Flexible Dry Electrode Based on a Wrinkled Surface That Uses Carbon Nanotube/Polymer Composites for Recording Electroencephalograms
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
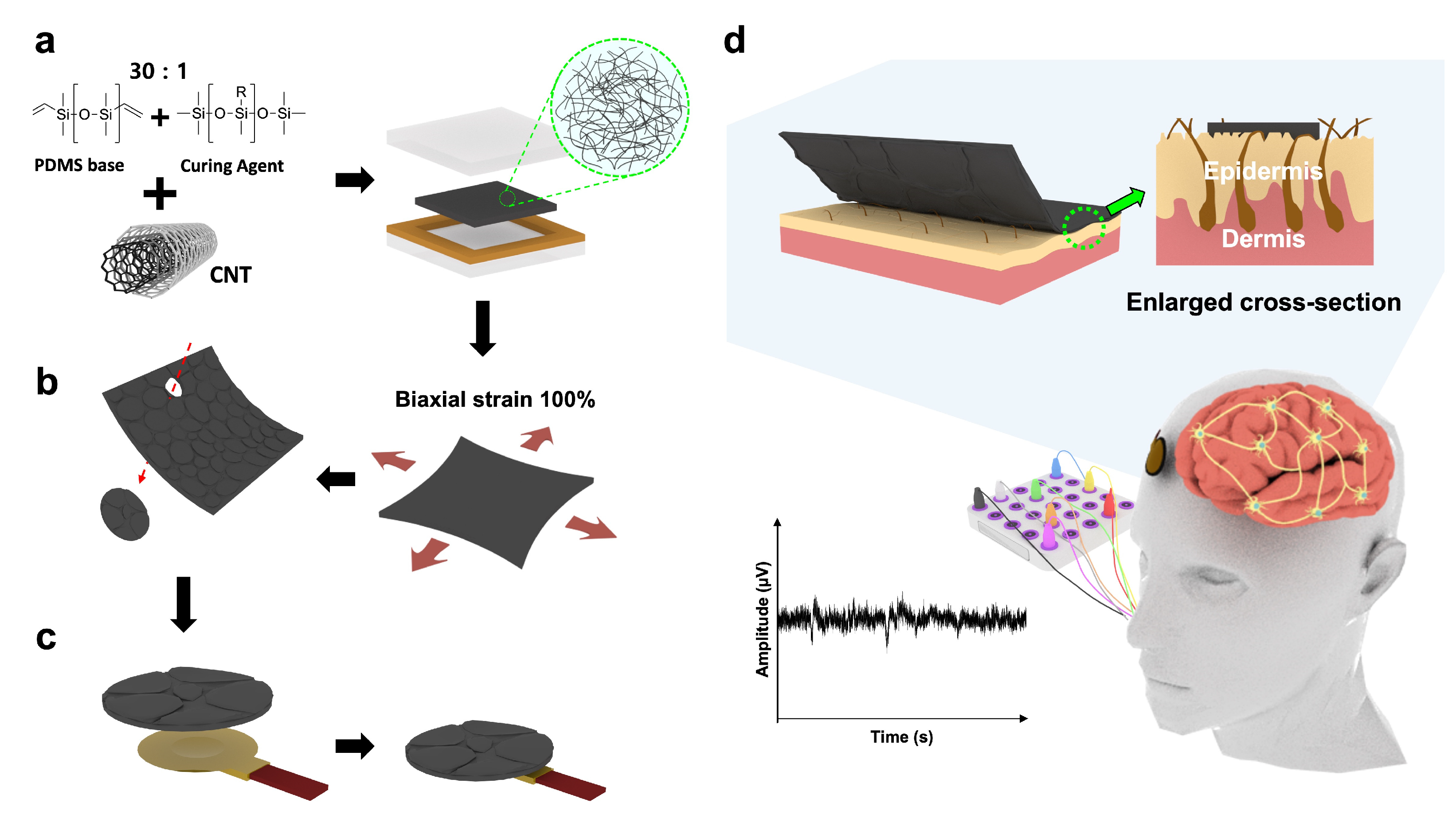
2.2. Preparing CNT/PDMS Composites and Fabricating EEG Electrodes
2.3. Characterization
3. Results and Discussion
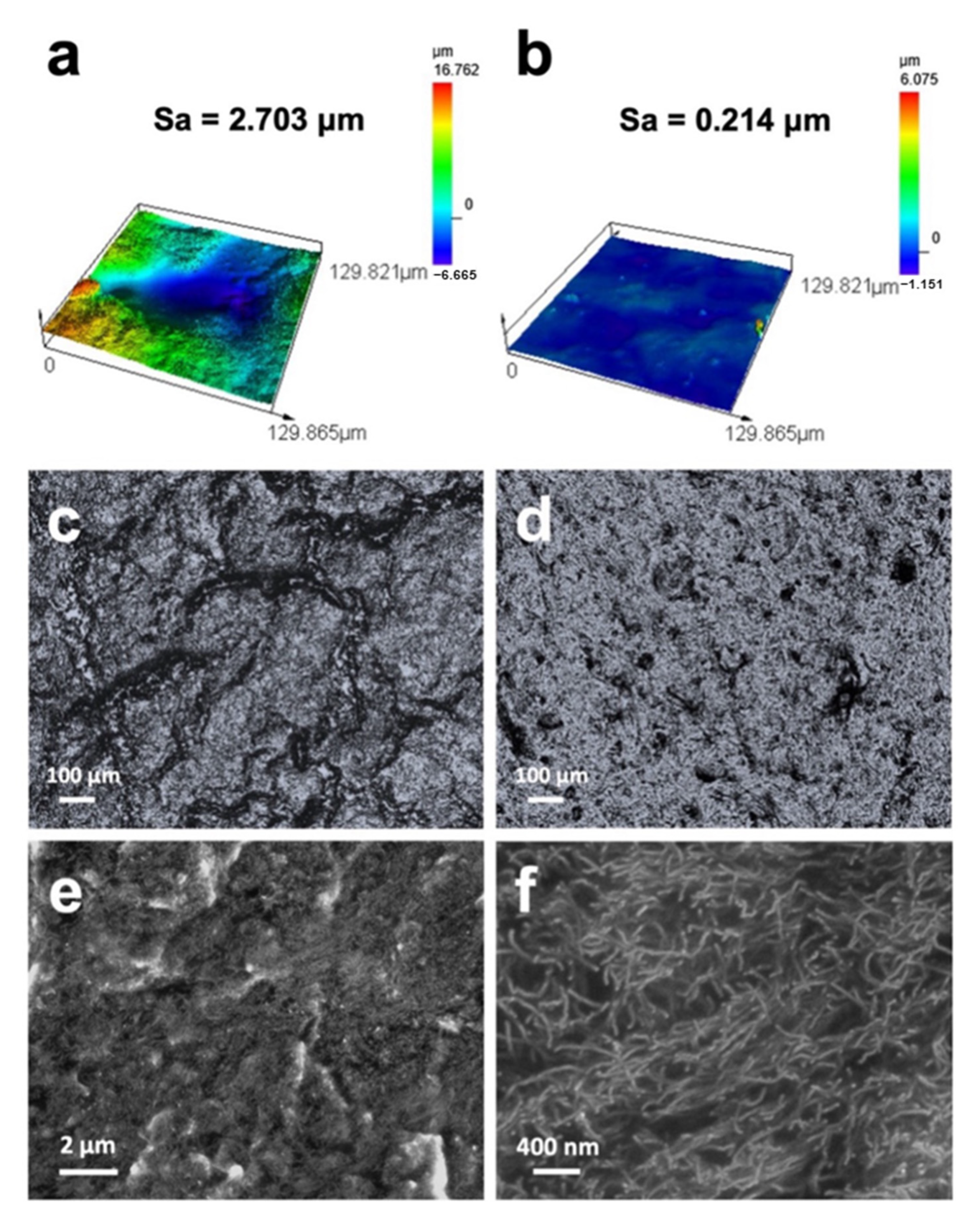
3.1. Morphologies
3.2. Electrical Properties
3.2.1. Electrical Conductivity and Percolation Threshold
3.2.2. Evaluating Dynamic Artifacts and Measuring Impedance
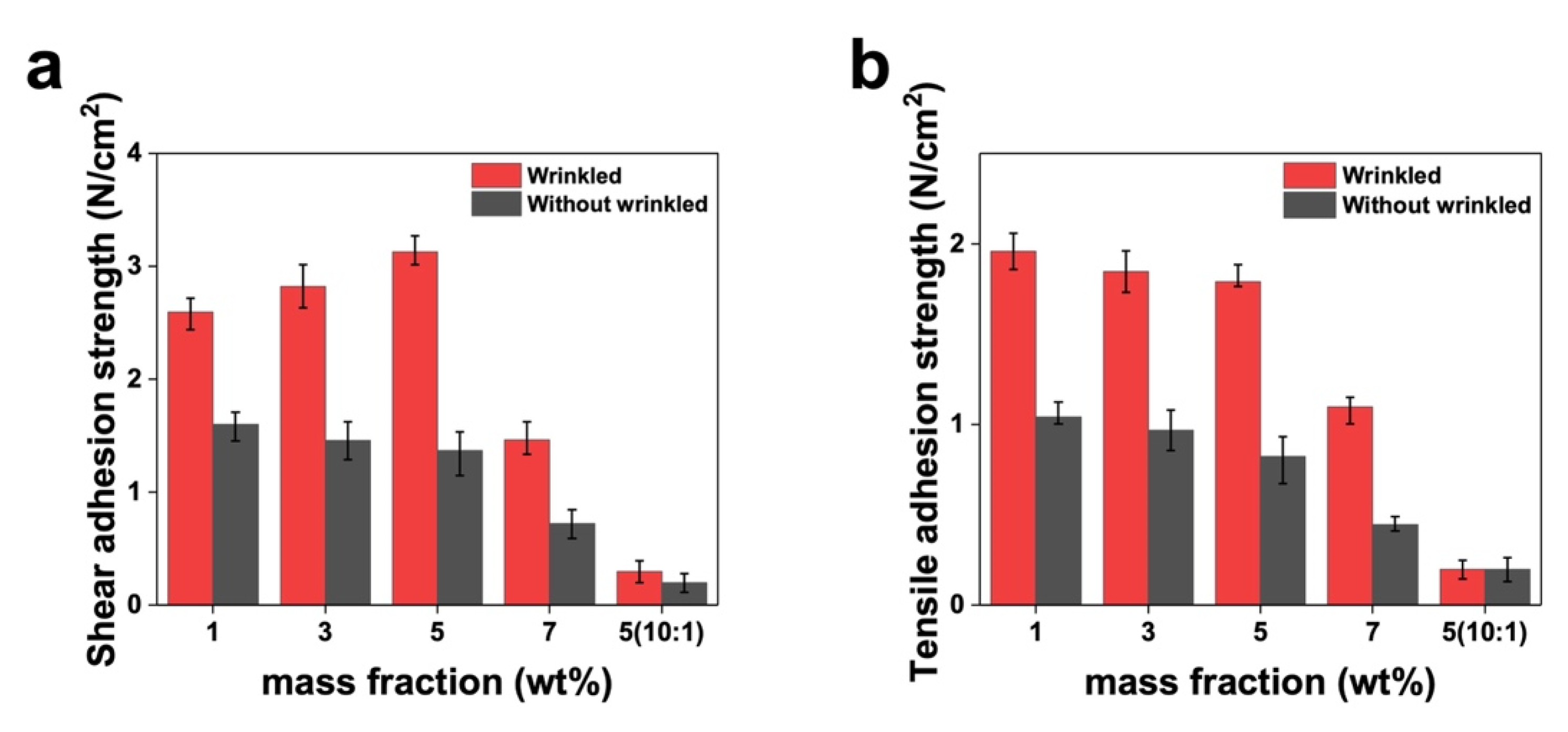
3.3. Mechanical Properties: Adhesion Testing
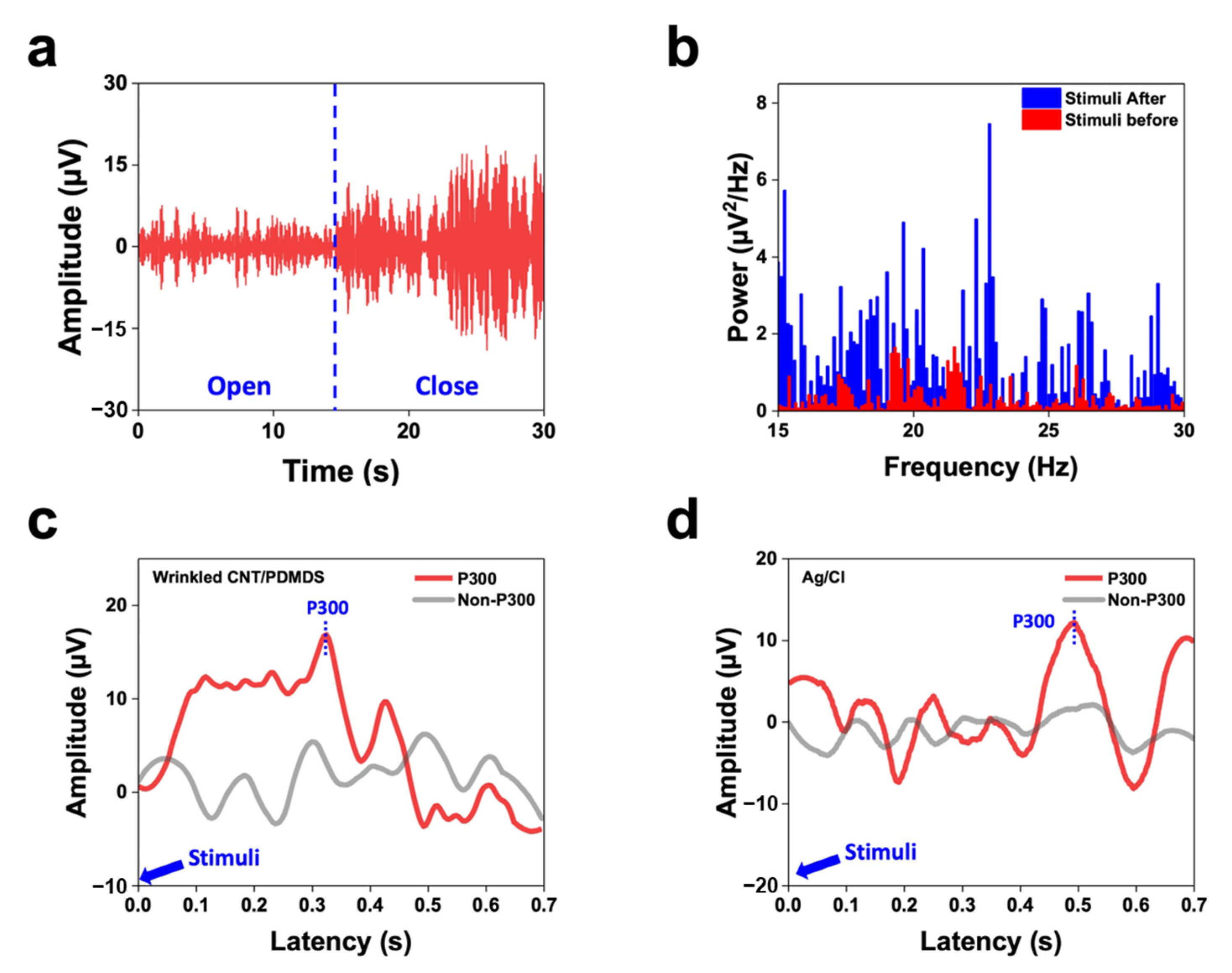
3.4. Recording EEG Signals
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schaul, N. The Fundamental Neural Mechanisms of Electroencephalography. Electroencephalogr. Clin. Neurophysiol. 1998, 106, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Lopes da Silva, F.L. Neural Mechanisms Underlying Brain Waves: From Neural Membranes to Networks. Electroencephalogr. Clin. Neurophysiol. 1991, 79, 81–93. [Google Scholar] [CrossRef]
- Anjum, M.F.; Dasgupta, S.; Mudumbai, R.; Singh, A.; Cavanagh, J.F.; Narayanan, N.S. Linear Predictive Coding Distinguishes Spectral EEG Features of Parkinson’s Disease. Park. Relat. Disord. 2020, 79, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Patidar, S.; Pachori, R.B.; Upadhyay, A.; Rajendra Acharya, U.R. An Integrated Alcoholic Index Using Tunable-Q Wavelet Transform Based Features Extracted from EEG Signals for Diagnosis of Alcoholism. Appl. Soft Comput. 2017, 50, 71–78. [Google Scholar] [CrossRef]
- Jeong, J. EEG Dynamics in Patients with Alzheimer’s Disease. Clin. Neurophysiol. 2004, 115, 1490–1505. [Google Scholar] [CrossRef]
- Zerr, I.; Pocchiari, M.; Collins, S.; Brandel, J.-P.; de Pedro Cuesta, J.; Knight, R.S.; Bernheimer, H.; Cardone, F.; Delasnerie-Lauprêtre, N.; Corrales, N.C. Analysis of EEG and CSF 14-3-3 Proteins as Aids to the Diagnosis of Creutzfeldt–Jakob disease. Neurology 2000, 55, 811–815. [Google Scholar] [CrossRef]
- Sturm, I.; Lapuschkin, S.; Samek, W.; Müller, K.R. Interpretable Deep Neural Networks for Single-Trial EEG Classification. J. Neurosci. Methods 2016, 274, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Brito, N.H.; Fifer, W.P.; Myers, M.M.; Elliott, A.J.; Noble, K.G. Associations Among Family Socioeconomic Status, EEG Power at Birth, and Cognitive Skills During Infancy. Dev. Cogn. Neurosci. 2016, 19, 144–151. [Google Scholar] [CrossRef]
- Thut, G.; Pascual-Leone, A. A Review of Combined TMS-EEG Studies to Characterize Lasting Effects of Repetitive TMS and Assess Their Usefulness in Cognitive and Clinical Neuroscience. Brain Topogr. 2010, 22, 219–232. [Google Scholar] [CrossRef]
- Nunez, P.L.; Srinivasan, R. Electric Fields of the Brain: The Neurophysics of EEG; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- Debener, S.; Ullsperger, M.; Siegel, M.; Engel, A.K. Single-trial EEG–fMRI Reveals the Dynamics of Cognitive Function. Trends Cogn. Sci. 2006, 10, 558–563. [Google Scholar] [CrossRef]
- Shin, J.H.; Kwon, J.; Kim, J.U.; Ryu, H.; Ok, J.; Joon Kwon, S.; Park, H.; Kim, T.-I. Wearable EEG Electronics for a Brain–AI Closed-Loop System to Enhance Autonomous Machine Decision-Making. Npj Flex. Electron. 2022, 6, 32. [Google Scholar] [CrossRef]
- Rivera, M.J.; Teruel, M.A.; Maté, A.; Trujillo, J. Diagnosis and Prognosis of Mental Disorders by Means of EEG and Deep Learning: A Systematic Mapping Study. Artif. Intell. Rev. 2022, 55, 1209–1251. [Google Scholar] [CrossRef]
- He, Y.; Eguren, D.; Azorín, J.M.; Grossman, R.G.; Luu, T.P.; Contreras-Vidal, J.L. Brain–Machine Interfaces for Controlling Lower-Limb Powered Robotic Systems. J. Neural Eng. 2018, 15, 021004. [Google Scholar] [CrossRef] [PubMed]
- Sabeti, M.; Katebi, S.; Boostani, R. Entropy and Complexity Measures for EEG Signal Classification of Schizophrenic and Control Participants. Artif. Intell. Med. 2009, 47, 263–274. [Google Scholar] [CrossRef]
- Lebedev, M.A.; Nicolelis, M.A. Brain–machine Interfaces: Past, Present and Future. Trends Neurosci. 2006, 29, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Andersen, R.A.; Musallam, S.; Pesaran, B. Selecting the Signals for a Brain–Machine Interface. Curr. Opin. Neurobiol. 2004, 14, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Hinrichs, H.; Scholz, M.; Baum, A.K.; Kam, J.W.Y.; Knight, R.T.; Heinze, H.J. Comparison Between a Wireless Dry Electrode EEG System With a Conventional Wired Wet Electrode EEG System for Clinical Applications. Sci. Rep. 2020, 10, 5218. [Google Scholar] [CrossRef] [PubMed]
- Hoon Lee, J.; Min Lee, S.; Jin Byeon, H.; Sook Hong, J.; Suk Park, K.; Lee, S.H. CNT/PDMS-based Canal-Typed Ear Electrodes for Inconspicuous EEG Recording. J. Neural Eng. 2014, 11, 046014. [Google Scholar] [CrossRef]
- Ruffini, G.; Dunne, S.; Fuentemilla, L.; Grau, C.; Farrés, E.; Marco-Pallarés, J.; Watts, P.C.P.; Silva, S.R.P. First Human Trials of a Dry Electrophysiology Sensor Using a Carbon Nanotube Array Interface. Sens. Actuators A 2008, 144, 275–279. [Google Scholar] [CrossRef]
- Li, Z.; Guo, W.; Huang, Y.; Zhu, K.; Yi, H.; Wu, H. On-skin Graphene Electrodes for Large Area Electrophysiological Monitoring and Human-Machine Interfaces. Carbon 2020, 164, 164–170. [Google Scholar] [CrossRef]
- Yun, Y.J.; Ju, J.; Lee, J.H.; Moon, S.H.; Park, S.J.; Kim, Y.H.; Hong, W.G.; Ha, D.H.; Jang, H.; Lee, G.H.; et al. Highly Elastic Graphene-Based Electronics Toward Electronic Skin. Adv. Funct. Mater. S 2017, 27, 1701513. [Google Scholar] [CrossRef]
- Chi, M.; Zhao, J.; Dong, Y.; Wang, X. Flexible carbon nanotube-based polymer electrode for long-term electrocardiographic recording. Materials 2019, 12, 971. [Google Scholar] [CrossRef]
- Jung, H.-C.; Moon, J.-H.; Baek, D.-H.; Lee, J.-H.; Choi, Y.-Y.; Hong, J.-S.; Lee, S.-H. CNT/PDMS composite flexible dry electrodesfor long-term ECG monitoring. IEEE Trans. Biomed. Eng. 2012, 59, 1472–1479. [Google Scholar] [CrossRef] [PubMed]
- Mao, P.; Li, H.; Yu, Z. A Review of Skin-Wearable Sensors for Non-Invasive Health Monitoring Applications. Sensors 2023, 23, 3673. [Google Scholar] [CrossRef]
- Mata, A.; Fleischman, A.J.; Roy, S. Characterization of Polydimethylsiloxane (PDMS) Properties for Biomedical Micro/Nanosystems. Biomed. Microdevices 2005, 7, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Lv, Z.; Batool, S.; Li, M.Z.; Zhao, P.; Guo, L.; Wang, Y.; Zhou, Y.; Han, S.T. Biocompatible Material-Based Flexible Biosensors: From Materials Design to Wearable/Implantable Devices and Integrated Sensing Systems. Small 2023, 19, e2207879. [Google Scholar] [CrossRef]
- Shetti, N.P.; Mishra, A.; Basu, S.; Mascarenhas, R.J.; Kakarla, R.R.; Aminabhavi, T.M. Skin-patchable Electrodes for Biosensor Applications: A Review. ACS Biomater. Sci. Eng. 2020, 6, 1823–1835. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Z.; Zhang, T. Flexible Sensing Electronics for Wearable/Attachable Health Monitoring. Small 2017, 13, 1602790. [Google Scholar] [CrossRef]
- Li, B.; Cao, Y.-P.; Feng, X.-Q.; Gao, H. Mechanics of Morphological Instabilities and Surface Wrinkling in Soft Materials: A Review. Soft Matter 2012, 8, 5728–5745. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, J. Wrinkled Interfaces: Taking Advantage of Surface Instabilities to Pattern Polymer Surfaces. Prog. Polym. Sci. 2015, 42, 1–41. [Google Scholar] [CrossRef]
- Schweikart, A.; Fery, A. Controlled Wrinkling as a Novel Method for the Fabrication of Patterned Surfaces. Microchim. Acta 2009, 165, 249–263. [Google Scholar] [CrossRef]
- Kim, D.H.; Lu, N.; Ma, R.; Kim, Y.S.; Kim, R.H.; Wang, S.; Wu, J.; Won, S.M.; Tao, H.; Islam, A.; et al. Epidermal Electronics. Science 2011, 333, 838–843. [Google Scholar] [CrossRef]
- Liu, Y.; Pharr, M.; Salvatore, G.A. Lab-On-Skin: A Review of Flexible and Stretchable Electronics for Wearable Health Monitoring. ACS Nano 2017, 11, 9614–9635. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, J.H.; Yoon, J.; Jung, K.Y. Influence of Task Difficulty on the Features of Event-Related Potential During Visual Oddball Task. Neurosci. Lett. 2008, 445, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Polich, J.; Margala, C. P300 and Probability: Comparison of Oddball and Single-Stimulus Paradigms. Int. J. Psychophysiol. 1997, 25, 169–176. [Google Scholar] [CrossRef]
- Lee, S.M.; Byeon, H.J.; Lee, J.H.; Baek, D.H.; Lee, K.H.; Hong, J.S.; Lee, S.H. Self-Adhesive Epidermal Carbon Nanotube Electronics for Tether-Free Long-Term Continuous Recording of Biosignals. Sci. Rep. 2014, 4, 6074. [Google Scholar] [CrossRef] [PubMed]
- Bauhofer, W.; Kovacs, J.Z. A Review and Analysis of Electrical Percolation in Carbon Nanotube Polymer Composites. Compos. Sci. Technol. 2009, 69, 1486–1498. [Google Scholar] [CrossRef]
- Oh, J.; Kim, D.Y.; Kim, H.; Hur, O.N.; Park, S.H. Comparative Study of Carbon Nanotube Composites as Capacitive and Piezoresistive Pressure Sensors Under Varying Conditions. Materials 2022, 15, 7637. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, Y.; Kang, M.; Hu, L.; Zhao, S.; Ahn, J.H. 2D Materials for Skin-Mountable Electronic Devices. Adv. Mater. 2021, 33, e2005858. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Son, J.; Kim, D.; Ko, H.J.; Lee, S.G.; Cho, K. Crocodile-skin-inspired Omnidirectionally Stretchable Pressure Sensor. Small 2022, 18, e2205643. [Google Scholar] [CrossRef] [PubMed]
- Mondal, R.K.; Dubey, K.A.; Kumar, J.; Jagannath, B.; Bhardwaj, Y.K.; Melo, J.S.; Varshney, L. Carbon Nanotube Functionalization and Radiation Induced Enhancements in ihe Sensitivity of Standalone Chemiresistors for Sensing Volatile Organic Compounds. ACS Appl. Nano Mater. 2018, 1, 5470–5482. [Google Scholar] [CrossRef]
- Du, J.; Wang, L.; Shi, Y.; Zhang, F.; Hu, S.; Liu, P.; Li, A.; Chen, J. Optimized CNT-PDMS Flexible Composite for Attachable Health-Care Device. Sensors 2020, 20, 4523. [Google Scholar] [CrossRef]
- Chen, G.; Matsuhisa, N.; Liu, Z.; Qi, D.; Cai, P.; Jiang, Y.; Wan, C.; Cui, Y.; Leow, W.R.; Liu, Z.; et al. Plasticizing Silk Protein for On-Skin Stretchable Electrodes. Adv. Mater. 2018, 30, e1800129. [Google Scholar] [CrossRef]
- Ji, B.; Wang, M.; Ge, C.; Xie, Z.; Guo, Z.; Hong, W.; Gu, X.; Wang, L.; Yi, Z.; Jiang, C.; et al. Flexible Bioelectrodes with Enhanced Wrinkle Microstructures for Reliable Electrochemical Modification and Neuromodulation In Vivo. Biosens. Bioelectron. 2019, 135, 181–191. [Google Scholar] [CrossRef]
- Hur, O.-N.; Ha, J.-H.; Park, S.-H. Strain-sensing Properties of Multi-Walled Carbon Nanotube/Polydimethylsiloxane Composites with Different Aspect Ratio and Filler Contents. Materials 2020, 13, 2431. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Yamamoto, D.; Takada, M.; Naito, H.; Arie, T.; Akita, S.; Takei, K. Efficient Skin Temperature Sensor and Stable Gel-Less Sticky ECG Sensor for a Wearable Flexible Healthcare Patch. Adv. Healthc. Mater. 2017, 6, 1700495. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Kumar, K.S.; He, H.; Cai, C.J.; He, X.; Gao, H.; Yue, S.; Li, C.; Seet, R.C.-S.; Ren, H.; et al. Fully Organic Compliant Dry Electrodes Self-Adhesive to Skin for Long-Term Motion-Robust Epidermal Biopotential Monitoring. Nat. Commun. 2020, 11, 4683. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.E.; Kim, S.; Yu, H.; Lee, K.N.; Do, Y.R.; Lee, S.M. Soft, adhesive and conductive composite for electroencephalogram signal quality improvement. Biomed. Eng. Lett. 2023, 13, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, E.; Choi, C.; Yeo, W.-H. Advances in soft and dry electrodes for wearable health monitoring devices. Micromachines 2022, 13, 629. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, J.; Nam, K.-W.; Kim, W.-J.; Kang, B.-H.; Park, S.-H. Flexible Dry Electrode Based on a Wrinkled Surface That Uses Carbon Nanotube/Polymer Composites for Recording Electroencephalograms. Materials 2024, 17, 668. https://doi.org/10.3390/ma17030668
Oh J, Nam K-W, Kim W-J, Kang B-H, Park S-H. Flexible Dry Electrode Based on a Wrinkled Surface That Uses Carbon Nanotube/Polymer Composites for Recording Electroencephalograms. Materials. 2024; 17(3):668. https://doi.org/10.3390/ma17030668
Chicago/Turabian StyleOh, Jihyeon, Kun-Woo Nam, Won-Jin Kim, Byung-Ho Kang, and Sung-Hoon Park. 2024. "Flexible Dry Electrode Based on a Wrinkled Surface That Uses Carbon Nanotube/Polymer Composites for Recording Electroencephalograms" Materials 17, no. 3: 668. https://doi.org/10.3390/ma17030668
APA StyleOh, J., Nam, K.-W., Kim, W.-J., Kang, B.-H., & Park, S.-H. (2024). Flexible Dry Electrode Based on a Wrinkled Surface That Uses Carbon Nanotube/Polymer Composites for Recording Electroencephalograms. Materials, 17(3), 668. https://doi.org/10.3390/ma17030668





