Systematic Review of Zinc’s Benefits and Biological Effects on Oral Health
Abstract
1. Introduction
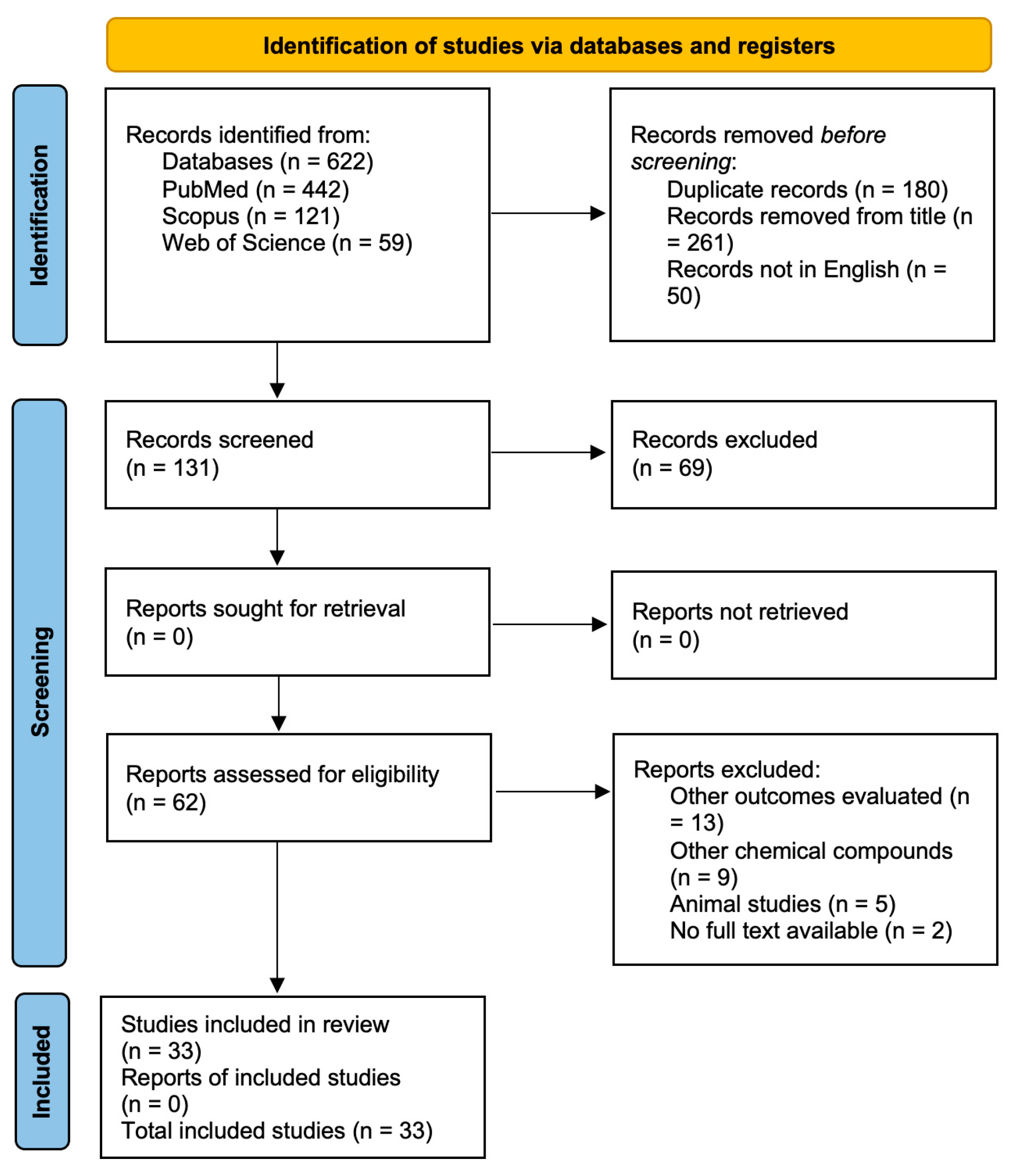
2. Materials and Methods
3. Results
3.1. In Vivo Studies
3.2. In Vitro Studies
| Author(s) and Year of Publication | Population Characteristics | Zinc Application and Chemical Composition | Control Group | Zinc Biological Properties and Effects on Oral Health | Principal Findings | Fundings | Quality Assessment Score |
|---|---|---|---|---|---|---|---|
| Doneria et al. 2017 [27] | N = 64 primary molars of 43 children (aged between 4 and 8 years) | Zinc oxide–ozonated oil (ZnO-OO): zinc oxide powder (DPI) (0.2 g, arsenic free) and ozonated castor oil (0.007 cc Ozonil, Ozone Forum of India) using motor-driven lentulospirals | Vitapex (Neo Dental Co.) and modified 3Mix MP paste | Clinical and radiographic success, effectiveness as pulpectomy agents: follow up at 1, 6, and 12 months (RX at 6 and 12 months). | Clinical success of ZnO-OO and Vitapex were comparable at 6 and 12 months (100%). Radiologically, success rates for ZnO-OO were 100% at 6 and 12 months, with significant differences between three groups after 12 months (p = 0.029 and p = 0.011). | None | 32/36 |
| Hagenfeld et al. 2019 [37] | N = 41 nonsmokers patient (mean age 54.86 ± 10.19 years; 25 females and 16 males) with PPD ≥ 4 mm in a minimum of 4 teeth, aged ≥ 18–75 years, and with at least 10 natural teeth | Zinc-substituted carbonated hydroxyapatite dentifrice: HA group N = 20 (BioRepair, Wolff) | dentifrice containing an amine fluoride/stannous fluoride: AmF/SnF2 group N = 21 (Meridol, CP GABA) | Microbiome variation analysis with paired-end Illumina Miseq 16S rDNA sequencing: plaque samples from buccal/lingual, interproximal, and subgingival sites at baseline, 4 weeks after oral hygiene, and 8 weeks after periodontal therapy. | No significant difference observed in terms of changes on community level (alpha and beta diversity) and on the level of single agglomerated ribosomal sequence variants (aRSV). In interproximal and subgingival sites: Fusobacterium and Prevotella species associated with periodontitis. | Kurt Wolff GmbH | 35/39 |
| Moura et al. 2021 [48] | N = 88 primary molars with pulp necrosis (mean age 5.5 ± 1.2 SD, 35 males and 35 females) | Zinc oxide eugenol paste (ZOE) (N = 44), with the zinc oxide packed into 250 mg capsules and mixed with 0.1 mL eugenol (Biodynamics); chemical–mechanical canal debridement and disinfection with 2% chlorhexidine solution (LT Rioquímica) and K-files (sizes 15 to 25; Dentsply); restoration with high-viscosity glass ionomer cement (Gold Label 9R, GC) | CTZ group (N = 44): 62.5 mg of chloramphenicol, 62.5 mg of tetracycline, and 125 mg of zinc oxide | Effectiveness of lesion sterilization and tissue repair (LSTR), evaluation every 3 months for 12 months, and clinical and radiographic evaluation. | No significant difference observed. The mean time taken to perform was 145.1 (±53.2) minutes for ZOE (p < 0.001). At 12 months, the clinical success rate was 90.9% and the radiographic success rate 72.7% for ZOE. | N/A | 36/36 |
| Prasad et al. 2018 [29] | 173 subjects | New fluoride toothpastes with Dual Zinc plus Arginine formulations: zinc (zinc oxide, zinc citrate) 0.96%, 1.5% Arginine, and 1450 ppm fluoride; zinc (zinc oxide, zinc citrate) 0.96%, 1.5% Arginine, and 1000 ppm fluoride (Dual Zinc plus Arginine; Colgate-Palmolive Company) | Regular fluoride dentifrice containing 1450 ppm fluoride (Colgate dentifrice; Colgate-Palmolive Company) | Effect on reducing bacteria in oral biofilm (CFU): oral samples collected from teeth, tongue, oral buccal mucosa, gingiva, mand saliva at baseline and 12 h after 14- and 29-days of assigned product use. | Subjects using the Dual Zinc plus Arginine Toothpaste with 1450 ppm F exhibited significant reductions in bacteria on buccal (35.4%, p < 0.001), teeth (38.3%, p < 0.001), gingiva (25.9%, p = 0.043), tongue (39.7, p = 0.001), and in saliva (41.1%, p < 0.001) 12 h after 29 days of product use. Toothpastes containing 0.96% zinc (zinc oxide, zinc citrate), 1.5% L-arginine, and either 1450 ppm or 1000 ppm fluoride as sodium fluoride in a silica base provide significant reductions in oral bacteria compared to toothpaste with fluoride alone. | Colgate-Palmolive Company | 37/39 |
| Sreenivasan et al. 2020 [41] | N = 44 adults (19-63 years) with at least 20 natural teeth, in good general health, and with a plaque index scores of 1.5 or more and gingival index scores of 1.0 or more | Herbal toothpaste incorporating zinc (N = 22, mean age 46.2) | Commercially available fluoride toothpaste (Colgate Dental Cream, Great Regular Flavor) (N = 22) | Microbiologic analysis (CFU) on anaerobic organisms, Gram-negative bacteria and malodor bacteria of dental plaque, tongue scrapings, and cheek surfaces. | Significant reductions in functional bacterial groups from distinct oral niches compared to control group (p < 0.05). Reductions between 42 and 68% for anaerobic bacteria 12 h after brushing, increasing to 46–80% 4 h after brushing; and between 49 and 61% for Gram-negative bacteria, that increased to 54–69% 4 h post-brushing. | Colgate-Palmolive Company | 34/39 |
| Author(s) and Year of Publication | Fundings | Cell Types or Microbial Strains | Zinc Application and Chemical Composition: Specimen Characteristics | Control Group | Zinc Biological Properties and Effects on Oral Health | Principal Findings | Quality Assessment Score |
|---|---|---|---|---|---|---|---|
| Conservative and Cariology | |||||||
| Barma et al. 2021 [49] | None | Human liver cancer (Hep G2) and human embryonic kidney 293 (HEK-293T) cell lines | Zinc oxide nanoparticles synthetized (ZnO-NP) varnish | None | Inhibition of S. mutans growth, biofilm, acid production, and antioxidant potential with 2,2-diphenyl-2-picrylhydrazyl hydrate (DDPH) assay, and cytotoxicity; ZnO-NP characterized using UV spectroscopy, x-ray diffraction spectroscopy, and transmission electron microscopy; secondary metabolites assessed using fourier transform infrared spectroscopy. | Excellent antimicrobial properties against S. mutans; minimum inhibitory and bactericidal concentrations were 0.53 μg/mL and 1.3 μg/mL, respectively. A total of 0.1 mg/μL had the greatest zone of inhibition (24 mm). A total of 0.1 mg/μL inhibited 90% of S. mutans biofilms and exhibited antioxidant capacity in a dose-dependent manner (94% inhibition, 100 μg/mL). A total of 0.1 mg/μL ZnO-NP caused very low cytotoxicity to Hep G2 cells and was non-cytotoxic to HEK-293T cells. | 35/36 |
| Eskandarizadeh et al. 2019 [38] | None | S. mutans strain | ZDMA powder: 100 mL of Hexan, 0.03 mL of Triton100,and 8.4 gr of ZnO; 5 test groups with dental resin adhesive containing zinc dimethacrylate ionomer (ZDMA) in different concentrations into resin bonding: 0.5, 1, 2.5, and 5 wt.% | Pure resin adhesive (Tetric N-Bond, voclar Vivodent) | Antibacterial test (CFU): bacterial strains and growth conditions (S. mutans PTCC 1683, Persian Type Culture Collection, IROST) for direct contact and material aging; physical test: degree of conversion (DC) and Zinc ion release amount in aqueous medium; mechanical test: compressive strength and shear bond test (enamel and dentine separately). | Significantly reduced amount of S. Mutans (p < 0.05); DC was enhanced; ion release analysis revealed stability of Zn2+ (as in the 5 wt.% group); even after 9 cycles of a 24 h wash. Compressive strength was significantly reduced (p < 0.05) just in the 5% ZDMA group, while the other groups were superior in comparison to the control. For the dentine shear bond strength, only the 5% ZDMA group was significantly higher than the control (p = 0.000). | 33/36 |
| Garcia et al. 2021 [50] | None | None | ZnO incorporated at 2.5 (G2.5%), 5 (G5%), and 7.5 (G5%) wt.% in an experimental dental adhesive | ZnO incorporated at 0 (GCTRL) in dental adhesive | Chemical and mechanical properties: degree of conversion (DC), flexural strength (FS), and elastic modulus (E). Antibacterial response in 48 h microcosm biofilm model after the formation of acquired pellicle on samples’ surfaces: colony-forming units (CFU), metabolic activity, and live/dead staining. | DC ranged from 62.21 (±1.05)% for GCtrl to 46.15 (±1.23)% for G7.5%; G7.5% showed lower FS; G2.5% showed higher E; G7.5% had lower CFU/mL compared to GCtrl for S. mutans and total microorganisms, despite presenting lower metabolic activity and higher dead bacteria (p < 0.05). | 34/36 |
| Huang et al. 2020 [42] | National Natural Science Foundation of China (21371139) and the Graduate Innovation Foundation of Wuhan Institute of Technology (CX2017125) | S. mutans (Ingbritt) | Ag/ZnO nanocomposite | None | Effects at sub-minimum inhibitory concentrations (sub-MICs) on virulence factors of S. mutans and related genes expressions by growth curves and MTT staining method; biofilm formation with crystal violet staining method and scanning electron microscopy; adherence, cell-surface hydrophobicity, acidogenicity, and extracellular polysaccharides (EPS) of S. mutans. Virulence factors related genes expressions by qRT-PCR. | Decrease of 69.00% biofilm formation, 31.78% sucrose-independent and 48.08% sucrose-dependent adherence, 69.44% cell-surface hydrophobicity, and 72.45% water-soluble and 90.60% water-insoluble EPS with Ag/ZnO at 1/2 MIC. Expression of virulence factors-related genes were significantly suppressed. | 36/39 |
| Lavaee et al. 2018 [30] | Vice-Chancellory of Shiraz University of Medical Science (Grant No. 8794121) | Standard strain of S. mutans (ATCC 35668, PTCC 1683) | Different concentrations of zinc sulfate (Merck) and zinc acetate (Falcon) solutions were prepared in concentrations of 6.25, 12.5, 25, and 50 μg/mL | Penicillin and chlorhexidine | Inhibitory and bactericidal effects on S. mutans: diameters of zone of inhibition detected by the disc diffusion method; minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC). | Zinc sulfate and zinc acetate salts with 37.19 and 31.25 µgr/mL concentration had an inhibitory effect on S. mutans, respectively. MIC and MBC of zinc sulfate solution were higher than penicillin and chlorhexidine (p < 0.001), but no priority in antibacterial activity of the studied zinc salts was determined. | 37/39 |
| Manus et al. 2018 [31] | N/A | In vitro oral epithelial tissue and saliva-derived biofilm models | Zinc citrate dentifrice formulations prepared with increasing replacement of zinc citrate with zinc oxide (a water insoluble source of zinc ions) to generate a dual-zinc active system | None | Bioavailability enhancement of zinc, zinc delivery, and antibacterial efficacy; zinc penetration and retention with imaging mass spectrometry (I-MS) | Enhanced antibacterial performance observed through significant reductions in metabolic activity as measured through bacterial glycolytic function (p = 0.0001) and total oxygen consumption (p = 0.0001). | 35/36 |
| Mirhosseini et al. 2019 [39] | None | Dtrains of S. mutans (ATCC 35668), E. faecalis (ATCC 29212), L. fermentum (ATCC 14931), and C. albicans (ATCC 10231) | Solutions at the concentration of 10 μg/mL were prepared using 20-nm, 40-nm, and 140-nm nano ZnO (nZnO) powder (Research Nanomaterials Inc.) | None | Antimicrobial effect of various sizes and concentrations of zinc oxide (ZnO) nanoparticles on S. mutans, E. faecalis, L. fermentum, and C. albicans with a spectrophotometer (UV-150-02; Shimadzu C0) for microbial growth, disk diffusion method, and minimum inhibitory concentrations (MICs), and minimum bactericidal concentrations (MBCs) using broth microdilution method. | The antimicrobial activity of nZnO increases with the decreasing particle size against S. mutans (p = 0.00), L. fermentum, and E. faecalis (p < 0.02). The greatest antimicrobial effect was observed against S. mutans and E. faecalis. | 33/36 |
| Peralta et al. 2018 [32] | CAPES (Coordination for the Improvement of Higher Education Personnel) and CNPq (National Council for Scientific and Technological Development (Grant No. 313294/2014-3) | S. mutans UA159, Enterococcus faecalis ATTC4083; fibroblast (NCTC clone 929) cells | Elastomeric temporary resin-based filling materials (TFMs) containing zinc methacrylate (ZM, Aldrich Chemical Co.), with concentration of 0.5% (Z0.5); 1% (Z1), 2% (Z2), or 5% (ZM5) wt% was added to the TFMs; N = 10 disk-shaped (6.0 mm in diameter and 1 mm thick) specimens per group for water sorption and solubility; N = 10 dumbbell-shaped specimens (10 × 5 × 1 mm) for UTS; N = 4 specimens 15 × 6 mm for hardness; N = 8 specimens 6 × 1 mm for biofilm accumulation test | ZM concentration of 0% control and the Fermit-N (Ivoclar Vivadent) (F) used as a commercial reference | Physical and mechanical properties, antibacterial effect, and biocompatibility: microleakage, water sorption/solubility, degree of conversion with Fourier transform infrared spectroscopy, depth of cure with scraping method, ultimate tensile strength (UTS), and the Shore D scale hardness tester were determined and compared. A modified direct contact test (DCT) with E. faecalis and a S. mutans’ biofilm accumulation assay were carried out to evaluate the antimicrobial effect and cytotoxicity (MTT). | Physical, mechanical, and biological properties are comparable with the properties of the commercial reference. Some properties were improved: lower microleakage and water sorption, and higher ultimate tensile strength values (p < 0.001). After the 24 h in direct contact test, all TFMs with ZM were similar (p = 0.058), also considering DC (p < 0.034). | 38/39 |
| Steiger et al. 2020 [43] | None | S. mutans (ATCC 25175) and S. mutans clinical isolate | Divalent cation Zn2+ and in combination ZnCl 1, 3, 10, 30, 100, and 200 mM for antibacterial properties, 1 mM ZnCl2 for biofilm analysis, 1, 10, and 100 mM for HA dissolution; hydroxyapatite (HA) disks (5 mm, HiMed Inc.) on biofilm formation | No divalent ions | Biofilm formations and growth using confocal laser scanning microscopy (CLSM) with Leica SP8 microscope (Leica SP8), cariogenic dissolution of hydroxyapatite, EPS extraction of S. mutans cultures with the phenol–sulphuric acid assay, analysis of the binding of ZnCl2 to EPS via isothermal titration calorimetry (ITC). | No significant effects observed. Zinc inhibited bacterial adhesion was also at low concentrations and had a strong antibacterial effect on the strains as well as on calcium dissolution with less biofilm and less EPS. Zn2+ had the lowest affinity to all EPS; the unbound zinc could also still remain in the environment and keep its antimicrobial properties. | 34/36 |
| Suzuki et al. 2018 [33] | Supported in part by Grants in Aid of Scientific Research (Nos. 26463175, 15K14423, and 16K07205) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, from the Sato Fund (2015–2016) of the Nihon University School of Dentistry | P. gingivalis FDC 381, P. gingivalis W83, P. gingivalis ATCC 33277, F. nucleatum ATCC 25586, P. intermedia ATCC 25611, S. mutans JCM 5705, S. sobrinus JCM 5176, S. salivarius GTC 0215, and S. anginosus FW73 | metal chlorides, ZnCl2, and metal acetates, (CH3COO)2Zn | Other metal ions | Binding of zinc ions to gaseous hydrogen sulfide (H2S); minimum concentration needed to inhibit H2S volatilization determined with serial dilution methods; six oral bacterial strains related to volatile sulfur compound (VSC) production and three strains not related to VSC were evaluated; inhibitory effects on growth of oral bacteria. | Zinc ions’ effect on the growth of oral bacteria was strain-dependent. F. nucleatum was the most sensitive and suppressed by media containing 0.001% zinc ions. There was an inhibitory effect on oral malodor with direct binding with gaseous H2S and suppressing the growth of VSC-producing oral bacteria. | 35/39 |
| Tabatabaei et al. 2019 [40] | N/A | Human gingival fibroblasts (HGFs) | 16 toothpastes and 4 mouthwashes available in Iranian market containing sodium fluoride (NaF), sodium lauryl sulfate, cocamidopropyl betaine, zinc lactate, paraben, and sodium benzoate | None | MTT assay was used to assess cytotoxicity. | Fifference in cytotoxicity was statistically significant (p < 0.001). Cytotoxicity was time- and concentration-dependent. Cytotoxicity of all concentrations of zinc lactate was <50%. | 38/39 |
| Toledano-Osorio et al. 2018 [13] | Supported by the Ministry of Economy and Competitiveness (MINECO) and the European Regional Development Fund (FEDER) (Project MAT2017-85999-P) | N = 30 and N = 15 sound single-rooted teeth obtained with informed consent from donors (18 to 25 yr of age); each root was removed 5 mm below the cement-enamel junction using a low-speed diamond saw (Accutom-50 Struers); N = 15 sound single-rooted teeth; from each tooth, two dentin blocks were obtained from the buccal surface of the root, just below the cementodentinal junction; the tooth was cut perpendicular to the axial axis using a diamond saw (Accutom-50 Struers) | Zinc-doped NPs (Zn-NPs), PolymP-n active nanoparticles (NanoMyP): aqueous solutions of ZnCl2 (containing zinc at 40 ppm at pH 6.5) | None | Dentin hypersensitivity: field emission scanning electron microscopy (FESEM Gemini, Carl Zeiss), energy dispersive analysis using an X-ray detector system (EDX Inca 300, Oxford Instruments), atomic force microscope (AFM Nanoscope V, Digital Instruments, Veeco Metrology group) and Nano-DMA analysis; complex, storage, loss modulus, and tan delta (δ). | Treating dentin with Zn-nanoparticles, complex modulus values attained at intertubular and peritubular dentin were higher. | 36/36 |
| Xu et al. 2020 [44] | Financial support from the National Natural Science Foundation of China (Grant Nos. 51973133, 51925304, 51773128, and 21534008) | Human oral keratinocyte (HOK) cells | Zinc-substituted hydroxyapatite/alendronate-grafted polyacrylic acid hybrid nanoneedles (ZHA@ALN-PAA) 286 mg of Zn(CH3COO)2%2H2O | None | Cytotoxicity ws assessed via Cell Counting Kit-8 (CCK-8 assay). | Cell viability higher than 80%. The acceptable cytocompatibility of the nanomaterials guarantees their further applications. | 36/39 |
| Endodontics | |||||||
| Fan et al. 2018 [34] | Financially supported by the National Natural Science Foundation of China (Grant Nos. 81570969 and 81470732) | E. faecalis (ATCC 29212, ATCC), human extracted wisdom teeth | A serial of Ag+-Zn2+ atomic combination ratios (1:1, 1:3, 1:6, 1:9, or 1:12) tested on both planktonic and biofilm-resident E. faecalis: 1 mL suspension (1 × 103 CFUs/mL) for CFU for 24 h, 4 mL suspension (1 × 103 CFUs/mL) for dynamic antimicrobial effect at 1, 2, 3, 4, and 5 h (2, 4, 6, 8, and 10 h for the Zn2+ only group); dentin slices (4 × 4 × 1 mm) prepared from human teeth for biofilm formation | CHX | Co-work pattern and optimum ratio between Ag+ and Zn2+ synergy, antibacterial activity against E. faecalis (CFU), dynamic growth curve method using spectrometer, serial microdilution assay for MIC and MBC, inhibition of biofilm on dentin, using a Zn2+ pretreatment study, membrane potential-permeability measurement (BacLight bacterial membrane potential kit (Molecular Probes, Invitrogen)) and flow cytometry; cytotoxicity of various Ag+-Zn2+ atomic ratios with cell counting kit-8 (CCK-8) (Dojindo Laboratories). | Ag+-Zn2+ (1:9 and 1:12) had the most powerful ability against planktonic and biofilm-resident E. faecalis (p < 0.05). This co-work could be attributed to the depolarization of E. faecalis cell membrane via the addition of Zn2+. | 34/39 |
| Garcia et al. 2018 [12] | Coordination for the Improvement of Higher Education Personnel (CAPES) for the scholarship of GARCIA IM (n° 1678704) | Pulp fibroblasts and S. mutans (NCTC 10449) | Zinc oxide quantum dots (ZnOQDs) into adhesive resin: ZnOQDs synthesized by sol–gel process and incorporated into HEMA; wxperimental adhesive resin formulated mixing 66.6 wt.% BisGMA and 33.3 wt.% HEMA | Photoinitiator system as control group | Antibacterial activity against S. mutans with direct contact inhibition evaluation and cytotoxicity with SRB assay. | Antibacterial activity assay indicated a significant difference (p = 0.003), with a reduction of more than 50% of biofilm formation on ZnOQDs group. | 35/39 |
| Garcia et al. 2020 [45] | Financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001, and Conselho Nacional de Desenvolvimento Científico e Tecnológico—Brasil (CNPq)—n° 307095/2016-9. | Human pulp cells collected from a third molar extracted; S. mutans strain (NCTC 10449) | Zinc-based particle with ionic liquid as filler for an experimental adhesive resin: zinc chloride (ZnCl2) used to synthesize 1-n-butyl-3-methylimidazolium trichlorozincate (BMI.ZnCl3), hydrolyzed under basic conditions to produce simonkolleite (SKT) particles, incorporated at 1, 2.5, or 5 wt.% in adhesive, containing bisphenol A glycerolate dimethacrylate (Bis-GMA) mixed with 2-hydroxyethyl methacrylate (HEMA) at a proportion of 66:33 wt.%; disc-shaped samples (1 × 4 mm); N = 3 for antibacterial activity; N = 5 for cytotoxicity; N = 5 for DC; N = 5 for hardness; hourglass-shaped samples (8 × 2 × 1 mm) N = 10 for UTS | Group without SKT as control | Scanning electron microscopy and transmission electron microscopy for SKT analysis.; antibacterial activity against S. mutans (CFU), cytotoxicity (SRB assay), degree of conversion (DC) using FTIR-ATR, ultimate tensile strength (UTS), Knoop hardness, and softening in solvent. | SKT addition provided antibacterial activity against biofilm formation and planktonic bacteria (p < 0.05). No changes in pulp cells’ viability. DC extended to 64.44 (± 1.55)% for 2.5 wt.%, but was not significant, like UTS, and softened in solvent. Physicochemical properties of adhesives were not affected by SKT incorporation. | 36/39 |
| Pilownic et al. 2017 [28] | N/A | Dental pulp cells | Zinc oxide eugenol (ZOE), Vitapex, and Calen paste thickened with zinc oxide (ZO); N = 5 for every analysis: 1, 4, and 12 h and 1, 3, 7, 15, and 30 days for ph; 1, 4, 12, and 24 h for direct contact test; 1, 3, and 7 days for MTT assay | Experimental MTA-based material | pH, radiopacity, and antimicrobial effect (direct contact test) against E. faecalis, cytotoxicity (MTT assay), and biocompatibility test | No significant effects observed. Vitapex presented the highest cell viability. | 32/36 |
| Periodontology and Implantology | |||||||
| Chen et al. 2021 [51] | N/A | rBMSCs; bone marrow mesenchymal stem cells; S. aureus and P. gingivalis | Co-incorporated zinc- (Zn-) and strontium- (Sr-) nanorod coating on sandblasted and acid-etched (SLA) titanium (SLA-Zn/Sr) fabricated by hydrothermal synthesis | SLAactive titanium | Osteogenesis and inhibition of biofilm formation | Sufficient interface bonding strength (42.00 ± 3.00 MPa). SLA-Zn/Sr enhanced the corrosion resistance property of Ti. SLA-Zn/Sr promoted cellular initial adhesion, proliferation, and osteogenic differentiation while inhibiting the adhesion of S. aureus and P. gingivalis and down-regulating icaA gene expression, reducing polysaccharide secretion and suppressing S. aureus biofilm formation. | 32/36 |
| Fröber et al. 2020 [46] | N/A | Fusobacterium nucleatum, Porphyromonas gingivalis, Prevotella intermedia, Aggregatibacter actinomycetemcomitans, Enterococcus faecalis, Staphylococcus aureus, Lactobacillus paracasei, and Candida albicans | Glucose-1-phosphate (Glc-1P) biofunctionalized zinc peroxide (ZnO2) nanoparticles of four different synthesis ratio (1–10:1) and sizes (4–5 nm) | Nanoparticles stabilized with o-phosphorylethanolamine, bis [2-(methacryloyloxy)ethyl] phosphate or dioctyl sulfosuccinate used as controls | Antimicrobial properties: minimal inhibitory (MIC) and minimal microbicidal concentration (MBC or MFC) determined under different pH conditions; transmission electron (TEM) and fluorescence microscopy after live-dead-staining performed on selected combinations of pathogens and nanoparticles to visualize interactions. | Inhibitory effect on Gram-negative anaerobes and on A. actinomycetemcomitans with a pH-dependent MIC ≥ 25 μg/mL and MBC ≥ 50 μg/mL. In TEM images, the attachment of nanoparticle chains to the bacterial outer membrane and the subsequent penetration were found together with an intracellular oxygen release. | 33/36 |
| Lin et al. 2021 [52] | N/A | MG-63 cells; human osteosarcoma cell line; Escherichia coli | Inner layer of nanoporous TiO2 and the outer layer of the chitosan matrix with ZnO nanoparticles | Chitosan coating alone or pure Ti | Dental implant-related infections: antibacterial activity against E. coli; the effects of the amount of ZnO coating on wettability, anti-scratch ability, bioactivity, and corrosion resistance. | Improvement in antibacterial properties and bioactivity of the chitosan/ZnO coating attributed to Zn2+ ions release. The critical force of scratching was approximately twice that of the chitosan coating. The potentiodynamic polarization confirmed that the corrosion resistance was improved. Good cytocompatibility in MG-63 cells as compared to pure Ti. | 32/36 |
| Sánchez et al. 2018 [35] | N/A | Static subgingival biofilm model with Streptococcus oralis, Actinomyces naeslundii, Veillonella parvula, Fusobacterium nucleatum, Porphyromonas gingivalis, and Aggregatibacter actinomycetemcomitans | Polymeric PolymP-n Active nanoparticles; hydroxyapatite discs coated with nanoparticles (NPs) doped with zinc (12, 24, 48, and 72 h) | PBS as control; NPs alone and doped with calcium, silver, and doxycycline | Nano-roughness of the different disc surfaces (SRa, in nm); morphological characteristic of the biofilms (thickness (μm) and bacteria viability) studied with different microscopy modalities; q-PCR to assess the effect of the nanoparticles on the bacterial load CFUmL−1. | Surfaces containing nanoparticles showed significant increments in roughness compared to controls (p < 0.05). Reductions in bacterial viability was more pronounced with silver and doxycycline NPs. | 34/36 |
| Vergara-Llanos et al. 2021 [53] | Viscerrectoría de Investigación y Desarrollo, Universidad de Concepción, Chile, grant nº 216.102.024-1.0, andViscerrectoría de Investigación, Desarrollo y Creación Artística, Universidad Austral de Chile | Human gingival fibroblasts(HGFs) and mono and multispecies bacterial models | Zinc oxide nanoparticles (ZnO-NPs) | Copper nanoparticles (Cu-NPs) | MIC and spectral confocal laser scanning microscopy; cytotoxic effects by MTT, LDH assays, production of ROS, and the activation of caspase-3 | After 24 h, ZnO-NPs are biocompatible between 78 and 100 μg/mL. Antibacterial activity in a monospecies model is strain-dependent, and in a multispecies model was in lower doses after 10 min of exposure. With induced mitochondrial dose-dependent cytotoxicity, ZnO-NPs increase LDH release and intracellular ROS generation. In a multispecies model, a significant decrease in the total biomass volume (μ3) and bactericidal activity was observed with 125 μg/mL (p < 0.05). | 38/39 |
| Orthodontics | |||||||
| Kachoei et al. 2021 [54] | Iran National Science Foundation (INSF) (Grant No. 92033574) | HGF (human gingival fibroblast) cells. N = 120 extracted human maxillary first premolars for for four groups of bracket bonding evaluation: composite without nanoparticles (O); with ZnO (Z) nanoparticles; with ZnO nanoparticles and silver ions (A&Z); and with Ag/ZnO nanoparticles (AZ) synthesized using optical precipitation | New bioactive orthodontic composite resin containing silver/zinc oxide (Ag/ZnO) nanoparticles; disc-shaped specimens (5 mm × 1 mm, N = 5) from composite for ion release e valuation | None | Wettability, shear bond strength (SBS) test, Zn and Ag release (inductively coupled plasma optical emission spectrometry (ICP-OEP, 700, Agilent Technologies)), cytotoxicity (MTT assay), biocompatibility, and antimicrobial properties with microbroth dilution (MIC) method against S. mutans, Lactobacillus, and C. albicans. | Significant antimicrobial properties (p < 0.05). Based on the MTT cell viability test, the concentration of ZnO nanoparticles up to 0.1 mg/mL was biocompatible and had no major and significant damaging effect to the human cells. Ag/ZnO nanoparticles exhibited the best antimicrobial activity and highest shear bond strength. | 37/39 |
| Kim et al. 2018 [36] | National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (NRF2015R1C1A1A01051832). | Human gingival fibroblasts (HGFs; ATCC, Manassas); S. mutans (Ingbritt); N = 60 human premolars for 6 groups for bracket SBS and ARI index | Orthodontic bonding agents containing zinc-doped bioactive glass (BAG; orthodontic bonding agents containing BAG were prepared by mixing BAG with flowable resin; resin disk specimen | TransbondTM XT (TXT, 3M) and CharmfilTM Flow (CF, A2 shade, Denkist) | Mechanical and biological properties: Ion release, cytotoxicity, antibacterial properties, microhardness, shear bond strength, adhesive remnant index (ARI), and micro-computed tomography was performed after pH cycling to analyze remineralization. | Bonding agents with zinc-doped BAG have stronger antibacterial and remineralization effects compared with conventional adhesives (p < 0.05). | 38/39 |
| Zeidan et al. 2022 [57] | The Science, Technology, and Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB) | S. mutans strain (ATCC 25175) | Brackets coated with nanoparticles of Ag, ZnO, and a combination of both Ag/ZnO; N = 48 brackets, stainless steel “American orthodontics 0.018′’ slot size of lower premolars | Brackets as received without modifications | Antibacterial activity on S. mutans and L. acidophilus using CFU, evaluated immediately after coating and after 3 months. | Combination of silver and zinc oxide nanoparticles had the highest bacterial reduction. The coating of orthodontic brackets could be further assessed in clinical application to prevent decalcification. | 37/39 |
| Other Aspects | |||||||
| Chen et al. 2021b [55] | N/A | Human gingival fibroblasts (hGF) cells | Pure Zn used in barrier membrane in GBR therapy | None | Degradation behavior in artificial saliva solution, cytotoxicity, and antibacterial activity investigated to explore Zn degradation and associated biocompatibility in the case of premature membrane exposure. | Zn degradation rate in artificial saliva was 31.42 μm (year-1) after 28 days of immersion. Zn presented an acceptable HGF cytocompatibility and a high antibacterial activity against P. gingivalis, exhibiting appropriate degradation behavior, adequate cell compatibility, and favorable antibacterial properties in the oral environment. | 36/36 |
| Mishra et al. 2020 [47] | N/A | Human dental pulp stem cells and human red blood corpuscles, Staphylococcus aureus (ATCC 9144) | Biomaterial composed of zinc–carboxymethyl chitosan(CMC)–genipin synthesized and transformed to porous scaffolds using freeze drying method; the scaffolds were cross-linked and stabilized with genipin and zinc (2 M zinc acetate) | Redundant controls | FTIR spectroscopic data, scanning electron microscopy, compressive strength, biodegradation, and antibacterial properties. | The scaffolds seemed to support the adhesion and proliferation of human dental pulp stem cells and were hemocompatible with human red blood corpuscles. The scaffolds were found to be antibacterial and mildly antibiofilm against S. aureus. | 32/36 |
| Wiesmann et al. 2021 [56] | Max Planck Graduate Center, Mainz, Germany, and by the research focus group “BiomaTiCS—Biomaterials, Tissues and Cells in Surgery” of the University Medical Center, Mainz, Germany | Human gingival fibroblasts (Provitro AG, HFIB-G) and human umbilical vein endothelial cells (HUVECs) isolated from human umbilical cord veins | Incorporation of zinc oxide nanoparticles 20 nm (ZnO NPs) in biomaterials for tissue regeneration; zinc oxide nanoparticles were obtained from IoLiTec Ionic Liquids Technologies GmbH (Product Nr.: NO-0011-HP) | None | Antibacterial effects and metabolic activity on fibroblasts and endothelial cells, and biocompatibility with chicken chorioallantoic membrane assay (CAM)l flow cytometry for cell death; cell viability assay for cellular metabolic activity (CMA) with the alamarBlue™ Cell Viability Reagent. | ZnO NPs had favorable properties for biomaterials modification and could help to guide the tissue reaction and promote complication-free healing (p ≤ 0.001). | 35/39 |
3.2.1. Conservative and Cariology
Antibacterial Properties
Cytotoxicity
Physical, Chemical, and Mechanical Properties
3.2.2. Endodontics
3.2.3. Periodontology and Implantology
3.2.4. Orthodontics
3.2.5. Other Aspects
4. Discussion
Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fatima, T.; Haji Abdul Rahim, Z.B.; Lin, C.W.; Qamar, Z. Zinc: A precious trace element for oral health care? J. Pak. Med. Assoc. 2016, 66, 1019–1023. [Google Scholar]
- Gaur, S.; Agnihotri, R. Trace mineral micronutrients and chronic periodontitis—A review. Biol. Trace Elem. Res. 2017, 176, 225–238. [Google Scholar] [CrossRef]
- Lynch, R.J. Zinc in the mouth, its interactions with dental enamel and possible effects on caries; a review of the literature. Int. Dent. J. 2011, 61 (Suppl. S3), 46–54. [Google Scholar] [CrossRef]
- Sejdini, M.; Begzati, A.; Salihu, S.; Krasniqi, S.; Berisha, N.; Aliu, N. The role and impact of salivary Zn levels on dental caries. Int. J. Dent. 2018, 2018, 8137915. [Google Scholar] [CrossRef]
- Devi, C.B.; Nandakishore, T.; Sangeeta, N.; Basar, G.; Devi, N.O.; Jamir, S.; Singh, M.A. Zinc in human health. IOSR J. Dent. Med. Sci. 2014, 13, 18–23. [Google Scholar] [CrossRef]
- Rahman, M.T.; Hossain, A.; Pin, C.H.; Yahya, N.A. Zinc and metallothionein in the development and progression of dental caries. Biol. Trace Elem. Res. 2019, 187, 51–58. [Google Scholar] [CrossRef]
- Uwitonze, A.M.; Ojeh, N.; Murererehe, J.; Atfi, A.; Razzaque, M.S. Zinc Adequacy Is Essential for the Maintenance of Optimal Oral Health. Nutrients 2020, 12, 949. [Google Scholar] [CrossRef] [PubMed]
- Glenske, K.; Donkiewicz, P.; Köwitsch, A.; Milosevic-Oljaca, N.; Rider, P.; Rofall, S.; Franke, J.; Jung, O.; Smeets, R.; Schnettler, R.; et al. Applications of metals for bone regeneration. Int. J. Mol. Sci. 2018, 19, 826. [Google Scholar] [CrossRef] [PubMed]
- Brandao, N.L.; Portela, M.B.; Maia, L.C.; Antonio, A.; Silva, V.L.M.E.; Silva, E.M.D. Model resin composites incorporating ZnO-NP: Activity against S. mutans and physicochemical properties characterization. J. Appl. Oral. Sci. 2018, 26, e20170270. [Google Scholar] [CrossRef] [PubMed]
- Garcia, P.P.N.S.; Cardia, M.F.B.; Francisconi, R.S.; Dovigo, L.N.; Spolidorio, D.M.P.; de Souza Rastelli, A.N.; Botta, A.C. Antibacterial activity of glass ionomer cement modified by zinc oxide nanoparticles. Microsc. Res. Tech. 2017, 80, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Osorio, R.; Yamauti, M.; Osorio, E.; Román, J.S.; Toledano, M. Zincdoped dentin adhesive for collagen protection at the hybrid layer. Eur. J. Oral Sci. 2011, 119, 401–410. [Google Scholar] [CrossRef]
- Garcia, I.M.; Leitune, V.C.B.; Visioli, F.; Samuel, S.M.W.; Collares, F.M. Influence of zinc oxide quantum dots in the antibacterial activity and cytotoxicity of an experimental adhesive resin. J. Dent. 2018, 73, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Toledano-Osorio, M.; Osorio, E.; Aguilera, F.S.; Medina-Castillo, A.L.; Toledano, M.; Osorio, R. Improved reactive nanoparticles to treat dentin hypersensitivity. Acta Biomater. 2018, 72, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.; Nikaido, T.; Abdou, A.; Matin, K.; Burrow, M.F.; Tagami, J. Inhibitory effect of zinc-containing desensitizer on bacterial biofilm formation and root dentin demineralization. Dent. Mater. J. 2019, 38, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Du, L.; Fu, Y.; Jiang, P.; Wang, X. ZnO nanoparticles inhibit the activity of Porphyromonas gingivalis and Actinomyces naeslundii and promote the mineralization of the cementum. BMC Oral. Health 2019, 19, 84. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Li, L.; Liu, H.; Tan, L.; Liu, T.; Meng, X. Solvothermal synthesis of ZnO nanoparticles and anti-infection application in vivo. ACS Appl. Mater. Interfaces 2015, 7, 1308–1317. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; He, Y.; Irwin, P.L.; Jin, T.; Shi, X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against campylobacter jejuni. Appl. Environ. Microbiol. 2011, 77, 2325–2331. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.G.; Cho, M.H.; Lee, J. ZnO nanoparticles inhibit Pseudomonas aeruginosa biofilm formationand virulence factor production. Microbiol. Res. 2014, 169, 888–896. [Google Scholar] [CrossRef]
- Sarwar, S.; Chakraborti, S.; Bera, S.; Sheikh, I.A.; Hoque, K.M.; Chakrabarti, P. The antimicrobial activity of ZnO nanoparticles against Vibrio cholerae: Variation in response depends on biotype. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1499–1509. [Google Scholar] [CrossRef]
- Gomes-Filho, J.E.; Silva, F.O.; Watanabe, S.; Cintra, L.T.A.; Tendoro, K.V.; Dalto, L.G.; Pacanaro, S.V.; Lodi, C.S.; de Melo, F.F.F. Tissue reaction to silver nanoparticles dispersion as an alternative irrigating solution. J. Endod. 2010, 36, 1698–1702. [Google Scholar] [CrossRef]
- Saunders, S.A. Current practicality of nanotechnology in dentistry. Part 1: Focus on nanocomposite restoratives and biomimetics. Clin. Cosmet. Investig. Dent. 2009, 30, 47–61. [Google Scholar] [CrossRef]
- Guerreiro-Tanomaru, J.M.; Pereira, K.F.; Nascimento, C.A.; Bernardi, M.I.B.; TanomaruFilho, M. Use of Nanoparticulate zinc oxide as Intracanal medication in endodontics:PH and antimicrobial activity. Acta Odontol. Latinoam. 2013, 26, 144–148. [Google Scholar] [PubMed]
- Winnik, F.M.; Maysinger, D. Quantum dot cytotoxicity and ways to reduce it. Acc. Chem. Res. 2013, 46, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Maia, L.C.; Antonio, A.G. Systematic reviews in dental research. A guideline. J. Clin. Pediatr. Dent. 2012, 37, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.; Jones, B.; Gardner, P.; Lawton, R. Quality assessment with diverse studies (QuADS): An appraisal tool for methodological and reporting quality in systematic reviews of mixed- or multi-method studies. BMC Health Serv. Res. 2021, 21, 144, Erratum in BMC Health Serv. Res. 2021, 21, 231. [Google Scholar] [CrossRef]
- Doneria, D.; Thakur, S.; Singhal, P.; Chauhan, D. Comparative evaluation of clinical and radiological success of zinc oxide-ozonated oil, modified 3mix-mp antibiotic paste, and vitapex as treatment options in primary molars requiring pulpectomy: An in vivo study. J. Indian Soc. Pedod. Prev. Dent. 2017, 35, 346–352. [Google Scholar] [CrossRef]
- Pilownic, K.J.; Gomes, A.P.N.; Wang, Z.J.; Almeida, L.H.S.; Romano, A.R.; Shen, Y.; Felix, A.d.O.C.; Haapasalo, M.; Pappen, F.G. Physicochemical and Biological Evaluation of Endodontic Filling Materials for Primary Teeth. Braz. Dent. J. 2017, 28, 578–586. [Google Scholar] [CrossRef]
- Prasad, K.V.; Therathil, S.G.; Agnihotri, A.; Sreenivasan, P.K.; Mateo, L.R.; Cummins, D. The Effects of Two New Dual Zinc plus Arginine Dentifrices in Reducing Oral Bacteria in Multiple Locations in the Mouth: 12-Hour Whole Mouth Antibacterial Protection for Whole Mouth Health. J. Clin. Dent. 2018, 29, A25–A32. [Google Scholar]
- Lavaee, F.; Ghapanchi, J.; Motamedifar, M.; Sharifzade Javidi, M. Experimental Evaluation of the Effect of Zinc Salt on Inhibition of Streptococcus mutans. J. Dent. 2018, 19, 168–173. [Google Scholar]
- Manus, L.M.; Daep, C.A.; Begum-Gafur, R.; Makwana, E.; Won, B.; Yang, Y.; Huang, X.Y.; Maloney, V.; Trivedi, H.M.; Wu, D.; et al. Enhanced In Vitro Zinc Bioavailability through Rational Design of a Dual Zinc plus Arginine Dentifrice. J. Clin. Dent. 2018, 29, A10–A19. [Google Scholar] [PubMed]
- Peralta, S.L.; Dutra, A.L.; de Leles, S.B.; Ribeiro, J.S.; Ogliari, F.A.; Piva, E.; Lund, R.G. Development and characterization of a novel bulk-fill elastomeric temporary restorative composite. J. Appl. Oral Sci. 2018, 27, e20180183. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Nakano, Y.; Watanabe, T.; Yoneda, M.; Hirofuji, T.; Hanioka, T. Two mechanisms of oral malodor inhibition by zinc ions. J. Appl. Oral Sci. 2018, 26, e20170161. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Sun, Q.; Li, Y.; Tay, F.R.; Fan, B. Synergistic mechanism of Ag+-Zn2+ in anti-bacterial activity against Enterococcus faecalis and its application against dentin infection. J. Nanobiotechnology 2018, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.C.; Toledano-Osorio, M.; Bueno, J.; Figuero, E.; Toledano, M.; Medina-Castillo, A.L.; Osorio, R.; Herrera, D.; Sanz, M. Antibacterial effects of polymeric PolymP-n Active nanoparticles: An in vitro biofilm study. Dent. Mater. 2019, 35, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-M.; Kim, D.-H.; Song, C.W.; Yoon, S.-Y.; Kim, S.-Y.; Na, H.S.; Chung, J.; Kim, Y.-I.; Kwon, Y.H. Antibacterial and remineralization effects of orthodontic bonding agents containing bioactive glass. Korean J. Orthod. 2018, 48, 163–171. [Google Scholar] [CrossRef]
- Hagenfeld, D.; Prior, K.; Harks, I.; Jockel-Schneider, Y.; May, T.W.; Harmsen, D.; Schlagenhauf, U.; Ehmke, B. No differences in microbiome changes between anti-adhesive and antibacterial ingredients in toothpastes during periodontal therapy. J. Periodontal Res. 2019, 54, 435–443. [Google Scholar] [CrossRef]
- Eskandarizadeh, A.; Sharokhi, F.; Hamze, F.; Kalantari, M.; Hoseiniffar, R.; Khaleghi, M.; Shadman, N.; Ramezani, F. Antibacterial, physical and mechanical properties of bonding agent containing synthesized Zinc Dimethacrylate. J. Clin. Exp. Dent. 2019, 11, e686–e694. [Google Scholar] [CrossRef]
- Mirhosseini, F.; Amiri, M.; Daneshkazemi, A.; Zandi, H.; Javadi, Z.S. Antimicrobial Effect of Different Sizes of Nano Zinc Oxide on Oral Microorganisms. Front. Dent. 2019, 16, 105–112. [Google Scholar] [CrossRef]
- Tabatabaei, M.H.; Mahounak, F.S.; Asgari, N.; Moradi, Z. Cytotoxicity of the Ingredients of Commonly Used Toothpastes and Mouthwashes on Human Gingival Fibroblasts. Front. Dent. 2019, 16, 450–457. [Google Scholar] [CrossRef]
- Sreenivasan, P.K.; Haraszthy, V.I.; Rayela, C.C. Antimicrobial effects in oral microenvironments by a novel herbal toothpaste. Contemp. Clin. Trials Commun. 2020, 21, 100680. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, S.; Sun, Y.; Shi, C.; Yang, H.; Lu, Z. Effects of Ag/ZnO nanocomposite at sub-minimum inhibitory concentrations on virulence factors of Streptococcus mutans. Arch. Oral. Biol. 2020, 111, 104640. [Google Scholar] [CrossRef]
- Steiger, E.L.; Muelli, J.R.; Braissant, O.; Waltimo, T.; Astasov-Frauenhoffer, M. Effect of divalent ions on cariogenic biofilm formation. BMC Microbiol. 2020, 20, 287. [Google Scholar] [CrossRef]
- Xu, X.; Wang, N.; Wu, M.; Wang, J.; Wang, D.; Chen, Z.; Xie, J.; Ding, C.; Li, J. Programmed antibacterial and mineralization therapy for dental caries based on zinc-substituted hydroxyapatite/alendronate-grafted polyacrylic acid hybrid material. Colloids Surf. B Biointerfaces 2020, 194, 111206. [Google Scholar] [CrossRef]
- Garcia, I.M.; Souza, V.S.; Souza, J.D.; Visioli, F.; Leitune, V.C.B.; Scholten, J.D.; Collares, F.M. Zinc-based particle with ionic liquid as a hybrid filler for dental adhesive resin. J. Dent. 2020, 102, 103477. [Google Scholar] [CrossRef]
- Fröber, K.; Bergs, C.; Pich, A.; Conrads, G. Biofunctionalized zinc peroxide nanoparticles inhibit peri-implantitis associated anaerobes and Aggregatibacter actinomycetemcomitans pH-dependent. Anaerobe 2020, 62, 102153. [Google Scholar] [CrossRef]
- Mishra, A.H.; Mishra, D. Evidences of Biomimetic and Nonantibiotic Characteristics of the Zinc–Carboxymethyl Chitosan–Genipin Organometallic Complex and Its Biocompatibility Aspects. Biomacromolecules 2020, 21, 688–700. [Google Scholar] [CrossRef]
- Moura, J.; Lima, M.; Nogueira, N.; Castro, M.; Lima, C.; Moura, M.; Moura, L. LSTR Antibiotic Paste Versus Zinc Oxide and Eugenol Pulpectomy for the Treatment of Primary Molars with Pulp Necrosis: A Randomized Controlled Trial. Pediatr. Dent. 2021, 43, 435–442. [Google Scholar] [PubMed]
- Barma, M.D.; Muthupandiyan, I.; Samuel, S.R.; Amaechi, B.T. Inhibition of Streptococcus mutans, antioxidant property and cytotoxicity of novel nano-zinc oxide varnish. Arch. Oral. Biol. 2021, 126, 105132. [Google Scholar] [CrossRef] [PubMed]
- Garcia, I.M.; Balhaddad, A.A.; Ibrahim, M.S.; Weir, M.D.; Xu, H.H.; Collares, F.M.; Melo, M.A.S. Antibacterial response of oral microcosm biofilm to nano-zinc oxide in adhesive resin. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2021, 37, e182–e193. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, C.; Xie, Y.; Xu, A.; Guan, Y.; Lu, W.; Wang, X.; He, F. Zinc- and strontium- co-incorporated nanorods on titanium surfaces with favorable material property, osteogenesis, and enhanced antibacterial activity. J. Biomed. Mater. Res. Part B 2021, 109, 1754–1767. [Google Scholar] [CrossRef]
- Lin, M.-H.; Wang, Y.-H.; Kuo, C.-H.; Ou, S.-F.; Huang, P.-Z.; Song, T.-Y.; Chen, Y.-C.; Chen, S.-T.; Wu, C.-H.; Hsueh, Y.-H.; et al. Hybrid ZnO/chitosan antimicrobial coatings with enhanced mechanical and bioactive properties for titanium implants. Carbohydr. Polym. 2021, 257, 117639. [Google Scholar] [CrossRef]
- Vergara-Llanos, D.; Koning, T.; Pavicic, M.F.; Bello-Toledo, H.; Díaz-Gómez, A.; Jaramillo, A.; Melendrez-Castro, M.; Ehrenfeld, P.; Sánchez-Sanhueza, G. Antibacterial and cytotoxic evaluation of copper and zinc oxide nanoparticles as a potential disinfectant material of connections in implant provisional abutments: An in-vitro study. Arch. Oral. Biol. 2021, 122, 105031. [Google Scholar] [CrossRef]
- Kachoei, M.; Divband, B.; Rahbar, M.; Esmaeilzadeh, M.; Ghanizadeh, M.; Alam, M. A Novel Developed Bioactive Composite Resin Containing Silver/Zinc Oxide (Ag/ZnO) Nanoparticles as an Antimicrobial Material against Streptococcus mutans, Lactobacillus, and Candida albicans. Evid.-Based Complement. Altern. Med. 2021, 2021, 4743411. [Google Scholar] [CrossRef]
- Chen, K.; Zhou, G.; Li, Q.; Tang, H.; Wang, S.; Li, P.; Gu, X.; Fan, Y. In vitro degradation, biocompatibility and antibacterial properties of pure zinc: Assessing the potential of Zn as a guided bone regeneration membrane. J. Mater. Chem. B 2021, 9, 5114–5127. [Google Scholar] [CrossRef]
- Wiesmann, N.; Mendler, S.; Buhr, C.R.; Ritz, U.; Kämmerer, P.W.; Brieger, J. Zinc Oxide Nanoparticles Exhibit Favorable Properties to Promote Tissue Integration of Biomaterials. Biomedicines 2021, 9, 1462. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, N.K.; Enany, N.M.; Mohamed, G.G.; Marzouk, E.S. The antibacterial effect of silver, zinc-oxide and combination of silver/zinc oxide nanoparticles coating of orthodontic brackets (an in vitro study). BMC Oral Health. 2022, 22, 230. [Google Scholar] [CrossRef] [PubMed]
- McBean, L.D.; Dove, J.T.; Halsted, J.A.; Smith, J.C., Jr. Zinc concentration in human tissues. Am J Clin Nutr. 1972, 25, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Smaïl-Faugeron, V.; Glenny, A.M.; Courson, F.; Durieux, P.; Muller-Bolla, M.; Fron Chabouis, H. Pulp treatment for extensive decay in primary teeth. Cochrane Database Syst. Rev. 2018, 5, CD003220. [Google Scholar] [CrossRef] [PubMed]
- Rajendiran, M.; Trivedi, H.M.; Chen, D.; Gajendrareddy, P.; Chen, L. Recent Development of Active Ingredients in Mouthwashes and Toothpastes for Periodontal Diseases. Molecules 2021, 26, 2001. [Google Scholar] [CrossRef] [PubMed]
- Moradpoor, H.; Safaei, M.; Mozaffari, H.R.; Sharifi, R.; Imani, M.M.; Golshah, A.; Bashardoust, N. An overview of recent progress in dental applications of zinc oxide nanoparticles. RSC Adv. 2021, 11, 21189–21206. [Google Scholar] [CrossRef]
- Pushpalatha, C.; Suresh, J.; Gayathri, V.S.; Sowmya, S.V.; Augustine, D.; Alamoudi, A.; Zidane, B.; Mohammad Albar, N.H.; Patil, S. Zinc Oxide Nanoparticles: A Review on Its Applications in Dentistry. Front. Bioeng. Biotechnol. 2022, 10, 917990. [Google Scholar] [CrossRef]
- Almoudi, M.M.; Hussein, A.S.; Abu Hassan, M.I.; Mohamad Zain, N. A systematic review on antibacterial activity of zinc against Streptococcus mutans. Saudi Dent. J. 2018, 30, 283–291. [Google Scholar] [CrossRef]
- Griauzdyte, V.; Jagelaviciene, E. Antimicrobial Activity of Zinc against Periodontal Pathogens: A Systematic Review of In Vitro Studies. Medicina 2023, 59, 2088. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Liu, S.; Du, J.; Xu, J.; Liu, Y.; Guo, L. The changes and potential effects of zinc homeostasis in periodontitis microenvironment. Oral. Dis. 2023, 29, 3063–3077. [Google Scholar] [CrossRef]
- López-García, S.; Pecci-Lloret, M.P.; García-Bernal, D.; Guerrero-Gironés, J.; Pecci-Lloret, M.R.; Rodríguez-Lozano, F.J. Are Denture Adhesives Safe for Oral Cells? J. Prosthodont. 2021, 30, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Lee, H.-H.; Kim, H.-W.; Yu, J.-W.; Kim, K.-N.; Kim, K.-M. Immunomodulatory/anti-inflammatory effect of ZOE-based dental materials. Dent. Mater. 2017, 33, e1–e12. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Lee, H.-H.; Kim, K.-N.; Kim, K.-M. Cytotoxicity and anti-inflammatory effects of zinc ions and eugenol during setting of ZOE in immortalized human oral keratinocytes grown as three-dimensional spheroids. Dent. Mater. 2016, 32, e93–e104. [Google Scholar] [CrossRef] [PubMed]
- Best, M.; Phillips, G.; Fowler, C.; Rowland, J.; Elsom, J. Characterisation and cytotoxic screening of metal oxide nanoparticles putative of interest to oral healthcare formulations in non-keratinised human oral mucosa cells in vitro. Toxicol. In Vitro 2015, 30, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Seker, S.; Elçin, A.E.; Yumak, T.; Sınağ, A.; Elçin, Y.M. In vitro cytotoxicity of hydrothermally synthesized ZnO nanoparticles on human periodontal ligament fibroblast and mouse dermal fibroblast cells. Toxicol. Vitro 2014, 28, 1349–1358. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
| PICOS | |
|---|---|
| Population/patient | Children and adult patients |
| Intervention/indicator | Zinc exposure |
| Comparator/control | No zinc exposure |
| Outcomes | Oral health |
| Study design | In vivo (clinical trials: controlled clinical trials (CCTs) and randomized controlled trials (RCTs); and observational studies: case control and cohoort studies) and in vitro |
| Other inclusion criteria | English or Italian studies published in the last 10 years |
| Exclusion criteria | Studies on other chemical compounds or with no full-text available, systematic reviews, animal studies, case reports, editorials, opinions, surveys, guidelines, conferences, commentaries |
| Pubmed (2 March 2022) |
| Query |
| ((“zinc”[MeSH Terms] OR “zinc”[All Fields]) AND (“anti bacterial agents”[Pharmacological Action] OR “anti bacterial agents”[MeSH Terms] OR (“anti bacterial”[All Fields] AND “agents”[All Fields]) OR “anti bacterial agents”[All Fields] OR “antibacterial”[All Fields] OR “antibacterials”[All Fields] OR “antibacterially”[All Fields]) AND (“mouth”[MeSH Terms] OR “mouth”[All Fields] OR “oral”[All Fields])) NOT (Systematic Review [Publication Type] OR Review [Publication Type] OR Meta-Analysis [Publication Type] OR Comment [Publication Type] OR Congress [Publication Type] OR Editorial [Publication Type] OR Case Reports [Publication Type] OR Clinical Conference [Publication Type] OR Comment [Publication Type] OR Consensus Development Conference [Publication Type]) Filters: English, Italian, 10 years. |
| Scopus (2 March 2022) |
| Query |
| (TITLE-ABS-KEY (“zinc”) AND TITLE-ABS-KEY (“anti bacterial agent*” OR “antibacterial*”) AND TITLE-ABS-KEY (“mouth” OR “oral”) AND NOT TITLE-ABS-KEY (systematic AND review AND [doctype] OR review AND [doctype] OR meta-analysis AND [doctype] OR comment AND [doctype] OR congress AND [doctype] OR editorial AND [doctype] OR case AND reports AND [doctype] OR clinical AND conference AND [doctype] OR comment AND [doctype] OR consensus AND development AND conference AND [doctype])) AND PUBYEAR > 2011 AND PUBYEAR < 2023 AND (LIMIT-TO (DOCTYPE, “ar”)) |
| Web of Science (2 March 2022) |
| Query |
| TS = (((“zinc”) AND (“anti-bacterial agents” OR “anti-bacterial*” OR “antibacterial*”)) AND (“mouth” OR “oral”)) AND LANGUAGE: (English OR Italian) AND DOCUMENT TYPES: (Article) Timespan = 2012–2022 |
| Dental Products | Zinc Advantages |
|---|---|
| In vivo studies | |
| Zinc oxide-ozonated oil (ZnO-OO) (0.007 cc Ozonil, Ozone Forum of India) | Pulpectomy agent |
| Zinc-substituted carbonated hydroxyapatite dentifrice (BioRepair, Wolff) | Antimicrobial activity in co-adiuvant periodontal therapy |
| Zinc oxide eugenol paste (ZOE) (Biodynamics) | Endodontic lesion sterilization and tissue repair |
| New fluoride toothpastes with Dual Zinc plus Arginine formulations (Dual Zinc plus Arginine; Colgate-Palmolive Company) | Antibacterial activity |
| Herbal toothpaste incorporating zinc | Antibacterial activity |
| In vitro studies | |
| Conservative and Cariology | |
| Zinc oxide nanoparticles synthetized (ZnO-NP) varnish | Antimicrobial, bactericidal, and antioxidant activity |
| Dental resin adhesive with zinc dimethacrylate ionomer (ZDMA) | Antibacterial activity, higher dentine shear bond strength, and lower compressive strength |
| ZnO incorporated in dental adhesive | Antibacterial activity, higher degree of conversion, and elastic modulus |
| Ag/ZnO nanocomposite | Antimicrobial activity and suppression of virulence factors related genes expression |
| Zinc sulfate (Merck) and zinc acetate (Falcon) solutions | Antibacterial activity |
| Dual Zinc active system dentifrice, with zinc citrate and zinc oxide | Antibacterial activity |
| Solutions with nano ZnO (nZnO) powder (Research Nanomaterials Inc.) | Antimicrobial activity |
| Elastomeric temporary resin-based filling materials with zinc methacrylate (ZM, Aldrich Chemical Co.) | Antibacterial activity, lower microleakage and water sorption, and higher ultimate tensile strength |
| Divalent cation Zn2+ and in combination ZnCl, ZnCl2 | Antibacterial activity |
| metal chlorides, ZnCl2, and metal acetates, (CH3COO)2Zn | Antimicrobial activity |
| toothpastes and mouthwashes with sodium fluoride, sodium lauryl sulfate, cocamidopropyl betaine, zinc lactate, paraben, and sodium benzoate | Cytocompatibility |
| Zinc-doped NPs (Zn-NPs), PolymP-n Active nanoparticles (NanoMyP): aqueous solutions of ZnCl2 | Higher intertubular and peritubular complex modulus values |
| Zinc-substituted hydroxyapatite/alendronate-grafted polyacrylic acid hybrid nanoneedles (ZHA@ALN-PAA) of Zn(CH3COO)2%2H2O | No cytotoxcity |
| Endodontics | |
| Ag+-Zn2+ atomic combination ratios | Antibacterial activity |
| Zinc oxide quantum dots (ZnOQDs) into adhesive resin | Antibacterial activity |
| Zinc-based particle with ionic liquid as filler for experimental adhesive resin | Antibacterial activity and no pulp cytotoxcity |
| Zinc oxide eugenol (ZOE), Vitapex, and Calen paste thickened with zinc oxide (ZO) | Biocompatibility and antimicrobial activity |
| Periodontology and Implantology | |
| co-incorporated zinc and strontium nanorod coating on sandblasted and acid-etched titanium (SLA-Zn/Sr) | Good interface bonding strength; enhanced corrosion resistance, biofilm inhibition, and cellular initial adhesion; and proliferation and osteogenic differentiation promotion |
| Glucose-1-phosphate (Glc-1P) biofunctionalized zinc peroxide (ZnO2) nanoparticles | Antimicrobial activity |
| Implant with inner layer of nanoporous TiO2 and outer layer of chitosan matrix with ZnO nanoparticles | Antibacterial activity, bioactivity, cytocompatibility, and improved corrosion resistance |
| Hydroxyapatite discs coated with nanoparticles (NPs) doped with zinc | Antibacterial activity and icrements in roughness |
| ZnO-NPs | Antibacterial activity and biocompatibility |
| Orthodontics | |
| New bioactive orthodontic composite resin containing silver/zinc oxide (Ag/ZnO) nanoparticles | Antimicrobial activity, biocompatibility, and higher shear bond strength |
| Orthodontic bonding agents containing zinc-doped bioactive glass (BAG) | Antimicrobial activity and remineralization effects |
| Brackets coated with nanoparticles of Ag, ZnO and a combination of both Ag/ZnO | Antibacterial activity |
| Other Aspects | |
| Pure Zn used in barrier membrane in GBR therapy | Antibacterial activity and cytocompatibility |
| Biomaterial composed of zinc-carboxymethyl chitosan (CMC)-genipin synthesized and transformed to porous scaffolds | Antibacterial activity, good adhesion and proliferation of human dental pulp stem cells, and hemocompatibility |
| Incorporation of zinc oxide nanoparticles (ZnO NPs) (IoLiTec Ionic Liquids Technologies GmbH) in biomaterials for tissue regeneration | Antibacterial activity, tissue reaction, and complication-free healing promotion |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caruso, S.; Valenti, C.; Marinucci, L.; Di Pasquale, F.; Truppa, C.; Di Benedetto, G.; Caruso, S.; Pagano, S. Systematic Review of Zinc’s Benefits and Biological Effects on Oral Health. Materials 2024, 17, 800. https://doi.org/10.3390/ma17040800
Caruso S, Valenti C, Marinucci L, Di Pasquale F, Truppa C, Di Benedetto G, Caruso S, Pagano S. Systematic Review of Zinc’s Benefits and Biological Effects on Oral Health. Materials. 2024; 17(4):800. https://doi.org/10.3390/ma17040800
Chicago/Turabian StyleCaruso, Silvia, Chiara Valenti, Lorella Marinucci, Francesca Di Pasquale, Claudia Truppa, Giulia Di Benedetto, Sara Caruso, and Stefano Pagano. 2024. "Systematic Review of Zinc’s Benefits and Biological Effects on Oral Health" Materials 17, no. 4: 800. https://doi.org/10.3390/ma17040800
APA StyleCaruso, S., Valenti, C., Marinucci, L., Di Pasquale, F., Truppa, C., Di Benedetto, G., Caruso, S., & Pagano, S. (2024). Systematic Review of Zinc’s Benefits and Biological Effects on Oral Health. Materials, 17(4), 800. https://doi.org/10.3390/ma17040800










