The Use of Thermoporometry in the Study of Frost Resistance of Rocks
Abstract
1. Introduction
- The first of them is spontaneous nucleation. When the temperature is lowered below the phase transition temperature, liquid water does not immediately undergo a phase change. In supercooled water, small clusters of water molecules appear, forming the basis for crystal-initiating nuclei (homogenous nucleation) [7]. The spontaneous phase transition begins when the nuclei of the new phase reach a critical size “r*” [8,9]. In a water-filled pore, ice can only appear if the critical diameters of the nuclei are not larger than the pore diameter.
- The second mechanism controlling the freezing process is derived by penetration of the ice front in pore spaces [11]. By removing energy from the system, an increasing volume of water gradually freezes. The freezing process of pore liquid after spontaneous nucleation is dependent on the liquid–ice interface and ice–gas interface [12,13,14]. When there is only liquid and solid water in the pores, the following equation can be used:where: rK is the solid–liquid surface curvature; M is the molar mass of the liquid; Θ is the contact angle; ΔHfus is the heat of fusion; ρl is the density of liquid; T is the temperature of phase transition; and T0 is the phase transition temperature of the bulk liquid.
2. Materials and Methods
2.1. Materials
2.2. Differential Scanning Calorimetry (DSC)
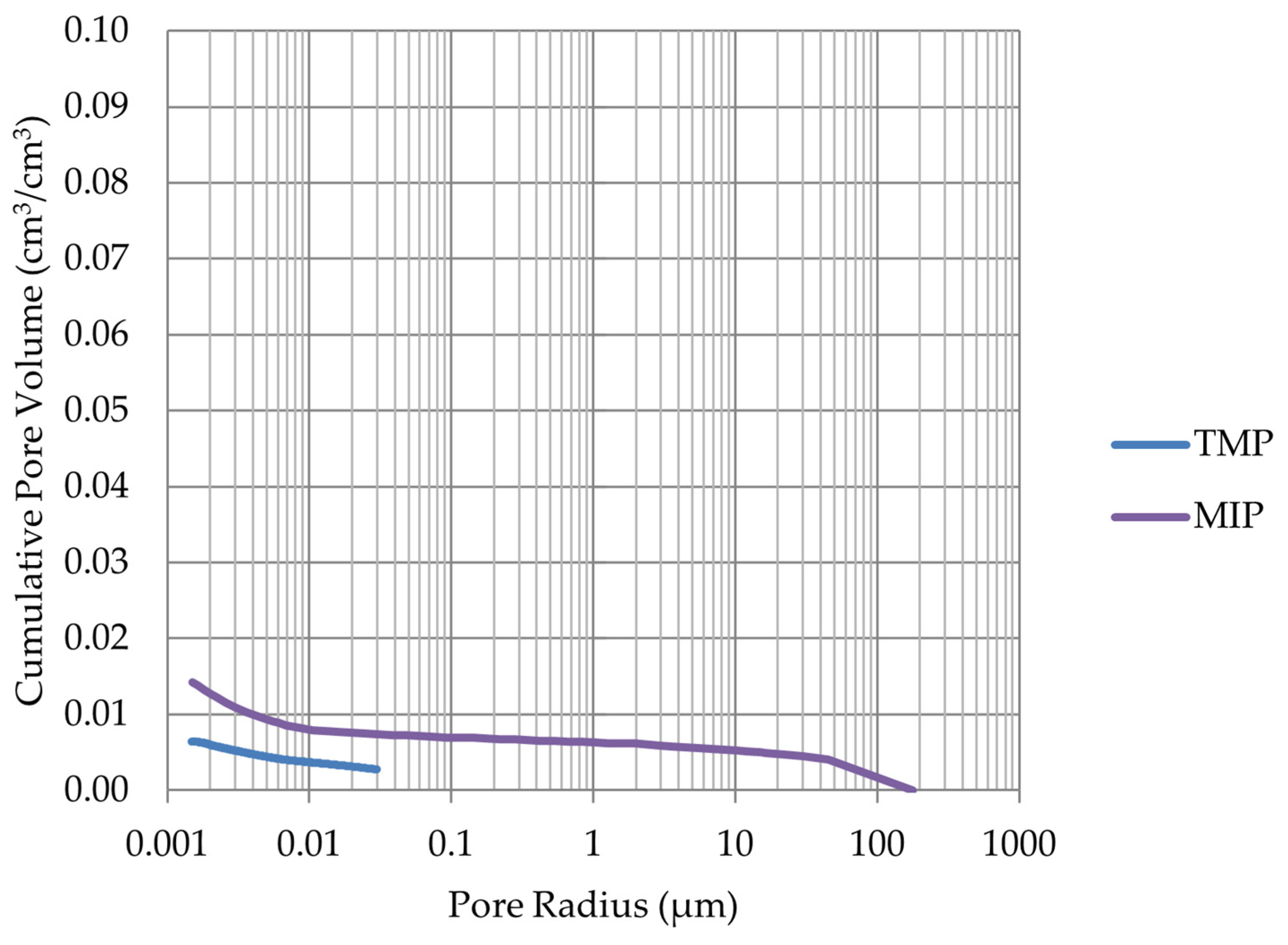
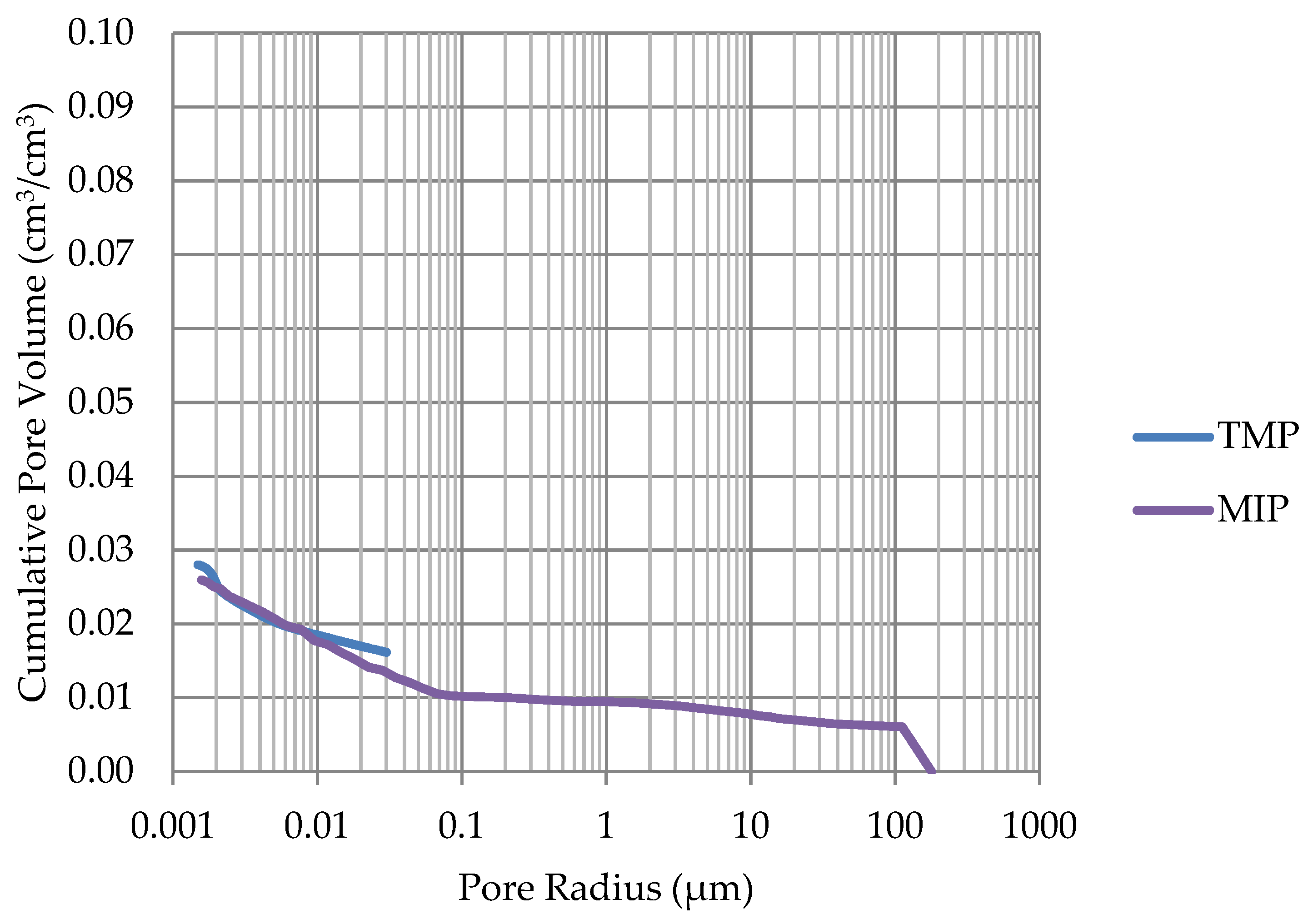
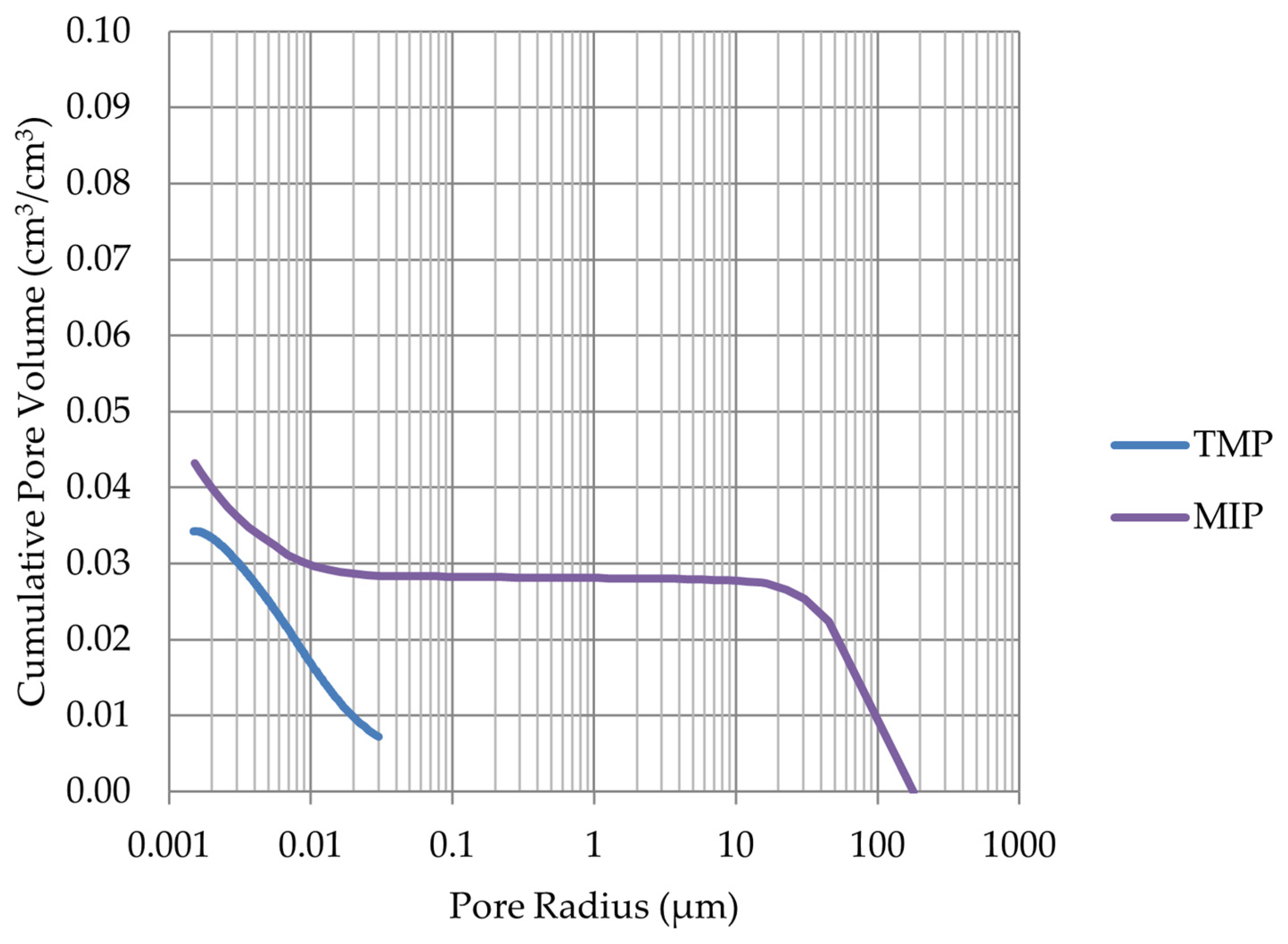
2.3. Mercury Intrusion Porosimetry (MIP)
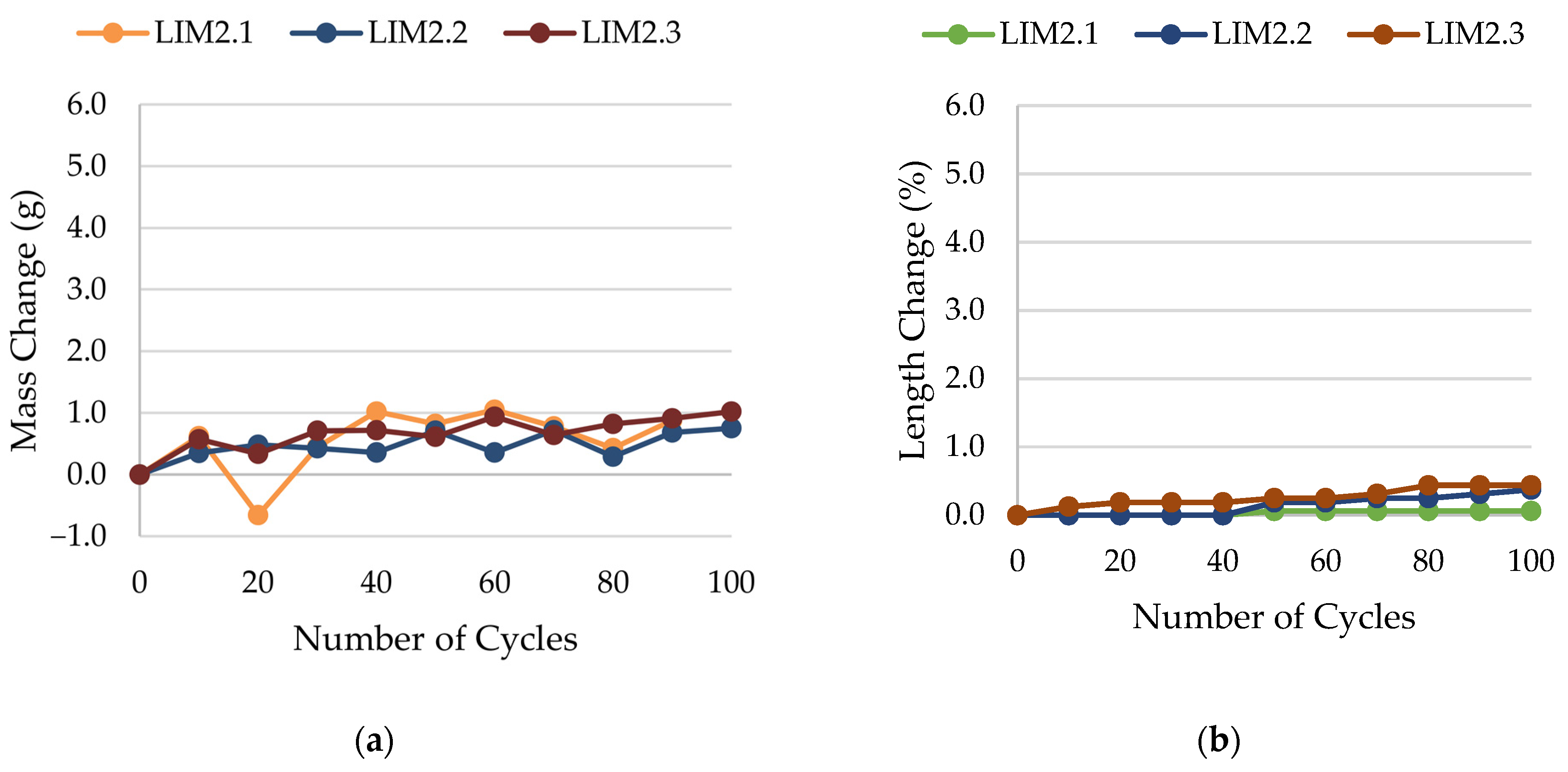
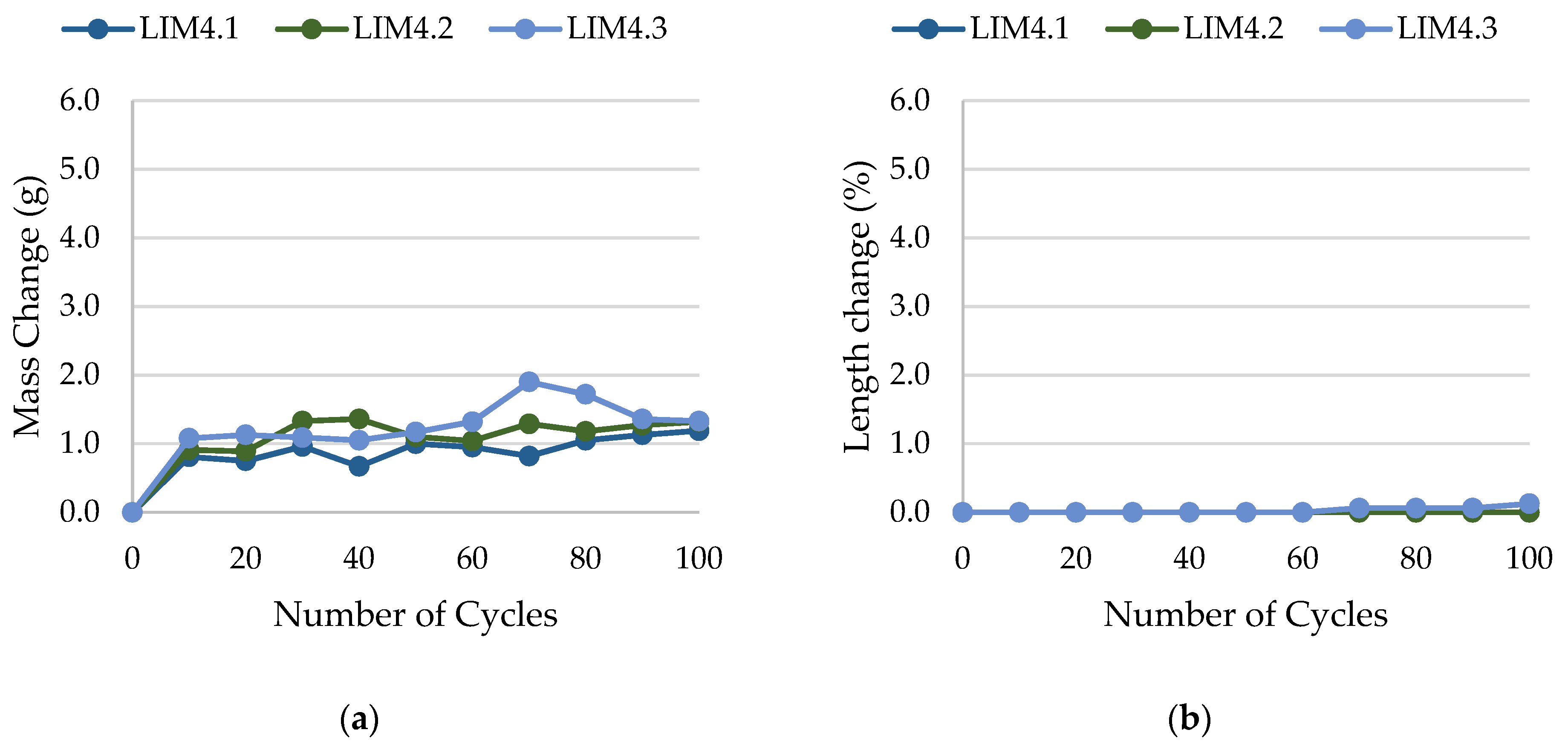
2.4. Frost Resistance Test
3. Results and Discussion
3.1. Results of Differential Scanning Calorimetry Research and Thermoporometry Method
3.2. Comparison Results of Differential Scanning Calorimetry Method and Thermoporometry with Results of Mercury Intrusion Porosimetry Method and Frost Resistance Test
4. Conclusions
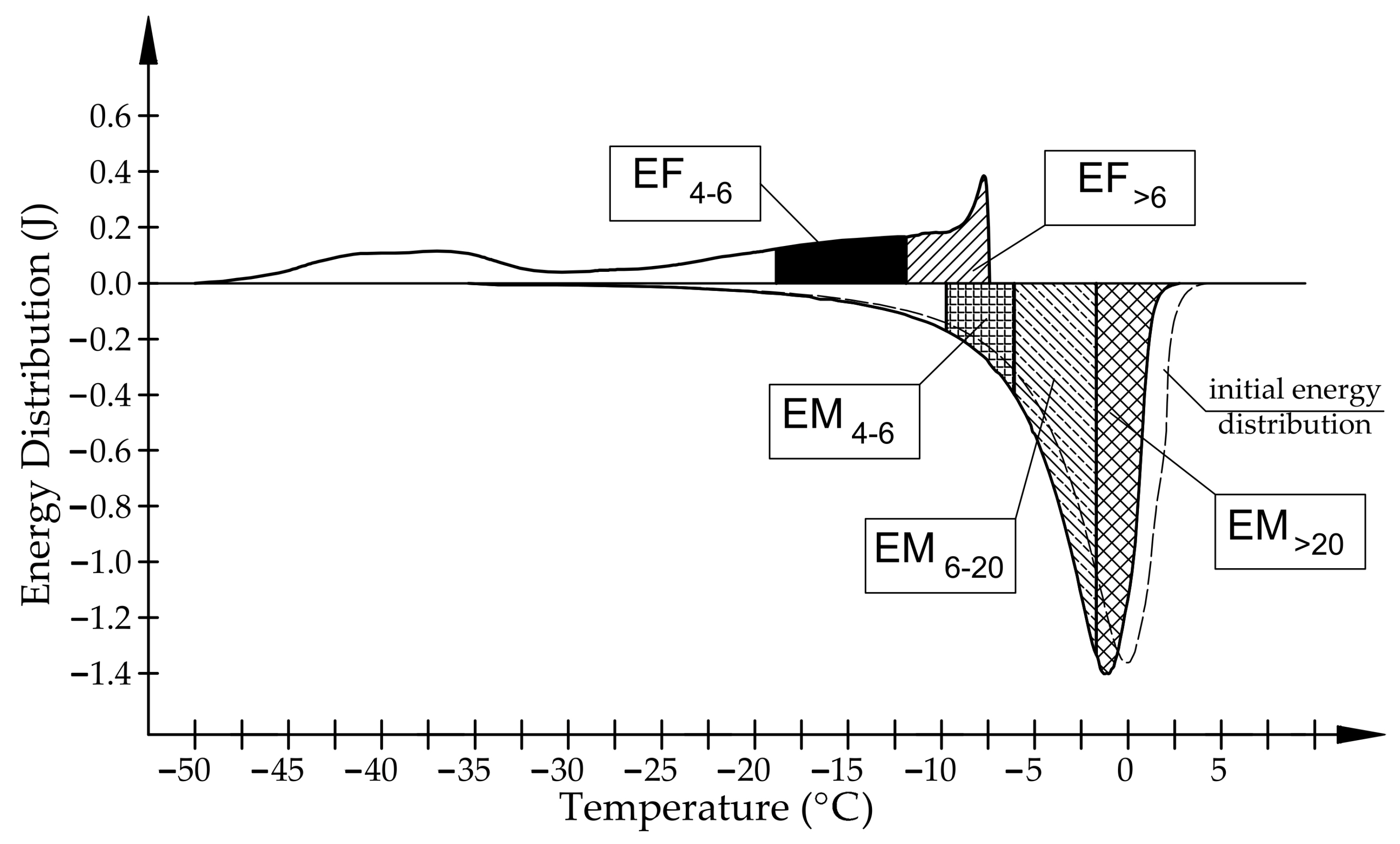
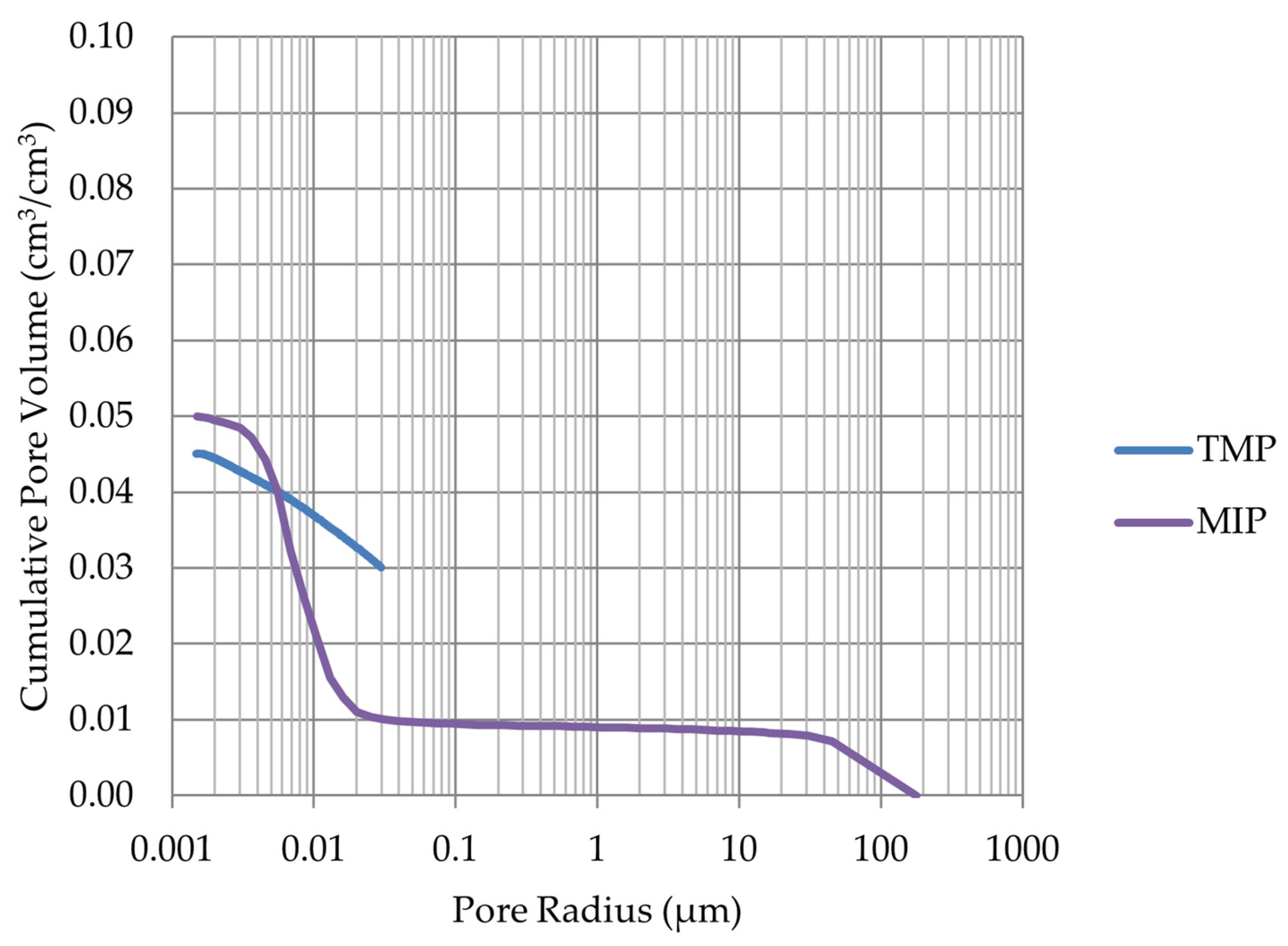
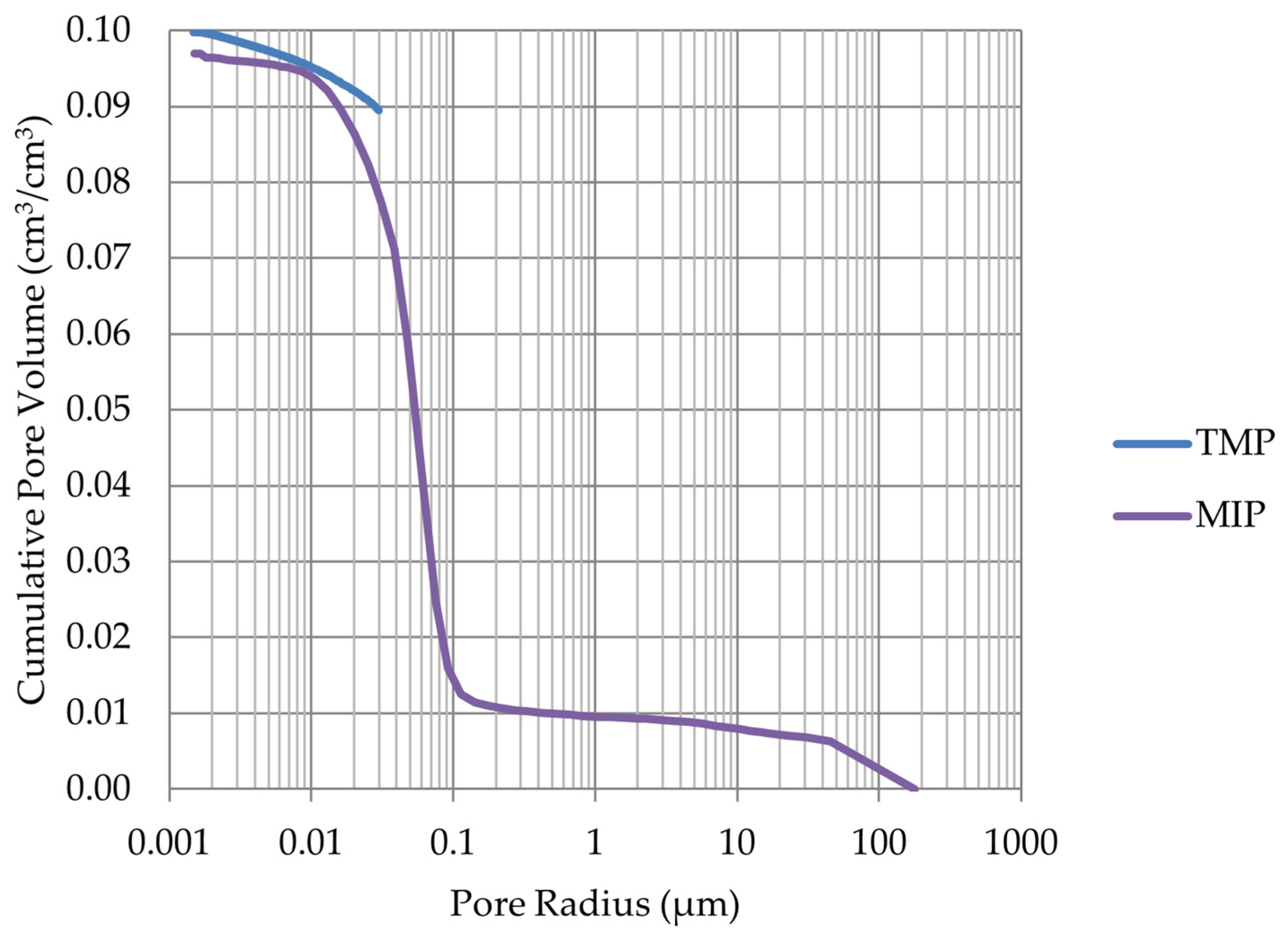
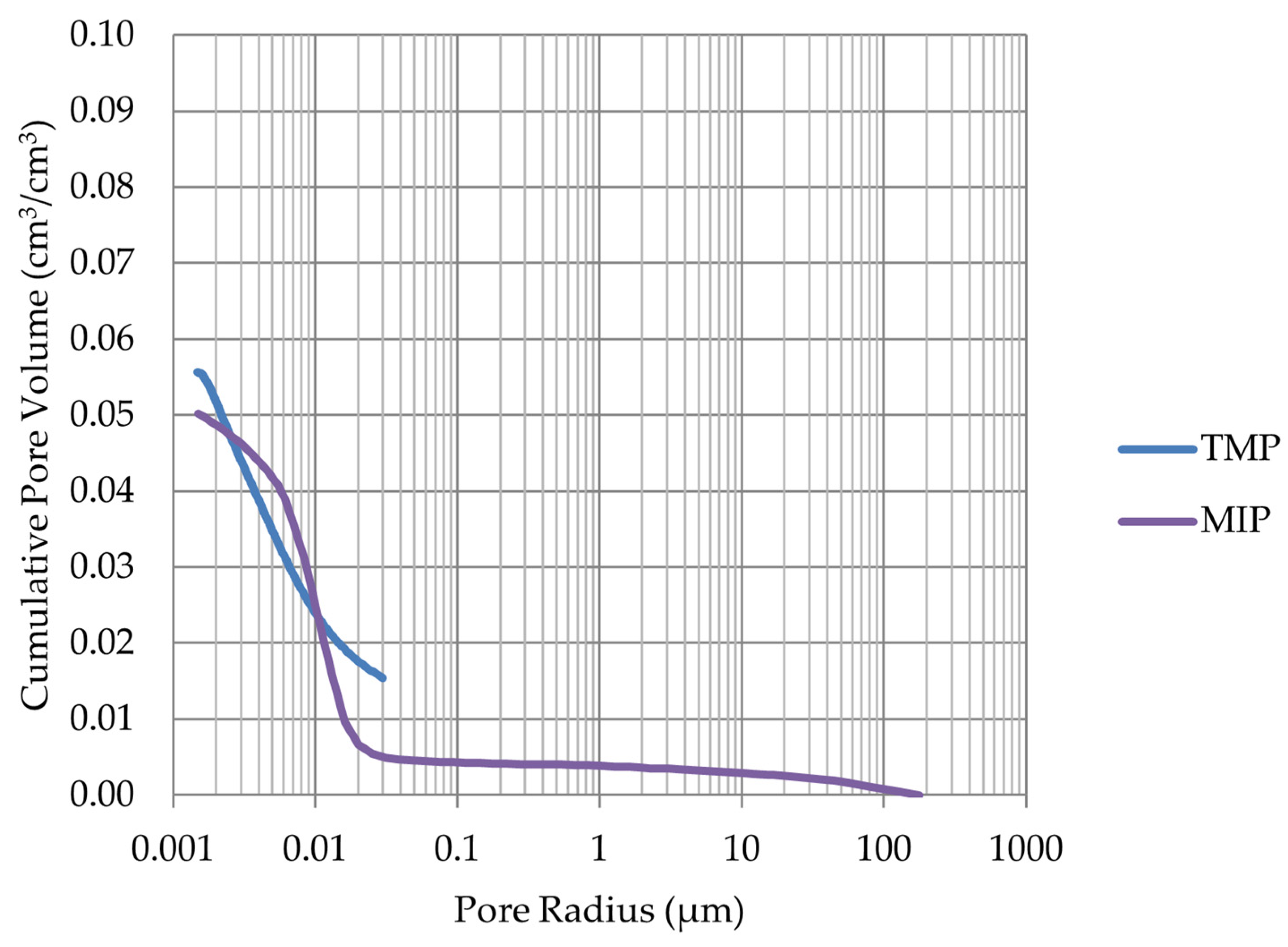
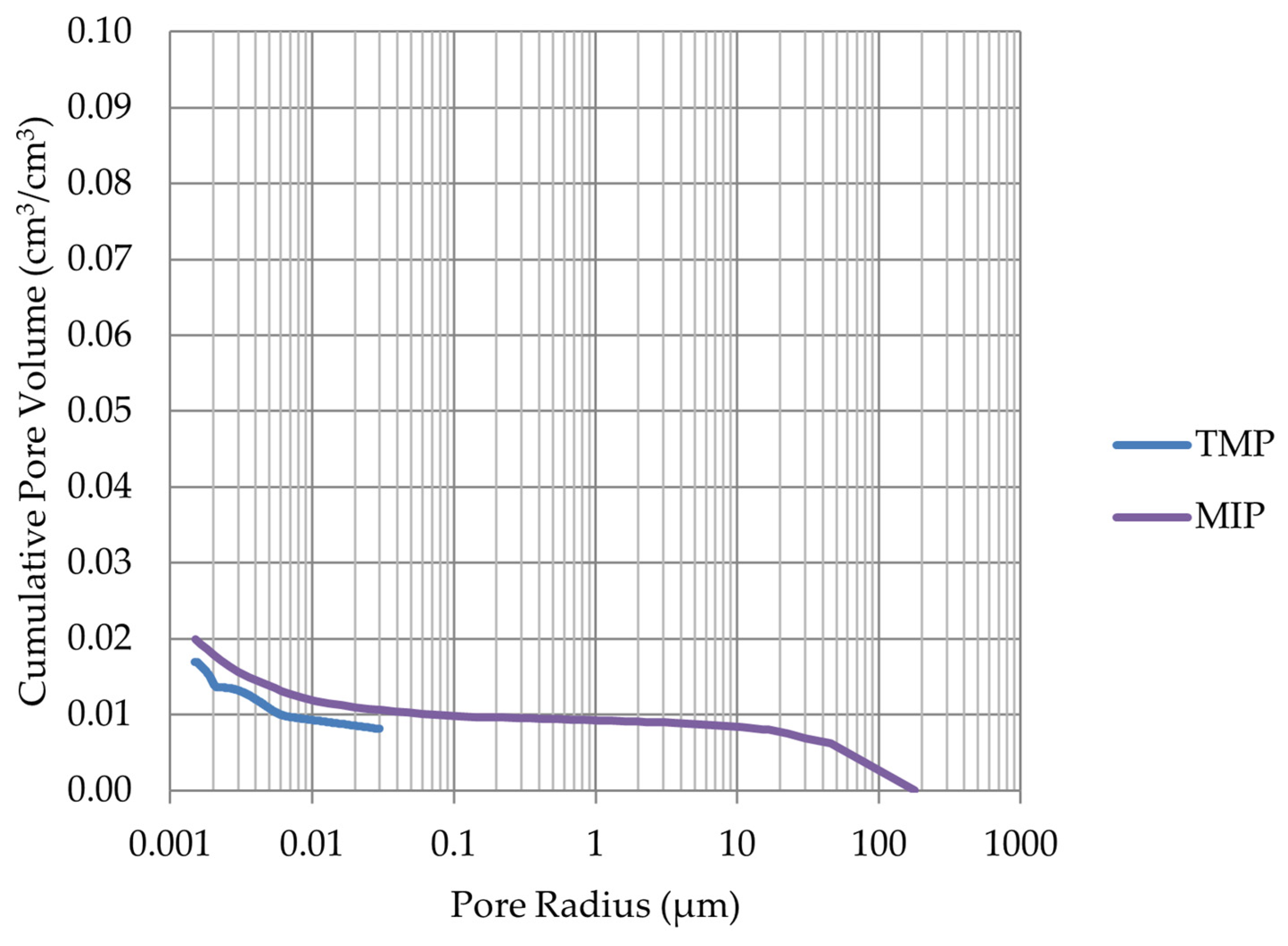
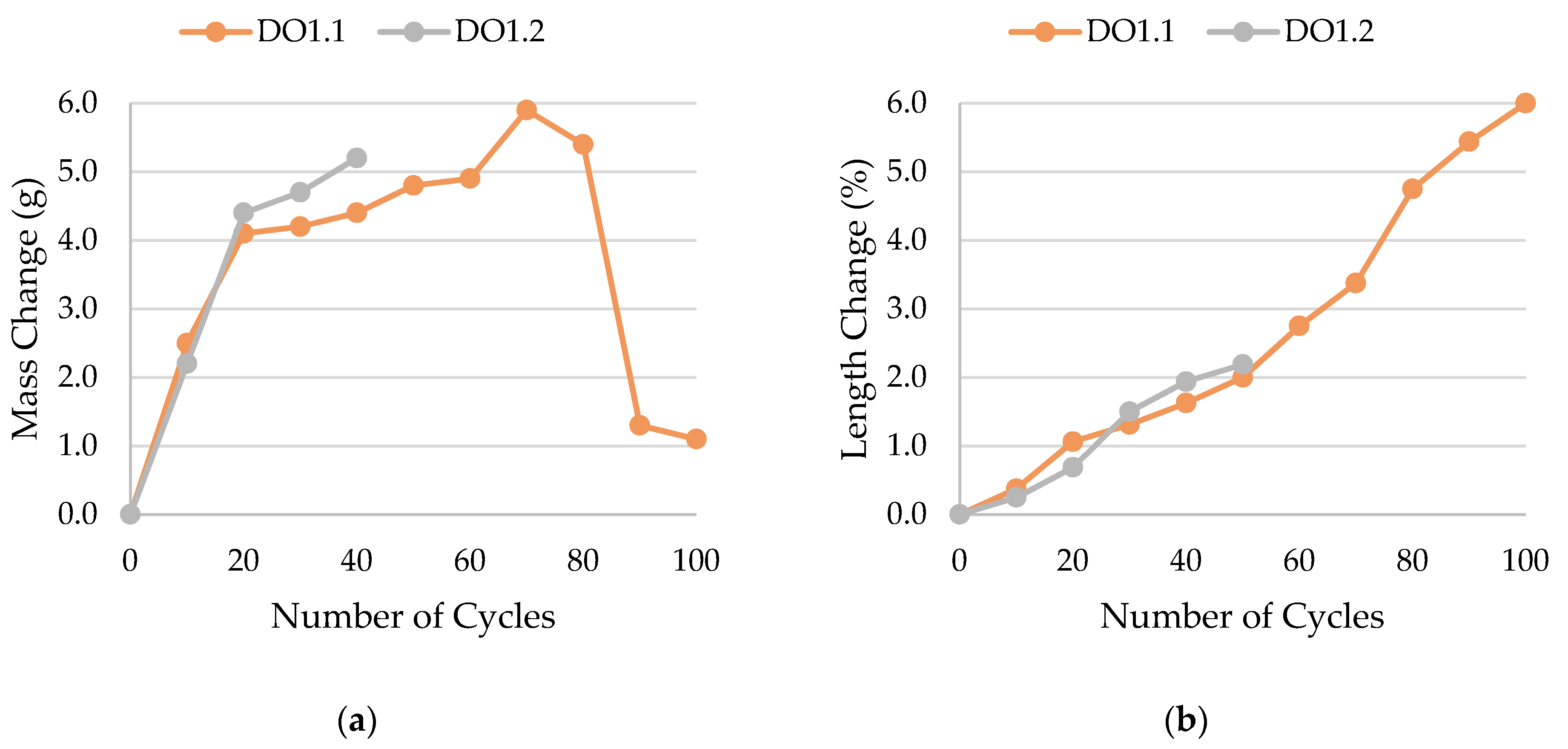
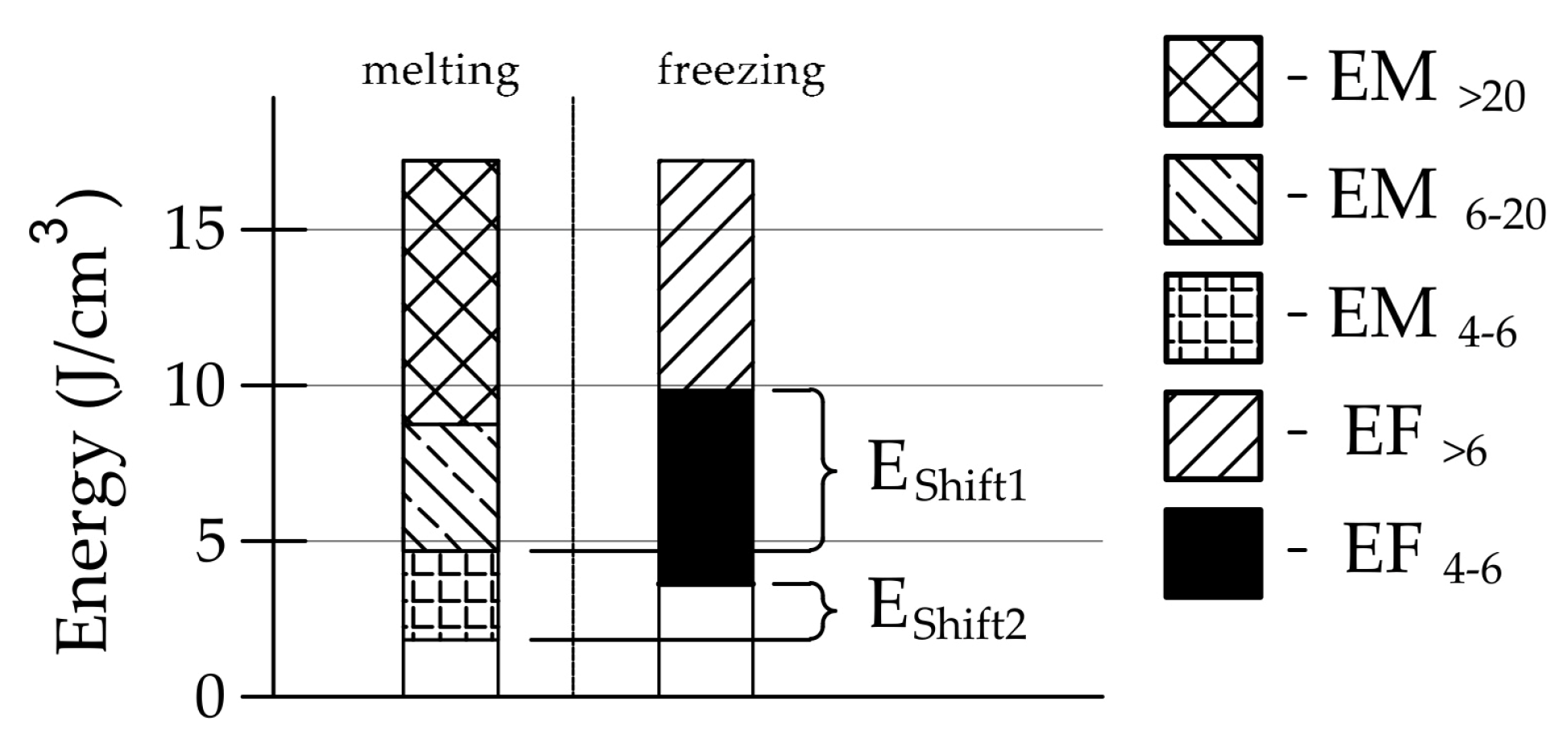
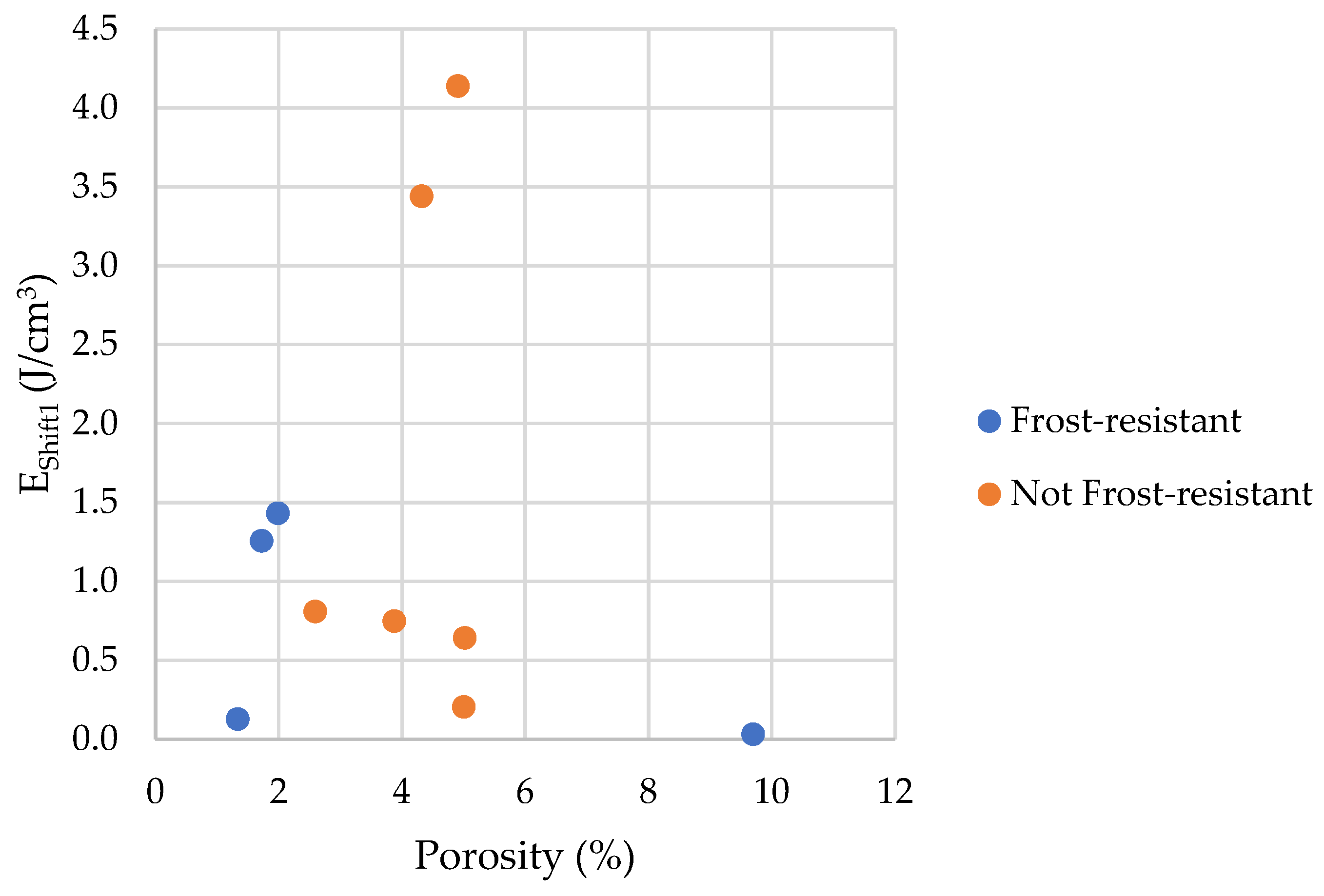
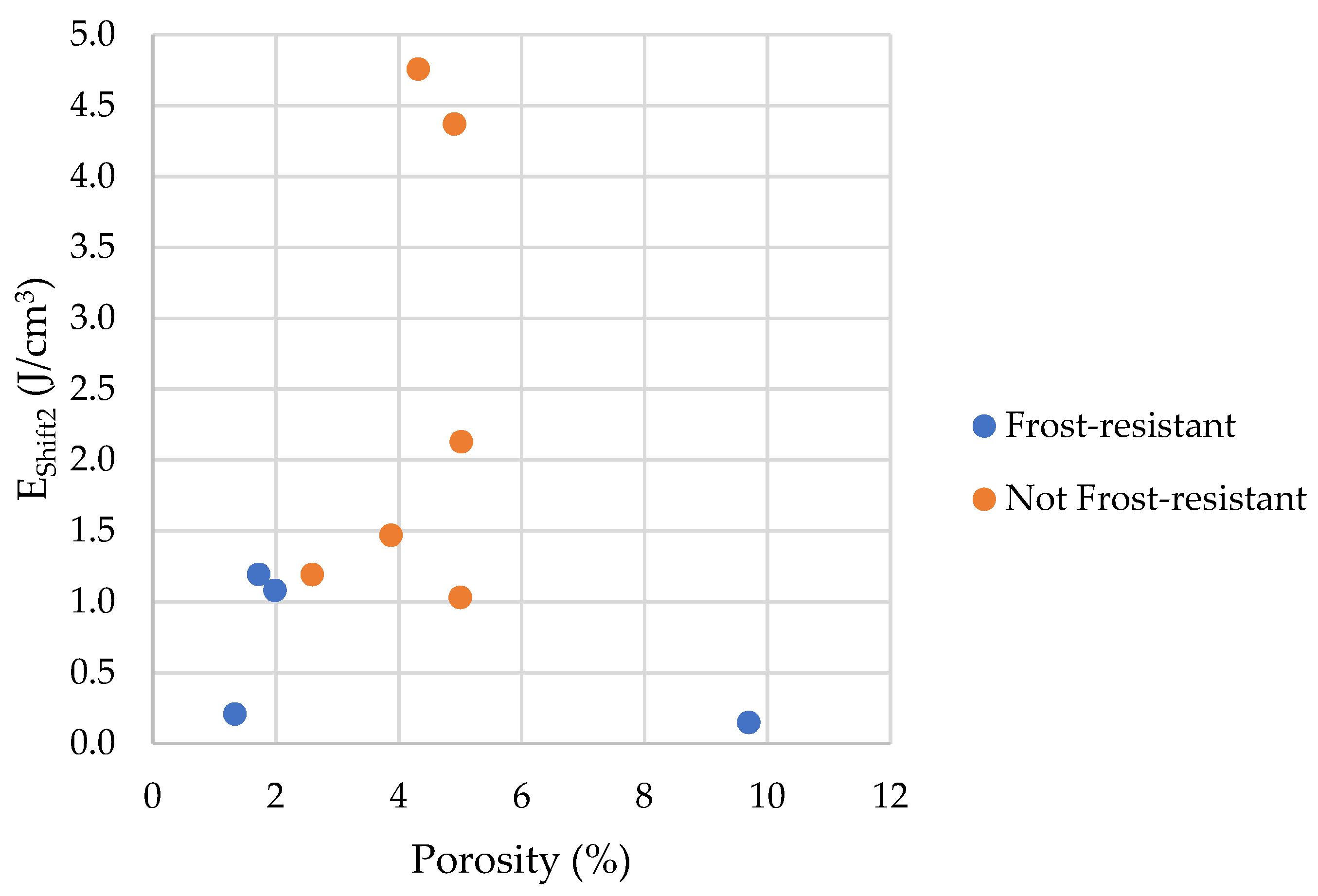
- Frost resistance research of rocks was conducted on cylindrical samples subjected to 100 freeze–thaw cycles. In the case of samples LIM2, LIM3, BA1, BA2, DO1, and DO4, visible cracks and damage on the samples were observed during frost resistance tests. For the DO1 and BA1 samples, this is expected to be due to a significant amount of water freezing in the temperature range from –9 °C to –20 °C (Figure 4, EF4–6 value). The EF4–6 value for all non-frost durable samples was higher than 0.68 J/cm3.
- The investigation confirms good frost resistance for rocks with porosity below 2% (LIM1, DO2, DO3). In the case of non-frost-resistant rocks, based on the analysis of test results from the differential scanning calorimetry and thermoporometry methods, a significant share of pores with a radius of less than 10 nm was found, with a larger value than 0.008 cm3/cm3. The LIM4 sample with a porosity of 9.70% has good frost resistance, which can be explained by it having a VR10 smaller than 0.008 cm3/cm3 and an insignificant amount of absorbed water during freeze–thaw cycles.
- The use of algorithms taking into account the thermal inertia of the DSC measuring system may be a useful tool for assessing the pore space of rocks and interpreting the obtained results. The scanning calorimetry method (DSC) and thermoporometry method (TMP) are valuable supplements to information about the frost resistance of rocks determined by commonly used methods. The use of these methods allowed us to expand our knowledge about the frost resistance of rocks, especially those with porosity in the range of 2–12%. The usefulness of the DSC and TMP methods in assessing the phase transitions of the tested materials was indicated.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DSC | differential scanning calorimetry method |
| EShift1 | the index of the influence of pore connections with a radius above 6 nm |
| EShift2 | the index of the influence of pore connections with a radius above 4 nm |
| ESUM | the total amount of water that undergoes a phase change in the DSC research |
| EF>6 | the total energy corresponding to the contamination of water in pores with a radius above 6 nm |
| EF4–6 | the total energy corresponding to the contamination of water in pores with a radius of 4 to 6 nm |
| EM4–6 | the total energy corresponding to the melting of ice in pores with a radius of 4 to 6 nm |
| EM6–20 | the total energy corresponding to the melting of ice in pores with a radius of 4 to 6 nm |
| EM>20 | the total energy corresponding to the melting of ice in pores with radii above 20 nm |
| M | molar mass of the liquid |
| MIP | mercury intrusion porosimetry method |
| NMR | nuclear magnetic resonance techniques |
| r* | critical size of the nuclei |
| rK | solid–liquid surface curvature |
| rp | pore radius |
| T | temperature of phase change |
| T0 | freezing temperature of bulk liquid |
| TMP | thermoporometry method |
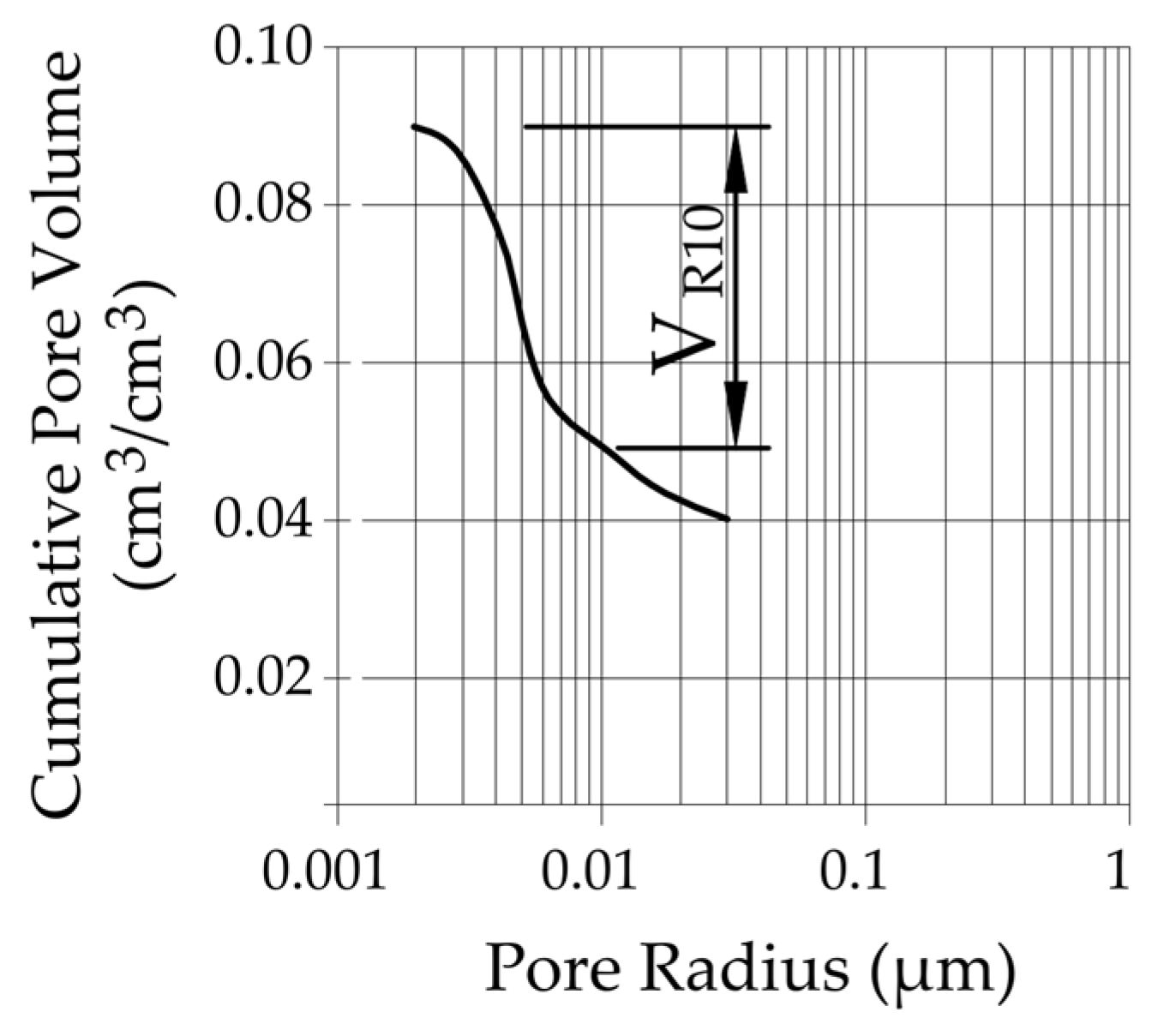
| VR10 | cumulative pore volume of pores with radii under 10 nm |
| γlc | solid–liquid surface curvature |
| ΔG | thermodynamic potential |
| ΔHfus | heat of fusion |
| Θ | contact angle |
| ρl | density of liquid |
References
- Nasri, F.; Boumezbeur, A.; Benavente, D. Influence of the petrophysical and durability properties of carbonate rocks on the deterioration of historic constructions in Tebessa (northeastern Algeria). Bull. Eng. Geol. Environ. 2019, 78, 3969–3981. [Google Scholar] [CrossRef]
- Fico, D.; Rizzo, D.; Casciaro, R.; Esposito Corcione, C. Historically accurate reconstruction of the materials and conservation technologies used on the facades of the artistic buildings in Lecce (Apulia, Italy). Materials 2022, 15, 3658. [Google Scholar] [CrossRef] [PubMed]
- Benavente, D. Why pore size is important in the deterioration of porous stones used in the built heritage. Macla 2011, 15, 41–42. Available online: https://rua.ua.es/dspace/handle/10045/19869 (accessed on 13 December 2023).
- Stelmaszczyk, G.; Świercz, P. Evaluation of calcareous aggregate usability for frost resistance concretes. Struct. Environ. 2009, 1, 5–10. [Google Scholar]
- Yates, T.; Mauko, A. Freeze thaw susceptibility of natural stone—Characterization of the mechanical strength and microstructure during frost cycling. In Proceedings of the DBMC International Conference on Durability of Building Materials and Components, Istanbul, Turkey, 11–14 May 2008. [Google Scholar]
- Skowera, K. Analysis of frost resistance and physical properties of devonian compact limestones deriving from one deposit. Struct. Environ. 2016, 8, 31–36. [Google Scholar]
- Karel, M.; Lund, D.B. Physical Principles of Food Preservation: Revised and Expanded, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar] [CrossRef]
- Klemm, P.; Przyrowska, A. Przejście fazowe woda-lód w materiałach kapilarno-porowatych przy ponadsorbcyjnym zawilgoceniu. In Proceedings of the XXVIII Konferencja Naukowa Komitetu Inżynierii Lądowej i Wodnej i Komitetu Nauki PZITB, Warsaw, Poland, 17–22 September 1982; pp. 79–83. (In Polish). [Google Scholar]
- Vali, G. Repeatability and randomness in heterogeneous freezing nucleation. Atmos. Chem. Phys. 2008, 8, 5017–5031. [Google Scholar] [CrossRef]
- Kozłowski, T. Some factors affecting supercooling and the equilibrium freezing point in soil-water systems. Cold Reg. Sci. Technol. 2009, 59, 25–33. [Google Scholar] [CrossRef]
- Khokhlov, A.; Valiullin, R.; Kärger, J.; Steinbach, F.; Feldhoff, A. Freezing and melting transitions of liquids in mesopores with ink-bottle geometry. New J. Phys. 2007, 9, 272. [Google Scholar] [CrossRef]
- Brun, M.; Lallemand, A.; Quinson, J.-F.; Eyraud, C. A new method for the simultaneous determination of the size and shape of pores: The thermoporometry. Thermochim. Acta 1977, 21, 59–88. [Google Scholar] [CrossRef]
- Clausse, D.; Wardhono, E.Y.; Lanoiselle, J.-L. Formation and determination of the ice formed in water dispersed in various materials. Colloids Surf. A Physiochem. Eng. Asp. 2014, 460, 519–526. [Google Scholar] [CrossRef]
- Fagerlund, G. Determination of pore-size distribution from freezing-point depression. Mater. Struct. 1973, 6, 215–225. [Google Scholar] [CrossRef]
- Wu, M.; Johannesson, B. Impact of sample saturation on the detected porosity of hardened concrete using low temperature calorimetry. Thermochim. Acta 2014, 580, 66–78. [Google Scholar] [CrossRef]
- Yamamoto, T.; Endo, A.; Inagi, Y.; Ohmori, T.; Nakaiwa, M. Evaluation of thermoporometry for characterization of mesoporous materials. J. Colloid Interface Sci. 2005, 284, 614–620. [Google Scholar] [CrossRef]
- Morishige, K.; Yasunaga, H.; Denoyel, R.; Wernert, V. Pore-blocking-controlled freezing of water in cagelike pores of KIT-5. J. Phys. Chem. C 2007, 111, 9488–9495. [Google Scholar] [CrossRef]
- PN-EN 1341:2013-05; Slabs and Natural Stone for External Paving—Requirements and Test Methods. Polish Committee for Standardization: Warsaw, Poland, 2013. (In Polish)
- PN-EN 1342:2013-05; Setts of Natural Stone for External Paving—Requirements and Test Methods. Polish Committee for Standardization: Warsaw, Poland, 2013. (In Polish)
- PN-EN 1343:2013-05; Kerbs of Natural Stone for External Paving—Requirements and Test Methods. Polish Committee for Standardization: Warsaw, Poland, 2013. (In Polish)
- Rusin, Z.; Owsiak, Z.; Stelmaszczyk, G.; Świercz, P. The Use of Differential Analysis of Deformations in the Diagnosis of Aggregates and Stones for Frost Durability; Development project N R04 006 10; Kielce University of Technology: Kielce, Poland, 2014. [Google Scholar]
- Rusin, Z.; Świercz, P.; Owsiak, Z. Effect of microstructure on frost durability of rock in the context of diagnostic needs. Proc. Eng. 2015, 108, 177–184. [Google Scholar] [CrossRef]
- PN-B-11204:1996; Stone Materials. Stone Elements—External Stud Slabs. Polish Committee for Standardization: Warsaw, Poland, 1996. (In Polish)
- Martínez-Martínez, J.; Benavente, D.; Gomez-Heras, M.; Marco-Castaño, L.; García-del-Cura, M.A. Non-linear decay of building stones during freeze-thaw weathering processes. Constr. Build. Mat. 2013, 38, 443–454. [Google Scholar] [CrossRef]
- Wessman, L. Studies on the Frost Resistance of Natural Stone. Ph.D. Thesis, Lund University, Lund, Sweden, 1997. [Google Scholar]
- Fan, L.; Fan, Y.; Xi, Y.; Gao, J. Spatially distributed damage in sandstone under stress-freeze-thaw coupling conditions. J. Rock Mech. Geotech. Eng. 2022, 14, 1910–1922. [Google Scholar] [CrossRef]
- Li, B.; Zhang, G.; Ma, W.; Liu, M.; Li, A. Damage mechanism of sandstones subjected to cycling freeze-thaw (FT) actions based on high-resolution computed tomography (CT). Bull. Eng. Geol. Environ. 2022, 81, 374. [Google Scholar] [CrossRef]
- Nicholson, D.T.; Nicholson, F.H. Physical deterioration of sedimentary rocks subjected to experimental freeze-thaw weathering. Earth Surf. Process. Landf. 2000, 25, 1295–1307. [Google Scholar] [CrossRef]
- Momeni, A.; Abdilor, Y.; Khanlari, G.R.; Heidari, M.; Sepahi, A.A. The effect of freeze-thaw cycles on physical and mechanical properties of granitoid hard rocks. Bull. Eng. Geol. Environ. 2016, 75, 1649–1656. [Google Scholar] [CrossRef]
- Benavente, D.; Martínez-Martínez, J.; Algozzini, G.; García del Cura, M.A. Influence of water transport and pore structure on the durability of porous building stones. In Proceedings of the Proceedings 10th IAEG International Congress, Nottingham, UK, 6–10 September 2006; IAEG2006 paper number 233. The Geological Society of London: London, UK, 2006. [Google Scholar]
- Skowera, K.; Rusin, Z. Differential analysis of volumetric strain method characterization in the context of phase change of water in carbonate rocks. Materials 2022, 15, 308. [Google Scholar] [CrossRef] [PubMed]
- Rusin, Z.; Świercz, P. Frost resistance of rock materials. Constr. Build. Mater. 2017, 148, 704–714. [Google Scholar] [CrossRef]
- Nartowska, E.; Kozłowski, T. The effect of the concentration of copper ions on the unfrozen water content in bentonites measured with the use of DSC method. Minerals 2022, 12, 632. [Google Scholar] [CrossRef]
- Ishikiriyama, K.; Todoki, M. Pore size distribution measurements of silica gels by means of differential scanning calorimetry. J. Coll. Interf. Sci. 1995, 171, 103–111. [Google Scholar] [CrossRef]
- Rasmussen, D.H.; MacKenzie, A.P. Clustering in supercooled water. J. Chem. Phys. 1973, 59, 5003–5013. [Google Scholar] [CrossRef]
- Landry, M.R. Thermoporometry by differential scanning calorimetry: Experimental consideration and applications. Thermochim. Acta 2005, 433, 27–55. [Google Scholar] [CrossRef]
- Beurroies, I.; Denoyel, R.; Llewellyn, P.; Rouquerol, J. A comparison between melting-solidification and capillary condensation hysteresis in mesoporous materials: Application to the interpretation of thermoporometry data. Thermochim. Acta 2004, 421, 11–18. [Google Scholar] [CrossRef]
- Bager, D.H.; Sellevold, E.J. Ice formation in hardened cement paste, Part I—Room temperature cured pastes with variable moisture contents. Cem. Concr. Res. 1986, 16, 709–720. [Google Scholar] [CrossRef]
- Jacobsen, S.; Sellevold, E.J.; Matala, S. Frost durability of high strength concrete: Effect of internal cracking on ice formation. Cem. Concr. Res. 1996, 6, 919–931. [Google Scholar] [CrossRef]
- Sun, Z.; Scherer, G.W. Pore size and shape in mortar by thermoporometry. Cem. Concr. Res. 2010, 40, 740–751. [Google Scholar] [CrossRef]
- Stępień, P.; Rusin, Z.; Skowera, K. Cement mortar porosity by modified analysis of differential scanning calorimetry records. Materials 2020, 13, 1080. [Google Scholar] [CrossRef] [PubMed]

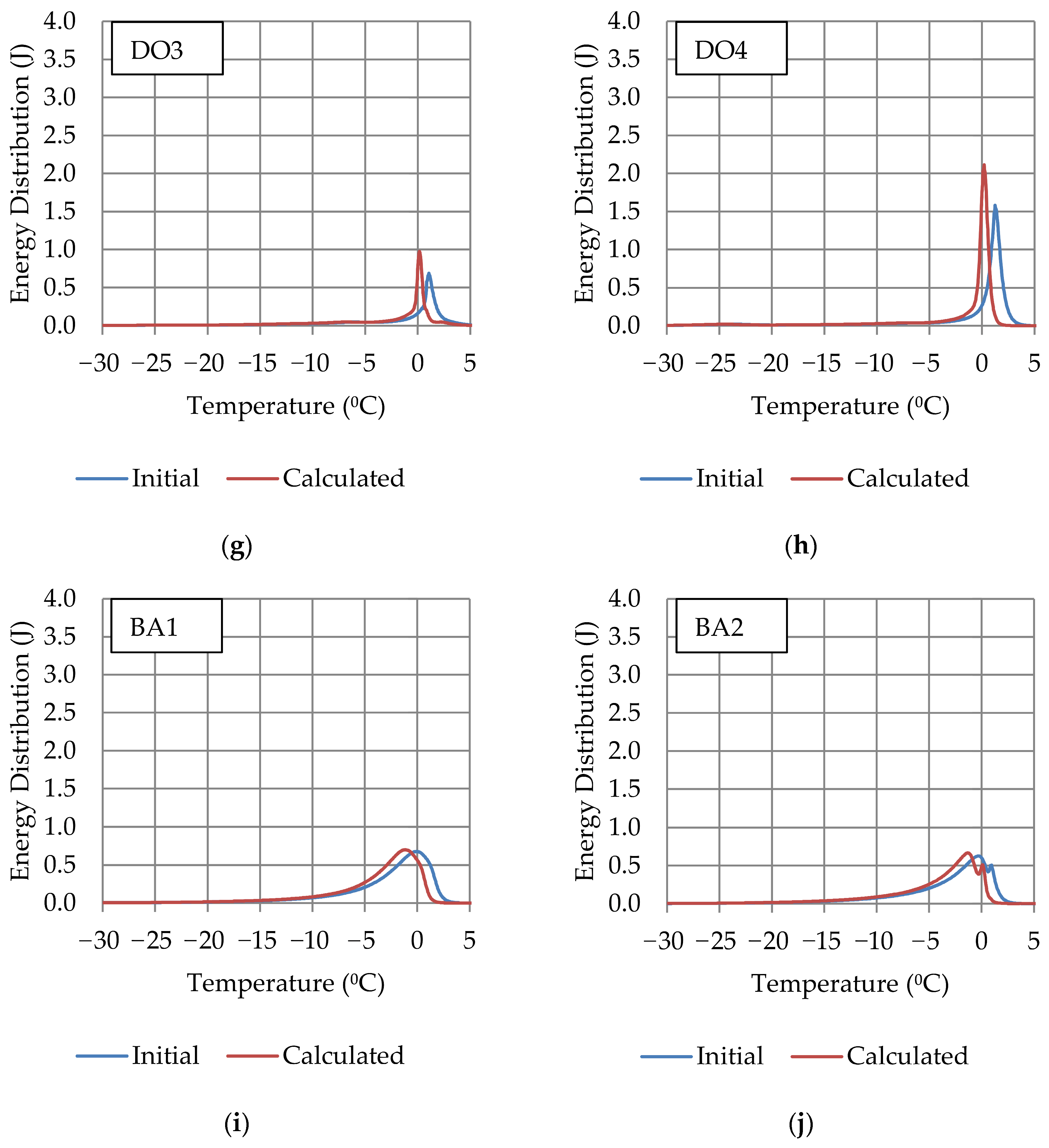








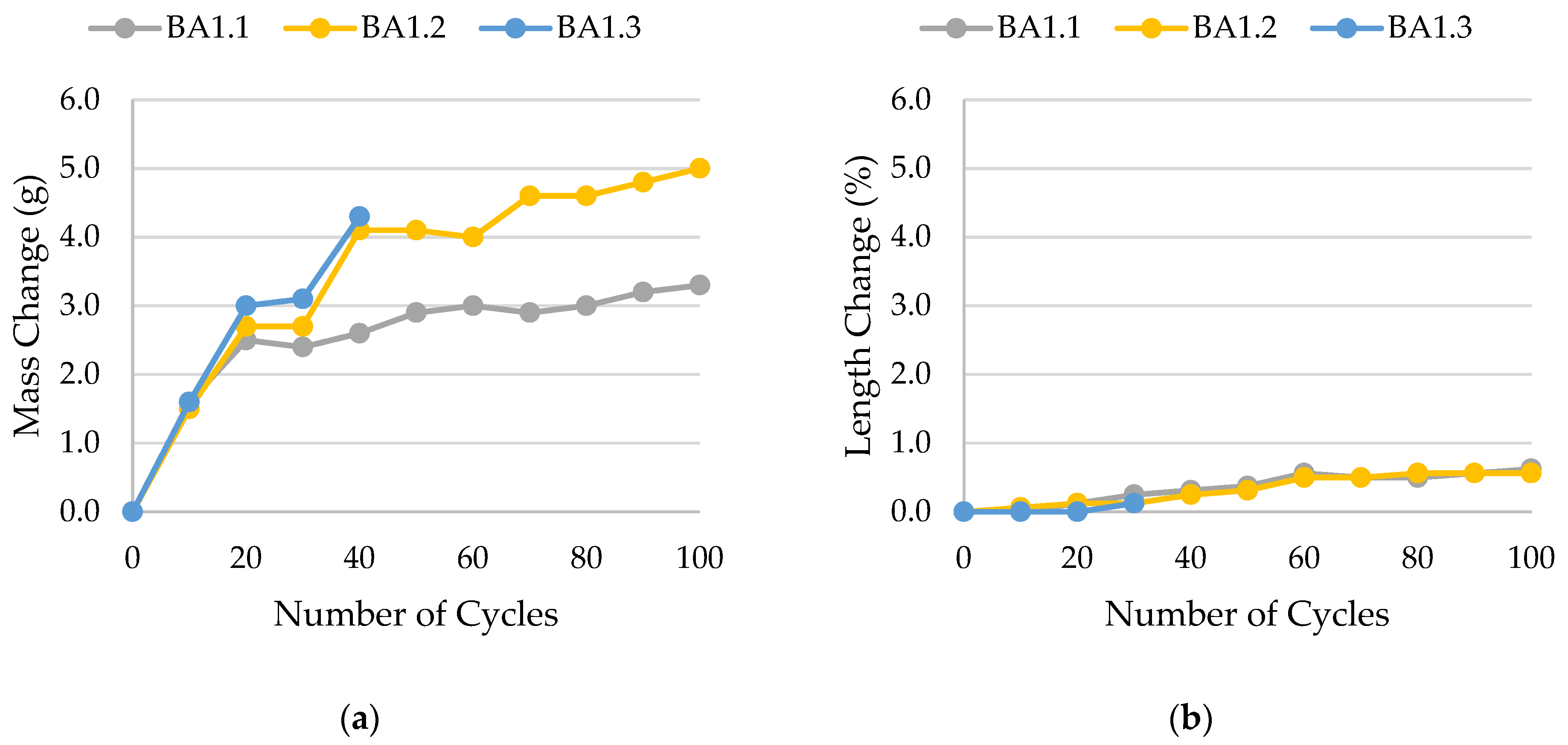


| Rock Code | Rock Type | Dominant Minerals |
|---|---|---|
| LIM1 | Devonian Limestone | 100% Calcite |
| LIM2 | Jurassic Limestone | 100% Calcite |
| LIM3 | Jurassic Limestone | 100% Calcite |
| LIM4 | Jurassic Limestone | 90% Calcite, 10% Iron Compounds |
| DO1 | Dolostone | 90% Dolomite, 10% Calcite |
| DO2 | Dolostone | 60% Dolomite, 35% Calcite |
| DO3 | Dolostone | 87% Dolomite, 13% Calcite |
| DO4 | Dolostone | 60% Dolomite, 35% Calcite |
| BA1 | Olivine Basalt | Plagioclase and Pyroxene, approx. 10% Hematite and Magnetite |
| BA2 | Olivine Basalt | Plagioclase and Pyroxene, <10% Hematite and Magnetite |
| Rock Code | Bulk Density (g/cm3) | Density (g/cm3) | Porosity (%) |
|---|---|---|---|
| LIM1 | 2.68 | 2.71 | 1.34 |
| LIM2 | 2.60 | 2.71 | 3.87 |
| LIM3 | 2.57 | 2.71 | 5.00 |
| LIM4 | 2.44 | 2.70 | 9.70 |
| DO1 | 2.70 | 2.84 | 5.02 |
| DO2 | 2.71 | 2.77 | 1.99 |
| DO3 | 2.79 | 2.84 | 1.72 |
| DO4 | 2.77 | 2.84 | 2.59 |
| BA1 | 2.81 | 2.94 | 4.32 |
| BA2 | 2.80 | 2.94 | 4.91 |
| Rock Code | Cumulative Pore Volume VR10 (cm3/cm3) | Information about Frost Resistance |
|---|---|---|
| LIM1 | 0.0027 | frost-resistant |
| LIM2 | 0.0145 | not frost-resistant, rapid decay after 90 freeze–thaw cycles |
| LIM3 | 0.0082 | not frost-resistant, rapid decay after 90 freeze–thaw cycles |
| LIM4 | 0.0046 | frost-resistant |
| DO1 | 0.0317 | not frost-resistant, gradual decay |
| DO2 | 0.0077 | frost-resistant |
| DO3 | 0.0080 | frost-resistant |
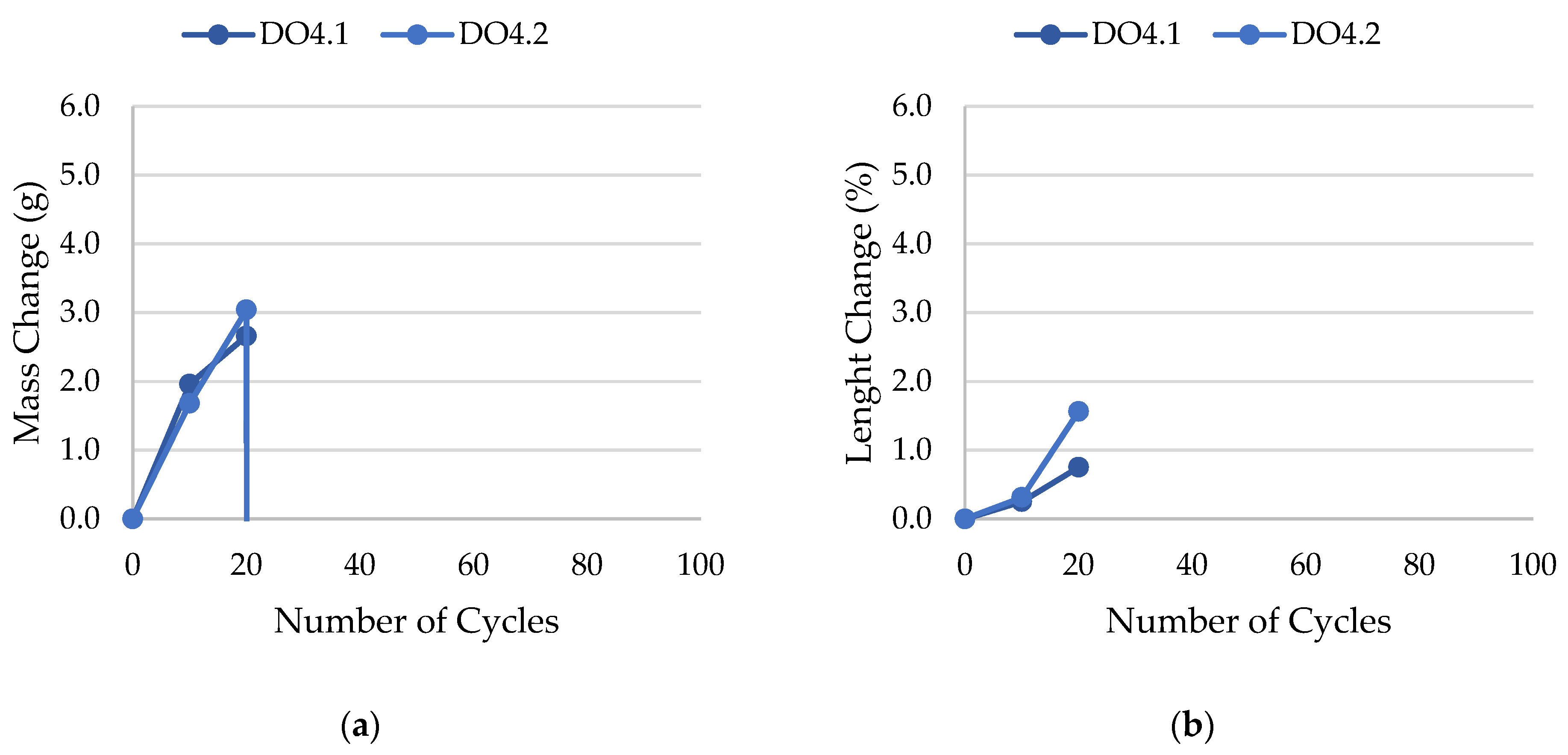
| DO4 | 0.0095 | not frost-resistant, gradual decay |
| BA1 | 0.0174 | not frost-resistant, gradual decay |
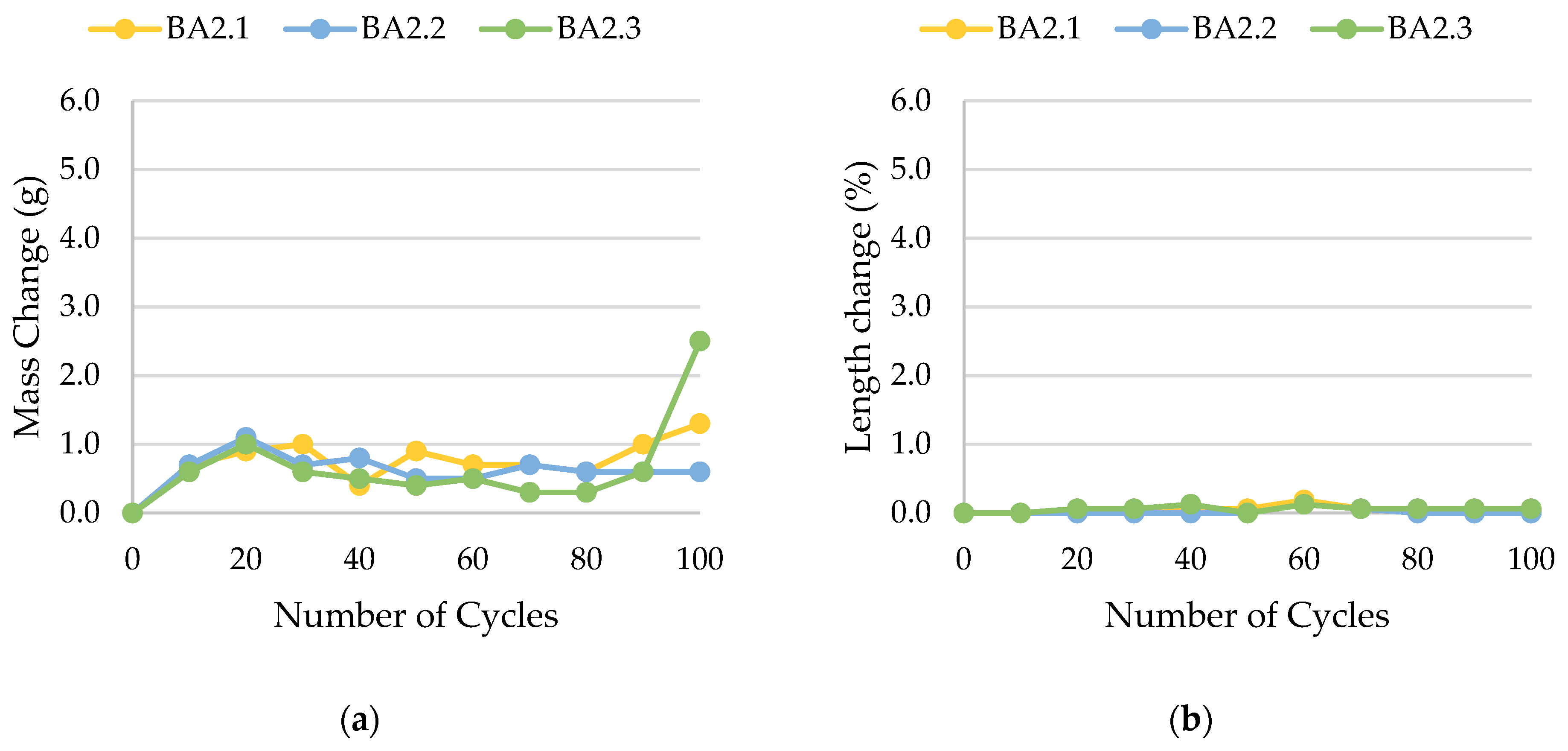
| BA2 | 0.0174 | not frost-resistant, rapid decay after 90 freeze–thaw cycles |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stępień, P.; Spychał, E. The Use of Thermoporometry in the Study of Frost Resistance of Rocks. Materials 2024, 17, 620. https://doi.org/10.3390/ma17030620
Stępień P, Spychał E. The Use of Thermoporometry in the Study of Frost Resistance of Rocks. Materials. 2024; 17(3):620. https://doi.org/10.3390/ma17030620
Chicago/Turabian StyleStępień, Piotr, and Edyta Spychał. 2024. "The Use of Thermoporometry in the Study of Frost Resistance of Rocks" Materials 17, no. 3: 620. https://doi.org/10.3390/ma17030620
APA StyleStępień, P., & Spychał, E. (2024). The Use of Thermoporometry in the Study of Frost Resistance of Rocks. Materials, 17(3), 620. https://doi.org/10.3390/ma17030620






