Preparation and Characterization of Composites Based on ABS Modified with Polysiloxane Derivatives
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- Polymethylhydrosiloxane, trimethylsilyl-terminated, 15–25 cSt from Gelest (Morrisville, PA, USA); styrene (STYR), octadecene (OD), toluene, chloroform-d, Karstedt’s catalyst xylene solution from Merck KGaA (Darmstadt, Germany); vinyltrimethoxysilane (VT) from BRB; and P2O5 from Avantor Performance Materials Poland S.A. (Gliwice, Poland)
- Toluene was degassed and dried by distilling it from P2O5 under an argon atmosphere.
2.2. Analyses
2.3. The Procedure for Synthesis of Polysiloxane Derivatives
2.4. The Procedure of Mixing Nanosilicate with a Modifier
2.5. Injection Molding
2.6. Preparation of Filament
2.7. 3D Printing (FDM)
3. Results and Discussion
3.1. Chemical Characterization of Modifiers
- VT:6STYR
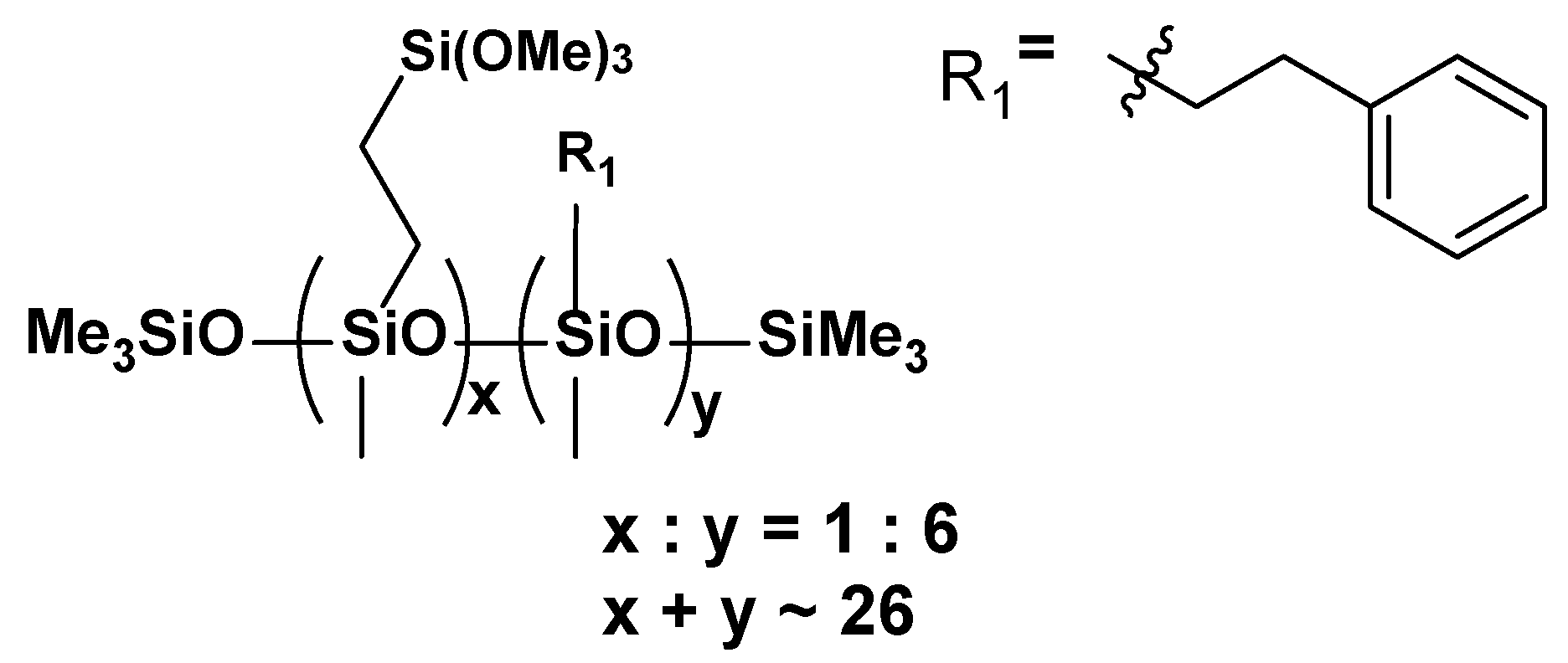
- VT:4STYR:2OD
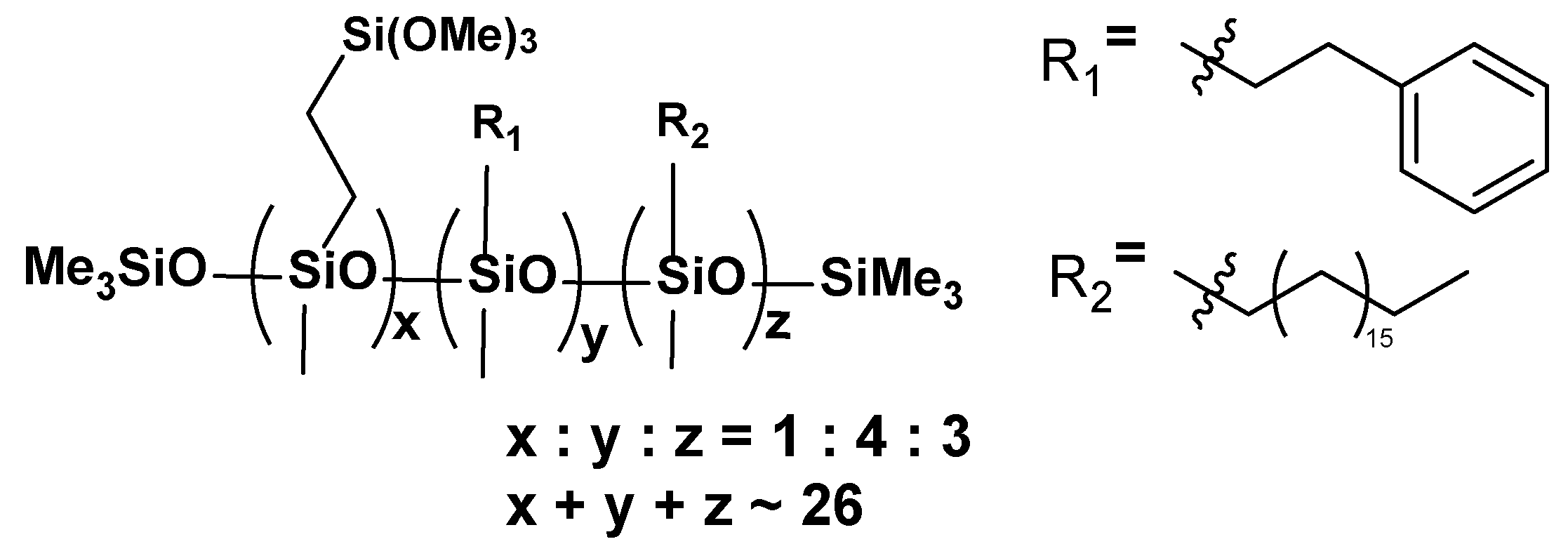
- VT:3STYR:3OD

- VT:2STYR:4OD
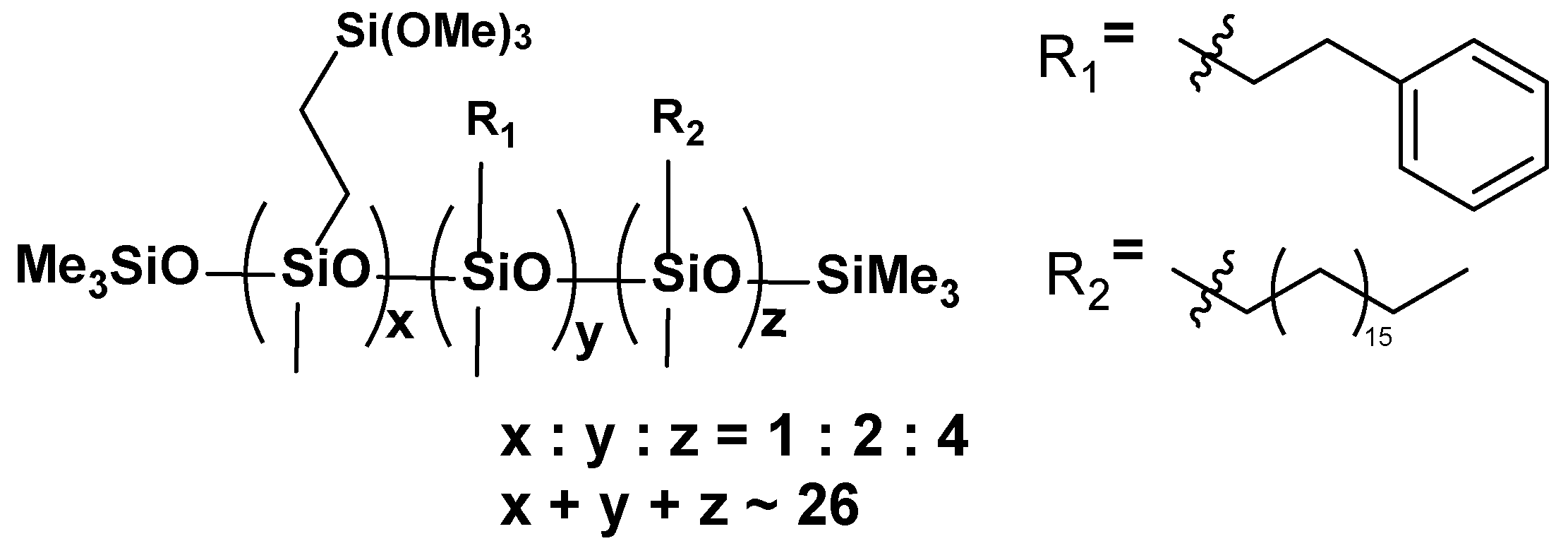
- VT:6OD
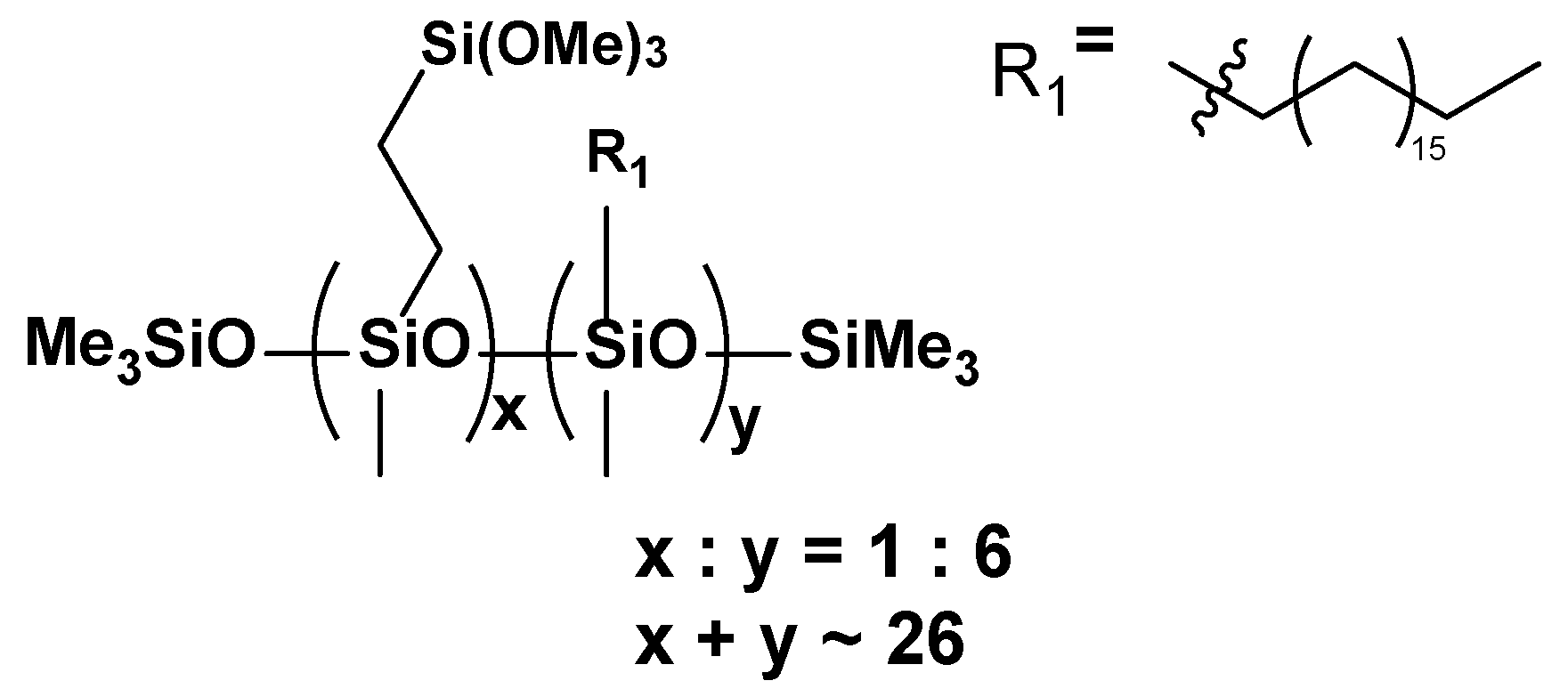
3.2. Thermal Analysis (TGA and DSC)
3.2.1. Thermogravimetric Analysis (TGA)
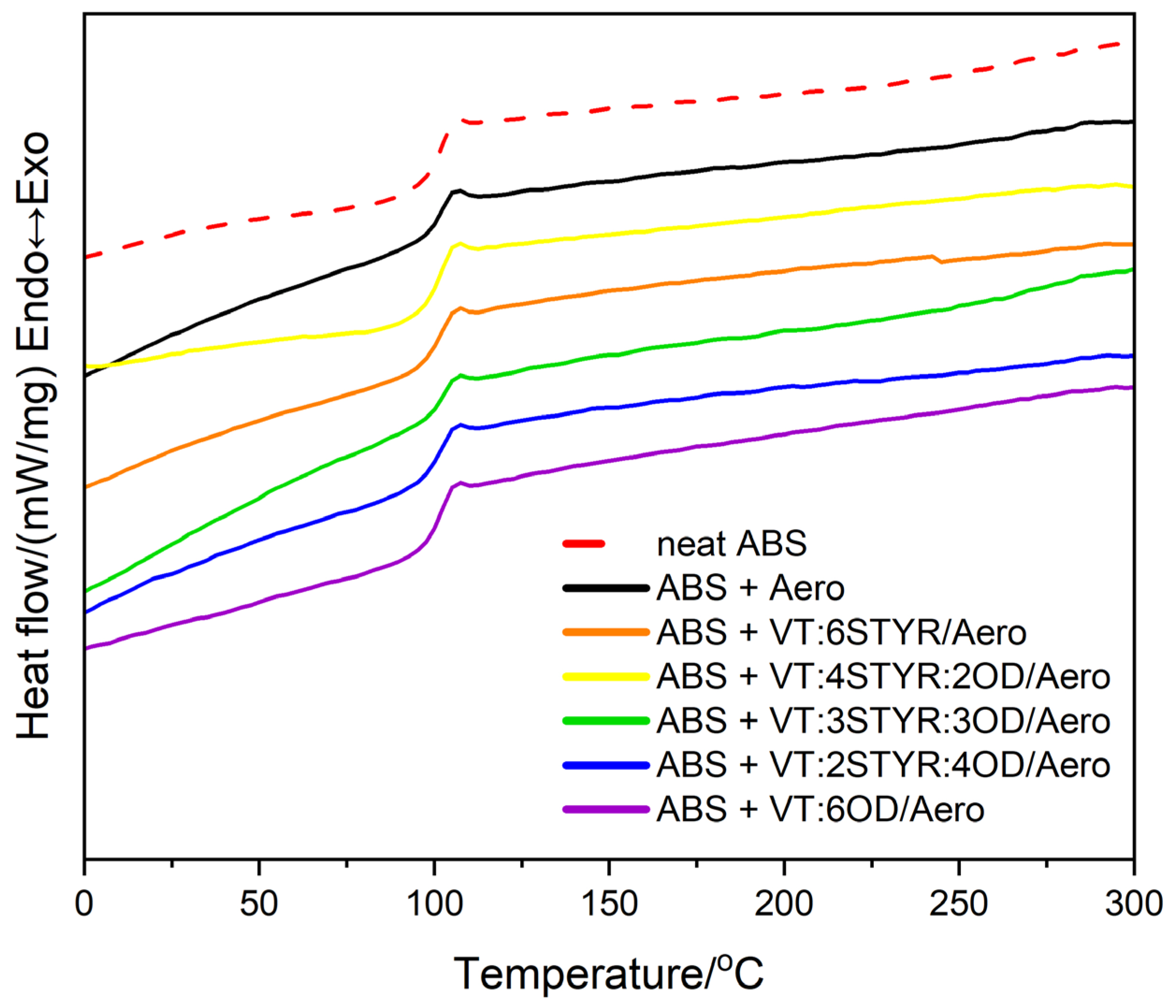
3.2.2. Differential Scanning Calorimetry (DSC)
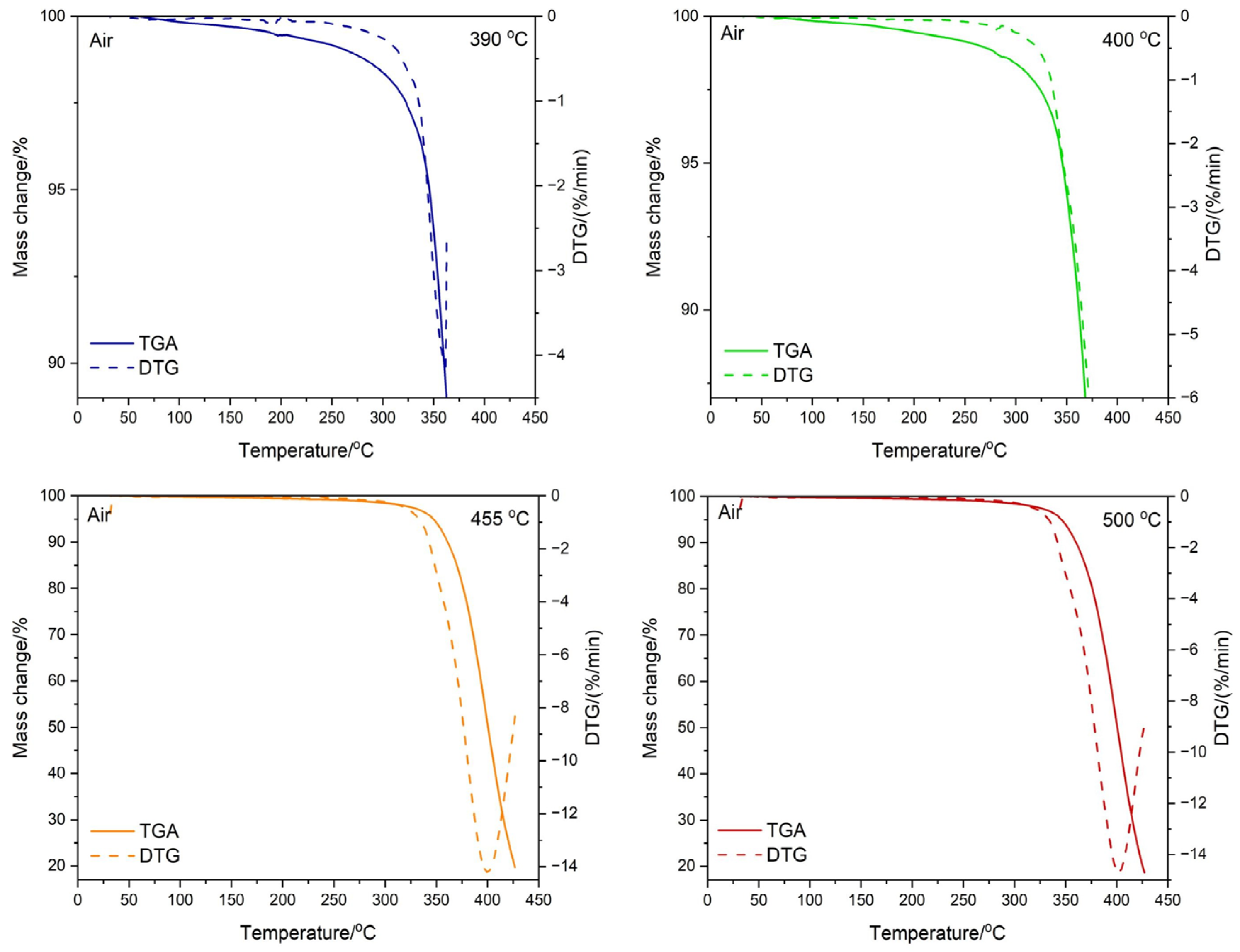
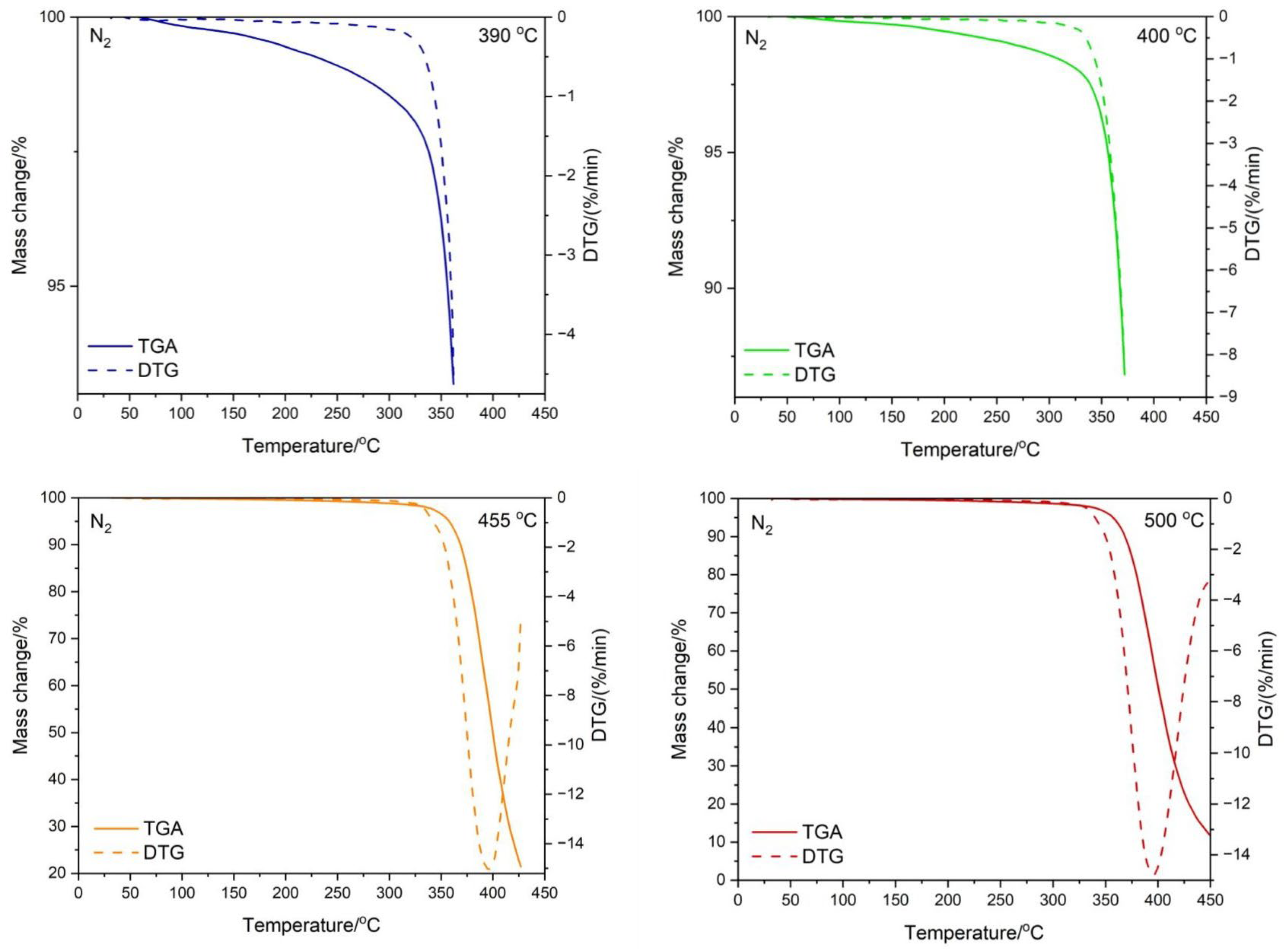
3.3. Testing the Composition of the Sample during Temperature Decomposition
3.4. Rheology
3.5. Microscopy
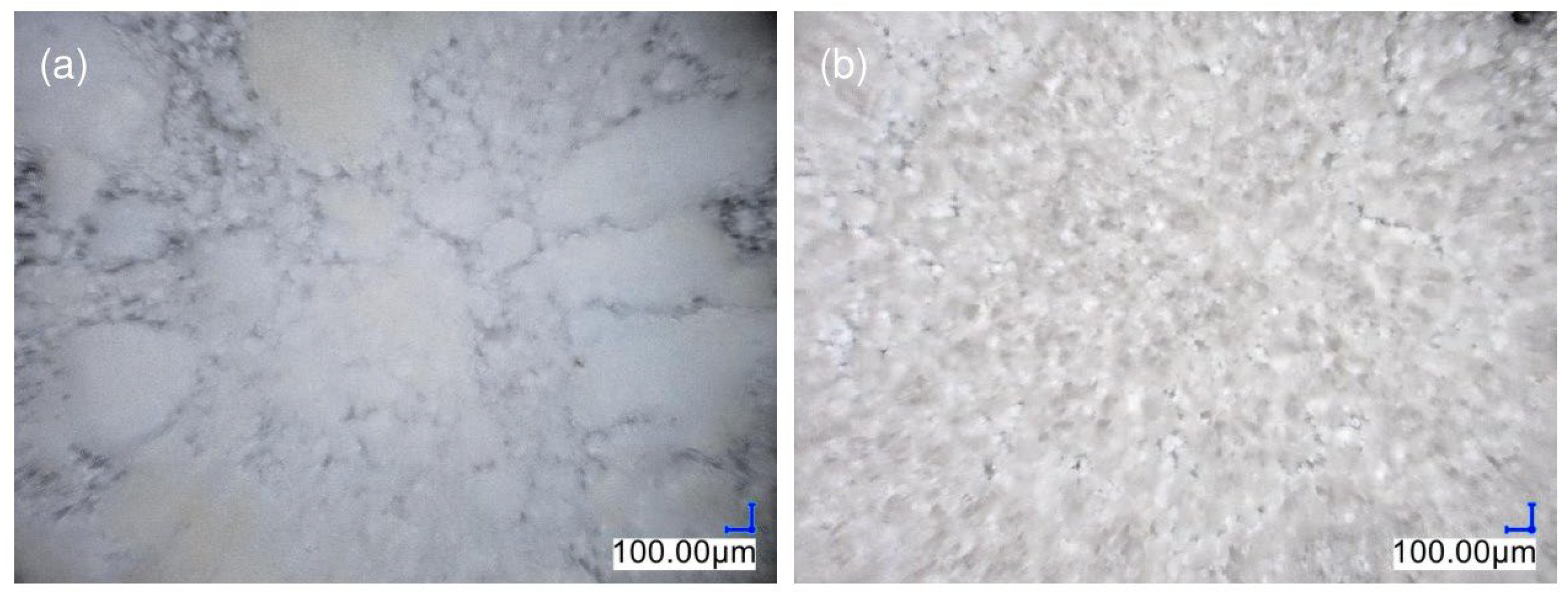
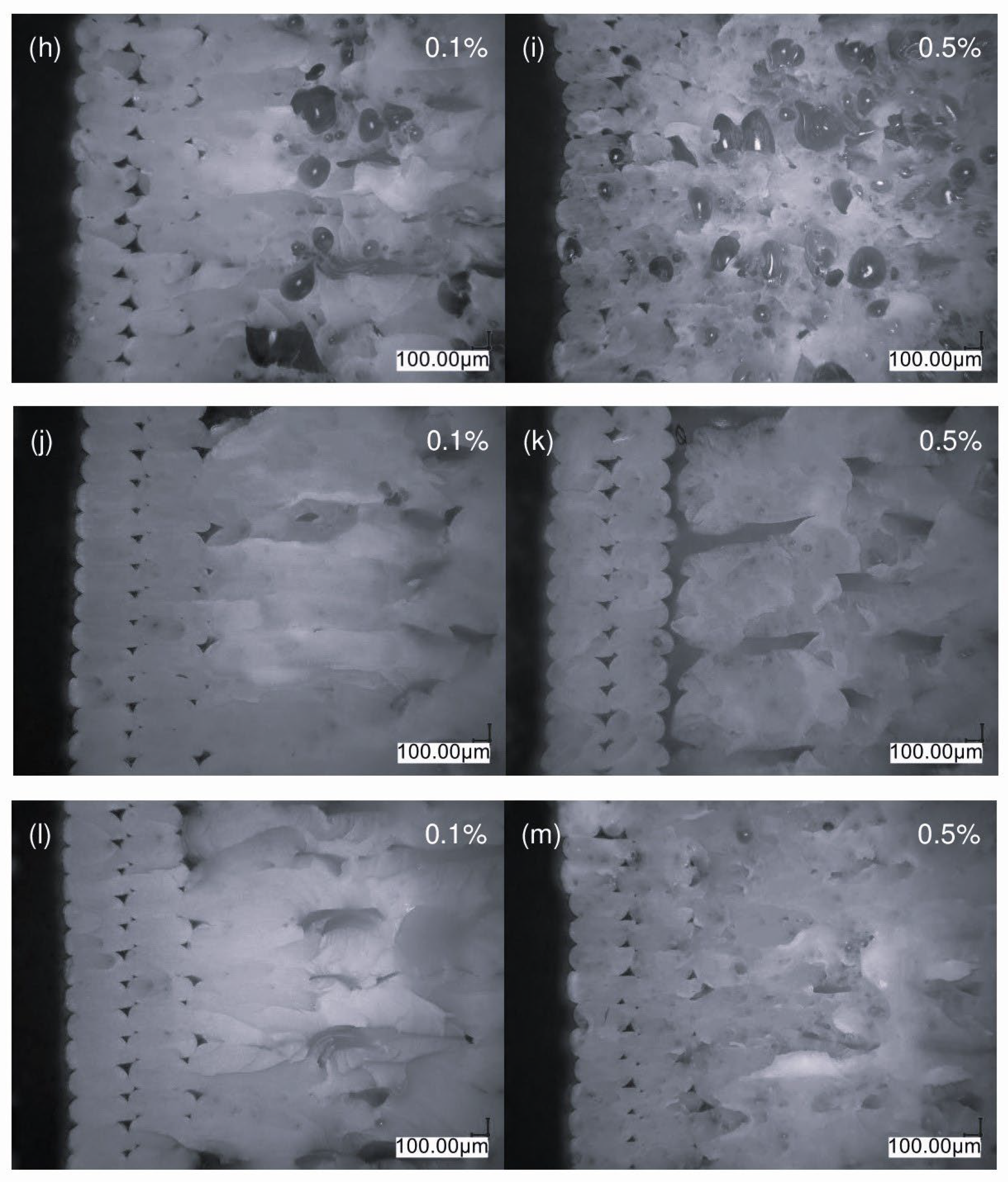
3.5.1. Optical Microscopy
3.5.2. SEM-EDS
3.6. Contact Angle Measurements
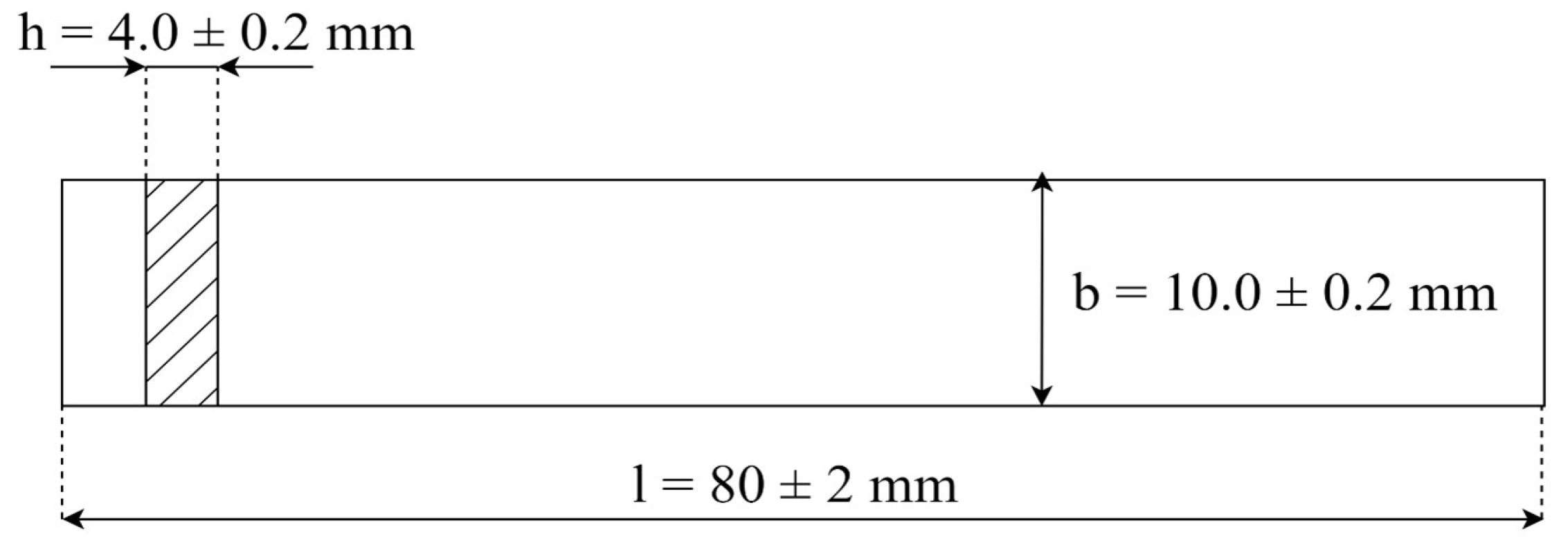
3.7. Mechanical Properties
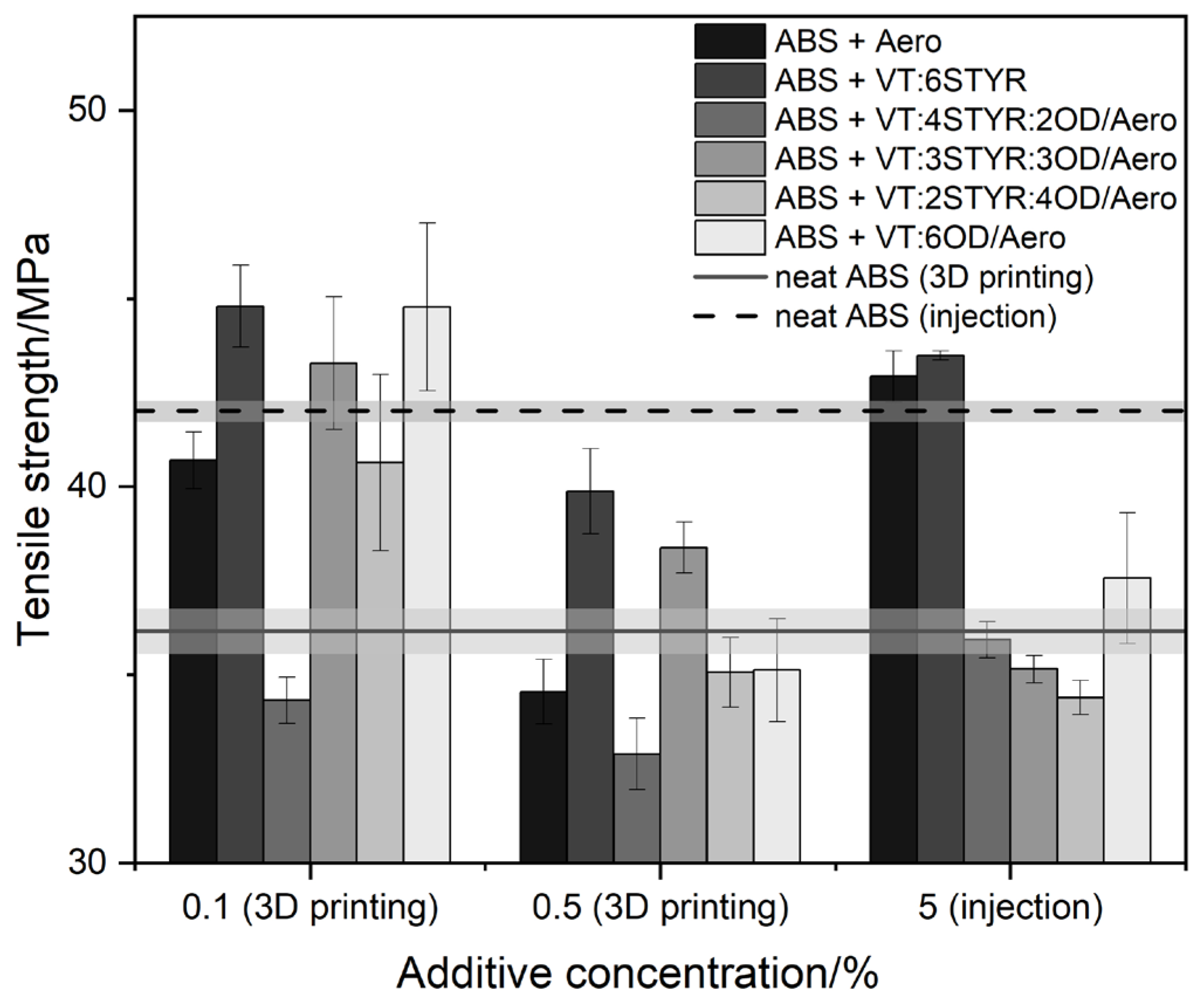
3.7.1. Tensile Strength
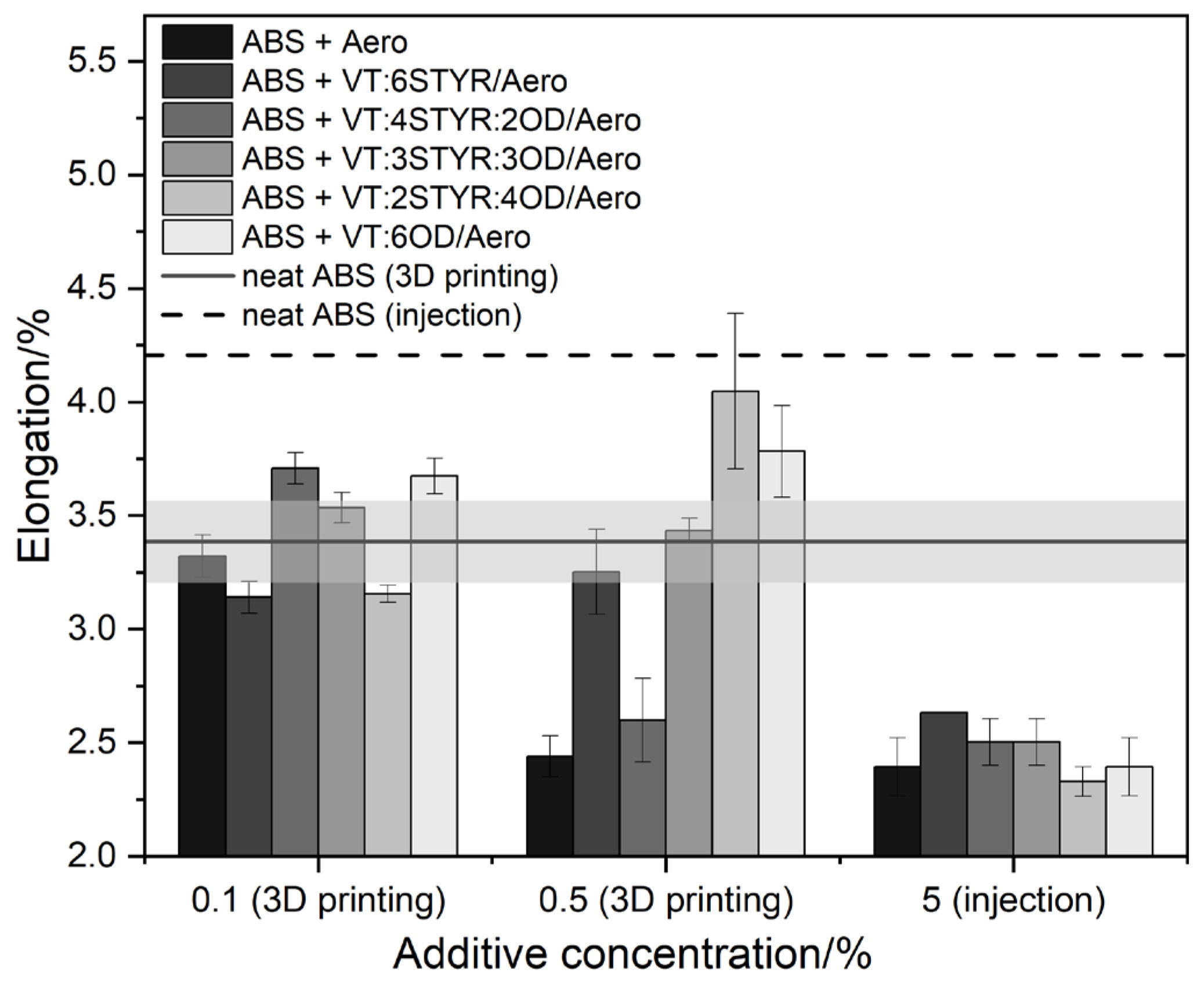
3.7.2. Elongation
3.7.3. Young’s Modulus
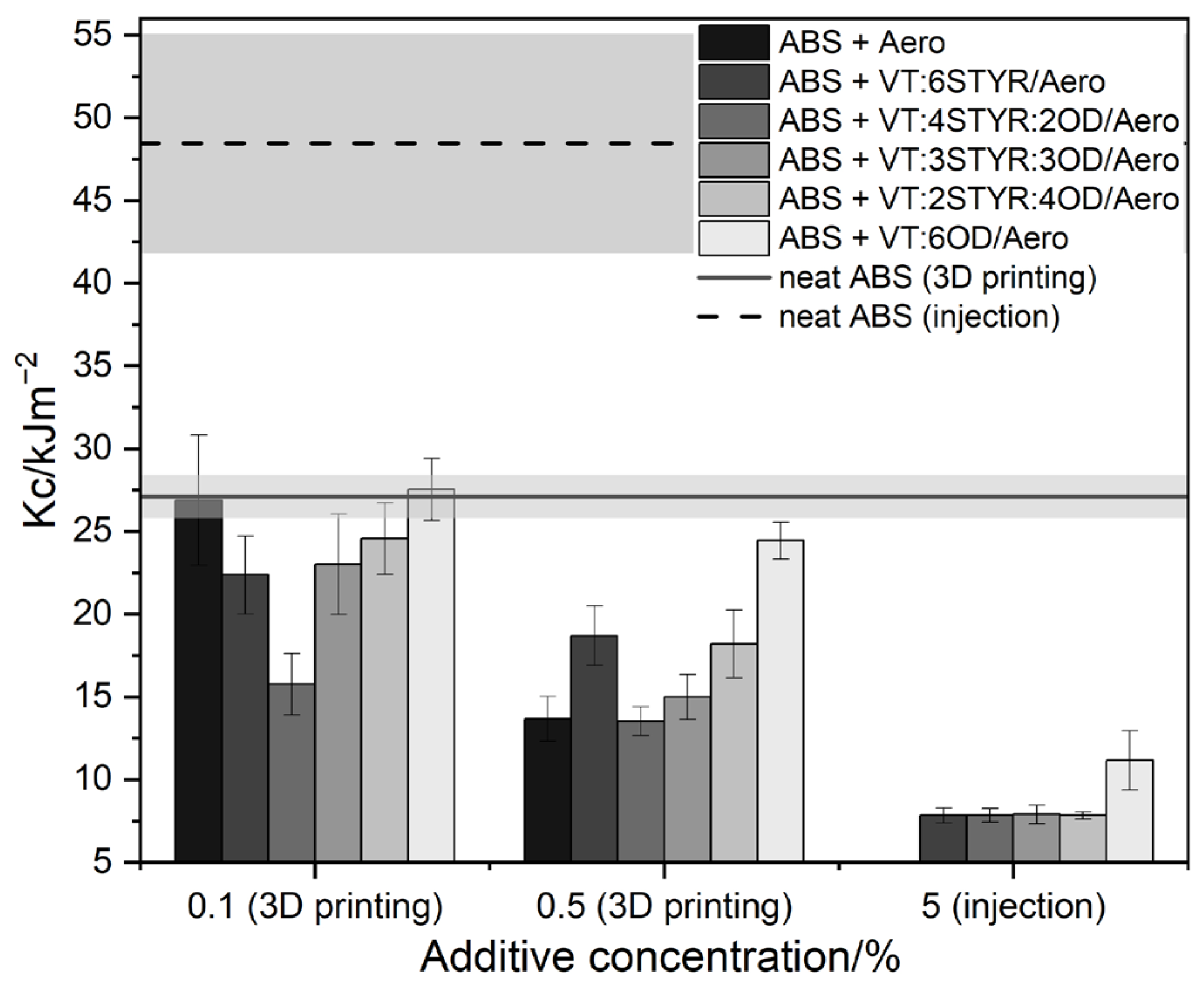
3.7.4. Impact Test and Hardness
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matias, E.; Rao, B. 3D Printing: On Its Historical Evolution and the Implications for Business. In Proceedings of the Portland International Conference on Management of Engineering and Technology (PICMET), Portland, OR, USA, 2–6 August 2015. [Google Scholar] [CrossRef]
- Hossain, M.A.; Zhumabekova, A.; Paul, S.C.; Kim, J.R. A Review of 3D Printing in Construction and Its Impact on the Labor Market. Sustainability 2020, 12, 8492. [Google Scholar] [CrossRef]
- Holt, C.; Edwards, L.; Keyte, L.; Moghaddam, F.; Townsend, B. Construction 3D Printing. In 3D Concrete Printing Technology; Butterworth-Heinemann: Oxford, UK, 2019; pp. 349–370. [Google Scholar] [CrossRef]
- Palinkas, I.; Pekez, J.; Desnica, E.; Rajic, A.; Nedelcu, D. Analysis and Optimization of UAV Frame Design for Manufacturing from Thermoplastic Materials on FDM 3D Printer. Mater. Plast. 2022, 58, 238–249. [Google Scholar] [CrossRef]
- Chambon, P.; Curran, S.; Huff, S.; Love, L.; Post, B.; Wagner, R.; Jackson, R.; Green, J. Development of a Range-Extended Electric Vehicle Powertrain for an Integrated Energy Systems Research Printed Utility Vehicle. Appl. Energy 2017, 191, 99–110. [Google Scholar] [CrossRef]
- Pugliese, R.; Beltrami, B.; Regondi, S.; Lunetta, C. Polymeric Biomaterials for 3D Printing in Medicine: An Overview. Ann. 3D Print. Med. 2021, 2, 100011. [Google Scholar] [CrossRef]
- Liaw, C.-Y.; Guvendiren, M. Current and Emerging Applications of 3D Printing in Medicine. Biofabrication 2017, 9, 024102. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.-J.; Lin, C.-L.; Tsai, J.-Y.; Lin, R.-M. Clinical Assessment of Customized 3D-Printed Wrist Orthoses. Appl. Sci. 2022, 12, 11538. [Google Scholar] [CrossRef]
- Vakharia, V.S.; Singh, M.; Salem, A.; Halbig, M.C.; Salem, J.A. Effect of Reinforcements and 3-D Printing Parameters on the Microstructure and Mechanical Properties of Acrylonitrile Butadiene Styrene (ABS) Polymer Composites. Polymers 2022, 14, 2105. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, G.; Lyu, H.; Ko, F. Experimental Investigation of Compression Properties of Composites with Printed Braiding Structure. Materials 2018, 11, 1767. [Google Scholar] [CrossRef]
- Mohamed, O.A.; Masood, S.H.; Bhowmik, J.L. Optimization of Fused Deposition Modeling Process Parameters: A Review of Current Research and Future Prospects. Adv. Manuf. 2015, 3, 42–53. [Google Scholar] [CrossRef]
- Valino, A.D.; Dizon, J.R.C.; Espera, A.H.; Chen, Q.; Messman, J.; Advincula, R.C. Advances in 3D Printing of Thermoplastic Polymer Composites and Nanocomposites. Prog. Polym. Sci. 2019, 98, 101162. [Google Scholar] [CrossRef]
- Yadav, D.K.; Srivastava, R.; Dev, S. Design & Fabrication of ABS Part by FDM for Automobile Application. Mater. Today Proc. 2020, 26, 2089–2093. [Google Scholar] [CrossRef]
- Aung, T.K.; Churei, H.; Tanabe, G.; Kinjo, R.; Togawa, K.; Li, C.; Tsuchida, Y.; Tun, P.S.; Hlaing, S.; Takahashi, H.; et al. Air Permeability, Shock Absorption Ability, and Flexural Strength of 3D-Printed Perforated ABS Polymer Sheets with 3D-Knitted Fabric Cushioning for Sports Face Guard Applications. Polymers 2021, 13, 1879. [Google Scholar] [CrossRef] [PubMed]
- Galatas, A.; Hassanin, H.; Zweiri, Y.; Seneviratne, L. Additive Manufactured Sandwich Composite/ABS Parts for Unmanned Aerial Vehicle Applications. Polymers 2018, 10, 1262. [Google Scholar] [CrossRef]
- Reggio, D.; Saviello, D.; Lazzari, M.; Iacopino, D. Characterization of Contemporary and Historical Acrylonitrile Butadiene Styrene (ABS)-Based Objects: Pilot Study for Handheld Raman Analysis in Collections. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 242, 118733. [Google Scholar] [CrossRef]
- Whelan, A. Polymer Technology Dictionary; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Kumar, V.; RamKumar, J.; Aravindan, S.; Malhotra, S.K.; Vijai, K.; Shukla, M. Fabrication and Characterization of ABS Nano Composite Reinforced by Nano Sized Alumina Particulates. Int. J. Plast. Technol. 2009, 13, 133–149. [Google Scholar] [CrossRef]
- Rosli, A.A.; Shuib, R.K.; Ishak, K.M.K.; Hamid, Z.A.A.; Abdullah, M.K.; Rusli, A. Influence of Bed Temperature on Warpage, Shrinkage and Density of Various Acrylonitrile Butadiene Styrene (ABS) Parts from Fused Deposition Modelling (FDM). In Proceedings of the 3rd International Postgraduate Conference on Materials, Minerals & Polymer (Mamip), Penang, Malaysia, 31 October–1 November 2019; AIP Publishing: Long Island, NY, USA, 2020. [Google Scholar] [CrossRef]
- Alhallak, L.M.; Tirkes, S.; Tayfun, U. Mechanical, Thermal, Melt-Flow and Morphological Characterizations of Bentonite-Filled ABS Copolymer. Rapid Prototyp. J. 2020, 26, 1305–1312. [Google Scholar] [CrossRef]
- Andrzejewski, J.; Misra, M. Development of Hybrid Composites Reinforced with Biocarbon/Carbon Fiber System. The Comparative Study for PC, ABS and PC/ABS Based Materials. Compos. B Eng. 2020, 200, 108319. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Maniadi, A.; Koudoumas, E.; Kenanakis, G.; Romanitan, C.; Tutunaru, O.; Suchea, M.; Kechagias, J. The Mechanical and Physical Properties of 3D-Printed Materials Composed of ABS-ZnO Nanocomposites and ABS-ZnO Microcomposites. Micromachines 2020, 11, 615. [Google Scholar] [CrossRef]
- Vidakis, N.; Moutsopoulou, A.; Petousis, M.; Michailidis, N.; Charou, C.; Papadakis, V.; Mountakis, N.; Dimitriou, E.; Argyros, A. Rheology and Thermomechanical Evaluation of Additively Manufactured Acrylonitrile Butadiene Styrene (ABS) with Optimized Tungsten Carbide (WC) Nano-Ceramic Content. Ceram. Int. 2023, 49, 34742–34756. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Velidakis, E.; Maniadi, A. Mechanical Properties of 3D-Printed ABS with Combinations of Two Fillers: Graphene Nanoplatelets, TiO2, ATO Nanocomposites, and Zinc Oxide Micro (ZnOm). In Recent Advances in Manufacturing Processes and Systems: Select Proceedings of RAM 2021; Springer Nature: Singapore, 2022; pp. 635–645. [Google Scholar]
- Kim, I.-J.; Kwon, O.-J.; Park, J.B.; Joo, H. Synthesis and Characterization of ABS/Silica Hybrid Nanocomposites. Curr. Appl. Phys. 2006, 6, e43–e47. [Google Scholar] [CrossRef]
- Meng, S.; He, H.; Jia, Y.; Yu, P.; Huang, B.; Chen, J. Effect of Nanoparticles on the Mechanical Properties of Acrylonitrile-Butadiene-Styrene Specimens Fabricated by Fused Deposition Modeling. J. Appl. Polym. Sci. 2017, 134, 44470. [Google Scholar] [CrossRef]
- Huang, B.; He, H.; Meng, S.; Jia, Y. Optimizing 3D Printing Performance of Acrylonitrile-Butadiene-Styrene Composites with Cellulose Nanocrystals/Silica Nanohybrids. Polym. Int. 2019, 68, 1351–1360. [Google Scholar] [CrossRef]
- Brząkalski, D.; Przekop, R.E.; Sztorch, B.; Jakubowska, P.; Jałbrzykowski, M.; Marciniec, B. Silsesquioxane Derivatives as Functional Additives for Preparation of Polyethylene-Based Composites: A Case of Trisilanol Melt-Condensation. Polymers 2020, 12, 2269. [Google Scholar] [CrossRef] [PubMed]
- Brząkalski, D.; Sztorch, B.; Frydrych, M.; Pakuła, D.; Dydek, K.; Kozera, R.; Boczkowska, A.; Marciniec, B.; Przekop, R.E. Limonene Derivative of Spherosilicate as a Polylactide Modifier for Applications in 3D Printing Technology. Molecules 2020, 25, 5882. [Google Scholar] [CrossRef]
- ISO 1133; Plastics—Determination of the Melt Mass-Flow Rate (MFR) and Melt Volume-Flow Rate (MVR) of Thermoplastics. International Organization for Standardization: Geneva, Switzerland, 2005.
- ISO 179; Plastics—Determination of Charpy Impact Properties. International Organization for Standardization: Geneva, Switzerland, 2010.
- ISO 527; Plastics—Determination of Tensile Properties. International Organization for Standardization: Geneva, Switzerland, 2019.
- Sztorch, B.; Brząkalski, D.; Głowacka, J.M.; Pakuła, D.J.; Frydrych, M.; Przekop, R.E. Trimming flow, plasticity, and mechanical proper-ties by cubic silsesquioxane chemistry. Sci. Rep. 2023, 13, 14156. [Google Scholar] [CrossRef]
- Dawoud, M.; Taha, I.; Ebeid, S.J. Mechanical Behaviour of ABS: An Experimental Study Using FDM and Injection Moulding Techniques. J. Manuf. Process. 2016, 21, 39–45. [Google Scholar] [CrossRef]











| Code | Amount of VT/g | Amount of STYR/g | Amount of OD/g |
|---|---|---|---|
| VT:6STYR | 11.0 | 46.5 | - |
| VT:4STYR:2OD | 11.0 | 31.0 | 37.6 |
| VT:3STYR:3OD | 11.0 | 23.2 | 56.4 |
| VT:2STYR:4OD | 11.0 | 15.5 | 75.1 |
| VT:6OD | 11.0 | - | 112.6 |
| Properties | Parameters |
|---|---|
| Maximum dispensing time | 15.0 s |
| Dispensing volume | 31.00 cm3 |
| Holding time | 7 s |
| Cooling time | 35.00 s |
| Holding pressure | 500.0–1100.0 bar |
| Mold temperature | 70 °C |
| Dosing efficiency | 0.71 cm3/s |
| Properties | Parameters |
|---|---|
| Zone 1 temperature | 215 °C |
| Zone 2 temperature | 240 °C |
| Zone 3 temperature | 230 °C |
| Filling zone temperature | 90 °C |
| Voltage | 20–25 V |
| Current | 1–1.5 A |
| Properties | Parameters |
|---|---|
| Nozzle diameter | 0.4 mm |
| Extruder temperature | 225 °C |
| Bed temperature | 105 °C |
| Layer height | 0.2 mm |
| Bottom and top layer style | linear |
| Fill style | linear |
| Infill density | 100% |
| Printing speed | 60 mm/s |
| Code | 1% of Weight Loss/°C | 5% of Weight Loss/°C | Onset Temperature/°C | Temperature at the Maximum Rate of Mass Loss/°C |
|---|---|---|---|---|
| neat ABS | 235.7 | 346.6 | 363.7 | 392.6 |
| ABS + 0.1%Aero | 205.5 | 342.8 | 364.0 | 396.3 |
| ABS + 0.1% VT:6STYR/Aero | 191.9 | 341.2 | 365.6 | 397.1 |
| ABS + 0.1% VT:4STYR:2OD/Aero | 217.2 | 342.1 | 363.9 | 395.0 |
| ABS + 0.1% VT:3STYR:3OD/Aero | 207.8 | 341.0 | 366.9 | 399.7 |
| ABS + 0.1% VT:2STYR:4OD/Aero | 187.3 | 340.7 | 365.3 | 397.1 |
| ABS + 0.1% VT:6OD/Aero | 187.1 | 340.6 | 364.5 | 396.3 |
| Code | 1% of Weight Loss/°C | 5% of Weight Loss/°C | Onset Temperature/°C | Temperature at the Maximum Rate of Mass Loss/°C |
|---|---|---|---|---|
| neat ABS | 212.9 | 350.2 | 361.9 | 384.3 |
| ABS + 0.1%Aero | 200.6 | 349.7 | 367.9 | 393.2 |
| ABS + 0.1% VT:6STYR/Aero | 209.6 | 351.3 | 365.1 | 389.7 |
| ABS + 0.1% VT:4STYR:2OD/Aero | 196.3 | 350.1 | 365.4 | 390.0 |
| ABS + 0.1% VT:3STYR:3OD/Aero | 191.3 | 349.1 | 370.3 | 400.1 |
| ABS + 0.1% VT:2STYR:4OD/Aero | 200.2 | 352.5 | 366.7 | 391.5 |
| ABS + 0.1% VT:6OD/Aero | 205.8 | 353.8 | 368.5 | 393.2 |
| Residual Mass/°C | ||
|---|---|---|
| Conditions | N2 | Air |
| 390 °C | 92.75 | 88.86 |
| 400 °C | 86.40 | 84.35 |
| 455 °C | 21.27 | 18.60 |
| 500 °C | 5.14 | 17.46 |
| Code | Contact Angle/° | ||||||
|---|---|---|---|---|---|---|---|
| Concentration of Additives/% | |||||||
| - | 0.1 | 0.25 | 0.5 | 1 | 1.5 | 2.5 | |
| neat ABS | 70.9 ± 3.2 | - | - | - | - | - | - |
| ABS + Aero | - | 86.2 ± 1.3 | 89.6 ±1.4 | 93.9 ± 2.0 | 89.8 ± 3.5 | 81.2 ± 0.9 | 91.7 ± 2.4 |
| ABS + VT:6STYR/Aero | - | 76.5 ± 0.5 | 76.6 ±0.6 | 74.9 ± 2.0 | 79.2 ± 0.5 | 86.4 ± 3.1 | 92.9 ± 2.0 |
| ABS + VT:4STYR:2OD/Aero | - | 90.5 ± 0.5 | 86.8 ± 1.2 | 87.4 ± 3.7 | 84.8 ± 3.9 | 85.0 ± 0.5 | 92.9 ± 1.9 |
| ABS + VT:3STYR:3OD/Aero | - | 86.6 ± 2.5 | 84.1 ± 2.1 | 88.6 ± 2.6 | 82.1 ± 0.8 | 73.0 ± 1.6 | 71.7 ± 1.2 |
| ABS + VT:2STYR:4OD/Aero | - | 85.3 ± 0.8 | 85.5 ± 3.2 | 83.2 ± 1.2 | 82.8 ± 1.4 | 72.1 ± 2.6 | 79.1 ± 3.3 |
| ABS + VT:6OD/Aero | - | 74.9 ±0.5 | 77.4 ± 1.8 | 76.6 ± 1.1 | 79.5 ± 2.6 | 77.2 ± 0.4 | 79.1 ± 2.6 |
| Code | Shore Hardness | ||||||
|---|---|---|---|---|---|---|---|
| Concentration of Additives/% | |||||||
| - | 0.1 | 0.25 | 0.5 | 1 | 1.5 | 2.5 | |
| neat ABS | 59 | - | - | - | - | - | - |
| ABS + Aero | - | 71 | 66 | 71 | 71 | 66 | 63 |
| ABS + VT:6STYR/Aero | - | 68 | 66 | 72 | 73 | 66 | 68 |
| ABS + VT:4STYR:2OD/Aero | - | 72 | 74 | 79 | 74 | 73 | 74 |
| ABS + VT:3STYR:3OD/Aero | - | 75 | 77 | 79 | 78 | 77 | 79 |
| ABS + VT:2STYR:4OD/Aero | - | 76 | 69 | 75 | 76 | 72 | 78 |
| ABS + VT:6OD/Aero | - | 78 | 68 | 76 | 77 | 78 | 77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sztorch, B.; Konieczna, R.; Pakuła, D.; Frydrych, M.; Marciniec, B.; Przekop, R.E. Preparation and Characterization of Composites Based on ABS Modified with Polysiloxane Derivatives. Materials 2024, 17, 561. https://doi.org/10.3390/ma17030561
Sztorch B, Konieczna R, Pakuła D, Frydrych M, Marciniec B, Przekop RE. Preparation and Characterization of Composites Based on ABS Modified with Polysiloxane Derivatives. Materials. 2024; 17(3):561. https://doi.org/10.3390/ma17030561
Chicago/Turabian StyleSztorch, Bogna, Roksana Konieczna, Daria Pakuła, Miłosz Frydrych, Bogdan Marciniec, and Robert E. Przekop. 2024. "Preparation and Characterization of Composites Based on ABS Modified with Polysiloxane Derivatives" Materials 17, no. 3: 561. https://doi.org/10.3390/ma17030561
APA StyleSztorch, B., Konieczna, R., Pakuła, D., Frydrych, M., Marciniec, B., & Przekop, R. E. (2024). Preparation and Characterization of Composites Based on ABS Modified with Polysiloxane Derivatives. Materials, 17(3), 561. https://doi.org/10.3390/ma17030561











