Material Design of Porous Hydroxyapatite Ceramics via Inverse Analysis of an Estimation Model for Bone-Forming Ability Based on Machine Learning and Experimental Validation of Biological Hard Tissue Responses
Abstract
1. Introduction
2. Materials and Methods
2.1. Dataset
2.2. Construction of Models and Verification of Predictability
2.3. Feature Importance
- Calculate the correlation coefficients for all the features.
- Calculate the absolute correlation coefficient for all the feature and set the coefficient to zero when there is no correlation.
- During CV, for n = 1, 2, ⋯, N, where N is the number of CV folds, the following procedures were performed using the training and validation data (VD) at each fold.
- 4.
- Integrate the y values estimated during the CV for VD1, VD2, ⋯, and VDN and calculate the reference score, rscv, with the integrated y. This score represents the determination coefficient, r2, for a regressor.
- 5.
- Integrate the y estimated during the CV for CVD1,I,j, CVD2,I,j, ⋯, and CVDN,I,j and calculate the score, scvi,j, with the actualy.
- 6.
- Calculate the importance, CVPFIi, for the ith feature as follows:
2.4. Materials Designs for New Bioceramics by Inverse Analysis of the Model
2.5. Fabrication of Materials
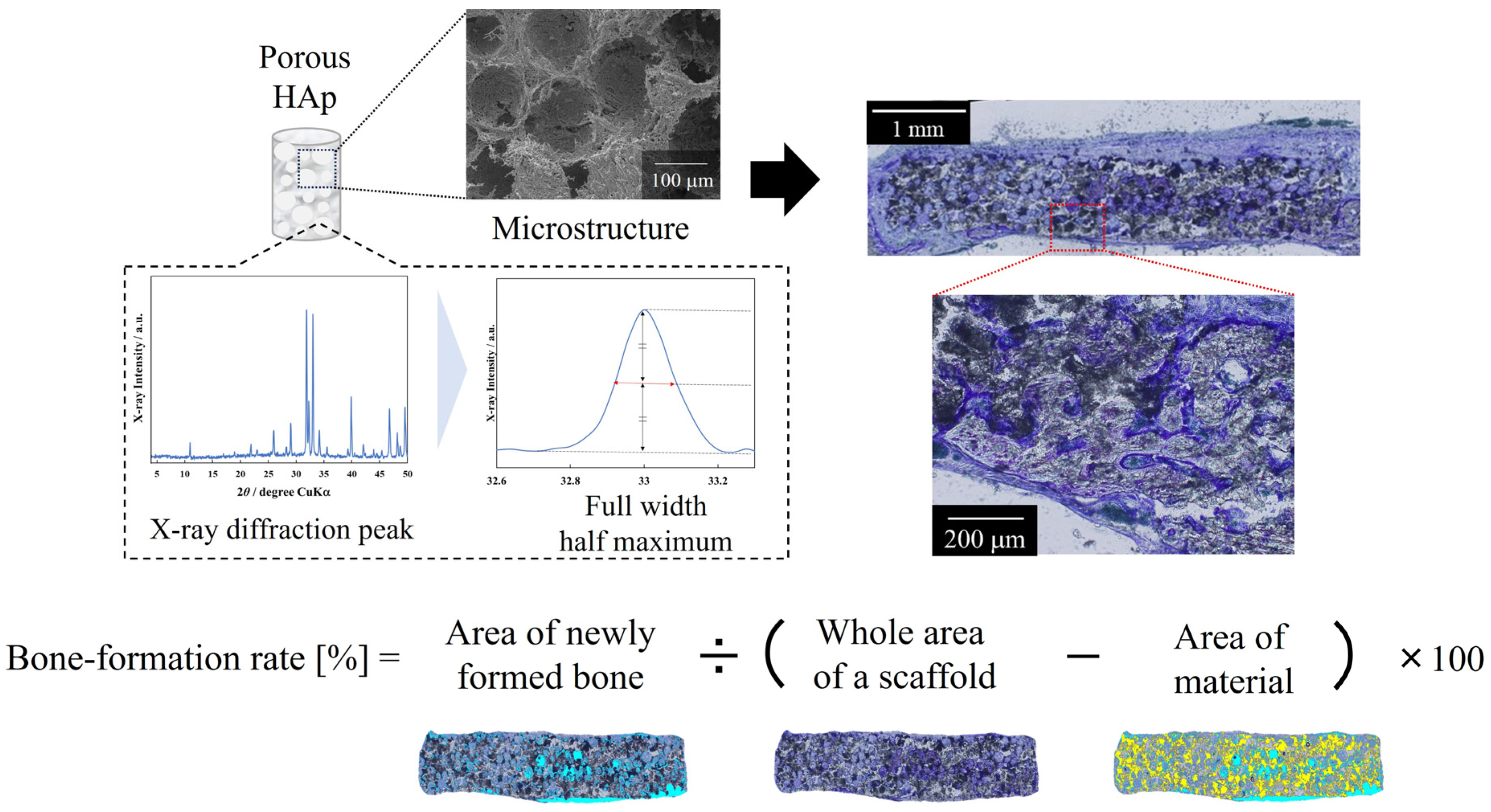
2.6. Material Characterization and Validation
2.7. In Vivo Evaluation of Materials Using a Pig Tibia Model and Validation
3. Results and Discussion
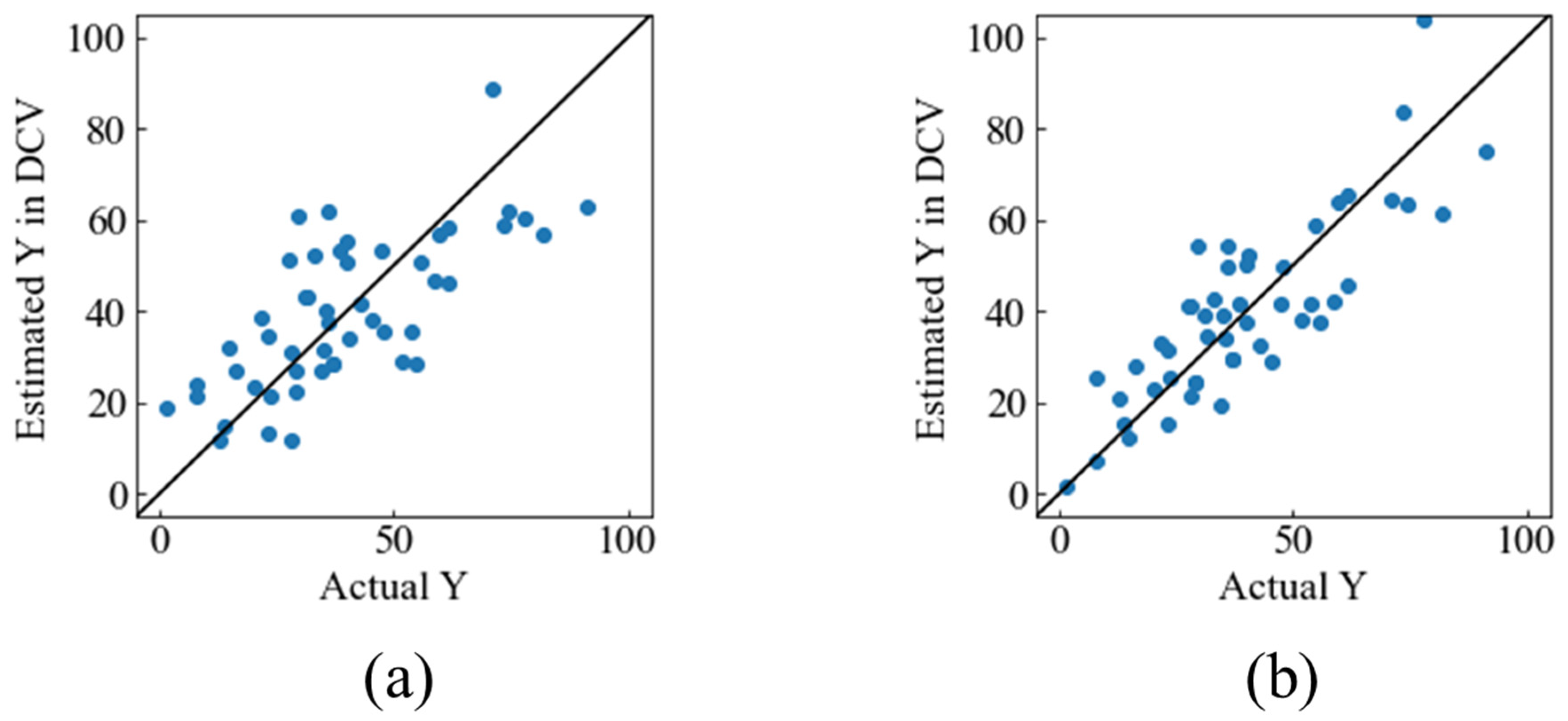
3.1. Construction of Model 1
3.2. Construction of Model 2
3.3. Feature Importance
3.4. Materials Designs for New Bioceramics by Inverse Analysis of Model
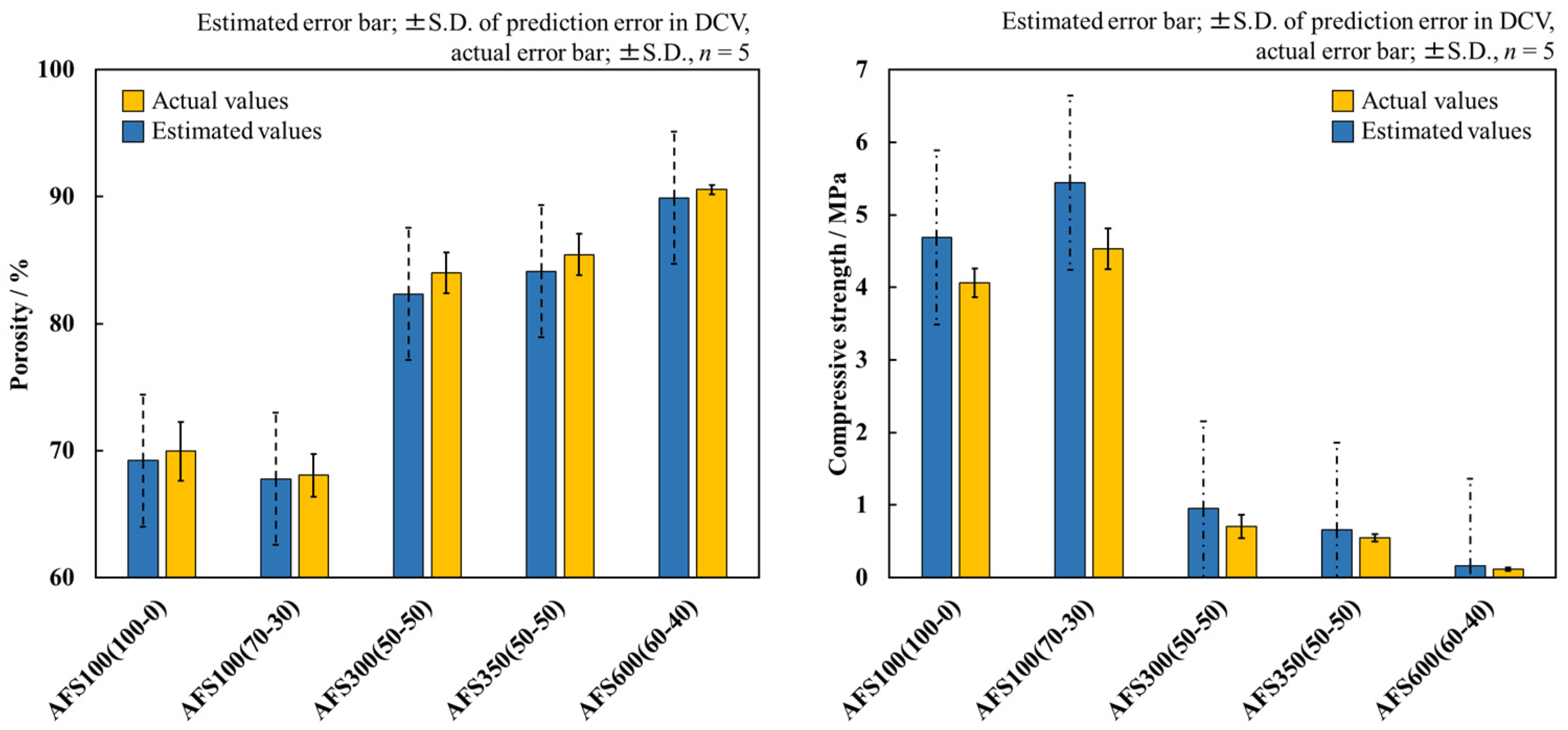
3.5. Experimental Validation of Material Properties
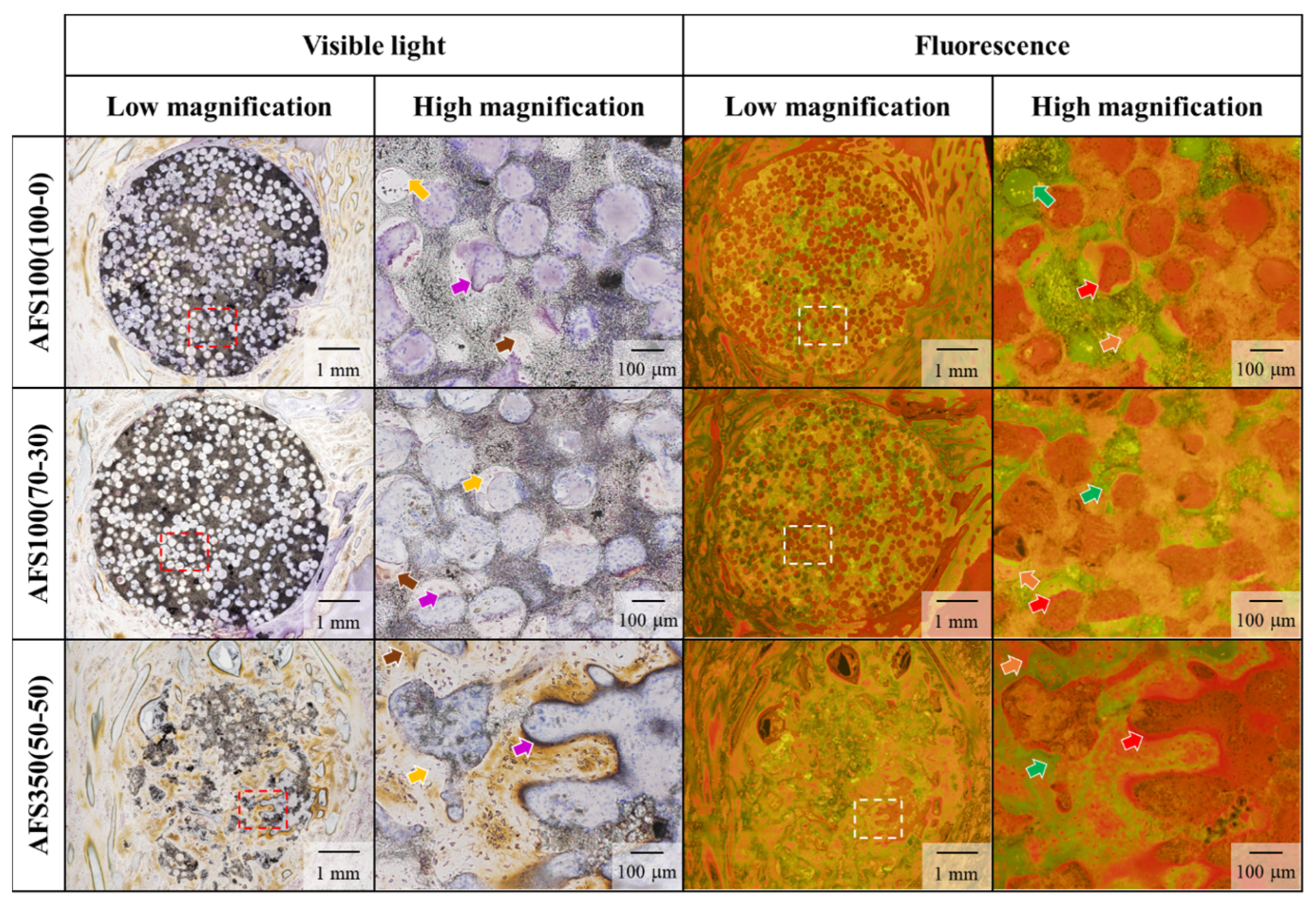
3.6. In Vivo Evaluation of Materials and Validation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alajlouni, A.D.; Bliuc, D.; Tran, S.T.; Blank, D.R.; Center, R.J. Muscle strength and physical performance contribute to and improve fracture risk prediction in older people: A narrative review. Bone 2023, 172, 116755. [Google Scholar] [CrossRef] [PubMed]
- Gibon, E.; Lu, Y.L.; Nathan, K.; Goodman, B.S. Inflammation, aging, and bone regeneration. J. Orthop. Translat. 2017, 10, 28–35. [Google Scholar] [CrossRef]
- St John, A.T.; Vaccaro, R.A.; Sah, P.A.; Schaefer, M.; Berta, C.S.; Albert, T.; Hilibrand, A. Physical and monetary costs associated with autogenous bone graft harvesting. Am. J. Orthop. 2003, 32, 18–23. [Google Scholar] [PubMed]
- Gristina, G.A. Biomaterial-centered infection: Microbial adhesion versus tissue integration. Science 1987, 237, 1588–1595. [Google Scholar] [CrossRef] [PubMed]
- Banwart, C.J.; Asher, A.M.; Hassanein, S.R. Iliac crest bone graft harvest donor site morbidity. A statical evaluation. Spine 1995, 20, 1055–1060. [Google Scholar] [CrossRef]
- Jiao, C.; Xie, D.; He, Z.; Liang, H.; Shen, L.; Yang, Y.; Tian, Z.; Wu, G.; Wang, C. Additive manufacturing of bio-inspired ceramic bone scaffolds: Structural design, mechanical properties and biocompatibility. Mater. Des. 2022, 217, 110610. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Yamasaki, N.; Hirao, M.; Nanno, K.; Sugiyasu, K.; Tamai, N.; Hashimoto, N.; Yoshikawa, H.; Myoui, A. A comparative assessment of synthetic ceramic bone substitutes with different composition and microstructure in rabbit femoral condyle model. J. Biomed. Mater. Res. Part B 2009, 91, 788–798. [Google Scholar] [CrossRef]
- Bohner, M.; Döbelin, N.; Santoni, B.L.G. β-tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef]
- von Doernberg, M.C.; von Rechenberg, B.; Bohner, M.; Grünenfelder, S.; van Lenthe, G.H.; Müller, R.; Gasser, B.; Mathys, R.; Baroud, G.; Auer, J. In vivo behavior of calcium phosphate scaffolds with four different pore sizes. Biomaterials 2006, 27, 5186–5198. [Google Scholar] [CrossRef]
- Dias, M.; Fernandes, P.; Guedes, J.; Hollister, S. Permeability analysis of scaffolds for bone tissue engineering. J. Biomech. 2012, 45, 938–944. [Google Scholar] [CrossRef]
- Shibahara, K.; Hayashi, K.; Nakashima, Y.; Ishikawa, K. Effects of channels and micropores in honeycomb scaffolds on the reconstruction of segmental bone defects. Bioact. Mater. 2022, 6, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, M.; Porter, E.A.; Best, M.S.; Bonefield, W. Ultrastructural observation of single-crystal apatite fibres. Biomaterials 2005, 26, 3427–3433. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, M.; Ueno, H.; Itatani, K.; Okada, I. Synthesis of calcium-deficient apatite fibers by a homogeneous precipitation method and their characterizations. J. Eur. Ceram. Soc. 2006, 26, 501–507. [Google Scholar] [CrossRef]
- Yamada, Y.; Inui, T.; Kinoshita, Y.; Shigemitsu, Y.; Honda, M.; Nakano, K.; Matsunari, H.; Nagaya, M.; Nagashima, H.; Aizawa, M. Silicon-containing apatite fiber scaffolds with enhanced mechanical property express osteoinductivity and high osteoconductivity. J. Asian Ceram. Soc. 2019, 7, 101–108. [Google Scholar] [CrossRef]
- Shigemitsu, Y.; Nagashima, H.; Matsunari, H.; Aizawa, M. In vivo evaluation of calcium-phosphate ceramics with highly-interconnected pores using porcine tibia defect model. Solid State Phenom. 2022, 340, 113–117. [Google Scholar] [CrossRef]
- Yokota, T.; Miki, T.; Honda, M.; Ikeda, T.; Ishii, K.; Matsumoto, M.; Aizawa, M. Fabrication and biological evaluation of hydroxyapatite ceramics including bone minerals. J. Ceram. Soc. Jpn. 2018, 126, 99–108. [Google Scholar] [CrossRef]
- Fontana, F.; Figueiredo, P.; Martins, J.P.; Santos, H.A. Requirements for animal experiments: Problems and challenges. Small 2020, 17, e2004182. [Google Scholar] [CrossRef]
- Kaufmann, K.; Maryanovsky, D.; Mellor, M.W.; Zhu, C.; Rosengarten, S.A.; Oses, C.; Toher, C.; Curtarolo, S.; Vecchio, S.K. Discovery of high-entropy ceramics via machine learning. Npj Comput. Mater. 2020, 6, 42. [Google Scholar] [CrossRef]
- Huang, D.; Li, Z.; Wang, K.; Zhou, H.; Zhao, X.; Zhang, R.; Wu, J.; Liang, J.; Zhao, L. Probing the efffect of photovoltaic material on Voc in ternary polymer solar cells with non-fullerene acceptors by machine learning. Polymers 2023, 15, 2954. [Google Scholar] [CrossRef]
- Guo, Y.; Rui, S.; Xu, W.; Sun, C. Machine learning method for fatigue strength prediction of nickel-based superalloy with various influencing factors. Materials 2023, 16, 46. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Y.; Dai, Z.; Yang, F.; Fallahpour, A.; Nasiri-Tabrizi, B. Structual features modeling of substituted hydroxyapatite nanopowders as bone fillers via machine learning. Ceram. Int. 2021, 47, 9034–9047. [Google Scholar] [CrossRef]
- Kwaria, J.R.; Mondarte, Q.A.E.; Tahara, H.; Chang, R.; Hayashi, T. Data-driven prediction of protein adsorption on self-assembled monolayers toward material screening and design. ACS Biomater. Sci. Eng. 2020, 6, 4949–4956. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Entezari, A.; Zheng, K.; Fang, J.; Zreiqat, H.; Steven, G.P.; Swain, M.V.; Li, Q. A machine learning-based multiscale model to predict bone formation in scaffolds. Nat. Comput. Sci. 2021, 1, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Motojima, K.; Shiratsuchi, R.; Suzuki, K.; Aizawa, M.; Kaneko, H. Machine learning model for predicting the material properties and bone formation rate and direct inverse analysis of the model for new synthesis conditions of bioceramics. Ind. Eng. Chem. Res. 2023, 62, 5898–5906. [Google Scholar] [CrossRef]
- Shimizu, N.; Kaneko, H. Direct inverse analysis based on gaussian mixture regression for multiple objective variables in material design. Mater. Des. 2020, 196, 109168. [Google Scholar] [CrossRef]
- Bishop, C.M. Pattern Recognition and Machine Learning; Springer: New York, NY, USA, 2006. [Google Scholar]
- Filzmoser, P.; Liebmann, B.; Varmuza, K. Repeated Double cross validation. J. Chemom. 2009, 23, 160–171. [Google Scholar] [CrossRef]
- Kaneko, H. Cross-validated permutation feature importance considering correlation between features. Anal. Sci. Adv. 2022, 3, 278–287. [Google Scholar] [CrossRef]
- Rabiei, M.; Palevicius, A.; Monshi, A.; Nasiri, S.; Vilkauskas, A.; Janusas, G. Comparing methods for calculating nano crystal size of natural hydroxyapatite using X-ray diffraction. Nanomaterials 2020, 10, 1627. [Google Scholar] [CrossRef]
- Tetko, V.I.; Sushko, I.; Pandey, K.A.; Zhu, H.; Tropsha, A.; Papa, E.; Oberg, T.; Todeschini, R.; Fourches, D.; Varnek, A. Critical assessment of QSAR models of environmental toxicity against tetrahymena pyriformis: Focusing on applicability domain and overftting by variable selection. J. Chem. Inf. Model. 2008, 48, 1733–1746. [Google Scholar] [CrossRef]
- Cover, T.; Hart, P. Nearest neighbor pattern classification. IEEE Trans. Inf. Theory 1967, 13, 21–27. [Google Scholar] [CrossRef]
- Kasten, P.; Beyen, I.; Niemeyer, P.; Luginbühl, R.; Bohner, M.; Richter, W. Porosity and pore size of β-tricalcium phosphate scaffold can influence protein production and osteogenic differentitation of human mesenchymal stem cells: An in vitro and in vivo study. Acta Biomater. 2008, 4, 1904–1915. [Google Scholar] [CrossRef]




| Variables | Variable Names | Variables | Variable Names |
|---|---|---|---|
| X1 | Ca(OH)2/mol·dm−3 | X1 | 20 μm CB rate/% |
| MgCl2·6H2O/mol·dm−3 | Molding pressure/MPa | ||
| NaCl/mol·dm−3 | Firing tempreture/°C | ||
| KCl/mol·dm−3 | Firing atmosphere | ||
| (NH4)2CO3/mol·dm−3 | (0: air, 1: steam, 2: steam in carbonate gas) | ||
| H3PO4/mol·dm−3 | Y1 | Porosity/% | |
| NH4F/mol·dm−3 | Compressive strength/MPa | ||
| Ball mill grinting time/h | Half-width of 4 peaks/degree | ||
| (NH4)2HPO4/mol·dm−3 | X2 | Implantation periods/weeks | |
| Si(OC2H5)4/mol·dm−3 | Implantation animals | ||
| Heating tempreture/°C | (0: pig, 1: rat) | ||
| Heating time/h | Vascular endothelial growth factor adding | ||
| Amount of carbon beads (CB)/mass% | (0: without, 1: with) | ||
| 150 μm CB ratio/% | Y2 | Bone-formation rate/% |
| Material Properties | r2DCV | RMSEDCV |
|---|---|---|
| Porosity | 0.933 | 3.02 |
| Compressive strength | 0.745 | 0.12 |
| FWHM211 | 0.935 | 0.02 |
| FWHM220 | 0.996 | 0.01 |
| Feature | r2DCV | RMSEDCV |
|---|---|---|
| Without FWHM | 0.497 | 14.33 |
| With FWHM | 0.689 | 11.30 |
| X1 | Y1 | X2 | Y2 | ||||
|---|---|---|---|---|---|---|---|
| Amount of CB [mass%] | 150 μm CB Rate [%] | Porosity [%] | Compressive Strength [MPa] | Crystalline Phase | Implantation Weeks | Implantation Animal | Bone-Formation Rate [%] |
| 100 | 100 | 70.0 | 4.7 | HAp | 12 | Pig | 37.0 |
| 100 | 70 | 68.1 | 5.4 | 36.8 | |||
| 300 | 50 | 84.0 | 0.9 | 49.6 | |||
| 350 | 50 | 85.4 | 0.7 | 51.6 | |||
| 600 | 60 | 90.5 | 0.2 | 59.4 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horikawa, S.; Suzuki, K.; Motojima, K.; Nakano, K.; Nagaya, M.; Nagashima, H.; Kaneko, H.; Aizawa, M. Material Design of Porous Hydroxyapatite Ceramics via Inverse Analysis of an Estimation Model for Bone-Forming Ability Based on Machine Learning and Experimental Validation of Biological Hard Tissue Responses. Materials 2024, 17, 571. https://doi.org/10.3390/ma17030571
Horikawa S, Suzuki K, Motojima K, Nakano K, Nagaya M, Nagashima H, Kaneko H, Aizawa M. Material Design of Porous Hydroxyapatite Ceramics via Inverse Analysis of an Estimation Model for Bone-Forming Ability Based on Machine Learning and Experimental Validation of Biological Hard Tissue Responses. Materials. 2024; 17(3):571. https://doi.org/10.3390/ma17030571
Chicago/Turabian StyleHorikawa, Shota, Kitaru Suzuki, Kohei Motojima, Kazuaki Nakano, Masaki Nagaya, Hiroshi Nagashima, Hiromasa Kaneko, and Mamoru Aizawa. 2024. "Material Design of Porous Hydroxyapatite Ceramics via Inverse Analysis of an Estimation Model for Bone-Forming Ability Based on Machine Learning and Experimental Validation of Biological Hard Tissue Responses" Materials 17, no. 3: 571. https://doi.org/10.3390/ma17030571
APA StyleHorikawa, S., Suzuki, K., Motojima, K., Nakano, K., Nagaya, M., Nagashima, H., Kaneko, H., & Aizawa, M. (2024). Material Design of Porous Hydroxyapatite Ceramics via Inverse Analysis of an Estimation Model for Bone-Forming Ability Based on Machine Learning and Experimental Validation of Biological Hard Tissue Responses. Materials, 17(3), 571. https://doi.org/10.3390/ma17030571






