Ryu, H. Experimental Investigations for Recycling of Silicon and Glass from Waste Photovoltaic Modules. Renew. Energy 2012, 47, 152–159. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, J. Dissolution of Ethylene Vinyl Acetate in Crystalline Silicon PV Modules Using Ultrasonic Irradiation and Organic Solvent. Sol. Energy Mater. Sol. Cells 2012, 98, 317–322. [Google Scholar] [CrossRef]
- Doi, T.; Tsuda, I.; Unagida, H.; Murata, A.; Sakuta, K.; Kurokawa, K. Experimental Study on PV Module Recycling with Organic Solvent Method. Sol. Energy Mater. Sol. Cells 2001, 67, 397–403. [Google Scholar] [CrossRef]
- Brenes, G.H.; Riech, I.; Giácoman-Vallejos, G.; González-Sánchez, A.; Rejón, V. Chemical Method for Ethyl Vinyl Acetate Removal in Crystalline Silicon Photovoltaic Modules. Sol. Energy 2023, 263, 111778. [Google Scholar] [CrossRef]
- Abdo, D.M.; Mangialardi, T.; Medici, F.; Piga, L. D-Limonene as a Promising Green Solvent for the Detachment of End-of-Life Photovoltaic Solar Panels under Sonication. Processes 2023, 11, 1848. [Google Scholar] [CrossRef]
- Prasad, D.S.; Sanjana, B.; Kiran, D.S.; Srinivasa Kumar, P.P.; Ratheesh, R. Process Optimization Studies of Essential Parameters in the Organic Solvent Method for the Recycling of Waste Crystalline Silicon Photovoltaic Modules. Sol. Energy Mater. Sol. Cells 2022, 245, 111850. [Google Scholar] [CrossRef]
- Dias, P.; Javimczik, S.; Benevit, M.; Veit, H. Recycling WEEE: Polymer Characterization and Pyrolysis Study for Waste of Crystalline Silicon Photovoltaic Modules. Waste Manag. 2017, 60, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.H.; Shin, W.J.; Wang, L.; Sun, W.C.; Tao, M. Strategy and Technology to Recycle Wafer-Silicon Solar Modules. Sol. Energy 2017, 144, 22–31. [Google Scholar] [CrossRef]
- Pagnanelli, F.; Moscardini, E.; Granata, G.; Abo Atia, T.; Altimari, P.; Havlik, T.; Toro, L. Physical and Chemical Treatment of End of Life Panels: An Integrated Automatic Approach Viable for Different Photovoltaic Technologies. Waste Manag. 2017, 59, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Spinella, L.; Bosco, N. FTIR Investigation of EVA Chemical Bonding Environment and Its Impact on Debond Energy. IEEE J. Photovolt. 2019, 9, 790–795. [Google Scholar] [CrossRef]
- Klemchuk, P.; Ezrin, M.; Lavigne, G.; Holley, W.; Galica, J.; Agro, S. Investigation of the Degradation and Stabilization of EVA-Based Encapsulant in Field-Aged Solar Energy Modules. Polym. Degrad. Stab. 1997, 55, 347–365. [Google Scholar] [CrossRef]
- Coulter, D.R.; Cuddihy, E.F.; Plueddeman, E.P. Chemical Bonding Technology for Terrestrial Photovoltaic Modules; NASA: Washington, DC, USA, 1983. [Google Scholar]
- Feng, Y.; He, Y.; Zhang, G.; Wang, S.; Wei, N.; Zhang, T. A Promising Method for the Liberation and Separation of Solar Cells from Damaged Crystalline Silicon Photovoltaic Modules. Sol. Energy Mater. Sol. Cells 2023, 262, 112553. [Google Scholar] [CrossRef]
- Huang, Q.; Yuan, W.; Guo, Y.; Ke, Q. Thermal Separation of Plastic Components from Waste Crystalline Silicon Solar Cells: Thermogravimetric Characteristics and Thermokinetics. J. Air Waste Manag. Assoc. 2023, 73, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Chitra; Sah, D.; Lodhi, K.; Kant, C.; Saini, P.; Kumar, S. Structural Composition and Thermal Stability of Extracted EVA from Silicon Solar Modules Waste. Sol. Energy 2020, 211, 74–81. [Google Scholar] [CrossRef]
- Han, H.; Yan, H.; Wang, X.; Zhang, K.; Huang, J.; Sun, Y.; Liu, J.; Verlinden, P.J.; Altermatt, P.; Liang, Z.; et al. Analysis of the Degradation of Encapsulant Materials Used in Photovoltaic Modules Exposed to Different Climates in China. Sol. Energy 2019, 194, 177–188. [Google Scholar] [CrossRef]
- Tsocheva, D.; Tsanov, T.; Terlemezyan, L. Structure of Composite Films Containing Polyaniline Studied by DSC. J. Therm. Anal. Calorim. 2001, 66, 415–422. [Google Scholar] [CrossRef]
- Agroui, K.; Maallemi, A.; Boumaour, M.; Collins, G.; Salama, M. Thermal Stability of Slow and Fast Cure EVA Encapsulant Material for Photovoltaic Module Manufacturing Process. Sol. Energy Mater. Sol. Cells 2006, 90, 2509–2514. [Google Scholar] [CrossRef]
- Marcilla, A.; Reyes-Labarta, J.A.; Sempere, F.J. DSC Kinetic Study of the Transitions Involved in the Thermal Treatment of Polymers. Methodological Considerations. Polymer 2001, 42, 5343–5350. [Google Scholar] [CrossRef]
- Oreski, G.; Wallner, G.M. Aging Mechanisms of Polymeric Films for PV Encapsulation. Sol. Energy 2005, 79, 612–617. [Google Scholar] [CrossRef]
| Polymer | Element (%) | Observed | Calculated |
|---|---|---|---|
| EVA | C | 71.63 | 70.58 |
| H | 10.76 | 10.59 | |
| PET | C | 63.10 | 62.50 |
| H | 4.33 | 4.17 | |
| PVDF | C | 47.23 | 37.51 |
| H | 5.31 | 3.15 |
| Polymer | Tg (°C) | ΔgCp (J·g−1·K−1) | Tm (°C) | ΔmH (J·g−1) |
|---|---|---|---|---|
| EVA | - | - | (1) 47.4, (2) 66.9 (1) 49, (2) 72 [34] | 55.0 |
| PVDF | 32.3 | 0.57 | 101.4 | 78.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Królikowski, M.; Fotek, M.; Żach, P.; Michałowski, M. Development of a Recycling Process and Characterization of EVA, PVDF, and PET Polymers from End-of-Life PV Modules. Materials 2024, 17, 821. https://doi.org/10.3390/ma17040821
Królikowski M, Fotek M, Żach P, Michałowski M. Development of a Recycling Process and Characterization of EVA, PVDF, and PET Polymers from End-of-Life PV Modules. Materials. 2024; 17(4):821. https://doi.org/10.3390/ma17040821
Chicago/Turabian StyleKrólikowski, Marek, Michał Fotek, Piotr Żach, and Marcin Michałowski. 2024. "Development of a Recycling Process and Characterization of EVA, PVDF, and PET Polymers from End-of-Life PV Modules" Materials 17, no. 4: 821. https://doi.org/10.3390/ma17040821
APA StyleKrólikowski, M., Fotek, M., Żach, P., & Michałowski, M. (2024). Development of a Recycling Process and Characterization of EVA, PVDF, and PET Polymers from End-of-Life PV Modules. Materials, 17(4), 821. https://doi.org/10.3390/ma17040821






